Abstract
Purpose
To identify and rank areas of treatment burden in chronic heart failure (CHF), including solutions, that should be discussed during the clinical encounter from a patient, and doctors’ perspective.
Patients and Methods
Patients with CHF and clinicians managing heart failure were invited. Nominal group technique sessions held either face to face or online in 2021–2022, with individual identification of priorities and voting on ranking.
Results
Four patient groups (N=22) and one doctor group (N=5) were held. For patients with heart failure, in descending order of priority Doctor-patient communication, Inefficiencies of the healthcare system, Healthcare access issues, Cost implications of treatment, Psychosocial impacts on patients and their families, and Impact of treatment work were the most important treatment burdens. Priorities independently identified by the doctors aligned with the patients’ but ranking differed. Patient solutions ranged from involvement of nurses or pharmacists to enhance understanding of discharge planning, through to linkage between health information systems, and maintaining strong family or social support networks. Doctors’ solutions covered timing medicines with activities of daily living, patient education on the importance of compliance, medication reviews to overcome clinical inertia, and routine clinical audits.
Conclusion
The top treatment burden priorities for CHF patients were related to interaction with clinicians and health system inefficiencies, whereas doctors were generally aware of patients’ treatment burden but tended to focus on the complexity of the direct treatment work. Addressing the priority issues identified here can commence with clinicians becoming aware of the issues that matter to patients and proactively discussing feasible immediate and longer-term solutions during clinical encounters.
Keywords: patient priorities, barriers, qualitative studies, older adults
Introduction
The population-wide prevalence of chronic heart failure (CHF) has been reported at 1–2%1 but the estimate increases three-fold for older people aged ≥45 years.2 The incidence of CHF is increasing in the developed and developing world,2–4 leading to progressive disability,5 as well as high costs for health care systems.6,7 As a result, attention has focused on its early detection, intensive treatment, patient education and self-management. Heart failure (HF) can be asymptomatic8 or present without alterations of ventricular ejection fraction (detectable on echocardiography),9 so a large proportion of patients can experience delayed diagnosis and subsequent worsened prognosis.10
A challenge less evident than the burden of symptoms for patients with chronic disease11 and their families is the burden of treatment. With the rapidly ageing population, the prevalence of comorbidities and the lack of readiness of health systems to deal with the increasing workload of repeat hospital presentations and admissions, the delegation of some disease management tasks to patients has escalated. While giving patient autonomy and capacity to self-manage is a worthy initiative, the physical, cognitive and emotional impact of these added responsibilities without matching health education or social networks may be detrimental.12 These disruptions to patients’ lifestyle routine is known to affect treatment compliance and quality of life13 as not all patients have the same level of literacy, financial resources or competence to meet the expected duties or access informal support.14 Treatment burden encompasses the tasks given by healthcare professionals to a patient and/or their informal caregiver for optimal management of a chronic disease, a workload that also impacts on the quality of life of patients’ families.15,16 A systematic review of qualitative studies reported that treatment burden includes a wide range of issues from patients receiving incomplete or conflicting information on prognosis, discordant expectations between patients and healthcare providers, poor availability of support services, to the need to self-monitoring treatment progress and disease milestones.17 In the case of heart failure, treatment burden has been described as the combined workload imposed on patients by multiple appointment, lifestyle changes, medications and the suboptimal communication and organisation of care.18 These pressures, including lack of relevant information and reduced service access, affect social interactions, emotional health and ability to self-manage the disease.19
People living with heart failure may not be fully aware of their illness trajectory, their goals of care or the actions required in a health crisis.20 To compound matters, the sometimes extensive list of recommendations delivered by doctors, while aligned with best practice guidelines, can negatively impact on patients’ social life,14 emotional wellbeing, quality of life,16 motivation for treatment adherence, satisfaction with treatment, and subsequent health outcomes.21 While measurement of this burden is subjective and patient-specific,21 treatment burden has a significant impact on patients and reflects the quality of patient-centered care (or lack thereof) that needs attention and remedial action.
In an ideal world, treatment burden would routinely be discussed in the patient-clinician encounter. However, despite this concept being acknowledged already a decade ago, it has not yet fully been incorporated in routine care or clinical practice guidelines.22 In an effort to address this gap, we aimed to elucidate treatment burden topics that patients and clinicians thought were important to discuss in the clinical encounter.23 It is also important to ascertain clinician’s awareness and understanding of the implications that self-management activities can have for their patients. The results may help to clarify barriers to treatment adherence, address patients’ treatment burden, including strategies that can be implemented in the clinical relationship (eg, de-prescribing, tailoring information to patients’ needs), as well as planning health service improvements, inform education programs and resource allocation.
The nominal group technique (NGT) was considered the method of choice as it is widely recommended to achieve consensus on priorities,24 and it has previously been used in patients with multiple chronic health conditions.25
Material and Methods
Specific Objectives
To identify and rank areas of treatment burden in chronic heart failure, including solutions, that should be discussed during the clinical encounter from a patient, and doctors’ perspective. This manuscript reports the findings from nominal group technique sessions, including patients with heart failure and physicians treating patients with heart failure, and follows the Standards for Reporting Qualitative Research guidelines.26
Eligibility
For the patient NGT sessions, adults (≥18 years) with chronic heart failure with or without comorbidities who had received in- and/or outpatient care at The Gold Coast Hospital and Health Service, Australia were invited to participate. Those with inability to give informed consent or unable to communicate in English were excluded. For the doctor NGT sessions, physicians in the Australian States of New South Wales and Queensland, who had managed patients with heart failure for at least two years, were invited to contribute.
