Abstract
Oral squamous cell carcinoma (OSCC) is an epithelial malignant tumor with great challenges of tumor metastasis and drug resistance. Nutlin-3 is a MDM2 inhibitor that can potently activate tumor suppressor gene p53. However, the exact role of Nutlin-3 in OSCC has not been identified yet. SCC-9 cells were treated with 0, 2.5, 5, 10, 20 μM Nutlin3. MDM2 and p53 protein levels were assessed using western blot analysis. Then, CCK8 assay, clone formation assay, TUNEL staining, wound healing and transwell assays were conducted to analyze the influences of Nutlin3 on the proliferation, apoptosis, migration, and invasion in SCC-9 cells. Moreover, SCC-9 cells were co-treated with 0, 0.5, 1, 2.5, 5 μM cisplatin and Nutlin3 to determine the effect of Nutlin3 on cisplatin chemosensitivity in OSCC. As expected, Nutlin-3 inhibited MDM2 but restored p53 in OSCC in a concentration-dependent manner. Meanwhile, Nutlin-3 suppressed the proliferation, clone formation, migration, invasion and epithelial-mesenchymal transition of SCC-9 cells and both boosted the apoptosis. In addition, Nutlin-3 caused a reduced cell viability and an elevated cell apoptosis rate in cisplatin-treated SCC-9 cells, indicating that Nutlin-3 enhanced cisplatin chemosensitivity in OSCC cells. Taken together, Nutlin-3 may suppress tumorigenesis and progression of OSCC and enhance chemosensitivity to cisplatin in OSCC.
Keywords: Nutlin-3, Oral squamous cell carcinoma, Cisplatin chemosensitivity
Introduction
Oral squamous cell carcinoma (OSCC) is an epithelial malignant tumor, which mainly occurs in maxillofacial skin and oral mucosa (Chen et al. 2017). In recent years, the morbidity of OSCC has gradually increased and about 355,000 new cases are diagnosed every year in the world (Bray et al. 2018). Most patients are often diagnosed in middle or advanced stage, accompanied by a fairly poor prognosis (Thomson 2018). At present, OSCC treatment is mainly based on surgical oriented comprehensive sequence therapy, especially the triple therapy of surgery, radiotherapy and chemotherapy (Bulsara et al. 2018). However, the overall survival outcomes have not markedly improved (Biddle et al. 2016). Therefore, researching pathogenesis and therapeutics of OSCC is in urgent need.
Tumor metastasis and drug resistance are great challenges in OSCC treatment (Major et al. 2013; Yan et al. 2017). Matrix metalloproteinases 2 (MMP2) and MMP9 can degrade extracellular matrix and destroy the complete basement membrane. MMP2 and MMP9 are closely related to OSCC metastasis (Feng et al. 2019). Epithelial to mesenchymal transition (EMT) plays crucial roles in the tumorigenesis and metastasis of OSCC. EMT endows epithelial cells with migratory, invasive properties and the ability to degrade ECM, by which polarized epithelial cells lose the adhesion property and obtain mesenchymal cell phenotypes (Acloque et al. 2009; Zeisberg and Neilson 2009). An increasing amount of evidence suggests that EMT plays an important role in the development of OSCC and promotes chemotherapy resistance (Wang et al. 2020). Hence, prevention and therapeutics against tumor metastasis and chemotherapy resistance are of great importance for of OSCC therapy.
Tumor suppressor gene p53, also termed as the guardian of the genome, functions in cell cycle regulation, DNA repair, cell differentiation and apoptosis (Kastenhuber and Lowe 2017). Murine double minute 2 (MDM2), an oncoprotein, is highly expressed in most tumor cells. MDM2 can block p53 tumor suppressor-mediated transcriptional transactivation (Zhao et al. 2014). Blocking the interaction between wild-type p53 and its negative regulator MDM2 has become an important approach in oncology for restoring p53 antitumor activity (Kussie et al. 1996). Meanwhile, suppression of p53-MDM2 interaction is closely related to tumor drug resistance (Zhu et al. 2013; Hou et al. 2019). Nutlin-3 is a MDM2 inhibitor that can potently activate p53. Besides, it has been proved in previous studies that Nutlin-3 not only suppresses the tumorigenicity and progression of different types of cancers, but also overcomes the resistance to chemotherapies, highlight the importance of Nutlin-3 concerning on cancer treatment (Zheng et al. 2014; Voon et al. 2015). Nevertheless, the precise role of Nutlin-3 in OSCC has not been identified yet, and the effects of Nutlin-3 on OSCC progression has not been examined to date.
