Highlights
-
•
With PSMA-PET we observed high rates of metastatic PrCa response to RT.
-
•
The regulation of PSMA expression by RT remains unclear.
-
•
We found that PSMA expression in PrCa tumors is highly heterogeneous.
-
•
Loss of PSMA expression was observed in human PrCa models surviving high-dose RT.
-
•
This work suggests cautious interpretation of PSMA-PET results in irradiated metastatic sites and need for further studies.
Keywords: PSMA-PET, FOLH1 expression, Immunohistochemistry, qPCR, Radio-resistance, Tumor heterogeneity
Abbreviations: ADT, Androgen Deprivation Therapy; AMACR, Alpha-Methylacyl-CoA Racemase; ARAT, Androgen Receptor Axis-Targeted; BED, Biological Effective Dose; CRPC, Castration Resistant Prostate Cancer; FOLH1, Folate Hydrolase 1; H&E, Hematoxylin and Eosin; H-Score, Histologic Score; HSPC, Hormone Sensitive Prostate Cancer; IHC, Immunohistochemistry; LHRH, Luteinizing Hormone Releasing Hormone; mCRPC, Metastatic Castration Resistant Prostate Cancer; MDRT, Metastasis Directed Radiotherapy; mRNA, Messenger Ribonucleic Acid; NH, Hormone Naïve; PET, Positron Emission Tomography; P-H3, Phosphorylated Histone-H3; PrCa, Prostate Cancer; PSA, Prostate Specific Antigen; PSMA, Prostate Specific Membrane Antigen; qPCR, Quantitative Polymerase Chain Reaction; Rec, Recurrence; RP, Radical Prostatectomy; RT, Radiation Therapy; SUV, Standardized Uptake Value
Abstract
Background
Prostate Specific Membrane Antigen (PSMA) – positron emission tomography (PET) guides metastasis-directed radiotherapy (MDRT) in prostate cancer (PrCa). However, its value as a treatment response assessment tool after MDRT remains unclear. Importantly, there is limited understanding of the potential of radiotherapy (RT) to alter PSMA gene (folate hydrolase 1; FOLH1) expression.
Methodology
We reviewed a series of 11 men with oligo-metastatic PrCa (25 metastasis sites) treated with MDRT before re-staging with 18F-DCFPyL (PSMA) PET upon secondary recurrence. Acute effects of RT on PSMA protein and mRNA levels were examined with qPCR and immunoblotting in human wild-type androgen-sensitive (LNCap), castrate-resistant (22RV1) and castrate-resistant neuroendocrine (PC3 and DU145) PrCa cell lines. Xenograft tumors were analyzed with immunohistochemistry. Further, we examined PSMA expression in untreated and irradiated radio-resistant (RR) 22RV1 (22RV1-RR) and DU145 (DU145-RR) cells and xenografts selected for survival after high-dose RT.
Results
The majority of MDRT-treated lesions showed lack of PSMA-PET/CT avidity, suggesting treatment response even after low biological effective dose (BED) MDRT. We observed similar high degree of heterogeneity of PSMA expression in both human specimens and in xenograft tumors. PSMA was highly expressed in LNCap and 22RV1 cells and tumors but not in the neuroendocrine PC3 and DU145 models. Single fraction RT caused detectable reduction in PSMA protein but not in mRNA levels in LNCap cells and did not significantly alter PSMA protein or mRNA levels in tissue culture or xenografts of the other cell lines. However, radio-resistant 22RV1-RR cells and tumors demonstrated marked decrease of PSMA transcript and protein expression over their parental counterparts.
Conclusions
PSMA-PET may be a promising tool to assess RT response in oligo-metastatic PrCa. However, future systematic investigation of this concept should recognize the high degree of heterogeneity of PSMA expression within prostate tumors and the risk for loss of PSMA expression in tumor surviving curative courses of RT.
Introduction
Positron emission tomography (PET) using low-molecular-weight ligands of prostate-specific membrane antigen (PSMA) provides significant improvements in prostate cancer (PrCa) detection over conventional imaging with computed tomography (CT) and bone scan (BS) [1]. Studies utilizing PSMA-PET in the initial staging or at biochemical failure have demonstrated superior sensitivity and specificity compared to conventional imaging [[2], [3], [4], [5], [6]]. PSMA is the product of folate hydrolase 1 (FOLH1) gene, a type II transmembrane protein expressed in prostate, kidney, small intestine, as well central and peripheral nervous system [7]. PSMA is suggested to have metabolic roles in the nervous and gastrointestinal systems [[8], [9]]. Studies showed that PSMA expression is higher in malignant prostate glands compared to normal cells [10]; however, potential contribution of PSMA to PrCa oncogenesis is unclear. Nonetheless, development of neuroendocrine features in PrCa, an aggressive state with poor prognosis [11], is associated inversely with PSMA expression [12].
PSMA expression was investigated in a limited number of studies, which showed significant heterogeneity in its expression, raising questions about its reliability as a disease marker. Most reports showed PSMA staining in most PrCa patients [[13], [14]]; however, variation amongst patients is significant. Further, Mannweiler et al. [14] found discordance in the percentage of the tumour expressing PSMA between primary prostate tumor and metastatic tissues from the same patients. The proportion of tumor expressing PSMA appears to have clinical consequences, as Ferraro et al. [13] found that high percentage of PSMA-negative tumour regions correlated strongly with the probability of negative PSMA-PET, despite high PSA values.
Today, PSMA-PET is regarded as the preferred imaging modality for the detection of recurrent disease, but its specific utility in response assessment after local therapy is less clear. Few reports address PSMA kinetics post radiotherapy. In a small case series of 5 oligo-metastatic PrCa patients treated with metastasis-directed RT (MDRT), high rates of SUV-based response were recorded [15]. Of 18 lesions reported, only 2 showed progression. Despite the small sample size, the authors noted a relationship between decrease in SUVmax and time interval between RT and PSMA.
Regulation of PSMA expression is not well understood. Early pre-clinical studies showed that treatment of PrCa cell lines with androgens suppresses PSMA expression in an androgen receptor-dependent manner [16]. Concordantly, in vitro expression of PSMA was increased in response to commonly used androgen-receptor axis-targeted treatments such as enzalutamide [16] and abiraterone [[17], [18]]. The potential of cytotoxic therapy to regulate PSMA expression had not been investigated until recently. While this study was prepared for publication, Sheehan et al. [19] reported evidence that PSMA expression can be upregulated by some DNA damaging agents, including topoisomerase-2 inhibitors (daunorubicin) and ionizing radiation. They detected upregulation of PSMA protein expression in castration-sensitive LNCap cells and castration-resistant LNCap95 and 22RV1 cells and PDX tumor models by low BED irradiation but no significant change at the mRNA (FOLH1).
In this report, we show a series of 11 patients who received RT to metastatic lesions (MDRT) detected using either 18F-DCFPyL (PSMA) PET and/or conventional imaging followed by PSMA-PET after secondary biochemical progression. Elimination of PET avidity in the majority of cases suggests either, i) a high rate of metastatic PrCa response to RT (supported by the associated CT findings), and/or ii) that RT could potentially down-regulate PSMA/FOLH1 levels in treated tissues. Given the significant challenge of obtaining tissue from sites of metastatic disease, we pursued a preclinical analysis of the regulation of FOLH1/PSMA expression by RT. FOLH1/PSMA transcript and protein levels were examined in established human parental and radio-resistant PrCa cell lines and xenografts after RT to elucidate the effects of RT at the cellular and tumor level.