Recruitment
We obtained ethics approval from our institutional human ethics board and our study complies with the Declaration of Helsinki. Separate nominal groups were conducted for patients and doctors. Patients were identified from outpatient clinic lists, the hospital’s electronic medical records, or through direct referrals from hospital doctors. Participants received an information sheet, and a brief set of questions on demographics, number of comorbidities, number of daily medications, and after considering the number of hours spent on self-care activities they were asked their self-perceived treatment burden as a single question using a 4-point Likert scale (from not at all burdened to overwhelmingly burdened). They signed a consent form prior to participation in the NG session. Participants in any of the groups were allowed to withdraw consent at any time. Sampling was not determined by saturation but by availability of patients as this project was conducted during the pandemic and experienced several interruptions. We had planned a minimum of 8 participants and three groups. Physician participants were directly identified and contacted by one of the investigators (CCD) and contacted via email using a brief screening interview (gender, specialty and whether managed CHF patients).
Nominal Group Meetings
We conducted four face-to-face NGT sessions lasting approximately 2 hours each with patient participants at Gold Coast Hospital, and one NGT session via a videoconference platform with physician participants. The NGT sessions had the following structure: 1) idea generation; 2) round robin sharing of ideas and discussion of tentative themes, and 3) ranking, as previously reported by others.27 All sessions were audio-recorded, and the discussion part was transcribed.
At least two facilitators were present at each NGT session, one facilitator was nominated as scribe and the other facilitator focused on moderating the discussion. For the patient NGTs, three facilitators had health services research background and two were physicians not managing any of the participating patients. After an initial introduction to the project objectives, participants spent the first 10 minutes reflecting on the main treatment concerns and listing treatment burden issues they would like to discuss during a patient-physician encounter. Subsequently, each participant took turns in a round-robin process to list a single treatment burden priority, until all priorities were recorded. The scribe documented the issues in an Excel spreadsheet, which was projected onto a wide screen for all participants to see. Any misunderstandings were rectified during the session before priority voting was undertaken. The same process was followed when conducting the virtual NGT sessions, except that the scribe shared their excel spreadsheet on screen.
The facilitators grouped similar issues into preliminary themes. The preliminary themes were reviewed by participants and after an opportunity to provide feedback, the agreed themes were finalised and approved by all members of the NGT session. Each participant then individually selected and ranked the five most important burden of treatment topics from the list. Voting and ranking was recorded on a customised anonymous form which was then provided to the scribe to record and tally. The NGT session resumed with a summary of the collective top five priorities for confirmation, and the facilitator encouraged patients to reflect on and discuss potential solutions to the priority concerns, which their doctor could assist with at a clinical encounter.
The process undertaken for doctors included two prompts to consider -from their own perspective: a) What issues related to patients’ burden of treatment do you think should be discussed between doctor and patient/carer? b) Please think about the elements of treatment that are likely the most burdensome to your patients, and why you think it would be good to discuss these during a consultation. The round robin proceeded in the same fashion as per patients’ NGTs, with another medical specialist as facilitator, individual doctor reflections being shared, and the collective list of issues prioritised.
Data Processing and Analysis
Electronic files were password protected and saved in the secure university server only designated researchers had access to them. All data was anonymized at collection time, with only indication for the date of NGT. Two authors (MC, AS) participated in the collation, interpretation and final decisions on the priorities identified in the NGT sessions using both scores and numbers of votes as previously recommended by others.28 The process for collation and analysis of the multiple patient NGs is described in Table 1.
Table 1.
Analysis Steps for Decisions on Priority Topics for the Patient NGT Sessions
| Steps | Description of Analysis Process |
|---|---|
| 1 | One author grouped all individual priorities raised during each of the individual patient NGT sessions under themes (coding).51 The initial coding framework was developed independently by this author based on the content of each emerging theme, irrespective of sum of scores. Priorities documented by the scribe were abstracted and similar concepts or experiences clustered into categories in a spreadsheet and a brief descriptive label was assigned (coding).51 Interpretation of these clusters (themes) relied on the detailed situations expressed by participants. Themes were analyses for all patient groups. |
| 2 | A second investigator reviewed the coding framework. In case of differences of opinion between the investigators, consensus was reached through iterative discussions and refinement of the framework of themes. |
| 3 | The scores were added from all patient NGT sessions for each individual treatment burden priority. |
| 4 | All priorities were grouped in into themes, independently of the scores they received. The scores from all priorities grouped under the same theme were combined to obtain an overall score for each theme. |
| 5 | Themes were ranked based on their combined score from all individual priorities. If different themes had the same score, frequency of voting (how often the topic had been ranked in the top 5) was used to determine the final ranking of themes.28 |
Abbreviation: NGT, nominal group technique.
For the doctors’ NGT session conducted, we used the same procedure but only had priorities grouped under themes from one session. The naming to themes from the doctors’ (D) NG that were similar to those emerging from patients (P) NG were mapped, arriving at consensus on themes. The mapping is presented graphically in the results section, with patients’ themes represented as 1P-6P and doctors’ themes represented as 1D-4D) and common themes cross-referenced.
Results
Five nominal groups were conducted over 12 months, four with 22 patients (10 female, 12 male), and one group with five doctors. The mean number of participants across the four patient groups was 5 (range 4–8), all but 3 were retired, and the overall mean age was 70.5 years. Of the 22 patients, five reported been significantly or overwhelmingly burdened by their treatment. They were taking a median of 14 medications per day (mean 13), and four of them had comorbidities including diabetes, kidney disease or cancer. Two thirds (13 patients) were somewhat burdened by their treatment. They were taking a median of 8 medications per day (mean 10), and seven of them had comorbidities (chronic obstructive pulmonary disease, asthma, diabetes, chronic kidney disease, cancer, sleep apnoea).
Of the 5 physicians, 3 were women, and all managed patients with heart failure in an in-and outpatient setting at consultant level (no information on years’ experience with CHF); two were geriatricians, two were general medicine physicians, and one was a general medicine physician and endocrinologist.
