Abstract
Objective:
Rates of ventilator-associated pneumonia (VAP) in low- and middle-income countries (LMIC) are several times above those of high-income countries. The objective of this study was to identify risk factors (RFs) for VAP cases in ICUs of LMICs.
Design:
Prospective cohort study.
Setting:
This study was conducted across 743 ICUs of 282 hospitals in 144 cities in 42 Asian, African, European, Latin American, and Middle Eastern countries.
Participants:
The study included patients admitted to ICUs across 24 years.
Results:
In total, 289,643 patients were followed during 1,951,405 patient days and acquired 8,236 VAPs. We analyzed 10 independent variables. Multiple logistic regression identified the following independent VAP RFs: male sex (adjusted odds ratio [aOR], 1.22; 95% confidence interval [CI], 1.16–1.28; P < .0001); longer length of stay (LOS), which increased the risk 7% per day (aOR, 1.07; 95% CI, 1.07–1.08; P < .0001); mechanical ventilation (MV) utilization ratio (aOR, 1.27; 95% CI, 1.23–1.31; P < .0001); continuous positive airway pressure (CPAP), which was associated with the highest risk (aOR, 13.38; 95% CI, 11.57–15.48; P < .0001); tracheostomy connected to a MV, which was associated with the next-highest risk (aOR, 8.31; 95% CI, 7.21–9.58; P < .0001); endotracheal tube connected to a MV (aOR, 6.76; 95% CI, 6.34–7.21; P < .0001); surgical hospitalization (aOR, 1.23; 95% CI, 1.17–1.29; P < .0001); admission to a public hospital (aOR, 1.59; 95% CI, 1.35-1.86; P < .0001); middle-income country (aOR, 1.22; 95% CI, 15–1.29; P < .0001); admission to an adult-oncology ICU, which was associated with the highest risk (aOR, 4.05; 95% CI, 3.22–5.09; P < .0001), admission to a neurologic ICU, which was associated with the next-highest risk (aOR, 2.48; 95% CI, 1.78–3.45; P < .0001); and admission to a respiratory ICU (aOR, 2.35; 95% CI, 1.79–3.07; P < .0001). Admission to a coronary ICU showed the lowest risk (aOR, 0.63; 95% CI, 0.51–0.77; P < .0001).
Conclusions:
Some identified VAP RFs are unlikely to change: sex, hospitalization type, ICU type, facility ownership, and country income level. Based on our results, we recommend focusing on strategies to reduce LOS, to reduce the MV utilization ratio, to limit CPAP use and implementing a set of evidence-based VAP prevention recommendations.
The International Nosocomial Infection Control Consortium (INICC) published international reports providing data on ventilator-associated pneumonia (VAP) and clinical outcomes of low- and middle-income countries (LMICs) in 2006, 1 2008, 2 2010, 3 2012, 4 2014, 5 2016, 6 2019, 7 and 2021. 8 Device utilization in INICC ICUs was comparable to that reported by the US Centers for Disease Control and Prevention National Healthcare Safety Network (CDC-NHSN) for ICUs, but INICC VAP rates were greater. 3 According to the CDC-NHSN, the VAP rate in medical surgical ICUs and all other ICUs with ≤15 beds in United States is 1.1 VAP cases per 1,000 mechanical ventilator (MV) days. 9 In the most recent international data for INICC ICUs, the pooled VAP rate was 10 times greater than those reported for CDC-NHSN ICUs (11.47 vs 1.1 per 1,000 ventilator days). 8 In INICC reports, the crude mortality rate in ICU patients without healthcare-associated infection (HAI) is 17.12% (95% CI, 16.93–17.32); for those with VAP it is 42.32% (95% CI, 40.61–44.09); and for those with VAP plus CLABSI plus CAUTI it is 63.44% (95% CI, 55.99–71.60). 8 A recent study demonstrated that VAP is an independent risk factor for mortality in a multiple logistic regression analysis (adjusted OR [aOR], 1.48; P < .0001). 10
The appropriate interventions to prevent VAP in LMICs have yet to be analyzed thoroughly and data are very limited. It is necessary to develop more definitive approaches for VAP prevention for implementation in LMICs. Researchers have identified the following VAP risk factors (RFs): tracheostomy, 11,12 length of stay (LOS), 13,14 older age, 15 trauma patients, 16 postsurgical patients, 17 burns patients, 17 longer duration of surgery, 18 history of smoking, 18 low serum albumin concentration, 17 high score on the American Society of Anesthesiologists (ASA) Physical Status Classification System, 17 Acute Physiology and Chronic Health Evaluation (APACHE II) score >20, 14 acute respiratory distress syndrome, 19 lung injury, 19 chronic obstructive pulmonary disease, 16 upper respiratory tract colonization, 16 sinusitis, 16 PaO2:FiO2 ratio <200 mmHg, 14 oropharyngeal colonization, 15 biofilm on the surface and within lumen of the endotracheal tube, 16 duration of mechanical ventilation (MV), 14,15 frequent change in ventilator circuit, 16 lack of use of heat and moist exchange humidifiers, 16 supine position, 15,20 frequent reintubation, 16 enteral feeding, 16 multiple central venous line insertions, 12 presence of catheter-related infection, 14 paralytic agents, 16 previous use of broad-spectrum antibiotics, 13,15 and patients transported out of an ICU. 16
Additional epidemiological studies need to be conducted to achieve an understanding of VAP risk factors in LMICs. Currently, no study has analyzed multiple countries simultaneously to identify VAP RFs in ICUs, nor has any study been conducted prospectively with a standardized form over 24 years. Also, no study has analyzed any of the following variables and their association with VAP: income level of the country according to the World Bank; facility ownership; hospitalization type; and ICU type. And all of these factors are important in understanding the unique challenges in LMICs.
The objective of this study was to simultaneously analyze the following 10 variables to identify VAP RFs in LMICs: (1) age, (2) sex, (3) duration of MV, (4) MV utilization ratio as marker of severity of illness of patients, (5) LOS, (6) type of respiratory support, (7) type of hospitalization, (8) ICU type, (9) facility ownership, and (10) income level according to the World Bank.
Methods
Study population and design
This prospective observational cohort study included patients admitted to 743 ICUs of 282 hospitals in 144 cities in 42 Asian, African, European, Latin American, and Middle Eastern countries across 24 years between July 1, 1998, and February 12, 2022.
