Abstract
In mammals, sleep duration is highest in the early postnatal period of life and is critical for shaping neural circuits that control the development of complex behaviors. The prairie vole is a wild, highly social rodent that serves as a unique model for the study of complex, species-typical social behaviors. Previous work in our laboratory has found that early life sleep disruption (ELSD) in prairie voles during a sensitive window of postnatal development leads to long lasting changes in social and cognitive behaviors as well as structural changes in excitatory and inhibitory neural circuits in the brain. However, it is currently unknown how later sleep is impacted by ELSD, both shortly after ELSD and over the long term. Therefore, the aim of this study was to describe the effects of ELSD on later life sleep, compared to sleep in normally developing prairie voles. First, we conducted tethered electroencephalogram/electromyogram (EEG/EMG) recordings in juvenile prairie voles undergoing ELSD, compared to Control conditions. Second, we conducted 24 h of home cage tethered EEG/EMG recordings in either adolescent or adult male and female prairie voles that had previously undergone ELSD or Control conditions as juveniles. We found that, as adults, male ELSD prairie voles showed persistently lower REM sleep duration and female ELSD prairie voles showed persistently higher NREM sleep duration compared to Controls, but no other sleep parameters differed. We concluded that 1) persistent effects of ELSD on sleep into adulthood may contribute to the social and cognitive deficits observed in adult voles, and 2) sleep disruption early in life can influence later sleep patterns in adulthood.
Keywords: Autism, Sleep fragmentation, Rapid eye movement, Social behavior, Vole
Highlights
-
•
Early life sleep disruption (ELSD) persistently reduces Rapid Eye Movement (REM) sleep time in juvenile prairie voles.
-
•
Adult males subjected to ELSD in their 3rd postnatal week had reduced REM sleep compared to age and sex matched Controls.
-
•
There were no persistent effects of ELSD on REM sleep in female prairie voles.
Abbreviations
- REM
Rapid Eye Movement
- ELSD
Early Life Sleep Disruption
- EEG
Electroencephalography
- EMG
Electromyography
- NREM
Non Rapid Eye Movement
- VA
Veterans Affairs
- ANOVA
Analysis of Variance
- SEM
Standard Error of the Mean
- ASD
Autism Spectrum Disorder
1. Introduction
In mammals, sleep duration, and in particular, rapid eye movement (REM) sleep, is highest in the early postnatal period of life. This period of increased REM may be necessary to shape neural circuits that control the development of complex behaviors, such as species-typical social behaviors. Prairie voles (Microtus ochrogaster) are an altricial rodent species commonly used to study social behavior. In both the wild and the laboratory, prairie voles are believed to form long-lasting pair bonds with opposite sex mates, making them an ideal model species to study the underlying neural circuitry of social bonding (Insel et al., 1995; Williams et al., 1992), as well as the various genetic and environmental factors that affect formation and maintenance of social bonds (DeVries et al., 1997; Lim et al., 2004).
We have previously shown that early life sleep disruption (ELSD) during a sensitive window in prairie vole development (postnatal week 3) results in long lasting changes in both social and cognitive behavior as adults (Jones et al., 2019, 2021). Our method of ELSD during postnatal day (P)14 to 21 using automated, gentle cage agitation at timed intervals produced selective reduction in rapid eye movement (REM) sleep, as well as fragmentation in non-REM (NREM) sleep, while allowing pups to remain otherwise undisturbed in their home cages without significant alterations in stress hormones or quantity of parental care received (Jones et al., 2019).
We found that ELSD resulted in long-term changes in social and cognitive behaviors in prairie voles as adults, including reduced affiliative huddling during the partner preference test (widely regarded as a laboratory proxy of the pair bond), as well as impaired fear extinction (a proxy for cognitive flexibility) (Jones et al., 2019, 2021). At the neuronal level, and consistent with the social and cognitive behavioral deficits above, we reported that ELSD altered markers of both excitation and inhibition within the cortex, including increased immunoreactivity of inhibitory parvalbumin positive interneurons within the primary somatosensory cortex (Jones et al., 2019), and increased dendritic spine density in layer 2/3 of prefrontal cortex (Jones et al., 2021).
However, it is currently unknown how ELSD alters sleep early in life and whether ELSD results in long-term sleep changes in prairie voles. Understanding the long-term effects of ELSD on sleep later in life would provide insight into the factors that shape the development and neural control of healthy sleep. Furthermore, characterizing the normal ontogeny of sleep in developing prairie voles would provide insight into the underlying biology of sleep in this naturally occurring, highly social species, and could also potentially enhance generalization and translation of results to human sleep and behavior. In order to address these scientific gaps, the aim of this study was to examine the effects of ELSD in prairie voles on their sleep patterns later in life.
2. Methods
Using skull-based EEG combined with nuchal EMG, we collected objective sleep measures analogous to the human polysomnography at 3 time points in prairie vole development: 1) during early life sleep disruption, 2) early adolescence (7 days after Early Life Sleep Disruption (ELSD)), and 3) young adulthood (11+ weeks after ELSD). All procedures were approved by the Institutional Animal Care and Use Committee at the Portland VA Medical Center and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
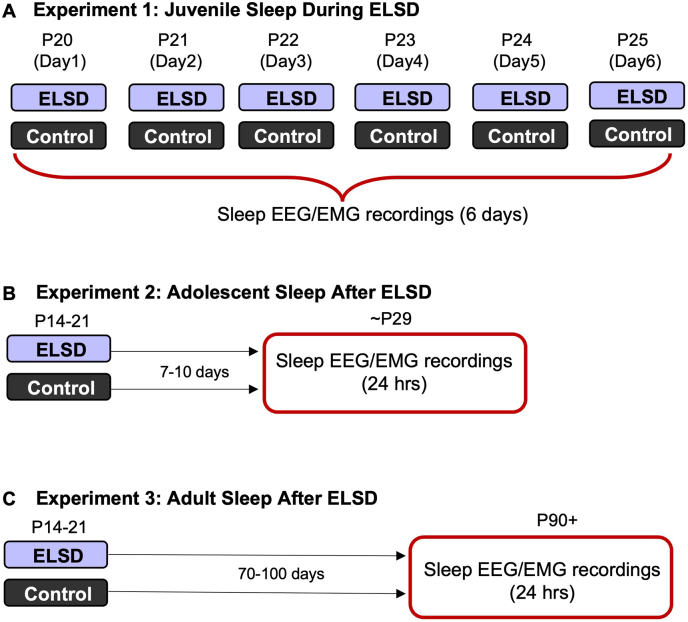
Experimental Design: Data was collected across three experiments. In Experiment 1, juvenile prairie voles were implanted with EEG/EMG electrodes and sleep was recorded for 6 consecutive days in the midst of either ELSD (n = 2 female and n = 3 male) or Control (n = 3 female and n = 2 male) conditions (Fig. 1A). This experiment was conducted in order to describe the full sleep phenotype produced by ELSD, including any potential habituation to the ELSD paradigm, in young voles. In Experiment 2, a separate cohort of ELSD (n = 6 female and n = 8 male) or Control (n = 5 female and n = 5 male) prairie voles were implanted with EEG/EMG electrodes and sleep was recorded for 24 h during early adolescence (or approximately 7 Days after the completion of ELSD or Control sleep manipulation; Fig. 1B). In Experiment 3, ELSD (n = 9 female and n = 10 male) or Control (n = 9 female and n = 10 male) prairie voles were implanted with EEG/EMG electrodes and sleep was recorded for 24 h during adulthood (approximately 11+ weeks after the completion of ELSD or Control sleep manipulation; Fig. 1C).