In this study, we investigated the role of Nutlin-3 in OSCC proliferation, migration, invasion and EMT, and further explored the association between Nutlin-3 and sensitivity of OSCC cell to cisplatin in vitro.
Materials and methods
Cell culture and treatment
Human OSCC cell line (SCC-9) was obtained from Shanghai cell bank, Chinese academy of sciences. Dulbecco's modified Eagle medium (DMEM; Hyclone, UT, USA) with 10% fetal bovine serum (FBS; Hyclone, UT, USA) and 1% penicillin/streptomycin was used for the incubation of SCC-9 cells. Cells were cultured in an incubator at 37 °C with 5% CO2.
To investigate the function of p53-MDM2 interaction in OSCC, SCC-9 cells were treated with 0, 2.5, 5, 10, 20 μM Nutlin3 (a MDM2 inhibitor). Furthermore, to determine the effects of Nutlin3 on cisplatin (CDDP) chemosensitivity in OSCC, SCC-9 cells were co-treated with 0, 0.5, 1, 2.5, 5 μM cisplatin and Nutlin3.
Cell counting kit-8 (CCK-8) assay
Briefly, SCC-9 cells were seeded in 96-well plates (5 × 103 cells/well). After treatment with different concentrations of Nutlin-3 with or without 1 μM CDDP for 48 h, cells were incubated for additional 2 h with CCK-8 reagent (Beyotime, Shanghai, China). Absorbance was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, MA, USA).
Clone formation assay
SCC-9 cells were seeded in 6-well plates (500 cells/well). Two weeks later, clones were fixed with 4% paraformaldehyde (PFA) and stained with 0.1% crystal violet (Beyotime, Shanghai, China) for 20 min. The images of colonies was captured by an optical microscope (Leica, Wetzlar, Germany).
TUNEL staining
SCC-9 cells were fixed with 4% PFA for 30 min and washed three times with PBS. Then, cells were incubated with TUNEL staining reagent (Roche, Basel, Switzerland) in dark for 60 min. Immediately, 4,6-diamidino-2-phenyl-indole (DAPI; Solarbio, Beijing, China) staining solution was added for nuclei staining. Fluorescence pictures were captured using a fluorescence microscope (Leica, Wetzlar, Germany).
Wound healing assay
Cells were cultured in 6-well plates until the confluence reached 80–90%. A scratch was made using a sterile 10 μl pipette tip and the wells were gently washed with PBS. Then, cells were incubated with serum-free DMEM. Pictures of wound gaps at different time interval (0 and 24 h) were recorded by microscopic photograph (Leica, Wetzlar, Germany).
Transwell assay
Cell invasion assay was performed using Matrigel-coated Transwell chambers. Briefly, a total of 2 × 104 cells were suspended with serum-free DMEM and added to the upper chamber. The lower chamber was plated DMEM medium with 10% FBS (600 µl). Following a 24 h-incubation, the upper chamber cells were wiped off and the invading cells were fixed with 4% PFA and stained with 0.1% crystal violet (Beyotime, Shanghai, China). The invasive cells was imaged by an optical microscope (Leica, Wetzlar, Germany).