Prospective cohort in ICUs and surveillance of HAIs
Each patient’s data were gathered at the time of ICU admission. An infection prevention professional (IPP) visited each patient’s bedside daily from the time of admission until discharge. This analysis prospectively included all adult and pediatric patients hospitalized in an ICU with or without HAI, and their data were gathered utilizing the INICC Surveillance Online System (ISOS). 21 An IPP brings a tablet to each hospitalized patient’s bedside in the ICU, signs in to the ISOS, and simultaneously uploads patient data. 21
Information provided at the time of admission includes setting (eg, nation, city, name of the hospital, and the ICU type) as well as information about the patient such as age, type of hospitalization, use of invasive devices (central line [CL], MV, urinary catheter [UC]), and presence of infection. 21 Every day until the patient is discharged, an IPP uploads details regarding invasive devices (CL, MV, and/or UC) and positive cultures (blood, urine, and respiratory samples) for each patient. 21
If the patient has signs or symptoms of infection, an infectious diseases specialist approaches the patient to determine the presence of an HAI (CLABSI, VAP, or CAUTI). According to the CDC-NHSN, an IPP looks at a patient’s signs and symptoms, cultures, radiographs, and other criteria that fulfill definitions of HAI. 22
Over the 24 years of this study, all IPPs of all participant hospitals have been applying the current and updated CDC definitions of HAI. That is, whenever the CDC updated their definitions, our IPPs began using the new updated definitions.
When IPPs upload the results of a culture to the ISOS, the ISOS immediately displays a message and directs the IPP to an online module of the ISOS where the IPP can check all the CDC-NHSN criteria to determine the presence of a HAI and the type of HAI (CLABSI, VAP, or CAUTI). 21
Daily device utilization checks are performed by ISOS. When a bias in patient days or device use is detected from admission to discharge, the ISOS notifies an IPP. The ISOS data may show that the patient has been hospitalized in the ICU without any devices in place, most likely because the IPP forgot to upload the use of devices or forgot to upload the discharge of the patient. If the ISOS detects the lack of use of any kind of device on any given day, it sends a message to the IPP to upload missing devices or upload the discharge of the patient. In other words, the ISOS asks the IPP to investigate why a patient in an ICU does not have any devices in place. 21 This approach significantly reduces biases associated with device utilization, patient days, and discharge conditions. 21
Patients with missing data were excluded from this study. The institutional review boards of the participating hospitals approved this study. Patient and hospital identities have been excluded for confidentiality.
INICC surveillance online system
Standard CDC-NSHN methodologies state that HAI denominators are device days gathered from all patients as pooled data, without mentioning the characteristics of particular patients or the quantity of device days associated with particular patients. 22 INICC HAI surveillance is carried out through an online platform, the ISOS, which includes CDC-NHSN criteria and methods. 22
Additionally, ISOS includes the gathering of patient-specific information on all patients, including those with and those without HAI, with a several variables per patient. 21 The ability to match data from all patients admitted to ICUs by different variables allows for the estimation of the VAP RFs. The CDC-NHSN criteria and methods are used in the data uploaded to ISOS to identify HAIs, to estimate HAI rates, and to determine device utilization ratios. 22
Validation of diagnosis of healthcare-associated infections
Validation of an HAI is a unique feature of the ISOS and is considered essential for maximizing the sensitivity and accuracy of surveillance data. Each HAI reported by an IPP is validated, that is, scrutinized to ascertain that criteria are fulfilled to justify its recording as an HAI. All necessary corrections and additions are indicated with a clear red sign on the screen. The validation process also includes the scrutiny of data reported for putatively uninfected patients to permit detection of unreported but true HAI. To accomplish this, should the ISOS suspect an HAI when the IPP uploads a culture to the ISOS but does not confirm an HAI (based on the uploaded culture, the date that the culture was taken, and the result of the culture), the ISOS automatic validation system sends an online message to the IPP requesting a check of the CDC-NHSN criteria for that putative HAI. Also, the ISOS sends a Excel (.xls) file (Microsoft, Redmond, WA) to the IPP every month with a list of biases regarding HAIs that have not been confirmed. 21
Study definitions
Ventilator was defined as any device used to support, assist, or control respiration through the application of positive pressure to the airway when delivered via an artificial airway, specifically an oral or nasal endotracheal or tracheostomy tube. Definitions of VAP used during surveillance were those published by the CDC in 199123 and included all subsequent updates through 2022. 24 VAP was defined as pneumonia in which the patient had been on MV for >2 consecutive calendar days on the date of the event, with the day of ventilator placement being day 1, and the ventilator had been in place on the date of the event or the day before. 24
Clinical pneumonia was defined as 2 or more serial chest-imaging results with at least 1 of the following: new and persistent or progressive and persistent, infiltrate, consolidation, cavitation, pneumatoceles (in infants aged ≤1 year). In addition, for any patient, at least 1 of the following must be present: fever, leukopenia or leukocytosis, or altered mental status with no other recognized cause (only in adults aged ≥70 years). Also, at least 2 of the following must be present: new onset of purulent sputum or change in character of sputum, increased respiratory secretions, or increased suctioning requirements; new-onset or worsening cough, dyspnea, or tachypnea; rales or bronchial breath sounds; worsening gas exchange; increased oxygen requirements; and/or increased ventilator demand. 24
Pneumonia with common bacterial or filamentous fungal pathogens and specific laboratory findings was defined as 2 or more serial chest imaging test results with at least 1 of the following: new and persistent or progressive and persistent infiltrate; consolidation; cavitation; pneumatoceles (in infants aged ≤1 year). Also, at least 1 of the following must be present: fever, leukopenia or leukocytosis, or altered mental status with no other recognized cause (only in adults aged ≥70 years). In addition, for any patient, at least 1 of the following must be present: new onset of purulent sputum, change in character of sputum, or increased respiratory secretions, or increased suctioning requirements; new onset or worsening cough, dyspnea, or tachypnea; rales or bronchial breath sounds; worsening gas exchange; increased oxygen requirements; or increased ventilator demand. In addition, at least 1 of the following must be present: organism identified from blood; organism identified from pleural fluid; positive quantitative culture or corresponding semiquantitative culture result from minimally contaminated LRT specimen; ≥5% BAL-obtained cells contain intracellular bacteria on direct microscopic exam; positive quantitative culture or corresponding semiquantitative culture result of lung tissue; or histopathologic exam showing evidence of pneumonia. 24
World Bank country classifications were defined in 4 income groups: low income, lower–middle income, upper–middle income, and high income. These classifications are based on gross national income (GNI) per capita expressed in current US dollars (USD). Low-income countries are those countries with a GNI <1,045 USD. Lower–middle income countries are those with a GNI between 1,046 and 4,095 USD. Upper–middle-income countries are those with a GNI between 4,096 and 12,695 USD. High-income countries are those with a GNI >12,695 USD. 25
Device utilization was calculated as the ratio of device days to patient days for each location type. As such, the device utilization of a location measures the use of invasive devices and constitutes an extrinsic RF for HAI. Device utilization may also serve as a marker for the severity of illness of patients (ie, severely ill patients are more likely to require an invasive device), which is an intrinsic RF for infection. 26
Facility and institution ownership type were defined as follows: publicly owned facilities owned or controlled by a governmental unit or another public corporation (where control is defined as the ability to determine the general corporate policy); not-for-profit privately owned facilities that are legal or social entities created for the purpose of producing goods and services, whose status does not permit them to be a source of income, profit or other financial gains for the unit(s) that establish, control or finance them; and, for-profit privately owned facilities that are legal entities set up for the purpose of producing goods and services and are capable of generating a profit or other financial gains for their owners. 27
Statistical analysis
Patients with and without VAP were compared using multiple logistic regression. Statistically significant variables were independently associated with an increased risk for VAP. The test statistic used was the Wald test, and the statistical significance level was set at 0.05. Calculated from the outputs of multiple logistic regression, adjusted odds ratios (aORs) and the corresponding 95% confidence intervals (CIs) of statistically significant variables were also reported.
