Abstract
Purpose
This study investigated the clinical implications of neutrophil extracellular trap (NET) formation (NETosis) and eosinophil extracellular trap (EET) formation (EETosis) regarding refractoriness in chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP).
Methods
Nasal polyp specimens were obtained from 117 patients with CRSwNP who received endoscopic sinus surgery. Disease control status at postoperative 1 year was assessed. Refractory cases were defined as partly controlled or uncontrolled cases according to the EPOS 2020 guidelines. NETosis and EETosis were evaluated through immunofluorescence staining (citrullinated histone H3-human neutrophil elastase and citrullinated histone-galectin-10, respectively) followed by manual counting. The z-score of NET and EET counts was used to define the following four groups: low extracellular trap formation (ETosis), NETosis-predominant, EETosis-predominant, and high-ETosis.
Results
The refractory and non-refractory groups showed significant differences in the tissue eosinophil count (P = 0.005) and EET count (P = 0.029). The tissue neutrophil count and the NET/neutrophil ratio were significantly different between the refractory and non-refractory groups of patients with neutrophilic CRS (P = 0.045, 0.031, respectively). Refractoriness significantly differed among the low-ETosis (30.77%), NETosis-predominant (47.83%), EETosis-predominant (56.67%), and high-ETosis (83.33%) groups (P = 0.005).
Conclusions
The results of this study suggest that tissue Eosinophilia and EETosis may play a prognostic role, primarily in CRSwNP and thattissue neutrophilia and NETosis can play as prognostic biomarkers in neutrophilic CRSwNP.
Keywords: Sinusitis, nasal polyps, extracellular DNA traps, eosinophils, neutrophils, allergy, immunology, biomarker
INTRODUCTION
Chronic rhinosinusitis (CRS) is a heterogeneous disease characterized by local inflammation of the nasal cavity and sinuses that persists for at least 12 weeks.1 CRS is classified into two distinctive phenotypes: CRS with nasal polyps (CRSwNP) and without nasal polyps (CRSsNP).1 In Western countries, CRSwNP shows high eosinophilic infiltration.2,3 In contrast, in Asia, CRSwNP has been predominantly associated with neutrophil infiltration.4,5,6,7 Since the presence of mucosal eosinophilia is correlated with disease severity, endotyping has been emphasized in both phenotypes.8 However, type 2 CRS shows an even higher recurrence rate in the presence of staphylococcal enterotoxin-specific immunoglobulin E (IgE), Charcot-Leyden crystals (CLCs), and higher neutrophilic infiltration with neutrophil extracellular trap formation (NETosis), as well as higher eosinophilic infiltration with eosinophil extracellular trap formation (EETosis).3,8,9 It is understood that neutrophils and eosinophils are recruited at sites of damaged epithelium and capture pathogens at defect sites.10,11 When a cell reaches the limit of its phagocytic capacity, it instead tries to capture and immobilize pathogens by releasing a mixture of DNA, histones, and granular enzymes, which forms a structure called an extracellular DNA trap.12,13 Extracellular DNA traps were first reported in neutrophils,14 but this process has also been observed in other immune cells, including eosinophils.12,13 We define the mechanism of neutrophil extracellular DNA trap (NET) formation as NETosis and that of eosinophil extracellular trap (EET) formation as EETosis.15,16 The excess production of DNA traps from either eosinophils or neutrophils increases the viscosity of the secretions.17,18 Difficult-to-remove secretions with a high level of cytotoxic granular enzymes can further damage the epithelium or impair the barrier repair, thereby exacerbating disease severity.11,19 The clinical importance of EETosis and NETosis in CRS is increasingly emerging, but the roles and relationships of these phenomena remain vaguely understood. Some previous studies of NETosis or EETosis in CRS attempted to distinguish NETosis versus EETosis based on the shapes of the formations. However, neutrophils and eosinophils have the ability to change themselves into various shapes,20 and their physical morphology can be affected by the adjacent tissue environment and density.21,22 Therefore, we instead analyzed NETosis and EETosis using citrullinated histone H3 staining. Since the anti-histone antibody does not penetrate the preserved plasma membrane and nuclear envelope, citrullinated histone H3 is not stained in fixed, non-permeabilized eosinophils with intact nuclei.16 In this study, we aimed to investigate the clinical role of citrullinated histone H3-positive NETosis and EETosis in refractory CRSwNP.
MATERIALS AND METHODS
Sample collection and clinical data
Sinonasal polyp tissues were obtained from CRSwNP patients during endoscopic sinus surgery. All participants were included in the study after institutional review board (IRB) approval and informed consent. Patients with 1) age younger than 18 years; 2) unilateral rhinosinusitis, antrochoanal polyp, allergic fungal sinusitis, cystic fibrosis, or immotile ciliary disease; 3) a history of antibiotics, systemic or topical corticosteroids, or other immunomodulatory treatment within 4 weeks prior to surgery were excluded.
CRSwNP was diagnosed by clinical history, physical examination, and findings on nasal endoscopy and ostiomeatal unit computed tomography (CT) of the sinuses according to the guidelines of the European Position Paper on Rhinitis and Nasal Polyps 2020 (EPOS 2020).1 Disease control status at postoperative 1 year was assessed, as previously described.23 The clinical control involved symptoms, endoscopic findings, and the usage of rescue medications as criteria. Non-refractory cases involved one who attained and maintained clinical control described as follows. The symptoms including nasal blockage, rhinorrhea/postnasal drip, facial pain/pressure, smell impairment, and sleep disturbance/fatigue should be less than 5 points on the visual analog scale. Healthy mucosa showing no abnormality such as nasal polyps, mucopurulent secretions, or swelling, should be observed under nasal endoscopic exam.24 In addition, rescue treatment should not be required for the last 6 months. Conversely, refractory patients have more than one of these problems and are defined as partly controlled or uncontrolled according to the EPOS 2020 guidelines. Lund-Mackay (LM) CT scores25, Global Osteitis Scoring Scale (GOSS) scores26, and olfactory cleft opacification (OCO) scores27 were calculated based on preoperative CT.
