Abstract
Escherichia coli type IIa heat-labile enterotoxin (LTIIa) binds in vitro with highest affinity to ganglioside GD1b. It also binds in vitro with lower affinity to several other oligosialogangliosides and to ganglioside GM1, the functional receptor for cholera toxin (CT). In the present study, we characterized receptor-mediated signal transduction by LTIIa in the cultured T84 cell model of human intestinal epithelium. Wild-type LTIIa bound tightly to the apical surface of polarized T84 cell monolayers and elicited a Cl− secretory response. LTIIa activity, unlike CT activity, was not blocked by the B subunit of CT. Furthermore, an LTIIa variant with a T14I substitution in its B subunit, which binds in vitro to ganglioside GM1 but not to ganglioside GD1b, was unable to bind to intact T84 cells and did not elicit a Cl− secretory response. These findings show that ganglioside GM1 on T84 cells is not a functional receptor for LTIIa. The LTIIa receptor on T84 cells was inactivated by treatment with neuraminidase. Furthermore, LTIIa binding was blocked by tetanus toxin C fragment, which binds to gangliosides GD1b and GT1b. These findings support the hypothesis that ganglioside GD1b, or possibly a glycoconjugate with a GD1b-like oligosaccharide, is the functional receptor for LTIIa on T84 cells. The LTIIa-receptor complexes from T84 cells were associated with detergent-insoluble membrane microdomains (lipid rafts), extending the correlation between toxin binding to lipid rafts and toxin function that was previously established for CT. However, the extent of association with lipid rafts and the magnitude of the Cl− secretory response in T84 cells were less for LTIIa than for CT. These properties of LTIIa and the previous finding that enterotoxin LTIIb binds to T84 cells but does not associate with lipid rafts or elicit a Cl− secretory response may explain the low pathogenicity for humans of type II enterotoxin-producing isolates of E. coli.
Type I and type II heat-labile enterotoxins (LTI and LTII) from Escherichia coli and cholera toxin (CT) from Vibrio cholerae belong to a family of structurally and functionally related AB5 enterotoxins (9). In each member of this toxin family, the A polypeptide assembles noncovalently with five identical B polypeptides to form the holotoxin. The enzymatically active fragment A1, which corresponds to the amino-terminal domain of polypeptide A, is generated from holotoxin by proteolytic cleavage of the A polypeptide and reduction of the disulfide bond that links fragment A1 to the carboxyl-terminal fragment A2. Fragment A1 acts inside target cells by catalyzing ADP-ribosylation of the α subunit of the heterotrimeric protein Gs, leading to activation of adenylyl cyclase and stimulation of intracellular cyclic AMP (cAMP) production (2, 12).
The pentameric B subunit exhibits lectin-like activity and tethers the toxin to target cell membranes by binding to specific cell surface receptors with high affinity and stability. The action of CT and LTI on target cells is characterized by a lag phase between toxin binding to receptors at the cell surface and the first detectable signs of toxicity. Results from multiple laboratories indicate that several events occur during this lag phase in polarized epithelial cells, including retrograde trafficking of toxin from apical cell surface receptors into Golgi cisternae and the endoplasmic reticulum (ER), translocation of fragment A1 into the cytosol, movement of fragment A1 to the cytoplasmic surface of the basolateral membrane, and interaction of fragment A1 with the adenylyl cyclase complex (16). The subsequent accumulation of intracellular cAMP results in toxicity.
The family of heat-labile enterotoxins is classified into two serogroups (10). CT and LTI belong to serogroup I, and LTII belongs to serogroup II. Minor antigenic differences are found among the members of serogroup I and also among the members of serogroup II. LTIIa and LTIIb are variants of LTII from different enterotoxigenic isolates of E. coli. Antiserum against any variant of CT or LTI can neutralize all enterotoxins in serogroup I but not those in serogroup II, and vice versa. The structures of CT, LTI, and LTIIb were determined by x-ray crystallography (21, 23, 26), and there are close similarities in their overall folds despite significant differences in their amino acid sequences and biological properties (10). The three-dimensional structure of LTIIa has not yet been determined.
The A polypeptides of the type I and type II enterotoxins are structurally and functionally homologous. Their A1 fragments exhibit similar NAD-dependent ADP-ribosyltransferase activity in vitro, with Gsα as the acceptor, but the A1 fragments of the type II toxins are less active than those of the type I toxins for ADP ribosylation of alternative substrates such as agmatine (2, 12). The B polypeptides of the type I enterotoxins have little amino acid sequence homology with the B subunits of the type II enterotoxins, and the type I and type II enterotoxins exhibit striking differences in receptor-binding specificity (8). CT binds in vitro with highest affinity to ganglioside GM1 and with lower affinity to ganglioside GD1b, and in polarized human intestinal T84 cells binding of CT to ganglioside GM1 induces a Cl− secretory response. In contrast, LTIIb binds in vitro with highest affinity to ganglioside GD1a, and it does not bind at all to ganglioside GM1.
Although LTIIb binds avidly to T84 cells, it fails to elicit any Cl− secretory response (24). For chimeric CT/LTIIb enterotoxins, toxicity in T84 cells is determined primarily by the origin and receptor-binding specificity of the B subunit and not by the origin of the A1 polypeptide. Toxin binding to ganglioside GM1 but not to ganglioside GD1a associates CT or the appropriate chimeric toxins with detergent-insoluble membrane microdomains (also called lipid rafts or DIGs), and this correlates closely with toxicity (24). These findings indicate that ganglioside GD1a does not serve as a functional receptor for LTIIb in T84 cells. We and others proposed that association with lipid rafts may be necessary for efficient trafficking of CT into Golgi cisternae and ER of target epithelial cells (19, 24). We also proposed that association of CT with lipid rafts may depend on the specific structure and function of its ganglioside GM1 receptor.
