Abstract
Purpose
Migraine is a relatively common neurologic disorder. A possible link between atopic disorders and migraine has been suggested. This study investigated atopic disorders and their risks of migraine in the Korean population.
Methods
From the Korean National Health Insurance Service database, patients aged ≥ 20 years who underwent health screening between January and December of 2009 were enrolled. To evaluate the risk of migraine, Cox proportional hazards regression analyses were performed.
Results
In multivariable analysis, the atopic dermatitis group (adjusted hazard ratio [aHR], 1.28; 95% confidence interval [CI], 1.23–1.33), asthma group (aHR, 1.32; 95% CI, 1.30–1.34) and allergic rhinitis group (aHR, 1.45; 95% CI, 1.44–1.46) had significantly increased risks of migraine compared to their respective control groups (P < 0.001). The patients with 1 (aHR, 1.43; 95% CI, 1.42–1.44), 2 (aHR, 1.50; 95% CI, 1.47–1.53), and 3 (aHR, 1.64; 95% CI, 1.43–1.88) atopic disorders had significantly increased risks of migraine compared to the control group (P < 0.001).
Conclusions
Our results demonstrate that patients with atopic disorders may have increased risk of migraine and that the larger the number of concomitant atopic disorders, the higher the risk of migraine.
Keywords: Epidemiology, atopic dermatitis, allergic rhinitis, asthma, migraine
INTRODUCTION
Atopic dermatitis (AD) is a common, chronic inflammatory skin disorder that has complex pathogenesis including barrier dysfunction and immune dysregulation driven by interactions between genetic and environmental factors.1 Recently, it has been suggested that AD is associated with various systemic diseases including atopic comorbidities.2,3 The spectrum of atopic disorders includes AD, allergic rhinitis (AR), asthma, and eosinophilic esophagitis related to type 2-dominant immune response.4
Migraine is a common neurologic disorder that affects approximately 1 billion people worldwide.5 It is characterized by severe episodic unilateral headache and can be accompanied by nausea, vomiting, photophobia, and phonophobia.6 Although the pathogenesis of migraine is not clearly elucidated, it could be associated with the activation and sensitization of the trigeminovascular system, vasoactive peptides, and neurogenic inflammation.5
The possible link between atopic disorders and migraine has been suggested but controversial.7,8 The similarities between the 2 disorders are that both have [common triggering factors such as food, exercise,] as well as [environmental factors, and common inflammatory environments.]7 Several case series studies on the relationship between the 2 diseases have been reported. Moreover, studies have reported improvement of migraine after immunotherapy in patients with atopic disorders.8,9,10 Most of the previous studies were case series, and there was a limitation in that they were held in a hospital-based setting with a limited number of study participants.11,12,13 Recently, large-scale and population-based studies have been published; however, most of them were limited to children or targeted only asthma or AR.14,15,16
In this study, we investigated the relationship between atopic disorders and risk of migraine in the Korean population using the National Health Insurance System (NHIS) claims database.
MATERIALS AND METHODS
Data source
The Korean NHIS database is managed by the Korean government and covers approximately 97% of the Korean population. Medical aid program covers the remaining 3% of the population with low income, which has been integrated into the NHIS database. The Korean population is encouraged to undergo a regular health check-up every 2 years, and their medical care needs are monitored throughout the life cycle. The health screening database of the Korean NHIS was used in this study. The NHIS data are based on the International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes.
Study population
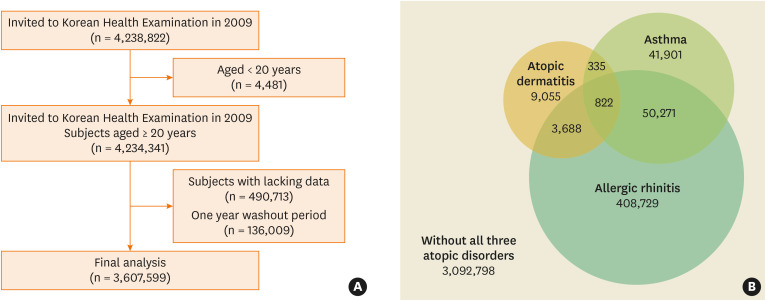
From the NHIS database, patients aged ≥ 20 years who underwent health screening from January and December of 2009 were included. In addition, patients diagnosed with migraine prior to enrollment were excluded. Finally, 3,607,599 subjects included in this study were at ≥ 20 years of age had visited a clinic or a hospital at least 3 times during the study period, and had ICD-10 diagnostic code for either AD (L20), AR (J301–304), or asthma (J45–46) (Figure A).17 Subjects with AD (n = 13,900), asthma (n = 93,329) or AR (n = 463,510) and controls with none of the 3 atopic disorders (n = 3,092,798), controls without AD (n = 3,593,699), controls without asthma (n = 3,514,270), and controls without AR (n = 3,144,089) were analyzed (Figure B). The subjects were followed up from 2009 to 2019 to identify newly diagnosed migraine (G43).
Figure. (A) Flowchart of enrolled subjects. (B) Distribution of the study population enrolled in the study.
Data and baseline comorbidities
Information on height (m), weight (kg), waist circumference (cm), systolic blood pressure, diastolic blood pressure, fasting plasma glucose, total cholesterol, low-density lipoprotein, high-density lipoprotein, body mass index (BMI) (kg/m2), smoking status (non-smoker, ex-smoker, or current smoker), alcohol consumption (non-drinker, mild drinker [< 30 g/day], or heavy drinker [≥ 30 g/day]), physical activity (yes/no), and socioeconomic status (dichotomized as ≤ 20% vs. > 20% of the median) were obtained from the NHIS health check-up data. Comorbid diseases including hypertension, dyslipidemia, diabetes mellitus were defined based on both ICD-10 codes and prescribed medications, and myocardial infarction (MI), ischemic stroke, anxiety disorder, bipolar disorder and depression were defined based on ICD-10 codes during the study period.