Patient’s Priorities
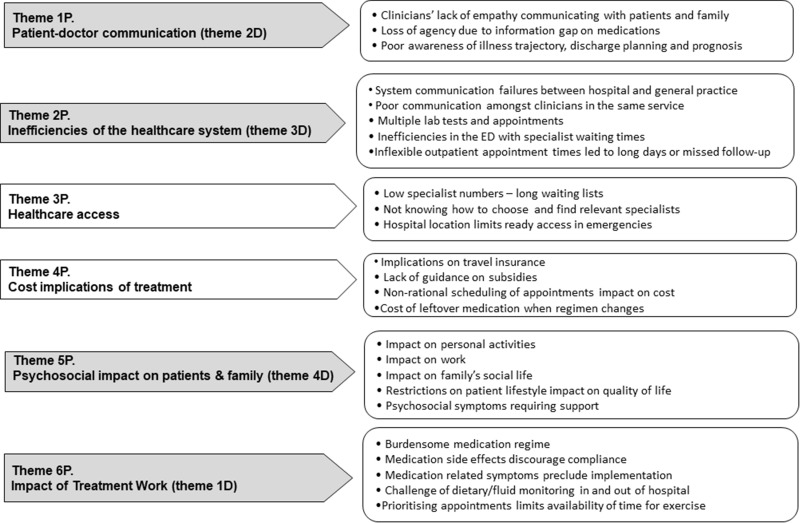
The priority issues raised are summarised in six themes and presented in descending ranking order in Figure 1. The most important priority was Doctor-patient Communication (1P), covering patient perception of unempathetic doctor conversations on the phone, poor understanding of patient circumstances, including language barriers and lack of inclusion of families in the discussions, ignoring patients’ suggestions based on their personal knowledge of their disease and their preferences, and use of medical jargon. Additional examples of poor communication included lack of information on the rationale for many medications, their potential risks, interactions, and side effects. Finally, the patients’ acknowledgment of their low literacy on own condition emerged repeatedly, resulting from having insufficient information from healthcare professionals on the disease progression/trajectory, the irreversible nature of the condition, and explanation for changes in medication during hospital admissions and details of discharge planning. These, compounded by lack of communication between different types of doctors, impacted on loss of faith in clinicians.
Figure 1.
Treatment burden themes emerging from the patient (P).
Note: Numbers in brackets (#D) signal the themes common between doctor (D) and patient (P) groups (see Figure 2).
Inefficiencies of the healthcare system (2P) ranked as the second priority theme for patients. This recurrent theme encompassed multiple testing requirements, poor communication across healthcare providers from different sectors of the health system leading to conflicting advice and fragmented care. Additionally, medication changes by junior doctors without consultation with managing specialists, waiting times for test results and action plans, and waiting in emergency department to be seen by specialists was also discussed.
The arrows in dark shades in Figure 1 indicate concordance of priorities with the doctors’ (D) group, which will be discussed in the next section. Table S1.1, Supplement 1 presents examples of quotes to illustrate the concepts as expressed by participating patients.
The third most important priority, Healthcare Access (3P), was a concern in terms of challenges finding appropriate specialists and being able to book timely appointments with them, and lack of hospitals close to patient’s home in case of emergencies.
Cost Implications of Treatment (4P) was the fourth priority covering the lack of information on government subsidies for medications and disability-related health service entitlements, as well as non-streamlining of appointments impacting on travel and parking costs.
The Psychosocial impact on patients and family (5P) manifested as a series of disruptions to usual activities, work, social interactions, and general quality of life stemming from the driving to multiple appointments, waiting times, and hospitalisations. Patients felt frustrated and refused to accept their predicament, and guilty about their inability to plan social life, and the imposition of time on their companions and needing support at home because of the associated treatments and the anxiety of not being able to self-manage.
Finally, the theme Impact of Treatment Work (6P) reflected the burden of implementing medication regimen and non-pharmacological aspects of management in and out of hospital. Concerns about the physical impact of treatment side effects, such as disrupted sleep, falls from hypotension, difficulties performing activities of daily living, weight gain precluding physical activity and the fluid and salt intake demands, discouraged compliance with recommendations. Although this theme was discussed at length, it emerged as the sixth priority.
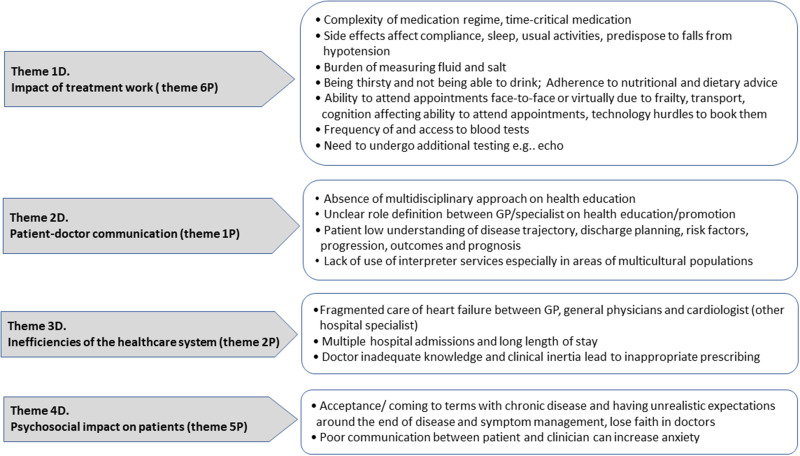
Doctors’ Perception of Treatment Burden
All four doctors’ themes for perceived patient treatment burden (Figure 2) coincided with four of the six themes identified by the patient groups (highlighted as darker arrows on the themes in both Figures 1 and 2. Impact of Treatment Work (1D) was the treatment burden topic with the highest priority for doctors and included pill burden (high number of different medications), having time-critical drugs, demands of repeat tests and appointments, and difficulties following advice on diet, fluid and salt restriction, and adverse effects of polypharmacy affecting activities of daily living.