In the present study, we characterized the functional receptors on T84 cells for the LTIIa variant of type II heat-labile enterotoxin. In contrast to CT and LTIIb, LTIIa binds in vitro with highest affinity to ganglioside GD1b, but it can also bind with lower affinity to gangliosides GD1a, GT1b, GQ1b, GM1, and GD2 (8). Our studies demonstrated, surprisingly, that ganglioside GM1 in T84 cells cannot mediate signal transduction by LTIIa, although it serves as a functional receptor for CT. Furthermore, we showed that binding of LTIIa to its functional receptor on human intestinal T84 cells, most likely ganglioside GD1b, associated LTIIa with lipid rafts and mediated retrograde trafficking of the toxin into the secretory pathway to elicit a Cl− secretory response.
MATERIALS AND METHODS
Biologicals and chemicals
CT, CT B subunit, and tetanus toxin C fragment were obtained from Calbiochem (San Diego, Calif.), and purified gangliosides GM1 and GD1b were from Matreya, Inc. (Pleasant Gap, Pa.). All other commercially available reagents were from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise stated. Anthrax protective antigen was a gift from John Collier at Harvard Medical School.
Cloning and expression of toxins.
Plasmid pTC201 encoding LTIIa holotoxin was made by subcloning a 2.2-kb EcoRI-KpnI fragment from pCP3727 (20) into pBluescript KS− (Stratagene, La Jolla, Calif.). Plasmid pTC401 encoding the B subunit of LTIIa and the pTC401T14I derivative encoding the T14I B subunit variant of LTIIa were described previously (3). A clone expressing LTIIa holotoxin with the T14I variant B subunit was made by cloning KpnI-SacI and SacI-BamHI fragments from pTC201 and pTC401T14I, respectively, into KpnI- and BamHI-cut pBluescript KS+ to create pMGJ204. To enable production of LTIIa A only, a 0.6-kbp PstI restriction fragment encoding the carboxyl half of LTIIa B was deleted from pTC201 to create pMGJ205.
Production of LTIIa holotoxin, LTIIa A or LTIIa B only, or variants of holotoxin or B subunit in E. coli TE1 containing the appropriate plasmid was induced with IPTG (isopropyl-β-d-thiogalactopyranoside), and 20-fold-concentrated periplasmic extracts were made in phosphate-buffered saline (PBS) with polymyxin B (1 mg/ml). Filtered extracts containing wild-type LTIIa were affinity purified by using immunoglobulin (Ig) from rabbit polyclonal anti-LTIIa serum coupled to CNBr-activated Sepharose 4B. All other filtered extracts were affinity purified by using (NH4)2SO4-fractionated Ig from goat anti-LTIIa serum coupled to Affigel-10 (Bio-Rad Laboratories, Hercules, Calif.). After repeated washing with PBS, toxin was eluted in 3.5 M MgCl2. Finally, the eluted toxin was concentrated and buffer exchanged into PBS in a Biomax Ultrafree-15 (10,000 nominal molecular weight limit [NMWL]) concentrator (Millipore Corp., Bedford, Mass.). Purity of wild-type and mutant forms of toxin and B subunits, assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue staining, was estimated to be greater than 90%. Toxin concentration was determined by A280 and/or bicinchoninic acid (BCA) assay (Pierce Chemical Co., Rockford, Ill.) of total protein, using known concentrations of CT B subunit to determine a standard curve. Values obtained by the two methods were similar.
Biotinylation of LTIIa B subunit.
LTIIa B subunit was biotinylated with sulfo-N-hydroxysuccinimide-biotin using a protocol recommended by the manufacturer (Pierce Chemical Co., Rockford, Ill.), and the biotinylated protein was exchanged into PBS by using a Centricon-20 centrifugal filter (30,000 NMWL; Millipore Corp., Bedford, Mass.). Final toxin concentration was determined by BCA assay. The binding Km for biotinylated LTIIa B subunits on T84 cells was determined to be 100 nM, compared to 203 nM for commercially available biotinylated CT B subunits and 4.1 nM for unmodified LTIIa B and CT B subunits.
Cell culture.
Human colonic T84 and mouse Y1 adrenal cells obtained from the American Type Culture Collection (Rockville, Md.) were cultured and passaged as previously described (13). T84 cell passages 70 to 90 were used for these experiments.
Electrophysiology.
Measurements of short circuit current (Isc) were performed with 0.33-cm2 monolayers as previously described (15). Polarized T84 monolayers were incubated with the appropriate toxin diluted in HEPES-buffered saline with glucose (HBS) for 40 to 60 min on ice to allow steady-state binding. Monolayers were then transferred to 37°C HBS, and the time course of toxin-induced Cl− secretion was determined. In most experiments, calibration of the Cl− secretory response was obtained using the cAMP agonist vasoactive intestinal peptide (5 nM) at the end of the study.
Toxin-binding assays.
Toxin binding to purified gangliosides on plastic substratum (PolySorp enzyme-linked immunosorbent assay [ELISA] plates [Nalge Nunc International, Rochester, N.Y.] incubated overnight with 100 ng of ganglioside per well and blocked with PBS–0.1% bovine serum albumin [BSA]) was determined after 1-h incubations at room temperature by ELISA, as previously described (24). Toxin binding to T84 cells grown to confluency on plastic substratum (96-well plates; Corning Costar Corp., Cambridge, Mass.) was determined in the same manner except that all incubations were performed on ice.
Toxin binding to purified gangliosides or T84 cell monolayers was detected by using primary rabbit polyclonal antibodies raised against LTIIa (20) followed by horseradish peroxidase (HRP)-conjugated secondary antibody, and the assays were developed by incubation with citrate-phosphate buffer, pH 4.2, containing 2.5 mg of ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] and 0.25 μl of H2O2 per ml for 30 min. Absorbance was read at 414 nm in a SpectraMax 250 plate reader (Molecular Devices Corp., Sunnyvale, Calif.).
Competition studies.
Polarized T84 cell monolayers were incubated with the indicated concentrations of competing ligand diluted in HBS containing either 0.5% BSA or gelatin for 40 min on ice. At that time, an additional volume of blocker in HBS-BSA (or gelatin) containing twice the desired final concentration of toxin was added, bringing the final toxin concentration to the desired amount. Incubation continued on ice for an additional 40 min. Monolayers were then transferred to 37°C in HBS for electrophysiological measurements or processed as described above to measure binding of toxin to the monolayers. In competition studies using biotinylated B subunits of LTIIa, the biotinylated proteins were detected and quantified using streptavidin-HRP conjugate.
