Abstract
Background
Differences in sex hormone levels contribute to differences in cardiovascular disease (CVD) risk. Adipokines play a role in cardiometabolic pathways and have differing associations with CVD. Adipokine levels differ by sex; however, the association between sex hormone profiles and adipokines is not well established. We hypothesized that a more androgenic sex hormone profile would be associated with higher leptin and resistin and lower adiponectin levels among postmenopausal women, with the opposite associations in men.
Methods
We performed an analysis of 1,811 adults in the Multi-Ethnic Study of Atherosclerosis who had both sex hormones and adipokines measured an average of 2.6 years apart. Sex hormones [Testosterone (T), estradiol (E2), sex hormone binding globulin (SHBG), and dehydroepiandrosterone (DHEA)] were measured at exam 1; free T was estimated. Serum adipokines (leptin, resistin, adiponectin) were measured at exams 2 or 3. We used multivariable linear regression to examine the cross-sectional associations between sex hormones and adipokines.
Results
The mean (SD) age was 63 (10) years, 48% were women; 59% non-White participants. For leptin, after adjusting for demographics only, higher free T and lower SHBG, were associated with higher leptin in women; this association was attenuated after further covariate adjustment. However in men, higher free T and lower SHBG were associated with greater leptin levels in fully adjusted models. For adiponectin, lower free T and higher SHBG were associated with greater adiponectin in both women and men after adjustment for CVD risk factors. For resistin, no significant association was found women, but an inverse association with total T and bioT was seen in men.
Conclusion
Overall, these results further suggest a more androgenic sex profile (higher free T and lower SHBG) is associated with a less favorable adipokine pattern. These findings may provide mechanistic insight into the interplay between sex hormones, adipokines, and CVD risk.
Keywords: sex hormones, adipokines, biomarkers, cardiovascular disease, visceral fat
Introduction
Sex differences exist in the risk for cardiometabolic diseases (1), which may, in part, be due to biological differences in sex hormone levels between the sexes. While the effects of exogenous hormone treatment remains controversial, multiple studies have suggested a higher age-adjusted incidence of cardiovascular disease (CVD) among men (compared to women) and an increased CVD risk in post-menopausal women compared to similarly aged pre-menopausal women, which implicate an adverse effect of endogenous androgens on the development of CVD (2–5). A prior study from the Multi-Ethnic Study of Atherosclerosis (MESA) found that a more androgenic (“male-like”) sex hormone pattern [i.e., higher testosterone to estradiol (T/E2) ratio] among post-menopausal women was associated with increased rates of CVD, coronary heart disease (CHD), and heart failure (6). Other studies have linked higher androgen levels (free T) in post-menopausal women with endothelial dysfunction, subclinical atherosclerosis, adverse cardiac remodeling, aortic stiffness, and visceral adiposity (7–11). In contrast to women where higher androgen levels have been associated with a worse cardiometabolic profile, the opposite pattern has been noted in men. Rather, low testosterone levels in men have been linked to insulin resistance, diabetes, visceral adiposity, and CVD risk (11–16).
Men have a greater prevalence of diabetes than women; however, diabetes confers a greater relative risk of CHD and heart failure in women compared to men (17, 18). Other work from MESA has found that in women greater androgen levels were associated with a more insulin resistant phenotype (19), whereas the opposite association was seen in men (13). This parallels patterns seen with adiposity, with higher bioavailable T (bio T) being associated with greater visceral fat in women, with the reverse association in men (11). Additionally, studies have shown that lower levels of sex-hormone binding globulin (SHBG) in women have been associated with central adiposity, diabetes, and CVD risk (20–23).
Obesity may attenuate the “female-advantage” of sex differences in CVD. Adipose tissue, particularly in the visceral space, is associated with an increased risk of CVD (24–28). This fat tissue releases hormones called adipokines, which play a role in metabolism and inflammation (29, 30). Adipokines, including adiponectin, leptin, and resistin, have differing relationships with CVD risk. While adiponectin is thought to be protective against CVD, leptin and resistin are associated with increased CVD risk (31–34). Adipokine levels differ by sex (35, 36); however, the relationship of sex hormones and adipokine levels has not been well-established.
Prior studies in MESA have looked at associations between both sex hormone levels with CVD risk and adipokine levels with CVD risk (6, 32, 33). However, the association between sex hormones and adipokine levels with each other has not been examined. Therefore, we investigated the relationship between sex hormone profiles and adipokine levels in men and post-menopausal women, to provide mechanistic insight into their relationship with increased CVD risk. We hypothesized that a more androgenic sex hormone pattern would be associated with an adverse adipokine profile in women, with an opposite relationship seen in men.
Materials and methods
Study population
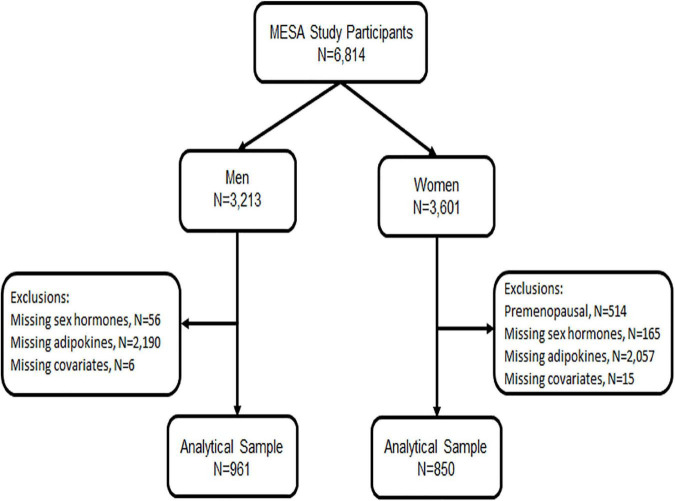
Between the years of 2000–2002, the MESA enrolled 6,814 men and women aged 45–84 years who were free of clinical CVD (37). Participants were recruited from 6 United States regions and 4 self-reported racial/ethnic groups (Black, Hispanic, Chinese, and non-Hispanic White individuals). MESA activities were approved by the institutional review boards at all field centers, and participants provided written informed consent. This study design has been previously described in detail (37).