Western blot analysis
Cells were lysed in RIPA buffer containing proteinase inhibitors (Beyotime, Shanghai, China) for total protein extraction. Total protein concentrations were quantified using bicinchoninic acid (BCA) protein assay kits (Beyotime, Shanghai, China). Cell lysates (30 µg) were resolved by 12% SDS–polyacrylamide gel electrophoresis, and subsequently transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, MA, USA). After that, membranes were blocked in 5% BSA for 1 h and incubated overnight at 4 °C with the following primary antibodies: anti-MDM2, anti-p53, anti-MMP2, anti-MMP9, anti-Bax, anti-Bcl-2, anti-Cleaved Caspase-3, anti-Caspase-3, anti-Cleaved PARP, anti-PARP, anti-N-cad, anti-Vimentin, anti-GAPDH. The next day, membranes were washed with TBST and then incubated with secondary antibody at room temperature for 1 h. After washing with TBST, protein bands were detected by ECL substrate (Bio-Rad, CA, USA) and Image Lab software (Bio-Rad, CA, USA) was used to quantify protein levels.
Statistical analysis
Experimental data were analyzed by one-way analysis of variance followed by Tukey’s post hoc test using GraphPad Prism 6.0 (GraphPad Software, Inc., CA, USA). All pairs of columns were compared and data were presented as mean ± standard deviation (SD). P < 0.05 represented a statistically significant difference.
Results
Nutlin-3 reduced cell viability and restored p53 expression in OSCC
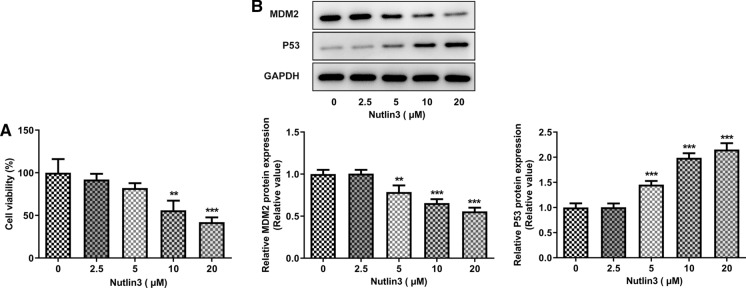
To determine the influence of Nutlin-3 on OSCC cell viability, SCC-9 cells were treated with 0, 2.5, 5, 10, 20 μM Nutlin3. Nutlin-3 dose-dependently inhibited OSCC cell viability, compared to that of untreated cells (Fig. 1A). Besides, in comparison with the control, Nutlin-3 downregulated MDM2 expression and upregulated p53 expression in a dose-dependent manner (Fig. 1B, C).
Fig. 1.
Nutlin-3 reduced cell viability, downregulated MDM2 expression and upregulated p53 expression in SCC-9 cells. A Viability of SCC-9 cells treated with 0, 2.5, 5, 10, 20 μM Nutlin3 was measured using CCK8 assay. B MDM2 and p53 protein expression in SCC-9 cells treated with 0, 2.5, 5, 10, 20 μM Nutlin3 were assessed using western blot analysis. **p < 0.01, ***p < 0.001 vs 0 μM
Nutlin-3 suppressed OSCC cell proliferation and clone formation ability
Since Nutlin-3 treatment at 5, 10, 20 μM showed strong inhibitory effects on the viability of SCC-9 cells, 5, 10, 20 μM were chosen as the representative doses for the subsequent studies. In comparison with untreated cells, viability of SCC-9 cells treated with 5, 10, and 20 μM Nutlin3 was significantly reduced (Fig. 2A). In addition, Nutlin3 dose-dependently suppressed the colony formation of SCC-9 cells (Fig. 2B).
Fig. 2.