Immunohistochemical staining and endotyping
We measured tissue neutrophils and eosinophils using the previously described method.28 Nasal polyp tissues were fixed with 4% paraformaldehyde. Following ethyl alcohol dehydration, tissues were embedded in paraffin and sectioned (4 μm). Fixed paraffin-embedded tissue sections were then deparaffinized. After deparaffinization, 3% hydrogen peroxidase incubation and heat-antigen retrieval were done with citrate buffer (pH 6.0). The prepared tissue sections were incubated with rabbit anti-human neutrophil elastase (HNE; 1:500, Abcam, Cambridge, UK) primary antibody, a broad antibody enhancer, and polymerized horseradish peroxidase in order. The DAB detection system (Golden Bridge International Labs, Bothell, WA, USA) was used for staining. Hematoxylin counterstaining was then performed. The number of eosinophils and neutrophils were counted in the five densest visual fields (×400) by two independent observers, and the average values were calculated. Eosinophilic CRS was defined as ≥ 55 tissue eosinophils (hematoxylin and eosin) per high-power field (HPF)29 and neutrophilic CRS as ≥ 20 tissue neutrophils (HNE-positive cells)/HPF30.
Immunofluorescence staining
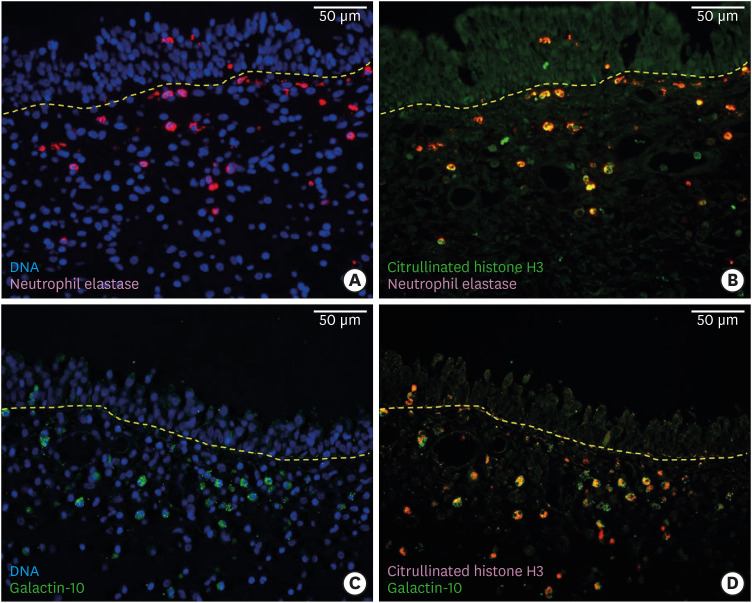
Tissue sections were prepared as described above. Each slide for NETosis and EETosis evaluation was prepared from immediately adjacent sections. The tissues were permeabilized by treatment with 0.3% Triton-X100 for 15 minutes at room temperature (RT), followed by blocking with 5% BSA solution (Bovogen Biologicals Pty Ltd., Kellor East, Australia) for 1 hour at RT. The slides were then incubated overnight at 4°C with antibodies against citrullinated histone H3 (cit-H3, 1:200, #ab174992, Abcam Inc., Cambridge, MA, USA), elastase (1:300, #MAB-91671, R&D Systems, Minneapolis, MN, USA) for NETosis evaluation and cit-H3, galactin-10 (gal-10, 1:100, #AF5447, R&D Systems) for EETosis evaluation. After being washed with 1× phosphate-buffered saline, the section slides were incubated with Cy3-conjugated IgG (1:1,000, #ab97035, Abcam; #ab6939, Abcam) and Alexa Fluor® 488-conjugated IgG (1:1,000, #ab150113, Abcam; A-11008, Invitrogen, Waltham, MA, USA). The cell nucleus was stained with Hoechst (1:1,000, #H1399, Invitrogen). Each slide was analyzed by CELENA® S Digital Cell Imaging System (Logos Biosystems, Anyang, Korea). Acquired images were merged using Image J (version 1.8.0; Bharti Airtel Ltd, New Delhi, India) for the quantification. For neutrophil and NETosis analysis, positivity for both DNA (blue) and elastase (red) staining was used to identify a neutrophil. Co-expression of cit-H3 (green) and elastase (red) indicated NETosis. For eosinophil and EETosis analysis, eosinophils were determined with DNA (blue) and gal-10 (green) double positivity, and EETosis was defined as cit-H3 (red) and gal-10 (green) positivity (Fig. 1). The average count of neutrophils, eosinophils, NETs and EETs in three representative fields containing the greatest cell infiltration throughout the tissue was analyzed by two independent observers. We held a consensus meeting to resolve controversies by inviting third independent observer. The average number of NETs (or EETs) was divided by the average number of neutrophils (or eosinophils) which analyzed in the same field; these parameters were described as the NET/neutrophil (%) and EET/eosinophil (%) ratios. Negative and healthy controls are indicated in the Supplementary Figs. S1 and S2.
Fig. 1. Representative images of the neutrophils, NETosis, eosinophils, EETosis are shown. (20× Magnification, Scale bars = 50 µm). (A) Neutrophils are stained with DNA (blue) and elastase (red). (B) NETosis is stained with citrullinated histone H3 (green) and elastase (red). (C) Eosinophils are stained with DNA (blue) and galactin-10 (green). (D) EETosis is stained with citrullinated histone H3 (red) and galactin-10 (green).
NET, neutrophil extracellular trap; EET, eosinophil extracellular trap.
