Introduction
Chronic spontaneous urticaria (CSU) is characterized by mast cell-driven wheals, angioedema, or both. Two endotypes of CSU have been identified, ie, autoallergic and autoimmune CSU. The latter comes with high disease activity as well as poor response to antihistamine and omalizumab treatment.1 Dupilumab was recently demonstrated to be effective in patients with antihistamine-resistant CSU.2 Here, we report a patient with CSU who did not tolerate or benefit from omalizumab, but showed complete response to dupilumab.
Case history
A 31-year-old man presented with a 3-year history of CSU, ie, recurrent spontaneous itchy wheals with worsening in the past 6 months, without angioedema or systemic symptoms. No exacerbating factors were identified. He had low total IgE, a positive autologous serum skin test, and elevated D-dimer levels, consistent with autoimmune CSU (Table I).3,4 Various antihistamines (standard-dosed and up-dosed) were previously used without any improvement. A year ago, the patient had received omalizumab, 300 mg every 4 weeks, which did not improve his urticaria. He developed cough and asthma 6 weeks after the start of omalizumab treatment. Both improved with corticosteroid inhalation and disappeared gradually after stopping omalizumab treatment.
Table I.
Patient’s treatment, disease activity, control and impact, blood markers, and autologous serum skin test reactivity
| Time | Dupilumab | UAS7 | UCT | CU-QoL | IgE1 | EOS2 | BASO3 | D-dimer4 | ASST |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | ※ | 30/42 | 6/16 | 35 | 34 | NA | NA | NA | NA |
| Wk 1 | 600 mg | 42/42 | 2/16 | NA | NA | 590 | 20 | 0.69 | +++ |
| Wk 3 | 300 mg | 4/42 | 13/16 | NA | NA | NA | NA | NA | + |
| Wk 6 | 300 mg | 4/42 | NA | 3 | NA | 660 | 50 | 0.30 | NA |
| Wk 10 | 300 mg | 2/42 | 14/16 | 2 | 71 | 590 | 50 | NA | NA |
| Wk 16 | 300 mg | 0/42 | 16/16 | 0 | 10 | 570 | 50 | NA | − |
| Wk 20 | ※ | 0/42 | 16/16 | 0 | NA | NA | NA | NA | NA |
| Wk 24 | 300 mg | 0/42 | 16/16 | 0 | 22 | 4620 | 70 | NA | NA |
| Wk 36 | ※ | 0/42 | 16/16 | 0 | NA | 6210 | 130 | NA | NA |
| Wk 37 | ※ | 0/42 | NA | NA | NA | 7930 | 130 | NA | NA |
| Wk 38 | ※ | 0/42 | 16/16 | 0 | NA | 50 | 50 | NA | NA |
| Wk 39 | ※ | 0/42 | NA | NA | NA | NA | NA | NA | NA |
| Wk 42 | ※ | 0/42 | 16/16 | 0 | NA | 460 | 50 | NA | NA |
+++, Strong positive; +, positive; −, negative; ※, not given.
1Total IgE in IU/ml, upper level of normal = 165 IU/ml; 2in cubic millimeters, upper level of normal = 500; 3in cubic millimeters, upper level of normal = 100; 4in mg/L, upper level of normal = 0.256.
ASST, Autologous serum skin test; BASO, basophils; CU-QoL, chronic urticaria quality of life questionnaire; EOS, eosinophils; NA, not assessed; UAS7, Urticaria Activity Score 7; UCT, urticaria control test.