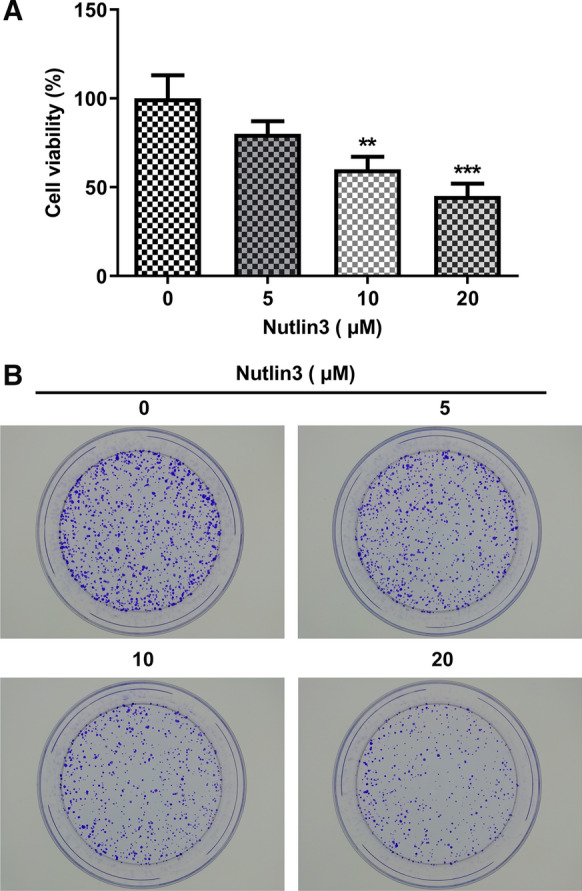
Nutlin-3 suppressed the proliferation and clone formation ability of SCC-9 cells. A Viability of SCC-9 cells treated with 0, 5, 10, 20 μM Nutlin3 was measured using CCK8 assay. B Representative photographs of the colony formation of SCC-9 cells treated with 0, 5, 10, 20 μM Nutlin3. **p < 0.01, ***p < 0.001 vs 0 μM
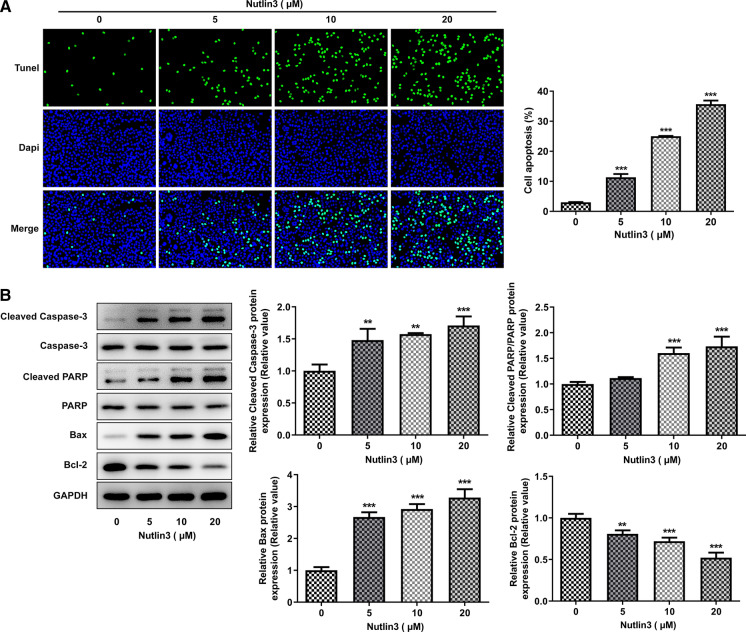
Nutlin-3 boosted OSCC cell apoptosis
As we observed a significant inhibitory effect of Nutlin3 on SCC-9 cell proliferation, we investigated whether Nutlin3 could induce OSCC cell apoptosis. As detected by TUNEL staining, Nutlin3 caused a notable elevation of TUNEL-positive cells (Fig. 3A). Meanwhile, Nutlin3 resulted in the upregulation of pro-apoptotic proteins (Cleaved Caspase-3, Cleaved PARP, Bax) and downregulation of anti-apoptotic protein (Bcl-2) (Fig. 3B). These findings suggested that Nutlin3 promoted apoptosis of SCC-9 cells.
Fig. 3.