Measurement of cytokines and IgE levels in tissue homogenates
We measured various tissue homogenate cytokine levels from 25 patients with CRSwNP using a previously described method.28 Protein concentrations of tissue extracts were determined using the Pierce 660 nm Protein Assay Kit (Thermo Fischer Scientific Inc., Waltham, MA, USA). Multiplex cytokine assay analysis kits of interleukin (IL)-5, IL-6, IL-1α, IL-1β, IL-1Rα, IL-17α, IL-18, IL-22, IL-23, IL-33, platelet-activating factor, B cell-activating factor (BAFF), matrix metallopeptidase (MMP)-9, myeloperoxidase (MPO), C-X-C motif ligand (CXCL)-1, CXCL-8, granulocyte-macrophage colony-stimulating factor, interferon-γ, oncostatin M, plasminogen activator inhibitor-1, tumor necrosis factor (TNF)-α, and vascular endothelial growth factor from R&D Systems were used. The data were measured using a Luminex 100 reader (Luminex, Austin, TX, USA) and analyzed using MasterPlex QT version 2.0 (MiraiBio, Alameda, CA, USA). Human neutrophil elastase (DY9167-05; R&D Systems), α1 anti-trypsin (DY1268-15; R&D Systems), IL-36α, β, γ (DY1078-05, DY1099-05, DY2320-05; R&D Systems), and IL38 (DY9110-05; R&D Systems) measurements were performed using commercially available enzyme-linked immunosorbent assay (ELISA) kits. IgE levels were measured using the Human IgE ELISA kit (K3231066: KOMA, Seoul, Korea). All assays were performed in duplicate, and protein levels were normalized to the concentration of total protein.
Statistical analyses
Continuous variables are presented with medians with interquartile ranges. The χ2 test was used for categorical data. Nonparametric methods (the two-tailed Mann-Whitney U test and the Kruskal-Wallis test) are performed for multiple independent samples. The correlations between variables were assessed using the Spearman correlation test. Target values were normalized by applying log(1+x) data transformation. Linear regression analysis was performed, using the least-squared method. Multivariate linear regression was performed with the stepwise selection method. Values that were statistically significant in the univariate analysis for potential prognostic factors were included in the binary logistic regression analysis for multivariate analysis. The z-score was calculated to evaluate the relationship between NET and EET counts, and the following groups were defined using the z-score: low extracellular trap formation (ETosis), NETosis-predominant, EETosis-predominant, and high-ETosis. P < 0.05 were considered significant. Missing values were pairwise deleted. All statistical analyses were performed using R (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
Study approval
This study was performed according to the Helsinki Declaration. The study was approved by the Ethical Committee of Boramae Medical Center, Seoul, Korea (IRB No.20-2019-100). Written informed consent was obtained from all participants before sample collection.
RESULTS
EETosis plays a prognostic role in CRSwNP
The clinical characteristics of the 117 CRSwNP patients enrolled in this study are listed in Table 1. The majority of the participants (76.07%) were men, and the median age was 52 years (interquartile range [IQR] = 23.00 years). Asthma was present as a comorbidity in 12.82%. There were 63 (53.85%) refractory cases and 54 (46.15%) non-refractory cases (Supplementary Table S1). Their median follow-up period was 23.50 (mean = 25.96, IQR = 26.00) and 16.00 (mean = 23.42, IQR = 23.25), respectively. The patients’ tissues were analyzed for neutrophils, eosinophils, NET counts, and EET counts. Eosinophilic CRS (≥ 55 tissue eosinophils/HPF) was present in 27.35% of patients. The median EET count was 10.00 (mean = 21.56, IQR = 30.33) and the median percentage of EET/eosinophil ratio was 72.22% (mean = 60.27%, IQR = 50.15%). The median NET count was 13.33 (mean = 13.33, IQR = 26.67) and the median NET/neutrophil ratio was 58.06% (mean = 57.34%, IQR = 47.86%). Fifteen cases (12.82%) were revision surgery and included in this study.
Table 1. Patient demographics.
| Characteristics | Values (n = 117) | |
|---|---|---|
| Sex (male:female) | 89 (76.07):28 (23.93) | |
| Age (yr) | 52.00 (60.00–37.00) | |
| Comorbidity | ||
| Asthma | 15 (12.82) | |
| AR | 52 (44.44) | |
| Lab | ||
| Total IgE (IU/mL) | 118.0 (286.00–48.20) | |
| Blood eosinophil (%) | 3.40 (6.40–1.80) | |
| Absolute eosinophil count (cell/μL) | 236.7 (391.60–119.30) | |
| Type 2 CRS (≥ 55 eosinophils/HPF) | 32 (27.35) | |
| Tissue eosinophil (/HPF) | 13.00 (70.00–3.50) | |
| Tissue neutrophil (/HPF) | 16.00 (38.00–6.00) | |
| EET count (/×200) | 10.00 (32.00–1.67) | |
| NET count (/×200) | 13.33 (32.00–5.33) | |
| EET/eosinophil (%) | 72.22 (88.61–38.46) | |
| NET/neutrophil (%) | 58.06 (83.14–35.28) | |
Values were stated as numbers (percentages) or median (Q3-Q1).
CRSwNP, chronic rhinosinusitis with nasal polyp; AR, allergic rhinitis; Ig, immunoglobulin; EET, eosinophil extracellular trap; NET, neutrophil extracellular trap; HPF, high power field.
We investigated the factors correlated with refractoriness. As expected, type 2 biomarkers (tissue eosinophils/HPF: ρ = 0.261, P = 0.004; absolute blood eosinophil count: ρ = 0.275, P = 0.003; asthma: ρ = 0.209, P = 0.024), LM CT score (ρ = 0.333, P < 0.001), GOSS (ρ = 0.295, P = 0.001), and OCO score (ρ = 0.217, P = 0.019) showed statistically significant correlations with refractoriness. Age showed a negative correlation (ρ = −0.272, P = 0.003) (Supplementary Table S2).