For the analysis presented below, we included all men and post-menopausal women who had endogenous sex hormone levels measured at baseline exam 1 (2000–2002) and who had adipokine levels measured at either exam 2 or 3 (2002–2005), randomly assigned, as part of an ancillary study (n = 1,811). We excluded pre-menopausal women given their small sample size and due to differential sex hormone distribution by menopause status (Figure 1). Post-menopausal status was determined using a previously published algorithm in MESA which included self-report, history of prior oophorectomy, and/or age > 55 years (8, 38).
FIGURE 1.
Study flow chart.
Sex hormone measurement
In an ancillary study in MESA, sex hormones were measured from fasting blood samples that were collected and stored at the time of exam 1. Serum hormone levels were measured at the Steroid Hormone Laboratory at the University of Massachusetts Medical Center (Worcester, MA). Estradiol was measured using an ultrasensitive radioimmunoassay kit (Diagnostic System Laboratories, Webster, TX). Testosterone and dehydroepiandrosterone (DHEA) were measured using radioimmunoassay kits and SHBG was measured using chemiluminescence enzyme immunometric assay using Immulite kits (Diagnostic Products Corporation, Los Angeles, CA). Bio T and free T were calculated using the total T and SHBG, as described by Sodergard (39).
Adipokine measurement
A random subset of all MESA participants (n = 1,970) underwent abdominal computed tomography (CT) scans at either exam 2 or exam 3 (randomly assigned) to measure abdominal aortic calcium (40). In a subsequent ancillary study related to body composition, adipokine levels were measured among these same participants from stored frozen serum samples obtained at the respective exams (2 or 3) of their CT scan (30). The adipokines (adiponectin, leptin, and resistin) were analyzed using a Bio-Rad Luminex flow cytometry (Millepore, Billerica, MA) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT), as previously reported (31, 32). The coefficients of variation for these assays ranged from 6 to 13%.
Covariate measurement
For this analysis, we included covariates from exam 1, the time of the sex hormone level measurement including: age, race/ethnicity, sex, smoking status (never, former, current), body mass index (BMI), highest education level achieved, physical activity (moderate + vigorous in METS-min-week), current use of hormone therapy for women, total cholesterol, high density lipoprotein cholesterol (HDL-C), lipid-lowering medication, systolic blood pressure, use of anti-hypertensive medication, diabetes status (normoglycemia being fasting glucose of 99 mg/dL or less, impaired fasting glucose 100–125 mg/dL, and diabetes ≥ 126 mg/dL or the use of diabetes medications), and estimated glomerular filtration rate (eGFR). Using the abdominal CT data acquired as part of the aforementioned ancillary study at exam 2 or 3, visceral adipose tissue (VAT) was measured as the total adipose tissue in the abdominal cavity and subcutaneous adipose tissue (SAT) measured as the total adipose tissue outside of the abdominal cavity but not within muscle tissue. VAT and SAT were measured from the average of 2 CT slices obtained at L2–L3 and adjusted for height, as previously performed (30).
Statistical analysis
Sex hormone levels were the exposure (independent) variable for this analysis, including total T, bio T, free T, estradiol, DHEA, SHBG, and the ratio of T/E2. Adipokine levels were the outcome (dependent) variables for this analysis, including leptin, resistin, and adiponectin.
We used a cross-sectional design as both sex hormones and adipokines were each only measured once in MESA, although it should be noted that measurements were non-concurrent with sex hormones being from the baseline exam and adipokines from exams 2 or 3, with average follow-up being 2.6 years from baseline exam. As sex hormone profiles differ by sex and are not over-lapping, all analyses were stratified separately by sex. We used descriptive statistics to report the baseline characteristics of the study population by sex, with continuous variables reported as means with their standard deviation (SD) or medians with interquartile range (IQR) for skewed distributions and categorical variables as frequencies with percentages.
We examined the unadjusted Pearson’s correlations (r) between sex hormones and adipokines. We used multivariable linear regression to examine the separate associations of the endogenous sex hormone levels of total T, bio T, free T, estradiol, DHEA, SHBG, and T/E2 ratio with adipokine levels (leptin, resistin, and adiponectin) separately. As some of the sex hormone levels and adipokines were not normally distributed, they were natural log-transformed for analysis, and then we exponentiated the beta-coefficients from the regression models to reflect their geometric mean and presented as percent differences. The sex hormones were compared continuously per 1SD increment. A priori, we decided to focus on free T and SHBG as our primary sex hormones of interest, as these have been shown to be most associated with subclinical CVD in prior MESA studies (7–10), but we report the findings for all sex hormones for comparison.
For all analyses, we used progressively adjusted regression models. Model 1 adjusted for age, race/ethnicity and study center. Model 2a included model 1 variables as well as lifestyle and behavioral factors such as education, physical activity, smoking status, and BMI. For women, this model was also adjusted for the use of hormone therapy. Model 2b included model 2a variables plus VAT and SAT. Model 3 included model 2 variables and CVD risk factors and medications such as systolic blood pressure, use of anti-hypertensive medication, total cholesterol, HDL-C, use of lipid-lowing medication, diabetes mellitus status, and eGFR.
In supplemental analysis, we examined for interaction by BMI (<30 vs. ≥ 30 kg/m2) and stratified results if a significant interaction was found.
Two-sided P-values of < 0.05 were considered to be statistically significant, including for interaction terms. We used STATA version 15.0 [StataCorp. (41). Stata Statistical Software: Release 15, College Station, TX, StataCorp LLC] for performing the analyses.
Results
Characteristics of study population
There were 1,811 participants with endogenous sex hormone levels and adipokine levels measured that were included in the analysis (Table 1). The mean (SD) age of participants was 63 (10) years; 47% were postmenopausal women. There were 41% White, 13% Chinese, 20% Black, and 26% Hispanic adults. Half the participants were never smokers. The average BMI was 28 (5) kg/m2. In terms of medication use, 37% of participants were on antihypertensives, 17% were on lipid-lowering medications, and 35% of women were currently using hormone therapy. As anticipated, men had higher T levels, as well as E2, than post-menopausal women, whereas women have higher SHBG levels. Women had higher leptin and adiponectin levels than men, with similar levels of resistin. The scatterplot of the unadjusted adipokine levels by free testosterone and SHBG, stratified by sex, are shown in Figures 2, 3, respectively.
TABLE 1.