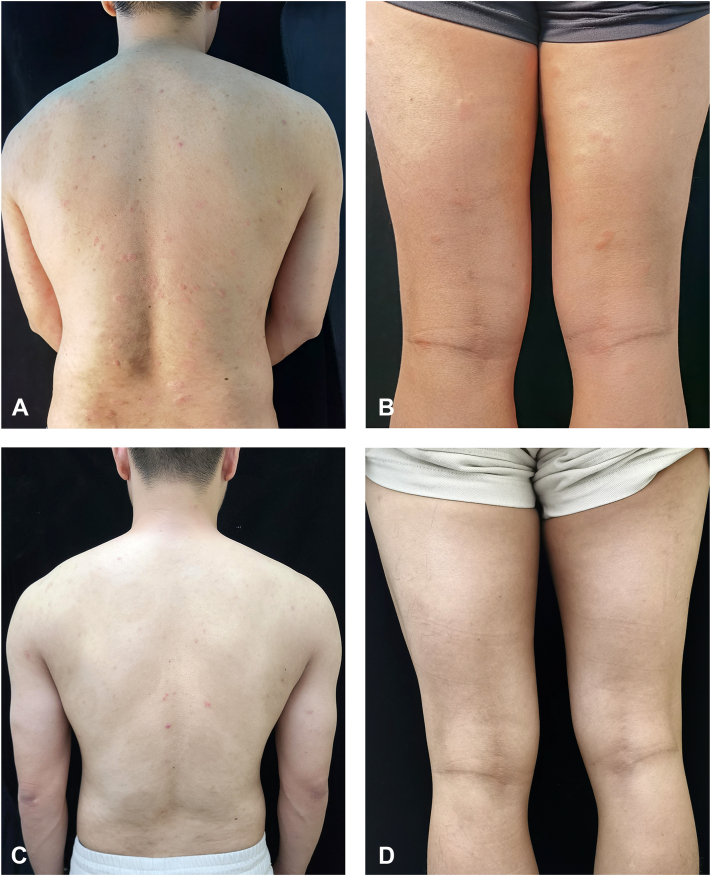
When the patient came to our clinic, he had high disease activity and was markedly impaired in his daily activities. We re-initiated omalizumab treatment (300 mg), but his urticaria worsened after 1 week (Fig 1, A and B), ie, the Urticaria Activity Score 7, increased from 30 to 42, and he developed inguinal lymph node swelling. Because of this, we discontinued omalizumab and recommended treatment with cyclosporine, which the patient declined due to concerns with the side effect profile. Dupliumab was initiated with a dose of 600 mg followed by 300 mg at weeks 3, 6, 10, 16, and 24 (Table I). Within 2 weeks after the initial dose, his wheals and pruritus improved significantly. After 16 weeks of treatment, the patient's lesions disappeared (Fig 1, C and D), he first achieved complete response: His urticaria control test score was 16, his Urticaria Activity Score 7 and chronic urticaria quality of life questionnaire values were both 0, and his autologous serum skin test was negative. At week 24, the patient showed moderate eosinophilia, with 4620/mm3 blood eosinophils (normal range: 300-500/mm3), and was scheduled for retesting 2 weeks later. He delayed this consultation until week 36, when his CSU was still in complete remission, but his blood eosinophil levels had increased to 6210/mm3 (= severe eosinophilia) and his bone marrow also showed eosinophilia (Table I). We explored possible underlying causes.5 Stool tests for parasites were negative as was testing for myeloid/lymphoid neoplasms with eosinophilia and rearrangement of pericentriolar material 1-JAK2 and factor interacting with PAPOLA and CPSF1-platelet derived growth factor receptor alpha;PAPOLA :poly(A) polymerase alpha [Homo sapiensCPSF-1:cleavage and polyadenylation specific factor 1 (FIP1L1-PDGFRA). His blood interleukin 5 levels was mildly increased (4.56 pg/ml; normal range: ≤3.1pg/ml). Pulmonary and cardiac function was normal. Dupilumab-induced eosinophilia was considered, dupilumab treatment was stopped, and oral prednisone was started at 60 mg/d and then slowly tapered, which led to a normalization of eosinophil levels. The patient maintained complete remission of his CSU at week 42, 16 weeks after the last treatment with dupilumab.
Fig 1.
Clinical features of the patient: Severe erythema and whealing were seen on the back (A) and thigh (B) before dupilumab treatment; after treatment, the erythema and wheal on the back (C) and thigh (D) completely disappeared.
Discussion
CSU can be very treatment-resistant, especially in patients with autoimmune CSU. Our patient had high disease activity, low IgE, a positive autologous serum skin test, and elevated d-Dimers, all of which have been linked to autoimmune CSU. This may explain why the patient did not respond to antihistamines and omalizumab, although he only received the latter treatment for 12 weeks the first time and only once the second time. Why this patient experienced worsening of his urticaria both times and cough and asthma upon his first treatment with omalizumab remains unexplained.
