Abstract
Coronaviruses can cause serious respiratory tract infections and may also impact other end organs such as the central nervous system, the lung and the heart. The coronavirus disease 2019 (COVID-19) has had a devastating impact on humanity. Understanding the mechanisms that contribute to the pathogenesis of coronavirus infections, will set the foundation for development of new treatments to attenuate the impact of infections with coronaviruses on host cells and tissues. During infection of host cells, coronaviruses trigger an imbalance between increased production of reactive oxygen species (ROS) and reduced antioxidant host responses that leads to increased redox stress. Subsequently, increased redox stress contributes to reduced antiviral host responses and increased virus-induced inflammation and apoptosis that ultimately drive cell and tissue damage and end organ disease. However, there is limited understanding how different coronaviruses including SARS-CoV-2, manipulate cellular machinery that drives redox responses. This review aims to elucidate the redox mechanisms involved in the replication of coronaviruses and associated inflammation, apoptotic pathways, autoimmunity, vascular dysfunction and tissue damage that collectively contribute to multiorgan damage.
Keywords: SARS-CoV-2, coronavirus, inflammation, oxidative stress, tissue damage, apoptosis
Introduction
The coronavirus disease 2019 (COVID-19) has had a devastating impact on humanity. Coronaviruses can cause serious respiratory tract infections and may impact other end organs such as the central nervous system. Coronaviruses are enveloped single-stranded positive-sense RNA viruses named after their crown-like appearance of their spike proteins on their surface (Singhal, 2020). To date, there has been seven human coronaviruses (HCoVs) identified: severe acute respiratory syndrome coronavirus (SARS-CoV-2), SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), Human coronavirus 229E (HCoV-229E), HCoV-OC43, HCoV-NL63, and HKU-1. Four of them including HCoV-OC43, HCoV-NL63, HCoV-229E, and HKU-1, typically trigger only mild respiratory illnesses in humans. On the other hand, SARS-CoV-2, SARS and MERS are known to cause more severe illness, acute respiratory distress syndrome (ARDS) or multi-organ dysfunction, especially in aged people with comorbidities (Li et al., 2021a). Understanding the mechanisms that contribute to the pathogenesis of coronavirus infections, will set the foundation for development of new treatments to attenuate the impact of coronaviruses on host cells and tissues. However, there is limited understanding how different coronaviruses including SARS-CoV-2, manipulate cellular machinery to drive host cell responses.
Emerging evidence suggests that human diseases including viral infections often disrupt the host natural balance between increased production of reactive oxygen species (ROS) and reduced antioxidant host responses that collectively increases redox stress (Amini et al., 2022; Figure 1). ROS are free radical and nonradical byproducts of metabolic processes in organelles such as plasma and nuclear membranes, the mitochondria, peroxisomes and the endoplasmic reticulum (ER; Reshi et al., 2014). ROS are necessary for cellular processes like mitochondrial energy production, host defense, cellular signaling, and the regulation of gene expression. Mitochondria are the main location of production of ROS (mito-ROS) during energy production. Increased ROS during viral infections have not only detrimental impact on the cells and tissues but are also important for antiviral immune function (Yang et al., 2007; Finkel, 2011) during viral infections like influenza (To et al., 2014), respiratory syncytial virus (RSV; Fink et al., 2008) and rhinoviruses (Kaul et al., 2000; Fink et al., 2008).
Figure 1.
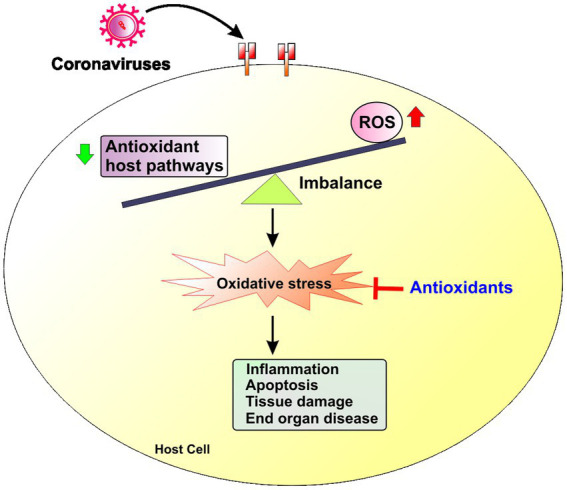
Redox imbalance in coronavirus infections. Coronavirus infection triggers an imbalance between increased production of reactive oxygen species (ROS) and reduced antioxidant host responses that leads to increased redox stress in the host cell. Increased redox stress induces inflammation, apoptosis and ultimately tissue damage and end organ disease.
However, an excess of ROS can damage cellular components including lipids, proteins, and DNA, alter immune functions, inflammatory responses and induce organ and tissue dysfunction (Preiser, 2012; Reshi et al., 2014; Labarrere and Kassab, 2022). Indeed, several studies have shown that oxidative stress contributes to the pathogenesis of respiratory viral infections (Khomich et al., 2018), influenza and RSV. Increased oxidative stress in severe COVID-19 contributes to inflammation, endothelial cell dysfunction, thrombosis that can lead to multiorgan damage (Li et al., 2021a; Alam and Czajkowsky, 2022). Oxidative stress, induced by coronavirus, also interferes with inflammatory pathways that may lead to more long-lasting tissue damage. However, there is limited understanding how different coronaviruses including SARS-CoV-2, manipulate cellular machinery that drives redox responses.
In this review, we summarize the scientific evidence regarding the cellular and molecular pathways modulated by oxidative stress that are implicated in the pathogenesis of coronavirus infections. We specifically review the role of redox pathways in major pathophysiological underpinnings that contribute to cell and tissue damage in coronavirus infection: (1) virus replication, (2) virus-associated inflammation, (3) virus-associated apoptosis, (4) redox-related end organ disease. We review the scientific evidence related to these redox pathways, separately for SARS-CoV-2 versus all the other coronaviruses [SARS-CoV, MERS, respiratory coronaviruses and other coronaviruses used to model SARS-CoV-2 infection such as the murine hepatitis virus (MHV)]. Finally, we discuss the relevance of these redox pathways with regards to acute severe COVID-19 and Post-Acute Sequelae of SARS-CoV-2 infection (PASC) and potential antioxidant treatments.
Redox mechanisms that regulate replication of coronaviruses
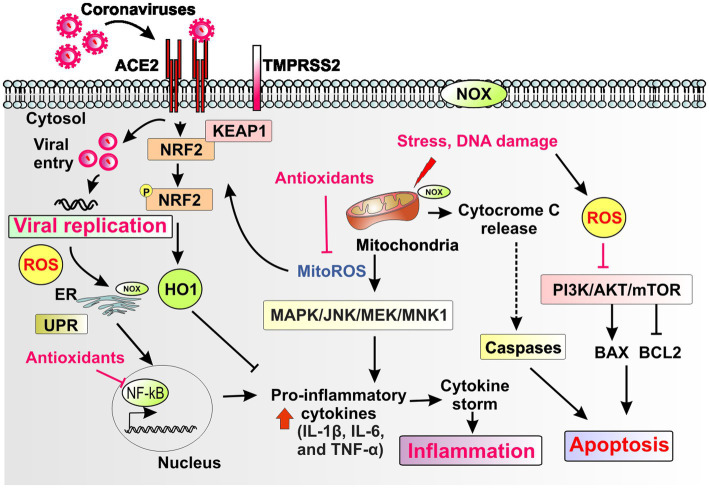
Several redox mechanisms can regulate both viral entry and cytosolic replication of coronaviruses (Figure 2; Table 1; Wang and Zhang, 1999; Kulisz et al., 2002; Halestrap et al., 2004; Mizutani et al., 2004; Emerling et al., 2005; Kefaloyianni et al., 2006; Doughan et al., 2008; Lucas et al., 2008; Cho et al., 2009; Garrido and Griendling, 2009; Hosakote et al., 2009; Jamaluddin et al., 2009; Wosniak et al., 2009; de Wilde et al., 2011; Kesic et al., 2011; Xia et al., 2011; Kosmider et al., 2012; Yamada et al., 2012; Kim et al., 2012b; Lee et al., 2013; Nguyen Dinh Cat et al., 2013; Komaravelli and Casola, 2014; Hyser and Estes, 2015; Kindrachuk et al., 2015; Komaravelli et al., 2015; Paszti-Gere et al., 2015; Shirihai et al., 2015; Simon et al., 2015; Demers-Lamarche et al., 2016; Kau et al., 2016; Morris et al., 2016; Zhang et al., 2016; Daiber et al., 2017; Trempolec et al., 2017; Khomich et al., 2018; Tu et al., 2019; Olagnier et al., 2020; Tao et al., 2020; Verdecchia et al., 2020; Herengt et al., 2021; Moghimi et al., 2021; Youn et al., 2021).
Figure 2.
Schematic representation of redox pathways that contribute to viral replication, inflammation, and apoptosis during coronavirus infection. Coronaviruses bind to the ACE2 receptor and replicate through host proteases such as TMPRSS2 and by hijacking cytosolic cellular machinery such as the mitochondria and the endoplasmic reticulum (ER), which engages the unfolded protein response (UPR). The plasma membrane, the ER and mitochondria harbor different isoforms of the NADPH oxidase (NOX) enzyme. Coronaviruses induce cellular oxidative stress with generation of reactive oxygen species (ROS) and mitochondrial ROS (mito-ROS) and impairment of stress-inducible, antioxidant, anti-inflammatory and antiviral responses such as the Nrf2 pathway and other key downstream mediators such as Heme oxygenase-1 (HO-1). Mito-ROS induce downstream signaling pathways such as MAPK, JNK, MEK/MNK1 that induce both viral replication and proinflammatory pathways such as induction of cytokines (e.g., IL-1b, IL-6, and TNF-a). Mito-ROS, ROS and ER stress response induce the proinflammatory pathway NF-κB. ROS and mito-ROS also induce apoptosis through alterations in apoptotic pathways such as PI3K/AKT, mTOR and induction of mitochondrial apoptosis. Collectively, redox mediated pathways that drive viral replication, inflammation and apoptosis contribute to cell and tissue damage that drive end organ disease in coronavirus infection. Endogenous antioxidant host pathways and exogenous therapeutic antioxidants could attenuate redox mediated pathways that drive pathogenesis of coronavirus infections.
Table 1.
Redox mechanisms that regulate replication of coronaviruses.
| Mediators | Effect on redox balance | References |
|---|---|---|
| Redox mechanisms that may regulate viral entry of coronaviruses | ||
| Bidirectional cross talk between virus and the ACE2-AngII (ligand of ACE2)-NOX axis |
|
Doughan et al. (2008), Garrido and Griendling (2009), Wosniak et al. (2009), Xia et al. (2011), Kim et al. (2012b), Lee et al. (2013), Nguyen Dinh Cat et al. (2013), Daiber et al. (2017), Verdecchia et al. (2020) |
| ||
| ||
| ||
| TMPRSS2 (host protease essential for replication of coronavirus) |
|
Lucas et al. (2008), Paszti-Gere et al. (2015), Kau et al. (2016), Moghimi et al. (2021), Youn et al. (2021) |
| Mito-ROS |
|
Demers-Lamarche et al. (2016), Morris et al. (2016) |
| ||
| Redox mechanisms regulating cytoplasmic replication of coronaviruses | ||
| Mito-ROS |
|
Wang and Zhang (1999), Kulisz et al. (2002), Halestrap et al. (2004), Mizutani et al. (2004), Emerling et al. (2005), Kefaloyianni et al. (2006), Jamaluddin et al. (2009), de Wilde et al. (2011), Hyser and Estes (2015), Kindrachuk et al. (2015), Shirihai et al. (2015), Zhang et al. (2016), Trempolec et al. (2017), Tao et al. (2020) |
| ||
| ||
| ||
| ||
| ||
| ||
| Keap1-Nrf2-ARE pathway |
|
Cho et al. (2009), Hosakote et al. (2009), Kesic et al. (2011), Yamada et al. (2012), Kosmider et al. (2012), Komaravelli and Casola (2014), Komaravelli et al. (2015), Simon et al. (2015), Khomich et al. (2018), Tu et al. (2019), Olagnier et al. (2020), Herengt et al. (2021) |
| ||
| ||
| ||
| ||
| ||
Abbreviations: AAK, adaptor-associated kinase; ACE2, Angiotensin-converting enzyme 2; AngII, Angiotensin II; ARE, antioxidant response element; Ca2+, Calcium (II) ion; ER, endoplasmic reticulum; HIV, human immunodeficiency virus; HO-1, Heme oxygenase 1; Keap1, Kelch-like ECH-associated protein 1; MAPK, mitogen-activated protein kinase; MEK, Mitogen-activated protein kinase; Mito-ROS, Mitochondrial reactive oxygen species; Mnk1, mitogen-activated protein kinase (MAPK) interacting protein kinase 1; mPTP, mitochondrial permeability transition pore; NOX, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; Nrf2, nuclear factor erythroid 2–related factor 2; NQo-1, NAD(P)H quinone oxidoreductase; RSV, Respiratory Syncytial Virus; SOD, Superoxide dismutase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, Transmembrane serine protease 2; UPR, unfolded protein response.
Redox mechanisms that regulate virus entry of coronaviruses
The spike S proteins on the surface of coronaviruses are responsible to their attachment to host receptors in airway epithelial cells such as the angiotensin-converting enzyme 2 (ACE2) receptors that interact with host cell proteases, such as transmembrane protease serine 2 (TMPRSS2; Hamming et al., 2004; Irigoyen et al., 2016; Lukassen et al., 2020; Xu et al., 2020). While many coronaviruses utilize peptidases, such as ACE2, dipeptidyl peptidase 4, aminopeptidase N, as their cellular receptors, SARS-CoV, SARS-CoV-2 and HCoV-NL63 utilize ACE2 as their receptors thus disrupting the renin-angiotensin system (Verdecchia et al., 2020).