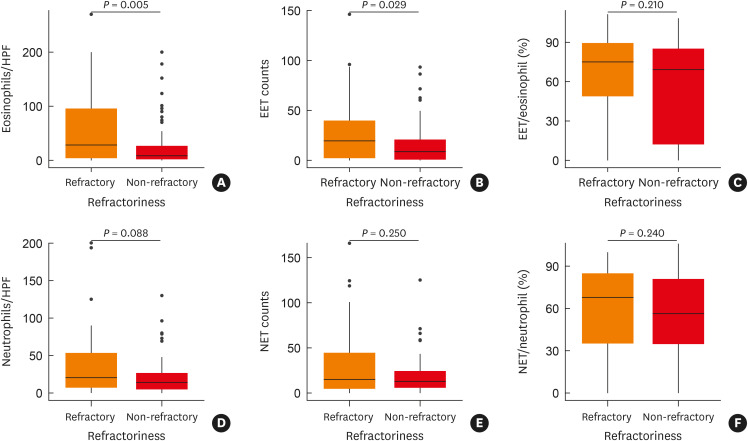
Significant differences were also found between the refractory and non-refractory polyp groups in the tissue eosinophil count (P = 0.005) and EET count (P = 0.029) (Fig. 2A-C). The median tissue eosinophil counts of refractory and non-refractory groups were 28.20/HPF and 8.60/HPF. The mean values were 56.05/HPF and 27.99/HPF, respectively, and the IQRs were 91.22/HPF and 24.10/HPF each. The median, mean, and IQR EET counts for the refractory group were 19.50/HPF, 28.08/HPF, and 37.25/HPF, respectively. In the non-refractory group, the median, mean, and IQR values for the EET count were 8.67/HPF, 15.97/HPF, and 19.83/HPF, respectively. The median EET/eosinophil ratio was 75.00% and 69.23% for the refractory and non-refractory groups (mean values: 65.71% and 55.61%; IQR: 40.45% and 73.19%). However, the tissue neutrophil count was not significantly different (P = 0.088) between the refractory and non-refractory polyp groups using the Mann-Whitney test (Fig. 2D-F). The median tissue neutrophil count was 20.00/HPF and 13.80/HPF for the refractory and non-refractory groups (mean: 35.81/HPF and 21.68/HPF; IQR: 46.38/HPF and 21.75/HPF, respectively). The median NET count was 14.83/HPF and 12.67/HPF for the refractory and non-refractory groups (mean: 30.98/HPF and 18.65/HPF; IQR: 39.75/HPF and 18.33/HPF, respectively). The median NET/neutrophil ratio was 67.86% and 56.52% in the refractory and non-refractory groups (mean: 60.56% and 54.58%; IQR: 49.38% and 45.93%, respectively). The χ2 test was performed to check whether there was a significant difference in prognosis between the eosinophilic and non-eosinophilic CRS groups or between the neutrophilic and non-neutrophilic CRS groups. A significant difference in refractoriness was found between the eosinophilic and non-eosinophilic groups (P = 0.010), but not between the neutrophilic and non-neutrophilic groups (P = 0.196) (Supplementary Table S3). Regarding the prognosis of CRSwNP, the results pointed to eosinophils as the most important main effector cells. EETosis was also found to play a prognostic role in CRSwNP. The Spearman correlation analysis confirmed that EET count was related to refractoriness, the blood eosinophil count, and the Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis (JESREC) classification (Table 2).
Fig. 2. Comparison of refractory and non-refractory groups regarding tissue eosinophils, EETs, EETs/eosinophil (%), neutrophils, NETs, and NET/neutrophil (%). (A) Tissue eosinophil counts (eosinophils per high-power field) were significantly different between the refractory and non-refractory chronic rhinosinusitis with nasal polyps (CRSwNP) groups (P = 0.005). (B) EET counts were significantly higher in the refractory CRSwNP group (P = 0.029). (C) The percentage of EET-forming eosinophils (%) was not different between the refractory and non-refractory CRSwNP groups (P = 0.210). (D) Tissue neutrophil counts (neutrophils per high-power field) in refractory and non-refractory groups (P = 0.088). (E) Neutrophil extracellular trap (NET) counts in the refractory and non-refractory groups (P = 0.250). (F) The percentage of NET-forming neutrophils in the refractory and non-refractory groups (P = 0.240).
EET, eosinophil extracellular trap; NET, neutrophil extracellular trap; CRSwNP, chronic rhinosinusitis with nasal polyp.
Table 2. EET count and clinical data correlations.
| Variables | ρ | P value |
|---|---|---|
| Tissue eosinophil (/HPF) | 0.809 | < 0.001* |
| EET/eosinophil (%) | 0.629 | < 0.001* |
| Tissue neutrophil (/HPF) | −0.252 | 0.006* |
| NET count | −0.129 | 0.167 |
| NET/neutrophil (%) | 0.017 | 0.856 |
| Refractoriness | 0.197 | 0.033* |
| Blood eosinophils (%) | 0.465 | < 0.001* |
| Absolute blood eosinophil count | 0.482 | < 0.001* |
| JESREC classification | 0.467 | < 0.001* |
| Lund-Mackay score | 0.010 | 0.918 |
| Global Osteitis Score Scale | −0.032 | 0.735 |
| Olfactory cleft opacification score | 0.091 | 0.329 |
| Smoking (pack-yr) | −0.119 | 0.200 |
| Alcohol (g/mon) | −0.055 | 0.559 |
EET, eosinophil extracellular trap; HPF, high power field; NET, neutrophil extracellular trap; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis.
*Statistically significant (P < 0.05).