Characteristics of study participants.
| Total, N = 1,811 | Women, n = 850 | Men, n = 961 | |
| Leptin, ng/mL | 12.8 (5.4, 27.8) | 25.0 (12.6, 42.9) | 7.0 (3.3, 14.3) |
| Resistin, ng/mL | 15.1 (11.9, 19.1) | 15.4 (11.9, 19.5) | 14.8 (11.9, 18.7) |
| Adiponectin, mcg/mL | 17.4 (11.8, 26.2) | 21.9 (14.8, 31.5) | 14.5 (10.1, 21.1) |
| Total T, nmoI/L | 8.1 (0.9, 14.7) | 0.9 (0.6, 1.3) | 14.3 (11.5, 17.8) |
| Free T, percent | 1.7 (1.3, 2.1) | 1.3 (0.9, 1.7) | 2.0 (1.7, 2.4) |
| Bio T, nmoI/L | 2.9 (0.2, 5.5) | 0.2 (0.1, 0.3) | 5.3 (4.3, 6.6) |
| Estradiol, nmoI/L | 0.10 (0.07, 0.14) | 0.07 (0.05, 0.16) | 0.11 (0.09, 0.14) |
| SHBG, nmoI/L | 45.9 (33.6, 66.3) | 58.4 (39.3, 95.9) | 40.2 (31.6, 51.5) |
| DHEA, nmoI/L | 11.5 (8.3, 16.1) | 10.5 (7.1, 14.9) | 12.5 (9.2, 16.9) |
| Total T: Estradiol ratio | 68.8 (12.8, 133.8) | 12.3 (4.9, 22.1) | 128.8 (97.2, 169.8) |
| Age, years | 63 (10) | 64 (9) | 62 (10) |
| Race/Ethnicity | |||
| White | 736 (41%) | 332 (39%) | 404 (42%) |
| Chinese-American | 242 (13%) | 111 (13%) | 131 (14%) |
| Black | 359 (20%) | 186 (22%) | 173 (18%) |
| Hispanic | 474 (26%) | 221 (26%) | 253 (26%) |
| Education | |||
| ≥ Bachelor’s degree | 654 (36%) | 251 (30%) | 403 (42%) |
| <Bachelor’s degree | 1,157 (64%) | 599 (70%) | 558 (58%) |
| Smoking status | |||
| Never | 905 (50%) | 499 (59%) | 406 (42%) |
| Former | 682 (38%) | 258 (30%) | 424 (44%) |
| Current | 224 (12%) | 93 (11%) | 131 (14%) |
| BMI, kg/m2 | 28 (5) | 28 (6) | 28 (4) |
| Physical activity, MET-minute/week | 4,050 (2,100, 7,530) | 3,783 (1,965, 6,645) | 4,410 (2,205, 8,693) |
| Systolic blood pressure, mmHg | 127 (22) | 129 (24) | 126 (20) |
| Total cholesterol, mg/dL | 196 (34) | 202 (34) | 191 (34) |
| HDL-C, mg/dL | 51 (15) | 57 (16) | 45 (12) |
| Use of antihypertensive medication | 664 (37%) | 336 (40%) | 328 (34% |
| Use of lipid-lowering medication | 299 (17%) | 162 (19%) | 137 (14%) |
| Current use of HT* | − | 294 (35%) | − |
| SAT, cm2 | 142 (101, 202) | 166 (116, 225) | 126 (92, 176) |
| VAT, cm2 | 154 (96, 227) | 115 (71, 171) | 201 (139, 264) |
| Diabetes | 212 (12%) | 89 (10%) | 123 (13%) |
| eGFR, mL/min/1.73 m2 | 73 (15) | 71 (15) | 75 (15) |
Bio, bioavailable; BMI, body mass index; DHEA, dehydroepiandrosterone; eGFR, estimated glomerular filtration rate; HDL-C, high density lipoprotein cholesterol; HT, hormone therapy; MESA, Multi-Ethnic Study of Atherosclerosis; MET, metabolic equivalent of task; SHBG, sex hormone binding globulin; SAT, subcutaneous adipose tissue; T, testosterone; VAT, visceral adipose tissue.
Data are presented as mean (SD) or median (IQR) or frequency (percentage).
*Data were collected only in women.
FIGURE 2.
Scatterplots of adipokines vs. free testosterone. T, Testosterone.
FIGURE 3.
Scatterplots of adipokines vs. sex hormone binding globulin. SHBG, sex hormone binding globulin.
Sex hormones and adipokines in women
Table 2 shows the multivariable-adjusted association between sex hormones and adipokines (leptin, resistin, and adiponectin) in post-menopausal women.
TABLE 2.
Multivariable-adjusted association between sex hormones and adipokines in women.
| Percent difference (95% CI) | ||||
| Model 1, n = 850 | Model 2a, n = 850 | Model 2b, n = 668 | Model 3, n = 668 | |
| Leptin | ||||
| Total T | 9 (2, 15) | 0 (−4, 5) | −2 (−7, 3) | 0 (−5, 5) |
| Free T | 26 (19, 34) | 6 (0, 12) | −1 (−7, 5) | 0 (−7, 6) |
| Bio T | 19 (12, 26) | 2 (−3, 7) | −3 (−8, 3) | −2 (−7, 4) |
| E2 | 10 (3, 17) | 7 (1, 14) | 4 (−2, 11) | 3 (−3, 10) |
| DHEA | 5 (−1, 11) | 0 (−5, 5) | −3 (−8, 2) | −1 (−6, 5) |
| SHBG | −21 (−26,−17) | −6 (−11, 0) | 1 (−5, 8) | 0 (−6, 7) |
| T/E2 | −3 (−8, 3) | −5 (−10, 1) | −5 (−10, 1) | −3 (−8, 3) |
| Resistin | ||||
| Total T | 2 (0, 5) | 1 (−1, 4) | 2 (−1, 5) | 2 (−2, 5) |
| Free T | 2 (−1, 5) | −1 (−4, 3) | 0 (−4, 4) | −1 (−5, 3) |
| Bio T | 2 (−1, 5) | 0 (−3, 3) | 1 (−3, 5) | 1 (−3, 4) |
| E2 | 0 (−3, 3) | −2 (−5, 2) | −2 (−5, 2) | −2 (−5, 2) |
| DHEA | −1 (−3, 2) | −1 (−4, 2) | −2 (−5, 2) | −1 (−4, 2) |
| SHBG | −2 (−4, 1) | 1 (−3, 4) | 1 (−3, 5) | 1 (−3, 5) |
| T/E2 | 1 (−1, 4) | 2 (−1, 6) | 3 (−1, 7) | 3 (−1, 6) |
| Adiponectin | ||||
| Total T | −5 (−8,−2) | −3 (−7, 0) | 0 (−3, 4) | 1 (−3, 5) |
| Free T | −16 (−19,−13) | −18 (−21,−14) | −14 (−18,−10) | −11 (−16,−7) |
| Bio T | −12 (−16,−9) | −11 (−15,−8) | −7 (−11,−3) | −5 (−9, 0) |
| E2 | −6 (−10,−3) | −7 (−11,−2) | −5 (−10,−1) | −7 (−11,−2) |
| DHEA | −6 (−9,−2) | −5 (−9,−1) | −2 (−6, 2) | −2 (−6, 2) |
| SHBG | 19 (15, 24) | 22 (17, 27) | 18 (12, 23) | 14 (8, 20) |
| T/E2 | 2 (−1, 6) | 3 (−2, 7) | 5 (0, 10) | 6 (1, 11) |
Bio, Bioavailable; E2, Estrogen; DHEA, Dehydroepiandrosterone; SHBG, Sex hormone binding globulin; T, Testosterone.