Y1 cell assay.
Toxin action on mouse Y1 adrenal cells was evaluated as described previously (24) except that shape change was assessed after incubation for 2 h at 37°C rather than overnight. The 50% effective dose (ED50) was determined to be the dose at which 50% of Y1 cells changed from elongated to rounded.
Neuraminidase treatment of T84 cells.
Polarized T84 cell monolayers were treated apically with α2–3,6,8-neuraminidase from V. cholerae at 0.5 U/ml in HBS for 1 h on ice. After extensive washing with HBS, toxin was applied, and toxic effects were measured as described above.
Detergent extractions of tissue culture cells and sucrose equilibrium density centrifugation.
Extraction of polarized T84 cells and mouse Y1 adrenal cells and subsequent sucrose equilibrium centrifugation were done as previously described (24). One confluent T84 monolayer or plate of confluent Y1 cells (45-cm2 each) was used for isolation of detergent-insoluble membranes. All steps were performed at 4°C. Cells were rinsed in ice-cold TBS (150 mM NaCl, 10 mM Tris, pH 7.5) and incubated on ice with the indicated concentration of toxin diluted in TBS for 40 min. After rinsing with TBS, cells were scraped into 1 or 2 ml of ice-cold TTBS (150 mM NaCl, 10 mM Tris, 1% Triton X-100, pH 7.5) and homogenized with five strokes in a tight-fitting Dounce homogenizer on ice. The homogenate was adjusted to 40% sucrose by addition of 80% sucrose in 1% TTBS, layered under a linear 5 to 30% sucrose gradient, and centrifuged at 48,000 rpm for 16 to 20 h in a swinging-bucket rotor (model SW41; Beckman Instruments, Palo Alto, Calif.). Sequential 0.5- or 1-ml fractions were collected from the top of the gradient, and 20 μl from each fraction was analyzed by SDS-PAGE and Western blot. Sucrose density was monitored by refractometry.
Statistics.
Data were analyzed using Statview 512+ software (Brainpower, Inc., Calabasas, Calif.).
RESULTS
Signaling by LTIIa.
Initial studies showed that purified LTIIa induced a physiologic Cl− secretory response from the polarized human intestinal cell line T84. Figure 1 shows the time course of LTIIa action. LTIIa (100 nM) applied to either the apical or basolateral cell surface induced a modest Cl− secretory response after a lag period of between 60 and 90 min. Compared to purified CT (50 nM), LTIIa induced a much smaller secretory response (peak Isc = 13 versus >100 μA/cm2) after a longer lag phase (>60 versus 30 min). With LTIIa, we did not observe the differentially faster time course following basolateral versus apical application of the toxin that is characteristic of CT (15). When LTIIa was applied to mouse Y1 adrenal cells, it was more potent than CT, as assessed by cAMP-dependent cell rounding after 2 h (ED50 = 0.2 nM versus 1.2 nM for CT). This was in agreement with previous observations that LTIIa is more toxic than CT for Y1 adrenal cells, based on morphological changes after overnight incubation (11).
FIG. 1.
Time course of secretion induced by LTIIa applied apically or basolaterally and CT applied apically in cultured polarized human epithelial T84 cells. Short circuit current (Isc) is plotted against minutes at 37°C; note that the Isc scale is both broken and expanded to clearly display all data sets on the same graph. Bars indicate SD. Results are from a typical experiment, n = 2.
To determine if proteolytic activation of the purified LTIIa A subunit was rate-limiting for LTIIa action on T84 cell monolayers, we treated the holotoxin with trypsin in vitro. Trypsin treatment increased the fraction of nicked A subunit by at least twofold but did not result in a detectable increase in Cl− secretion (data not shown). We also used brefeldin A to demonstrate that LTIIa, like CT, required endocytosis and retrograde trafficking of toxin into the Golgi and ER to elicit signal transduction. Brefeldin A (20 μM), which blocks retrograde Golgi-to-ER traffic in T84 cells (14), completely inhibited LTIIa-induced Cl− secretion (data not shown). These data showed that purified LTIIa could induce a physiological Cl− secretory response in polarized human intestinal T84 cell monolayers, although the lag phase of the response to LTIIa was longer and the magnitude of the response to LTIIa was smaller than for CT.
In subsequent experiments we investigated whether these differences in physiological responses could be explained by differences in the interactions of purified LTIIa and CT with their cellular receptors.
Toxin binding.
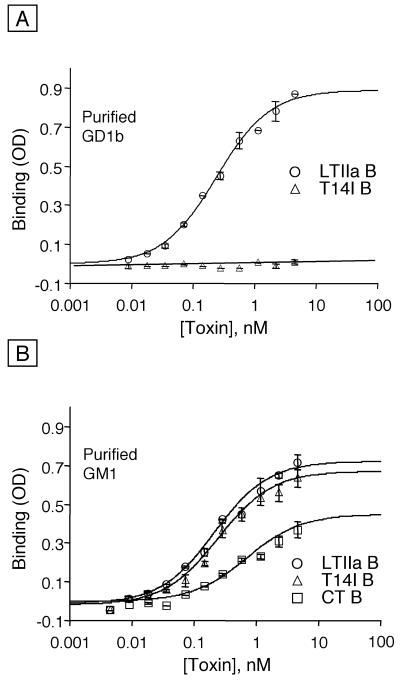
First, we compared the binding of purified wild-type and mutant forms of the B subunit of LTIIa to purified gangliosides immobilized on plastic. Initial studies showed that the B subunit of wild-type LTIIa bound in vitro to purified ganglioside GD1b with high affinity (Fig. 2A and Table 1). To demonstrate specificity, we used the T14I variant of the B subunit of LTIIa, which has a single residue substitution of isoleucine for threonine 14 in the oligosaccharide binding site. Although the LTIIa B T14I variant did not bind in vitro to ganglioside GD1b (Fig. 2A), both the wild-type and T14I variant of the LTIIa B subunit bound in vitro with high affinity to purified ganglioside GM1, as did CT B (Fig. 2B and Table 1). These findings with the purified toxin B subunits confirmed and extended previous experiments that were performed with the corresponding recombinant proteins in crude periplasmic extracts from E. coli (3).