NETosis plays a prognostic role in neutrophilic CRSwNP
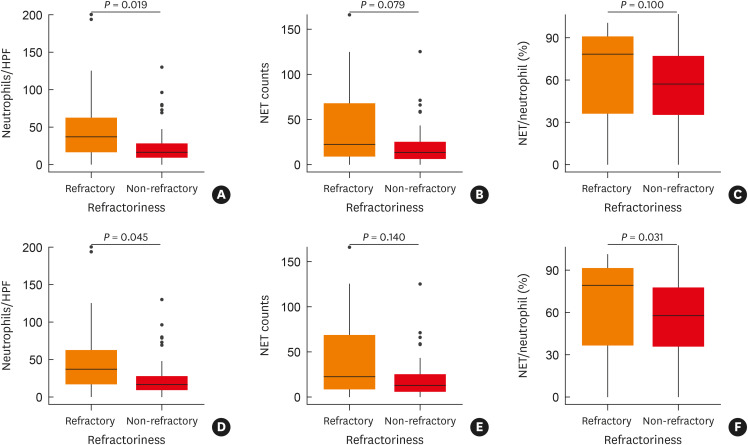
As mentioned above, there was no significant difference in the prognosis according to whether polyps were neutrophilic or not. However, among non-eosinophilic CRSwNP patients (< 55 tissue eosinophils/HPF), the tissue neutrophil and NET counts were higher in the refractory group. The tissue neutrophil counts were significantly different between the refractory and non-refractory groups of patients with non-eosinophilic CRSwNP (median: 37.00/HPF and 15.95/HPF, mean: 47.06/HPF and 24.54/HPF, IQR: 45.60/HPF and 18.78/HPF, respectively; P = 0.019) The NET count in the refractory group tended to be higher than in the non-refractory group (median: 22.33/HPF and 13.33/HPF, mean: 40.07/HPF and 19.81/HPF, IQR: 59.00/HPF and 19.00/HPF; P =0.079) (Fig. 3A-C). Among neutrophilic CRSwNP patients (≥ 20 tissue neutrophils /HPF), the tissue neutrophil count and the NET/neutrophil ratio were significantly different between the refractory group and the non-refractory group (P = 0.045, 0.031, respectively) (Fig. 3D-F).
Fig. 3. Comparison of refractory and non-refractory groups in non-type 2 CRS (non-eosinophilic, < 55 eosinophils/HPF) and neutrophilic CRS (≥ 20 neutrophils/HPF). (A) Comparison of tissue neutrophil counts comparison in non-type 2 CRS (P = 0.019). (B) Comparison of neutrophil extracellular trap (NET) counts in non-type 2 CRS (P = 0.079). (C) Comparison of the NETs/neutrophil ratio (%) in non-type 2 CRS (P = 0.100). (D) Comparison of tissue neutrophil counts in neutrophilic CRS (P = 0.045). (E) Comparison of NET counts comparison in neutrophilic CRS (P = 0.140). (F) Comparison of the NETs/neutrophils ratio (%) in neutrophilic CRS (P = 0.031).
NET, neutrophil extracellular trap; CRS, chronic rhinosinusitis; HPF, high-power field.
NETosis was found to be related to the LM CT score (Spearman correlation ρ = 0.207, P = 0.025; linear regression P = 0.040) and GOSS (Spearman correlation ρ = 0.287, P = 0.002; linear regression P = 0.002) (Table 3, Supplementary Table S4)
Table 3. NET count and clinical data correlations.
| Variables | ρ | P value |
|---|---|---|
| Neutrophil count (/HPF) | 0.576 | < 0.001* |
| NET/neutrophil (%) | 0.505 | < 0.001* |
| Eosinophil count (/HPF) | −0.103 | 0.268 |
| EET count | −0.129 | 0.167 |
| EET/eosinophil count (%) | 0.004 | 0.964 |
| Refractoriness | 0.108 | 0.248 |
| Lund-Mackay score | 0.207 | 0.025* |
| Global Osteitis Score Scale | 0.287 | 0.002* |
| Olfactory cleft opacification score | 0.113 | 0.225 |
| Blood eosinophil (%) | −0.251 | 0.006* |
| Absolute blood eosinophil count | −0.204 | 0.027* |
| JESREC classification | −0.235 | 0.012* |
| Smoking (pack-yr) | 0.147 | 0.114 |
| Alcohol (g/mon) | 0.071 | 0.447 |
NET, neutrophil extracellular trap; HPF, high power field; EET, eosinophil extracellular trap; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis.
*Statistically significant (P < 0.05).
The possibility that NETosis had the greatest effect on the GOSS or LM score was supported via modeling the tissue eosinophil count, neutrophil count, NET count, EET count, NET/neutrophil proportion, and EET/eosinophil proportion variables by using multivariate linear regression analysis (Supplementary Table S4). We infer from these findings that not only NETosis would affect the LM score and GOSS, but also the proportion of NETosis occurrence would predict a poor prognosis in neutrophilic CRS.
CRSwNP showing high extracelluar trap formation is strongly associated with refractoriness
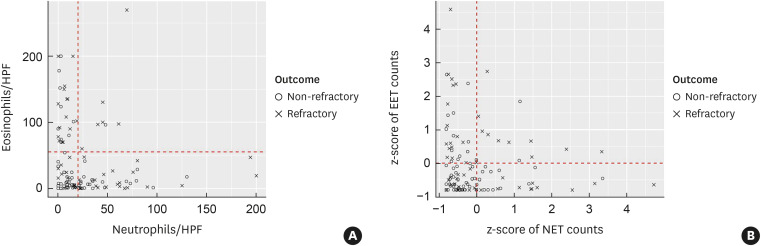
Summarizing the previous analysis, refractoriness in CRSwNP was found to be significantly correlated to the tissue eosinophil and EET counts (Fig. 2). Tissue neutrophil and NET counts were also correlated with refractoriness in non-eosinophilic or neutrophilic CRSwNP patients (Fig. 3). Since both eosinophils and neutrophils affect the pathophysiology of CRSwNP, we drew a scatter plot to visualize the relationship between tissue eosinophils and neutrophils (Fig. 4A) and a scatter plot for z-scored NETosis versus z-scored EETosis (Fig. 4B). Both scatter plots showed inverse correlations. When we normalized the distribution of NET and EET counts showing the relationship to mean values and classified the results into four groups according to a z-score of 0 (Fig. 4B, Table 4), the proportion of refractoriness was higher in high-ETosis group than in any other groups (refractory rate: 30.77% in the low-ETosis group, 47.83% in the NETosis-prominent group, 56.67% in the EETosis-prominent group, and 83.33% in the high-ETosis group; P = 0.005).