Figure 2.
Themes emerging from doctors’ (D) perceived treatment burden of their patients.
Note: Numbers in brackets signal the themes common between patients (P) and doctor (D) groups (See Figure 1).
Doctors recognised that there was poor Patient-doctor Communication (2D) affecting health system coordination and leading to communication breakdowns between health sectors, absence of multidisciplinary approaches and lack of clarity on what role the primary care provider plays. They also raised patients’ lack of knowledge about the disease process and patients not seeing a connection between disease symptoms and prognosis or risk factors and outcomes. However, doctors did not mention that this second treatment burden might be due to them not providing sufficient and appropriate information to patients.
The theme inefficiencies of Healthcare System (3D) also emerged for doctors and referred to fragmented care across different occupational groups, absence of medication monitoring by different colleagues managing patients, and repeat or lengthy hospitalisations.
Psychosocial impact on patients and family (4D) of treatment burden ranked among the top priority themes for both patient and doctor groups (5P, 4D). It covered the need for multiple appointments, long waiting times at services, and repeat testing was also prioritised. Work absenteeism, and constantly needing family members stressed relatives and interfered with other caring responsibilities, such as child minding. These were mentioned as an important point of discussion in a clinical encounter whereby anxiety, frustration, and irritability about the many lifestyle limitations and how the uncertainty of coping alone led to poor quality of life and need for psychological support in several cases. Doctors perceived the impact extending to patients struggling to accept the irreversible nature of chronic heart failure and anxiety (4D) resulting from communication breakdowns with clinicians in relation to medication changes and conflicting advice about fluid restrictions. Table S1.2, Supplement 1 provides exemplar priorities raised to support the above themes.
Issues such as the lack of clinician’s knowledge or CHF management leading to inappropriate prescribing (clinical inertia, lack of titrating, no tailoring) and lack of use of interpreter services posing a burden on multicultural populations were raised in the NG but attained zero points during voting. However, they fitted within the inefficiencies of healthcare system and patient-doctor communication themes respectively.
The ranking of individual priorities for both participant groups based on sum of scores and frequency of votes is shown in Table 2.
Table 2.
Comparative Priorities of Patients and Doctors
| Sum of Scores for Each Theme (Groups 1–4) >>> | Group 1 | Group 2 | Group 3 | Group 4 | Overall Score for all Themes Across All Groups | Frequency of Voting for All Themes Across All Groups | Ranked Priority (Based on Scores and Frequency) |
|---|---|---|---|---|---|---|---|
| Umbrella Theme on patients priorities | |||||||
| Patient-doctor communication | 24 | 49 | 20 | 18 | 111 | 37 | 1st |
| Inefficiency of healthcare system | 49 | 21 | 27 | 9 | 106 | 34 | 2nd |
| Healthcare access | 32 | 2 | 34 | 13 | 3rd | ||
| Cost implications of treatment | 15 | 3 | 4 | 11 | 33 | 12 | 4th |
| Psychosocial impacts on patient and families | 35 | 35 | 9 | 5th | |||
| Treatment Work | 9 | 13 | 22 | 9 | 6th | ||
| Priorities identified by doctor participants | Group 1 | Overall score | Frequency of voting* | Ranked priority (scores and frequency) | |||
| Impact of treatment work | 49 | 49 | 11 | 1st | |||
| Patient-doctor communication | 19 | 19 | 8 | 2nd | |||
| Inefficiencies of healthcare system | 13 | 13 | 5 | 3rd | |||
| Psychosocial impacts on patient | 3 | 3 | 3 | 4th |
Solutions Proposed by All Groups to Highest Ranked Treatment Burden Priorities
The solutions proposed by patients and doctors were summarised from the respective spreadsheets used during the NGT sessions.
To overcome poor Patient-doctor Communication (theme 1P) on discharge planning and prognosis, patients proposed the involvement of nurses prior to hospital discharge or CHF educators after specialist appointments to inform patients about diet and medications (equivalent to diabetes educators). Doctors were also supportive of the concept of nurses or pharmacists directly providing information to patients, thus freeing up doctors for other tasks. In addressing this theme doctors also proposed (theme 2D) monitoring indicators of deterioration, such as functional decline and hospital readmissions and involving the patient as part of the coordination process including referrals back to GP and discussions on advance care planning.
The perceived inefficiencies of the Healthcare System (theme 2P), such as poor communication between clinicians in different services, could be potentially addressed through email communication between hospital and general practice, or linkage between information systems to prevent delays in transmission of test results and discharge summaries. These are expectations from patients who have signed up to consent to hold all healthcare related information in the mobile electronic health records in the Australian health system. Some but not all GP services have already implemented this strategy to streamline communication. Doctors’ suggestions to alleviate fragmented care (theme 3D) included improved communication across different specialists for a holistic approach to care and medication update across body systems.
Patients did not perceive consultations with trainee doctors or telehealth as valid alternatives to face-to-face appointments to address challenges of Healthcare Access (theme 3P). Patients acknowledged that there are already mechanisms in place in the Australian universal health insurance scheme to mitigate the Cost implications of treatment (theme 4P), such as partial government subsidies and coverage of some medications and tests. However, patients suggested that clinicians would provide them with information on how to access subsidised services and reduce cost.
To mitigate the Psychosocial Impacts (theme 5P) of treatment burden, patients acknowledged the importance of a strong family/ social support network. To prevent the prescription of unnecessary or inappropriate medications by junior doctors or doctors usually not involved in the patients’ care, and to be better informed about the reasons for having to take specific drugs (theme 6P, Impact of Treatment Work) patients suggested that pharmacists directly communicate with clinicians to improve prescribing practices. In addition, brochures or printouts, double-sided cards with medication list and allergies, and a personalised letter with reasons for medications prescribed and its benefits, and explanations on the rationale for repeat testing, were proposed.