We estimated variables independently associated with the outcome (VAP), adjusted to the following prospectively collected data: (1) sex (female or male), (2) age, (3) MV days before acquisition of VAP, (4) MV utilization ratio as a marker of severity of illness of patient, (5) type of respiratory support (continuous positive airway pressure [CPAP], endotracheal tube connected to a mechanical ventilator, tracheostomy connected to a mechanical ventilator, tracheostomy without connection to a mechanical ventilator, (6) hospitalization type (medical or surgical), (7) LOS, (8) ICU type (medical-surgical, medical, pediatric, surgical, coronary, neurosurgical, cardiothoracic, neurologic, trauma, pediatric oncology, or adult oncology), (9) facility ownership (publicly owned facility, not-for-profit privately owned facility, for-profit privately owned facility, or university hospital), 27 and (10) income per country according to the World Bank classification (ie, low, lower-middle, upper-middle, or high). 25
The evaluated outcome was the acquisition of VAP according to the CDC-NHSN definitions. All statistical analyses were performed using R version 4.1.3 software (R Foundation for Statistical Computing, Vienna, Austria).
To estimate VAP rates per country and per continent, we used the full database. To estimate risk factors for VAP, we included only those patients with data available for sex, age, and MV utilization ratio.
Results
A cohort, prospective, multicenter, surveillance study of VAP was conducted in 743 ICUs of 282 hospitals in 144 cities in 42 countries from Asia, Africa, Europe, Latin America, and Middle East currently participating in the INICC: Argentina, Bahrain, Brazil, Bulgaria, China, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Egypt, El Salvador, Greece, India, Jordan, Kingdom of Saudi Arabia, Kosovo, Kuwait, Lebanon, Malaysia, Mexico, Mongolia, Morocco, Nepal, Pakistan, Palestine, Panama, Papua New Guinea, Peru, Philippines, Poland, Romania, Russia, Slovakia, Serbia, Sri Lanka, Sudan, Thailand, Tunisia, Turkey, United Arab Emirates, and Vietnam.
In this is a cohort study, the length of participation by hospitals ranged from 1.17 to 226.07 months (mean, 38.47; SD 42.62). Between July 1, 1998, and February 12, 2022, over 24 years, 289,643 patients admitted to 743 ICUs were followed for 1,951,405 patient days, and these patients acquired 8,236 VAPs.
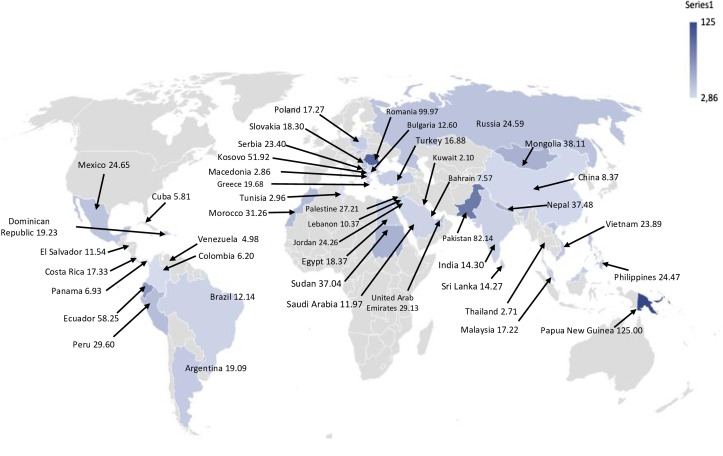
Table 1 shows data on facility ownership, ICU type, and other participating hospital and patient characteristics. Rates of VAP stratified per country and per region are shown in Table 2 and Figure 1. VAP rates stratified per ICU type and per type of respiratory support are shown in Table 3. VAP rates stratified per World Bank country classification by income level (lower–middle income, upper–middle income, and high income) and by facility ownership type (publicly owned facilities, for-profit privately owned facilities, Teaching hospitals, and not-for-profit privately owned facilities) are shown in Table 3.
Table 1.