Logarithmically transformed adipokines and one SD of logarithmically transformed sex hormones were used for the analysis.
Percent difference was calculated from ([Exp (β) −1]*100) derived from linear regression models.
Statistically significant results at p < 0.05 are in bold font.
If 0 value is bolded in table, this is because with additional digits, the value excluded 0.
Model 1: age, race/ethnicity, and MESA field site. Model 2a: model 1 covariates plus smoking, BMI, education, physical activity, and current use of hormone therapy.
Model 2b: model 2a covariates plus subcutaneous adipose tissue and visceral adipose tissue.
Model 3: model 2b covariates plus total cholesterol, HDL-C, use of lipid-lowering medication, systolic blood pressure, use of anti-hypertensive medication, diabetes, and eGFR. Premenopausal women were excluded from the analysis.
Sample sizes for Bio T: models 1 and 2a = 845, models 2b and 3 = 663.
Leptin
When adjusting for age, race/ethnicity, and MESA field site (model 1) we found that total T [percent difference 9% (95% CI, 2, 15)], free T [26% (19, 34)], bio T [19% (12, 26)], and E2 [10% (3, 17)] all had positive associations with leptin levels, while SHBG [−21% (−26, −17)] had an inverse association. However, after further adjusting for smoking, BMI, education, physical activity, and current use of hormone therapy (model 2a), only the positive association of E2 [7% (1, 14)], and the inverse association of SHBG [−6% (−11, 0)], with leptin remained statistically significant. Additional adjustment for CVD risk factors further attenuated these associations which became no longer statistically significant.
Resistin
There were no significant associations between the sex hormones and resistin for post-menopausal women.
Adiponectin
Free T, bio T, and E2 had a significant inverse association with adiponectin across all models. In the fully adjusted model for CVD risk factors (model 3), the relationship with lower adiponectin was as follows: free T [−11% (−16, −7)], bio T [−5% (−9, 0)], and E2 [−7% (−11, −2)]. On the other hand, SHBG was positively associated with adiponectin, even after full covariate adjustment for CVD risk factors in model 3 [14% (8, 20)]. Although free T was associated with lower adiponectin, the T/E2 ratio was associated with higher adiponectin level [6% (1, 11)].
Sex hormones and adipokines in men
Table 3 shows the multivariable-adjusted association between sex hormones and adipokines in men.
TABLE 3.
Multivariable-adjusted association between sex hormones and adipokines in men.
| Percent difference (95% CI) | ||||
| Model 1, n = 961 | Model 2a, n = 961 | Model 2b, n = 668 | Model 3, n = 668 | |
| Leptin | ||||
| Total T | −20 (−25,−15) | −8 (−13,−3) | −8 (−13,−2) | −8 (−14,−2) |
| Free T | 21 (13, 31) | 6 (0, 13) | 8 (1, 15) | 8 (1, 15) |
| Bio T | −13 (−9,−7) | −5 (−10, 0) | −4 (−10, 3) | −5 (−11, 2) |
| E2 | 7 (1, 15) | 2 (−4, 7) | 3 (−2, 8) | 3 (−2, 9) |
| DHEA | −12 (−18,−5) | −8 (−13,−2) | −6 (−11, 0) | −5 (−11, 1) |
| SHBG | −20 (−26,−15) | −6 (−12,−1) | −8 (−13,−2) | −8 (−13,−2) |
| T/E2 | −22 (−27,−17) | −9 (−13,−3) | −8 (−13,−3) | −9 (−14,−3) |
| Resistin | ||||
| Total T | −3 (−6,−1) | −3 (−5, 0) | −5 (−8,−1) | −5 (−8,−1) |
| Free T | −1 (−3, 2) | −1 (−4, 2) | 0 (−4, 3) | −3 (−6, 1) |
| Bio T | −4 (−6,−1) | −3 (−6,−1) | −5 (−9,−2) | −6 (−9,−3) |
| E2 | 1 (−1, 4) | 1 (−2, 4) | 1 (−2, 4) | 0 (−3, 3) |
| DHEA | 0 (−3, 3) | 0 (−3, 3) | 1 (−3, 4) | 1 (−3, 4) |
| SHBG | 0 (−2, 3) | 1 (−2, 4) | 0 (−4, 3) | 2 (−2, 5) |
| T/E2 | −4 (−6,−2) | −3 (−6,−1) | −4 (−7,−1) | −3 (−6, 0) |
| Adiponectin | ||||
| Total T | 7 (4, 11) | 5 (2, 9) | 8 (3, 13) | 6 (2, 11) |
| Free T | −11 (−14,−8) | −10 (−13,−6) | −8 (−12,−4) | −7 (−11,−2) |
| Bio T | 2 (−1, 6) | 1 (−3, 4) | 3 (−2, 7) | 2 (−2, 7) |
| E2 | −3 (−6, 0) | −2 (−5, 1) | −1 (−5, 2) | −1 (−5, 2) |
| DHEA | −2 (−2, 6) | 1 (−3, 5) | 2 (−2, 7) | 2 (−2, 6) |
| SHBG | 13 (9, 17) | 11 (7, 15) | 9 (4, 14) | 7 (3, 12) |
| T/E2 | 9 (5, 12) | 7 (3, 10) | 7 (3, 11) | 6 (2, 10) |
Bio, Bioavailable; E2, Estrogen; DHEA, Dehydroepiandrosterone; SHBG, Sex hormone binding globulin; T, Testosterone. Logarithmically transformed adipokines and one SD of logarithmically transformed sex hormones were used for the analysis.