Nutlin3 promoted apoptosis of SCC-9 cells. A Representative photographs and quantitative analysis of the apoptosis of SCC-9 cells treated with 0, 5, 10, 20 μM Nutlin3 by TUNEL staining. B Representative protein bands and quantitative analysis of pro-apoptotic proteins (Cleaved Caspase-3, Cleaved PARP, Bax) and anti-apoptotic protein (Bcl-2) in SCC-9 cells treated with 0, 5, 10, 20 μM Nutlin3 by western blot analysis. **p < 0.01, ***p < 0.001 vs 0 μM
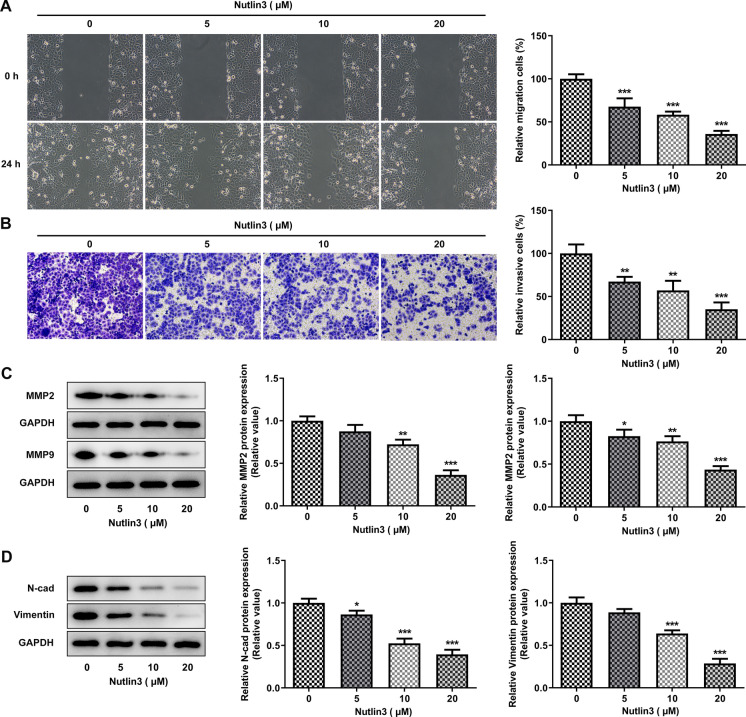
Nutlin-3 arrested OSCC cell migration, invasion and EMT
To evaluate the impacts of Nutlin-3 on cell migration and invasion, wound healing and transwell assays were performed. It was found that Nutlin-3 suppressed the migration of SCC-9 cells (Fig. 4A). Consistent with this finding, Nutlin-3 weakened the invasion capacity of SCC-9 cells (Fig. 4B). Reduction of MMP2 and MMP9 expression further evidenced that Nutlin-3 inhibited OSCC cell metastasis (Fig. 4C). Furthermore, downregulation of N-cad and Vimentin levels indicated that Nutlin-3 could repress EMT in OSCC (Fig. 4D).
Fig. 4.
Nutlin-3 suppressed migration, invasion and EMT in SCC-9 cells. A Representative photographs and quantitative analysis of the migration of SCC-9 cells treated with 0, 5, 10, 20 μM Nutlin3 by wound healing assays. B Representative photographs and quantitative analysis of the invasion of SCC-9 cells treated with 0, 5, 10, 20 μM Nutlin3 by transwell assays. C Representative protein bands and quantitative analysis of MMP2 and MMP9 in SCC-9 cells treated with 0, 5, 10, 20 μM Nutlin3 by western blot analysis. D Representative protein bands and quantitative analysis of N-cad and Vimentin in SCC-9 cells treated with 0, 5, 10, 20 μM Nutlin3 by western blot analysis. *p < 0.05, **p < 0.01, ***p < 0.001 vs 0 μM
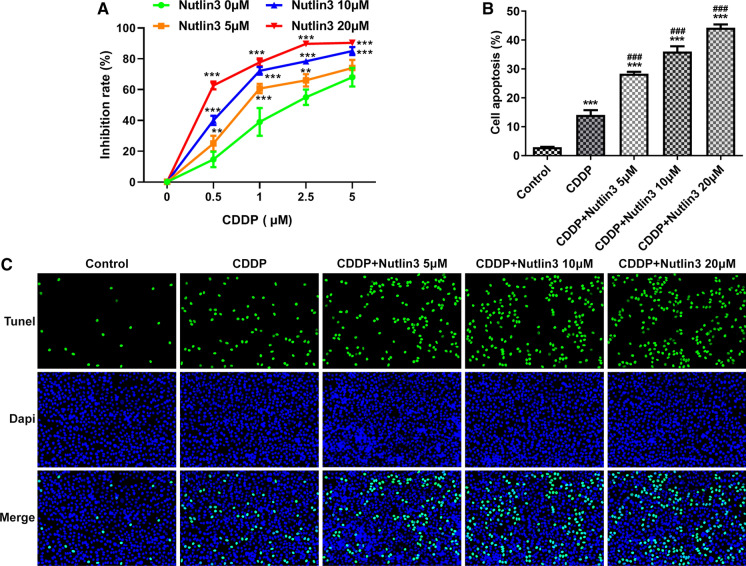
Nutlin-3 enhanced cisplatin chemosensitivity in OSCC
SCC-9 cells were co-treated with Nutlin3 and CDDP (0, 0.5, 1, 2.5, 5 μM) to investigate the influence of Nutlin3 on CDDP chemosensitivity in OSCC. At a given concentration of CDDP, Nultin3 treatment resulted in the enhanced chemosensitivity of OSCC cells (Fig. 5A). In addition, compared to the CDDP treatment alone, a high rate of OSCC cell apoptosis was found following additional treatment of Nutlin-3 in a concentration-dependent manner (Fig. 5C). The findings supported that Nutlin-3 might be a positive regulator of CDDP chemosensitivity in OSCC.