ACE2, a peptidase that exists on the cell surfaces of most organs (Hamming et al., 2004), is one of the most crucial key players in induction of redox stress (Shatizadeh Malekshahi et al., 2022). Angiotensin II (AngII), the ligand of ACE2, is a potent activator of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and an inducer of ROS production in the vasculature, kidney and brain (Garrido and Griendling, 2009). Typically, ACE2 helps avert NAPDH oxidase activity by converting Ang II into angiotensin 1–7, thereby reducing ROS levels; Ang II stimulates NAPDH oxidase. ACE2 overexpression has been shown to reduce ROS, and ACE2 deficiency has been shown to induce oxidative stress (Xia et al., 2011; Pena Silva et al., 2012). The complex cross-talk between ACE2 and redox pathways is further emphasized by a possible bidirectional redox regulation of ACE2 levels. High ACE2 activity may reduce redox stress but vice versa high redox stress may regulate ACE2 activity. In vitro studies showed that NOX-driven ROS may reduce ACE2 in vascular smooth muscle cells (Lavrentyev and Malik, 2009). Consistent with this evidence, independent in vitro studies demonstrated that Ang II-induced activation of mitochondrial Nox4 is an important endogenous source of ROS and is related to cell survival in kidney epithelial cells (Kim et al., 2012b). The crosstalk between NOX and ACE2 has also been shown in vivo in mouse models of disease and increased levels of ACE2 are generally associated with reduced oxidative stress in mammalian cells (Xia et al., 2011).
Angiotensin II is often upregulated in viral infections (Doughan et al., 2008; Wosniak et al., 2009; Lee et al., 2013; Daiber et al., 2017). However, when cells are infected with coronavirus, there is a reduction of ACE2 receptors on the cell surface and this results in an increase of Ang II which binds to ACE1 and increases ROS levels through NADPH oxidase (Nguyen Dinh Cat et al., 2013). Experimental studies have demonstrated that in vitro exposure to S protein induces excessive oxidative stress in endothelial cells, which is mediated specifically by activation of NADPH oxidase isoform 2 (NOX2), but not NOX1 or NOX4 (Youn et al., 2021). However, it is unclear if there is bidirectional link between ACE2 levels and increased redox cellular pathways in the setting of SARS-CoV-2-induced ACE2 downregulation in airway epithelial cells.
TMPRSS2 is expressed in both the cytoplasm as well as in the cell membrane in epithelial cells (Lucas et al., 2008). In vitro studies with porcine intestinal epithelial cells have shown that acute excessive oxidative stress induces altered distribution pattern of TMPRSS2 and relocalized transmembrane serine protease activity that may contribute to weakening of epithelial barrier integrity (Paszti-Gere et al., 2015). However, a small study of COVID-19 patients and uninfected controls showed that measures of oxidative stress in sperm epithelial cells were not associated with levels of TMPRSS2 (Moghimi et al., 2021). Similarly, another experimental study showed that cigarette smoking extract (CSE) that is an established trigger of oxidative stress (Kau et al., 2016) had no effect on ACE2 and TMPRSS2 expression in endothelial cells (Youn et al., 2021). Overall, there is no solid evidence to support a role of increased redox stress in regulation of TMPRSS2.
Other than redox-dependent regulation of membrane receptors for coronaviruses, mito-ROS are also instigators of aberrant vacuole formation (Demers-Lamarche et al., 2016) by activation of adaptor-associated kinase 1 (AAK1), a regulator of endocytosis (Chen et al., 2006) that has been targeted therapeutically in SARS-CoV-2 infection with baricitinib (Stebbing et al., 2020). Mito-ROS can also induce alterations in membrane lipid rafts and lipid-based cellular signaling changing their properties (Morris et al., 2016) and these membrane changes may also impact viral entry of coronaviruses. Thus, redox mechanisms may regulate entry of coronaviruses in mammalian cells but these mechanisms need to be further studied specifically in airway epithelial cells and in vivo.
Redox mechanisms that regulate cytoplasmic replication of coronavirus
Viral infections may alter the mitochondrial dynamics leading to excessive mito-ROS generation, mitochondrial biogenesis, and altered mitochondrial β-oxidation (Elesela and Lukacs, 2021). Mitochondria are targeted by coronavirus (Shi et al., 2014). Coronaviruses may directly induce production of mito-ROS in cells. Non-structured viral proteins, such as coronavirus 3a protein directly activate NLRP3 inflammasome in macrophages, which is mediated by increased mito-ROS level (Zhou et al., 2011; Chen et al., 2019). Finally, redox pathways also regulate cellular machinery that propagates replication of coronaviruses through multiple pathways.
First, Mito-ROS regulate the endoplasmic reticulum stress and the unfolded protein response (UPR) that contribute to replication of coronaviruses (de Wilde et al., 2011; Hyser and Estes, 2015; Kindrachuk et al., 2015; Zhang et al., 2016) and associated Ca2+ signaling systems. Second, mito-ROS induce the mitochondrial permeability transition pore (mPTP) that is a proviral factor for replication of coronaviruses. Indeed, by blocking the mPTP, cyclosporin A impacts coronavirus replication (Halestrap et al., 2004). Mitochondria-targeted antioxidants inhibit mPTP, mito-ROS (Halestrap et al., 2004), and ROS (Dikalova et al., 2010; Dikalov et al., 2014). Third, mito-ROS regulate mitophagy that regulates replication of coronaviruses. Protein misfolding mitochondrial depolarization and ROS activate mitophagy (Shirihai et al., 2015). Viral proteins like SARS-CoV ORF-9 (Shi et al., 2014) interact with mitophagic machinery such as LC3 and Beclin1 (Zhang et al., 2018). Therapeutic targeting of aberrant autophagy through Beclin1 reduces MERS infection (Gassen et al., 2019). Fifth, mito-ROS trigger MEK (Zhang et al., 2016), MNK1 (Wang and Zhang, 1999) and MAPK signaling pathways (Kulisz et al., 2002; Emerling et al., 2005; Trempolec et al., 2017) that propagate viral protein synthesis and SARS-Co-V replication (Mizutani et al., 2004; Kefaloyianni et al., 2006; Jamaluddin et al., 2009). Sixth, ROS regulate cytoplasmic interferon host antiviral responses during coronavirus infection. ROS promotes MHV replication by downregulating interferon host responses during MHV infection (Tao et al., 2020). Lastly, preclinical studies suggest that mito-ROS may contribute to viral reservoirs and replication of SARS-CoV-2 in macrophages, but this has not been clearly demonstrated in vivo (Codo et al., 2020). Thus, mito-ROS induce multiple proviral cytoplasmic pathways.
Antioxidant mechanisms that regulate cytoplasmic replication of coronavirus
The primary transcription factor regulating the antioxidant response is the nuclear factor E2-related factor 2 (Nrf2), which regulates the Kelch-like ECH-associated protein 1 (Keap1)-Nrf2-antioxidant response elements (ARE) pathway (Khomich et al., 2018). Under normal circumstances, the Keap1-Nrf2-ARE pathway is activated by the oxidative stress resulting from ROS production. Nrf2, which is usually bound to Keap1 by ubiquitination or degraded by Keap1 in the absence of oxidative stress, is translocated to the nucleus when oxidative stress modifies the conformational structure of Keap1 and prevents it from binding Nrf2 (Komaravelli and Casola, 2014; Han et al., 2021). Mito-ROS activate Nrf2 through protein kinases, and induce production of antioxidant proteins and genes involved in mitochondrial quality control (Kasai et al., 2020). The activation of Nrf2 results in the upregulation of antioxidant gene expression as Nrf2 binds to antioxidant response element (ARE) sites, leading to the expression of key players of the antioxidant response, including heme oxygenase-1 (HO-1), NADPH quinone oxidoreductase 1 (NQO-1), superoxide dismutases (SOD), and glutathione derived molecules catalase, peroxiredoxins, and glutathione peroxidases which collectively attenuate oxidative stress (Khomich et al., 2018; Tu et al., 2019).
Several studies have found that respiratory viruses downregulate the expression of antioxidant genes by inhibiting Nrf2, preventing it from mobilizing to the nucleus and binding to ARE sites (Komaravelli and Casola, 2014). The Nrf2 pathway that mediates pathogenesis and tissue damage of several viral infections including HIV, RSV (Cho et al., 2009; Hosakote et al., 2009; Komaravelli et al., 2015), influenza (Kesic et al., 2011; Kosmider et al., 2012; Yamada et al., 2012; Simon et al., 2015), and SARS-CoV-2 (Olagnier et al., 2020). Induction of the Nrf2 pathway and key downstream mediators such as Heme oxygenase-1 (HO-1) triggers stress-inducible, anti-inflammatory, and antiviral responses present in most human cells (Espinoza et al., 2017). NRF2 has antiviral properties but, it remains unclear which genes mediate these effects and how they exert antiviral effect (Herengt et al., 2021).
Emerging evidence has increased our understanding of the role of Nrf2 activation in SARS-CoV-2 infection. In vitro experiments with Vero hTMPRSS2 cells, Calu-3 and primary human airway epithelial cell lines and using gene silencing of Keap1 and Nrf2 agonists 4-octyl-itaconate (4-OI) and dimethyl fumarate (DMF), it was shown that the Nrf2 pathway has a critical role in inhibiting SARS-CoV-2 replication, in addition to limiting the host inflammatory response. SARS-CoV-2 reduced in vitro basal levels of HO-1 and NQO-1 in lung cells. Notably, considering Nrf2’s known role in inhibiting anti-viral IFN responses, it was shown that the antiviral effect of Nrf2 is independent of interferon responses (Olagnier et al., 2020). Mechanistic preclinical studies showed that Nrf2 activation reduced SARS-CoV-2 replication by inducing the metabolite biliverdin, whereas SARS-CoV-2 altered the NRF2 axis through the cross-talk between the nonstructural viral protein NSP14 and the NAD-dependent deacetylase Sirtuin 1 (SIRT1; Olagnier et al., 2020; Zhang et al., 2022).
Experimental studies have also shown that downregulation of antioxidant genes by SARS-CoV-2 and SARS-CoV-1 is combined with an upregulation of oxidative stress genes like myeloperoxidase (MPO), calprotectin (S100A8 and S100A9), sulfiredoxin-1 (SRXN1), glutamate cysteine ligase modifier subunit (GCLM), sestrin2 (SESN2), and thioredoxin-1 (TXN; Saheb Sharif-Askari et al., 2021). The results of these studies have revealed key aspects of SARS-CoV-2 infection: such as downregulation of host’s antioxidant pathway as an important role in viral replication, and possible utility of activators of antioxidant pathways as specific therapeutic targets.
Redox mechanisms that regulate replication of coronavirus through apoptotic pathways
Many viruses alter apoptosis or programmed cell death of the infected cell as a mechanism of increased production of virus progeny, cell killing and virus spread (Roulston et al., 1999). Apoptosis is the programmed cell death that involves the activation of proteases called caspases and a cascade of events that link apoptosis-initiating stimuli to final death of the cell. ROS (Pierce et al., 1991; Kasahara et al., 1997) and mitochondria play pivotal roles in induction of apoptosis under both physiologic and pathologic conditions. Increased mito-ROS induce apoptosis and cell death (Orrenius et al., 2007). Excessive ROS can activate pro-apoptotic Bcl-2 family proteins by increasing mitochondrial permeability to drive the mitochondrial membrane potential, release cytochrome c, mtDNA (Santos et al., 2003), and pro-apoptotic caspase-3 and-9. This leads to the activation of intrinsic or mitochondrial driven cell death by apoptosis (Green and Llambi, 2015). Coronaviruses impact apoptosis through several pathways. Notably, mitochondrial apoptosis is directly and uniquely induced by SARS-CoV (Pfefferle et al., 2011) triggering viral replication (Supinski et al., 2009; Maiti et al., 2017). SARS-CoV-2 infection also downregulates the Nrf2 pathway (Olagnier et al., 2020; Zhang et al., 2022) which has antiapoptotic cellular effect (Niture and Jaiswal, 2012; Khan et al., 2018). Thus, coronaviruses induce apoptosis through multiple pathways, either directly (Pfefferle et al., 2011), or indirectly by inducing production of mito-ROS and downregulating antiapoptotic pathways such as Nrf2 and the virus-induced alteration of mitochondrial apoptosis contributes to increased replication of coronaviruses (Supinski et al., 2009; Pfefferle et al., 2011; Maiti et al., 2017).
Redox mechanisms that regulate replication of coronavirus through the complement system
The complement system is a major host defense mechanism against viral replication. Several viruses hijack the complement system for cellular entry and spread (Agrawal et al., 2017). The role of the complement system in the pathogenesis of coronavirus infections is complex and contradictory (Santiesteban-Lores et al., 2021). During SARS-CoV-2 infection, the complement system is a host defense mechanism against viral replication in asymptomatic or mild cases (Santiesteban-Lores et al., 2021). However, complement activation has also potent proinflammatory effect and can increase local and systemic damage in severe COVID-19 (Santiesteban-Lores et al., 2021). As outlined above, coronavirus induce production of mito-ROS during infection. Mito-ROS induce the “complement–metabolism–inflammasome axis”(Arbore and Kemper, 2016). MERS-CoV can also directly induce the complement system (Chen et al., 2010). Collectively, limited evidence suggests that complement activation through redox pathways may have a more important role in cell and tissue damage in severe coronavirus infections rather than a major regulatory role in replication of coronaviruses.
Redox mechanisms that regulate replication of coronavirus through mitophagy
Mitophagy, the cellular process that clears excess or damaged mitochondria, has a key role in function of mitochondria and mammalian cells and regulates severeal physiological and pathological processes, including apoptosis, immunity and inflammation. Emerging evidence suggests that several viruses hijack mitophagy to enable viral replication and escape host immune responses (Li et al., 2022). SARS-CoV can encode open reading frame-9b (ORF-9b), which is localized in mitochondria and induces mitochondrial elongation which further triggers mitophagy and coronavirus replication (Shi et al., 2014). Preclinical studies have shown that SARS-CoV-2 directly causes mitochondrial dysfunction and mitophagy impairment (Shang et al., 2021). Notably, defects in autophagy and mitophagy processes may regulate host response to coronavirus infection (Pacheco et al., 2021). Coronaviruses also induce production of mito-ROS that have an established complex crosstalk with mitophagy (Schofield and Schafer, 2021). Overall, further evidence is needed to clearly link the role of aberrant redox pathways and mitophagy in the regulation of replication of coronaviruses.