Percent difference was calculated from ([Exp (β) −1]*100) derived from linear regression models. Statistically significant results at p < 0.05 are in bold font.
Model 1: age, race/ethnicity, and MESA field site.
Model 2a: model 1 covariates plus smoking, BMI, education, and physical activity. Model 2b: model 2a covariates plus subcutaneous adipose tissue and visceral adipose tissue.
Model 3: model 2b covariates plus total cholesterol, HDL-C, use of lipid-lowering medication, systolic blood pressure, use of anti-hypertensive medication, diabetes, and eGFR.
Leptin
Total T [−8% (−14, −2)], SHBG [−8% (−13, −2)], and T/E2 ratio [−9% (−14, −3)] were significantly inversely associated with leptin in men even in the fully adjusted model (model 3), adjusted for CVD risk factors. A positive relationship between free T [8% (1, 15)] and leptin was also statistically significant in model 3. Bio T was inversely associated with leptin in demographic adjusted model, but not in further adjusted models.
Resistin
Total T [−5% (−8, −1)], bio T [−6% (−9, −3)], and T/E2 ratio [−3% (−6, 0)] had an inverse relationship with resistin in the fully adjusted model (model 3).
Adiponectin
Total T [6% (2, 11)], SHBG [7% (3, 12)], and T/E2 ratio [6% (2, 20)] had a positive relationship with adiponectin, while free T [−7% (−11, −2)] had an inverse relationship in the fully adjusted model (model 3), adjusted for CVD risk factors.
Interaction testing
In our supplemental analysis, we found significant interactions in women between obesity status and sex hormone levels (p < 0.05 for leptin, resistin, and adiponectin) (Supplementary Table 1). Among women with a BMI < 30 kg/m2, free T [20% (11, 30)] and bio T [15% (6, 24)] were significantly positively associated with leptin when adjusting for age, race/ethnicity, field site, smoking status, education, physical activity, and current use of hormone therapy (model 2a). This association was attenuated in models further adjusted for CVD risk factors. In this BMI < 30 kg/m2 group, there was also a significant positive association between total T [4% (0, 8)] and resistin across models 1, 2a, and 2b. Lastly, for post-menopausal women with a BMI ≥ 30 kg/m2 there is a significant inverse association between total T [−7% (−13, −1)] and adiponectin across models 1 and 2a. There was no significant interaction between obesity status and sex hormone levels in men.
Discussion
Overall summary of finding
Adipokines have been implicated in cardiometabolic diseases, with higher leptin and resistin levels being associated with elevated CVD risk (33, 42), and adiponectin showing favorable associations (31). However, the relationship of sex hormones with adipokine levels had not been well established previously. In this cross-sectional study from a multi-ethnic cohort, we found mixed associations of sex hormones with adipokine levels. In both post-menopausal women and men, a more androgenic sex profile (characterized by higher free T and lower SHBG) was significantly associated with lower adiponectin, a cardio-protective adipokine, even after adjusting for other CVD risk factors. But unexpectedly, a higher T/E2 ratio was associated with higher adiponectin levels in both sexes. Additionally in men, higher free T was associated with higher leptin levels, whereas higher total T, SHBG, and T/E2 were associated with lower leptin levels. Also in men higher total T, bio T, and T/E2 were associated with lower resistin levels. No association was seen between sex hormones with leptin and resistin levels in women, after full covariate adjustment.
Sex hormones and adipokines in women
Prior MESA studies have suggested that a more androgenic sex hormone pattern (with higher free T levels) is associated with an adverse cardiometabolic profile and increased CVD risk in post-menopausal women (6–10). Women with higher androgen levels have more visceral fat (11); thus we had hypothesized that a more androgenic sex hormone profile would be associated with an adverse adipokine profile in postmenopausal women. Some of our findings were consistent with this hypothesis. At least in a limited adjusted model, higher androgen levels (i.e., free T and bio T) were associated with higher leptin levels in women, although this was no longer significant after adjusting for other CVD risk factors. In our supplemental analysis, this positive association between free T and leptin appeared stronger for women with a BMI < 30 compared to ≥ 30 kg/m2. We did not find any relationship of sex hormones with resistin in women.
Also consistent with our hypothesis, greater androgens such as free T and bio T were associated with lower levels of adiponectin, a favorable adipokine, in women. On the other hand, surprisingly and contrary to our hypothesis, higher total T/E2 ratio and lower E2 levels were positively associated with higher adiponectin. Although estrogen is thought to be cardioprotective in pre-menopausal women, its role is further complicated in our population of post-menopausal women. After menopause, endogenous E2 levels are low in women not on hormone therapy, even lower than in men. Circulating E2 levels in post-menopausal women, as well as in men, reflect peripheral conversion of testosterone to estradiol, which may be why the E2 results track with the results of free T. Interactions of E2 with adipokines in conferring cardiovascular risk or protection remain complicated and warrant further study. Total T includes both T in the bound and unbound states, and higher SHBG was also positively associated with adiponectin. It may be that free (unbound) T is a better marker of CVD risk in women. In our supplemental analysis, the inverse relationship between free T and adiponectin was stronger for post-menopausal women with BMI ≥ 30 kg/m2, compared to women with BMI < 30. Overall, taken together, our results further suggest that a more androgenic sex profile is inversely associated with an adipokine thought to be protective against cardiovascular risk in women.
Sex hormones and adipokines in men
Prior studies have suggested that a low androgen (hypogonadal state) is associated with visceral adiposity (11) and CVD risk (15, 16) in men, so we had hypothesized that higher androgens in men would be associated with a favorable adipokine profile. For men, higher androgen levels in the form of total T and T/E2 ratio, as well as lower SHBG levels, were associated with lower leptin levels, even after adjustment for other CVD risk factors. Higher androgen levels in the form of total T, bio T, and T/E2 ratio were also associated with lower resistin levels. Higher androgen levels in the form of total T and T/E2 were also associated with higher adiponectin levels, a cardio-protective adipokine. These results were consistent with our hypothesis of androgens being associated with a favorable adipokine profile in men.