For the challenges of dietary restrictions (theme 6P) patients proposed specific recommendations from dietitians on food preparation or resources, such as a take-home book of easy recipes, which could be used by the relatives supporting them. This was preferable to vague recommendations on reducing salt and fluid intake. Patients recognised that the need to cook following specific dietary requirements could potentially negatively impact on the family’s social life (6P), as could having to accompany patients to multiple appointments. Many practical suggestions emerged from the doctors’ NG to address the Impact of Treatment Work (theme 1D) including: Timing with other activities of daily living to ensure adherence, patients setting a timer, compliance checks (eg pill score) at the community pharmacy; blister packs; education of patients on the importance of adherence and self-management of titration; having realistic dietary goals in the context of patient preference and religious needs. More broadly, critical medication review (doctors’ role), rationalising and tailoring of medications to the individual balancing optimal treatment effect vs quality of life; and Clinical audit (system level solution).
Discussion
Summary of Results
This study based on NGT sessions with patients with CHF and physicians treating physicians with CHF, found that the burdens of heart failure treatment are numerous and complex. From a patient’s perspective, Patient-doctor communication, Inefficiency of healthcare system, Healthcare access limitations, Cost implications of treatment, Psychosocial impacts and Impact of Treatment Work were the most important burdens they wanted discussed in a clinical encounter. Over half of the burdens prioritized by patients were also identified by doctors, suggesting aligned awareness. However, the priority rankings differed, indicating that doctors’ awareness of their patients’ treatment burden tends to focus on the complexity of medication and testing demands and health system coordination issues. The perception that health professionals’ focus during the clinical encounter is the pharmacological and laboratory component of treatment rather than patient education for optimal self-care has been identified by CHF patients before.29 Patient-doctor communication was an emerging theme in both our groups, but the contents of the themes differed: patients focused on low empathy and loss of autonomy due to information gaps, while doctors emphasized underuse of interpreter services and blurred role definition across healthcare providers on whose duty was to provide patient health education about illness trajectories. Likewise, the common theme of inefficiencies of the healthcare system was built from disconnection between different healthcare providers, multiple service encounters or testing numbers and long waiting times. In turn, doctors interpreted this theme as fragmented care across specialties, deficiencies of medication reviews, and overuse of hospitals. Overall, doctors were largely aware of the treatment burdens affecting their patients with heart failure and what they wanted to discuss with them, they rarely considered the broader impact this care has on the patients care givers and family. We believe the discrepancies in the content of themes by patients and doctors was due to differing roles, perceptions and priorities rather than any methodological issues.
Results in Context with Other Literature
It is reassuring that all the identified priorities in our NGs align with the components of the treatment burden questionnaire applicable across chronic conditions, which summarises burden items into domains of medication adherence, dietary and physical activity advice, self-monitoring with laboratory tests, healthcare provider visits, and social impact.30 Our study broadened this scope of understanding by not only investigating the component of treatment burden for people with CHF, but also focusing on which of these components should be discussed as a priority in the clinical encounter. Several of these treatment burdens have been highlighted in other health systems such as in the UK.18 Likewise in the US, patients with deteriorating heart failure were more likely to be non-adherent and attain high treatment burden scores using a questionnaire that included the domains of side effects, disruption of daily living activities, convenience, and global satisfaction.31
The lack of older patients’ awareness about the nature of their chronic illness due to Patient-clinician Communication problems (1P, 2D) was also reported in heart failure in a European survey of nurses. They perceived that conversations on illness trajectory was more appropriate if left for relevant moments, such as during exacerbations or towards the end of life, and acknowledged that patient education on this was not commonplace in routine care.32 Patients in a UK qualitative study complained about the medicalized models of care and consistent with our NGs also mentioned time-limited consultations diminishing understanding of their disease prognosis and precluding discussions on concerns when they would prefer an inclusive partnership approach and better communication.33 Contrary to our patients’ suggestion of coordinated discharge planning in the hospital setting itself, others have promoted transitional healthcare34–36 post-discharge as a beneficial interface with community providers to ensure optimal treatment adherence and support to manage the burden. Patient-centered care in chronic disease management may be delivered at either or both time points.
Inefficiency of healthcare system, was also described as “fragmented care” in a UK consultation that reported multiple clinicians not communicating with one another; this led to restricted patient relationships with the management team and lower patient confidence in health professionals.18 Poor care coordination leading to loss care continuity was also raised by by CHF patients in a qualitative survey in Norway.29 Overall, our findings align with this body literature and given that people who experience chronic disease are more likely to be older from disadvantaged backgrounds, there needs to be more coordinated care and focus during the clinical encounter on communication that caters for physical disability and social disparity.
Healthcare access limitations mentioned by patients appear to occur across countries as acknowledged in a UK retrospective analysis of qualitative data from 47 patients with CHF where waiting times for specialist advice and challenges of transport arrangements were highlighted,18 and in an Iranian qualitative consultation of 14 CHF patients where lack of continuity and access to health professionals hindered adherence to self-management.37 What is interesting in our study and worth considering is that patients did not perceive consultations via telehealth as a valid alternative to face-to-face appointments as a means of addressing Healthcare Access. Although telehealth been promoted as an alternative to face-to-face consultation and may even overcome challenges of access for some people,38,39 our findings suggest that possibility that it may not be suitable for all patients, all ages, or all topics to be discussed.
Within the Cost implications of Treatment theme (4P), a review within the US healthcare system found that advanced age and other underlying comorbidities predisposed patients with heart failure to higher costs of treatment, with hospitalization being one of the major financial burdens.40 While this does not necessarily translate to all health systems, it is important to consider as our NG patients may not have identified or emphasized this association with concurrent illnesses. On the other hand, the absence of information on subsidised services that may benefit disadvantaged patients has been previously reported,41 although the link with access to care found by others did not emerge in our NGs.