Fig. 5.
Nutlin-3 enhanced cisplatin chemosensitivity in SCC-9 cells. A CCK8 assay was performed to determine the influence of Nutlin-3 om cisplatin chemosensitivity in OSCC cell proliferation. **p < 0.01, ***p < 0.001 vs 0 μM. B and C Representative photographs and quantitative analysis of the apoptosis of SCC-9 cells co-treated with cisplatin and Nutlin3 by TUNEL staining. ***p < 0.001 vs control; ###p < 0.001 vs CDDP
Discussion
In normal cells, MDM2 and wild-type p53 has a delicate balance. Interaction between MDM2 and wild-type p53 forms a negative feedback loop named p53-MDM2, which promotes p53 ubiquitination and then degradation by protease (Kussie et al. 1996; Zhao et al. 2014; Kastenhuber and Lowe 2017). A growing number of studies verifies that mutation or deletion of human cancer suppressor gene p53 result in function loss in about 50% of human cancers (Best et al. 2020). Therefore, suppression of p53-MDM2 interaction is a promising approach for activating p53. Protein–protein interaction between p53-MDM2 is an important target for antitumor drugs (Hainaut and Milner 1993). Importantly, our present study disclosed the critical role of Nutlin-3 in OSCC tumorigenesis, progression and drug resistance.
p53 plays an important role in the regulation of DNA repair, cell cycle arrest, apoptosis and senescence (Kastenhuber and Lowe 2017). Chemical inhibitor Nutlin-3 was applied to specifically abrogate the interaction between MDM2 and p53. Our study confirmed that Nutlin-3 could downregulate MDM2 expression and upregulate p53 expression in SCC-9 cells in a dose-dependent manner. Meanwhile, Nutlin3 significantly suppressed the proliferation and clonal growth of SCC-9 cells. Cleaved PARP and cleaved caspase-3 are two representative biomarkers of apoptosis. Caspase-3 is a critical protease in the process of apoptosis. The activation of caspase 3 requires proteolytic processing of its inactive zymogen into activated fragments (cleaved caspase-3). PARP is a cleavage targets of activated caspase-3, and cleavage of PARP promotes cellular disassembly and facilitates cell apoptosis (Fernandes-Alnemri et al. 1994; Nicholson et al. 1995). It has been reported that p53 activation can induce apoptosis of cancer cells (Chipuk et al. 2003). Our study further supported that Nutlin-3 elevated protein expression levels of cleaved PARP and cleaved caspase-3 in SCC-9 cells, contributing to SCC-9 cell apoptosis.