Statistical analyses and ethics statement
The baseline demographic characteristics of the study population are described as numbers and percentages or as means ± standard deviations (SDs). Differences in clinical characteristics according to the presence of concurrent atopic disorders were analyzed using Student’s t-test or χ2 test. To evaluate the risk for migraine, Cox proportional hazards regression analyses were performed. Multivariable hazard ratio (HR) and 95% confidence interval (CI) were calculated to assess the risk of migraine after adjusting for confounding factors. Model 1 was not adjusted. Model 2 was adjusted for age and sex. Model 3 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, and dyslipidemia. Model 4 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, MI and ischemic stroke. Model 5 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, anxiety disorder, bipolar disorder and depression. Model 6 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, MI, ischemic stroke anxiety disorder, bipolar disorder and depression. Subgroup analyses were performed according to age and sex. Statistical analyses were performed using SAS software ver. 9.4 (SAS Institute, Cary, NC, USA). Two-sided P < 0.05 was considered to indicate statistical significance. This study was approved by the Ethics Committee of Seoul St. Mary’s Hospital, The Catholic University of Korea (IRB No. KC21ZISI0784) and conducted in accordance with the principles of the Declaration of Helsinki.
RESULTS
Baseline characteristics of the study population
Subjects with AD (n = 13,900), asthma (n = 93,329), or AR (n = 463,510), and controls with none of the 3 atopic disorders (n = 3,092,798), controls without AD (n = 3,593,699), controls without asthma (n = 3,514,270), and controls without AR (n = 3,144,089) were included and the baseline clinical characteristics are summarized in Table 1.
Table 1. Characteristics of the study population.
| Characteristics | Atopic dermatitis | Asthma | Allergic rhinitis | One or more atopic disorders | |||||
|---|---|---|---|---|---|---|---|---|---|
| No (n = 3,593,699) | Yes (n = 13,900) | No (n = 3,514,270) | Yes (n = 93,329) | No (n = 3,144,089) | Yes (n = 463,510) | No (n = 3,092,798) | Yes (n = 514,801) | ||
| Age group (yr) | |||||||||
| 20–29 | 469,110 (13.05) | 2,136 (15.37) | 467,702 (13.31) | 3,544 (3.8) | 428,854 (13.64) | 42,392 (9.15) | 426,005 (13.77) | 45,241 (8.79) | |
| 30–39 | 721,950 (20.09) | 2,246 (16.16) | 715,569 (20.36) | 8,627 (9.24) | 641,454 (20.4) | 82,742 (17.85) | 637,098 (20.60) | 87,098 (16.92) | |
| 40–49 | 950,159 (26.44) | 2,662 (19.15) | 936,587 (26.65) | 16,234 (17.39) | 834,193 (26.53) | 118,628 (25.59) | 826,472 (26.72) | 126,349 (24.54) | |
| 50–59 | 741,520 (20.63) | 2,746 (19.76) | 723,942 (20.60) | 20,324 (21.78) | 642,813 (20.45) | 101,453 (21.89) | 632,269 (20.44) | 111,997 (21.76) | |
| 60–60 | 463,126 (12.89) | 2,427 (17.46) | 441,809 (12.57) | 23,744 (25.44) | 389,684 (12.39) | 75,869 (16.37) | 376,572 (12.18) | 88,981 (17.28) | |
| ≥ 70 | 247,834 (6.90) | 1,683 (12.11) | 228,661 (6.51) | 20,856 (22.35) | 207,091 (6.59) | 42,426 (9.15) | 194,382 (6.28) | 55,135 (10.71) | |
| Sex | |||||||||
| Male | 2,080,158 (57.88) | 7,246 (52.13) | 2,044,787 (58.19) | 42,617 (45.66) | 1,868,233 (59.42) | 219,171 (47.29) | 1,843,330 (59.60) | 244,074 (47.41) | |
| Female | 1,513,541 (42.12) | 6,654 (47.87) | 1,469,483 (41.81) | 50,712 (54.34) | 1,275,856 (40.58) | 244,339 (52.71) | 1,249,468 (40.40) | 270,727 (52.59) | |
| Smoking | |||||||||
| Non | 2,059,360 (57.30) | 8,542 (61.45) | 2,005,490 (57.07) | 62,412 (66.87) | 1,762,109 (56.05) | 305,793 (65.97) | 1,729,053 (55.91) | 338,849 (65.82) | |
| Ex | 535,803 (14.91) | 2,201 (15.83) | 523,606 (14.90) | 14,398 (15.43) | 465,456 (14.80) | 72,548 (15.65) | 457,678 (14.80) | 80,326 (15.60) | |
| Current | 998,536 (27.79) | 3,157 (22.71) | 985,174 (28.03) | 16,519 (17.70) | 916,524 (29.15) | 85,169 (18.37) | 906,067 (29.30) | 95,626 (18.58) | |
| Drinker | |||||||||