Methods
Patients
Eleven patients with oligo-metastatic PrCa (five or less metastatic sites) participating in a Hamilton Integrated Health Research Board (HiREB)-approved PSMA-PET registry were included in this study. Patients were treated with MDRT to one or more oligometastatic lesions, and subsequent biochemical failure had imaging with 18F-DCFPyL-PET performed 5–72 months after radiotherapy (Table 1). 18F-DCFPyL (333 MBq [9 mCi]) was administered intravenously 60+/-10 min before imaging. PET/CT was performed using a 64-slice Discovery RX scanner (GE Healthcare). All cases reported in this study had their baseline conventional / PET imaging and post-MDRT PSMA-PET scans reviewed by two nuclear medicine experts (the reporting physician and Dr. KZ co-author). For each PET/CT study acquired post-radiation therapy, each site of irradiated disease was evaluated for 18F-DCFPyL avidity (SUVmax). In addition, the PET/CT scans were compared with conventional imaging available prior to MDRT. In patients who underwent both baseline and secondary or tertiary (2nd/3rd) recurrence PSMA-PET/CT around MDRT, the change in avidity and morphology of each radiated lesion between the PET studies was determined by both CT and PET images and the presence of new lesions was documented.
Table 1.
Cohort of patients treated with MDRT and subsequent PSMA-PET. Ages at first MDRT, initial diagnoses, site(s) of metastases, associated MDRT dose/fractionation (and BED1.5), concurrent systemic therapy, interval between MDRT and PSMA-PET scan (in months, for each course), interpretation of response based on PSMA-PET, overall pattern of recurrence at PSMA, and subsequent management synopsis are given.
|
Patient Number (Age at MDRT:years) |
Initial Diagnosis (Dx) and treatment [Dx before initial MDRT] (Dx at further progression) |
Sites of Metastases |
Radiotherapy (MDRT) Regimen (BED1.5) |
Concurrent Systemic therapy at time of PSMA-PET |
Time from RT to PSMA (in mo) | Response (SUVmax) |
Order of Imaging modalities PSMA Findings [1st line metastasis management] (2nd line metastasis management]) |
|---|---|---|---|---|---|---|---|
| 1 (68) | mCRPC, GG5, PSA 12, cT3b ADT + ARAT [mCRPC] |
|
|
Yes ADT + ARAT |
7.6 7.6 7.6 |
|
Baseline BS/CT &: [MDRT] 2nd Rec. PSMA-PET &: Oligo-metastatic recurrence; (MDRT) |
| 2 (71) | mHSPC, GG4, PSA 40, cT3b Pelvic RT + ADT [mHSPC] |
|
|
No | 72.2 |
|
Baseline BS/CT: [MDRT + ADT × 36mo] 2nd Rec PSMA-PET: Mixed local prostate, regional and oligometastatic recurrence; (MDRT + ADT) |
| 3 (66) | Localized PrCa GG3, PSA 5.5, pT3a, RP: positive margin Salvage RT [mHSPC] |
|
|
No | 17.9 47.3 27 27 27 |
|
Baseline CT/BS [MDRT] 2nd Rec. PSMA-PET: Oligo-metastatic recurrence, [MDRT] 3rd Rec. PSMA-PET: Oligo-metastatic recurrence $ (MDRT, ADT + MDRT) |
| 4 (77) | Localized PrCa GG3, PSA 10, pT2b RP, Salvage RT [HN-mHSPC] |
|
|
No | 21.2 |
|
Baseline BS-CT: [MDRT] 2nd Rec. PSMA-PET: Poly-metastatic recurrence; (palliative RT and ADT) |
| 5 (69) | Localized PrCa, GG3, PSA 7.6, cT2b RT [HN-HSPC] (mHSPC) (mCRPC) |
|
|
No | 11.4 11.4 11.4 |
|
Baseline BS/CT: [MDRT] 2nd. Rec. PSMA-PET: Oligo-metastatic recurrence. Later: Poly-metastatic recurrence (MDRT, ADT + ARAT) |
| 6 (69) | Localized PrCa GG3, PSA 7.4 RT [HN-HSPC] (mHSPC) |
|
|
No | 8.4 8.4 8.4 8.4 |
|
Baseline BS/CT and PSMA-PET: Oligometastatic recurrence; [MDRT] 2nd Rec. PSMA-PET: Poly-metastatic recurrence; (MDRT, ADT + ARAT) |
| 7 (65) | Localized PrCa GG5, PSA 51, RT + ADT [HN-HSPC] (mHSPC) |
|
|
No | 15.5 |
|
Baseline BS/CT and PSMA-PET: Oligometastatic recurrence; [MDRT] 2nd Rec. PSMA-PET: Poly-metastatic recurrence |
| 8 (69) | Localized PrCa GG2, PSA 11.2, cT2c RP, Salvage RT, [CRPC] (mCRPC) |
|
|
Yes ADT+ ARAT |
6.2 6.2 |
|
Baseline BS/CT &: [MDRT] 2nd Rec. PSMA-PET&: Poly-metastatic recurrence confined to paraaortic nodes; (MDRT) |
| 9 (74) | Localized PrCa GG3, PSA 12.7, pT3a RP, Salvage RT [CRPC] (mCRPC) |
|
|
Yes ADT+ ARAT |
5.3 5.6 5.6 |
|
Baseline BS/CT &: 2nd Rec. PSMA-PET &: Oligometastatic recurrence. 3rd Rec. PSMA-PET &: Oligometastatic recurrence; (repeat MDRT) |
| 10 (70) | mHSPC, GG2, PSA 178, cT2b MDRT + ADT [mHSPC] |
|
|
ADT | 23.6 |
|
Baseline BS/CT: [MDRT + brief course ADT] 2nd Rec. PSMA-PET: Oligometastatic recurrence. (ADT) |
| 11 (69) | Localized PrCa GG3, PSA 5.2, cT2a RT [mHSPC] |
|
|
No | 15.6 15.6 |
|
Baseline BS/CT: [MDRT] 2nd Rec. PSMA-PET: Poly-metastatic recurrence; (ADT) |
RP: radical prostatectomy; RT: radiotherapy; ADT: androgen deprivation therapy; ARAT: androgen receptor axis therapies; 2nd: secondary; 3rd: tertiary; Rec.: recurrence; m: metastatic; NH: hormone naïve; HSPC: hormone sensitive prostate cancer; CRPC: castrate-resistant prostate cancer.
*No uptake above background.
**MDRT given after 2nd PSMA scan.
$Metastatic lesion response detected with PSMA-PET at time of secondary (2nd) or tertiary (3rd) biochemical recurrence, one lesion showed initially response (2nd Rec. PSMA-PET) but avidity at tertiary progression (3rd Rec. PSMA-PET).
&: indicates cases that were on systemic therapy (ADT + ARAT) at the time of baseline and repeated conventional imaging or PSMA-PET.
Cells
Wildtype LNCap, PC3, 22RV1 and DU145 PrCa cell lines were purchased from ATCC. Radiation-resistant 22RV1-RR and DU145-RR were generated by serially treating wild-type DU145 and 22RV1 cells with 2 Gy daily fractions (Monday-Friday) to a total of 118 Gy, as described [20]. Treatments: Cells were grown in culture to 70–80 % confluence, were irradiated (RT) 0, 2 or 8 Gy with a clinical linear accelerator and parallel-opposed beams (6MV), using established methodology (as described [21]), followed by incubation for 24 or 48 h post-treatment for qPCR and immunoblotting experiments, respectively.
Xenografts
Using protocols approved by the McMaster University Animal Research Ethics Board, cells (1 × 106/100 μL), suspended in 50:50 mixture of ice-cold Matrigel: phosphate, were injected subcutaneously into the flanks of 8-week old male BALB/c nude mice (CAnN.Cg-Foxnnu/Crl; Charles River: Wilmington, MA). Growth kinetics were monitored using a caliper and tumor volume was calculated using V=½(length × width2). When tumors reached 100 mm3, mice were randomly assigned to control or RT (5 Gy), delivered by a clinical linear accelerator and parallel-opposed beams (6MV; equally weighted left and right lateral) using established methodology, as described [21]. Tumor progression was monitored until each tumor reached endpoint of 2200 mm3. Extracted tumors were bisected and were either formalin-fixed paraffin-embedded (FFPE) or snap frozen with liquid N2.