Fig. 1.
Experimental design. Three experiments were conducted to describe sleep in prairie voles during and after ELSD using EEG and EMG recordings over 24-h bins during different developmental windows. A) In experiment 1, sleep was recorded during ELSD or Control conditions for 6 consecutive days. B) In experiment 2, sleep was recorded after ELSD or Control conditions during early adolescence. C) In experiment 3, sleep was recorded after ELSD or Control conditions during adulthood.
Subjects: Subjects were male and female prairie voles bred in house at the VA Portland Health Care System and reared by both parents. Subjects were housed in clear polycarbonate cages (27 cm × 27 cm × 13 cm) and housed in temperature and humidity controlled rooms on an automated 14:10 light:dark cycle (lights on at 0700h). Animals had ad libitum access to a mixed diet of rabbit chow (LabDiet Hi-Fiber Rabbit), corn (Nutrena Cleaned Grains), and oats (Grainland Select Grains) and water (water bottles and/or hydrogel) throughout the entirety of all three experiments. Cotton nestlets and a wooden block or stick for chewing enrichment were added to each cage and replaced weekly with cage change. No litter contributed more than n = 2 animals of the same sex to any given experiment. The prairie vole colony originated from Emory University derived from field caught prairie voles in Illinois. Colony diversity was maintained through generous bi-annual donations from researchers across the United States, including Dr. Lisa McGraw at North Carolina State University in 2014, Dr. Karen Bales at UC Davis in 2015, Dr. Zoe Donaldson at CU Boulder in 2017, and Dr. Zuoxin Wang at Florida State University in 2019. Breeder pairs were checked each morning at lights on for the presence of pups and the day of pup discovery was designated as postnatal day (P)1. Voles were weaned at P21 and socially housed with same sex littermates (2–4/cage) until testing (∼P29, ∼P100). Females and males were housed in separate colony rooms and tested on separate days to avoid male induced estrus in female prairie voles.
In Experiment 1, two voles were removed from analysis because they did not produce a full 6 days of useable sleep data due to equipment malfunction.
Early Life Sleep Disruption: To disrupt sleep early in life (ELSD group), home cages containing prairie vole litters with both parents present were placed on a standard laboratory orbital shaker connected to a timer programmed to turn on every 110 s for 10 s, thus providing gentle agitation to the entire cage (110 rotations per minute). These parameters of sleep disruption are based on previously validated studies conducted in mice (Li et al., 2014; Sinton et al., 2009) and prairie voles (Jones et al., 2019). With this method, prairie vole pups remain otherwise undisturbed, parental care is not disrupted, and markers of stress are not increased (Jones et al., 2019). Cage card holders were locked into place to limit auditory disruption during cage agitation. Water bottles were removed and hydrogel was provided as an alternative to prevent spillage during shaking. Cages containing control animals were housed in the same room as the shakers and supplemented with hydrogel but cages were not physically disturbed. In Experiment 1, sleep was recorded during ELSD starting on P20, as such, young voles were housed individually, without parents, on the orbital shaker along with hydrogel and softened food.
EEG/EMG electrode implantation: Custom made electrodes were built for each animal prior to implantation. Electrodes consisted of an 8-pin female header socket (MillMax) with 4 silver EEG leads (A-M Systems, Inc) soldered to the top row of pins and 3 insulated silver wire EMG leads soldered to the bottom row. To implant electrodes, voles were anesthetized with isoflurane (3–5% induction, 1–3% maintenance) and affixed in a stereotaxic frame (Kopf) customized for prairie voles. During surgery, the skull was exposed and 4 small holes drilled (2 frontal and 2 parietal) for the insertion of stainless-steel screws soldered to the EEG wires. EMG wires were partially stripped of their insulation before being routed through the dorsal neck muscles. The entire electrode was secured to the skull using both dental cement and a small amount of cyanoacrylate glue. The animal received subdermal fluids and carprofen (5 mg/kg, i.p.) before being placed into a clean home cage on top of a heating pad until alert and ambulatory. Support heat, softened food, hydrogel, and extra nestlets were provided during recovery (3–5 days).
Sleep Recordings: Animals were connected to custom built lightweight recording cables (Cooner wire) via male header sockets and given enough slack to move freely. Data was collected from a Grass amplifier via AcqKnowledge software (BIOPAC). In Experiment 1, sleep signals were recorded for 6 consecutive days starting on P20. In Experiments 2 and 3, animals were recorded for 3 consecutive days starting on approximately either P29 (Experiment 2) or P100 (Experiment 3) with the middle 24 h used for analysis. In all experiments, animals were allowed to acclimate to the tethers for one day prior to the start of recordings.