| Non | 1,780,130 (49.53) | 7,920 (56.98) | 1,726,048 (49.12) | 62,002 (66.43) | 1,521,279 (48.39) | 266,771 (57.55) | 1,487,913 (48.11) | 300,137 (58.30) | |
| Mild | 1,512,417 (42.09) | 5,132 (36.92) | 1,491,070 (42.43) | 26,479 (28.37) | 1,347,608 (42.86) | 169,941 (36.66) | 1,332,736 (43.09) | 184,813 (35.90) | |
| Heavy | 301,152 (8.38) | 848 (6.10) | 297,152 (8.46) | 4,848 (5.19) | 275,202 (8.75) | 26,798 (5.78) | 272,149 (8.80) | 29,851 (5.80) | |
| Exercise | 653,557 (18.19) | 2,597 (18.68) | 638,804 (18.18) | 17,350 (18.59) | 566,712 (18.02) | 89,442 (19.30) | 557,562 (18.03) | 98,592 (19.15) | |
| BMI (kg/m2) | |||||||||
| < 18.5 | 134,279 (3.74) | 548 (3.94) | 131,395 (3.74) | 3,432 (3.68) | 118,292 (3.76) | 16,535 (3.57) | 116,183 (3.76) | 18,644 (3.62) | |
| < 23 | 1,403,983 (39.07) | 5,455 (39.24) | 1,377,866 (39.21) | 31,572 (33.83) | 1,232,384 (39.20) | 177,054 (38.20) | 1,214,618 (39.27) | 194,820 (37.84) | |
| < 25 | 884,770 (24.62) | 3,330 (23.96) | 865,206 (24.62) | 22,894 (24.53) | 771,881 (24.55) | 116,219 (25.07) | 759,651 (24.56) | 128,449 (24.95) | |
| < 30 | 1,043,730 (29.04) | 4,077 (29.33) | 1,017,067 (28.94) | 30,740 (32.94) | 910,762 (28.97) | 137,045 (29.57) | 894,176 (28.91) | 153,631 (29.84) | |
| ≥ 30 | 126,937 (3.53) | 490 (3.53) | 122,736 (3.49) | 4,691 (5.03) | 110,770 (3.52) | 16,657 (3.59) | 108,170 (3.50) | 19,257 (3.74) | |
| Diabetes | 232,473 (6.47) | 1,086 (7.81) | 224,790 (6.40) | 8,769 (9.40) | 202,353 (6.44) | 31,206 (6.73) | 197,256 (6.38) | 36,303 (7.05) | |
| Hypertension | 561,854 (15.63) | 2,325 (16.73) | 544,239 (15.49) | 19,940 (21.37) | 491,570 (15.63) | 72,609 (15.67) | 480,211 (15.53) | 83,968 (16.31) | |
| Dyslipidemia | 456,536 (12.70) | 2,060 (14.82) | 443,463 (12.62) | 15,133 (16.21) | 395,164 (12.57) | 63,432 (13.69) | 386,906 (12.51) | 71,690 (13.93) | |
| MI | 14,519 (0.40) | 109 (0.78) | 13,604 (0.39) | 1,024 (1.10) | 12,085 (0.38) | 2,543 (0.55) | 11,515 (0.37) | 3,113 (0.60) | |
| Stroke | 50,924 (1.42) | 372 (2.68) | 47,921 (1.36) | 3,375 (3.62) | 42,315 (1.35) | 8,981 (1.94) | 40,379 (1.31) | 10,917 (2.12) | |
| Anxiety | 196,179 (5.46) | 1,359 (9.78) | 184,286 (5.24) | 13,252 (14.20) | 151,824 (4.83) | 45,714 (9.86) | 145,376 (4.70) | 52,162 (10.13) | |
| Bipolar disorder | 5,533 (0.15) | 36 (0.26) | 5,310 (0.15) | 259 (0.28) | 4,673 (0.15) | 896 (0.19) | 4,520 (0.15) | 1,049 (0.20) | |
| Depression | 94,862 (2.64) | 702 (5.05) | 89,292 (2.54) | 6,272 (6.72) | 74,797 (2.38) | 20,767 (4.48) | 71,707 (2.32) | 23,857 (4.63) | |
| Age (yr) | 46.50 ± 14.02 | 48.88 ± 15.96 | 46.23 ± 13.91 | 56.87 ± 14.33 | 46.15 ± 13.98 | 48.94 ± 14.11 | 45.96 ± 13.88 | 49.78 ± 14.43 | |
| BMI (kg/m2) | 23.70 ± 3.48 | 23.67 ± 3.28 | 23.69 ± 3.48 | 24.12 ± 3.43 | 23.69 ± 3.51 | 23.76 ± 3.22 | 23.68 ± 3.51 | 23.79 ± 3.25 | |
| WC (cm) | 80.30 ± 9.48 | 80.43 ± 9.53 | 80.25 ± 9.46 | 82.21 ± 9.90 | 80.30 ± 9.46 | 80.25 ± 9.55 | 80.27 ± 9.45 | 80.46 ± 9.62 | |
| SBP (mmHg) | 122.43 ± 15.00 | 122.55 ± 15.22 | 122.37 ± 14.98 | 124.83 ± 15.78 | 122.50 ± 15.00 | 121.96 ± 14.98 | 122.46 ± 14.98 | 122.30 ± 15.12 | |
| DBP (mmHg) | 76.34 ± 10.05 | 76.10 ± 9.98 | 76.32 ± 10.05 | 76.91 ± 10.10 | 76.41 ± 10.07 | 75.83 ± 9.95 | 76.40 ± 10.07 | 75.96 ± 9.97 | |
| FPG (mg/dL) | 97.26 ± 24.08 | 97.41 ± 24.55 | 97.19 ± 24.05 | 99.83 ± 25.17 | 97.27 ± 24.25 | 97.24 ± 22.95 | 97.22 ± 24.22 | 97.54 ± 23.27 | |
| TC (mg/dL) | 194.90 ± 41.37 | 195.53 ± 43.20 | 194.83 ± 41.30 | 197.62 ± 43.96 | 194.84 ± 41.30 | 195.29 ± 41.83 | 194.80 ± 41.26 | 195.47 ± 42.01 | |
| HDL (mg/dL) | 56.37 ± 32.68 | 57.50 ± 35.44 | 56.37 ± 32.53 | 56.78 ± 38.28 | 56.36 ± 32.00 | 56.48 ± 37.00 | 56.35 ± 31.91 | 56.52 ± 36.99 | |
| LDL (mg/dL) | 121.03 ± 217.52 | 123.54 ± 262.05 | 121.10 ± 219.77 | 118.79 ± 115.77 | 121.32 ± 224.42 | 119.18 ± 165.14 | 121.33 ± 225.22 | 119.28 ± 165.54 | |
| TG* (mg/dL) | 112.73 (112.67–112.8) | 112.40 (111.33–113.47) | 112.65 (112.58–112.72) | 116.06 (115.65–116.46) | 113.02 (112.94–113.09) | 110.83 (110.65–111.01) | 112.96 (112.89–113.03) | 111.39 (111.22–111.56) | |
Values are presented as number (%) or mean ± standard deviation.