Redox pathways that regulate inflammation during infection with coronaviruses
Several redox mechanisms regulate inflammation during infection with coronaviruses (Figure 2; Table 2; Shono et al., 1996; Wesselborg et al., 1997; Chua et al., 1998; Canty et al., 1999; Tenjinbaru et al., 1999; Wang and Zhang, 1999; Cooke and Davidge, 2002; Pearlstein et al., 2002; Takada et al., 2003; Mizutani et al., 2004; Desouki et al., 2005; Mukherjee et al., 2005; Kefaloyianni et al., 2006; Xie and Shaikh, 2006; Schrader et al., 2007; Doughan et al., 2008; Nanduri et al., 2008; Cho et al., 2009; Hosakote et al., 2009; Jamaluddin et al., 2009; Martinon et al., 2009; Wosniak et al., 2009; Dikalova et al., 2010; Bulua et al., 2011; Kesic et al., 2011; Kosmider et al., 2012; Yamada et al., 2012; Lee et al., 2013; Nakajima and Kitamura, 2013; Nguyen Dinh Cat et al., 2013; Komaravelli and Casola, 2014; Zinovkin et al., 2014; Komaravelli et al., 2015; Simon et al., 2015; Sun et al., 2016; Zhang et al., 2016; Daiber et al., 2017; Espinoza et al., 2017; Khomich et al., 2018; Tu et al., 2019; Valle et al., 2019; Connors and Levy, 2020; Mahmud-Al-Rafat et al., 2020; Olagnier et al., 2020; Herengt et al., 2021; Saheb Sharif-Askari et al., 2021; Toro et al., 2022).
Table 2.
Redox mechanisms that regulate cell and tissue damage during infection with coronaviruses.
| Mediators | Effect on redox balance | References |
|---|---|---|
| Redox NF-kB |
|
Nakajima and Kitamura (2013) |
| ||
| ||
| ROS |
|
Pearlstein et al. (2002), Mukherjee et al. (2005), Zinovkin et al. (2014) |
| ||
| ||
| ||
| ||
| Mito-ROS |
|
Shono et al. (1996), Wesselborg et al. (1997), Chua et al. (1998), Canty et al. (1999), Tenjinbaru et al. (1999), Wang and Zhang (1999), Cooke and Davidge (2002), Mizutani et al. (2004), Desouki et al. (2005), Kefaloyianni et al. (2006), Xie and Shaikh (2006), Doughan et al. (2008), Jamaluddin et al. (2009), Martinon et al. (2009), Wosniak et al. (2009), Bulua et al. (2011), Lee et al. (2013), Sun et al. (2016), Zhang et al. (2016), Daiber et al. (2017), Saheb Sharif-Askari et al. (2021) |
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| Keap1-Nrf2-ARE pathway |
|
Cho et al. (2009), Hosakote et al. (2009), Kesic et al. (2011), Yamada et al. (2012), Kosmider et al. (2012), Komaravelli and Casola (2014), Komaravelli et al. (2015), Simon et al. (2015), Espinoza et al. (2017), Khomich et al. (2018), Tu et al. (2019), Olagnier et al. (2020), Herengt et al. (2021), Toro et al. (2022) |
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| Ang II |
|
Nguyen Dinh Cat et al. (2013), Mahmud-Al-Rafat et al. (2020) |
| Type I IFNs |
|
Dikalova et al. (2010) |
| Cytokines (bidirectional link with redox stress) |
|
Takada et al. (2003), Schrader et al. (2007), Nanduri et al. (2008), Valle et al. (2019), Connors and Levy (2020) |
|
Abbreviations: ACE2, Angiotensin-converting enzyme 2; AngII, Angiotensin II; ARE, antioxidant response element; COVID, COrona Virus Disease; Ca2+, Calcium (II) ion; ER, endoplasmic reticulum; HIV, human immunodeficiency virus; HO-1, Heme oxygenase 1; IFNs, Interferons; IL, interleukin; Keap1, Kelch-like ECH-associated protein 1; MAPK, mitogen-activated protein kinase; MEK, Mitogen-activated protein kinase; Mito-ROS, Mitochondrial reactive oxygen species; Mnk1, mitogen-activated protein kinase (MAPK) interacting protein kinase 1; NF-κB, Nuclear factor kappa B; NOX, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; Nrf2, nuclear factor erythroid 2–related factor 2; NQo-1, NAD(P)H quinone oxidoreductase; ROS, reactive oxygen species; RSV, Respiratory Syncytial Virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, Tumor necrosis factor; UPR, unfolded protein response.
NF-κB pathway
Nuclear factor-κB (NF-κB) is a redox-sensitive transcription factor that is regulated by ROS through the classical IkB kinase (IKK)-dependent canonical pathway (Liu et al., 2017) and coordinates innate and adaptive immunity, inflammation, and apoptosis (Piette et al., 1997). The redox regulation of the NF-κB pathway has been reviewed elsewhere and varies between different mammalian cells and in the setting of cancer (Gloire et al., 2006). Although it is established that cytokines and lipopolysaccharides induce proinflammatory activation of NF-κB (Schreck and Baeuerle, 1991), ROS may also reduce NF-κB activity (Nakajima and Kitamura, 2013). Oxidative stress in the early phase may induce activation of NF-κB in epithelial cells (Wesselborg et al., 1997; Tenjinbaru et al., 1999; Thevenod et al., 2000) and endothelial cells (Shono et al., 1996; Chua et al., 1998; Canty et al., 1999; Cooke and Davidge, 2002) which are targets of coronaviruses. Redox stress in epithelial cells in the late phase may also inhibit basal and inducible activation of NF-κB (Xie and Shaikh, 2006; Yang et al., 2007; Nakajima and Kitamura, 2013). The regulation of NF-κB by ROS is dependent not only on the phase of responses and the pattern of stimulation, but also depends on specific cell types (Nakajima and Kitamura, 2013). However, most of the evidence regarding redox regulation of the NF-κB pathway is not based on airway epithelial cells, the main target of SARS-CoV-2, and heterogeneous redox stimuli have been utilized in several experimental studies, often in supraphysiological concentrations. Thus, it is not well defined how ROS regulate activity of NF-κB in a bidirectional fashion in airway epithelial cells (Nakajima and Kitamura, 2013).
Overall, cumulative evidence suggests that there is context-dependent regulation of NF-κB by ROS (Nakajima and Kitamura, 2013). Preclinical studies have shown that ROS trigger NF-κB activation in airway epithelial cells (Jany et al., 1995; Ito et al., 2004). In contrast, inhibition of cytokine-triggered NF-κB activation under pre-exposure to ROS has been described in distal airway alveolar epithelial cells (Korn et al., 2001; Reynaert et al., 2006). The oxidative stress– unfolded protein response (UPR) pathway and redox ER responses play a key role in the bidirectional control of NF-κB (Nakajima and Kitamura, 2013). Thus, the opposite, bidirectional effects of redox stimuli on NF-κB seem to depend on the phase of response, the context, the type of cells and the specific redox stimuli. Overall, this bidirectional crosstalk is not well characterized specifically in coronavirus infections.
Viruses may hijack cellular signaling pathways and transcription factors and control them to their own advantage. In particular, the NF-κB pathway appears to be an attractive target for common human viral pathogens (Santoro et al., 2003). Distinct viral proteins encoded by viruses such as HCV, rotavirus, EBV, HBV, HTLV-1, and HIV-1 activate NF-κB by interacting with cellular signaling pathways including calcium-or redox-regulated signals or through ER stress mechanisms. Accumulation of viral dsRNA activates PKR, which in turn stimulates IKK. However, most of the evidence regarding virus-induced regulation of the NF-κB pathway is based on chronic viral infections or infections with DNA viruses (Santoro et al., 2003). There is limited evidence regarding the direct impact of coronaviruses on this pathway.
Evidence has suggested that proteins of SARS-CoV-2 can directly or indirectly impact NF-kB activation. In vitro studies showed that the spike protein of SARS-CoV induces a strong cytokine response through the NF-kB pathway (Dosch et al., 2009). It was also shown that SARS-CoV nucleocapsid protein activated NF-kB in Vero E6 cells in a dose dependent manner (Liao et al., 2005). ORF7a protein of SARS-CoV-2 mediates activation of NF-kB and induced proinflammatory expression of cytokines (Su et al., 2021). Similarly, Nsp5 in SARS-CoV-2 activated NF-kB pathway through upregulation of SUMOylation of mitochondrial antiviral-signaling proteins (Li et al., 2021b). Notably, studies show that the NF-κB signal pathway is a central pathway involved in induction of pro-inflammatory cytokines and chemokines in respiratory virus infection, including SARS-CoV-2-triggered COVID-19 (Kircheis et al., 2020; Hariharan et al., 2021; Kandasamy, 2021). Thus, the pharmacological inactivation of the NF-κB signaling pathway can represent a potential therapeutic target to treat severe COVID-19 (Kircheis et al., 2020; Hariharan et al., 2021; Kandasamy, 2021).
Mito-ROS pathways
As outlined above, coronavirus induce production of mito-ROS during infection. Mito-ROS have been shown to inhibit interferons and induce aberrant alterations of lipids, membranes, proteins and ultimately tissue damage. Mito-ROS induce inflammasome activation (Dashdorj et al., 2013; Han et al., 2018) and the “complement–metabolism–inflammasome axis”(Arbore and Kemper, 2016), Mito-ROS indirectly regulate inflammatory caspases 1 and 12, as well as the cytokines IL-1β and IL-18 in macrophages through the NLRP3 inflammasome (Martinon et al., 2009). Mito-ROS induce NFκB (Imai et al., 2008) which drives a cytokine storm, triggering lung damage during viral infection. Mito-ROS also induce activate MAPK pathways and promote production of IL-6 and TNF-α (Wang and Zhang, 1999; Mizutani et al., 2004; Kefaloyianni et al., 2006; Jamaluddin et al., 2009; Bulua et al., 2011; Zhang et al., 2016). Mito-ROS directly induce release of IL-1β (Dashdorj et al., 2013; Han et al., 2018), IL-6 (Lowes et al., 2008, 2013; Bulua et al., 2011; Li et al., 2019). Consistent with this evidence it has been shown that Mito-ROS induce inflammatory response and lung injury in mouse models of viral infections (Hu et al., 2019a; Hu et al., 2019b). Thus, mito-ROS may regulate redox cytoplasmic proinflammatory responses in respiratory viral infections.
Nf2 pathways
Heme oxygenase 1 (HO-1), a downstream protein of the Nrf2 pathway, contributes to anti-inflammatory and antiviral responses, removes toxic heme, protects against oxidative injury and also regulates apoptosis, inflammation and angiogenesis (Espinoza et al., 2017). While the exact mechanism by which SARS-CoV-2 affects HO-1 and, conversely, how HO-1 exerts its antiviral effects against SARS-CoV-2 is still being studied, there is an established association between HO-1 and a reduction of tissue damage through its anti-inflammatory and antioxidative functions throughout the body (Toro et al., 2022). This makes HO-1 an important target for developing novel COVID-19 therapeutics.
Angiotensin II and NOX
During SAS-CoV-2 infection, the reduction of ACE2 on the cell surface leads to increase of Ang II and NOX (Nguyen Dinh Cat et al., 2013). Bidirectional crosstalk between mitochondria and NOX, markedly affects redox responses to angiotensin II, the ligand of ACE2 that is upregulated in viral infections (Doughan et al., 2008; Wosniak et al., 2009; Lee et al., 2013; Daiber et al., 2017). Indeed, therapeutic targeting of NOX, triggered by mito-ROS (Desouki et al., 2005), increased the survival of mice with post-influenza pneumonia (Sun et al., 2016). Thus, as a result of increased NOX, NF-κβ activation there is activation of the pro-inflammatory response and release of cytokines like IL-6, IL-8, and TNFα (Mahmud-Al-Rafat et al., 2020). Pro-inflammatory cytokines like IL-1, IL-6, and TNFα activate macrophages, neutrophils, and endothelial cells through NADPH oxidase, resulting in a greater production of superoxide and H2O2 (Takada et al., 2003; Nanduri et al., 2008; Connors and Levy, 2020).
The complement system
As outlined above, coronavirus induce production of mito-ROS which trigger the “complement–metabolism–inflammasome axis”(Arbore and Kemper, 2016). MERS-CoV can also directly induce the complement system (Chen et al., 2010). The complement activation has also potent proinflammatory effect and can increase local and systemic damage in severe COVID-19 (Santiesteban-Lores et al., 2021). Preclinical in vitro studies have shown controversial data regarding the role of the complement system in binding coronaviruses (Santiesteban-Lores et al., 2021). Experimental studies with animals have shown that complement activation induces a systemic pro-inflammatory response during experimental infection with SARS-CoV and MERS that drives disease progression (Gralinski et al., 2018; Jiang et al., 2018). Small human cohorts also show that complement activation is associated with disease progression of SARS (Wang et al., 2005). Collectively, limited and often controversial evidence suggests that complement activation through redox pathways may have an important role in cell and tissue damage in severe coronavirus infections.
Other proinflammatory mechanisms in coronavirus infections
Other than activation of proinflammatory NF-kB, mito-ROS and NOX pathways and downregulation of anti-inflammatory ACE2 and Nrf2 pathways, different coronaviruses may also directly induce other proinflammatory effects. MERS-CoV can induce the complement system and increase inflammatory response, pyroptosis and eventually lung tissue damage (Chen et al., 2010). MERS-CoV infected macrophages increase pro-inflammatory cytokines and chemokines (Pruijssers and Denison, 2019). SARS-CoV and MERS-CoV may also attenuate levels of endogenous Type I IFNs that are immunomodulatory (Dikalova et al., 2010). Finally, mouse hepatitis virus (MHV) directly upregulated interleukin signaling such as IL-27 during acute encephalomyelitis.
Redox pathways that regulate apoptosis during infection with coronaviruses
As described above, coronaviruses induce apoptosis through multiple pathways, either directly (Pfefferle et al., 2011), or indirectly by inducing production of mito-ROS and downregulating antiapoptotic pathways such as Nrf2. Excessive ROS generation can lead to loss of mitochondrial function and apoptosis of lung epithelial cells (Sun et al., 2013). Increased mito-ROS also directly contribute to acute injury in lung tissue in mouse models of viral infections (Hu et al., 2019a,b). Indeed, increased apoptosis of epithelial cells is associated with lung injury in COVID-19 (Hussman, 2020). Studies have also shown that CD4 and CD8 T cells in patients with COVID-19 are more likely to get affected by apoptosis (Nieto-Torres et al., 2015). Thus, increased apoptosis during coronavirus infection contributes to increased tissue damage and pathogenesis of coronavirus infections.