Our findings ratify the psychosocial impacts (5P, 4D) of both symptoms and CHF management on patients and families daily life reported in a recent ethnographic study.42 This predicament -termed “biographical disruption”- was described for chronic conditions in general some two decades ago.43 A recent literature review on chronic heart failure also referred to the impacts of treatment burden on work, life and social relationships.23 Likewise a US survey of 125 older patients with heart failure reported psychological distress and mental exhaustion resulting from the social restrictions of treatment and self-management.19 Despite the psychosocial impact of CHF being acknowledged some two decades ago, it is concerning that recent literature, including our findings, suggest that this burden is still an ongoing issue for patients. Recently, studies reported in a systematic review indicate that symptom burden can also lessen patient ability and opportunity for adherence to treatment recommendations.44 However, the impact of symptoms mentioned by our participants was mostly referred to as side effects of treatment, and did not emerge as a strong hurdle for participants’ confidence in self-management or capacity for health service seeking behaviour.
As the detrimental consequences of treatment non-adherence on heart failure trajectories has been previously identified,45 the Impact of Treatment Work (6P, 1D) needs to be addressed. A narrative review described its scope in line with our findings as the demands of medication management and coordination of information, resources and health service interaction for testing and follow-up. Additionally, treatment work encompassed coping with multiple side effects of medication and dietary restrictions, particularly onerous for those with multimorbidity and in end stages.46 Another review highlighted the difficulties achieving assistance from clinicians and families to deal with the challenges of monitoring and adjusting HF treatment regimens.47
Several solutions to treatment burden emerging from our participants have been investigated by others in the literature to ensure patients’ adherence to medication. A narrative review on patient education to reduce re-hospitalisation also recommends educational interventions tailored to older patient’s education, motivation and cognitive levels.48 Further, a randomised trial of patient education for general practitioners via a 2-day workshop, however, did not achieve enhanced quality of care for CHF patients compared to usual management, despite 3-monthly personalised instructions to patients on identifying risk factors, alarm signs, self-care and patient goals.49
Patients’ and their family’s reluctance to accept heart failure prognosis and adapt to changing lifestyle (4D) has also been reported before.50 The authors of that qualitative consultation recommended monitoring of the barriers and enablers for successful management processes.
Our study ratified and prioritised perceptions of the unanticipated practical, financial, and psychosocial demands heart failure patients’ and their families experience19,51 and the aspects they want to discuss in a clinical encounter. These burdens have also been reported for other chronic conditions52 and might be amenable to a holistic approach. Treatment burden has been shown to affect patients’ compliance with treatment and their satisfaction with care, hence it is important to address it. Recent calls for inclusion of the implications of this burden in clinical practice guidelines set a direction where patients and caregivers’ values and preferences are considered in a shared decision-making process.22 We support others in this area to suggest that patients’ families and caregivers should not be excluded from treatment and decision-making.53,54
Traditionally, the strategies of motivation and education have been used to attempt to improve patients’ adherence with prescribed treatments. Given the numerous additional responsibilities self-management and self-monitoring of heart failure brings on patients, we agree that there is a role for enhancing clinician awareness of the concept of patients’ treatment burden 1518 as an important source of non-adherence55 and address some of the treatment burden challenges outlined in this study during the clinical encounter.
Strengths and Limitations
Our research aimed to highlight, prioritise and change patient-doctor discussions about treatments and to provide a framework for patients’ non-adherence issues based on a patient-centred perspective. The use of the NGT process based on a published approach,28 minimised the impact of any dominant participants (which can be a challenge with other focus group formats). Two reviewers independently analysed the content of the discussions and agreed on the final themes and sub-themes. While our target number of participant patients was 8–10 for each NG session in accordance with previous recommendations in the expert literature,56 the actual group sizes were smaller (range: 4–8). This was due to unforeseen acute patient illness, COVID-19 related social distancing restrictions, or participants changing their mind about attending the session at the last minute. COVID-19 regulations also meant that patient sessions had to be rescheduled or postponed several times to comply with distancing and this lengthened our study duration. Our doctors’ NGT session had to be conducted online instead of face to face, but the session dynamics or contributions were not affected by this modality.
Conclusion
The burden of heart failure treatment in and out of hospital is multidimensional and extends beyond the direct impact of treatment work. This study compared and contrasted perceived patient burden from two perspectives. For patients, the impact of poor communication with clinician is the most burdensome as it impairs their autonomy and understanding of disease trajectory. Inefficiencies of the healthcare system, for example, the lack of care coordination that impacts on their time spent on appointments and tests, follow closely. Doctors were generally aware of patients’ treatment burden but tended to focus on the complexity of the direct treatment work. They need to better understand the financial and psychosocial impacts of the recommendations they give in routine care. While participating patient numbers were fewer than planned due to COVID-19 restrictions, their insights were collectively prioritized to inform future discussions at clinical encounters. In addressing the burdens identified by patients, future guidelines could accommodate patient concerns, preferences and financial capacity and should ideally incorporate a section on treatment burden and recommendations for evidence-based interventions. Meanwhile, strategies to address the priority issues identified here can commence with clinicians becoming aware of the issues that matter to patients and their families/caregivers and proactively discussing feasible immediate and longer-term solutions during clinical encounters.
Acknowledgments
We thank the patients and doctors for their participation, and nurse Helen McEvoy for her valuable assistance with participant identification, recruitment and logistic support in the conduct of the NGs.
Funding Statement
This study was funded by a grant from the Gold Coast Health Collaborative Research Grant Scheme (application ID RGS20190034). The funding body did not have any role in the design, planning or implementation of the study. CCD had salary support (independent of the study) from the Australian National Health and Medical Research Council (NHMRC) (application ID APP1123733).
Data Sharing Statement
Raw datasets are not publicly available, but requests for processed tables can be made from the senior author CCD (c.dobler@unsw.edu.au) and may require further ethics approval.