BMI, body mass index; MI, myocardial infarction; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides
*Data were log-transformed before analysis.
Risk of migraine in patients with atopic disorders
Table 2 shows the incidence rates of migraine and the risk of migraine in the presence of atopic disorders. The incidence rates of migraine per 1,000 person-years were 23.06 and 16.49 in the AD group vs control group without AD, 27.12 and 16.25 in the asthma group vs control group without asthma, and 25.23 and 15.29 in the AR group vs control group without AR.
Table 2. Risks and incidence rates of migraine in patients with atopic disorders.
| Characteristics | Number | Migraine | Person-years | IR | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atopic dermatitis | |||||||||||
| No | 3,593,699 | 505,738 | 30,673,370 | 16.49 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 13,900 | 2,625 | 113,815 | 23.06 | 1.40 (1.35–1.46) | 1.32 (1.28–1.38) | 1.32 (1.27–1.38) | 1.32 (1.27–1.37) | 1.28 (1.23–1.33) | 1.28 (1.23–1.33) | |
| Asthma | |||||||||||
| No | 3,514,270 | 488,551 | 30,056,578 | 16.25 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 93,329 | 19,812 | 730,607 | 27.12 | 1.67 (1.65–1.693) | 1.39 (1.37–1.41) | 1.39 (1.37–1.41) | 1.38 (1.36–1.40) | 1.32 (1.30–1.34) | 1.32 (1.30–1.34) | |
| Allergic rhinitis | |||||||||||
| No | 3,144,089 | 412,756 | 26,998,339 | 15.29 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 463,510 | 95,607 | 3,788,846 | 25.23 | 1.65 (1.64–1.66) | 1.50 (1.49–1.51) | 1.50 (1.48–1.51) | 1.49 (1.48–1.50) | 1.45 (1.44–1.46) | 1.45 (1.44–1.46) | |
| One or more atopic disorders | |||||||||||
| No | 3,092,798 | 402,939 | 26,594,549 | 15.15 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 514,801 | 105,424 | 4,192,636 | 25.15 | 1.66 (1.65–1.67) | 1.49 (1.48–1.50) | 1.49 (1.48–1.50) | 1.49 (1.48–1.50) | 1.44 (1.43–1.45) | 1.44 (1.43–1.45) | |
Model 1 was not adjusted.
Model 2 was adjusted for age and sex.
Model 3 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, and dyslipidemia.
Model 4 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, myocardial infarction and stroke.
Model 5 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, anxiety disorder, bipolar disorder and depression.
Model 6 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, myocardial infarction, stroke, anxiety disorder, bipolar disorder and depression.
IR, incidence rate (per 1,000 person-years).
In multivariable Cox proportional hazards regression analyses after adjusting for age, sex, smoking, drinking, physical activity, hypertension, diabetes, and dyslipidemia (model 3), the AD group (HR, 1.32; 95% CI, 1.27–1.38), asthma group (HR, 1.39; 95% CI, 1.37–1.41) and AR group (HR, 1.50; 95% CI, 1.48–1.51) showed a significantly increased risk of migraine compared to their respective control groups (P < 0.001). In model 4 which was additionally adjusted for MI and ischemic stroke, the results were similar to those of model 3. After additionally adjusting for mental disorders in model 6, the risk of migraine was slightly lower than in models 3 and 4, but significantly higher than in the AD group (HR, 1.28; 95% CI, 1.23–1.33), asthma group (HR, 1.32; 95% CI, 1.30–1.34) and AR group (HR, 1.45; 95% CI, 1.44–1.46) compared to their respective control group (P < 0.001).
Risk of migraine according to concurrent atopic disorders
Tables 3 and 4 show the risk of migraine in the presence of concurrent atopic disorders. In multivariable Cox proportional hazards regression analyses (model 4), the aHRs of migraine in the presence of 1, 2, and 3 atopic disorders were 1.48 (95% CI, 1.46–1.49), 1.58 (95% CI, 1.56–1.61), and 1.79 (95% CI, 1.56–2.06), respectively (P < 0.001) (Table 3). In model 6, the aHRs were 1.43 (95% CI, 1.42–1.44), 1.50 (95% CI, 1.47–1.53), and 1.64 (95% CI, 1.43–1.88), respectively (P < 0.001). Among them, the risk of migraine was the highest in patients with both AD and asthma (model 6: aHR, 1.79; 95% CI, 1.45–2.22) (P < 0.001) (Table 4).