FIG. 2.
Binding of toxin B subunits to individual gangliosides as determined by ELISA. Each well was coated overnight with 100 ng of the respective ganglioside in PBS at 4°C. Bars indicate SD. Binding to (A) purified ganglioside GD1b (LTIIa wild-type and T14I variant B subunits, n = 2, one of three experiments) or (B) ganglioside GM1 (LTIIa wild-type and T14I variant B subunits and CT B subunits, n = 2, one of three experiments) is shown.
TABLE 1.
Summary of binding constants for LTIIa wild-type B subunit, LTIIa variant T14I B subunit, and CT B subunit for purified ganglioside GD1b, ganglioside GM1, and cultured T84 cells
| Toxin subunit |
Km (nM) for receptor:
|
||
|---|---|---|---|
| Ganglioside GD1b | Ganglioside GM1 | T84 cells | |
| LTIIa B | 0.24a | 0.22a | 4.1b |
| CT B | 0.13a | 0.41a | 4.1c |
| LTIIa T14I B | NDd | 0.31a | ND |
n = 2.
Five experiments, SEM = 1.6.
Two experiments, SEM = 3.0.
ND, not determined.
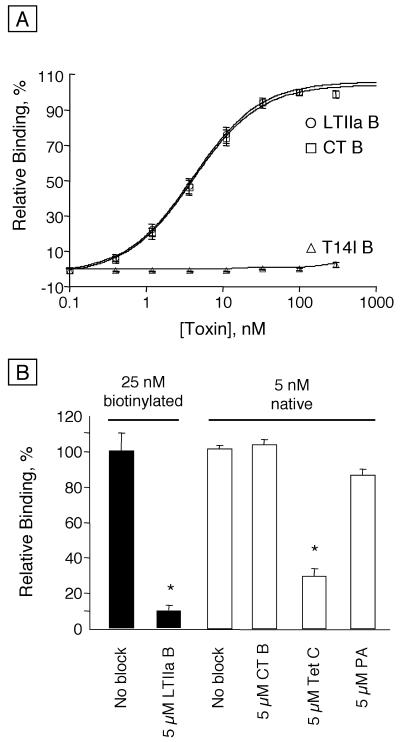
Next, we compared the binding of these toxin B subunits to receptors on T84 cell monolayers. The B subunits of wild-type LTIIa and CT exhibited comparable binding affinities to apical cell surface receptors on T84 cells (Fig. 3A and Table 1). In striking contrast, however, the T14I variant of the LTIIa B subunit did not bind at all to T84 cells. This result was remarkable, because ganglioside GM1 is known to be present and to serve as the functional receptor for CT on T84 cells (24), and also because the B subunits of wild-type LTIIa and the T14I variant of LTIIa bind in vitro to ganglioside GM1 with high affinity (Fig. 2B and Table 1).
FIG. 3.
Binding of toxin B subunits to T84 cells. (A) Bars indicate standard error of the mean (SEM) (five different experiments, with n ≤ 2). (B) Binding of 25 nM biotinylated (solid bars) or 5 nM native (open bars) LTIIa B subunit with or without blocking by an excess (5 μM) of LTIIa wild-type B subunit, CT B subunit, tetanus toxin C fragment (Tet C), or anthrax protective antigen (PA, an unrelated negative control). Values significantly different from the control (no blocker) are indicated by an asterisk (P < 0.05, paired t test). Bars indicate SEM. Each value represents two experiments, each with n = 3.
The mass of LTIIa and CT bound to T84 cells relative to the exposure concentration was also examined. T84 cells incubated with 150 nM LTIIa bound roughly the same mass of toxin B subunit as cells incubated with 20 nM CT (data not shown), suggesting that, at near saturating levels of toxin, there are similar numbers of LTIIa and CT receptors on T84 cells. Even with LTIIa concentrations up to 450 nM, when T84 cells bound a slightly greater mass of LTIIa compared to the mass of CT bound at 20 nM, LTIIa still elicited a smaller Cl− secretory response (data not shown), indicating that factors other than decreased toxin binding are responsible for the decreased toxicity of LTIIa for T84 cells.
The binding of LTIIa B subunits to T84 cell surface receptors was specific. We biotinylated LTIIa B subunit in order to examine the ability of LTIIa B to compete with itself for binding to T84 cells. Figure 3B shows that the binding of 25 nM biotinylated LTIIa B subunit to T84 cells was effectively blocked by an excess of unlabeled LTIIa B subunit (at 5 μM). Although biotinylation increased the Km of LTIIa B binding, 25 nM biotinylated LTIIa B subunit was within the linear range of detection. In contrast, the binding of native, unmodified LTIIa B subunit (at 5 nM) was not diminished by an excess of competing CT B subunit (5 μM) or anthrax protective antigen (5 μM, a negative control). However, tetanus toxin C fragment (at 5 μM), which can bind to gangliosides GD1b and GT1b, inhibited the binding of the native LTIIa B subunit to T84 cells by approximately 65%. Taken together, these data showed that LTIIa exhibited little or no binding to ganglioside GM1 on T84 cells, and they suggested that ganglioside GD1b is likely the principal receptor for LTIIa on T84 cells.
Analysis of functional LTIIa receptors.