Sleep Scoring: EEG/EMG signals were converted to European Data Format before being scored offline for NREM and REM sleep and Wake in 4-s epochs across the full 24-h period (SleepSign, Kissei Comtec). EEG channels were filtered with a high-pass filter at 0.75 Hz and EMG channels with a band-pass filter from 20 to 50 Hz. Wake was defined as high amplitude mixed frequency EEG paired with high amplitude EMG. NREM was defined by high amplitude, low frequency EEG paired with low amplitude EMG. REM was defined by low amplitude, high frequency EEG paired with low amplitude EMG with occasional muscle twitches. EDF files were scored per the above criteria automatically (SleepSign) and then manually verified and corrected by trained experimenters. Brief arousals were defined as ≤ 3 epochs (12 s) of Wake immediately following ≥ 3 epochs (12 s) of sleep (NREM + REM). Wake bouts were defined as ≥ 4 epochs of Wake immediately following ≥ 3 epochs of sleep (NREM + REM) (Li et al., 2014). Two measures of sleep fragmentation were used following the methods of an identical method of sleep disruption on adult mice (Li et al., 2014): 1) Average bout length for NREM and REM sleep and 2) arousal index, which was calculated by combining the total frequency of brief arousals and wake bouts per hour of sleep (NREM + REM) over the full 24 h of recording for each day.
Statistics: Assumptions were met for parametric testing and group differences were analyzed with ANOVA (between group factors: Sex, Sleep Group). When the same animal was tested at multiple time points, repeated measures ANOVA were used (within group factor: day or age). If sphericity tests were violated (Mauchly's), F values were Greenhouse-Geisser corrected. For ANOVA, alpha values were set at 0.05. In Experiment 1, we were not powered to detect sex differences and males and females were analyzed together. Significant interactions were followed up with independent sample t-tests (two-tailed) with alpha values set at 0.0083 for Experiment 1 and 0.025 for Experiments 2 and 3 (Bonferroni corrected for multiple comparisons).
3. Results
3.1. Experiment 1–6 days of shaker sleep disruption
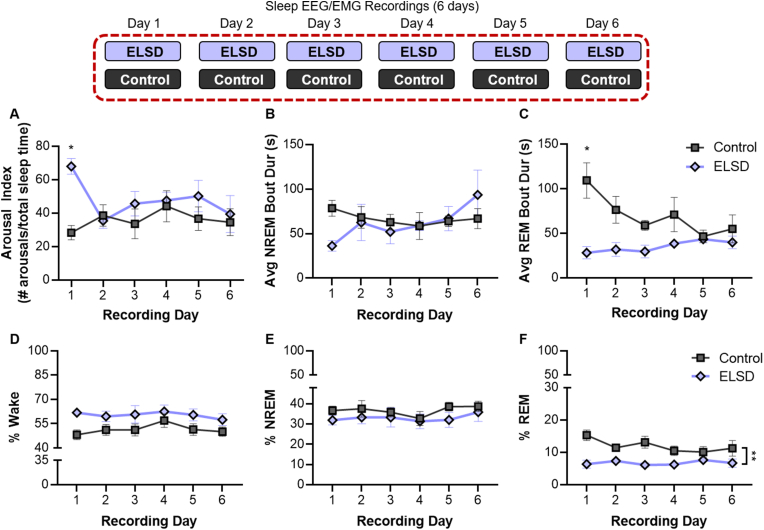
Young prairie voles acclimate to sleep fragmentation during ELSD: Sleep fragmentation was quantified using a combination of Arousal Index (# Brief arousals + # Wake Bouts/Total Sleep Time) and average NREM and REM bout length for each 24-h period.
Arousal Index: Repeated measures ANOVA (between group factor = sleep group; within subjects factor = recording day) indicated a significant day by sleep group interaction F(5,40) = 4.022, p = 0.005. Follow-up independent sample t-tests revealed that arousal index was only increased during ELSD on the first full day that animals were housed on the shaker (t(8) = 6.202, p < 0.001) with no significant differences detected on other shaker days (all p values > 0.15; p < 0.0083 required for statistical significance after multiple comparisons) (Fig. 2).
NREM Average Bout Length: There were no significant day by sleep group interactions on NREM average bout length (repeated measures ANOVA, Greenhouse-Geisser corrected: F(2.3,18.3) = 2.808, p = 0.167) and no significant main effects (between group effect of sleep group: F(1,8) = 0.135, p = 0.723) (Fig. 2B).
REM Average Bout Length: There was a significant day by sleep group interaction on REM average bout length (repeated measures ANOVA, Greenhouse-Geisser corrected: F(1.84,14.72) = 4.37, p = 0.035) as well as a main effect of group (between subjects factor: F(1,8) = 9.828, p = 0.014). Follow up t-tests revealed that the average REM bout length was decreased on the shaker for the first day only (day 1: t(8) = 3.854, p = 0.005; day 2: t(8) = 2.622, p = 0.031; day 3: t(8) = 3.237, p = 0.012; day 4: t(8) = 1.658, p = 0.136; day 5: t(8) = 0.402, p = 0.698; day 6: t(8) = 0.886, p = 0.401; p < 0.0083 required for statistical significance) (Fig. 2C).
A
Fig. 2.
Experiment 1: ELSD in juvenile voles acutely fragments REM and NREM sleep and chronically reduces REM sleep duration. A) Arousal Index (# arousals/total sleep time) was increased only on the first day of ELSD. B) Average length of NREM sleep bout each day of recording was not significantly reduced. C) Average length of REM sleep bout was decreased the first 3 days of ELSD. D) Time spent in Wake across the entire 6 days of ELSD was not significantly increased. E) No change in NREM sleep amounts. F) REM sleep amount were reduced across the entire 6 days of ELSD. N = 5/group. Symbol represents mean, error bars ± SEM. *p < 0.0083 (p value 0.0083 required for statistical significance after Bonferroni correction); **p < 0.01 significant effect of sleep group (repeated measures ANOVA) REM = rapid eye movement; NREM = non-rapid eye movement; ELSD = early life sleep disruption.
REM sleep duration is reduced for entire ELSD period: Duration in each vigilance state was calculated as a percentage by dividing the total time spent in each state (Wake, NREM, REM) by the total recording time for each day.