Dupilumab is a monoclonal antibody that blocks interleukin 4/13 and is used for type 2 inflammatory diseases including atopic dermatitis. CSU exhibits features of type 2 inflammation, and dupilumab has recently been reported to benefit omalizumab-naive patients with antihistamine-refractory CSU.2 Our patient had failed omalizumab treatment, twice, but did show complete response to dupilumab, after 16 weeks of treatment. The exact mechanisms of action of dupilumab in CSU remain to be characterized but may include effects on mast cells and infiltrating cells in CSU skin lesions.6 Dupilumab may also be effective in CSU by acting on B cells and downregulating IgE, with subsequent effects on IgE receptor expression.
Four months into his treatment, our patient developed eosinophilia, a rare but well-known adverse effect of dupilumab. In patients treated with dupilumab for conditions other than CSU, the rates of eosinophilia-related treatment-emergent adverse events range from 0% to 13.6%.7 In line with published guidance, we assessed our patient for other causes of eosinophilia (and found none), stopped dupilumab, and started short-term oral glucocorticoid treatment, which normalized eosinophil levels.8 It is important for physicians who treat patients with CSU with dupilumab to know that eosinophilia can occur and to monitor patients for this.
Our patient showed sustained complete response, more than 4 months after the last treatment with dupilumab. Possible reasons for this include disease-modifying effects of dupilumab upstream of skin mast cell activation as well as spontaneous remission, a hallmark feature of CSU. Our case demonstrates that patients with CSU, who can not be helped with omalizumab, may benefit from treatment with dupilumab. This further supports the development of dupilumab as a novel treatment for CSU.
Conflicts of interest
Dr Maurer is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, GSK, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Pfizer, Roche, Sanofi/Regeneron, Third Harmonic Bio, UCB, and Uriach. Drs Zhu, Fok, Lin, and Su have no conflicts of interest to declare.
Acknowledgments
We are most grateful to the patient reported here for his cooperation and providing detailed information.
Footnotes
Drs Zhu and Su contributed equally to this article.
Drs Lin and Su are cofirst authors.
Funding sources: Intramural funds of Fujian Medical University Union Hospital2021XH026.
IRB approval status: Approval.
Patient consent: The patient gave consent for his photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
Contributor Information
Lihang Lin, Email: 460879404@qq.com.
Huichun Su, Email: suhuichun@163.com.
References
- 1.Zuberbier T., Abdul Latiff A.H., Abuzakouk M., et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77(3):734–766. doi: 10.1111/all.15090. [DOI] [PubMed] [Google Scholar]
- 2.Munoz-Bellido F.J., Moreno E., Davila I. Dupilumab: a review of present indications and off-label uses. J Investig Allergol Clin Immunol. 2022;32(2):97–115. doi: 10.18176/jiaci.0682. [DOI] [PubMed] [Google Scholar]
- 3.Maurer M., Khan D.A., Elieh Ali Komi D., Kaplan A.P. Biologics for the use in chronic spontaneous urticaria: when and which. J Allergy Clin Immunol Pract. 2021;9(3):1067–1078. doi: 10.1016/j.jaip.2020.11.043. [DOI] [PubMed] [Google Scholar]
- 4.Altrichter S., Fok J.S., Jiao Q., et al. Total IgE as a marker for chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2021;13(2):206–218. doi: 10.4168/aair.2021.13.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shomali W., Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94(10):1149–1167. doi: 10.1002/ajh.25617. [DOI] [PubMed] [Google Scholar]
- 6.Kay A.B., Clark P., Maurer M., Ying S. Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous ('idiopathic') urticaria. Br J Dermatol. 2015;172(5):1294–1302. doi: 10.1111/bjd.13621. [DOI] [PubMed] [Google Scholar]
- 7.Wechsler M.E., Klion A.D., Paggiaro P., et al. Effect of dupilumab on blood eosinophil counts in patients with asthma, chronic rhinosinusitis with nasal polyps, atopic dermatitis, or eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2022;10(10):2695–2709. doi: 10.1016/j.jaip.2022.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Caminati M., Olivieri B., Dama A., et al. Dupilumab-induced hypereosinophilia: review of the literature and algorithm proposal for clinical management. Expert Rev Respir Med. 2022;16(7):713–721. doi: 10.1080/17476348.2022.2090342. [DOI] [PubMed] [Google Scholar]