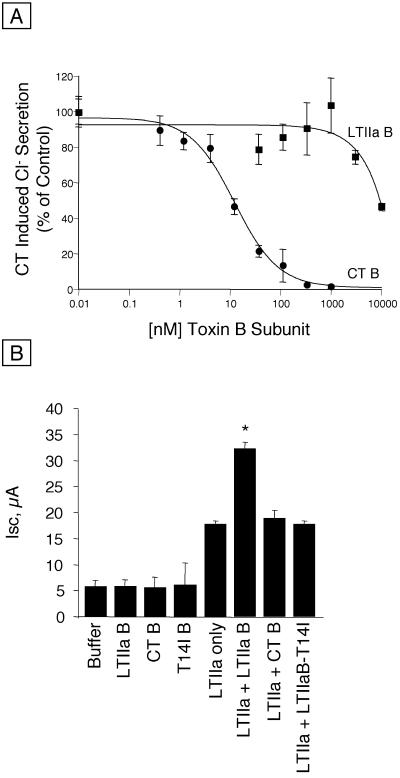
To confirm by a functional assay that LTIIa did not bind to ganglioside GM1 on T84 cells, we compared the ability of the B subunits of CT and of LTIIa to block intoxication by 0.5 nM CT. Figure 4A shows the specific inhibition of CT-induced Cl− secretion when the ganglioside GM1 receptors were blocked by CT B. The CT-induced Isc decreased by approximately 50% when CT B was present at 10 nM, and CT B at 300 nM completely inhibited the response. In contrast, the LTIIa B subunit did not interfere with CT-induced Cl− secretion until concentrations of >3 μM were used, and the inhibition of CT-induced Cl− secretion by 10 μM LTIIa B was only 50%. These results indicated that the affinity of the LTIIa B subunit for ganglioside GM1 on T84 cells was approximately 1,000-fold less than that of CT.
FIG. 4.
Blocking of secretion induced by CT or LTIIa in T84 cells by enterotoxin B subunits or other ligands. (A) Blocking of secretion induced by 0.5 nM CT by the B subunits of LTIIa and of CT. The concentration of the blocking ligand is indicated on the horizontal axis. Mean results of five experiments; bars indicate SEM. (B) Blocking of secretion induced by 75 nM LTIIa. The concentration of the blocking ligand was 10 μM for LTIIa B subunit, CT B subunit, and LTIIa T14I B subunit variant. Results shown are from a typical experiment (n = 3). Values significantly different from LTIIa secretion without added B subunits are indicated by an asterisk (P < 0.05, paired t test).
Next, we performed the reciprocal experiment of competing for LTIIa-induced Cl− secretion with an excess of the wild-type or T14I variant of LTIIa B subunit or of CT B (Fig. 4B). LTIIa holotoxin (75 nM) elicited a mean peak Isc of 18 ± 0.6 μA (mean ± standard deviation [SD], n = 3). Competition with 10 μM CT B, which completely blocked the physiological response to CT in the previous experiment, did not alter the response to LTIIa. This finding confirmed that GM1 is not the functional receptor for LTIIa in T84 cells. We also found, however, that high concentrations (10 μM) of the LTIIa B subunit enhanced the toxic effect of LTIIa (mean peak Isc = 32 ± 1.2 μA, mean ± SD, n = 3) rather than inhibiting it. This enhancement occurred at several different concentrations of LTIIa holotoxin, and the magnitude of the enhancement increased as the dose of LTIIa holotoxin increased (data not shown).
These results are likely explained by the ability of free LTIIa A subunits to assemble with free LTIIa B subunits, originally described by Connell and Holmes (4). To test this, we performed additional studies with Y1 cells and crude periplasmic extracts from recombinant strains of E. coli producing only LTIIa A and LTIIa B. We added LTIIa B subunits at saturating concentrations, washed the cells to remove the unbound B subunits, and then added serial dilutions of free LTIIa A subunit extracts. A dose-dependent rounding of the Y1 cells showed that free LTIIa A subunits interacted with the receptor-bound LTIIa B subunits to form holotoxin and cause intoxication. Neither free A subunits alone nor free B subunits alone caused rounding of the Y1 cells. Finally, as expected, the secretory response to LTIIa was not blocked by the T14I variant of LTIIa B subunit.
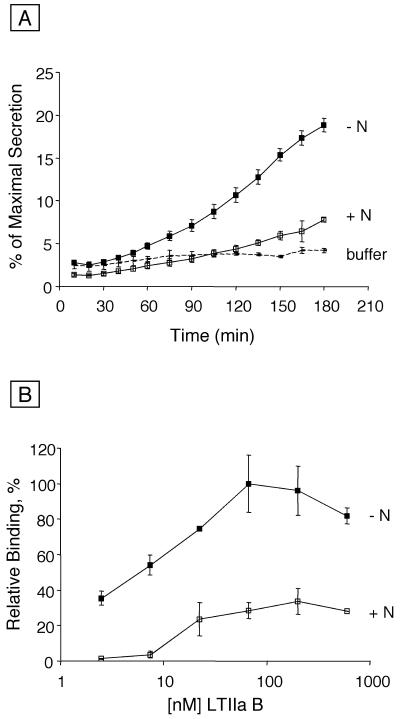
To confirm the specificity of LTIIa binding to T84 cell surface receptors, we examined the effects of neuraminidase treatment of T84 cells on LTIIa binding and LTIIa-mediated signal transduction. Neuraminidase from V. cholerae trims sialic acid residues from oligosialogangliosides and other glycoconjugates, and it converts oligosialogangliosides of the G1b series to ganglioside GM1. Consequently, treatment of T84 cells with V. cholerae neuraminidase was predicted to decrease their subsequent capacity to bind LTIIa and to be intoxicated by LTIIa. These predicted results were obtained. Treatment of T84 cell monolayers with neuraminidase strongly inhibited both the Cl− secretory response induced by 75 nM LTIIa (Fig. 5A) and LTIIa binding to T84 cells (Fig. 5B). As expected, neuraminidase treatment of T84 cells increased CT binding, demonstrating that the number of ganglioside GM1 receptors for CT had increased (data not shown).
FIG. 5.
Effect of neuraminidase treatment of T84 cells on LTIIa function and binding. (A) Polarized T84 monolayers were treated with 0.5 U of neuraminidase per ml for 1 h on ice before being incubated with 75 nM LTIIa on ice for 40 min. Monolayers were then moved to 37°C. Values shown are percent of maximum secretion, determined by addition of vasoactive intestinal peptide at 185 min, with (+N, n = 4) or without (−N, n = 3) neuraminidase treatment. Bars indicate SD. (B) T84 cells cultured in 96-well plates were incubated on ice with 0.5 U of neuraminidase per ml for 1 h. ELISA analysis of LTIIa B subunit binding was done as described in the text. Values for cells treated with neuraminidase (+N) are shown as a percentage of maximal binding, determined on cells not treated with neuraminidase (−N). Bars indicate SD, n = 2.