Metastasis is the main cause for high mortality rate of patients with OSCC, which seriously affects the prognosis (Yan et al. 2017). It has been proved that EMT and extracellular matrix remodeling are important influencing factors for OSCC cell metastasis (Liu et al. 2019). MMPs are a family of zinc-dependent proteases, including multiple subpopulations, of which MMP2 and MMP9 can decompose proteins such as gelatin protein, fibrin and type IV collagen in extracellular matrix to promote tumor metastasis (Feng et al. 2019). EMT is a complex molecular procedure, which can endow cancer cells with migratory, invasive properties and the ability to degrade extracellular matrix (Acloque et al. 2009; Zeisberg and Neilson 2009). Yao et al. indicates that downregulation of p53 suppresses metastasis in OSCC (Yao et al. 2019). Besides, inhibition of p53 reduces E-cadherin expression and elevates N-cadherin, vimentin and α-SMA expression in OSCC cells, indicating that p53 can repress EMT in OSCC (Ouyang et al. 2017). In the present study, Nutlin-3 markedly suppressed the metastatic ability and downregulated MMP2, MMP9, N-cadherin and vimentin expression in SCC-9 cells. Chemoresistance is one of the major hurdles in OSCC therapy and management (Huang et al. 2020). Lin et al. indicates that p53 activation can facilitate CDDP-induced apoptosis of OSCC cells. In addition, our results confirmed that Nutlin-3 obviously enhanced chemosensitivity of SCC-9 cells to CDDP, suggesting the role of Nutlin-3 in OSCC chemoresistance.
Conclusion
To sum up, it is the first time in this study to elucidate the direct effects of Nutlin-3 on the biological activities of OSCC cells, as Nutlin-3 boosted apoptosis, arrested cell proliferation and metastasis in OSCC, and enhanced cisplatin chemosensitivity of OSCC cells. The findings provided novel insights into the mechanism by which Nutlin-3 suppressed OSCC progression and enhanced the chemosensitivity of OSCC cells to cisplatin. Thus, Nutlin-3 might be a promising therapeutic option for OSCC patients.
Acknowledgements
Not applicable.
Author contribution
KZ, ZL, XD and HL designed the study, performed the experiments, analyzed the data, wrote the manuscript and revised the manuscript.
Funding
A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, 2018-87).
Data availability
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
No competing interest exists in this manuscript.
Ethical approval
Not applicable. This study only contains in vitro experiments.
Consent for publication
All authors approve to submit the manuscript to your journal for publication.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best SA, Vandenberg CJ, Abad E, Whitehead L, Guiu L, Ding S, Brennan MS, Strasser A, Herold MJ, Sutherland KD, Janic A. Consequences of Zmat3 loss in c-MYC- and mutant KRAS-driven tumorigenesis. Cell Death Dis. 2020;11:877. doi: 10.1038/s41419-020-03066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle A, Gammon L, Liang X, Costea DE, Mackenzie IC. Phenotypic plasticity determines cancer stem cell therapeutic resistance in oral squamous cell carcinoma. EBioMedicine. 2016;4:138–145. doi: 10.1016/j.ebiom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Bulsara VM, Worthington HV, Glenny AM, Clarkson JE, Conway DI, Macluskey M. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database Syst Rev. 2018;12:CD006205. doi: 10.1002/14651858.CD006205.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Cao Y, Huang J, Yan L, Lin L, Liu F, Liu F, Wu J, Qiu Y, Cai L, He B. A novel prognostic index for oral squamous cell carcinoma patients with surgically treated. Oncotarget. 2017;8:55525–55533. doi: 10.18632/oncotarget.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Maurer U, Green DR, Schuler M. Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell. 2003;4:371–381. doi: 10.1016/s1535-6108(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Feng J, Xu J, Xu Y, Xiong J, Xiao T, Jiang C, Li X, Wang Q, Li J, Li Y. CLIC1 promotes the progression of oral squamous cell carcinoma via integrins/ERK pathways. Am J Transl Res. 2019;11:557–571. [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–30764. doi: 10.1016/S0021-9258(18)47344-9. [DOI] [PubMed] [Google Scholar]