Setting and Patient Characteristics, July 1, 1998, to February 12, 2022
| Variable | Total |
|---|---|
| Study years, no. | 24 |
| ICUs, no. | 743 |
| Hospitals, no. | 282 |
| Cities, no. | 144 |
| Countries, no. | 42 |
| Total patients, no. | 289,643 |
| Total patients days, no. | 1,951,405 |
| Average LOS, mean (SD) | 6.74 (8.33) |
| VAP cases, no. | 8,236 |
| Survival status, no. (%) | |
| Alive | 249,461 (86.13) |
| Death | 40,182 (13.87) |
| Countries, stratified per income level according to the World Bank, no. (%) | |
| Lower–middle-income country | 11 (30.56) |
| Upper–middle-income country | 19 (52.78) |
| High-income country | 6 (16.67) |
| Patients admitted per facility ownership, no. (%) | |
| Publicly owned facilities | 68,437 (23.63) |
| For-profit, privately owned facilities | 121,792 (42.05) |
| Teaching hospitals | 87,030 (30.05) |
| Not-for-profit, privately owned facilities | 12,384 (4.27) |
| Patients per hospitalization type, no. (%) | |
| Medical hospitalization | 210,427 (72.65) |
| Surgical hospitalization | 79,216 (27.35) |
| Patients admitted per type of ICU, no. (%) | |
| Medical-surgical ICU | 174,396 (60.21) |
| Medical ICU | 32,212 (11.12) |
| Coronary ICU | 26,940 (9.30) |
| Pediatric ICU | 15,851 (5.47) |
| Surgical ICU | 15,437 (5.33) |
| Cardiothoracic ICU | 8,215 (2.84) |
| Neurosurgical ICU | 5,710 (1.97) |
| Adult oncology ICU | 3,573 (1.23) |
| Trauma ICU | 2,724 (0.94) |
| Neurologic ICU | 1,703 (0.59) |
| Pediatric oncology ICU | 1,501 (0.52) |
| Respiratory ICU | 1,381 (0.48) |
| Sex, no. (%) | |
| Male | 176,382 (60.90) |
| Female | 113,261 (39.10) |
| Age, mean (SD) | 52.14 (23.93) |
| Device days and device utilization ratio | |
| MV utilization ratio, mean (SD) | 0.27 (0.65) |
| Total MV days, no.; mean (SD) | 702,335; 2.42 (6.19) |
| Days using following types of respiratory support | |
| CPAP connected to a MV | 2,361 |
| Endotracheal tube connected to a MV | 93,574 |
| Tracheostomy connected to a MV | 3,068 |
| Tracheostomy without connection to MV | 719 |
Note. ICU, intensive care unit; MV, mechanical ventilator; LOS, length of stay; VAP, ventilator-associated pneumonia; SD, standard deviation; CPAP, continuous positive airway pressure.
Table 2.
Ventilator-Associated Pneumonia Rates Stratified per Country and per Region
| Country | Patients, No. | Patient Days, No. | VAP Cases, No. | MV Days, No. | VAP Rate, No. | 95% CI |
|---|---|---|---|---|---|---|
| Argentina | 23,590 | 168,234 | 829 | 43,431 | 19.09 | 19.04–19.12 |
| Bahrain | 1,224 | 11,205 | 48 | 6,338 | 7.57 | 7.50–7.64 |
| Brazil | 16,895 | 150,181 | 860 | 70,833 | 12.14 | 12.11–12.16 |
| Bulgaria | 992 | 9,544 | 79 | 6,272 | 12.60 | 12.50–12.68 |
| China | 4,324 | 35,999 | 99 | 11,827 | 8.37 | 8.31–8.42 |
| Colombia | 17,160 | 127,842 | 338 | 54,559 | 6.20 | 6.17–6.21 |
| Costa Rica | 1,469 | 6,413 | 37 | 2,135 | 17.33 | 17.15–17.50 |
| Dominican Republic | 1,418 | 10,569 | 52 | 2,704 | 19.23 | 19.06–19.39 |
| Ecuador | 944 | 16,826 | 250 | 4,292 | 58.25 | 58.02–58.47 |
| Egypt | 5,751 | 66,521 | 357 | 19,437 | 18.37 | 18.30–18.42 |
| El Salvador | 1,128 | 9,811 | 85 | 7,367 | 11.54 | 11.46–11.61 |
| Greece | 100 | 801 | 17 | 864 | 19.68 | 19.38–19.97 |
| India | 151,486 | 1,963,884 | 3,863 | 270,233 | 14.30 | 14.28–14.31 |
| Jordan | 5,106 | 39,200 | 244 | 10,058 | 24.26 | 24.16–24.35 |
| Kosovo | 248 | 3,462 | 58 | 1,117 | 51.92 | 51.50–52.34 |
| Kuwait | 7,047 | 101,688 | 85 | 40,409 | 2.10 | 2.08–2.11 |
| Lebanon | 6,292 | 54,448 | 210 | 20,244 | 10.37 | 10.32–10.41 |
| Macedonia | 3,550 | 21,939 | 28 | 9,783 | 2.86 | 2.82–2.89 |
| Malaysia | 5,748 | 43,913 | 468 | 26,409 | 17.72 | 17.67–17.77 |
| Mexico | 9,002 | 69,880 | 1,014 | 41,141 | 24.65 | 24.60–24.69 |
| Mongolia | 2,458 | 23,363 | 172 | 4,513 | 38.11 | 37.93–38.29 |
| Morocco | 3,583 | 25,061 | 225 | 7,197 | 31.26 | 31.13–31.39 |
| Nepal | 2,009 | 25,806 | 177 | 4,723 | 37.48 | 37.30–37.65 |
| Pakistan | 714 | 5,738 | 126 | 1,534 | 82.14 | 81.68–82.59 |
| Palestine | 1,264 | 64,988 | 123 | 4,521 | 27.21 | 27.05-27.35 |
| Panama | 948 | 8,771 | 48 | 6,929 | 6.93 | 6.86–6.98 |
| Papua New Guinea | 17 | 106 | 1 | 8 | 125.00 | 117.37–132.99 |
| Peru | 2,034 | 12,752 | 180 | 6,081 | 29.60 | 29.46–29.73 |
| Philippines | 5,480 | 33,028 | 392 | 16,055 | 24.42 | 24.34–24.49 |
| Poland | 1,908 | 23,011 | 264 | 15,287 | 17.27 | 17.20–17.33 |
| Romania | 977 | 8,465 | 397 | 3,971 | 99.97 | 99.66–100.28 |
| Russia | 98 | 1,116 | 3 | 122 | 24.59 | 23.71–25.48 |
| Saudi Arabia | 27,276 | 322,683 | 1,909 | 159,500 | 11.97 | 11.95–11.99 |
| Serbia | 186 | 1,862 | 15 | 641 | 23.40 | 23.02–23.77 |
| Slovakia | 938 | 9,293 | 107 | 5,846 | 18.30 | 18.19–18.41 |
| Sri Lanka | 327 | 2,398 | 14 | 981 | 14.27 | 14.03–14.51 |
| Sudan | 69 | 434 | 1 | 27 | 37.04 | 34.77–39.40 |
| Thailand | 649 | 2,774 | 2 | 739 | 2.71 | 2.58–2.82 |
| Tunisia | 221 | 1,909 | 4 | 1,351 | 2.96 | 2.86–3.05 |
| Turkey | 13,172 | 258,098 | 1,731 | 102,569 | 16.88 | 16.85–16.90 |
| United Arab Emirates | 385 | 53,273 | 6 | 206 | 29.13 | 28.39–29.87 |
| Vietnam | 4,280 | 51,644 | 442 | 18,504 | 23.89 | 23.81–23.95 |
| Region | ||||||
| Latin America | 74,578 | 581,279 | 3,683 | 239,472 | 15.38 | 15.36–15.40 |
| Asia | 177,155 | 2,186,255 | 5,732 | 354,545 | 16.17 | 16.15–16.18 |
| Eastern Europe | 8,988 | 79,493 | 959 | 43,903 | 21.84 | 21.80–21.88 |
| Middle East | 70,115 | 934,520 | 4,809 | 367,336 | 13.09 | 13.08–13.10 |
Note. MV, mechanical ventilator; VAP, ventilator-associated pneumonia; CI, confidence interval.