RT-qPCR
For tumor RNA extraction, frozen 22RV1 xenograft tumors were crashed and homogenized using a Precellys 24 tissue homogenizer (Rockville, MD). Total RNA from homogenized tissues, or cells collected from tissue cultures, was isolated using TRIzol (Life technologies, Grand Island, NY) and RNeasy kit (QIAGEN) column were utilized for RNA purification. cDNA was prepared and RT-qPCR was performed using a MBI Corbett Rotor Gene 6000 (Dorval, QC), as described [22]. Each sample was run in duplicate for a total of 45 cycles. Relative gene expression was calculated using Livak comparative Ct (2-ΔΔCt) method [23], where values were normalized to a housekeeping gene (s18). TaqMan probes used: Catalog ID: 18 s: Hs03003631_g1 and FOLH1 Hs00379515_m (Life Technologies, CA).
Immunoblotting
PrCa lines seeded in 6-well plates (at 3-7x105 cells/well) were treated with 0, 2 or 8 Gy in one fraction and incubated for 48 h. Cells were washed, lysed and subjected to immunoblotting, using specific primary antibodies against PSMA, neuron-specific enolase (NSE) or, GAPDH (Cell Signaling, as described earlier) [21]. Antibodies were purchased from Cell Signaling Technology (Whitby, ON). Immunoreactions were visualized with ECL (Bio-Rad, CA) and exposed to a Vilber fusion-FX imaging system (Marne-la-Vallée, France).
Immunohistochemistry (IHC)
FFPE tissue blocks were sectioned in 5 μM thickness slices, de-paraffinized and rehydrated in xylene and ethanol, followed by endogenous peroxidase removal, and heat antigen retrieval in citrate buffer. Tissues were blocked in 10 % goat serum and incubated with non-specific serum or anti-PSMA, anti-NSE, anti-phospho-histone H3 (P-H3-Ser-10) and anti-a-Methylacyl-CoA Racemase (AMACR) antibodies, Cell Signaling Technology (Whitby, ON). This was followed by incubation with biotinylated goat-anti-rabbit secondary antibody and streptavidin peroxidase and developed using Nova Red (Vector Labs, CA). Hematoxylin was used as counter stain.
Quantification of IHC markers: Marker expression was quantified in IHC specimens with the H-score system, which is considered as one of the “gold standard” methods in IHC quantification. H-Scores were determined by multiplication of the percentage of cells with staining intensity ordinal value (0 for no, 1 for weak, 2 for medium, 3 for strong), ranging from 0 to 300 possible values, [H-score = (% weak staining) (1) + (% medium staining) (2) + (% heavy staining) (3)]. Typically, ten random high-power fields were assessed from whole xenograft sections, 4–6 tumors per treatment group.
Statistical analysis
Unpaired T-test, one- or two-way ANOVA with post hoc Tukey’s multiple comparison tests were used for statistical analysis. Analysis was pursued using GraphPad prism v9.5. Significance was accepted at p ≤ 0.05 (*=p < 0.05, **=p < 0.01, ***=p < 0.001 and ****= p < 0.0001).
Results
PSMA-PET – Based assessment of treatment response to metastasis-directed RT (MDRT).
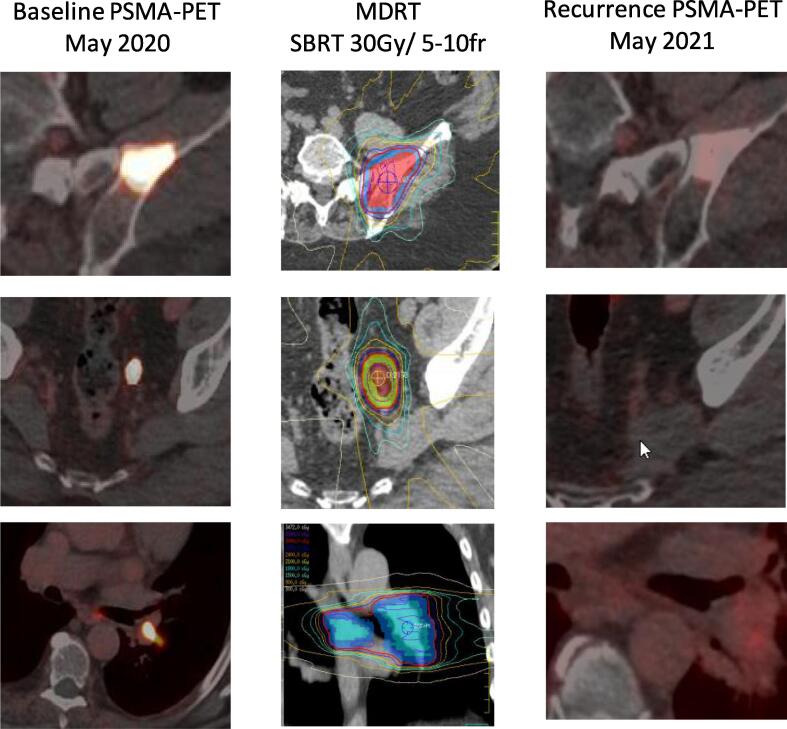
In a total of 11 patients, three of them presented with de novo metastatic disease and eight with localized PrCa. Four patients were initially treated with radical prostatectomy and four with radical radiotherapy to prostate. 26 courses of MDRT were given to a total of 25 metastatic lesions (one lesion was re-treated, see Table 1) followed by 18F-DCFPyL PET/CT (PSMA-PET) scan upon biochemical disease recurrence. All patients were offered standard of care ADT with or without androgen receptor-axis-targeted therapy (ARAT) at the time of MDRT, but 5 out of 11 patients declined. All patients were staged with CT of chest, abdomen, and pelvis and bone scans prior to MDRT, while 4 were also staged with PSMA-PET. Distribution of the lesions was: 1 regional lymph node (in the presence of other, metastatic lesions), 6 non-regional lymph nodal (M1a), 15 bone (M1b), 3 visceral (lung; M1c). One patient met the definition of metastatic castrate-resistant PC (mCRPC) at the time of PSMA-PET detected recurrence. A total of three patients were on combined androgen deprivation therapy (ADT: LHRH-agonist) and androgen receptor axis therapy (ARAT: abiraterone and prednisone or Enzalutamide) at the time of baseline as well as during the repeated conventional imaging or PSMA-PET, while the remaining eight patients were on no systemic therapy at either time. RT doses ranged from 20 Gy in 5 fractions (BED1.5 = 73) to 48 Gy in 4 fractions (BED1.5 = 432). No grade 3 or higher toxicity was noted in any of the patients. Interestingly, PSMA-PET revealed no uptake after radiotherapy in 18 out of the 26 courses. Fig. 1 illustrates representative images of metastatic sites imaged with baseline PSMA-PET/CT before MDRT, RT dose distributions, and secondary PSMA-PET/CT at biochemical progression after MDRT. CT and bone scans were concordant with improvement in all 18 lesions that responded. All patients showed biochemical response (decreasing PSA) in response to MDRT. However, eventually all cases experienced biochemical failure and developed additional metastatic sites (5 oligo-metastatic, 6 poly-metastatic). One patient had further loco-regional failure. All patients were offered systemic therapy while 7 received further MDRT.
Fig. 1.
Representative baseline PSMA-PET, metastasis-directed RT (MDRT) dose distributions, and secondary recurrence PSMA-PET images. Representative images of patient #6 are shown illustrating baseline and 2nd recurrence (Rec.) PSMA-PET (post-MDRT) of left iliac bone metastasis (top row), left perirectal lymph node metastasis (middle row) and hilar lymph node metastasis (bottom row). RT dose distribution is illustrated in the middle column.