These data provided direct evidence that the functional receptors for LTIIa were sensitive to hydrolysis by neuraminidase. Furthermore, LTIIa holotoxin containing the T14I variant of the B subunit, which was unable to bind to ganglioside GD1b in vitro (Fig. 2A) or to T84 cells in vivo (Fig. 3A), did not show biological activity against T84 cells even at the high concentration of 300 nM (data not shown). Taken together, these data support the conclusion that ganglioside GD1b, and possibly other glycoconjugates with similar carbohydrate moieties, serves as the functional receptor for LTIIa.
Association of LTIIa receptor with lipid rafts.
In human intestinal T84 and mouse Y1 adrenal cells, ganglioside GM1 functions to associate CT with lipid rafts, and this correlates strongly with toxicity (24). In the present study, we extended these observations by examining human T84 and mouse Y1 adrenal cells for association of LTIIa with lipid rafts (Fig. 6). In T84 cells, where LTIIa is less potent than CT, less than half of the LTIIa-receptor complex partitioned with the Triton-insoluble lipid raft fraction, whereas the CT-receptor complex was present primarily in the Triton-insoluble lipid raft fraction, as previously described (24). In contrast, in Y1 cells, where LTIIa exhibited greater potency than CT in eliciting cAMP-dependent cellular shape change, almost all of the LTIIa-receptor complex was found in the lipid raft fraction (Fig. 6). These data support the idea that receptor-mediated association with lipid rafts functions critically in toxin action for LTIIa as well as for CT. Thus, the LTIIa receptor (presumably ganglioside GD1b) can act like the CT receptor (ganglioside GM1) in T84 cells to drive toxin association with lipid rafts, and this correlates with toxin function.
FIG. 6.
Distribution of LTIIa and CT B subunits between detergent-soluble fraction and detergent-insoluble fraction (lipid rafts). Polarized T84 monolayers or mouse Y1 adrenal cells were subjected to detergent extraction and sucrose equilibrium centrifugation, followed by SDS-PAGE of the resulting fractions. D, detergent-insoluble fraction; S, detergent-soluble fraction; C, purified toxin (35 ng of LTIIa B subunit or CT) in buffer controls; 0.5% of the gradient total was loaded into lanes D and S.
DISCUSSION
We found that the receptor-binding activity of LTIIa distinguished between the oligosaccharide side chains of ganglioside GM1 immobilized on plastic and those displayed on the apical membranes of cultured intestinal T84 cells. The B subunits of CT and LTIIa bound with similar high apparent affinities to gangliosides GM1 and GD1b on plastic and with similar apparent affinities to the apical surface of T84 cells in culture. Unexpectedly, the T14I variant of the LTIIa B subunit, which binds to ganglioside GM1 with similar affinity as the wild-type LTIIa B subunit but not to ganglioside GD1b in vitro, was completely unable to bind to monolayers of T84 cells. Furthermore, the CT B subunit failed to block binding and signal transduction by wild-type LTIIa in T84 cells. Taken together, these findings demonstrated that ganglioside GM1 on T84 cells cannot act either as a ligand or as a functional receptor for LTIIa despite its high activity as a functional receptor for CT binding and CT-induced Cl− secretion on the same cells.
In previous studies based on immunoassays, LTIIa and CT were shown to interact differently with the oligosaccharide side chains of gangliosides (3, 8). Both LTIIa and CT can bind under appropriate conditions to gangliosides GM1 and GD1b, which have one and two sialic acid residues, respectively, on the internal galactose. When a small amount of immobilized ganglioside is used in immunoassays, LTIIa binds preferentially to ganglioside GD1b, but CT binds preferentially to ganglioside GM1. When a large amount of immobilized ganglioside is used, however, the binding of LTIIa to ganglioside GM1 is almost equal to its binding to ganglioside GD1b. The immunoassays in the present study used a large amount of immobilized ganglioside. Under these conditions, the apparent affinities of LTIIa and CT for gangliosides GD1a and GM1 varied within a range of only threefold, but the differences in binding of wild-type and T14I variants of LTIIa B subunit to ganglioside GD1b were dramatic. Furthermore, LTIIa but not CT can bind to gangliosides such as GD1a, GT1b, and GQ1b that have either one or two sialic residues on the terminal galactose (8).
Although the molecular interactions of CT with GM1 oligosaccharide have been determined by x-ray crystallography (17, 18), the precise mode of binding of LTIIa to the GM1 oligosaccharide is not yet known. Direct effects that could explain the inaccessibility of the binding site for LTIIa but not the binding site for CT on ganglioside GM1 on T84 cells include, but are not limited to, the orientation of the oligosaccharide moiety versus the cell surface and possible competitive binding interactions of the oligosaccharide with other cell membrane components. Indirect effects that could explain this phenomenon might be caused by differences in the ceramide components among gangliosides that exhibit similar binding to LTIIa in vitro but differential binding to LTIIa when they are displayed on cultured T84 cells. These results emphasize the structural and functional complexity of the apical surfaces of intestinal epithelial cells that are displayed to invading toxins and microbes and the importance of receptor context.
Our results indicate that ganglioside GD1b, or possibly a glycoconjugate with a similar oligosaccharide component, is a functional receptor for LTIIa on T84 cells. LTIIa binds ganglioside GD1b with the highest affinity among the known, purified gangliosides (3, 8). In our studies with T84 cells, only the LTIIa B subunit and tetanus toxin C fragment (which binds to gangliosides GD1b and GT1b [5, 7, 25]) were able to inhibit LTIIa binding. The B subunit of LTIIa did not block LTIIa signaling in T84 cells; instead, it enhanced signaling. This apparently paradoxical but repeatable increase in signaling was likely caused by the presence in our purified LTIIa of some free A subunits in addition to, or derived from, holotoxin, as discussed below.