However, not all findings aligned with our a priori hypothesis. Unexpectedly, higher free T was positively associated with leptin and inversely associated with adiponectin in men—an unfavorable adipokine pattern. It should be noted that approximately 68% of total T in circulation is tightly bound to SHBG and is biologically inactive in this form. The biologically active forms of T (i.e., bio T) consists of the ∼30% of T which is loosely bound to albumin and the 2% circulating as free T (43). In our analyses for men, the associations of free T were generally in the opposite directions of total T and SHBG. It may be that free T is a better estimate of available androgens in men and reflect the true relationship with cardiometabolic risk. This discordance highlights the complexity of understanding sex hormone patterns, when looking at a single hormone level. But generally the associations of free T with lower adiponectin levels mirrored that seen in women and further support the potentially adverse effects of endogenous androgens and the development of CVD in both sexes.
Putting findings into clinical context
We examined the association of sex hormones with adipokines, because a more unfavorable fat-distribution and adipokine profile may be an intermediary step linking an androgenic sex hormone profile with CVD risk. There are multiple proposed mechanisms by which adipokines influence CVD risk. Adipokines have been shown to influence chronic inflammation and metabolic disorders, including glucose intolerance, hyperlipidemia, and arterial hypertension (29, 44, 45), and vascular calcification (42, 46). Both estrogens and androgens have been implicated in the modulation of adipokines (47–50). Postmenopausal women, who have low estradiol levels, have increased central adiposity, increased leptin levels, and decreased adiponectin levels (49). Our findings suggest that a more androgenic sex hormone state after menopause is related to this unfavorable adipokine pattern. Reproductive age women with polycystic ovary syndrome also have hyperandrogenic sex hormone profiles that were associated with higher levels of leptin and lower levels of adiponectin (47, 48, 51). Although we studied post-menopausal women in our analysis, our findings are generally consistent with this findings by demonstrating higher free T and lower SHBG being associated with lower adiponectin.
Despite the associations of adipokines with cardiometabolic risk factors, the association of adipokines with future CVD events has been inconsistent, with both positive and negative (null) associations (32, 33, 52–54). In prior work from MESA, the association of leptin with incident CVD events was attenuated after accounting for BMI and traditional CVD risk factors (32), whereas greater resistin levels remained independently associated with CVD even after accounting for these factors (33). This underscores the complexity of our understanding of the pathways adipokines play in CVD risk.
Strengths and limitations
Our study has significant strengths. Prior studies from the MESA cohort have previously examined the independent associations between sex hormones and CVD risk (6) as well as adipokines and CVD risk (32, 33). We now newly examine the relationship of sex hormones and adipokines with each other. Our findings generally suggest there may be a link between more androgenic sex hormone profiles (captured by free T levels) and the adipokines that are thought to increase CVD risk, and a negative association with adipokines thought to be cardioprotective. Some findings though were inconsistent with our hypothesis. Although the clinical significance of our findings remains uncertain, this work suggests a possible pathophysiologic relationship between adipose-derived biomarkers and sex hormones, leading to differences in cardiovascular risk between the sexes. Little is known about this interaction and we provide data aimed at filling this need and hope future studies can provide additional clarity.
However, our study also has a number of limitations. Sex hormone levels and adipokines were only measured once in the MESA cohort, so we could not examine changes in these levels over time. Furthermore, we use a cross-sectional design and the sex hormones and adipokine levels were measured at different periods in time. Although we adjusted for confounding variables, residual confounders may still be present. Finally, we performed multiple analyses therefore some of our results may have been due to chance. However, the aim of our study was to be exploratory in nature with associations found to be further hypothesis-generating. Future studies should seek to understand mechanisms linking sex hormones, adipokines, and CVD risk.
Conclusion
In summary, we found in a diverse community-based cohort that an androgenic sex hormone profiles, characterized by higher free T and lower SHBG levels, were independently associated with the risk-enhancing adipokine of leptin and inversely associated with the more cardioprotective adipokine of adiponectin in both men and post-menopausal women. These findings may provide mechanistic insight in the interplay between sex hormones, adipokines, and CVD risk.
Data availability statement
The datasets presented in this article are not readily available because the data, methods, and materials used to conduct this study will be made available to other researchers for the purposes of reproducing or expanding on the results upon application to and approval by the MESA publications and presentations committee. Requests for the use of MESA data can also be done through the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Coordinating Center (https://biolincc.nhlbi.nih.gov/studies/mesa/). Requests to access the datasets should be directed to chsccweb@u.washington.edu.
Ethics statement
The studies involving human participants were reviewed and approved by the Johns Hopkins School of Medicine Institutional Review Board, IRB3 committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
BV and EM designed the study and wrote the initial draft. OOg performed the statistical analysis. MA secured grant funding for the measurement of adipokines and body composition. CN, BK, CR, OOs, MA, and AB provided critical revisions for important intellectual content. All authors approved the final draft for submission.
Acknowledgments
We thank the other investigators, the staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Statement
EM was supported by the Amato Fund for Women’s Cardiovascular Health at Johns Hopkins. The MESA study was supported by the contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart Lung and Blood Institute (NHLBI), and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from National Center for Advancing Translational Sciences (NCATS). The adipokine data used in this analysis were funded by the R01 HL088451. The sex hormone ancillary study was supported by the R01 HL074406 and R01 HL074338 from the NHLBI. CN and EM were additionally supported for this work by the American Heart Association Strategic Focused Research Network Grant 20SFRN35120152.