Ethics Approval
The Gold Coast Hospital and Health Service Human Research Ethics Committee provided approval for the study (LNR/2020/QGC/61202).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no financial or other conflicts of interests in this work.
References
- 1.Sahle BW, Owen AJ, Mutowo MP, et al. Prevalence of heart failure in Australia: a systematic review. BMC Cardiovasc Disord. 2016;16:32. doi: 10.1186/s12872-016-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan Y-K, Tuttle C, Ball J, et al. Current and projected burden of heart failure in the Australian adult population: a substantive but still ill-defined major health issue. BMC Health Serv Res. 2016;16:501. doi: 10.1186/s12913-016-1748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American heart association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 4.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlay SM, Manemann SM, Chamberlain AM, et al. Activities of daily living and outcomes in heart failure. Circ Heart Fail. 2015;8:261–267. doi: 10.1161/circheartfailure.114.001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord. 2018;18:74. doi: 10.1186/s12872-018-0815-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson SL, Tong X, King RJ, et al. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11:e004873–e004873. doi: 10.1161/CIRCHEARTFAILURE.117.004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sara JD, Toya T, Taher R, et al. Asymptomatic left ventricle systolic dysfunction. Eur Cardiol. 2020;15:e13–e13. doi: 10.15420/ecr.2019.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanderson JE. Heart failure with a normal ejection fraction. Heart. 2007;93:155–158. doi: 10.1136/hrt.2005.074187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson BS, Yancy CW. Immediate vs delayed diagnosis of heart failure: is there a difference in outcomes? Results of a Harris interactive® Patient Survey. J Card Fail. 2004;10:S125. doi: 10.1016/j.cardfail.2004.06.399 [DOI] [Google Scholar]
- 11.Zannad F, Agrinier N, Alla F. Heart failure burden and therapy. Europace. 2009;11:v1–9. doi: 10.1093/europace/eup304 [DOI] [PubMed] [Google Scholar]
- 12.May CR, Eton DT, Boehmer K, et al. Rethinking the patient: using burden of treatment theory to understand the changing dynamics of illness. BMC Health Serv Res. 2014;14(281):20140626. doi: 10.1186/1472-6963-14-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan P, Murphy M, Man MS, et al. Development and validation of the Multimorbidity Treatment Burden Questionnaire (MTBQ). BMJ Open. 2018;8(e019413):20180412. doi: 10.1136/bmjopen-2017-019413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sav A, Whitty JA, McMillan SS, et al. Treatment burden and chronic illness: who is at most risk? Patient. 2016;9:559–569. doi: 10.1007/s40271-016-0175-y [DOI] [PubMed] [Google Scholar]
- 15.Alsadah A, van Merode T, Alshammari R, et al. A systematic literature review looking for the definition of treatment burden. Heliyon. 2020;6:e03641–e03641. doi: 10.1016/j.heliyon.2020.e03641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahoz R, Proudfoot C, Fonseca AF, et al. Caregivers of patients with heart failure: burden and the determinants of health-related quality of life. Patient Prefer Adherence. 2021;15:1153–1164. doi: 10.2147/PPA.S297816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallacher K, Jani B, Morrison D, et al. Qualitative systematic reviews of treatment burden in stroke, heart failure and diabetes - Methodological challenges and solutions. BMC Med Res Methodol. 2013;13:10. doi: 10.1186/1471-2288-13-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallacher K, May CR, Montori VM, et al. Understanding patients’ experiences of treatment burden in chronic heart failure using normalization process theory. Ann Fam Med. 2011;9:235–243. doi: 10.1370/afm.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordfonn OK, Morken IM, Bru LE, et al. Burden of treatment in patients with chronic heart failure – a cross-sectional study. Heart Lung. 2021;50:369–374. doi: 10.1016/j.hrtlng.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 20.Klindtworth K, Oster P, Hager K, et al. Living with and dying from advanced heart failure: understanding the needs of older patients at the end of life. BMC Geriatr. 2015;15:125. doi: 10.1186/s12877-015-0124-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eton DT, Elraiyah TA, Yost KJ, et al. A systematic review of patient-reported measures of burden of treatment in three chronic diseases. Patient Relat Outcome Meas. 2013;4:7–20. doi: 10.2147/prom.S44694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobler CC, Harb N, Maguire CA, et al. Treatment burden should be included in clinical practice guidelines. BMJ. 2018;363:k4065. doi: 10.1136/bmj.k4065 [DOI] [PubMed] [Google Scholar]
- 23.Austin RC, Schoonhoven L, Kalra PR, et al. Burden of treatment in chronic heart failure: does symptom burden play a role? Br J Card Nurs. 2019;14:91–93. doi: 10.12968/bjca.2019.14.2.91 [DOI] [Google Scholar]
- 24.Potter M, Gordon S, Hamer P. The nominal group technique: a useful consensus methodology in physiotherapy research. N Z J Physiother. 2004;32:70–75. [Google Scholar]
- 25.Sav A, McMillan SS, Kelly F, et al. The ideal healthcare: priorities of people with chronic conditions and their carers. BMC Health Serv Res. 2015;15:551. doi: 10.1186/s12913-015-1215-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245–1251. doi: 10.1097/acm.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 27.Dening KH, Jones L, Sampson EL. Jones L and Sampson EL. Preferences for end-of-life care: a nominal group study of people with dementia and their family carers. Palliat Med. 2012;27:409–417. doi: 10.1177/0269216312464094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMillan SS, Kelly F, Sav A, et al. Using the nominal group technique: how to analyse across multiple groups. Health Serv Outcomes Res Methodol. 2014;14:92–108. doi: 10.1007/s10742-014-0121-1 [DOI] [Google Scholar]