For all 3 vigilance states there were no significant within subjects interactions over the 6 days of ELSD (all p values > 0.728). There was a trend towards increased time in Wake in animals on the shaker that did not reach significance (repeated measures ANOVA, between subjects effect of sleep group: F(1,8) = 5.055, p = 0.055) (Fig. 2D). There was not a significant effect of housing on the orbital shaker on NREM sleep duration (between subjects effect of sleep group: F(1,8) = 1.185, p = 0.308) (Fig. 2E). There was a main effect of sleep group on REM sleep duration with voles housed on the shaker during ELSD spending significantly less time in REM sleep compared to controls across the 6 days of recording (between subjects effect of sleep group: F(1,8) = 19.212, p = 0.002) (Fig. 2F; see Tables S1 and S2 for mean REM sleep duration by sex).
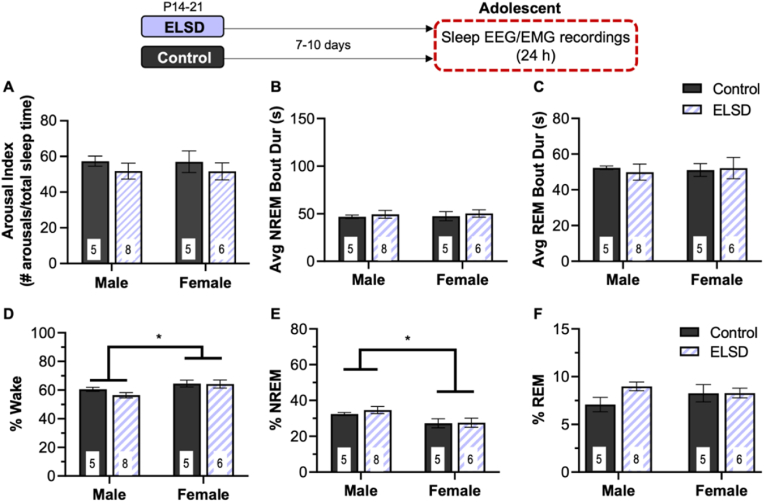
3.2. Experiment 2: ELSD does not affect adolescent sleep
Sleep fragmentation: There were no long term effects of Early Life Sleep Disruption on any of the sleep fragmentation metrics quantified here at adolescence (main effect of sleep group - arousal index: F(1,20) = 1.29, p = 0.270 (Fig. 3A); NREM Avg Bout: F(1,20) = 0.435, p = 0.517 (Fig. 3B); REM avg bout F(1,20) = 0.021, p = 0.886 (Fig. 3C)). There were also no significant effects of sex on sleep fragmentation in prairie voles in adolescence (arousal index, main effect of sex: F(1,20) = 0.002, p = 0.961; NREM avg bout F(1,20) = 0.042, p = 0.839; REM avg bout F(1,20) = 0.013, p = 0.911) and none of the group × sex interaction terms were significant (all p values > 0.709).
Fig. 3.
Experiment 2: Adolescent sleep was not altered after ELSD. There were no long-term effects of ELSD on measures of sleep fragmentation during early adolescence including arousal index (# arousals/total sleep time) (A), NREM average bout length (B), or REM average bout length (C). Adolescent females spent more time in wake than males (D) and less time in NREM (E) but these were not affected by the ELSD procedure. F) there were no significant changes in REM sleep time during adolescents after ELSD compared to Controls. Sleep and wake metrics totaled over 24 h of EEG/EMG recordings. Bar height is mean, number in bars is sample size, error bars ± SEM. *P < 0.00.05.
Wake Duration: ELSD did not have a significant effect on time spent in wake during adolescence (main effect of sleep group: F(1,20) = 0.952, p = 0.341) (Fig. 3D). Adolescent females spent more time in wake than adolescent males (main effect of sex: F(1,20) = 6.623, p = 0.018) and the interaction was not significant (F(1,20) = 0.689, p = 0.416).
NREM Sleep Duration: Adolescent female prairie voles spent significantly less time in NREM sleep than males (main effect of sex: F(1,20) = 7.576, p = 0.012)(Fig. 3E). There were no significant effects of early life sleep group on NREM sleep duration during adolescence (main effect of sleep group: F(1,20) = 0.385, p = 0.542; sleep group × sex interaction: F(1,20) = 0.232, p = 0.635).
REM Sleep Duration: The effects of ELSD on REM sleep duration in adolescent prairie voles was not significant (main effect of sex: F(1,20) = 0.162, p = 0.692; main effect of sleep group: F(1,20) = 2.405, p = 0.137; sleep group × sex interaction: F(1,20) = 2.266, p = 0.148)(Fig. 3F).
When stage percentage was compared between light and dark periods there were no significant within subjects effects (repeated measures ANOVA, all p values > 0.332) indicating that time in Wake, NREM sleep, or REM sleep did not differ between the light and dark cycle in adolescent prairie voles.
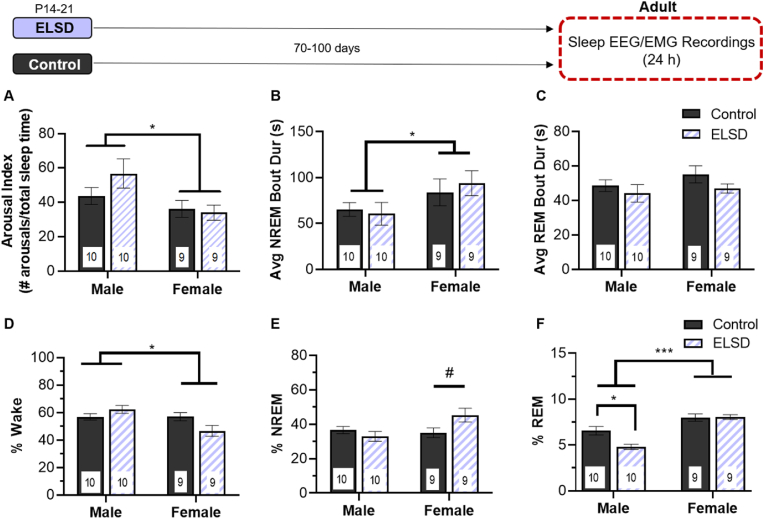
3.3. Experiment 3: ELSD reduces REM sleep duration in adult males
Sleep fragmentation: There were also no long term effects of Early Life Sleep Disruption on any of the sleep fragmentation metrics quantified here at adulthood (main effect of sleep group - arousal index: F(1,34) = 0.808, p = 0.375 (Fig. 4A); NREM Avg Bout: F(1,34) = 0.047, p = 0.829 (Fig. 4B); REM Avg Bout: F(1,34) = 2.298, p = 0.139) (Fig. 4C). However, in contrast to adolescence, in adulthood, females had more consolidated NREM sleep than males as evidenced by reduced arousal index (main effect of sex: F(1,34) = 6.173, p = 0.018) and increased average NREM bout length (F(1,34) = 4.565, p = 0.04) but not average REM bout length (main effect of sex: F(1,34) = 1.243, p = 0.273 (Fig. 4C)). Consistent with adolescence, there were no significant sex by early life sleep interactions in adulthood (all p values > 0.219).