PSMA expression patterns in human PrCa
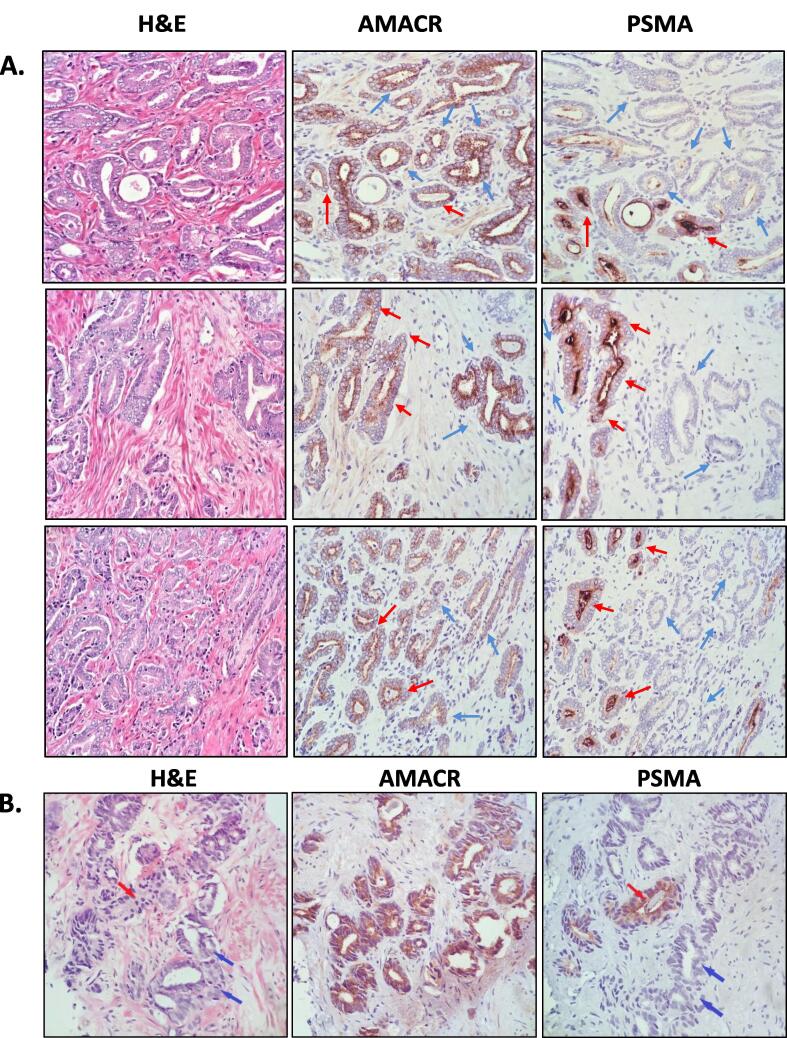
We questioned whether the observed “response” could be an artifact of altered PSMA-PET due to reduced PSMA expression in irradiated metastatic PrCa. Given the challenges involved in obtaining biopsies from metastases, we investigated effects of RT on PSMA expression in preclinical PrCa models. However, first, we assessed PSMA expression in a number of radical prostatectomy and diagnostic prostate biopsy specimens (obtained with standard transrectal ultrasound (TRUS)-guidance) to compare to preclinical models. Fig. 2 shows representative images of H&E, PSMA and α-methylacyl-CoA racemase (AMACR) IHC staining of prostatectomy and core needle biopsy tissue (ISUP grade group 2 adenocarcinoma, Gleason patterns 3 and 4), revealing heterogeneous PSMA expression, ranging from negative to strongly positive in malignant glands (high power 40x magnification). Malignant glands were confirmed by AMACR staining, commonly used for confirmation of carcinoma [24] (See Fig. s1 for low magnification images). PSMA expression was heterogeneous in the human tissue, with some glands expressing high levels (red arrows) and others no (negative, blue arrows) PSMA, confirming a significant intra-tumor heterogeneity.
Fig. 2.
SMA expression in human prostate tumor specimens. PSMA expression in human prostate tumors is highly heterogeneous. (A.) Representative images of a human prostatectomy specimen (40x magnification). The patient was diagnosed with ISUP Grade Group 2 adenocarcinoma, acinar type. Three representative tumor areas are shown in three rows. Tumor sections were stained with Hematoxylin & Eosin (H&E; left column) or subjected to IHC for α-methylacyl-CoA racemase (AMACR; middle column), and PSMA (right column). Red arrows indicate malignant glands with PSMA staining; blue arrows indicate malignant glands without PSMA staining. (B.) Representative sections of human diagnostic prostate core biopsies. The patient was diagnosed with ISUP Grade Group 2 (Gleason score (GS) 3 + 4) adenocarcinoma. Red arrows indicate fused GS4 glands with high PSMA expression; blue arrows indicate fused glands lacking PSMA expression. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Early and late effects of low BED radiotherapy on PSMA protein and mRNA levels
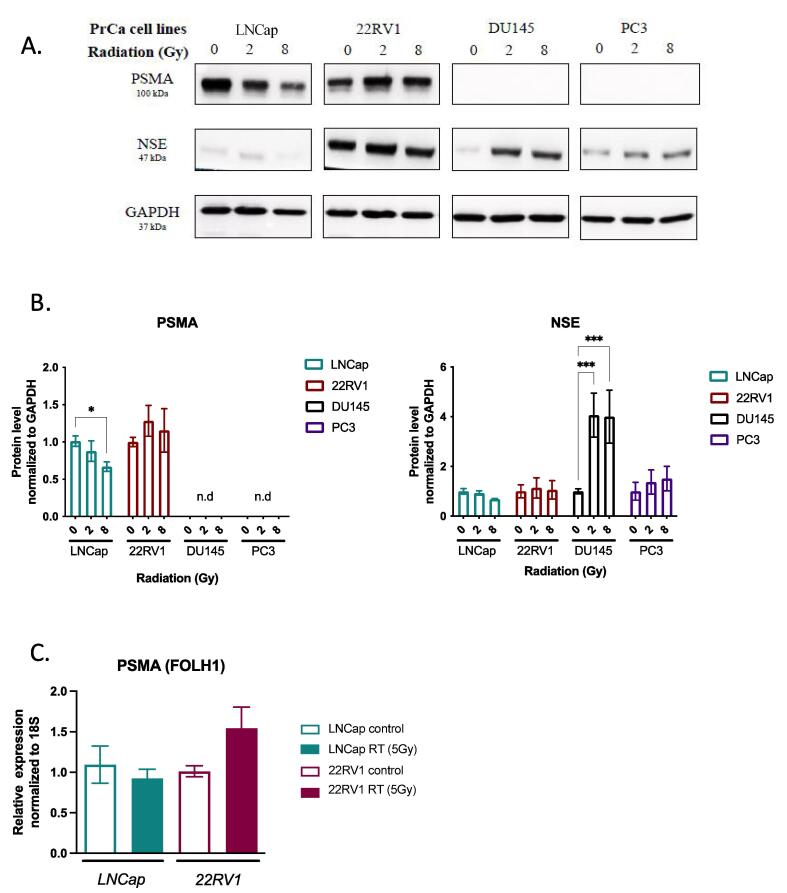
To study effects of radiation treatment on PSMA expression, we compared baseline expression of PSMA in commonly used castration-sensitive (LNCap), castrate-resistant (22RV1) and neuroendocrine castrate-resistant (PC3 and DU145) PrCa cell lines. Concordant with previous reports [25], immunoblotting showed that LNCap and 22RV1 cells expressed significant levels of PSMA protein but no expression in neuroendocrine PC3 and DU145 cells (Fig. 3A-B). To assess the influence of RT, cells were irradiated with 2 Gy or 8 Gy and analyzed 48 h later. In LNCap cells, 8 Gy RT resulted in a small but significant decrease of PSMA protein levels (0.88 ± 0.14-fold and 0.67 ± 0.06-fold for 2 Gy and 8 Gy, respectively). GAPDH levels were not altered in response to RT. Conversely, there was a non-significant trend for increase in PSMA levels of 22RV1 (1.28 ± 0.21 and -fold 1.16 ± 0.29-fold, for 2 and 8 Gy, respectively). Similarly, we detected non-significant trends for change in PSMA mRNA levels after RT (LNCAp: 0.92 ± 0.11-fold and 22RV1: 1.55 ± 0.26-fold; Fig. 3C) and no expression in DU145 or PC3 cells after RT (Fig. 3A).