Our LTIIa holotoxin was purified by affinity chromatography with polyclonal rabbit antibodies that recognized epitopes on both the A and the B subunits and could therefore have contained some free A and/or B subunits. Previous studies showed that biologically active LTIIa and LTIIb holotoxins can be formed when crude periplasmic extracts containing their recombinant A and B subunits are mixed together and tested by solid-phase immunoassays or bioassays (4). In contrast, in vitro assembly of CT or LTI holotoxins from their A and B subunits only occurs when the subunits are exposed to denaturing and renaturing conditions (6, 9, 22). In the present experiments, the activity of LTIIa on T84 cells was stimulated rather than inhibited even when LTIIa B subunits were added in large excess, and the magnitude of the stimulation increased as the dose of LTIIa holotoxin increased. These results, together with the previous findings of Connell and Holmes (4), raised the possibility that free LTIIa A subunits were present that interacted preferentially with LTIIa B subunits bound to ganglioside receptors on T84 cells.
Alternatively, since free LTIIa A and B subunits can spontaneously assemble into holotoxin, pure holotoxin may exist in equilibrium with free A and B subunits. Assembly of free A subunits in our purified holotoxin with B subunits bound on T84 cells would have resulted in exposure of the cells to an increased dose of holotoxin and a consequent increase in LTIIa-mediated signal transduction despite the presence of a large excess of LTIIa B subunits in the culture medium. Intoxication of Y1 cells by the addition of free LTIIa A subunits to cells previously incubated with and washed free of unbound LTIIa B subunits provided experimental evidence in support of this concept. Our present findings confirm and extend the conclusion of Connell and Holmes (4) that there appear to be striking differences in conditions that permit in vitro assembly of type II and type I heat-labile enterotoxins.
We used neuraminidase treatment of T84 cells to further establish that LTIIa function is receptor mediated. Neuraminidase treatment removes sialic acid residues from oligosialogangliosides and other oligosialylated glycoconjugates, and it converts many of the known oligosialogangliosides to the neuraminidase-resistant monosialoganglioside GM1. Neuraminidase treatment of T84 cells largely eliminated the ability of LTIIa to either bind to the cells or elicit a Cl− secretory response. These findings confirmed that LTIIa requires an oligosialylated receptor, which is most likely ganglioside GD1b.
Heat-labile enterotoxins ADP-ribosylate the regulatory protein Gsα, resulting in activatation of adenylyl cyclase, accumulation of intracellular cAMP, and activation of cAMP-dependent signals (2, 12). Because the A1 polypeptides of the type I and type II enterotoxins have similar ADP-ribosylating activity for Gsα in vitro but the toxicity of LTIIa for T84 cells is much less than that of CT, it seems likely that the LTIIa receptor is not as efficient as the CT receptor in directing its ligand into a pathway that results in intoxication of T84 cells. LTIIa also exhibits a significantly longer signaling lag time than CT when it is applied to the apical surface of polarized T84 cells and, unlike CT, does not show a significant decrease in signaling lag time when it is applied to the basolateral surface rather than the apical cell surface. These observations support the hypothesis that the pathways leading from receptor binding to the activation of adenylyl cyclase, while similar in outcome for these two toxins, must differ greatly in efficiency.
We and others have previously shown that specificity of CT action in model intestinal epithelia depends on the ability of the ganglioside GM1 receptor to partition CT into detergent-insoluble glycosphingolipid-rich membranes (lipid rafts) (19, 24). Additionally, LTIIb, which does not partition into lipid rafts in T84 cells but does so in Y1 cells (24), is inactive on T84 cells but highly active on Y1 cells. Together these studies provide evidence that toxin function (and presumably intracellular trafficking) correlates with association of the toxin-receptor complexes with lipid rafts. Similarly, the present study suggests that LTIIa function is correlated with the degree of association of LTIIa with lipid rafts. In T84 cells, where LTIIa is much less toxic than CT, a much smaller proportion of LTIIa than of CT was associated with lipid rafts. However, in mouse Y1 adrenal cells, where LTIIa is at least as potent as CT, nearly all of the LTIIa (this paper) or CT (24) was associated with lipid rafts. The partial to nearly complete exclusion of LTIIa and LTIIb from lipid rafts in human intestinal cells (this paper and references 1 and 24) may at least partially explain why isolates of enterotoxigenic E. coli that produce type II enterotoxins have not been clearly associated with diarrheal disease in humans.
These studies also demonstrate that ganglioside GM1 is not the only receptor that can partition heat-labile enterotoxins into lipid rafts. Furthermore, if ganglioside GD1b is indeed the receptor for LTIIa, then the differences that we found in distribution of the LTIIa receptor among cell types indicate that the oligosaccharide moiety cannot be the sole determinant of ganglioside partitioning into lipid rafts. Other factors that may play a role in partitioning of gangliosides into lipid rafts, such as the composition of the ceramide domain, possible interactions of the oligosaccharide moiety with other components of lipid rafts, or both, must also be considered and investigated.
In conclusion, our studies showed that entry of LTIIa into the polarized human intestinal cell line T84 and subsequent signal transduction depend on its binding to the cell surface receptor ganglioside GD1b or possibly to another glycoconjugate with a similar oligosaccharide moiety. Ganglioside GM1 on T84 cells, which functions as the receptor for CT, was not able to be bound by LTIIa or to serve as its receptor. Receptor binding partitioned LTIIa into apical membrane lipid rafts, which correlated with toxin function. Thus, LTIIa, like CT, must associate with lipid rafts for toxicity.
ACKNOWLEDGMENTS
The research reported here was supported in part by the following research grants from the National Institutes of Health: AI31940 (to R.K.H.), DK48106 (to W.I.L.), DK34854 (to the Harvard Digestive Disease Center), and individual NRSAs (to S.W.-M. and A.A.W.).