Fig. 4. Scatter plot for (A) tissue leukocyte counts (/HPF). (B) z-scores of EET and NET counts.
HPF, high-power field; EET, eosinophil extracellular trap; NET, neutrophil extracellular trap.
Table 4. Refractoriness related to ETosis.
| ETosis | Low ETosis | NETosis predominant | EETosis predominant | High ETosis | P value |
|---|---|---|---|---|---|
| Non-refractory | 36 (69.23) | 12 (52.17) | 13 (43.33) | 2 (16.67) | 0.005* |
| Refractory | 16 (30.77) | 11 (47.83) | 17 (56.67) | 10 (83.33) | |
| Total | 52 | 23 | 30 | 12 |
Four groups are classified into low ETosis (negative z-score of NETosis count and EETosis count), high NETosis (positive z-score of NETosis count and negative z-score of EETosis count), high EETosis (positive z-score of EETosis count and negative z-score of NETosis count), high ETosis (positive z-score of NETosis count and EETosis count).
ETosis, extracellular trap formation; NETosis, neutrophil extracellular trap formation; EETosis, eosinophil extracellular trap formation.
*Statistically significant (P < 0.05).
Cytokine correlations
To find potential substances affecting NETosis, a multiplex cytokine assay was performed from 25 samples to identify associations (Supplementary Table S5). The EET count showed significant correlations with IL-5 (ρ = 0.524, P = 0.007) and IgE (ρ = 0.547, P = 0.005) levels. The NET count showed significant correlations with elastase/serpina1 (ρ = 0.662, P < 0.001), BAFF (ρ = 0.641, P = 0.001), MMP-9 (ρ = 0.494, P = 0.012), CXCL-1 (ρ = 0.464, P = 0.020), and TNF-α (ρ = 0.508, P = 0.010).
DISCUSSION
The importance of the effects of NETosis and EETosis on clinical outcomes in CRS is increasingly recognized, but these relationships have not yet investigated on large-scale. This study is valuable in that it analyzed a relatively large number of patient samples using clinical data.
Our data showed that the tissue eosinophil and EET counts were the most important prognostic factors. The tissue eosinophil and EET counts seemed to have a similar tendency in correlation to clinical indicators such as the JESREC score and type 2 cytokines, including IL-5, and IgE.31,32 Eosinophils make up a relatively small percentage of circulating white blood cells (about 0.0–4.0%).33 The majority of eosinophils reside within mucosal tissues, identifying and responding rapidly to pathogens.33 Eosinophils have phagocytic capabilities similar to those of neutrophils, but are less efficient.33 Eosinophils in the tissue are regarded as highly active with certain immune responses, and it is thought that a high proportion of tissue eosinophils shows a tendency for EETosis. Previous studies have investigated the presence of EETosis in eosinophilic mucin secretions and raised the possibility that EETs may further damage the epithelium or prevent barrier repair by remaining around the tissue for a long time with a high level of cytotoxic granular enzymes.11,18,19 We expect highly active eosinophils and EETosis to play a similar role within the tissue and to be involved in disease and tissue damage pathogenesis.
In non-eosinophilic CRS (≤ 55 tissue eosinophils/HPF), neutrophils were related to refractoriness. In patients with a high tissue neutrophil count (neutrophilic CRS, ≥ 20 tissue neutrophils/HPF), a higher tissue neutrophil count and a higher NET/neutrophil ratio were associated with a worse prognosis. Unlike eosinophils, neutrophils maintain a precise balance of about 70% of all white blood cells and mainly patrol in the blood.34 Neutrophils display various phenotypes and have a high degree of functional heterogeneity determined by particular stimuli or various organs.35,36 Terminally migrated mucosal neutrophils have a hyperactive phenotype with increased adhesion and internalization of microbes and NET formation capacities.37,38 In 2014, Branzk and colleagues reported that neutrophils sensed pathogen size and regulated NETosis.39 Phagocytosis is a rapid process capable of engulfing pathogens within hours,33 but NETosis is a slow process.39 Therefore, phagocytosis, which can quickly remove pathogens, plays a major role when fewer and smaller pathogens have invaded, and NETosis seems to function as an alternative defense mechanism that occurs when a cell’s phagocytic capacity is overwhelmed.12,13
The outcomes of neutrophil responses are mostly context-dependent.36,40 We previously suggested that a high ratio of neutrophil elastase to α1 anti-trypsin (a potent inhibitor of neutrophil elastase) may play a role in refractoriness in Asian CRSwNP patients.28 This study showed that the ratio of elastase to α1 anti-trypsin was correlated with the NET count, suggesting that NETosis may influence refractoriness in Asian patients with CRSwNP. Moreover, the DNA, histones, and contents of NETs are highly detrimental to tissues, but they also have beneficial effects in certain conditions. For example, MPO diminishes toxic effects and protects the host from lipopolysaccharide-induced tissue injury.41 Aggregated NETs promote the resolution of neutrophilic inflammation by degrading cytokines and chemokines via serine proteases (including neutrophil elastase) and disrupting neutrophil recruitment and activation.42 Therefore, it seems that an imbalance between NET and neutrophils leads to tissue damage and play a prognosis-related role.