Redox pathways that regulate mitophagy during infection with coronaviruses
As outlined above, coronaviruses induce production of mito-ROS that have an established complex crosstalk with mitophagy (Schofield and Schafer, 2021). Mitochondrial ROS and damage-associated molecular patterns (DAMPs) activate inflammasomes to induce inflammatory responses and tissue injury. Emerging evidence suggests that mitophagy protects against the hyperinflammation induced by ROS and DAMPs and regulates inflammatory responses in several diseases (Zhao et al., 2015). Thus, by inducing production of mito-ROS, mitochondrial dysfunction and mitophagy impairment, SARS-CoV-2 may contribute to inflammation and tissue damage (Shang et al., 2021).
Redox pathways that regulate other instigators of tissue damage during infection with coronaviruses
Other than regulation of viral replication, inflammation and apoptosis, redox pathways may also contribute to regulation of other pathways that contribute to tissue damage such as autoimmunity and vascular dysfunction. Oxidative stress plays a central in autoimmune diseases (Ramani et al., 2020). Specifically, the antioxidant pathway Nrf2 has also a key role in regulation of autoimmunity (Freeborn and Rockwell, 2021). Given the possible role of autoimmunity in pathogenesis of COVID-19 and Long COVID, further understanding of the contribution of dysregulation redox pathways in development of autoimmunity during coronavirus infections is needed (Liu et al., 2021; Saad et al., 2021).
ROS induce levels of the adhesion molecules and increase permeability in endothelial cells (Mukherjee et al., 2005; Zinovkin et al., 2014). ROS also contribute to TNF-induced IL-6 expression and NF-κB activation (Pearlstein et al., 2002). Notably, IL-6 directly induces mito-ROS production and NOX in endothelial cells (Schrader et al., 2007; Valle et al., 2019) and impact NO bioavailability and endothelial function (Saura et al., 2006). SARS-CoV-2 S-protein binds to ACE2 and subsequently triggers reduction in ACE2 levels that cleaves ATII. High ATII level further leads to oxidative stress and endothelial dysfunction (Chernyak et al., 2020) and induces ROS production via NOX in endothelial cells. Thus, increased redox stress induced by SARS-CoV-2 may impact not only vascular permeability and vasodilation but also vascular inflammation.
Oxidative stress and end organ damage during infection with coronaviruses
All coronaviruses have the potential to induce tissue damage and end organ disease through viral replication, increased inflammation and apoptosis, induction of ROS and reduction of cytoprotective pathways such as the Nrf2 and HO-1 pathways. Increased redox stress is known instigator of lung dysfunction (Kellner et al., 2017), cardiovascular disease (Dubois-Deruy et al., 2020), central nervous system dysfunction such as neurodegeneration and neuropsychiatric disease (Reiter, 1998; Patel, 2016; Salim, 2017) and the metabolic syndrome (Ando and Fujita, 2009; Roberts and Sindhu, 2009; Carrier, 2017) which are all manifestations of both acute severe COVID-19 and post-acute sequelae of SARS-CoV-2 infection (often called Long COVID syndrome; Figure 3; Nalbandian et al., 2021). Coronaviruses differ in their potential to induce end organ damage (Table 3; Bonavia et al., 1997; De Albuquerque et al., 2006; de Wilde et al., 2013; Josset et al., 2013; Zhao et al., 2015; Li et al., 2016; Agostini et al., 2018; Coperchini et al., 2020; Huang et al., 2020; Petersen et al., 2020; Wang et al., 2020; Yi et al., 2020; Caldera-Crespo et al., 2021; Paidas et al., 2021; Tian et al., 2021; Jansen et al., 2022). Among the various human coronaviruses, end organ damage is observed in MERS, SARS-CoV-1, and SARS-CoV-2. These coronaviruses demonstrate a more severe pathology than HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1 in terms of their fatality and systemic effects on multiple organ systems. Multiple animal models have been used to uncover the ways coronaviruses lead to the end organ damage that presents in patient autopsies. The MHV mice model is the most studied model among the coronaviruses, and it has served as a useful proxy in understanding SARS-CoV-2 (Paidas et al., 2022); MHV is known to enteric and respiratory disease, hepatitis, encephalitis, and chronic demyelination and is useful in studying infection of the liver and brain (Weiss and Navas-Martin, 2005). Despite MHV-1 utilizing a different receptor than either MERS or the SARS coronaviruses (carcinoembryonic antigen-related cell adhesion molecule 1 instead of dipeptidylpeptidase 4 and angiotensin-converting enzyme 2), end organ damage in the MHV-1 model has been acclaimed as an appropriate model for MERS, SARS-CoV-1, and SARS-CoV-2 (De Albuquerque et al., 2006; Agostini et al., 2018; Caldera-Crespo et al., 2021; Paidas et al., 2021; Tian et al., 2021). Herein, we briefly summarize redox pathways that regulate damage of the lung and the brain, the two main target organs for end organ disease in acute COVID-19 and Long COVID.
Figure 3.
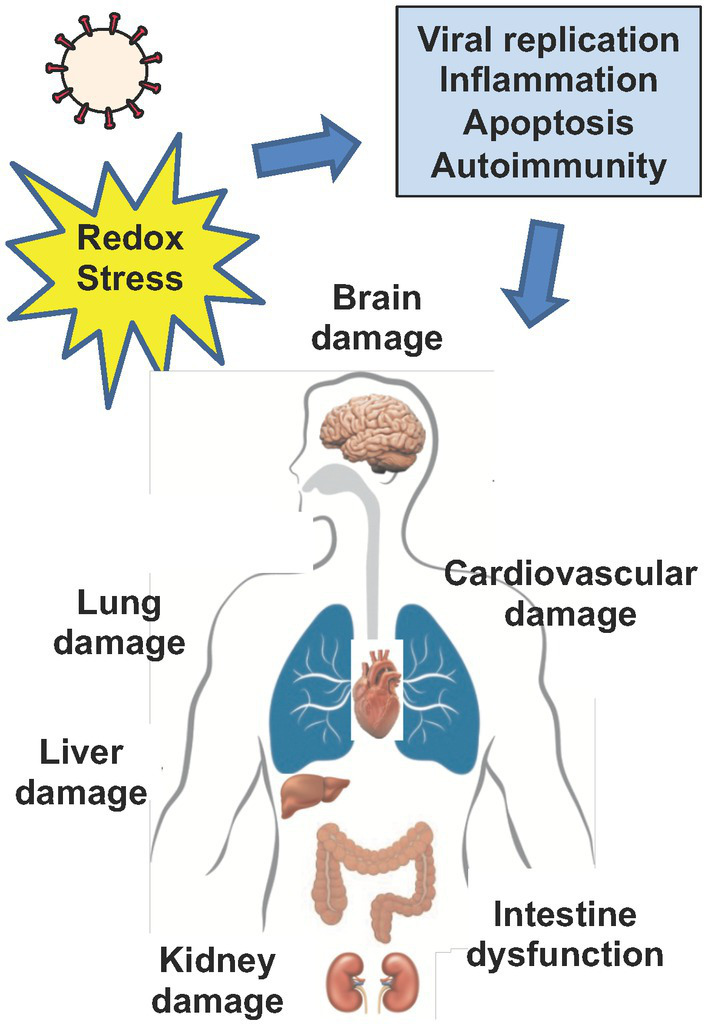
Viral infection and end organ damage. Increased redox stress drives viral replication, inflammation, apoptosis, vascular dysfunction and autoimmunity, in both acute infection with coronaviruses and in the setting of Post-Acute Sequelae of SARS-CoV-2 (PASC or Long COVID). Collectively, redox mediated pathways that drive viral replication, inflammation, apoptosis, autoimmunity, and vascular dysfunction contribute to cell and tissue damage that drives end organ disease in coronavirus infection. Cells enriched in mitochondria such as neurons, endothelial and epithelial cells may be particularly susceptible to increased redox stress driven by coronaviruses. Ultimately, increased redox stress during acute infection with coronaviruses and in the setting of PASC can directly or indirectly drive end organ disease such as brain, lung, liver, kidney and cardiovascular damage and induce intestinal dysfunction.
Table 3.
Comparison of coronaviruses with regards to impact on end organ disease.
| Differences SARS-CoV-2 with other coronaviruses | Similarities between SARS-CoV-2 with other coronaviruses |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviations: ACE2, Angiotensin-converting enzyme 2; HCoV-229E, Human coronavirus 229E; HCoV-OC43; Human coronavirus OC43; HCoV-NL63; HKU-1, HCoV-HKU1 = human coronavirus HKU1; MERS-CoV, Middle East respiratory syndrome coronavirusl MHV, mouse hepatitis virus; SARS-CoV, Severe acute respiratory syndrome coronavirus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TH1, Type 1 T helper
Lung damage
The excessive generation of oxygen radicals under pathological conditions such as acute lung injury (ALI) and its most severe form acute respiratory distress syndrome (ARDS) leads to increased endothelial permeability. Increased redox stress leads to increased permeability of lung blood vessels, increased infiltration of immune cells and increased accumulation of fluids in the alveolar system (Kellner et al., 2017). Mitochondria, NADPH oxidase (NOX), xanthine oxidase (Shasby et al., 1985; Barnard and Matalon, 1992), and eNOS are the major contributors of ROS in cells of vasculature during active metabolism that also contribute to the pathogenesis of ALI (Gross et al., 2015). Imbalance of antioxidant enzymes such as superoxide dismutase (SOD; Ndengele et al., 2005; Cai et al., 2014), catalase (Flick et al., 1988; Kozower et al., 2003) and glutathione peroxidase (GPx; Aggarwal et al., 2012; Kim et al., 2012a) and Nrf2 (Zhu et al., 2013; Peng et al., 2016) also contribute to pathogenesis of ALI and ARDS. Similarly, to MERS and SARS, severe SARS-CoV-2 infection presents with high levels of pro-inflammatory cytokines like IL-6, and can lead to ARDS, which is associated with acute renal injury, acute respiratory injury, and septic shock (Chen et al., 2020). COVID-19-related ARDS has a high prevalence and is different to ARDS due to other etiologies (Park et al., 2009).
SARS-CoV-2 directly impacts several of established instigators that contribute to pathogenesis of ALI/ARDS including mitochondrial function (Srinivasan et al., 2021), NOX (Damiano et al., 2020; Violi et al., 2020; de Oliveira and Nunes, 2021), xanthine oxidase (Pratomo et al., 2021; Al-Kuraishy et al., 2022), eNOS (Guimaraes et al., 2021), glutathione peroxidase (Labarrere and Kassab, 2022) and Nrf2 (Olagnier et al., 2020; Zhang et al., 2022). To date, there is no treatment for ARDS in COVID-19 disease (Jafari-Oori et al., 2021).
Brain damage
The brain is highly susceptible to oxidative stress due to enrichment for lipids, mitochondria, calcium, glutamate and increased redox stimuli (Cobley et al., 2018). Brain damage induced by oxidative stress may negatively impact normal functions of central nervous system and may contribute to the pathogenesis of neurodegenerative disorders such as Alzheimer and Parkinson disease and in the pathogenesis of neuropsychiatric disorders, including anxiety and depression (Salim, 2017). For these, increased oxidative stress through mitochondrial dysfunction, increased inflammation and energy imbalance has also been hypothesized to contribute to pathogenesis of neurocognitive dysfunction in Long COVID (Paul et al., 2021; Jarrott et al., 2022).
Antioxidant therapies in coronavirus infections
Multiple trials underway have tested antioxidants as therapeutic agents in COVID-19.1 Several therapies targeting redox imbalance already have been used for the treatment of COVID-19 including inhaled NO (Lotz et al., 2021), ubiquinol (Fukuda et al., 2016), combination of NADH and CoQ10 (Castro-Marrero et al., 2015), N-acetyl cysteine, mitochondria-targeted antioxidant MitoQ (Codo et al., 2020; Petcherski et al., 2022) and Nrf2 agonists (Zinovkin and Grebenchikov, 2020). Other potential antioxidant treatments that have been considered include, ubiquinol, nicotinamide, glutathione (and glutathione donors), cysteamine, sulforaphane, melatonin vitamin C, vitamin D, vitamin E, melatonin plus pentoxifylline and selenium. However, most of the proposed antioxidant treatments have either not been directly tested in humans in the setting of randomized control clinical trials or due to several methodological issues of heterogeneous studies, the data were inconclusive (Table 4). Many ongoing clinical trials regarding the use of antioxidants in treatment of COVID-19 have not been published. Notably, oral antioxidants have not produced dramatic improvements in conditions associated with redox imbalance (Barcelos et al., 2020). No single antioxidant can scavenge all the various ROS and reactive nitrogen species (RNS). Further validation with animal models and clinical trials are necessary to reveal therapeutic potential of combination therapies of antivirals, antioxidant and anti-inflammatory treatments.
Table 4.
Antioxidant treatments that have been tested in humans for treatment of coronavirus infections.
| Mediators | Effect | References |
|---|---|---|
| Inhaled NO | ↑ oxygenation in severe COVID-19, no effect on mortality | Lotz et al. (2021), Prakash et al. (2021) |
| Ubiquinol (CoQ10) | Does not ↓ the number or severity of PASC-related symptoms when compared to placebo | Hargreaves and Mantle (2021), Hansen et al. (2022) |
| N-acetyl cysteine | Oral high dose of N-acetyl cysteine may ↓ morbidity in severe COVID-19 in observational studies; many ongoing clinical trials with unpublished data | Wong et al. (2021), Izquierdo et al. (2022) |
| Glutathione | ↓ reduces dyspnea in COVID-19 in a case series | Horowitz et al. (2020) |
| Melatonin | May improve clinical outcomes in patients with COVID-19 based on RCTs | Lan et al. (2022) |
| Vitamin C | Controversial data may have some benefit in morbidity in COVID-19 based on clinical trials | Olczak-Pruc et al. (2022) |
| Vitamins | Controversial data overall weak/negative; supplementation with vitamins A, B, C, D, and E could improve the inflammatory response and decrease the severity of disease in ICU-admitted patients with COVID-19 | Beigmohammadi et al. (2021) |
| Zinc | Overall limited data/no major effect on morbidity in COVID-19, many ongoing clinical trials with unpublished data | Perera et al. (2020), Balboni et al. (2022) |
| Selenium | Overall limited data/no major effect on morbidity in COVID-19, many ongoing clinical trials with unpublished data | Alshammari et al. (2022), Balboni et al. (2022) |
| Pentoxifylline | May reduce lung inflammation, ongoing clinical trials with unpublished data | Feret et al. (2021) |
Abbreviations: RCT, Randomized control clinical trial; PASC, Post Acute Sequalae of SARS-CoV-2 infection.