Fig. 4.
Experiment 3: Persistent effects of ELSD on sleep duration are sex specific and age dependent. A-C) There were no significant effects of ELSD on adult sleep fragmentation metrics including arousal index (A), average NREM bout duration (B), or average REM bout duration (C) over 24 h. D) Adult females spent less time in wake than males. E) ELSD females spent more time in NREM sleep than Control females during adulthood and F) male ELSD voles spent less time in REM sleep than age and sex matched controls. Adult females spent more time in REM than adult males and have more consolidated NREM sleep (B). Sleep and wake metrics were totaled over 24 h of EEG/EMG recordings. Note y-axis limit is different on each panel. *p < 0.05 or p < 0.025 (after Bonferroni correction); ***p < 0.001; # 0.05 > p > 0.025 (p value of 0.025 required for statistical significance after Bonferroni correction). number at bottom of bar is sample size for each group, bar height is mean, error bars ± SEM.
Wake Duration: In adulthood, there was not a significant main effect of sleep group: F(1,34) = 0.658, p = 0.423 and adult females spent less time in wake than adult males (main effect of sex: F(1,34) = 6.448, p = 0.016). There was a significant sleep group by sex interaction in adult prairie voles (F(1,34) = 6.710, p = 0.014) but follow up t-tests did not reach significance (males t(18) = 1.461, p = 0.161; females t(16) = 2.101, p = 0.052; p value < 0.025 required for significance after Bonferroni correction) (Fig. 4D).
NREM Sleep Duration: In contrast to sex effects on NREM sleep observed in adolescence, there were no main effects of sex on NREM sleep duration in adults (main effect of sex: F(1,34) = 2.191, p = 0.148). There was a significant sleep group × sex interaction in adults (F(1,34) = 6.103, p = 0.019) with adult females showing a trend towards increased time in NREM sleep after ELSD than Controls (t(16) = 2.226, p = 0.041; p value < 0.025 required for statistical significance after Bonferroni correction) (Fig. 4E). We then looked at NREM sleep duration by hour and found a significant hour by sex interaction (repeated measures ANOVA, f(23,759) = 1.804, p = 0.012) but no within subjects effects of ELSD on REM sleep (all p values > 0.531) (Fig. S1).
REM Sleep Duration: Adult females spent significantly more time in REM sleep than adult males (main effect of sex: F(1,34) = 32.281, p < 0.001) and REM sleep was reduced after ELSD in males only (sleep group × sex interaction: F(1,34) = 9.734, p = 0.004; t(18) = 3.224, p = 0.005) (Fig. 4F). We then looked at REM sleep duration by hour and found a significant hour by sex interaction (repeated measures ANOVA, f(23,759) = 2.019, p = 0.003) but no within subjects effects of ELSD on REM sleep (all p values > 0.325) (Fig. S1).
Adult voles spent significantly more time awake during the dark portion of the light cycle (57.89% Wake) compared to the light portion (53.67% Wake) (repeated measures ANOVA, within subjects effect of light F(1,33) = 7.134, p = 0.012) and significantly less time in NREM sleep in the dark (35.51%) compared to the light (39.39%) (F(1,33) = 8.583, p = 0.006) but there were no significant interactions (all other p values > 0.395). REM sleep time did not differ between light and dark periods (p = 0.382) (see Fig. S1 for percentage duration in each sleep stage by hour) and interactions were not significant (all p values > 0.08).
4. Discussion
In these experiments, prairie vole pups underwent ELSD or Control conditions for one continuous week in postnatal development (postnatal week 3). We conducted sleep EEG/EMG recordings at three separate developmental timepoints in order to describe the effects of ELSD on sleep behavior later in life. We found that during the ELSD period itself, REM sleep quality and quantity are preferentially disrupted. Furthermore, we found that ELSD generates long-lasting effects on REM sleep development in male prairie voles. These findings may be relevant to human neurodevelopmental disorders such as autism spectrum disorder (ASD) that feature impairments in both sleep and social behavior that persist into adulthood.
To our knowledge, this study is the first description of prairie vole REM and NREM sleep across development. Our results from Experiment 1 suggest that the amounts of REM and NREM sleep in young prairie voles mirror sleep times found in mice, a similar altricial rodent species (Maret et al., 2011; Rensing et al., 2018). Interestingly, we observed several notable sex differences in sleep in prairie voles at both adolescent and adult time points. For example, female adolescent voles spent significantly more time in Wake and less time in NREM sleep than male adolescent voles. Although these sex differences in themselves deserve further exploration, they were not impacted by ELSD per se.
Furthermore, we found that adult female voles spent more time in REM sleep than adult male voles and their timing of both REM and NREM sleep throughout the day differed from males. This finding was surprising, and is in contrast to other published literature on rats and mice reporting either decreased REM sleep amounts in females compared to males (Fang and Fishbein, 1996; Yamaoka, 1980) or no sex differences in REM sleep amounts (Paul et al., 2006). REM sleep amounts are influenced by the estrous cycle in female rats (Yamaoka, 1980; Colvin et al., 1968; Kleinlogel, 1983; Schwierin et al., 1998; Swift et al., 2020) but the effects of estrus on female mice appears to be minimal and strain specific (Koehl et al., 2003). Unlike mice and rats, prairie voles are male induced ovulators, and are assumed to be in a persistent state of diestrus if not exposed to males (as was done in these sets of experiments). Also, adult female voles showed less arousals and longer NREM bouts compared to adult male voles, suggesting sex differences in NREM sleep consolidation.