Table 3. Risks and incidence rates of migraine according to concurrent atopic disorders.
| No. of atopic disorders* | Number | Migraine | Person-years | IR | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3,092,798 | 402,939 | 26,594,549 | 15.15 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 1 | 459,685 | 93,007 | 3,758,349 | 24.75 | 1.63 (1.62–1.65) | 1.48 (1.47–1.49) | 1.48 (1.47–1.49) | 1.48 (1.46–1.49) | 1.43 (1.42–1.44) | 1.43 (1.42–1.44) |
| 2 | 54,294 | 12,214 | 427,942 | 28.54 | 1.89 (1.85–1.92) | 1.59 (1.57–1.62) | 1.59 (1.56–1.62) | 1.58 (1.56–1.61) | 1.50 (1.48–1.53) | 1.50 (1.47–1.53) |
| 3 | 822 | 203 | 6,345 | 31.99 | 2.12 (1.84–2.43) | 1.81 (1.58–2.08) | 1.80 (1.57–2.07) | 1.79 (1.56–2.06) | 1.65 (1.44–1.89) | 1.64 (1.43–1.88) |
Model 1 was not adjusted.
Model 2 was adjusted for age and sex.
Model 3 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, and dyslipidemia.
Model 4 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, myocardial infarction and stroke.
Model 5 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, anxiety disorder, bipolar disorder and depression.
Model 6 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, myocardial infarction, stroke, anxiety disorder, bipolar disorder and depression.
IR, incidence rate (per 1,000 person-years).
*Number of atopic disorders per person.
Table 4. Risks and incidence rates of migraine according to combinations of atopic disorders.
| Atopic dermatitis | Asthma | Allergic rhinitis | Number | Migraine | Person-years | IR | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | No | No | 3,092,798 | 402,939 | 26,594,549 | 15.15 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 408,729 | 83,273 | 3,357,057 | 24.81 | 1.64 (1.63–1.65) | 1.50 (1.49–1.51) | 1.49 (1.48–1.51) | 1.49 (1.48–1.50) | 1.45 (1.44–1.46) | 1.45 (1.44–1.46) | ||
| Yes | No | 41,901 | 8,228 | 325,747 | 25.26 | 1.67 (1.63–1.71) | 1.36 (1.33–1.39) | 1.36 (1.33–1.39) | 1.35 (1.32–1.38) | 1.30 (1.27–1.33) | 1.30 (1.27–1.33) | |
| Yes | 50,271 | 11,298 | 396,017 | 28.53 | 1.88 (1.85–1.92) | 1.58 (1.55–1.61) | 1.58 (1.55–1.61) | 1.57 (1.55–1.60) | 1.49 (1.47–1.52) | 1.49 (1.46–1.52) | ||
| Yes | No | No | 9,055 | 1,506 | 75,545 | 19.94 | 1.32 (1.25–1.39) | 1.26 (1.19–1.32) | 1.25 (1.19–1.32) | 1.25 (1.19–1.32) | 1.23 (1.17–1.29) | 1.23 (1.17–1.29) |
| Yes | 3,688 | 833 | 29,427 | 28.31 | 1.87 (1.75–2.00) | 1.70 (1.59–1.82) | 1.69 (1.58–1.81) | 1.69 (1.58–1.81) | 1.61 (1.50–1.72) | 1.60 (1.50–1.72) | ||
| Yes | No | 335 | 83 | 2,498 | 33.23 | 2.20 (1.77–2.73) | 1.91 (1.54–2.36) | 1.90 (1.53–2.36) | 1.89 (1.53–2.35) | 1.80 (1.45–2.23) | 1.79 (1.45–2.22) | |
| Yes | 822 | 203 | 6,345 | 31.99 | 2.12 (1.84–2.43) | 1.81 (1.58–2.07) | 1.80 (1.57–2.07) | 1.79 (1.56–2.06) | 1.65 (1.44–1.89) | 1.64 (1.43–1.88) |
Model 1 was not adjusted.
Model 2 was adjusted for age and sex.
Model 3 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, and dyslipidemia.
Model 4 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, myocardial infarction and stroke.
Model 5 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, anxiety disorder, bipolar disorder and depression.
Model 6 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, dyslipidemia, myocardial infarction, stroke, anxiety disorder, bipolar disorder and depression.
IR, incidence rate (per 1,000 person-years).
Risk of migraine in subgroups by age and sex
As a result of analyzing the age subgroups according to atopic disorders, the risk of migraine was the highest in patients in their 30s and 40s in those with asthma, AR, and 1 or more atopic disorders (Supplementary Table S1). In the AD group, the risk was the highest in those in their 30s, but there was no statistical significance. Subgroup analysis by sex revealed a significantly higher risk of migraine in men for all 3 atopic disorders (Supplementary Table S2).
Follow-up duration until the diagnosis of migraine in patients with atopic disorders
As a result of analyzing the migraine onset time difference in patients with atopic disorders, mean duration (mean ± SD) was 8.14 ± 2.54 in patients with 1 or more atopic disorders, and the asthma group showed the shortest onset duration (7.83 ± 2.78) (Supplementary Table S3). The mean onset duration were 8.18 ± 2.51, 7.88 ± 2.75, and 7.72 ± 2.78, respectively compared to controls in the presence of 1, 2, and 3 atopic disorders.