Conclusion
There is limited understanding how different coronaviruses including SARS-CoV-2, manipulate cellular redox machinery to drive viral replication and associated host cell responses including inflammation, apoptosis and associated end organ disease. The crosstalk between NOX and ACE2 as well mito-ROS may impact viral entry of coronaviruses while mito-ROS may also induce multiple proviral cytoplasmic pathways. Experimental studies have also shown that coronaviruses induce downregulation of antioxidant genes such as Nrf2 in combination with an upregulation of oxidative stress genes like myeloperoxidase that may contribute to both increased viral replication and inflammation. Coronaviruses may induce several redox sensitive proinflammatory pathways such NF-kB, mito-ROS and NOX pathways and downregulate anti-inflammatory ACE2 and Nrf2 pathways. Coronaviruses may further trigger cell damage through activation of redox sensitive pyroptosis and apoptosis. Finally, other than regulation of viral replication, inflammation and apoptosis, redox pathways may also contribute to regulation of other pathways that contribute to tissue damage such as autoimmunity and vascular dysfunction. Thus, coronaviruses have the potential to induce tissue damage and end organ disease through viral replication, increased inflammation and apoptosis, induction of ROS and reduction of cytoprotective pathways such as the Nrf2 and HO-1 pathways. Coronaviruses differ in their potential to induce end organ damage. Among the various human coronaviruses, end organ damage is observed in MERS, SARS-CoV-1, and SARS-CoV-2. Increased redox stress is known instigator of lung dysfunction (Kellner et al., 2017), cardiovascular disease (Dubois-Deruy et al., 2020), central nervous system dysfunction such as neurodegeneration and neuropsychiatric disease (Reiter, 1998; Patel, 2016; Salim, 2017) and the metabolic syndrome (Ando and Fujita, 2009; Roberts and Sindhu, 2009; Carrier, 2017) which are all manifestations of both acute severe COVID-19 and Long COVID syndrome (Nalbandian et al., 2021). Given the complexity of the pathogenesis of coronavirus infections and that oral antioxidants have not produced dramatic improvements in conditions associated with redox imbalance, further validation with animal models and clinical trials are necessary to reveal therapeutic potential of combination therapies of antivirals, antioxidant and anti-inflammatory treatments. Understanding the mechanisms that contribute to the pathogenesis of coronavirus infections, will set the foundation for development of new treatments for coronavirus infections.
Author contributions
CG, SiS, TA, and TK wrote the manuscript, reviewed the literature, and collected the information. SaS revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by National Institute of Health grant R01AG059501 (TK), National Institute of Health grant R01AG059502 04S1 (TK), and California HIV/AIDS Research Program grant OS17-LA-002 (TK).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
- Aggarwal S., Dimitropoulou C., Lu Q., Black S. M., Sharma S. (2012). Glutathione supplementation attenuates lipopolysaccharide-induced mitochondrial dysfunction and apoptosis in a mouse model of acute lung injury. Front. Physiol. 3:161. doi: 10.3389/fphys.2012.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M. L., Andres E. L., Sims A. C., Graham R. L., Sheahan T. P., Lu X., et al. (2018). Coronavirus susceptibility to the antiviral Remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 9:2. doi: 10.1128/mBio.00221-18, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal P., Nawadkar R., Ojha H., Kumar J., Sahu A. (2017). Complement evasion strategies of viruses: an overview. Front. Microbiol. 8:1117. doi: 10.3389/fmicb.2017.01117, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M. S., Czajkowsky D. M. (2022). SARS-CoV-2 infection and oxidative stress: pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor Rev. 63, 44–57. doi: 10.1016/j.cytogfr.2021.11.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy H. M., Al-Gareeb A. I., Al-Niemi M. S., Aljowaie R. M., Almutairi S. M., Alexiou A., et al. (2022). The prospective effect of allopurinol on the oxidative stress index and endothelial dysfunction in Covid-19. Inflammation 45, 1651–1667. doi: 10.1007/s10753-022-01648-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari M. K., Fatima W., Alraya R. A., Khuzaim Alzahrani A., Kamal M., Alshammari R. S., et al. (2022). Selenium and COVID-19: A spotlight on the clinical trials, inventive compositions, and patent literature. J. Infect. Public Health 15, 1225–1233. doi: 10.1016/j.jiph.2022.09.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini M. A., Karimi J., Talebi S. S., Piri H. (2022). The association of COVID-19 and reactive oxygen species modulator 1 (ROMO1) with oxidative stress. Chonnam Med. J. 58, 1–5. doi: 10.4068/cmj.2022.58.1.1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K., Fujita T. (2009). Metabolic syndrome and oxidative stress. Free Radic. Biol. Med. 47, 213–218. doi: 10.1016/j.freeradbiomed.2009.04.030 [DOI] [PubMed] [Google Scholar]
- Arbore G., Kemper C. (2016). A novel "complement-metabolism-inflammasome axis" as a key regulator of immune cell effector function. Eur. J. Immunol. 46, 1563–1573. doi: 10.1002/eji.201546131, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni E., Zagnoli F., Filippini T., Fairweather-Tait S. J., Vinceti M. (2022). Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies. J. Trace Elem. Med. Biol. 71:126956. doi: 10.1016/j.jtemb.2022.126956, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelos I., Shadiack E., Ganetzky R. D., Falk M. J. (2020). Mitochondrial medicine therapies: rationale, evidence, and dosing guidelines. Curr. Opin. Pediatr. 32, 707–718. doi: 10.1097/MOP.0000000000000954, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard M. L., Matalon S. (1992). Mechanisms of extracellular reactive oxygen species injury to the pulmonary microvasculature. J. Appl. Physiol. 72, 1724–1729. doi: 10.1152/jappl.1992.72.5.1724 [DOI] [PubMed] [Google Scholar]
- Beigmohammadi M. T., Bitarafan S., Hoseindokht A., Abdollahi A., Amoozadeh L., Soltani D. (2021). The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial. Trials 22:802. doi: 10.1186/s13063-021-05795-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia A., Arbour N., Yong V. W., Talbot P. J. (1997). Infection of primary cultures of human neural cells by human coronaviruses 229E and OC43. J. Virol. 71, 800–806. doi: 10.1128/jvi.71.1.800-806.1997, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulua A. C., Simon A., Maddipati R., Pelletier M., Park H., Kim K. Y., et al. (2011). Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208, 519–533. doi: 10.1084/jem.20102049, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Yi F., Dai Z., Huang X., Zhao Y. D., Mirza M. K., et al. (2014). Loss of caveolin-1 and adiponectin induces severe inflammatory lung injury following LPS challenge through excessive oxidative/nitrative stress. Am. J. Phys. Lung Cell. Mol. Phys. 306, L566–L573. doi: 10.1152/ajplung.00182.2013, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldera-Crespo L. A., Paidas M. J., Roy S., Schulman C. I., Kenyon N. S., Daunert S., et al. (2021). Experimental models of COVID-19. Front. Cell. Infect. Microbiol. 11:792584. doi: 10.3389/fcimb.2021.792584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty T. G., Jr., Boyle E. M., Jr., Farr A., Morgan E. N., Verrier E. D., Pohlman T. H. (1999). Oxidative stress induces NF-kappaB nuclear translocation without degradation of IkappaBalpha. Circulation 100, II361–II364. [DOI] [PubMed] [Google Scholar]
- Carrier A. (2017). Metabolic syndrome and oxidative stress: A complex relationship. Antioxid. Redox Signal. 26, 429–431. doi: 10.1089/ars.2016.6929 [DOI] [PubMed] [Google Scholar]
- Castro-Marrero J., Cordero M. D., Segundo M. J., Saez-Francas N., Calvo N., Roman-Malo L., et al. (2015). Does oral coenzyme Q10 plus NADH supplementation improve fatigue and biochemical parameters in chronic fatigue syndrome? Antioxid. Redox Signal. 22, 679–685. doi: 10.1089/ars.2014.6181, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Krmar R. T., Dada L., Efendiev R., Leibiger I. B., Pedemonte C. H., et al. (2006). Phosphorylation of adaptor protein-2 mu2 is essential for Na+, K+-ATPase endocytosis in response to either G protein-coupled receptor or reactive oxygen species. Am. J. Respir. Cell Mol. Biol. 35, 127–132. doi: 10.1165/rcmb.2006-0044OC, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lau Y. F., Lamirande E. W., Paddock C. D., Bartlett J. H., Zaki S. R., et al. (2010). Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 84, 1289–1301. doi: 10.1128/JVI.01281-09, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. Y., Moriyama M., Chang M. F., Ichinohe T. (2019). Severe acute respiratory syndrome coronavirus Viroporin 3a activates the NLRP3 Inflammasome. Front. Microbiol. 10:50. doi: 10.3389/fmicb.2019.00050, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513. doi: 10.1016/S0140-6736(20)30211-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak B. V., Popova E. N., Prikhodko A. S., Grebenchikov O. A., Zinovkina L. A., Zinovkin R. A. (2020). COVID-19 and oxidative stress. Biochemistry (Mosc) 85, 1543–1553. doi: 10.1134/S0006297920120068, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. Y., Imani F., Miller-Degraff L., Walters D., Melendi G. A., Yamamoto M., et al. (2009). Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 179, 138–150. doi: 10.1164/rccm.200804-535OC, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua C. C., Hamdy R. C., Chua B. H. (1998). Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic. Biol. Med. 25, 891–897. doi: 10.1016/S0891-5849(98)00115-4, PMID: [DOI] [PubMed] [Google Scholar]
- Cobley J. N., Fiorello M. L., Bailey D. M. (2018). 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 15, 490–503. doi: 10.1016/j.redox.2018.01.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codo A. C., Davanzo G. G., Monteiro L. B., De Souza G. F., Muraro S. P., Virgilio-Da-Silva J. V., et al. (2020). Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab. 32:e435, 437–446.e5. doi: 10.1016/j.cmet.2020.07.007 [DOI] [Google Scholar]
- Connors J. M., Levy J. H. (2020). COVID-19 and its implications for thrombosis and anticoagulation. Blood 135, 2033–2040. doi: 10.1182/blood.2020006000, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke C. L., Davidge S. T. (2002). Peroxynitrite increases iNOS through NF-kappaB and decreases prostacyclin synthase in endothelial cells. Am. J. Phys. Cell Physiol. 282, C395–C402. doi: 10.1152/ajpcell.00295.2001, PMID: [DOI] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. (2020). The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 53, 25–32. doi: 10.1016/j.cytogfr.2020.05.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A., Di Lisa F., Oelze M., Kroller-Schon S., Steven S., Schulz E., et al. (2017). Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 174, 1670–1689. doi: 10.1111/bph.13403, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano S., Sozio C., La Rosa G., Santillo M. (2020). NOX-dependent signaling dysregulation in severe COVID-19: clues to effective treatments. Front. Cell. Infect. Microbiol. 10:608435. doi: 10.3389/fcimb.2020.608435, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashdorj A., Jyothi K. R., Lim S., Jo A., Nguyen M. N., Ha J., et al. (2013). Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 11:178. doi: 10.1186/1741-7015-11-178, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Albuquerque N., Baig E., Ma X., Zhang J., He W., Rowe A., et al. (2006). Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J. Virol. 80, 10382–10394. doi: 10.1128/JVI.00747-06, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira A. A., Nunes K. P. (2021). Crosstalk of TLR4, vascular NADPH oxidase, and COVID-19 in diabetes: what are the potential implications? Vasc. Pharmacol. 139:106879. doi: 10.1016/j.vph.2021.106879, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde A. H., Raj V. S., Oudshoorn D., Bestebroer T. M., Van Nieuwkoop S., Limpens R., et al. (2013). MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-alpha treatment. J. Gen. Virol. 94, 1749–1760. doi: 10.1099/vir.0.052910-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde A. H., Zevenhoven-Dobbe J. C., Van Der Meer Y., Thiel V., Narayanan K., Makino S., et al. (2011). Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 92, 2542–2548. doi: 10.1099/vir.0.034983-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers-Lamarche J., Guillebaud G., Tlili M., Todkar K., Belanger N., Grondin M., et al. (2016). Loss of mitochondrial function impairs lysosomes. J. Biol. Chem. 291, 10263–10276. doi: 10.1074/jbc.M115.695825, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouki M. M., Kulawiec M., Bansal S., Das G. M., Singh K. K. (2005). Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol. Ther. 4, 1367–1373. doi: 10.4161/cbt.4.12.2233, PMID: [DOI] [PubMed] [Google Scholar]
- Dikalov S. I., Nazarewicz R. R., Bikineyeva A., Hilenski L., Lassegue B., Griendling K. K., et al. (2014). Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 20, 281–294. doi: 10.1089/ars.2012.4918, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova A. E., Bikineyeva A. T., Budzyn K., Nazarewicz R. R., Mccann L., Lewis W., et al. (2010). Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 107, 106–116. doi: 10.1161/CIRCRESAHA.109.214601, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch S. F., Mahajan S. D., Collins A. R. (2009). SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 142, 19–27. doi: 10.1016/j.virusres.2009.01.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughan A. K., Harrison D. G., Dikalov S. I. (2008). Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 102, 488–496. doi: 10.1161/CIRCRESAHA.107.162800, PMID: [DOI] [PubMed] [Google Scholar]
- Dubois-Deruy E., Peugnet V., Turkieh A., Pinet F. (2020). Oxidative stress in cardiovascular diseases. Antioxidants 9, 864–879. doi: 10.3390/antiox9090864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elesela S., Lukacs N. W. (2021). Role of mitochondria in viral infections. Life 11, 232–247. doi: 10.3390/life11030232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling B. M., Platanias L. C., Black E., Nebreda A. R., Davis R. J., Chandel N. S. (2005). Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol. Cell. Biol. 25, 4853–4862. doi: 10.1128/MCB.25.12.4853-4862.2005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza J. A., Gonzalez P. A., Kalergis A. M. (2017). Modulation of antiviral immunity by Heme Oxygenase-1. Am. J. Pathol. 187, 487–493. doi: 10.1016/j.ajpath.2016.11.011, PMID: [DOI] [PubMed] [Google Scholar]