- Hainaut P, Milner J. A structural role for metal ions in the "wild-type" conformation of the tumor suppressor protein p53. Cancer Res. 1993;53:1739–1742. [PubMed] [Google Scholar]
- Hou H, Sun D, Zhang X. The role of MDM2 amplification and overexpression in therapeutic resistance of malignant tumors. Cancer Cell Int. 2019;19:216. doi: 10.1186/s12935-019-0937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhang Q, Wang Y, Chen R, Wang Y, Huang Z, Zhou G, Li H, Rui X, Jin T, Li S, Zhang Y, Huang Z. Inhibition of caspase-3-mediated GSDME-derived pyroptosis aids in noncancerous tissue protection of squamous cell carcinoma patients during cisplatin-based chemotherapy. Am J Cancer Res. 2020;10:4287–4307. [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber ER, Lowe SW. Putting p53 in context. Cell. 2017;170:1062–1078. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996;274:948–953. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- Liu X, Cao Y, Zhang Y, Zhou H, Li H. Regulatory effect of MiR103 on proliferation, EMT and invasion of oral squamous carcinoma cell through SALL4. Eur Rev Med Pharmacol Sci. 2019;23:9931–9938. doi: 10.26355/eurrev_201911_19559. [DOI] [PubMed] [Google Scholar]
- Major AG, Pitty LP, Farah CS. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013;2013:319489. doi: 10.1155/2013/319489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Jiang F, Zeng B, Wei C, Yu D. miR-222 knockdown suppresses epithelial-to-mesenchymal transitionin human oral squamous cell carcinoma. Int J Clin Exp Pathol. 2017;10:11251–11259. [PMC free article] [PubMed] [Google Scholar]
- Thomson PJ. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J Oral Pathol Med. 2018;47:803–807. doi: 10.1111/jop.12733. [DOI] [PubMed] [Google Scholar]
- Voon YL, Ahmad M, Wong PF, Husaini R, Ng WT, Leong CO, Lane DP, Khoo AS. Nutlin-3 sensitizes nasopharyngeal carcinoma cells to cisplatin-induced cytotoxicity. Oncol Rep. 2015;34:1692–1700. doi: 10.3892/or.2015.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Lu X, Yu R. lncRNA MALAT1 promotes EMT process and cisplatin resistance of oral squamous cell carcinoma via PI3K/AKT/m-TOR signal pathway. Onco Targets Ther. 2020;13:4049–4061. doi: 10.2147/OTT.S251518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Chen F, Liu F, Qiu Y, Wang J, Wu J, Bao X, Hu Z, Peng X, Lin X, Cai L, Lin L, He B. Differences in modifiable factors of oral squamous cell carcinoma in the upper and lower of oral fissure. Oncotarget. 2017;8:75094–75101. doi: 10.18632/oncotarget.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Zhou WY, He RX. Down-regulation of JMJD5 suppresses metastasis and induces apoptosis in oral squamous cell carcinoma by regulating p53/NF-κB pathway. Biomed Pharmacother. 2019;109:1994–2004. doi: 10.1016/j.biopha.2018.07.144. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yu H, Hu W. The regulation of MDM2 oncogene and its impact on human cancers. Acta Biochim Biophys Sin (shanghai) 2014;46:180–189. doi: 10.1093/abbs/gmt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Yin D, Lu Z, Wang J, Li Y, Chen X, Liang Y, Song X, Qi S, Sun B, Xie C, Meng X, Pan S, Liu J, Jiang H, Liu L. Nutlin-3 overcomes arsenic trioxide resistance and tumor metastasis mediated by mutant p53 in Hepatocellular Carcinoma. Mol Cancer. 2014;13:133. doi: 10.1186/1476-4598-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Regunath K, Jacq X, Prives C. Cisplatin causes cell death via TAB1 regulation of p53/MDM2/MDMX circuitry. Genes Dev. 2013;27:1739–1751. doi: 10.1101/gad.212258.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.