DISCUSSION
In this nationwide population-based cohort study, we found significantly increased risk of migraine among adult patients with AD, asthma, or AR compared to their respective control group. The risk of migraine was significantly increased in patients with a larger number of concurrent atopic disorders. Notably, patients with concurrent AD and asthma showed the highest risk compared to controls without any atopic disorder. In this study, various lifestyle habits and comorbidities that could potentially be related to migraine development were adjusted as variable, and we observed that lifestyle habits (smoking status, alcohol consumption, physical activity) and mental disorder (anxiety disorder, bipolar disorder and depression) had a more significant effect on the risk of migraine.
Although there is ongoing debate about a possible link between atopic disorders and migraine, several studies have demonstrated an association between the 2.7,18,19 Arguments about correlation between the 2 disorders began with a report that food allergy could trigger migraine.20 Previous reports have shown that allergens are associated with migraine, and that symptoms of migraine improve after immunotherapy, food avoidance, or anti-allergic therapies in patients with both migraine and allergy.8,9,20,21,22 There are also reports suggesting that patients with migraine have more personal or family history of atopic disorders, and that the incidence of migraine is increased in allergic patients.12,13,23,24,25 However, most of the previous studies have limitations in that they are case reports, questionnaire studies, or single-institution studies, and that they included a limited number of study participants.11,12,26 Recently, population-based studies have been performed to overcome these limitations whereas most of them have still targeted children and adolescents or have examined the correlation between migraine and atopic disorders other than AD.15,27 Previous studies have evaluated the risk of migraine in children with atopic disorders, and the increased risk of migraines has been explained by several stimuli in the brain development process caused by allergic diseases.27 However, in this study, we analyzed the risk of migraine in patients older than 20 years to determine the effect of atopic disorders on the risk of migraine in adults. This study is meaningful in that it is a large-scale, population-based study that analyzed the correlation between migraine and atopic disorders including AD in the adult population, which has not previously been conducted.
In a recent questionnaire study of an adult population, mild (adjusted relative risk ratio [aRRR], 1.50; 95% CI, 1.38–1.64) and severe migraine (aRRR, 1.80; 95% CI, 1.50–2.17) were associated with AD.18 Previous epidemiological studies in children suggested that atopic disorders at a pediatric age can affect brain development and increase the risk of migraine.15,27 A population-based study in Taiwan found higher association of migraine and AR (adjusted odds ratio [aOR], 2.17; 95% CI, 2.09–2.26), asthma (aOR, 1.76; 95% CI, 1.66–1.87), and AD (aOR, 1.74; 95% CI, 1.58–1.93) compared to controls in children aged 7 to 18 years.27 According to a previous study, boys showed greater risk of migraine than girls.27 Moreover, the risk of migraine increased with the number of concurrent atopic disorders, in consistent with our results.27
Although the exact mechanisms explaining the link between atopic disorders and migraine have not yet been elucidated, there is some evidence that the 2 diseases share a pathogenic process. First, the inflammatory environments in atopic disorders and migraine have similarities. Studies have suggested alteration in brain excitability, sensitization of the trigeminovascular system, dilatation of intracranial arteries, and sterile neuroinflammation as the main pathogenesis of migraine.28,29 There is some evidence that calcitonin gene-related peptide (CGRP) which plays a critical role in the pathophysiology of migraine, induces Langerhans cell cytokine polarization favoring T helper 2 cell recruitment, up-regulation of interleukin 4, and mast cell degranulation, and CGRP is increased in AD lesions.30,31,32,33 In addition, inflammatory mediators such as histamine, leukotrienes, and prostaglandins are elevated in migraine attack, which are also important immune modulators in atopic disorders.15,27
Previous studies have shown that allergen triggered allergic responses release inflammatory mediators or elevate neuropeptide mediated by mast cells, inducing airway hypersensitivity in patients with asthma.6 In migraine, mast cells in the dura matter may secrete pro-inflammatory and vasodilatory molecules, which could activate the trigeminal pain pathway underlying migraine pathogenesis.7,14 Furthermore, parasympathetic hyperactivity is considered to be involved in both asthma and migraine.6,24 Cholinergic stimulation could [trigger bronchospasm in asthma, and could activate trigeminal pain pathway] as well as [induce degranulation of meningeal mast cell in migraine.]6
Nasal congestion, discharge and sneezing in AR require sensitive trigeminal transmission which is associated with migraine, and the inflammatory response that occurs in AR are suggested to be involved in migraine aggravation and development through activation of immune responses.11
Secondly, atopic disorders and migraine have several common risk factors and comorbidities, which can serve as a link between the 2 disorders. Atopic disorders are associated with mental health-related diseases such as depression, anxiety, attention deficit and hyperactivity disorder, and sleep disorder, which are suggested to have a bidirectional association with migraine.34,35,36,37,38,39,40,41,42 Although the mechanism of these associations needs to be clarified, it can be explained by imbalance of the dopaminergic system and serotonin neurotransmitters, ovarian hormone fluctuation, altered autonomic regulation, and somatization.42,43 A recent large genome-wide study has shown that migraine has a genetic correlation with psychiatric disorders, which suggests a common genetic basis or shared pathways between the 2.42 Furthermore, interaction might be explained as a stressor that significantly increases the risk of each other, and stress as a trigger can induce migraine attacks, escalating in frequency and shortening the intervals of migraine.44
In subgroup analyses by age, no significant correlation was observed in patients with AD, whereas patients with asthma or AR showed the highest risk of migraine in those in their 30s and 40s. In subgroup analyses by sex, the risk of migraine was higher in both sexes with atopic disorders. However, the increased risk of migraine was higher in men than in women. Although this population-based study analyzed temporal associations between 2 disorders, whether sex and age affect the risk of migraine in patients with atopic disorders and the exact mechanisms need further research.