- Feret W., Nalewajska M., Wojczynski L., Witkiewicz W., Klos P., Dziedziejko V., et al. (2021). Pentoxifylline as a potential adjuvant therapy for COVID-19: impeding the burden of the cytokine storm. J. Clin. Med. 10, 5305–5313. doi: 10.3390/jcm10225305, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K., Duval A., Martel A., Soucy-Faulkner A., Grandvaux N. (2008). Dual role of NOX2 in respiratory syncytial virus-and Sendai virus-induced activation of NF-kappaB in airway epithelial cells. J. Immunol. 180, 6911–6922. doi: 10.4049/jimmunol.180.10.6911, PMID: [DOI] [PubMed] [Google Scholar]
- Finkel T. (2011). Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15. doi: 10.1083/jcb.201102095, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick M. R., Milligan S. A., Hoeffel J. M., Goldstein I. M. (1988). Catalase prevents increased lung vascular permeability during air emboli in unanesthetized sheep. J. Appl. Physiol. 64, 929–935. doi: 10.1152/jappl.1988.64.3.929 [DOI] [PubMed] [Google Scholar]
- Freeborn R. A., Rockwell C. E. (2021). The role of Nrf2 in autoimmunity and infectious disease: therapeutic possibilities. Adv. Pharmacol. 91, 61–110. doi: 10.1016/bs.apha.2020.10.003, PMID: [DOI] [PubMed] [Google Scholar]
- Fukuda S., Nojima J., Kajimoto O., Yamaguti K., Nakatomi Y., Kuratsune H., et al. (2016). Ubiquinol-10 supplementation improves autonomic nervous function and cognitive function in chronic fatigue syndrome. Biofactors 42, 431–440. doi: 10.1002/biof.1293, PMID: [DOI] [PubMed] [Google Scholar]
- Garrido A. M., Griendling K. K. (2009). NADPH oxidases and angiotensin II receptor signaling. Mol. Cell. Endocrinol. 302, 148–158. doi: 10.1016/j.mce.2008.11.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen N. C., Niemeyer D., Muth D., Corman V. M., Martinelli S., Gassen A., et al. (2019). SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat. Commun. 10:5770. doi: 10.1038/s41467-019-13659-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloire G., Legrand-Poels S., Piette J. (2006). NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 72, 1493–1505. doi: 10.1016/j.bcp.2006.04.011, PMID: [DOI] [PubMed] [Google Scholar]
- Gralinski L. E., Sheahan T. P., Morrison T. E., Menachery V. D., Jensen K., Leist S. R., et al. (2018). Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio 9, e01753–e01718. doi: 10.1128/mBio.01753-18, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Llambi F. (2015). Cell death signaling. Cold Spring Harb. Perspect. Biol. 7:7. doi: 10.1101/cshperspect.a006080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C. M., Rafikov R., Kumar S., Aggarwal S., Ham P. B., 3rd, Meadows M. L., et al. (2015). Endothelial nitric oxide synthase deficient mice are protected from lipopolysaccharide induced acute lung injury. PLoS One 10:e0119918. doi: 10.1371/journal.pone.0119918, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes L. M. F., Rossini C. V. T., Lameu C. (2021). Implications of SARS-Cov-2 infection on eNOS and iNOS activity: consequences for the respiratory and vascular systems. Nitric Oxide 111-112, 64–71. doi: 10.1016/j.niox.2021.04.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Clarke S. J., Javadov S. A. (2004). Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc. Res. 61, 372–385. doi: 10.1016/S0008-6363(03)00533-9, PMID: [DOI] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M. L., Lely A. T., Navis G., Van Goor H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637. doi: 10.1002/path.1570, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Lee D., Lee S. H., Kim T. H. (2021). Oxidative stress and antioxidant pathway in allergic rhinitis. Antioxidants 10, 1266–1281. doi: 10.3390/antiox10081266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Xu X., Tang C., Gao P., Chen X., Xiong X., et al. (2018). Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 16, 32–46. doi: 10.1016/j.redox.2018.02.013, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K. S., Mogensen T. H., Agergaard J., Schiottz-Christensen B., Ostergaard L., Vibholm L. K., et al. (2022). High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: A randomized, phase 2, crossover trial. Lancet Reg. Health Eur.,:100539, doi: 10.1016/j.lanepe.2022.100539 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Hargreaves I. R., Mantle D. (2021). COVID-19, coenzyme Q10 and selenium. Adv. Exp. Med. Biol. 1327, 161–168. doi: 10.1007/978-3-030-71697-4_13, PMID: [DOI] [PubMed] [Google Scholar]
- Hariharan A., Hakeem A. R., Radhakrishnan S., Reddy M. S., Rela M. (2021). The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology 29, 91–100. doi: 10.1007/s10787-020-00773-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herengt A., Thyrsted J., Holm C. K. (2021). NRF2 in viral infection. Antioxidants 10, 1491–1688. doi: 10.3390/antiox10091491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz R. I., Freeman P. R., Bruzzese J. (2020). Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases. Respir. Med. Case. Rep. 30:101063. doi: 10.1016/j.rmcr.2020.101063, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosakote Y. M., Liu T., Castro S. M., Garofalo R. P., Casola A. (2009). Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 41, 348–357. doi: 10.1165/rcmb.2008-0330OC, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Bogoyevitch M. A., Jans D. A. (2019a). Subversion of host cell mitochondria by RSV to favor virus production is dependent on inhibition of mitochondrial complex I and ROS generation. Cells 8, 1417–1433. doi: 10.3390/cells8111417, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Schulze K. E., Ghildyal R., Henstridge D. C., Kolanowski J. L., New E. J., et al. (2019b). Respiratory syncytial virus co-opts host mitochondrial function to favour infectious virus production. elife 8, 1–30. doi: 10.7554/eLife.42448, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. doi: 10.1016/S0140-6736(20)30183-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussman J. P. (2020). Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention. Front. Pharmacol. 11:1169. doi: 10.3389/fphar.2020.01169, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyser J. M., Estes M. K. (2015). Pathophysiological consequences of calcium-conducting Viroporins. Annu. Rev. Virol. 2, 473–496. doi: 10.1146/annurev-virology-100114-054846, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Neely G. G., Yaghubian-Malhami R., Perkmann T., Van Loo G., et al. (2008). Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cells 133, 235–249. doi: 10.1016/j.cell.2008.02.043, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irigoyen N., Firth A. E., Jones J. D., Chung B. Y., Siddell S. G., Brierley I. (2016). High-resolution analysis of coronavirus gene expression by RNA sequencing and ribosome profiling. PLoS Pathog. 12:e1005473. doi: 10.1371/journal.ppat.1005473, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hanazawa T., Tomita K., Barnes P. J., Adcock I. M. (2004). Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem. Biophys. Res. Commun. 315, 240–245. doi: 10.1016/j.bbrc.2004.01.046, PMID: [DOI] [PubMed] [Google Scholar]
- Izquierdo J. L., Soriano J. B., Gonzalez Y., Lumbreras S., Ancochea J., Echeverry C., et al. (2022). Use of N-acetylcysteine at high doses as an oral treatment for patients hospitalized with COVID-19. Sci. Prog. 105:003685042210745. doi: 10.1177/00368504221074574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari-Oori M., Ghasemifard F., Ebadi A., Karimi L., Rahimi-Bashar F., Jamialahmadi T., et al. (2021). Acute respiratory distress syndrome and COVID-19: A scoping review and meta-analysis. Adv. Exp. Med. Biol. 1321, 211–228. doi: 10.1007/978-3-030-59261-5_18, PMID: [DOI] [PubMed] [Google Scholar]
- Jamaluddin M., Tian B., Boldogh I., Garofalo R. P., Brasier A. R. (2009). Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J. Virol. 83, 10605–10615. doi: 10.1128/JVI.01090-09, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J., Reimer K. C., Nagai J. S., Varghese F. S., Overheul G. J., De Beer M., et al. (2022). SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 29:e218, 217–231.e8. doi: 10.1016/j.stem.2021.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jany B., Betz R., Schreck R. (1995). Activation of the transcription factor NF-kappa B in human tracheobronchial epithelial cells by inflammatory stimuli. Eur. Respir. J. 8, 387–391. doi: 10.1183/09031936.95.08030387, PMID: [DOI] [PubMed] [Google Scholar]
- Jarrott B., Head R., Pringle K. G., Lumbers E. R., Martin J. H. (2022). "LONG COVID"-A hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol. Res. Perspect. 10:e00911. doi: 10.1002/prp2.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Zhao G., Song N., Li P., Chen Y., Guo Y., et al. (2018). Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg. Microbes Infect. 7, 1–12. doi: 10.1038/s41426-018-0063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josset L., Menachery V. D., Gralinski L. E., Agnihothram S., Sova P., Carter V. S., et al. (2013). Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. MBio 4, e00165–e00113. doi: 10.1128/mBio.00165-13, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M. (2021). NF-kappaB signalling as a pharmacological target in COVID-19: potential roles for IKKbeta inhibitors. Naunyn Schmiedeberg's Arch. Pharmacol. 394, 561–567. doi: 10.1007/s00210-020-02035-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara Y., Iwai K., Yachie A., Ohta K., Konno A., Seki H., et al. (1997). Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood 89, 1748–1753. doi: 10.1182/blood.V89.5.1748, PMID: [DOI] [PubMed] [Google Scholar]
- Kasai S., Shimizu S., Tatara Y., Mimura J., Itoh K. (2020). Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomol. Ther. 10, 320–341. doi: 10.3390/biom10020320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau H. C., Wu S. B., Tsai C. C., Liu C. J., Wei Y. H. (2016). Cigarette smoke extract-induced oxidative stress and fibrosis-related genes expression in orbital fibroblasts from patients with Graves' Ophthalmopathy. Oxidative Med. Cell. Longev. 2016, 1–10. doi: 10.1155/2016/4676289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul P., Biagioli M. C., Singh I., Turner R. B. (2000). Rhinovirus-induced oxidative stress and interleukin-8 elaboration involves p47-phox but is independent of attachment to intercellular adhesion molecule-1 and viral replication. J. Infect. Dis. 181, 1885–1890. doi: 10.1086/315504, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefaloyianni E., Gaitanaki C., Beis I. (2006). ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell. Signal. 18, 2238–2251. doi: 10.1016/j.cellsig.2006.05.004, PMID: [DOI] [PubMed] [Google Scholar]
- Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S. M. (2017). ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv. Exp. Med. Biol. 967, 105–137. doi: 10.1007/978-3-319-63245-2_8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesic M. J., Simmons S. O., Bauer R., Jaspers I. (2011). Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic. Biol. Med. 51, 444–453. doi: 10.1016/j.freeradbiomed.2011.04.027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. M., Ahmad I., Haqqi T. M. (2018). Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic. Biol. Med. 116, 159–171. doi: 10.1016/j.freeradbiomed.2018.01.013, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomich O. A., Kochetkov S. N., Bartosch B., Ivanov A. V. (2018). Redox biology of respiratory viral infections. Viruses 10, 392–419. doi: 10.3390/v10080392, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. M., Kim Y. G., Jeong K. H., Lee S. H., Lee T. W., Ihm C. G., et al. (2012b). Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One 7:e39739. doi: 10.1371/journal.pone.0039739, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Suh G. J., Kwon W. Y., Kwak Y. H., Lee K., Lee H. J., et al. (2012a). Antioxidant effects of selenium on lung injury in paraquat intoxicated rats. Clin. Toxicol. 50, 749–753. doi: 10.3109/15563650.2012.708418, PMID: [DOI] [PubMed] [Google Scholar]
- Kindrachuk J., Ork B., Hart B. J., Mazur S., Holbrook M. R., Frieman M. B., et al. (2015). Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 59, 1088–1099. doi: 10.1128/AAC.03659-14, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircheis R., Haasbach E., Lueftenegger D., Heyken W. T., Ocker M., Planz O. (2020). NF-kappaB pathway as a potential target for treatment of critical stage COVID-19 patients. Front. Immunol. 11:598444. doi: 10.3389/fimmu.2020.598444, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaravelli N., Casola A. (2014). Respiratory viral infections and subversion of cellular antioxidant defenses. J. Pharmacogenomics Pharmacoproteomics 5, 1000141–1000161. doi: 10.4172/2153-0645.1000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaravelli N., Tian B., Ivanciuc T., Mautemps N., Brasier A. R., Garofalo R. P., et al. (2015). Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of Nrf2. Free Radic. Biol. Med. 88, 391–403. doi: 10.1016/j.freeradbiomed.2015.05.043, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. H., Wouters E. F., Vos N., Janssen-Heininger Y. M. (2001). Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J. Biol. Chem. 276, 35693–35700. doi: 10.1074/jbc.M104321200, PMID: [DOI] [PubMed] [Google Scholar]
- Kosmider B., Messier E. M., Janssen W. J., Nahreini P., Wang J., Hartshorn K. L., et al. (2012). Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir. Res. 13:43. doi: 10.1186/1465-9921-13-43, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozower B. D., Christofidou-Solomidou M., Sweitzer T. D., Muro S., Buerk D. G., Solomides C. C., et al. (2003). Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 21, 392–398. doi: 10.1038/nbt806, PMID: [DOI] [PubMed] [Google Scholar]
- Kulisz A., Chen N., Chandel N. S., Shao Z., Schumacker P. T. (2002). Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am. J. Phys. Lung Cell. Mol. Phys. 282, L1324–L1329. doi: 10.1152/ajplung.00326.2001, PMID: [DOI] [PubMed] [Google Scholar]