Fig. 3.
PSMA expression in prostate cancer cell lines and its modulation by radiation treatment. (A.) Immunoblotting analysis of PSMA, neuron-specific enolase (NSE) and GAPDH in untreated and irradiated (0, 2 or 8 Gy) LNCAp, 22RV1, DU145, and PC3 cells. Representative immunoblots of three independent experiments are shown. (B.) Immunoblot densitometric analysis of the experiments in (A.) for PSMA and NSE. Densitometry values for PSMA and NSE in each sample were normalized to GAPDH. Individual values were then normalized to the mean value of control (mock-radiated) samples. Graph shows Mean ± SE of 3 independent experiments; * p < 0.05, *** p < 0.001; two-way ANOVA test). (C.) RT-qPCR analysis of PSMA (FOLH1 gene). ΔCT values were obtained by normalization to 18S ribosomal RNA. Graph shows Mean ± SE values for LNCAp and 22RV1 cells treated with 0 Gy (mock) or 5 Gy RT of 3 independent experiments (differences were not statistically significant).
Since transformation of PrCa into neuroendocrine phenotype is associated with decreased PSMA levels [12], we examined in PrCa cells the levels of neuron-specific enolase (NSE). Consistent with other reports [26], LNCap cells expressed NSE weakly while 22RV1 cells showed high levels of NSE expression (Fig. 3A-B). Castrate-resistant neuroendocrine DU145 and PC3 cells showed significant levels of NSE, which increased with RT in DU145 (4.07 ± 0.88-fold for 2 Gy and 4.00 ± 1.07-fold expression for 8 Gy) and to a lesser degree, in PC3 cells (1.37 ± 0.49-fold for 2 Gy and 2.03 ± 0.49-fold expression for 8 Gy) (Fig. 3A-B).
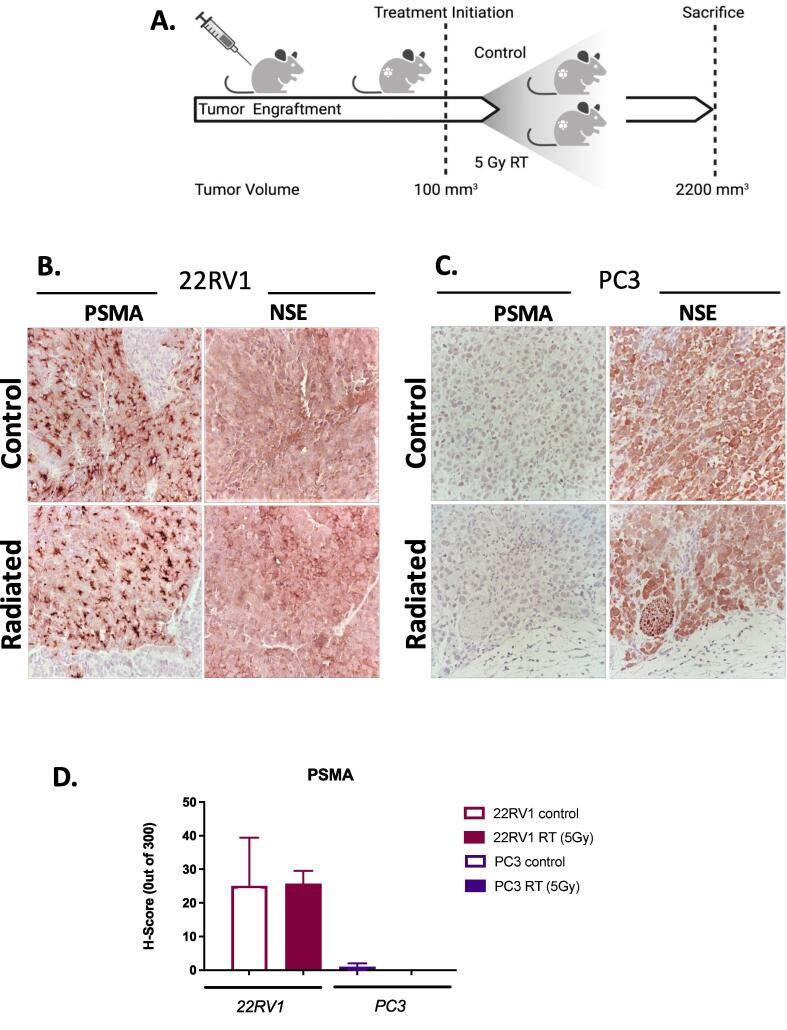
PSMA expression in xenografts of human PrCa
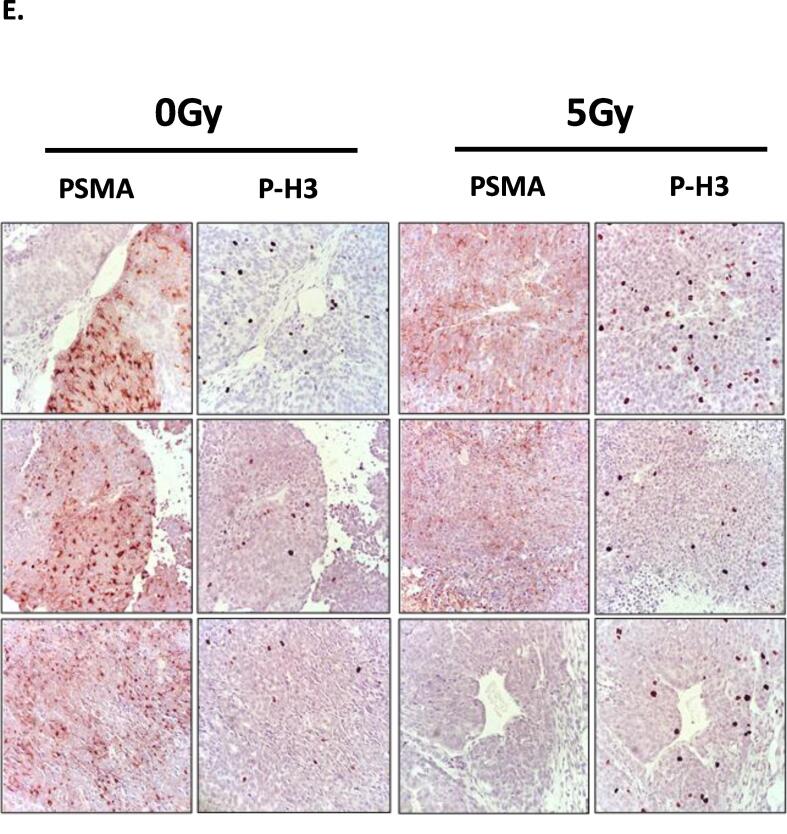
We generated xenografts of PC3 and 22RV1 cells in parallel and treated them with 0 Gy (mock) or 5 Gy of radiation treatment (schematic Fig. 4A). PSMA expression was readily detectable in 22RV1 but not PC3 xenografts (Fig. 4B-C). To appreciate the effects of RT treatment on PSMA expression, we quantified H-scores for each of the stains. Concordant with in vitro findings, RT (5 Gy) did not alter significantly PSMA protein levels in xenografts (Fig. 4D; H-scores: 22RV1: Control: 25.0 ± 14.4 vs RT: 25.7 ± 3.9; PC3: Control:1.0 ± 1.0 vs RT: 0.0 ± 0.0). Both 22RV1 and PC3 xenografts expressed NSE, with no spatial correlation between PSMA and NSE expression in the 22RV1 cells. To test if PSMA expression is associated with regions of tumor proliferating rapidly, we examined the distribution of phosphorylated histone-H3 (P-H3), an established marker of DNA replication and mitosis [27] (Fig. 4E). While PSMA staining showed heterogeneous expression, there was no detectable association of PSMA expression with the distribution of mitotically active foci (P-H3 stain). RT (5 Gy) did not alter PSMA expression nor its association with P-H3. Thus, in PrCa xenograft tumors we detected no evidence of acute regulation of PSMA by single fraction low BED RT and no clear correlation between the expression of neuroendocrine (NSE), mitosis (P-H3) markers and PSMA.