We expected that there might be some additive or synergistic effects between eosinophils and neutrophils. However, the scatter plot showed an inverse correlation between tissue eosinophil and neutrophil count, as well as between NETosis and EETosis. The same trend was reported in previous studies conducted in China.43 EETosis is known to be the basis of CLCs, which are found at the site of eosinophil infiltration and promote neutrophilic inflammation as well as NETosis.16,44,45 This is a possible explanation of why most patients with severe type 2 CRS show mixed neutrophilic-eosinophilic inflammation, and the formation of NETs ultimately aggravates type 2 inflammation.46 A previous study described positive correlations of tissue neutrophils with EETosis and the number of CLCs in patients with type 2 CRSwNP.46 Hexagonal bipyramidal autocrystallized CLCs are known to be formed from a galectin-10 (gal-10) base.47 In order to examine distinctive autocrystallized CLCs, we stained for gal-10, but CLCs were not observed. This likely occurred because there were few severe type 2 CRS cases in our study sample. In our study, the proportion of mixed eosinophilic and neutrophilic CRS cases was 5.13%. Bachert et al. reported that 40% of Western patients had severe type 2 CRSwNP.9 Studies in Asia reported that the prevalence of severe type 2 CRS was generally less than 10% (4.4%–11.2%), and our results are similar to those of previous studies.28,43 When investigating mixed eosinophilic-neutrophilic CRS in Asia, it is necessary to find causes of mixed inflammation other than autocrystallized CLCs.
Our study has several limitations. We could not adopt quantitative methods measuring EETosis or NETosis. Future prospective studies including in vitro experiments or collection of fresh tissues would be required and valuable for this topic. Furthermore, we demonstrated the potential interaction between EETosis and NETosis by describing high refractoriness in the high ETosis group, but we could not prove synergistic or additive effects between EETosis and NETosis. Further mechanistical studies are needed.
In summary, the results of this study suggest that tissue eosinophilia and EETosis primarily may affect refractoriness in CRSwNP patients, and that tissue neutrophilia and NETosis can affect the prognosis of non-eosinophilic CRSwNP or neutrophilic CRSwNP.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Research Foundation of Korea (NRF-2019R1A2C2087170 to D.W.K).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Demographic characteristics of refractory and non-refractory patients
Factors correlated with refractoriness
Refractoriness difference between each endotypes of chronic rhinosinusitis with nasal polyps (CRSwNP)
Regression analysis of LM score and GOSS versus NET count
Cytokine profile correlation
Negative control (20× magnification, scale bars = 50 µm). (A) Negative control of Fig. 1A. (B) Negative control of Fig. 1B. (C) Negative control of Fig. 1C. (D) Negative control of Fig. 1D.
The NETosis and EETosis staining in healthy control (20× magnification, scale bars = 50 µm). (A) Tissue eosinophils. (B) EETs. (C) Negative control of Supplementary Fig. S2A. (D) Negative control of Supplementary Fig. S2B. (E) Tissue neutrophils. (F) NETs. (G) Negative control of Supplementary Fig. S2E. (H) Negative control of Supplementary Fig. S2F.
References
- 1.Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- 2.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim DH, Kim SW. Considerations for the use of biologic agents in patients with chronic rhinosinusitis with nasal polyposis. Clin Exp Otorhinolaryngol. 2021;14:245–246. doi: 10.21053/ceo.2021.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–1353. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Kim DW. Can neutrophils be a cellular biomarker in Asian chronic rhinosinusitis? Clin Exp Otorhinolaryngol. 2019;12:325–326. doi: 10.21053/ceo.2019.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalmuratova R, Shin HW. Crosstalk between mucosal inflammation and bone metabolism in chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2021;14:43–49. doi: 10.21053/ceo.2020.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DW, Yang SK. Application of biologics in treating chronic rhinosinusitis with nasal polyps in Asian populations. Clin Exp Otorhinolaryngol. 2022;15:125–126. doi: 10.21053/ceo.2022.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 9.Bachert C, Desrosiers MY, Hellings PW, Laidlaw TM. The role of biologics in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2021;9:1099–1106. doi: 10.1016/j.jaip.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Hall CH, Campbell EL, Colgan SP. Neutrophils as components of mucosal homeostasis. Cell Mol Gastroenterol Hepatol. 2017;4:329–337. doi: 10.1016/j.jcmgh.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gevaert E, Zhang N, Krysko O, Lan F, Holtappels G, De Ruyck N, et al. Extracellular eosinophilic traps in association with Staphylococcus aureus at the site of epithelial barrier defects in patients with severe airway inflammation. J Allergy Clin Immunol. 2017;139:1849–1860.e6. doi: 10.1016/j.jaci.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Daniel C, Leppkes M, Muñoz LE, Schley G, Schett G, Herrmann M. Extracellular DNA traps in inflammation, injury and healing. Nat Rev Nephrol. 2019;15:559–575. doi: 10.1038/s41581-019-0163-2. [DOI] [PubMed] [Google Scholar]
- 13.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 14.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 15.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 16.Fukuchi M, Miyabe Y, Furutani C, Saga T, Moritoki Y, Yamada T, et al. How to detect eosinophil ETosis (EETosis) and extracellular traps. Allergol Int. 2021;70:19–29. doi: 10.1016/j.alit.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papayannopoulos V, Staab D, Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One. 2011;6:e28526. doi: 10.1371/journal.pone.0028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, et al. Eosinophil extracellular trap cell death-derived DNA traps: their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016;137:258–267. doi: 10.1016/j.jaci.2015.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueki S, Tokunaga T, Fujieda S, Honda K, Hirokawa M, Spencer LA, et al. Eosinophil ETosis and DNA traps: a new look at eosinophilic inflammation. Curr Allergy Asthma Rep. 2016;16:54. doi: 10.1007/s11882-016-0634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts RE, Hallett MB. Neutrophil cell shape change: mechanism and signalling during cell spreading and phagocytosis. Int J Mol Sci. 2019;20:1383. doi: 10.3390/ijms20061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borenstein A, Fine N, Hassanpour S, Sun C, Oveisi M, Tenenbaum HC, et al. Morphological characterization of para- and proinflammatory neutrophil phenotypes using transmission electron microscopy. J Periodontal Res. 2018;53:972–982. doi: 10.1111/jre.12595. [DOI] [PubMed] [Google Scholar]