- Labarrere C. A., Kassab G. S. (2022). Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease. Front. Microbiol. 13:979719. doi: 10.3389/fmicb.2022.979719, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan S. H., Lee H. Z., Chao C. M., Chang S. P., Lu L. C., Lai C. C. (2022). Efficacy of melatonin in the treatment of patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. J. Med. Virol. 94, 2102–2107. doi: 10.1002/jmv.27595, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrentyev E. N., Malik K. U. (2009). High glucose-induced Nox1-derived superoxides downregulate PKC-betaII, which subsequently decreases ACE2 expression and ANG(1-7) formation in rat VSMCs. Am. J. Physiol. Heart Circ. Physiol. 296, H106–H118. doi: 10.1152/ajpheart.00239.2008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. Y., Wauquier F., Eid A. A., Roman L. J., Ghosh-Choudhury G., Khazim K., et al. (2013). Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: role of mitochondrial reactive oxygen species. J. Biol. Chem. 288, 28668–28686. doi: 10.1074/jbc.M113.470971, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Qiao J., You Q., Zong S., Peng Q., Liu Y., et al. (2021b). SARS-CoV-2 Nsp5 activates NF-kappaB pathway by upregulating SUMOylation of MAVS. Front. Immunol. 12:750969. doi: 10.3389/fimmu.2021.750969, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Ren T., Zeng J. (2019). Mitochondrial coenzyme Q protects sepsis-induced acute lung injury by activating PI3K/Akt/GSK-3beta/mTOR pathway in rats. Biomed. Res. Int. 2019, 1–9. doi: 10.1155/2019/5240898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A. K., Reznikov L. R., et al. (2016). Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 213, 712–722. doi: 10.1093/infdis/jiv499, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wu K., Zeng S., Zou L., Li X., Xu C., et al. (2022). The role of Mitophagy in viral infection. Cells 11, 711–723. doi: 10.3390/cells11040711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhu D., Yang J., Yan L., Xiong Z., Lu J., et al. (2021a). Clinical treatment experience in severe and critical COVID-19. Mediat. Inflamm. 2021:9924542. doi: 10.1155/2021/9924542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q. J., Ye L. B., Timani K. A., Zeng Y. C., She Y. L., Ye L., et al. (2005). Activation of NF-kappaB by the full-length nucleocapsid protein of the SARS coronavirus. Acta Biochim. Biophys. Sin. Shanghai 37, 607–612. doi: 10.1111/j.1745-7270.2005.00082.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Sawalha A. H., Lu Q. (2021). COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 33, 155–162. doi: 10.1097/BOR.0000000000000776, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S. C. (2017). NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2, 1–9. doi: 10.1038/sigtrans.2017.23, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz C., Muellenbach R. M., Meybohm P., Mutlak H., Lepper P. M., Rolfes C. B., et al. (2021). Effects of inhaled nitric oxide in COVID-19-induced ARDS - is it worthwhile? Acta Anaesthesiol. Scand. 65, 629–632. doi: 10.1111/aas.13757, PMID: [DOI] [PubMed] [Google Scholar]
- Lowes D. A., Thottakam B. M., Webster N. R., Murphy M. P., Galley H. F. (2008). The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic. Biol. Med. 45, 1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003, PMID: [DOI] [PubMed] [Google Scholar]
- Lowes D. A., Webster N. R., Murphy M. P., Galley H. F. (2013). Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth. 110, 472–480. doi: 10.1093/bja/aes577, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. M., True L., Hawley S., Matsumura M., Morrissey C., Vessella R., et al. (2008). The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J. Pathol. 215, 118–125. doi: 10.1002/path.2330, PMID: [DOI] [PubMed] [Google Scholar]
- Lukassen S., Chua R. L., Trefzer T., Kahn N. C., Schneider M. A., Muley T., et al. (2020). SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 39:e105114. doi: 10.15252/embj.2020105114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud-Al-Rafat A., Muzammal Haque Asim M., Taylor-Robinson A. W., Majumder A., Muktadir A., Muktadir H., et al. (2020). A combinational approach to restore cytokine balance and to inhibit virus growth may promote patient recovery in severe COVID-19 cases. Cytokine 136:155228. doi: 10.1016/j.cyto.2020.155228, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti A. K., Saha N. C., More S. S., Panigrahi A. K., Paul G. (2017). Neuroprotective efficacy of mitochondrial antioxidant MitoQ in suppressing peroxynitrite-mediated mitochondrial dysfunction inflicted by lead toxicity in the rat brain. Neurotox. Res. 31, 358–372. doi: 10.1007/s12640-016-9692-7, PMID: [DOI] [PubMed] [Google Scholar]
- Martinon F., Mayor A., Tschopp J. (2009). The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265. doi: 10.1146/annurev.immunol.021908.132715, PMID: [DOI] [PubMed] [Google Scholar]
- Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. (2004). Phosphorylation of p38 MAPK and its downstream targets in SARS coronavirus-infected cells. Biochem. Biophys. Res. Commun. 319, 1228–1234. doi: 10.1016/j.bbrc.2004.05.107, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi N., Eslami Farsani B., Ghadipasha M., Mahmoudiasl G. R., Piryaei A., Aliaghaei A., et al. (2021). COVID-19 disrupts spermatogenesis through the oxidative stress pathway following induction of apoptosis. Apoptosis 26, 415–430. doi: 10.1007/s10495-021-01680-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G., Walder K., Puri B. K., Berk M., Maes M. (2016). The deleterious effects of oxidative and nitrosative stress on palmitoylation, membrane lipid rafts and lipid-based cellular signalling: New drug targets in neuroimmune disorders. Mol. Neurobiol. 53, 4638–4658. doi: 10.1007/s12035-015-9392-y, PMID: [DOI] [PubMed] [Google Scholar]
- Mukherjee T. K., Mukhopadhyay S., Hoidal J. R. (2005). The role of reactive oxygen species in TNFalpha-dependent expression of the receptor for advanced glycation end products in human umbilical vein endothelial cells. Biochim. Biophys. Acta 1744, 213–223. doi: 10.1016/j.bbamcr.2005.03.007, PMID: [DOI] [PubMed] [Google Scholar]
- Nakajima S., Kitamura M. (2013). Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response. Free Radic. Biol. Med. 65, 162–174. doi: 10.1016/j.freeradbiomed.2013.06.020, PMID: [DOI] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M. V., Mcgroder C., Stevens J. S., et al. (2021). Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615. doi: 10.1038/s41591-021-01283-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J., Yuan G., Kumar G. K., Semenza G. L., Prabhakar N. R. (2008). Transcriptional responses to intermittent hypoxia. Respir. Physiol. Neurobiol. 164, 277–281. doi: 10.1016/j.resp.2008.07.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndengele M. M., Muscoli C., Wang Z. Q., Doyle T. M., Matuschak G. M., Salvemini D. (2005). Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock 23, 186–193. doi: 10.1097/01.shk.0000144130.36771.d6, PMID: [DOI] [PubMed] [Google Scholar]
- Nguyen Dinh Cat A., Montezano A. C., Burger D., Touyz R. M. (2013). Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 19, 1110–1120. doi: 10.1089/ars.2012.4641, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Torres J. L., Verdia-Baguena C., Jimenez-Guardeno J. M., Regla-Nava J. A., Castano-Rodriguez C., Fernandez-Delgado R., et al. (2015). Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 485, 330–339. doi: 10.1016/j.virol.2015.08.010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture S. K., Jaiswal A. K. (2012). Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 287, 9873–9886. doi: 10.1074/jbc.M111.312694, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olagnier D., Farahani E., Thyrsted J., Blay-Cadanet J., Herengt A., Idorn M., et al. (2020). SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 11:4938. doi: 10.1038/s41467-020-18764-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olczak-Pruc M., Swieczkowski D., Ladny J. R., Pruc M., Juarez-Vela R., Rafique Z., et al. (2022). Vitamin C supplementation for the treatment of COVID-19: A systematic review and meta-analysis. Nutrients 14, 4217–4231. doi: 10.3390/nu14194217, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S., Gogvadze V., Zhivotovsky B. (2007). Mitochondrial oxidative stress: implications for cell death. Annu. Rev. Pharmacol. Toxicol. 47, 143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122 [DOI] [PubMed] [Google Scholar]
- Pacheco Y., Valeyre D., El Jammal T., Vallee M., Chevalier F., Lamartine J., et al. (2021). Autophagy and Mitophagy-related pathways at the crossroads of genetic pathways involved in familial sarcoidosis and host-pathogen interactions induced by coronaviruses. Cells 10, 1995–2024. doi: 10.3390/cells10081995, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidas M. J., Mohamed A. B., Norenberg M. D., Saad A., Barry A. F., Colon C., et al. (2021). Multi-organ histopathological changes in a mouse hepatitis virus model of COVID-19. Viruses 13. doi: 10.3390/v13091703, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidas M. J., Sampath N., Schindler E. A., Cosio D. S., Ndubizu C. O., Shamaladevi N., et al. (2022). Mechanism of multi-organ injury in experimental COVID-19 and its inhibition by a small molecule peptide. Front. Pharmacol. 13:864798. doi: 10.3389/fphar.2022.864798, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. S., Kim S. R., Lee Y. C. (2009). Impact of oxidative stress on lung diseases. Respirology 14, 27–38. doi: 10.1111/j.1440-1843.2008.01447.x [DOI] [PubMed] [Google Scholar]
- Paszti-Gere E., Barna R. F., Kovago C., Szauder I., Ujhelyi G., Jakab C., et al. (2015). Changes in the distribution of type II transmembrane serine protease, TMPRSS2 and in paracellular permeability in IPEC-J2 cells exposed to oxidative stress. Inflammation 38, 775–783. doi: 10.1007/s10753-014-9988-9, PMID: [DOI] [PubMed] [Google Scholar]
- Patel M. (2016). Targeting oxidative stress in central nervous system disorders. Trends Pharmacol. Sci. 37, 768–778. doi: 10.1016/j.tips.2016.06.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. D., Lemle M. D., Komaroff A. L., Snyder S. H. (2021). Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. U. S. A. 118, 1–10. doi: 10.1073/pnas.2024358118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein D. P., Ali M. H., Mungai P. T., Hynes K. L., Gewertz B. L., Schumacker P. T. (2002). Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arterioscler. Thromb. Vasc. Biol. 22, 566–573. doi: 10.1161/01.ATV.0000012262.76205.6A, PMID: [DOI] [PubMed] [Google Scholar]
- Pena Silva R. A., Chu Y., Miller J. D., Mitchell I. J., Penninger J. M., Faraci F. M., et al. (2012). Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke 43, 3358–3363. doi: 10.1161/STROKEAHA.112.667063, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Hang N., Liu W., Guo W., Jiang C., Yang X., et al. (2016). Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-kappaB pathways. Acta Pharm. Sin. B 6, 205–211. doi: 10.1016/j.apsb.2016.02.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera M., El Khoury J., Chinni V., Bolton D., Qu L., Johnson P., et al. (2020). Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: trial protocol. BMJ Open 10:e040580. doi: 10.1136/bmjopen-2020-040580, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petcherski A., Sharma M., Daskou M., Satta S., Vasilopoulos H., Hugo C., et al. (2022). Mitoquinone mesylate targets SARS-CoV-2 and associated lung inflammation through host pathways. bioRxiv [Google Scholar]
- Petersen E., Koopmans M., Go U., Hamer D. H., Petrosillo N., Castelli F., et al. (2020). Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 20, e238–e244. doi: 10.1016/S1473-3099(20)30484-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle S., Schopf J., Kogl M., Friedel C. C., Muller M. A., Carbajo-Lozoya J., et al. (2011). The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 7:e1002331. doi: 10.1371/journal.ppat.1002331, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. B., Parchment R. E., Lewellyn A. L. (1991). Hydrogen peroxide as a mediator of programmed cell death in the blastocyst. Differentiation 46, 181–186. doi: 10.1111/j.1432-0436.1991.tb00880.x, PMID: [DOI] [PubMed] [Google Scholar]
- Piette J., Piret B., Bonizzi G., Schoonbroodt S., Merville M. P., Legrand-Poels S., et al. (1997). Multiple redox regulation in NF-kappaB transcription factor activation. Biol. Chem. 378, 1237–1245. [PubMed] [Google Scholar]
- Prakash A., Kaur S., Kaur C., Prabha P. K., Bhatacharya A., Sarma P., et al. (2021). Efficacy and safety of inhaled nitric oxide in the treatment of severe/critical COVID-19 patients: A systematic review. Indian J. Pharm. 53, 236–243. doi: 10.4103/ijp.ijp_382_21, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratomo I. P., Noor D. R., Kusmardi K., Rukmana A., Paramita R. I., Erlina L., et al. (2021). Xanthine oxidase-induced inflammatory responses in respiratory epithelial cells: A review in immunopathology of COVID-19. Int. J. Inflamm. 2021, 1–10. doi: 10.1155/2021/1653392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiser J. C. (2012). Oxidative stress. JPEN J. Parenter. Enteral Nutr. 36, 147–154. doi: 10.1177/0148607111434963 [DOI] [PubMed] [Google Scholar]
- Pruijssers A. J., Denison M. R. (2019). Nucleoside analogues for the treatment of coronavirus infections. Curr. Opin. Virol. 35, 57–62. doi: 10.1016/j.coviro.2019.04.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani S., Pathak A., Dalal V., Paul A., Biswas S. (2020). Oxidative stress in autoimmune diseases: an under dealt malice. Curr. Protein Pept. Sci. 21, 611–621. doi: 10.2174/1389203721666200214111816, PMID: [DOI] [PubMed] [Google Scholar]
- Reiter R. J. (1998). Oxidative damage in the central nervous system: protection by melatonin. Prog. Neurobiol. 56, 359–384. doi: 10.1016/S0301-0082(98)00052-5 [DOI] [PubMed] [Google Scholar]
- Reshi M. L., Su Y. C., Hong J. R. (2014). RNA viruses: ROS-mediated cell death. Int. J. Cell Biol. 2014:467452. doi: 10.1155/2014/467452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaert N. L., Van Der Vliet A., Guala A. S., Mcgovern T., Hristova M., Pantano C., et al. (2006). Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc. Natl. Acad. Sci. U. S. A. 103, 13086–13091. doi: 10.1073/pnas.0603290103, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. K., Sindhu K. K. (2009). Oxidative stress and metabolic syndrome. Life Sci. 84, 705–712. doi: 10.1016/j.lfs.2009.02.026 [DOI] [PubMed] [Google Scholar]