Fig. 4.
PSMA expression in untreated and irradiated prostate cancer xenografts. (A.) Schematic diagram of xenograft treatments (Created using BioRender.com). Prostate cancer cells were grafted ectopically in the right flank. RT treatment (1x 5 Gy) was delivered when xenografts reached approximately 100 mm3. Animals were euthanized when tumours reached 2200 mm3, tumors were collected, bisected and were either formalin-fixed paraffin-embed (FFPE) or snap frozen for IHC or RNA extraction, respectively. (B., C.) PSMA and NSE IHC analyses of untreated and irradiated parental 22RV1 (B.) and PC3 (C.) xenografts (imaged at 40x magnification). (D.) H-score analysis of PSMA staining in untreated and irradiated xenografts. (E.) Representative images of PSMA and phospho-histone H3 (P-H3) IHC analysis of mock-treated and irradiated (1x 5 Gy) 22RV1 xenografts (at 40x magnification). No association could be detected between PSMA and P-H3 stain with analysis of whole xenograft section slides (10 random high-power fields, 4–6 xenografts per group were analyzed) (quantification not shown).
Long term effects of high BED RT on PSMA expression - models of radio-resistance
Next, we examined whether acquired radio-resistance, developed with survival after high BED RT, could modulate PSMA expression. For that, we analyzed two PrCa cellular models of acquired radio-resistance 22RV1-RR and DU145-RR previously developed and characterized [20] (see Methods). Fig. s2A illustrates the radio-resistant properties of 22RV1-RR cells to increasing RT doses compared to parental cells. Xenograft studies show similar growth kinetics for 22RV1 and 22RV1-RR (Fig. s2B). However, 22RV1-RR xenografts demonstrate resistance to acute radiation treatments (5 Gy) compared to parental, also reflected in the survival (time to tumor endpoint) of the host animal (Fig. s2C).
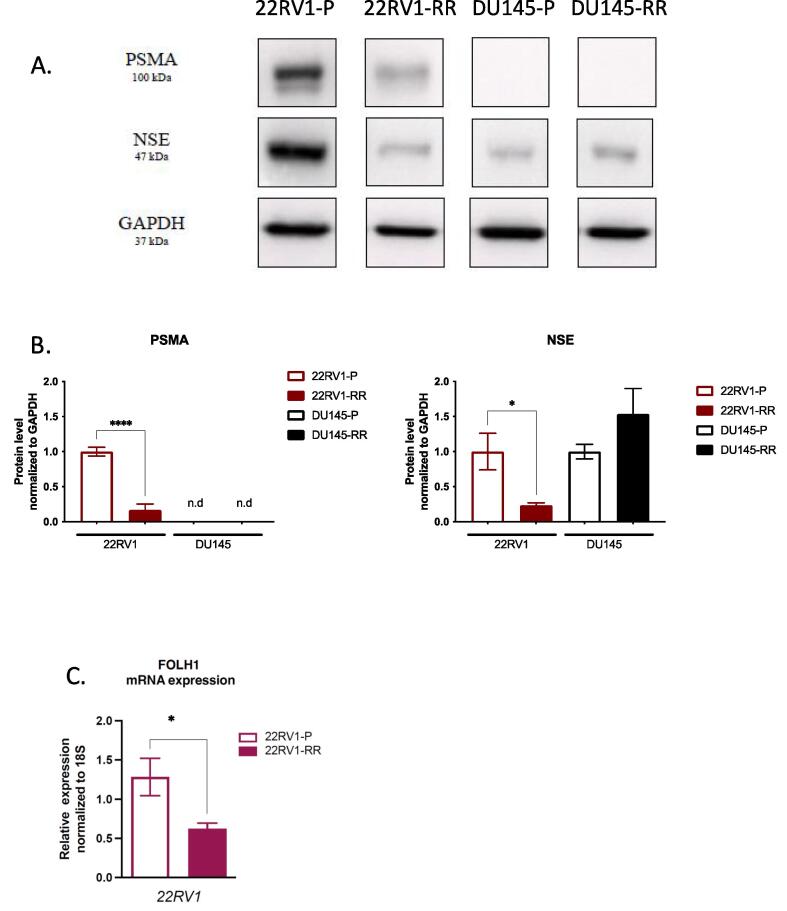
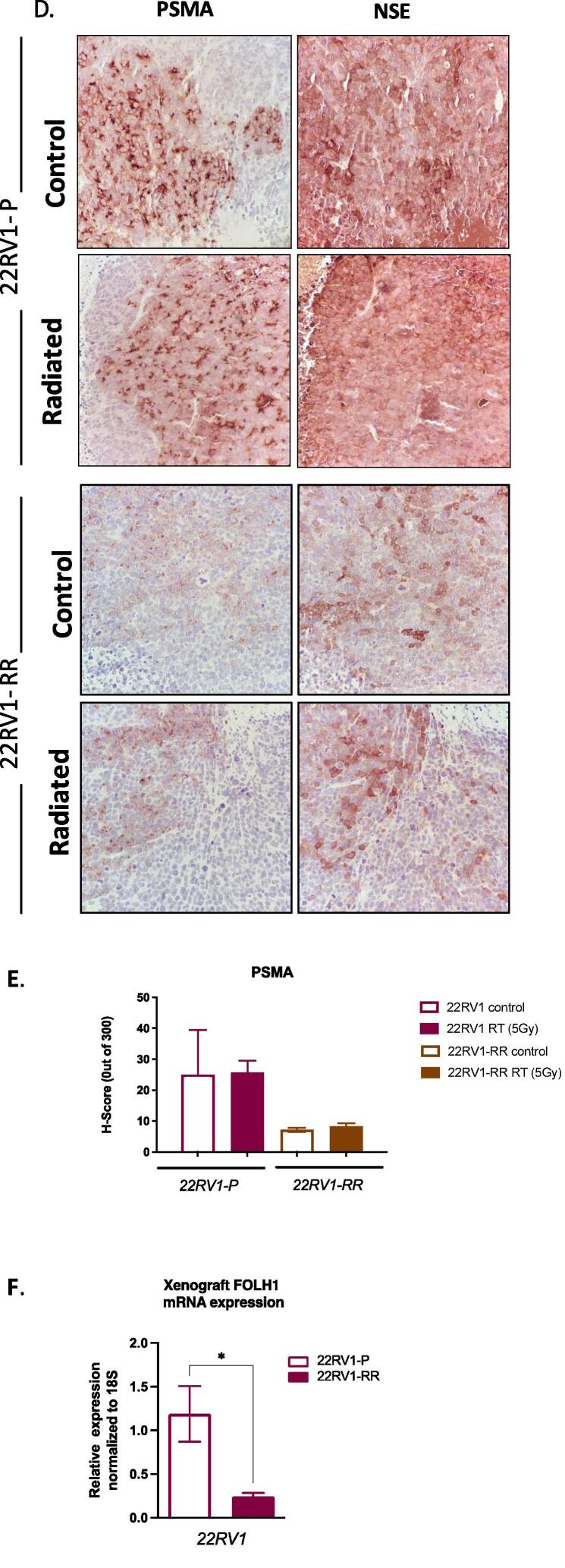
Immunoblotting and qPCR analysis of untreated 22RV1-RR cells showed substantially reduced PSMA levels compared to parental (22RV1: 0.17 ± 0.09-fold protein and 0.62 ± 0.07-fold transcript), while PSMA levels in DU145-RR cells remained undetectable (Fig. 5A-B). 22RV1-RR but not DU145-RR cells appeared to express lower levels of NSE compared to wild-type counterparts (Fig. 5A-B). Similarly, qPCR demonstrated 54 % lower PSMA (FOLH1) mRNA level in 22RV1-RR over 22RV1 (Fig. 5C). We confirmed these results with IHC and qPCR in xenografts (Fig. 5D-F). Similar to tissue culture results, 22RV1-RR xenografts expressed substantially lower levels of PSMA protein and mRNA levels (Fig. 5D-F). Further, low BED RT (5 Gy) did not acutely alter the expression levels of PSMA or NSE in 22RV1-RR tumors (Fig. 5D-E).