- 22.Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, Reichner JS, et al. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114:1387–1395. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JY, Lim S, Lim HS, Kim YS, Eun KM, Khalmuratova R, et al. Bone morphogenetic protein-2 as a novel biomarker for refractory chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2021;148:461–472.e13. doi: 10.1016/j.jaci.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Psaltis AJ, Li G, Vaezeafshar R, Cho KS, Hwang PH. Modification of the Lund-Kennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope. 2014;124:2216–2223. doi: 10.1002/lary.24654. [DOI] [PubMed] [Google Scholar]
- 25.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 26.Georgalas C, Videler W, Freling N, Fokkens W. Global Osteitis Scoring Scale and chronic rhinosinusitis: a marker of revision surgery. Clin Otolaryngol. 2010;35:455–461. doi: 10.1111/j.1749-4486.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim DW, Kim JY, Jeon SY. The status of the olfactory cleft may predict postoperative olfactory function in chronic rhinosinusitis with nasal polyposis. Am J Rhinol Allergy. 2011;25:e90–e94. doi: 10.2500/ajra.2011.25.3617. [DOI] [PubMed] [Google Scholar]
- 28.Kim DK, Kim JY, Han YE, Kim JK, Lim HS, Eun KM, et al. Elastase-positive neutrophils are associated with refractoriness of chronic rhinosinusitis with nasal polyps in an Asian population. Allergy Asthma Immunol Res. 2020;12:42–55. doi: 10.4168/aair.2020.12.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toro MD, Antonio MA, Alves Dos Reis MG, de Assumpcao MS, Sakano E. Achieving the best method to classify Eosinophilic Chronic Rhinosinusitis: a systematic review. Rhinology. 2021;59:330–339. doi: 10.4193/Rhin20.512. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013;123:E1–E9. doi: 10.1002/lary.24154. [DOI] [PubMed] [Google Scholar]
- 31.Cho SW, Kim DY. Lessons from localized chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol Res. 2021;13:827–829. doi: 10.4168/aair.2021.13.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DH, Kim SW, Basurrah MA, Hwang SH. Clinical and laboratory features for various criteria of eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2022;15:230–246. doi: 10.21053/ceo.2022.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343:57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58:705–715. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371:531–539. doi: 10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moonen CG, Hirschfeld J, Cheng L, Chapple IL, Loos BG, Nicu EA. Oral neutrophils characterized: chemotactic, phagocytic, and neutrophil extracellular trap (NET) formation properties. Front Immunol. 2019;10:635. doi: 10.3389/fimmu.2019.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Pan L, Liu Z. Neutrophils as a protagonist and target in chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2019;12:337–347. doi: 10.21053/ceo.2019.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ham J, Kim J, Ko YG, Kim HY. The dynamic contribution of neutrophils in the chronic respiratory diseases. Allergy Asthma Immunol Res. 2022;14:361–378. doi: 10.4168/aair.2022.14.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reber LL, Gillis CM, Starkl P, Jönsson F, Sibilano R, Marichal T, et al. Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide. J Exp Med. 2017;214:1249–1258. doi: 10.1084/jem.20161238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 43.Yao Y, Zeng M, Liu Z. Revisiting Asian chronic rhinosinusitis in the era of type 2 biologics. Clin Exp Allergy. 2022;52:231–243. doi: 10.1111/cea.14065. [DOI] [PubMed] [Google Scholar]
- 44.Ueki S, Tokunaga T, Melo RC, Saito H, Honda K, Fukuchi M, et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood. 2018;132:2183–2187. doi: 10.1182/blood-2018-04-842260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gevaert E, Delemarre T, De Volder J, Zhang N, Holtappels G, De Ruyck N, et al. Charcot-Leyden crystals promote neutrophilic inflammation in patients with nasal polyposis. J Allergy Clin Immunol. 2020;145:427–430.e4. doi: 10.1016/j.jaci.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 46.Delemarre T, Holtappels G, De Ruyck N, Zhang N, Nauwynck H, Bachert C, et al. A substantial neutrophilic inflammation as regular part of severe type 2 chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2021;147:179–188.e2. doi: 10.1016/j.jaci.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 47.Su J. A brief history of Charcot-Leyden crystal protein/galectin-10 research. Molecules. 2018;23:2931. doi: 10.3390/molecules23112931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic characteristics of refractory and non-refractory patients
Factors correlated with refractoriness
Refractoriness difference between each endotypes of chronic rhinosinusitis with nasal polyps (CRSwNP)
Regression analysis of LM score and GOSS versus NET count
Cytokine profile correlation
Negative control (20× magnification, scale bars = 50 µm). (A) Negative control of Fig. 1A. (B) Negative control of Fig. 1B. (C) Negative control of Fig. 1C. (D) Negative control of Fig. 1D.
The NETosis and EETosis staining in healthy control (20× magnification, scale bars = 50 µm). (A) Tissue eosinophils. (B) EETs. (C) Negative control of Supplementary Fig. S2A. (D) Negative control of Supplementary Fig. S2B. (E) Tissue neutrophils. (F) NETs. (G) Negative control of Supplementary Fig. S2E. (H) Negative control of Supplementary Fig. S2F.