4.1. During the ELSD paradigm itself, sleep fragmentation is only transiently increased, but total REM time is persistently decreased at the expense of wake
Previous work in our lab recorded sleep on the shaker for 1 day in young prairie voles and reported that ELSD reduces REM sleep time and fragments NREM sleep compared to 1 day of recordings in that same animal off the shaker (Jones et al., 2019). Here, we expanded these findings in two ways: 1) we recorded from younger animals more closely matched to the experimental age of our other ELSD experiments and 2) we recorded EEG/EMG for 7 consecutive days (6 full 24-h periods for analysis) from age-matched animals during either ELSD or Control conditions.
Consistent with our previous work, we found that juvenile prairie voles housed on an orbital shaker experienced significantly reduced REM sleep duration compared to Control voles (Jones et al., 2019). During ELSD in prairie voles, REM average sleep bout lengths were shorter than controls, but only transiently so. Furthermore, using an arousal index (Li et al., 2014) to quantify arousals from sleep, we found that the orbital shaker only increased arousals for the first day of sleep disruption in young prairie voles. This is in contrast to results reported by Li et al., 2014) in adult mice sleep disrupted for 4 continuous weeks. Our method of sleep disruption is identical to the method described by Li et al., 2014) in adult mice, however, Li et al. found chronic sleep fragmentation, resulting in shorter sleep bouts and increased arousal index sustained across the full period of sleep disruption. Here we report that juvenile prairie voles acclimate to the sleep fragmentation effect of ELSD but do not acclimate to the REM sleep reduction. This may be specific to prairie voles as a species, or it may be specific to this period in early life.
4.2. ELSD cause both age- and sex-specific changes in sleep later in life
We examined prairie voles at two time points after ELSD or Control conditions, and found that ELSD caused long-lasting effects on REM sleep later in life – but only in males. ELSD did not affect sleep patterns in adolescent animals. However, ELSD caused decreased REM sleep in adult males, compared to age- and sex-matched controls. Notably, ELSD effects on REM sleep were absent in females.
Previous research has shown that clomipramine treatment from P8–P21, a method that reduces REM sleep by 45–70%, in male rats also resulted in long lasting REM sleep abnormalities, although the sleep changes were in the opposite direction of our results (increased REM sleep in adults) (Vogel et al., 1990). Follow up work using instrumental instead of pharmacological REM sleep deprivation (>80% decrease in REM sleep) from P14–P21 in rats also leads to increased REM sleep as adults (Feng and Ma, 2003). While this could be due to species effects or differing degrees of early life sleep disruption, this work supports our hypothesis that early life REM sleep is important for the development of adult sleep and social behavior.
The timepoint chosen for both early life sleep disruption and adult sleep phenotyping in these studies mirrors the timing of our previous work describing social and cognitive deficits in adult males after ELSD. The same ELSD paradigm described in this work results in long lasting changes to social and cognitive behavior in prairie voles when tested as adults (reduced pair bond behavior in males and impaired cognitive flexibility in both sexes) (Jones et al., 2019, 2021). Pharmacological treatment with clomipramine to reduce REM sleep at varying neonatal windows has revealed that treatment during P12-17 and P14-20 resulted in impaired sexual behavior in adult male rats (reduced mounts, intromissions, and ejaculations) (Feng et al., 2001). Instrumental REM sleep deprivation in rats from P14–P21 also leads to sexual, aggressive, and locomotor behavior impairments (Feng and Ma, 2003). ELSD using an identical method of intermittent cage agitation from P14–P21 in mice led to adult impairments in social and anxiety like behavior in genetically vulnerable mice that were also sex specific (Lord et al., 2022).These results provide further support of the importance of early life REM sleep on the development of, not only social and cognitive behaviors, but also sleep behaviors. Despite transient effects of sleep fragmentation during the first day of ELSD, this effect resolved over the next 6 days of the ELSD paradigm while total REM time remained persistently decreased over the entirety of the weeklong ESLD protocol.
The exact role that ELSD plays in shaping the underlying neural circuitry relevant to both sleep and social behavior is still unknown. It is still unknown whether the combination of ontogenetic REM sleep changes observed in male prairie voles that underwent ELSD leads to long term REM sleep reduction in adulthood and subsequent impairments in social bonding behavior or if long lasting REM sleep disturbance exist after ELSD independent of social and cognitive impairments.
4.3. Possible relevance of early life sleep disruption to human ASD
There is a large and growing body of research describing sleep problems in patients diagnosed with ASD. The majority of this work describes difficulties with initiating and/or maintaining sleep (Krakowiak et al., 2008; Richdale and Prior, 1995; Schreck and Mulick, 2000) as well as decreased time in REM sleep (Buckley et al., 2010; Díaz-Román et al., 2018). This early life sleep phenotype in children with ASD mirrors the sleep disturbance experimentally created with the ELSD paradigm in the current study.
In patients with ASD, sleep problems persist throughout development and into adulthood (Gregory and Sadeh, 2016; Kocevska et al., 2017) and may increase as young children grow older (Humphreys et al., 2014; Verhoeff et al., 2018). Adults with ASD reveal problems falling asleep (longer sleep latency) and frequent night awakenings as well as reduced time in NREM sleep and reduced rapid eye movements during REM sleep. (Limoges et al., 2005). While our ELSD paradigm did not result in long term reductions in NREM sleep, ELSD males (but not females) spent less time in REM sleep than their age and sex matched counterparts. It is important to point out that we did not find reduced sleep time in adolescent prairie voles previously subjected to ELSD. This finding may be in line with human research examining sleep in older children and adolescents with ASD reporting increased daytime sleepiness and oversleeping combined with nighttime problems with sleep maintenance (Goldman et al., 2012; Tesfaye et al., 2021). Finally, other research has pointed to adolescence being a unique time that does not necessarily show linearity in other behavioral trajectories (reviewed in (Casey et al., 2008)).
Research related to sex differences in sleep and ASD is mixed. While some studies report increased sleep disturbances in females with ASD throughout development (Hartley and Sikora, 2009; Jovevska et al., 2020) others suggest that male sex contributes to the severity of sleep disruption or the association between sleep and ASD symptomology (Humphreys et al., 2014; Saré and Smith, 2020; Sivertsen et al., 2012). Our studies suggest that both males and females are susceptible to sleep disruption using the ELSD paradigm, however our results suggest that females may be more resilient to developing lasting behavioral impairments (both social and sleep) as a result of this early life sleep disruption than males.