Fig. 1.
Rate of ventilator-associated pneumonia per 1,000 mechanical ventilator day, stratified per country.
Table 3.
Ventilator-Associated Pneumonia Rates Stratified per ICU Type, per Type of Respiratory Support, per World Bank Country Classifications by Income Level, and per Facility Ownership Type
| Variable | Patients, No. | Patient Days, No. | VAP, No. | MV Days, No. | VAP Rate | 95% CI |
|---|---|---|---|---|---|---|
| ICU type a | ||||||
| Adult-oncology | 1,381 | 13,438 | 92 | 9,679 | 24.96 | 24.84–25.07 |
| Neurologic | 1,703 | 11,702 | 52 | 3,389 | 15.34 | 15.21–15.47 |
| Medical | 5,710 | 38,669 | 121 | 11,806 | 14.07 | 14.04–14.10 |
| Cardiothoracic | 174,396 | 1,181,406 | 5,790 | 477,062 | 12.50 | 12.44–12.54 |
| Pediatric oncology | 3,573 | 17,748 | 173 | 6,931 | 12.23 | 12.05–12.40 |
| Medical-surgical | 32,212 | 234,303 | 911 | 64,731 | 12.14 | 12.13–12.15 |
| Neurosurgical | 8,215 | 49,858 | 225 | 18,004 | 10.25 | 10.19–10.30 |
| Respiratory | 2,724 | 13,357 | 27 | 4,593 | 9.51 | 9.44–9.56 |
| Surgical | 15,851 | 124,703 | 388 | 48,049 | 8.23 | 8.19–8.25 |
| Pediatric | 26,940 | 154,734 | 209 | 28,696 | 8.08 | 8.04–8.10 |
| Coronary | 1,501 | 9,288 | 19 | 1,554 | 7.28 | 7.25–7.31 |
| Trauma | 15,437 | 102,199 | 229 | 27,841 | 5.88 | 5.80–5.94 |
| Pooled | 289,643 | 1,951,405 | 8,236 | 702,335 | 11.73 | 11.72–11.73 |
| Respiratory support type | ||||||
| CPAP connected to a MV | 2,361 | 18,187 | 252 | 4,092 | 61.58 | 61.34–61.82 |
| Tracheostomy connected to MV | 3,068 | 41,098 | 329 | 30,751 | 10.70 | 10.66–10.73 |
| Endotracheal tube connected to MV | 93,574 | 834,256 | 5,857 | 587,815 | 9.96 | 9.95–9.97 |
| Lower–middle income | ||||||
| Pooled | 154,646 | 907,515 | 3,453 | 256,999 | 13.44 | 13.42–13.45 |
| Publicly owned facilities | 14,333 | 91,176 | 606 | 33,562 | 18.06 | 18.01–18.10 |
| For-profit privately owned facilities | 76,555 | 442,987 | 1,996 | 121,341 | 16.45 | 16.43–16.47 |
| Teaching hospitals | 52,805 | 312,669 | 707 | 86,626 | 8.16 | 8.14–8.18 |
| Not-for-profit, privately owned facilities | 10,953 | 60,683 | 144 | 15,470 | 9.31 | 9.26–9.35 |
| Upper–middle income | ||||||
| Pooled | 98,839 | 699,513 | 3,277 | 273,755 | 11.97 | 11.96–11.98 |
| Publicly owned facilities | 22,515 | 167,783 | 963 | 75,883 | 12.69 | 12.66–12.71 |
| For-profit, privately owned facilities | 42,536 | 262,694 | 854 | 73,329 | 11.64 | 11.62–11.67 |
| University hospitals | 32,357 | 258,542 | 1,396 | 119,806 | 11.65 | 11.63–11.67 |
| Not-for-profit, privately owned facilities | 1,431 | 10,494 | 64 | 4,737 | 13.51 | 13.40–13.61 |
| High income | ||||||
| Pooled | 36,158 | 344,377 | 1,506 | 171,581 | 8.78 | 8.76–8.79 |
| Publicly owned facilities | 31,589 | 300,701 | 1,208 | 148,744 | 8.12 | 8.10–8.13 |
| For-profit, privately owned facilities | 2,701 | 26,083 | 133 | 10,358 | 12.84 | 12.77–12.91 |
| University hospitals | 1,868 | 17,593 | 165 | 12,479 | 13.22 | 13.15–13.28 |
Note. ICU, intensive care unit; CI, confidence interval; MV, mechanical ventilator; VAP, ventilator-associated pneumonia; CPAP, continuous positive airway pressure.
aICUs are listed in order of the highest to lowest ventilator-associated pneumonia rate.
Using multiple logistic regression, the following 6 variables were identified as statistically significantly independently associated with VAP: male sex; longer LOS, which increased the risk by 7% per day; MV utilization ratio; CPAP, which was associated with the highest risk; tracheostomy connected to a MV and endotracheal tube connected to a MV, which had the next-highest risk; surgical hospitalization instead of medical; public hospital; middle-income country; adult oncology ICU, which was associated with the highest risk; neurologic ICU and respiratory ICU, which had the next-highest risk. (Table 4). Coronary ICU showed the lowest risk.
Table 4.