- 29.Nordfonn OK, Morken IM, Bru LE, et al. Patients’ experience with heart failure treatment and self-care—A qualitative study exploring the burden of treatment. J Clin Nurs. 2019;28:1782–1793. doi: 10.1111/jocn.14799 [DOI] [PubMed] [Google Scholar]
- 30.Tran V-T, Harrington M, Montori VM, et al. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med. 2014;12:109. doi: 10.1186/1741-7015-12-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunbar SB, Tan X, Lautsch D, et al. Patient-centered outcomes in HFrEF following a worsening heart failure event: a survey analysis. J Card Fail. 2021;27:877–887. doi: 10.1016/j.cardfail.2021.05.017 [DOI] [PubMed] [Google Scholar]
- 32.Hjelmfors L, van der Wal MH, Friedrichsen MJ, et al. Patient-nurse communication about prognosis and end-of-life care. J Palliat Med. 2015;18:865–871. doi: 10.1089/jpm.2015.0037 [DOI] [PubMed] [Google Scholar]
- 33.Boyd KJ, Murray SA, Kendall M, et al. Living with advanced heart failure: a prospective, community based study of patients and their carers. Eur J Heart Fail. 2004;6:585–591. doi: 10.1016/j.ejheart.2003.11.018 [DOI] [PubMed] [Google Scholar]
- 34.Kalter-Leibovici O, Freimark D, Freedman LS, et al. Disease management in the treatment of patients with chronic heart failure who have universal access to health care: a randomized controlled trial. BMC Med. 2017;15:90. doi: 10.1186/s12916-017-0855-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16:195. doi: 10.1186/s12872-016-0371-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen LA, Fonarow GC, Liang L, et al. Medication initiation burden required to comply with heart failure guideline recommendations and hospital quality measures. Circulation. 2015;132:1347–1353. doi: 10.1161/circulationaha.115.014281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Negarandeh R, Aghajanloo A, Seylani K. Barriers to self-care among patients with heart failure: a qualitative study. J Caring Sci. 2021;10(196–204):20201020. doi: 10.34172/jcs.2020.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polinski JM, Barker T, Gagliano N, et al. Patients’ satisfaction with and preference for telehealth visits. J Gen Intern Med. 2016;31:269–275. doi: 10.1007/s11606-015-3489-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fien S, Dowsett C, Hunter CL, et al. Feasibility, satisfaction, acceptability and safety of telehealth for first nations and culturally and linguistically diverse people: a scoping review. Public Health. 2022;207:119–126. doi: 10.1016/j.puhe.2022.04.007 [DOI] [PubMed] [Google Scholar]
- 40.Urbich M, Globe G, Pantiri K, et al. A systematic review of medical costs associated with heart failure in the USA (2014–2020). PharmacoEconomics. 2020;38:1219–1236. doi: 10.1007/s40273-020-00952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson PN, Shetterly SM, Clarke CL, et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305:1695–1701. doi: 10.1001/jama.2011.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fry M, McLachlan S, Purdy S, et al. The implications of living with heart failure; the impact on everyday life, family support, co-morbidities and access to healthcare: a secondary qualitative analysis. BMC Fam Pract. 2016;17:139. doi: 10.1186/s12875-016-0537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bury M. Chronic illness as biographical disruption. Sociol Health Illn. 1982;4:167–182. doi: 10.1111/1467-9566.ep11339939 [DOI] [PubMed] [Google Scholar]
- 44.Austin RC, Schoonhoven L, Clancy M, et al. Do chronic heart failure symptoms interact with burden of treatment? Qualitative literature systematic review. BMJ Open. 2021;11:e047060. doi: 10.1136/bmjopen-2020-047060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J-R, Moser DK. Medication adherence mediates the relationship between heart failure symptoms and cardiac event-free survival in patients with heart failure. J Cardiovasc Nurs. 2018;33:40–46. doi: 10.1097/JCN.0000000000000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jani B, Blane D, Browne S, et al. Identifying treatment burden as an important concept for end of life care in those with advanced heart failure. Curr Opin Support Palliat Care. 2013;7(3–7):3–7. doi: 10.1097/SPC.0b013e32835c071f [DOI] [PubMed] [Google Scholar]
- 47.Sheehan OC, Leff B, Ritchie CS, et al. A systematic literature review of the assessment of treatment burden experienced by patients and their caregivers. BMC Geriatr. 2019;19:262. doi: 10.1186/s12877-019-1222-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strömberg A. The crucial role of patient education in heart failure. Eur J Heart Fail. 2005;7:363–369. doi: 10.1016/j.ejheart.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 49.Vaillant-Roussel H, Laporte C, Pereira B, et al. Impact of patient education on chronic heart failure in primary care (ETIC): a cluster randomised trial. BMC Fam Pract. 2016;17:80. doi: 10.1186/s12875-016-0473-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zippel-Schultz B, Palant A, Eurlings C, et al. Determinants of acceptance of patients with heart failure and their informal caregivers regarding an interactive decision-making system: a qualitative study. BMJ Open. 2021;11:e046160. doi: 10.1136/bmjopen-2020-046160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13:117. doi: 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sav A, Salehi A, Mair FS, et al. Measuring the burden of treatment for chronic disease: implications of a scoping review of the literature. BMC Med Res Methodol. 2017;17:140. doi: 10.1186/s12874-017-0411-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–1952. doi: 10.1161/CIR.0b013e31824f2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzsimons D, Doherty LC, Murphy M, et al. Inadequate communication exacerbates the support needs of current and bereaved caregivers in advanced heart failure and impedes shared decision-making. J Cardiovasc Nurs. 2019;34:11. [DOI] [PubMed] [Google Scholar]
- 55.May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology. 2009;43:535–554. doi: 10.1177/0038038509103208 [DOI] [Google Scholar]
- 56.Feldman A, Herbert A, Posch P, et al. Teachers Investigate Their Work: An Introduction to Action Research Across the Professions. 3rd. London: Routledge; 2018. [Google Scholar]