Conflict of interest
Unrelated to this work, EM served on a Medical Advisory Board for Amgen, Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Esperion, Novartis, Novo Nordisk, and Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1062460/full#supplementary-material
References
- 1.Broni E, Ndumele C, Echouffo-Tcheugui J, Kalyani R, Bennett W, Michos E. The diabetes-cardiovascular connection in women: understanding the known risks, outcomes, and implications for care. Curr Diab Rep. (2022) 22:11–25. 10.1007/s11892-021-01444-x [DOI] [PubMed] [Google Scholar]
- 2.Kannel W, Hjortland M, McNamara P, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. (1976) 85:447–52. 10.7326/0003-4819-85-4-447 [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Ding J, Bush T, Longenecker J, Nieto F, Golden S, et al. Relative androgen excess and increased cardiovascular risk after menopause: a hypothesized relation. Am J Epidemiol. (2001) 154:489–94. 10.1093/aje/154.6.489 [DOI] [PubMed] [Google Scholar]
- 4.Vitale C, Mendelsohn M, Rosano G. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. (2009) 6:532–42. 10.1038/nrcardio.2009.105 [DOI] [PubMed] [Google Scholar]
- 5.Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol. (2000) 52:595–600. 10.1046/j.1365-2265.2000.01000.x [DOI] [PubMed] [Google Scholar]
- 6.Zhao D, Guallar E, Ouyang P, Subramanya V, Vaidya D, Ndumele C, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol. (2018) 71:2555–66. 10.1016/j.jacc.2018.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathews L, Subramanya V, Zhao D, Ouyang P, Vaidya D, Guallar E, et al. Endogenous sex hormones and endothelial function in postmenopausal women and men: the multi-ethnic study of atherosclerosis. J Womens Health. (2019) 28:900–9. 10.1089/jwh.2018.7441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subramanya V, Zhao D, Ouyang P, Ying W, Vaidya D, Ndumele C, et al. Association of endogenous sex hormone levels with coronary artery calcium progression among post-menopausal women in the multi-ethnic study of atherosclerosis (MESA). J Cardiovasc Comput Tomogr. (2019) 13:41–7. 10.1016/j.jcct.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanya V, Zhao D, Ouyang P, Lima J, Vaidya D, Ndumele C, et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: the multi-ethnic study of atherosclerosis (MESA). Maturitas. (2018) 108:37–44. 10.1016/j.maturitas.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanya V, Ambale-Venkatesh B, Ohyama Y, Zhao D, Nwabuo C, Post W, et al. Relation of sex hormone levels with prevalent and 10-year change in aortic distensibility assessed by MRI: the multi-ethnic study of atherosclerosis. Am J Hypertens. (2018) 31:774–83. 10.1093/ajh/hpy024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongraw-Chaffin M, Anderson C, Allison M, Ouyang P, Szklo M, Vaidya D, et al. Association between sex hormones and adiposity: qualitative differences in women and men in the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. (2015) 100:E596–600. 10.1210/jc.2014-2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly D, Jones T. Testosterone and cardiovascular risk in men. Front Horm Res. (2014) 43:1–20. 10.1159/000360553 [DOI] [PubMed] [Google Scholar]
- 13.Colangelo L, Ouyang P, Liu K, Kopp P, Golden S, Dobs A, et al. Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: multi-ethnic study of atherosclerosis. Diabetes Care. (2009) 32:1049–51. 10.2337/dc08-2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menke A, Guallar E, Rohrmann S, Nelson W, Rifai N, Kanarek N, et al. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol. (2010) 171:583–92. 10.1093/aje/kwp415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corona G, Rastrelli G, Monami M, Guay A, Buvat J, Sforza A, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. (2011) 165:687–701. 10.1530/EJE-11-0447 [DOI] [PubMed] [Google Scholar]
- 16.Araujo A, Dixon J, Suarez E, Murad M, Guey L, Wittert G. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2011) 96:3007–19. 10.1210/jc.2011-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters S, Huxley R, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. (2014) 57:1542–51. 10.1007/s00125-014-3260-6 [DOI] [PubMed] [Google Scholar]
- 18.Ohkuma T, Komorita Y, Peters S, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia. (2019) 62:1550–60. 10.1007/s00125-019-4926-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden S, Dobs A, Vaidya D, Szklo M, Gapstur S, Kopp P, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. (2007) 92:1289–95. 10.1210/jc.2006-1895 [DOI] [PubMed] [Google Scholar]
- 20.Haffner S, Valdez R. Endogenous sex hormones: impact on lipids, lipoproteins, and insulin. Am J Med. (1995) 98:S40–7. 10.1016/S0002-9343(99)80058-8 [DOI] [PubMed] [Google Scholar]
- 21.Pugeat M, Moulin P, Cousin P, Fimbel S, Nicolas M, Crave J, et al. Interrelations between sex hormone-binding globulin (SHBG), plasma lipoproteins and cardiovascular risk. J Steroid Biochem Mol Biol. (1995) 53:567–72. 10.1016/0960-0760(95)00102-6 [DOI] [PubMed] [Google Scholar]
- 22.Rexrode K, Manson J, Lee I, Ridker P, Sluss P, Cook N, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. (2003) 108:1688–93. 10.1161/01.CIR.0000091114.36254.F3 [DOI] [PubMed] [Google Scholar]
- 23.Sutton-Tyrrell K, Wildman R, Matthews K, Chae C, Lasley B, Brockwell S, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the study of women across the nation (SWAN). Circulation. (2005) 111:1242–9. 10.1161/01.CIR.0000157697.54255.CE [DOI] [PubMed] [Google Scholar]
- 24.Bays H. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. (2011) 57:2461–73. 10.1016/j.jacc.2011.02.038 [DOI] [PubMed] [Google Scholar]
- 25.Bays H, Gonzalez-Campoy J, Bray G, Kitabchi A, Bergman D, Schorr A, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. (2008) 6:343–68. 10.1586/14779072.6.3.343 [DOI] [PubMed] [Google Scholar]
- 26.Fliotsos M, Zhao D, Rao V, Ndumele C, Guallar E, Burke G, et al. Body mass index from early-, mid-, and older-adulthood and risk of heart failure and atherosclerotic cardiovascular disease: MESA. J Am Heart Assoc. (2018) 7:e009599. 10.1161/JAHA.118.009599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gassett A, Sheppard L, McClelland R, Olives C, Kronmal R, Blaha M, et al. Risk factors for long-term coronary artery calcium progression in the multi-ethnic study of atherosclerosis. J Am Heart Assoc. (2015) 4:e001726. 10.1161/JAHA.114.001726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes-Austin J, Wassel C, Jiménez J, Criqui M, Ix J, Rasmussen-Torvik L, et al. The relationship between adiposity-associated inflammation and coronary artery and abdominal aortic calcium differs by strata of central adiposity: the multi-ethnic study of atherosclerosis (MESA). Vasc Med. (2014) 19:264–71. 10.1177/1358863X14537545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutheil F, Gordon B, Naughton G, Crendal E, Courteix D, Chaplais E, et al. Cardiovascular risk of adipokines: a review. J Int Med Res. (2018) 46:2082–95. 10.1177/0300060517706578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao V, Zhao D, Allison M, Guallar E, Sharma K, Criqui M, et al. Adiposity and incident heart failure and its subtypes: MESA (multi-ethnic study of atherosclerosis). JACC Heart Fail. (2018) 6:999–1007. 10.1016/j.jchf.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pischon T, Girman C, Hotamisligil G, Rifai N, Hu F, Rimm E. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. (2004) 291:1730–7. 10.1001/jama.291.14.1730 [DOI] [PubMed] [Google Scholar]