As research in adults with ASD suggest that the presence and/or degree of adult sleep disturbance correlates with the severity of autism symptomology (Saré and Smith, 2020; Schreck et al., 2004; Sikora et al., 2012; Taylor et al., 2012; Veatch et al., 2017) the milder social phenotype associated with female ELSD voles compared with males (Jones et al., 2019) is perhaps consistent with the lack of sleep disturbances in adult ELSD females.
4.4. Potential limitations, and implications
There are several limitations of the current work to be considered when interpreting the data presented here. Importantly, sleep was recorded from tethered EEG and EMG electrodes that require single housing in order to avoid damaging cables. Prairie voles are highly social rodents and it is possible that this period of single housing influenced their natural sleep behavior. We also did not conduct social or cognitive testing in these experiments, future work will examine correlations between social bonding and cognitive flexibility and REM sleep measurements. Finally, our studies conducted in Experiment 1 were not powered to detect sex differences during ELSD and juvenile EEG/EMG recordings were conducted at a later (P20-26), albeit overlapping time period as the ELSD conducted in Experiments 2 and 3 (P14-21). It is important to know if sleep is affected equally in males and females during the ELSD paradigm. Although the sample size was small for each sex, we did not observe any notable sex differences to support expanding the animal usage for this experiment. Future work using wireless telemetry devices to acquire EEG and EMG signals during sleep will be considered to more thoroughly track sleep ontogeny from the beginning of the ELSD period into young adulthood in both male and female prairie voles thereby expanding on the developmental time points chosen here.
5. Conclusion
We find that early life sleep disruption for 1 week during postnatal development in the highly social prairie vole rodent chronically reduces REM sleep duration and has long lasting effects on the later development of REM sleep in adult males. Combined with our previously published research describing long term effects on male social bonding behavior after early life sleep disruption, these results mirror many of the behavioral and sex specific hallmarks of autism spectrum disorder.
CRediT authorship contribution statement
Carolyn E. Jones-Tinsley: Methodology, Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Project administration, Supervision. Randall J. Olson: Methodology, Investigation, Data curation, Writing – review & editing. Miranda Mader: Investigation, Data curation. Peyton T. Wickham: Investigation, Writing – review & editing. Katelyn Gutowsky: Investigation, Data curation. Claire Wong: Investigation, Data curation. Sung Sik Chu: Writing – review & editing. Noah E.P. Milman: Writing – review & editing. Hung Cao: Funding acquisition, Writing – review & editing. Miranda M. Lim: Methodology, Conceptualization, Resources, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Declarations of competing interest
None.
Acknowledgments
The data in this work was supported with resources and the use of facilities at the VA Portland Health Care System, National Science Foundation NCS Foundations Award # 1926818 to H.C. and M.M.L., NIH NHLBI T32 HL083808-10 to C.E.T., NIH NIDA T32 DA007262 to R.J.O, NIH NIA T32 AG055378 to N.E.M., and Portland VA Research Foundation to M.M.L. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbscr.2022.100087.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Buckley A.W., et al. Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Arch. Pediatr. Adolesc. Med. 2010;164(11):1032–1037. doi: 10.1001/archpediatrics.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin G.B., et al. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res. 1968;7(2):173–181. doi: 10.1016/0006-8993(68)90095-4. [DOI] [PubMed] [Google Scholar]
- DeVries A.C., Johnson C.L., Carter C.S. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster) Can. J. Zool. 1997;75(2):295–301. [Google Scholar]
- Díaz-Román A., et al. Sleep in youth with autism spectrum disorders: systematic review and meta-analysis of subjective and objective studies. Evid. Base Ment. Health. 2018;21(4):146–154. doi: 10.1136/ebmental-2018-300037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734(1–2):275–285. [PubMed] [Google Scholar]
- Feng P., Ma Y. Instrumental REM sleep deprivation in neonates leads to adult depression-like behaviors in rats. Sleep. 2003;26(8):990–996. doi: 10.1093/sleep/26.8.990. [DOI] [PubMed] [Google Scholar]
- Feng P., Ma Y., Vogel G.W. The critical window of brain development from susceptive to insusceptive: effects of clomipramine neonatal treatment on sexual behavior. Dev. Brain Res. 2001;129(1):107–110. doi: 10.1016/s0165-3806(01)00158-4. [DOI] [PubMed] [Google Scholar]
- Goldman S.E., et al. Parental sleep concerns in autism spectrum disorders: variations from childhood to adolescence. J. Autism Dev. Disord. 2012;42(4):531–538. doi: 10.1007/s10803-011-1270-5. [DOI] [PubMed] [Google Scholar]
- Gregory A.M., Sadeh A. Annual research review: sleep problems in childhood psychiatric disorders–a review of the latest science. JCPP (J. Child Psychol. Psychiatry) 2016;57(3):296–317. doi: 10.1111/jcpp.12469. [DOI] [PubMed] [Google Scholar]
- Hartley S.L., Sikora D.M. Sex differences in autism spectrum disorder: an examination of developmental functioning, autistic symptoms, and coexisting behavior problems in toddlers. J. Autism Dev. Disord. 2009;39(12):1715–1722. doi: 10.1007/s10803-009-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys J.S., et al. Sleep patterns in children with autistic spectrum disorders: a prospective cohort study. Arch. Dis. Child. 2014;99(2):114–118. doi: 10.1136/archdischild-2013-304083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R., Preston S., Winslow J.T. Mating in the monogamous male: behavioral consequences. Physiol. Behav. 1995;57(4):615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Jones C.E., et al. Early-life sleep disruption increases parvalbumin in primary somatosensory cortex and impairs social bonding in prairie voles. Sci. Adv. 2019;5(1):eaav5188. doi: 10.1126/sciadv.aav5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.E., et al. Early life sleep disruption alters glutamate and dendritic spines in prefrontal cortex and impairs cognitive flexibility in prairie voles. Current Research in Neurobiology. 2021;2 doi: 10.1016/j.crneur.2021.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovevska S., et al. Sleep quality in autism from adolescence to old age. Autism in Adulthood. 2020;2(2):152–162. doi: 10.1089/aut.2019.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinlogel H. The female rat's sleep during oestrous cycle. Neuropsychobiology. 1983;10(4):228–237. doi: 10.1159/000118016. [DOI] [PubMed] [Google Scholar]