There are several limitations to this study. First, diagnosis of atopic disorders and migraine was made on the basis of NHIS claim data, without direct review of medical records. Secondly, some patients included in this study may have had migraine before enrollment at this study. In addition, since only patients who visited the hospital and were diagnosed were included in the study, patients who did not visit the hospital were not included, imparting possibility of selection bias. Thirdly, NHIS database records lack detailed information about family history, severity of diseases, presence of aura, and type of migraine.
Despite these limitations, the strengths of our study are its large sample size and nationally representative study population. The NHIS data used in this study are managed by the Korean government to provide public health information, indicating data stability. Furthermore, different from other studies, we controlled for confounding factors of BMI, smoking status, alcohol consumption status, physical activity level and various comorbidities which could affect the development of migraine.
Taken together, our results demonstrated that patients with atopic disorders may have an increased risk of migraine, and that the larger the number of concomitant atopic disorders, the higher the risk of migraine.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Risk of migraine in atopic subgroups stratified according to age group
Risk of migraine in atopic subgroups stratified according to sex
Follow-up duration until the migraine onset in each patient with atopic disorders
References
- 1.Ravnborg N, Ambikaibalan D, Agnihotri G, Price S, Rastogi S, Patel KR, et al. Prevalence of asthma in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2021;84:471–478. doi: 10.1016/j.jaad.2020.02.055. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250–258. doi: 10.1684/ejd.2019.3557. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144–151. doi: 10.1016/j.anai.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Bellanti JA, Settipane RA. The atopic disorders and atopy … “strange diseases” now better defined! Allergy Asthma Proc. 2017;38:241–242. doi: 10.2500/aap.2017.38.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashina M. Migraine. N Engl J Med. 2020;383:1866–1876. doi: 10.1056/NEJMra1915327. [DOI] [PubMed] [Google Scholar]
- 6.Ozge A, Ozge C, Oztürk C, Kaleagasi H, Ozcan M, Yalçinkaya DE, et al. The relationship between migraine and atopic disorders-the contribution of pulmonary function tests and immunological screening. Cephalalgia. 2006;26:172–179. doi: 10.1111/j.1468-2982.2005.01021.x. [DOI] [PubMed] [Google Scholar]
- 7.Özge A, Uluduz D, Bolay H. Co-occurrence of migraine and atopy in children and adolescents: myth or a casual relationship? Curr Opin Neurol. 2017;30:287–291. doi: 10.1097/WCO.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 8.Aupiais C, Wanin S, Romanello S, Spiri D, Moretti R, Boizeau P, et al. Association between migraine and atopic diseases in childhood: a potential protective role of anti-allergic drugs. Headache. 2017;57:612–624. doi: 10.1111/head.13032. [DOI] [PubMed] [Google Scholar]
- 9.Theodoropoulos DS, Katzenberger DR, Jones WM, Morris DL, Her C, Cullen NA, et al. Allergen-specific sublingual immunotherapy in the treatment of migraines: a prospective study. Eur Rev Med Pharmacol Sci. 2011;15:1117–1121. [PubMed] [Google Scholar]
- 10.Mitchell N, Hewitt CE, Jayakody S, Islam M, Adamson J, Watt I, et al. Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr J. 2011;10:85. doi: 10.1186/1475-2891-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forcelini CM, Ramos M, Santos IF, Brackmann G, Bernardon LG, Corbellini AP, et al. The influence of allergic rhinoconjunctivitis on migraine disability in children. Arq Neuropsiquiatr. 2019;77:418–423. doi: 10.1590/0004-282X20190058. [DOI] [PubMed] [Google Scholar]
- 12.Turan MO, Susuz CC, Turan PA. Presence of headache and migraine in asthma patients. Turk Thorac J. 2017;18:47–51. doi: 10.5152/TurkThoracJ.2017.16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ku M, Silverman B, Prifti N, Ying W, Persaud Y, Schneider A. Prevalence of migraine headaches in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:226–230. doi: 10.1016/S1081-1206(10)60018-X. [DOI] [PubMed] [Google Scholar]
- 14.Peng YH, Chen KF, Liao WC, Hsia TC, Chen HJ, Yin MC, et al. Association of migraine with asthma risk: a retrospective population-based cohort study. Clin Respir J. 2018;12:1030–1037. doi: 10.1111/crj.12623. [DOI] [PubMed] [Google Scholar]
- 15.Wang IC, Tsai JD, Shen TC, Lin CL, Li TC, Wei CC. Allergic conjunctivitis and the associated risk of migraine among children: a nationwide population-based cohort study. Ocul Immunol Inflamm. 2017;25:802–810. doi: 10.1080/09273948.2016.1178303. [DOI] [PubMed] [Google Scholar]
- 16.Graif Y, Shohat T, Machluf Y, Farkash R, Chaiter Y. Association between asthma and migraine: a cross-sectional study of over 110 000 adolescents. Clin Respir J. 2018;12:2491–2496. doi: 10.1111/crj.12939. [DOI] [PubMed] [Google Scholar]
- 17.Han JH, Bang CH, Han K, Ryu JY, Lee JY, Park YM, et al. The risk of psoriasis in patients with allergic diseases: a nationwide population-based cohort study. Allergy Asthma Immunol Res. 2021;13:638–645. doi: 10.4168/aair.2021.13.4.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirnova J, Montgomery S, Lindberg M, Svensson Å, von Kobyletzki L. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi: 10.1186/s12895-020-00117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shreberk-Hassidim R, Hassidim A, Gronovich Y, Dalal A, Molho-Pessach V, Zlotogorski A. Atopic dermatitis in Israeli adolescents from 1998 to 2013: trends in time and association with migraine. Pediatr Dermatol. 2017;34:247–252. doi: 10.1111/pde.13084. [DOI] [PubMed] [Google Scholar]