Multiple Logistic Regression Analysis of Risk Factors for Ventilator-Associated Pneumonia
| Variable | aOR | 95% CI | P Value |
|---|---|---|---|
| Age, y | 1 | 1.00–1.01 | .01 |
| Sex, male | 1.22 | 1.16–1.28 | <.0001 |
| Length of stay, d | 1.07 | 1.07–1.08 | <.0001 |
| MV days | 0.96 | 0.95–0.96 | <.0001 |
| MV utilization ratio | 1.27 | 1.23–1.31 | <.0001 |
| MV type | |||
| CPAP connected to a MV | 13.38 | 11.57–15.48 | <.0001 |
| Tracheostomy connected to a MV | 8.31 | 7.21–9.58 | <.0001 |
| Endotracheal tube connected to a MV | 6.76 | 6.34–7.21 | <.0001 |
| Tracheostomy not connected to a MV | 4.48 | 3.19–6.28 | <.0001 |
| Surgical hospitalization | 1.23 | 1.17–1.29 | <.0001 |
| Hospital type | |||
| Publicly owned facilities | 1.59 | 1.35–1.86 | <.0001 |
| For-profit privately owned facilities | 1.36 | 1.17–1.59 | <.0001 |
| Teaching hospitals | 1.05 | 0.91–1.24 | .48 |
| Country classification | |||
| Upper middle income country | 1.22 | 1.15–1.29 | <.0001 |
| High income country | 0.79 | 0.73–0.86 | <.0001 |
| ICU type | |||
| Adult oncology ICU | 4.05 | 3.22–5.09 | <.0001 |
| Neurologic ICU | 2.48 | 1.78–3.45 | <.0001 |
| Respiratory ICU | 2.35 | 1.79–3.07 | <.0001 |
| Medical-surgical ICU | 2.15 | 1.85–2.49 | <.0001 |
| Medical ICU | 1.99 | 1.68–2.34 | <.0001 |
| Neurosurgical ICU | 1.26 | 0.98–1.63 | .07 |
| Pediatric ICU | 1.19 | 0.97–1.43 | .08 |
| Pediatric oncology ICU | 1.09 | 0.64–1.83 | .76 |
| Surgical ICU | 1.02 | 0.83–1.24 | .87 |
| Trauma ICU | 0.91 | 0.59–1.39 | .67 |
| Coronary ICU | 0.63 | 0.51–0.77 | <.0001 |
Note. ICU, intensive care unit; MV, mechanical ventilation; LOS, length of stay; VAP, ventilator-associated pneumonia; CPAP, continuous positive airway pressure; aOR, adjusted odds ratio; CI, confidence interval.
Discussion
The VAP rates in the present study per country and per continent are significantly higher than those of the CDC-NHSN. 8 This finding has been reported by the INICC since 20061 and beyond. 2–8
In the present study, we identified an association between male sex and VAP. In 1997, Kollef et al 28 conducted a prospective cohort study in ICUs of Barnes-Jewish Hospital; they analyzed 521 ICU patients requiring MV for >12 hours. With multiple logistic regression analysis, they demonstrated that male sex was independently associated with the development of VAP. 28
We further identified an association between the MV utilization ratio and VAP. In 2000, Sofianou et al 14 conducted a prospective study to determine risk factors for VAP in 198 patients requiring MV for >48 hours. They found that MV for >10 days was a risk factor for VAP (OR, 44.4; 95% CI, 2.16–26.7; P < .0001). 14
In our study, CPAP was associated with risk of acquiring pneumonia. Strategies to prevent VAP published by the Society for Healthcare Epidemiology of America (SHEA)–Association for Professionals in Infection Control and Epidemiology (APIC)–Infectious Diseases Society of America (IDSA) include the recommendation of using high-flow nasal oxygen or noninvasive positive pressure ventilation based on high quality of evidence, but there is no recommendation to use CPAP. 29 A nationwide study conducted in Taiwan analyzed the impact of CPAP as a pneumonia RF. During 10 years, they identified adult patients with sleep apnea from the Taiwan National Health Insurance Research Database. A control cohort without sleep apnea, matched for age, sex and comorbidities, was selected for comparison. Of the 34,100 patients (6,816 study patients and 27,284 matched controls), 2,757 (8.09%) had pneumonia during a mean follow-up period of 4.50 years, including 638 (9.36%) study patients and 2,119 (7.77%) controls. Kaplan–Meier analysis showed a higher incidence of pneumonia among patients with sleep apnea (log rank test, P < .001). After multivariate adjustment, patients with sleep apnea experienced a 1.20-fold (95% CI, 1.10–1.31) increase in incident pneumonia. The risk was even higher among patients who use CPAP. 30
We did not find a difference in the risk of VAP associated with age. However, Jovanovic et al 31 conducted a prospective study to identify VAP RF, and age was independently associated with late-onset VAP. Furthermore, the Jovanovic study reported an association between surgical hospitalization and VAP compared with medical hospitalization. In our study, the ICUs with the highest risk for VAP were adult oncology ICU, respiratory ICU, and neurology ICU. The coronary ICU showed the lowest risk of VAP. The MV utilization ratio, as a marker of severity of illness of patients, is the highest in these types of ICUs, 32 which could explain why these ICUs are associated with the highest risk of VAP.