- 32.Martin S, Blaha M, Muse E, Qasim A, Reilly M, Blumenthal R, et al. Leptin and incident cardiovascular disease: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. (2015) 239:67–72. 10.1016/j.atherosclerosis.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muse ED, Feldman D, Blaha M, Dardari Z, Blumenthal R, Budoff M, et al. The association of resistin with cardiovascular disease in the multi-ethnic study of atherosclerosis. Atherosclerosis. (2015) 239:101–8. 10.1016/j.atherosclerosis.2014.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau W, Ohashi K, Wang Y, Ogawa H, Murohara T, Ma X, et al. Role of adipokines in cardiovascular disease. Circ J. (2017) 81:920–8. 10.1253/circj.CJ-17-0458 [DOI] [PubMed] [Google Scholar]
- 35.Ohman-Hanson R, Cree-Green M, Kelsey M, Bessesen D, Sharp T, Pyle L, et al. Ethnic and sex differences in adiponectin: from childhood to adulthood. J Clin Endocrinol Metab. (2016) 101:4808–15. 10.1210/jc.2016-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hellstrom L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P. Mechanisms behind gender differences in circulating leptin levels. J Intern Med. (2000) 247:457–62. 10.1046/j.1365-2796.2000.00678.x [DOI] [PubMed] [Google Scholar]
- 37.Bild D, Bluemke D, Burke G, Detrano R, Diez Roux A, Folsom A, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. (2002) 156:871–81. 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 38.Chu J, Michos E, Ouyang P, Vaidya D, Blumenthal R, Budoff M, et al. Coronary artery calcium and atherosclerotic cardiovascular disease risk in women with early menopause: the multi-ethnic study of atherosclerosis (MESA). Am J Prev Cardiol. (2022) 11:100362. 10.1016/j.ajpc.2022.100362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. (1982) 16:801–10. 10.1016/0022-4731(82)90038-3 [DOI] [PubMed] [Google Scholar]
- 40.Forbang N, McClelland R, Remigio-Baker R, Allison M, Sandfort V, Michos E, et al. Associations of cardiovascular disease risk factors with abdominal aortic calcium volume and density: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. (2016) 255:54–8. 10.1016/j.atherosclerosis.2016.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; (2017). [Google Scholar]
- 42.Varma B, Ogunmoroti O, Ndumele C, Zhao D, Szklo M, Sweeney T, et al. Higher leptin levels are associated with coronary artery calcium progression: the multi-ethnic study of atherosclerosis (MESA). Diabet Epidemiol Manag. (2022) 6:100047. 10.1016/j.deman.2021.100047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oskui P, French W, Herring M, Mayeda G, Burstein S, Kloner R. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. (2013) 2:e000272. 10.1161/JAHA.113.000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smekal A, Vaclavik J. Adipokines and cardiovascular disease: a comprehensive review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2017) 161:31–40. 10.5507/bp.2017.002 [DOI] [PubMed] [Google Scholar]
- 45.Kim W, Bae K, Lee S, Oh K. The latest insights into adipokines in diabetes. J Clin Med. (2019) 8:1874. 10.3390/jcm8111874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweeney T, Ogunmoroti O, Ndumele C, Zhao D, Varma B, Allison M, et al. Associations of adipokine levels with the prevalence and extent of valvular and thoracic aortic calcification: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. (2021) 338:15–22. 10.1016/j.atherosclerosis.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ardawi M, Rouzi A. Plasma adiponectin and insulin resistance in women with polycystic ovary syndrome. Fertil Steril. (2005) 83:1708–16. 10.1016/j.fertnstert.2004.11.077 [DOI] [PubMed] [Google Scholar]
- 48.Samy N, Hashim M, Sayed M, Said M. Clinical significance of inflammatory markers in polycystic ovary syndrome: their relationship to insulin resistance and body mass index. Dis Markers. (2009) 26:163–70. 10.1155/2009/465203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tankó L, Christiansen C. Adipose tissue, insulin resistance and low-grade inflammation: implications for atherogenesis and the cardiovascular harm of estrogen plus progestogen therapy. Climacteric. (2006) 9:169–80. 10.1080/13697130600738765 [DOI] [PubMed] [Google Scholar]
- 50.Van Sinderen M, Steinberg G, Jørgensen S, Honeyman J, Chow J, Herridge K, et al. Effects of estrogens on adipokines and glucose homeostasis in female aromatase knockout mice. PLoS One. (2015) 10:e0136143. 10.1371/journal.pone.0136143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guan C, Zahid S, Minhas A, Ouyang P, Vaught A, Baker V, et al. Polycystic ovary syndrome: a “risk-enhancing” factor for cardiovascular disease. Fertil Steril. (2022) 117:924–35. 10.1016/j.fertnstert.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 52.Sattar N, Wannamethee G, Sarwar N, Chernova J, Lawlor D, Kelly A, et al. Leptin and coronary heart disease: prospective study and systematic review. J Am Coll Cardiol. (2009) 53:167–75. 10.1016/j.jacc.2008.09.035 [DOI] [PubMed] [Google Scholar]
- 53.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace A, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. (2006) 114:623–9. 10.1161/CIRCULATIONAHA.106.618918 [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Guo W, Li J, Cao S, Zhang J, Pan J, et al. Leptin concentration and risk of coronary heart disease and stroke: a systematic review and meta-analysis. PLoS One. (2017) 12:e0166360. 10.1371/journal.pone.0166360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because the data, methods, and materials used to conduct this study will be made available to other researchers for the purposes of reproducing or expanding on the results upon application to and approval by the MESA publications and presentations committee. Requests for the use of MESA data can also be done through the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Coordinating Center (https://biolincc.nhlbi.nih.gov/studies/mesa/). Requests to access the datasets should be directed to chsccweb@u.washington.edu.