REFERENCES
- 1.Badizadegan K, Dickinson B L, Wheeler H E, Blumberg R S, Holmes R K, Lencer W I. Heterogeneity of detergent-insoluble membranes from human intestine containing caveolin-1 and ganglioside GM1. Am J Physiol Gastrointest Liver Physiol. 2000;278:G895–G904. doi: 10.1152/ajpgi.2000.278.6.G895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang P P, Moss J, Twiddy E M, Holmes R K. Type II heat-labile enterotoxin of Escherichia coli activates adenylate cyclase in human fibroblasts by ADP ribosylation. Infect Immun. 1987;55:1854–1858. doi: 10.1128/iai.55.8.1854-1858.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connell T D, Holmes R K. Molecular genetic analysis of ganglioside GD1b-binding activity of Escherichia coli type IIa heat-labile enterotoxin by use of random and site-directed mutagenesis. Infect Immun. 1992;60:63–70. doi: 10.1128/iai.60.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connell T D, Holmes R K. Characterization of hybrid toxins produced in Escherichia coli by assembly of A and B polypeptides from type I and type II heat-labile enterotoxins. Infect Immun. 1992;60:1653–1661. doi: 10.1128/iai.60.4.1653-1661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Critchley D R, Habig W H, Fishman P H. Reevaluation of the role of gangliosides as receptors for tetanus toxin. J Neurochem. 1986;47:213–222. doi: 10.1111/j.1471-4159.1986.tb02852.x. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein R A, Boesman M, Neoh S H, LaRue M K, Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen) J Immunol. 1974;113:145–150. [PubMed] [Google Scholar]
- 7.Fishman P H. Role of membrane gangliosides in binding and action of bacterial toxins. J Membrane Biol. 1982;69:85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- 8.Fukuta S, Magnani J L, Twiddy E M, Holmes R K, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirst T R. Cholera toxin and Escherichia coli heat-labile enterotoxins. In: Alouf J E, Freer J H, editors. The comprehensive sourcebook of bacterial protein toxins. 2nd ed. London, England: Academic Press Limited; 1999. pp. 104–129. [Google Scholar]
- 10.Holmes R K. Heat-labile enterotoxins (Escherichia coli) In: Rappuoli R, Montecucco C, editors. Guidebook to protein toxins and their use in cell biology. Oxford, England: Oxford University Press; 1997. pp. 30–33. [Google Scholar]
- 11.Holmes R K, Twiddy E M, Pickett C L. Purification and characterization of type II heat-labile enterotoxin of Escherichia coli. Infect Immun. 1986;53:464–473. doi: 10.1128/iai.53.3.464-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee C M, Chang P P, Tsai S C, Adamik R, Price S R, Kunz B C, Moss J, Twiddy E M, Holmes R K. Activation of Escherichia coli heat-labile enterotoxins by native and recombinant ADP-ribosylation factors, 20-kDa guanine nucleotide-binding proteins. J Clin Investig. 1991;87:1780–1786. doi: 10.1172/JCI115197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lencer W I, Constable C, Moe S, Jobling M, Webb H M, Ruston S, Madara J L, Hirst T, Holmes R. Targeting of cholera toxin and E. coli heat labile toxin in polarized epithelia: role of C-terminal KDEL. J Cell Biol. 1995;131:951–962. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lencer W I, de Almeida J B, Moe S, Stow J L, Ausiello D A, Madara J L. Entry of cholera toxin into polarized human intestinal epithelial cells: identification of an early brefeldin A sensitive event required for A1-peptide generation. J Clin Investig. 1993;92:2941–2951. doi: 10.1172/JCI116917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lencer W I, Delp C, Neutra M R, Madara J L. Mechanism of cholera toxin action on a polarized human epithelial cell line: role of vesicular traffic. J Cell Biol. 1992;117:1197–1209. doi: 10.1083/jcb.117.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lencer W I, Hirst T R, Holmes R K. Membrane traffic and the cellular uptake of cholera toxin. Biochim Biophys Acta. 1999;1450:177–190. doi: 10.1016/s0167-4889(99)00070-1. [DOI] [PubMed] [Google Scholar]
- 17.Merritt E A, Kuhn P, Sarfaty S, Erbe J L, Holmes R K, Hol W G. The 1.25 Å resolution refinement of the cholera toxin B-pentamer: evidence of peptide backbone strain at the receptor-binding site. J Mol Biol. 1998;282:1043–1059. doi: 10.1006/jmbi.1998.2076. [DOI] [PubMed] [Google Scholar]
- 18.Merritt E A, Sarfaty S, van der Akker F, L'Hoir C, Martial J A, Hol W G J. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 1994;3:166–175. doi: 10.1002/pro.5560030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlandi P A, Fishman P H. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickett C L, Twiddy E M, Belisle B W, Holmes R K. Cloning of genes that encode a new heat-labile enterotoxin of Escherichia coli. J Bacteriol. 1986;165:348–352. doi: 10.1128/jb.165.2.348-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sixma T K, Kalk K H, van Zanten B A, Dauter Z, Kingma J, Witholt B, Hol W G. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J Mol Biol. 1993;230:890–918. doi: 10.1006/jmbi.1993.1209. [DOI] [PubMed] [Google Scholar]
- 22.Takeda Y, Honda T, Taga S, Miwatani T. In vitro formation of hybrid toxins between subunits of Escherichia coli heat-labile enterotoxin and those of cholera enterotoxin. Infect Immun. 1981;34:341–346. doi: 10.1128/iai.34.2.341-346.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Akker F, Sarfaty S, Twiddy E M, Connell T D, Holmes R K, Hol W G. Crystal structure of a new heat-labile enterotoxin, LT-IIb. Structure. 1996;4:665–678. doi: 10.1016/s0969-2126(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 24.Wolf A A, Jobling M G, Wimer-Mackin S, Ferguson-Maltzman M, Madera J L, Holmes R K, Lencer W I. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J Cell Biol. 1998;141:917–927. doi: 10.1083/jcb.141.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yavin E. Gangliosides mediate association of tetanus toxin with neural cells in culture. Arch Biochem Biophys. 1984;230:129–137. doi: 10.1016/0003-9861(84)90093-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R G, Scott D L, Westbrook M L, Nance S, Spangler B D, Shipley G G, Westbrook E M. The three-dimensional crystal structure of cholera toxin. J Mol Biol. 1995;251:563–573. doi: 10.1006/jmbi.1995.0456. [DOI] [PubMed] [Google Scholar]