- 20.Kuzemko JA, Grant EC. Food allergy and migraine. Lancet. 1979;2:358–359. doi: 10.1016/s0140-6736(79)90368-4. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro RS, Eisenberg BC. Allergic headache. Ann Allergy. 1965;23:123–126. [PubMed] [Google Scholar]
- 22.Zhao Z, Jin H, Yin Y, Hou Y, Wang J, Tang C, et al. Association of migraine with its comorbidities and food specific immunoglobulin G antibodies and inflammatory cytokines: cross-sectional clinical research. J Pain Res. 2021;14:2359–2368. doi: 10.2147/JPR.S316619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davey G, Sedgwick P, Maier W, Visick G, Strachan DP, Anderson HR. Association between migraine and asthma: matched case-control study. Br J Gen Pract. 2002;52:723–727. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Deng ZR, Zu MD, Zhang J, Wang Y. The comorbid relationship between migraine and asthma: a systematic review and meta-analysis of population-based studies. Front Med (Lausanne) 2021;7:609528. doi: 10.3389/fmed.2020.609528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desalu OO, Sanya EO, Adeoti AO, Ojuawo OB, Aladesanmi AO, Olarinoye JK, et al. Increased risk of migraine among students with asthma: results of headache and asthma study among university students. West Afr J Med. 2022;39:256–261. [PubMed] [Google Scholar]
- 26.Muñoz-Jareño N, Fernández-Mayoralas DM, Martínez-Cervell C, Campos-Castelló J. Relationship between migraine and atopy in childhood: a retrospective case-control study. Rev Neurol. 2011;53:713–720. [PubMed] [Google Scholar]
- 27.Wei CC, Lin CL, Shen TC, Chen AC. Children with allergic diseases have an increased subsequent risk of migraine upon reaching school age. J Investig Med. 2018;66:1064–1068. doi: 10.1136/jim-2018-000715. [DOI] [PubMed] [Google Scholar]
- 28.Schwedt TJ. Chronic migraine. BMJ. 2014;348:g1416. doi: 10.1136/bmj.g1416. [DOI] [PubMed] [Google Scholar]
- 29.Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol. 2017;264:2031–2039. doi: 10.1007/s00415-017-8434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granstein RD, Wagner JA, Stohl LL, Ding W. Calcitonin gene-related peptide: key regulator of cutaneous immunity. Acta Physiol (Oxf) 2015;213:586–594. doi: 10.1111/apha.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16:76–87. doi: 10.1016/S1474-4422(16)30293-9. [DOI] [PubMed] [Google Scholar]
- 32.Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the trigeminal system in migraine. Headache. 2019;59:659–681. doi: 10.1111/head.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voisin T, Bouvier A, Chiu IM. Neuro-immune interactions in allergic diseases: novel targets for therapeutics. Int Immunol. 2017;29:247–261. doi: 10.1093/intimm/dxx040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng BT, Silverberg JI. Depression and psychological distress in US adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:179–185. doi: 10.1016/j.anai.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Paller A, Jaworski JC, Simpson EL, Boguniewicz M, Russell JJ, Block JK, et al. Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821–838. doi: 10.1007/s40257-018-0383-4. [DOI] [PubMed] [Google Scholar]
- 36.Bawany F, Northcott CA, Beck LA, Pigeon WR. Sleep disturbances and atopic dermatitis: relationships, methods for assessment, and therapies. J Allergy Clin Immunol Pract. 2021;9:1488–1500. doi: 10.1016/j.jaip.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.To T, Ryckman K, Zhu J, Williams D, Feldman LY, Larsen K, et al. Mental health services claims and adult onset asthma in Ontario, Canada. J Allergy Clin Immunol Pract. 2017;5:1388–1393.e3. doi: 10.1016/j.jaip.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Kim DH, Han K, Kim SW. Relationship between allergic rhinitis and mental health in the general Korean adult population. Allergy Asthma Immunol Res. 2016;8:49–54. doi: 10.4168/aair.2016.8.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17:174–182. doi: 10.1016/S1474-4422(17)30435-0. [DOI] [PubMed] [Google Scholar]
- 40.Dresler T, Caratozzolo S, Guldolf K, Huhn JI, Loiacono C, Niiberg-Pikksööt T, et al. Understanding the nature of psychiatric comorbidity in migraine: a systematic review focused on interactions and treatment implications. J Headache Pain. 2019;20:51. doi: 10.1186/s10194-019-0988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peres MF, Mercante JP, Tobo PR, Kamei H, Bigal ME. Anxiety and depression symptoms and migraine: a symptom-based approach research. J Headache Pain. 2017;18:37. doi: 10.1186/s10194-017-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Shao A, Jiang Z, Tsai H, Liu W. The exploration of mechanisms of comorbidity between migraine and depression. J Cell Mol Med. 2019;23:4505–4513. doi: 10.1111/jcmm.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29:504–515. doi: 10.1080/09540261.2017.1326882. [DOI] [PubMed] [Google Scholar]
- 44.Pavkovic IM, Kothare SV. Migraine and sleep in children: a bidirectional relationship. Pediatr Neurol. 2020;109:20–27. doi: 10.1016/j.pediatrneurol.2019.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of migraine in atopic subgroups stratified according to age group
Risk of migraine in atopic subgroups stratified according to sex
Follow-up duration until the migraine onset in each patient with atopic disorders