- Kocevska D., et al. The developmental course of sleep disturbances across childhood relates to brain morphology at age 7: the Generation R Study. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw022. [DOI] [PubMed] [Google Scholar]
- Koehl M., Battle S.E., Turek F.W. Sleep in female mice: a strain comparison across the estrous cycle. Sleep. 2003;26(3):267–272. doi: 10.1093/sleep/26.3.267. [DOI] [PubMed] [Google Scholar]
- Krakowiak P., et al. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population‐based study. J. Sleep Res. 2008;17(2):197–206. doi: 10.1111/j.1365-2869.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., et al. Effects of chronic sleep fragmentation on wake-active neurons and the hypercapnic arousal response. Sleep. 2014;37(1):51–64. doi: 10.5665/sleep.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.M., et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429(6993):754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Limoges E., et al. Atypical sleep architecture and the autism phenotype. Brain. 2005;128(5):1049–1061. doi: 10.1093/brain/awh425. [DOI] [PubMed] [Google Scholar]
- Lord J.S., et al. Early life sleep disruption potentiates lasting sex-specific changes in behavior in genetically vulnerable Shank3 heterozygous autism model mice. Mol. Autism. 2022;13(1):1–19. doi: 10.1186/s13229-022-00514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret S., et al. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat. Neurosci. 2011;14(11):1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K.N., et al. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29(9):1211–1223. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- Rensing N., et al. Longitudinal analysis of developmental changes in electroencephalography patterns and sleep-wake states of the neonatal mouse. PLoS One. 2018;13(11):e0207031. doi: 10.1371/journal.pone.0207031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richdale A.L., Prior M.R. The sleep/wake rhythm in children with autism. Eur. Child Adolesc. Psychiatr. 1995;4(3):175–186. doi: 10.1007/BF01980456. [DOI] [PubMed] [Google Scholar]
- Saré R.M., Smith C.B. Association between sleep deficiencies with behavioral problems in autism spectrum disorder: subtle sex differences. Autism Res. 2020;13(10):1802–1810. doi: 10.1002/aur.2396. [DOI] [PubMed] [Google Scholar]
- Schreck K.A., Mulick J.A. Parental report of sleep problems in children with autism. J. Autism Dev. Disord. 2000;30(2):127–135. doi: 10.1023/a:1005407622050. [DOI] [PubMed] [Google Scholar]
- Schreck K.A., Mulick J.A., Smith A.F. Sleep problems as possible predictors of intensified symptoms of autism. Res. Dev. Disabil. 2004;25(1):57–66. doi: 10.1016/j.ridd.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Schwierin B., Borbély A.A., Tobler I. Sleep homeostasis in the female rat during the estrous cycle. Brain Res. 1998;811(1–2):96–104. doi: 10.1016/s0006-8993(98)00991-3. [DOI] [PubMed] [Google Scholar]
- Sikora D.M., et al. The relationship between sleep problems and daytime behavior in children of different ages with autism spectrum disorders. Pediatrics. 2012;130(Suppl. ment_2):S83–S90. doi: 10.1542/peds.2012-0900F. [DOI] [PubMed] [Google Scholar]
- Sinton C.M., Kovakkattu D., Friese R.S. Validation of a novel method to interrupt sleep in the mouse. J. Neurosci. Methods. 2009;184(1):71–78. doi: 10.1016/j.jneumeth.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Sivertsen B., et al. Sleep problems in children with autism spectrum problems: a longitudinal population-based study. Autism. 2012;16(2):139–150. doi: 10.1177/1362361311404255. [DOI] [PubMed] [Google Scholar]
- Swift K.M., et al. Sex differences within sleep in gonadally intact rats. Sleep. 2020;43(5):zsz289. doi: 10.1093/sleep/zsz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.A., Schreck K.A., Mulick J.A. Sleep disruption as a correlate to cognitive and adaptive behavior problems in autism spectrum disorders. Res. Dev. Disabil. 2012;33(5):1408–1417. doi: 10.1016/j.ridd.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Tesfaye R., et al. Investigating longitudinal associations between parent reported sleep in early childhood and teacher reported executive functioning in school-aged children with autism. Sleep. 2021;44(9):zsab122. doi: 10.1093/sleep/zsab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch O.J., et al. Shorter sleep duration is associated with social impairment and comorbidities in ASD. Autism Res. 2017;10(7):1221–1238. doi: 10.1002/aur.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeff M.E., et al. The bidirectional association between sleep problems and autism spectrum disorder: a population-based cohort study. Mol. Autism. 2018;9(1):1–9. doi: 10.1186/s13229-018-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G., et al. REM sleep abnormalities in a new animal model of endogenous depression. Neurosci. Biobehav. Rev. 1990;14(1):77–83. doi: 10.1016/s0149-7634(05)80163-0. [DOI] [PubMed] [Google Scholar]
- Williams J.R., Catania K.C., Carter C.S. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav. 1992;26(3):339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Yamaoka S. Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res. 1980;185(2):385–398. doi: 10.1016/0006-8993(80)91076-8. [DOI] [PubMed] [Google Scholar]
Further reading
- Ibuka N. Ontogenesis of circadian sleep-wakefulness rhythms and developmental changes of sleep in the altricial rat and in the precocial Guinea pig. Behav. Brain Res. 1984;11(3):185–196. doi: 10.1016/0166-4328(84)90210-9. [DOI] [PubMed] [Google Scholar]
- Jouvet‐Mounier D., Astic L., Lacote D. Ontogenesis of the states of sleep in rat, cat, and Guinea pig during the first postnatal month. Dev. Psychobiol.: The Journal of the International Society for Developmental Psychobiology. 1969;2(4):216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Roffwarg H.P., Muzio J.N., Dement W.C. Ontogenetic Development of the Human Sleep-Dream Cycle: the prime role of" dreaming sleep" in early life may be in the development of the central nervous system. Science. 1966;152(3722):604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.