Fig. 5.
PSMA expression in radiation-resistant clones of prostate cancer cells and xenografts. (A.) Representative immunoblots of PSMA, neuron-specific enolase (NSE) and GAPDH expression in parental 22RV1 (22RV1-P), radio-resistant 22RV1 (22RV1-RR), parental DU145 (DU145-P), radio-resistant of DU145 (DU145-RR). (B.) Densitometric analysis of PSMA and NSE immunoblots (Mean ± SE of 3 independent experiments; * p < 0.05, **** p < 0.0001; one-way ANOVA). (C.) RT-qPCR analysis of PSMA gene (FOLH1) expression in 22RV1-P and 22RV1-RR cells. ΔCT values were obtained by normalization to 18S ribosomal RNA (Mean ± SE of 3 independent experiments; * p < 0.05; unpaired T-test). (D.) Representative images of IHC analysis of 22RV1-P and 22RV1-RR xenografts (40x magnification) (tumor growth curves are shown in Fig.s2, 6 animals per treatment group). PSMA and NSE staining are shown for mock- (0 Gy) or radiation-treated (1 × 5 Gy) tumors. (E.) H-score analysis of PSMA staining in mock-treated or irradiated xenografts above (Mean ± SE of 3 xenografts analyzed × 10 high power fields quantified per xenograft). (F.) RT-qPCR analysis of PSMA (FOLH1 gene) expression in 22RV1-P and 22RV1-RR xenografts. ΔCT values were obtained by normalization to 18S ribosomal RNA. (Mean ± SE, 6 xenograft tumors per group; * p < 0.05, **** p < 0.0001; unpaired T-test).
Discussion
Several reports have shown improved sensitivity and specificity of PSMA-PET compared to conventional imaging at various stages of PrCa. However, use of this modality in assessing response to MDRT is less commonly reported. Baumann et al. [15] reported a small series of patients assessed by 68Ga-PSMA-11 after ablative dose MDRT. They found a high response rate, similar to our report. In contrast, here we report PSMA-PET response to both ablative and palliative doses of RT (BED1.5 = 73.3–432 Gy). Regardless, our cohort also showed high rates of local disease control in treated metastases. Importantly, lesions showing residual PSMA avidity were not limited to those treated with conventional palliative RT doses. Further studies are required to determine whether high dose RT provides superior local control.
Baumann et al. [15] observed that a longer interval between RT and PSMA may result in a greater reduction of PSMA avidity. Interestingly, the interval between RT and PSMA-PET was generally longer in our study but the number of lesions showing residual PSMA signal was comparable (4/18 vs 8/26). The optimal timing of PSMA-PET imaging after RT is unclear and may influence observed response rates. It is possible that 18F-DCFPyL, used in this study, may offer improved sensitivity and signal-to-noise ratio over 68Ga-based tracers [[15], [28]]. However, we feel it is unlikely that the choice of specific tracer would have significantly changed the observations in these studies. Further, use of systemic therapy (ADT and/or ARATs) for the management of metastatic PrCa, before re-staging, may indeed influence the ability of PSMA-PET to detect residual surviving disease at metastatic sites treated with MDRT. Nevertheless, this factor did not contribute to the observations in our series since none of the patients received new systemic treatments at the time of evaluation with PSMA-PET.
To date, access to PSMA-PET imaging remains limited in many countries and PSMA-based imaging has not been routinely incorporated in clinical trial protocols. Therefore, similar to our series, the majority of patients with available PSMA-PET imaging would have scans mainly at the time of biochemical recurrence but not baseline PET. There is an increasing need for standardization of PSMA-PET reporting. Evaluation of response to RT presents an evolving challenge and reporting of sites with partial response would be more challenging. For that, in this report we did not provide rigid terms to describe the observed signals but give SUV values when residual signal is detected. Larger studies are needed to help standardize reporting for such patients. We hope that the findings of this study could help improve the biological prospective of reporting specialists.
PSMA expression levels in tumors were shown to have consequences for PSMA-PET detection [13] and understanding the mechanisms regulating PSMA expression is important. Our analysis of PSMA expression at the molecular level, in human PrCa xenografts, confirms a similar heterogeneous pattern of expression as in human prostate tumors. This heterogeneity suggests caution in considering PSMA as a universal tumour marker and guide for MDRT given the absence of PSMA expression from significant portions of tumors (Fig. 2,4,5). Previous reports also observed significant variation of PSMA expression within tumors, and between primary prostate tumors and PrCa metastases in the same patient [[14], [29]]. Tsourlakis et al. [30] found that PSMA expression was present at least weakly in 1144/1172 tissue spots derived from 173 prostatectomy patients, but noted significant expression variances (i.e. strong and weak staining present within the same tumour) in just over 50 % of the analyzed tumour samples. Technical factors (e.g. tissue processing, staining) may account for some differences between reports. Further studies are crucial in understanding the mechanisms regulating PSMA in tumors. Our attempts to correlate markers of neuroendocrine differentiation (NSE) or DNA replication (P-H3 histone) did not show correlation with PSMA expression (Fig. 4).
Despite widespread use of PSMA-PET after RT, the regulation of PSMA expression by RT was not investigated until recently. As discussed above, while this study was being prepared for publication, Sheehan et al. [19] reported upregulation of PSMA protein, but not mRNA levels, by fractionated RT in castrate-sensitive (LNCap) and castrate-resistant (22RV1) cells and PDX tumour models. They postulated that PSMA expression may be regulated by RT mostly at the post-transcriptional level. That study investigated effects of low BED fractionated RT (5–28 Gy, α/β ratio of 1.5) and analyzed cells and tumors 1–2 weeks after RT. In the present study, we also found trends of PSMA protein upregulation in 22RV1 cells 48 h after RT (BED: 4.6–50 Gy). However, we observed down-regulation of PSMA protein levels in LNCap in response to the same treatments, which reached statistical significance. The changes were associated with similar trends in mRNA levels that were not statistically significant (Fig. 3). Further, we observed no significant modulation of PSMA protein levels in 22RV1 xenografts analyzed 18–30 days after low BED RT and these treatments did not alter the undetectable levels of PSMA expression in neuroendocrine PrCa cells and xenografts (PC3 and DU145) (Fig. 3, Fig. 4). Although the observations in 22RV1 cells in culture were similar between the two studies, the findings of our study do not support a significant early regulation of PSMA levels in PrCa by low BED RT.
Importantly, here we show that PrCa cell clones that survived high BED RT express substantially reduced PSMA protein levels. The loss of PSMA expression was not only detected at the protein but also at the transcript level. This indicates that development of radio-resistance in PrCa tumor cells involves a transcriptional reprogramming that could include suppression of FOLH1 gene (Fig. 5). These findings highlight a significant concern in utilizing PSMA-PET as a response assessment tool after RT.
We feel that the results of this study are novel and of high clinical interest. It cautions clinicians to interpret negative PSMA-PET scans carefully in patients treated previously with RT. Consequently, our results and those by Baumann et al. [15] are subject to the same caveat; lack of PSMA-PET signal alone cannot be reliably equated to lack of surviving tumour. Numerous clinical trials have and continue to be designed to take advantage of PSMA-PET in PrCa therapeutics. Since this relies on tumor PSMA expression, a better understanding of the regulation of PSMA expression, at the molecular level, by PrCa therapies as well as driver mutations, is key to properly interpreting the results of such trials.