Moreover, we detected an association between the acquisition of VAP rates in public hospitals compared with Teaching hospitals. However, a study 33 conducted in neonatal ICUs found that the VAP rate per 1,000 MV days at Teaching hospitals was 13.2 (95% CI, 11.5–15.0). At public hospitals, this rate was 4.9 (95% CI, 2.5–8.6), and at private hospitals, this rate was 2.4 (95% CI, 1.3–3.9). Compared with public hospitals, Teaching hospitals showed a higher risk for VAP (relative risk [RR], 2.69; 95% CI, 1.50–4.80; P = .0001). 33 In a study 34 conducted in pediatric ICUs, the VAP rate per 1,000 MV days at Teaching hospitals was 8.3 (95% CI, 7.3–9.3). At public hospitals this rate was 4.7 (95% CI, 3.9–5.7), and at private hospitals this rate was 3.5 (95% CI, 2.6–4.5). 34 Compared with private or public hospitals, Teaching hospitals showed a highest risk for VAP. 34
In our study, patients admitted to ICUs in upper–middle-income countries were at higher risk for VAP than those admitted to ICUs in high-income countries. This finding could be explained by the lower quality of healthcare programs in middle-income countries participating in this study. A previous study 33 conducted in NICUs reported a VAP rate per 1,000 MV days at lower–middle-income countries of 11.8 (95% CI, 10.1–13.6). In upper–middle-income countries, this rate was 6.7 (95% CI, 5.2–8.5). Compared with upper–middle-income countries, lower–middle-income countries showed a higher risk for VAP (RR, 1.75; 95% CI, 1.32–2.32; P = .0001). 33 Another study 34 conducted in PICUs reported a VAP rate per 1,000 MV-days in lower–middle-income countries of 9.0 (95% CI, 7.5–10.6). In upper–middle-income countries, this rate was 5.4 (95% CI, 4.8–6.1). Compared with upper–middle-income countries, lower–middle-income countries showed a higher risk for VAP. 34
According with the most recent and also previous INICC reports 1–8 published from 2006 to 2021, VAP is the most prevalent HAI in LMICs, and VAP is associated with high mortality, extra LOS, costs, and high bacterial resistance. 1–8 To save countless lives in LMICs, it is essential to act quickly to control and prevent VAP. 10 To do so, we suggest first focusing on identifying an evidence-based set of VAP prevention recommendations, such as those of SHEA–APIC–IDSA. 29 Given that these organizations have already identified a number of evidence-based strategies to prevent the acquisition of VAP, it is crucial to be aware of this set of recommendations. 29 Second, it is also recommended to monitor healthcare worker compliance with this set of recommendations and to provide them with performance feedback. This strategy has been effective in reducing the very high rate of VAP in LMICs. 35–42 Last but not least, we suggest focusing on risk factors that can be changed to prevent VAPs. Some VAP risk factors are unlikely to change, such as sex, medical or surgical hospitalization, ICU type, facility ownership, and the country’s economy. Based on the our findings, addressing the following risk factors has the highest chance to reduce VAP: reducing LOS, limiting the duration of mechanical ventilation, limiting the use of CPAP. In addition, we suggest following a set of evidence-based recommendations to prevent VAP such as those published by the SHEA–APIC–IDSA. 29
This study had several strengths. We use a prospective cohort study design. We collected data prospectively using standardized forms with a checklist for diagnosis of VAP, an online platform with dropdown menus to select the options of type of devices used, criteria for VAP, and diagnosis of VAP, to avoid typos in collected data. We used an electronic system to avoid bias in data collection among denominators and VAPs. IPPs who collected the data were trained individually by our principal investigator. This study was conducted across 24 years in 42 countries. All hospitals worldwide are invited to join this surveillance and research network and to collect data on HAIs using the INICC online platform (ie, participation is free).
Our research also had several limitations. First, this study is not representative of all hospitals in the 42 participant countries because hospitals voluntarily join the INICC and use this surveillance system for free. Second, the hospitals that participate in our surveillance system likely have better-quality HAI surveillance and prevention programs. Thus, the HAI rates in our study may be lower than the HAI rates in other hospitals not participating in our research. Finally, participating hospitals have not collected data on disease severity scores and underlying diseases, but we collected mechanical ventilation utilization ratio as a marker of severity of illness of patients, and we adjusted the analysis to this independent variable.
Acknowledgments
The authors thank the many healthcare professionals who assisted in conducting surveillance in their hospitals, the INICC advisory board, country directors, and secretaries who have so generously supported this unique international infection control network.
Author contributions
Dr. Rosenthal was responsible for study conception and design, software development, technical support, drafting tutorials for surveillance process, training of data collectors, provision of study patients, data validation, data assembly, data interpretation, epidemiological analysis, drafting of the manuscript. Zhilin Jin and Ruijie Yin contributed equally to building machine-learning models and conducting statistical analyses, critical revision for important intellectual content, and final approval of the manuscript. The remaining authors were involved in the providing patient data. All authors were involved in critical revision of the manuscript for important intellectual content and final approval of the manuscript.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1. Rosenthal VD, Maki DG, Salomao R, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med 2006;145:582–591. [DOI] [PubMed] [Google Scholar]
- 2. Rosenthal VD, Maki DG, Mehta A, et al. International Nosocomial Infection Control Consortium report, data summary for 2002–2007, issued January 2008. Am J Infect Control 2008;36:627–637. [DOI] [PubMed] [Google Scholar]
- 3. Rosenthal VD, Maki DG, Jamulitrat S, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003–2008, issued June 2009. Am J Infect Control 2010;38:95–104. [DOI] [PubMed] [Google Scholar]
- 4. Rosenthal VD, Bijie H, Maki DG, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009, (in eng), Am J Infect Control 2012;40:396–407. [DOI] [PubMed] [Google Scholar]
- 5. Rosenthal VD, Maki DG, Mehta Y, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 43 countries for 2007–2012. Device-associated module. Am J Infect Control 2014;42:942–956. [DOI] [PubMed] [Google Scholar]
- 6. Rosenthal VD, Al-Abdely HM, El-Kholy AA, et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010–2015: device-associated module. Am J Infect Control 2016;44:1495–1504. [DOI] [PubMed] [Google Scholar]
- 7. Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012–2017: device-associated module. Am J Infect Control 2020;48:423–432. [DOI] [PubMed] [Google Scholar]
- 8. Rosenthal VD, Duszynska W, Bat-Erdene I, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2013–2018, adult and pediatric units, device-associated module. Am J Infect Control 2021;49:1267–1274. [DOI] [PubMed] [Google Scholar]
- 9. Dudeck M. National Healthcare Safety Network (NHSN) Report, data summary for 2011, device-associated module. https://typeset.io/pdf/national-healthcare-safety-network-nhsn-report-data-summary-2qz4c6rli3.pdf Accessed September 17, 2022. [DOI] [PubMed]
- 10. Rosenthal VD, Yin R, Lu Y, et al. The impact of healthcare-associated infections on mortality in ICU: a prospective study in Asia, Eastern Europe, Latin America, and the Middle East. Am J Infect Control 2022. doi: 10.1016/j.ajic.2022.08.024. [DOI] [PubMed]
- 11. Sugerman HJ, Wolfe L, Pasquale MD, et al. Multicenter, randomized, prospective trial of early tracheostomy. J Trauma 1997;43:741–747. [DOI] [PubMed] [Google Scholar]
- 12. Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest 2001;120:555–561. [DOI] [PubMed] [Google Scholar]
- 13. Talon D, Mulin B, Rouget C, Bailly P, Thouverez M, Viel JF. Risks and routes for ventilator-associated pneumonia with Pseudomonas aeruginosa . Am J Respir Crit Care Med 1998;157:978–984. [DOI] [PubMed] [Google Scholar]
- 14. Sofianou DC, Constandinidis TC, Yannacou M, Anastasiou H, Sofianos E. Analysis of risk factors for ventilator-associated pneumonia in a multidisciplinary intensive care unit. Eur J Clin Microbiol Infect Dis 2000;19:460–463. [DOI] [PubMed] [Google Scholar]
- 15. Bauer TT, Ferrer R, Angrill J, Schultze-Werninghaus G, Torres A. Ventilator-associated pneumonia: incidence, risk factors, and microbiology. Semin Respir Infect 2000;15:272–279. [DOI] [PubMed] [Google Scholar]
- 16. Charles MP, Kali A, Easow JM, et al. Ventilator-associated pneumonia. Australas Med J 2014;7:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garibaldi RA, Britt MR, Coleman ML, Reading JC, Pace NL. Risk factors for postoperative pneumonia. Am J Med 1981;70:677–680. [DOI] [PubMed] [Google Scholar]
- 18. Grobmyer SR, Maniscalco SP, Purdue GF, Alcohol Hunt JL., drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J Burn Care Rehabil 1996;17:532–529. [DOI] [PubMed] [Google Scholar]
- 19. Chastre J, Trouillet JL, Vuagnat A, et al. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1998;157:1165–1172. [DOI] [PubMed] [Google Scholar]
- 20. Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J 2002;20:1017–1028. [DOI] [PubMed] [Google Scholar]
- 21. Rosenthal VD. International Nosocomial Infection Control Consortium (INICC) resources: INICC multidimensional approach and INICC surveillance online system. Am J Infect Control 2016;44:e81–e90. [DOI] [PubMed] [Google Scholar]
- 22. National Healthcare Safety Network (NHSN) patient safety component manual. Centers for Disease Control and Prevention website. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed August 23, 2022.