- Roulston A., Marcellus R. C., Branton P. E. (1999). Viruses and apoptosis. Annu. Rev. Microbiol. 53, 577–628. doi: 10.1146/annurev.micro.53.1.577 [DOI] [PubMed] [Google Scholar]
- Saad M. A., Alfishawy M., Nassar M., Mohamed M., Esene I. N., Elbendary A. (2021). COVID-19 and autoimmune diseases: A systematic review of reported cases. Curr. Rheumatol. Rev. 17, 193–204. doi: 10.2174/1573397116666201029155856, PMID: [DOI] [PubMed] [Google Scholar]
- Saheb Sharif-Askari N., Saheb Sharif-Askari F., Mdkhana B., Hussain Alsayed H. A., Alsafar H., Alrais Z. F., et al. (2021). Upregulation of oxidative stress gene markers during SARS-COV-2 viral infection. Free Radic. Biol. Med. 172, 688–698. doi: 10.1016/j.freeradbiomed.2021.06.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S. (2017). Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 360, 201–205. doi: 10.1124/jpet.116.237503, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiesteban-Lores L. E., Amamura T. A., Da Silva T. F., Midon L. M., Carneiro M. C., Isaac L., et al. (2021). A double edged-sword - the complement system during SARS-CoV-2 infection. Life Sci. 272:119245. doi: 10.1016/j.lfs.2021.119245, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Rossi A., Amici C. (2003). NF-kappaB and virus infection: who controls whom. EMBO J. 22, 2552–2560. doi: 10.1093/emboj/cdg267, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. H., Hunakova L., Chen Y., Bortner C., Van Houten B. (2003). Cell sorting experiments link persistent mitochondrial DNA damage with loss of mitochondrial membrane potential and apoptotic cell death. J. Biol. Chem. 278, 1728–1734. doi: 10.1074/jbc.M208752200, PMID: [DOI] [PubMed] [Google Scholar]
- Saura M., Zaragoza C., Bao C., Herranz B., Rodriguez-Puyol M., Lowenstein C. J. (2006). Stat3 mediates interleukin-6 [correction of interelukin-6] inhibition of human endothelial nitric-oxide synthase expression. J. Biol. Chem. 281, 30057–30062. doi: 10.1074/jbc.M606279200, PMID: [DOI] [PubMed] [Google Scholar]
- Schofield J. H., Schafer Z. T. (2021). Mitochondrial reactive oxygen species and mitophagy: A complex and nuanced relationship. Antioxid. Redox Signal. 34, 517–530. doi: 10.1089/ars.2020.8058, PMID: [DOI] [PubMed] [Google Scholar]
- Schrader L. I., Kinzenbaw D. A., Johnson A. W., Faraci F. M., Didion S. P. (2007). IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler. Thromb. Vasc. Biol. 27, 2576–2581. doi: 10.1161/ATVBAHA.107.153080, PMID: [DOI] [PubMed] [Google Scholar]
- Schreck R., Baeuerle P. A. (1991). A role for oxygen radicals as second messengers. Trends Cell Biol. 1, 39–42. doi: 10.1016/0962-8924(91)90072-H [DOI] [PubMed] [Google Scholar]
- Shang C., Liu Z., Zhu Y., Lu J., Ge C., Zhang C., et al. (2021). SARS-CoV-2 causes mitochondrial dysfunction and mitophagy impairment. Front. Microbiol. 12:780768. doi: 10.3389/fmicb.2021.780768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. (1985). Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood 65, 605–614. doi: 10.1182/blood.V65.3.605.605, PMID: [DOI] [PubMed] [Google Scholar]
- Shatizadeh Malekshahi S., Yavarian J., Shafiei-Jandaghi N. Z. (2022). Usage of peptidases by SARS-CoV-2 and several human coronaviruses as receptors: A mysterious story. Biotechnol. Appl. Biochem. 69, 124–128. doi: 10.1002/bab.2087, PMID: [DOI] [PubMed] [Google Scholar]
- Shi C. S., Qi H. Y., Boularan C., Huang N. N., Abu-Asab M., Shelhamer J. H., et al. (2014). SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 193, 3080–3089. doi: 10.4049/jimmunol.1303196, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirihai O. S., Song M., Dorn G. W., 2nd (2015). How mitochondrial dynamism orchestrates mitophagy. Circ. Res. 116, 1835–1849. doi: 10.1161/CIRCRESAHA.116.306374, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shono T., Ono M., Izumi H., Jimi S. I., Matsushima K., Okamoto T., et al. (1996). Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol. Cell. Biol. 16, 4231–4239. doi: 10.1128/MCB.16.8.4231, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H. K., Libby P., Ridker P. M. (2021). COVID-19 - A vascular disease. Trends Cardiovasc. Med. 31, 1–5. doi: 10.1016/j.tcm.2020.10.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P. F., Mccorrister S., Hu P., Chong P., Silaghi A., Westmacott G., et al. (2015). Highly pathogenic H5N1 and novel H7N9 influenza A viruses induce More profound proteomic host responses than seasonal and pandemic H1N1 strains. J. Proteome Res. 14, 4511–4523. doi: 10.1021/acs.jproteome.5b00196, PMID: [DOI] [PubMed] [Google Scholar]
- Singhal T. (2020). A review of coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 87, 281–286. doi: 10.1007/s12098-020-03263-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K., Pandey A. K., Livingston A., Venkatesh S. (2021). Roles of host mitochondria in the development of COVID-19 pathology: could mitochondria be a potential therapeutic target? Mol. Biomed. 2:38. doi: 10.1186/s43556-021-00060-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., et al. (2020). COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 20, 400–402. doi: 10.1016/S1473-3099(20)30132-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. M., Wang L., Yoo D. (2021). Activation of NF-kappaB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 11:13464. doi: 10.1038/s41598-021-92941-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Sursal T., Adibnia Y., Zhao C., Zheng Y., Li H., et al. (2013). Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS One 8:e59989. doi: 10.1371/journal.pone.0059989, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Yajjala V. K., Bauer C., Talmon G. A., Fischer K. J., Kielian T., et al. (2016). Nox2-derived oxidative stress results in inefficacy of antibiotics against post-influenza S. aureus pneumonia. J. Exp. Med. 213, 1851–1864. doi: 10.1084/jem.20150514, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski G. S., Murphy M. P., Callahan L. A. (2009). MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am. J. Phys. Regul. Integr. Comp. Phys. 297, R1095–R1102. doi: 10.1152/ajpregu.90902.2008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Mukhopadhyay A., Kundu G. C., Mahabeleshwar G. H., Singh S., Aggarwal B. B. (2003). Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 278, 24233–24241. doi: 10.1074/jbc.M212389200, PMID: [DOI] [PubMed] [Google Scholar]
- Tao L., Lemoff A., Wang G., Zarek C., Lowe A., Yan N., et al. (2020). Reactive oxygen species oxidize STING and suppress interferon production. elife 9, 1–23. doi: 10.7554/eLife.57837, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenjinbaru K., Furuno T., Hirashima N., Nakanishi M. (1999). Nuclear translocation of green fluorescent protein-nuclear factor kappaB with a distinct lag time in living cells. FEBS Lett. 444, 1–4. doi: 10.1016/S0014-5793(99)00002-2, PMID: [DOI] [PubMed] [Google Scholar]
- Thevenod F., Friedmann J. M., Katsen A. D., Hauser I. A. (2000). Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-kappaB activation protects kidney proximal tubule cells from cadmium-and reactive oxygen species-induced apoptosis. J. Biol. Chem. 275, 1887–1896. doi: 10.1074/jbc.275.3.1887, PMID: [DOI] [PubMed] [Google Scholar]
- Tian J., Middleton B., Kaufman D. L. (2021). GABA(A)-receptor agonists limit pneumonitis and death in murine coronavirus-infected mice. Viruses 13, 966–978. doi: 10.3390/v13060966, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- To E. E., Broughton B. R., Hendricks K. S., Vlahos R., Selemidis S. (2014). Influenza A virus and TLR7 activation potentiate NOX2 oxidase-dependent ROS production in macrophages. Free Radic. Res. 48, 940–947. doi: 10.3109/10715762.2014.927579, PMID: [DOI] [PubMed] [Google Scholar]
- Toro A., Ruiz M. S., Lage-Vickers S., Sanchis P., Sabater A., Pascual G., et al. (2022). A journey into the clinical relevance of Heme oxygenase 1 for human inflammatory disease and viral clearance: why does it matter on the COVID-19 scene? Antioxidants 11, 276–298. doi: 10.3390/antiox11020276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempolec N., Munoz J. P., Slobodnyuk K., Marin S., Cascante M., Zorzano A., et al. (2017). Induction of oxidative metabolism by the p38alpha/MK2 pathway. Sci. Rep. 7:11367. doi: 10.1038/s41598-017-11309-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu W., Wang H., Li S., Liu Q., Sha H. (2019). The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 10, 637–651. doi: 10.14336/AD.2018.0513, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M. L., Dworshak J., Sharma A., Ibrahim A. S., Al-Shabrawey M., Sharma S. (2019). Inhibition of interleukin-6 trans-signaling prevents inflammation and endothelial barrier disruption in retinal endothelial cells. Exp. Eye Res. 178, 27–36. doi: 10.1016/j.exer.2018.09.009, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. (2020). The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 76, 14–20. doi: 10.1016/j.ejim.2020.04.037, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violi F., Oliva A., Cangemi R., Ceccarelli G., Pignatelli P., Carnevale R., et al. (2020). Nox2 activation in Covid-19. Redox Biol. 36:101655. doi: 10.1016/j.redox.2020.101655, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. S., Chu F. L., Jin L., Li Y. G., Zhang Z., Xu D., et al. (2005). Acquired but reversible loss of erythrocyte complement receptor 1 (CR1, CD35) and its longitudinal alteration in patients with severe acute respiratory syndrome. Clin. Exp. Immunol. 139, 112–119. doi: 10.1111/j.1365-2249.2005.02681.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu S., Liu H., Li W., Lin F., Jiang L., et al. (2020). SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 73, 807–816. doi: 10.1016/j.jhep.2020.05.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang X. (1999). The nucleocapsid protein of coronavirus mouse hepatitis virus interacts with the cellular heterogeneous nuclear ribonucleoprotein A1 in vitro and in vivo. Virology 265, 96–109. doi: 10.1006/viro.1999.0025, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Navas-Martin S. (2005). Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 69, 635–664. doi: 10.1128/MMBR.69.4.635-664.2005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselborg S., Bauer M. K., Vogt M., Schmitz M. L., Schulze-Osthoff K. (1997). Activation of transcription factor NF-kappaB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J. Biol. Chem. 272, 12422–12429. doi: 10.1074/jbc.272.19.12422, PMID: [DOI] [PubMed] [Google Scholar]
- Wong K. K., Lee S. W. H., Kua K. P. (2021). N-acetylcysteine as adjuvant therapy for COVID-19 - A perspective on the current state of the evidence. J. Inflamm. Res. 14, 2993–3013. doi: 10.2147/JIR.S306849, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosniak J., Jr., Santos C. X., Kowaltowski A. J., Laurindo F. R. (2009). Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid. Redox Signal. 11, 1265–1278. doi: 10.1089/ars.2009.2392, PMID: [DOI] [PubMed] [Google Scholar]
- Xia H., Suda S., Bindom S., Feng Y., Gurley S. B., Seth D., et al. (2011). ACE2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS One 6:e22682. doi: 10.1371/journal.pone.0022682, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Shaikh Z. A. (2006). Cadmium-induced apoptosis in rat kidney epithelial cells involves decrease in nuclear factor-kappa B activity. Toxicol. Sci. 91, 299–308. doi: 10.1093/toxsci/kfj131, PMID: [DOI] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., et al. (2020). High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 12:8. doi: 10.1038/s41368-020-0074-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Limmon G. V., Zheng D., Li N., Li L., Yin L., et al. (2012). Major shifts in the spatio-temporal distribution of lung antioxidant enzymes during influenza pneumonia. PLoS One 7:e31494. doi: 10.1371/journal.pone.0031494, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. M., Chen H. C., Tsai J. S., Lin L. Y. (2007). Cadmium induces Ca2+−dependent necrotic cell death through calpain-triggered mitochondrial depolarization and reactive oxygen species-mediated inhibition of nuclear factor-kappaB activity. Chem. Res. Toxicol. 20, 406–415. doi: 10.1021/tx060144c, PMID: [DOI] [PubMed] [Google Scholar]
- Yi Y., Lagniton P. N. P., Ye S., Li E., Xu R. H. (2020). COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 16, 1753–1766. doi: 10.7150/ijbs.45134, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J. Y., Zhang Y., Wu Y., Cannesson M., Cai H. (2021). Therapeutic application of estrogen for COVID-19: attenuation of SARS-CoV-2 spike protein and IL-6 stimulated, ACE2-dependent NOX2 activation, ROS production and MCP-1 upregulation in endothelial cells. Redox Biol. 46:102099. doi: 10.1016/j.redox.2021.102099, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Qin Y., Chen M. (2018). Viral strategies for triggering and manipulating mitophagy. Autophagy 14, 1665–1673. doi: 10.1080/15548627.2018.1466014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., et al. (2016). ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 1–18. doi: 10.1155/2016/4350965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang J., Wang L., Aliyari S., Cheng G. (2022). SARS-CoV-2 virus NSP14 impairs NRF2/HMOX1 activation by targeting Sirtuin 1. Cell. Mol. Immunol. 19, 872–882. doi: 10.1038/s41423-022-00887-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Jiang Y., Qiu H., Gao T., Zeng Y., Guo Y., et al. (2015). Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with Middle East respiratory syndrome-coronavirus. PLoS One 10:e0145561. doi: 10.1371/journal.pone.0145561, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225. doi: 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- Zhu T., Wang D. X., Zhang W., Liao X. Q., Guan X., Bo H., et al. (2013). Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-kappaB. PLoS One 8:e56407. doi: 10.1371/journal.pone.0056407, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinovkin R. A., Grebenchikov O. A. (2020). Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients. Biochemistry (Mosc) 85, 833–837. doi: 10.1134/S0006297920070111, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinovkin R. A., Romaschenko V. P., Galkin I., Zakharova V. V., Pletjushkina O. Y., Chernyak B. V., et al. (2014). Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium. Aging (Albany NY) 6, 661–674. doi: 10.18632/aging.100685, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]