Finally, our preclinical results, must also be interpreted with caution; biology of PrCa cell lines can differ from that of human tumors. Ultimately, this work supports further preclinical and clinical studies to elucidate the mechanisms regulating PSMA expression that remain poorly understood.
Conclusion
Consistent with conventional imaging and early biochemical control, PSMA-PET illustrates high rates of local control of oligo-metastatic PrCa after MDRT. However, clinicians should be cognizant of the high degree of heterogeneity of PSMA expression in PrCa tumors that is not necessarily associated with expression of neuroendocrine features. RT may acutely alter PSMA expression in some PrCa models but in our hands this modulation appears to be limited. Importantly, PrCa cell survival after high dose RT could be associated with significant loss of PSMA expression at both the protein and mRNA levels, raising concerns on the utilization of PSMA-PET as a long-term effective response assessment tool after MDRT. Future clinical protocol and translational study design should aim to provide biospecimens and analysis methods that can address reliably whether survival after curative RT indeed limits the sensitivity of PSMA-PET to detect residual or recurrent PrCa in the prostate or metastatic sites.
Funding
Supported by Hamilton Health Sciences Foundation grant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100583.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.McCarthy M., Francis R., Tang C., Watts J., Campbell A. A Multicenter prospective clinical trial of (68)Gallium PSMA HBED-CC PET-CT restaging in biochemically relapsed prostate carcinoma: oligometastatic rate and distribution compared with standard imaging. Int J Radiat Oncol Biol Phys. 2019;104(4):801–808. doi: 10.1016/j.ijrobp.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Hofman M.S., Hicks R.J., Maurer T., Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38(1):200–217. doi: 10.1148/rg.2018170108. [DOI] [PubMed] [Google Scholar]
- 3.Hofman M.S., Lawrentschuk N., Francis R.J., Tang C., Vela I., Thomas P., et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–1216. doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- 4.Pan K.H., et al. Evaluation of 18F-DCFPyL PSMA PET/CT for prostate cancer: a meta-analysis. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.597422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo Z., Mena E., Rowe S.P., Plyku D., Nidal R., Eisenberger M.A., et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17(4):565–574. doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rousseau E., Wilson D., Lacroix-Poisson F., Krauze A., Chi K., Gleave M., et al. A prospective study on 18F-DCFPyL PSMA PET/CT Imaging in biochemical recurrence of prostate cancer. J Nucl Med. 2019;60(11):1587–1593. doi: 10.2967/jnumed.119.226381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silver D.A., et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3(1):81–85. [PubMed] [Google Scholar]
- 8.Neale J.H., Bzdega T., Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75(2):443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- 9.Silhavy J., et al. Dissecting the role of Folr1 and Folh1 genes in the pathogenesis of metabolic syndrome in spontaneously hypertensive rats. Physiol Res. 2018;67(4):657–662. doi: 10.33549/physiolres.933932. [DOI] [PubMed] [Google Scholar]
- 10.Israeli R.S., et al. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54(7):1807–1811. [PubMed] [Google Scholar]
- 11.Conteduca V., Oromendia C., Eng K.W., Bareja R., Sigouros M., Molina A., et al. Clinical features of neuroendocrine prostate cancer. Eur J Cancer. 2019;121:7–18. doi: 10.1016/j.ejca.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakht M.K., Derecichei I., Li Y., Ferraiuolo R.-M., Dunning M., Oh S.W., et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr Relat Cancer. 2019;26(2):131–146. doi: 10.1530/ERC-18-0226. [DOI] [PubMed] [Google Scholar]
- 13.Ferraro D.A., Rüschoff J.H., Muehlematter U.J., Kranzbühler B., Müller J., Messerli M., et al. Immunohistochemical PSMA expression patterns of primary prostate cancer tissue are associated with the detection rate of biochemical recurrence with (68)Ga-PSMA-11-PET. Theranostics. 2020;10(14):6082–6094. doi: 10.7150/thno.44584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannweiler S., Amersdorfer P., Trajanoski S., Terrett J.A., King D., Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15(2):167–172. doi: 10.1007/s12253-008-9104-2. [DOI] [PubMed] [Google Scholar]
- 15.Baumann R., Koncz M., Luetzen U., Krause F., Dunst J. Oligometastases in prostate cancer: metabolic response in follow-up PSMA-PET-CTs after hypofractionated IGRT. Strahlenther Onkol. 2018;194(4):318–324. doi: 10.1007/s00066-017-1239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans M.J., Smith-Jones P.M., Wongvipat J., Navarro V., Kim S., Bander N.H., et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci USA. 2011;108(23):9578–9582. doi: 10.1073/pnas.1106383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathy C.S., Mayr T., Kürpig S., Meisenheimer M., Dolscheid-Pommerich R.C., Stoffel-Wagner B., et al. Antihormone treatment differentially regulates PSA secretion, PSMA expression and 68Ga–PSMA uptake in LNCaP cells. J Cancer Res Clin Oncol. 2021;147(6):1733–1743. doi: 10.1007/s00432-021-03583-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meller B., Bremmer F., Sahlmann C.O., Hijazi S., Bouter C., Trojan L., et al. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015;5(1) doi: 10.1186/s13550-015-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheehan B., et al. Prostate-Specific membrane antigen expression and response to DNA damaging agents in prostate cancer. Clin Cancer Res. 2022;28(14):3104–3115. doi: 10.1158/1078-0432.CCR-21-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghiam A.F., Taeb S., Huang X., Huang V., Ray J., Scarcello S., et al. Long non-coding RNA urothelial carcinoma associated 1 (UCA1) mediates radiation response in prostate cancer. Oncotarget. 2017;8(3):4668–4689. doi: 10.18632/oncotarget.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storozhuk Y., Hopmans S.N., Sanli T., Barron C., Tsiani E., Cutz J.-C., et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108(10):2021–2032. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lally J.S.V., Ghoshal S., DePeralta D.K., Moaven O., Wei L., Masia R., et al. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019;29(1):174–182.e5. doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Z., Woda B.A., Rock K.L., Xu Y., Savas L., Khan A., et al. P504S: a new molecular marker for the detection of prostate carcinoma. Am J Surg Pathol. 2001;25(11):1397–1404. doi: 10.1097/00000478-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Laidler P., Dulińska J., Lekka M., Lekki J. Expression of prostate specific membrane antigen in androgen-independent prostate cancer cell line PC-3. Arch Biochem Biophys. 2005;435(1):1–14. doi: 10.1016/j.abb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Marchiani S., Tamburrino L., Nesi G., Paglierani M., Gelmini S., Orlando C., et al. Androgen-responsive and -unresponsive prostate cancer cell lines respond differently to stimuli inducing neuroendocrine differentiation. Int J Androl. 2010;33(6):784–793. doi: 10.1111/j.1365-2605.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 27.Juan G., Traganos F., James W.M., Ray J.M., Roberge M., Sauve D.M., et al. Histone H3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G2 and mitosis. Cytometry. 1998;32(2):71–77. doi: 10.1002/(sici)1097-0320(19980601)32:2<71::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Dietlein M., Kobe C., Kuhnert G., Stockter S., Fischer T., Schomäcker K., et al. Comparison of [(18)F]DCFPyL and [(68)Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17(4):575–584. doi: 10.1007/s11307-015-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright G.L., Mayer Grob B., Haley C., Grossman K., Newhall K., Petrylak D., et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48(2):326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 30.Tsourlakis M.C., Klein F., Kluth M., Quaas A., Graefen M., Haese A., et al. PSMA expression is highly homogenous in primary prostate cancer. Appl Immunohistochem Mol Morphol. 2015;23(6):449–455. doi: 10.1097/PAI.0000000000000110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.