- 23. Emori TG, et al. National nosocomial infections surveillance system (NNIS): description of surveillance methods. Am J Infect Control 1991;19:19–35. [DOI] [PubMed] [Google Scholar]
- 24. National Healthcare Safety Network. General key terms. Centers for Disease Control and Prevention website. https://www.cdc.gov/nhsn/pdfs/pscmanual/16psckeyterms_current.pdf. Accessed August 23, 2022.
- 25. New World Bank country classifications by income level, 2021–2022. World Bank website. https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2021-2022. Accessed August 23, 2022.
- 26. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network report, data summary for 2013, device-associated module. Am J Infect Control 2015;43:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glossary of terms. WHO European Primary Health Care Impact Performance and Capacity Tool (PHC-IMPACT). World Health Organization website. https://www.euro.who.int/__data/assets/pdf_file/0006/421944/Glossary-web-171219.pdf. Accessed August 23, 2022.
- 28. Kollef MH, Von Harz B, Prentice D, et al. Patient transport from intensive care increases the risk of developing ventilator-associated pneumonia. Chest 1997;112:765–773. [DOI] [PubMed] [Google Scholar]
- 29. Klompas M, Branson R, Cawcutt K, et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 update. Infect Control Hosp Epidemiol 2022;43:687–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su VY, Liu C-J, Wang H-K, et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ 2014;186:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beardsley AL. Ventilator-associated infections need a new approach. Pediatr Crit Care Med 2016;17:587. [DOI] [PubMed] [Google Scholar]
- 32. National Healthcare Safety Network. The 2019 National and State Healthcare-Associated Infections (HAI) Progress Report. Centers for Disease Control and Prevention website. https://www.cdc.gov/nhsn/datastat/index.html. Accessed February 17, 2022. [Google Scholar]
- 33. Rosenthal VD, Lynch P, Jarvis WR, et al. Socioeconomic impact on device-associated infections in limited-resource neonatal intensive care units: findings of the INICC. Infection 2011;39:439–450. [DOI] [PubMed] [Google Scholar]
- 34. Rosenthal VD, Jarvis WR, Jamulitrat S, et al. Socioeconomic impact on device-associated infections in pediatric intensive care units of 16 limited-resource countries: International Nosocomial Infection Control Consortium findings. Pediatr Crit Care Med 2012;13:399–406. [DOI] [PubMed] [Google Scholar]
- 35. Leblebicioglu H, Yalcin AN, Rosenthal VD, et al. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in 11 adult intensive care units from 10 cities of Turkey: findings of the International Nosocomial Infection Control Consortium (INICC). Infection 2013;41:447–456. [DOI] [PubMed] [Google Scholar]
- 36. Mehta Y, Jaggi N, Rosenthal VD, et al. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in 21 adult intensive-care units from 10 cities in India: findings of the International Nosocomial Infection Control Consortium (INICC). Epidemiol Infect 2013;141:2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenthal VD, Rodrigues C, Álvarez-Moreno C, et al. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in adult intensive care units from 14 developing countries of four continents: findings of the International Nosocomial Infection Control Consortium. Crit Care Med 2012;40:3121–3128. [DOI] [PubMed] [Google Scholar]
- 38. Rosenthal VD, Álvarez-Moreno C, Villamil-Gómez W, et al. Effectiveness of a multidimensional approach to reduce ventilator-associated pneumonia in pediatric intensive care units of 5 developing countries: International Nosocomial Infection Control Consortium findings. Am J Infect Control 2012;40:497–501. [DOI] [PubMed] [Google Scholar]
- 39. Rosenthal VD, Rodríguez-Calderón ME, Rodríguez-Ferrer M, et al. Findings of the International Nosocomial Infection Control Consortium (INICC), part II: impact of a multidimensional strategy to reduce ventilator-associated pneumonia in neonatal intensive care units in 10 developing countries. Infect Control Hosp Epidemiol 2012;33:704–710. [DOI] [PubMed] [Google Scholar]
- 40. Tao L, Hu B, Rosenthal VD, Zhang Y, Gao X, He L. Impact of a multidimensional approach on ventilator-associated pneumonia rates in a hospital of Shanghai: findings of the International Nosocomial Infection Control Consortium. J Crit Care 2012:27:440–446. [DOI] [PubMed] [Google Scholar]
- 41. Al-Mousa HH, Omar AA, Rosenthal VD, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional approach on rates of ventilator-associated pneumonia in intensive care units of two hospitals in Kuwait. J Infect Prev 2018;19:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosenthal VD, Desse J, Maurizi DM, et al. Impact of the International Nosocomial Infection Control Consortium’s multidimensional approach on rates of ventilator-associated pneumonia in 14 intensive care units in 11 hospitals of 5 cities within Argentina. Am J Infect Control 2018;46:674–679. [DOI] [PubMed] [Google Scholar]