Abstract
Background
Continuous venovenous hemodiafiltration (CVVHDF) is one of the treatments of critically ill children presenting severe acute liver failure. This affliction might be induced by HSV infection requiring a treatment by acyclovir. Continuous kidney replacement therapy (CKRT) can alter its pharmacokinetics, according to its physicochemical properties and CVVHDF settings.
Case–Diagnosis/Treatment
The patient was a 21-month-old female presenting liver failure with hyperammonemia treated by acyclovir with presumed HSV infection. CKRT was initiated on day 1 with substantial replacement and dialysate flow rates (respectively 75 and 220 mL/kg/h). Acyclovir was intravenously administered every 8 h with a 1-h infusion of 500 mg/m2. Plasma and effluent concentrations were measured by liquid chromatography-tandem mass spectrometry assay to estimate the area under a curve (AUC) and CKRT clearance by 2 methods (one based on pre- and post-filter concentrations and the other one on dialysate flow rates). Clearance was estimated between 19.2 and 26.3 mL/min with the first method and between 27.6 and 44.3 mL/min with the second one. Concentrations were highly above the therapeutic index (peak concentration was measured at 28 mg/L), but AUC was appropriate.
Conclusions
This case describes acyclovir pharmacokinetics during CKRT in a pediatric patient treated by acyclovir. The patient was treated with adapted exposure with the usual dosing, but lower dosing should be investigated with complementary studies.
Trial registration
ClinicalTrials.gov NCT02539407.
Keywords: Therapeutic drug monitoring, Acyclovir, Pharmacokinetics, Continuous hemodiafiltration
Introduction
Severe acute liver injury is a rare affliction associated with high morbidity and mortality. The etiologies may be numerous such as metabolic and infectious diseases. Acute liver failure can result in hyperammonemia and acute kidney injury (AKI) requiring continuous kidney replacement therapy (CKRT) [1]. HSV infection is one of the potential etiologies in neonates, and acyclovir treatment is usually initiated before polymerase chain reaction (PCR) results.
Acyclovir inhibits viral DNA polymerase through viral thymidine kinase and acyclovir triphosphate phosphorylation and thus DNA replication of herpes viruses [2]. Acyclovir is mainly eliminated through kidney glomerular filtration and kidney tubules, requiring dose adaptation in patients with altered kidney function [3, 4]. Continuous venovenous hemodiafiltration (CVVHDF) is used in critically ill patients presenting severe acute liver failure. CKRT alters drug pharmacokinetics, depending on their physicochemical properties and CVVHDF settings [3]. Because of small molecular size, low protein-binding capacity, and water solubility, acyclovir is removed by CKRT [5]. A dosing of 5–7.5 mg/kg/day was recommended under CVVHDF in adult studies [5], while optimal dosing for acyclovir in children under CKRT remains unknown. Pediatric studies recommend individual therapeutic drug monitoring [6, 7]; however, no recommendations are established leading to variabilities in practice and risk of adverse events or therapeutic failure.
We describe an illustrative case of a pediatric patient receiving acyclovir with acute liver failure, requiring a CKRT, and we depict the acyclovir exposure and the individual pharmacokinetic parameters.
Case report
Clinical course
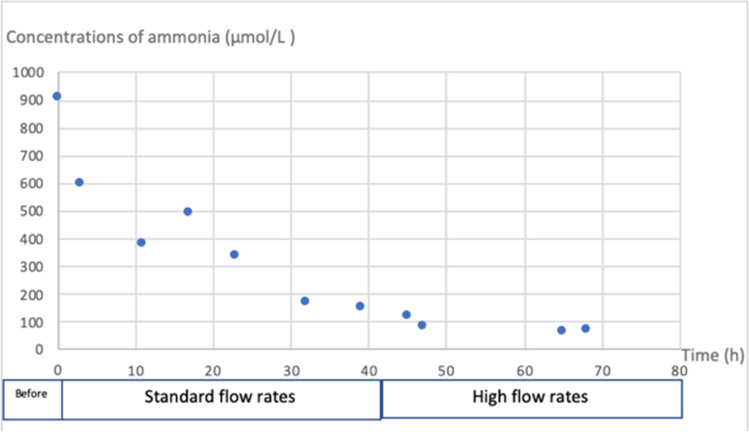
The patient was a 21-month-old female presenting acute liver failure (prothrombin < 10%) manifested by vomiting, asthenia, and hypotonia. Her weight was 10 kilos, and her body surface area was 0.46 m2. She presented altered consciousness with hyperammonemia (maximum of 907 µmol/L) motivating the initiation of CKRT. CVVHDF was started on day 1. The clearance of ammonia was inefficient, motivating the substantial increase of replacement and dialysate flow rates, respectively from 25 to 75 mL/kg/h and 50 to 220 mL/kg/h (Fig. 1).
Fig. 1.
Concentrations of ammonia before and during CKRT
Acyclovir was initiated on day 1 under the hypothesis of a herpes infection causing liver injury. No etiology was found, but COVID-19 serology was positive, and adenovirus was present in the fecal analysis. Acyclovir was stopped on day 4 when PCR and serology results for HSV came back negative. CKRT was stopped on day 7.
Kidney function was normal before CKRT initiation (creatinine 22 µmol/L). Urine output pre-CKRT was around 5.7 mL/kg/h 3; 9.5]; post-CKRT was around 1.7 mL/kg/h 1.52; 1.8] and decreased after initiation of ultrafiltrate flow to 0.88 mL/kg/h [0.61; 1.15].
The patient died after hemorrhagic shock following a hepatic biopsy with no etiology found and no complete liver recovery.
CKRT circuit and priming
CVVHDF was performed with the Prismaflex system machine™ (Baxter Int, SE) with a ST 60 set™. Pre-pump solute and dialysate fluid were Phoxilium. No anticoagulation was administrated given the hemorrhagic risk. During the whole period of drug monitoring, the blood flow rate was set to 100 mL/min (10 mL/kg/min), dialysate flow rate to 2200 mL/h (220 mL/kg/h), replacement flow rate to 750 mL/h (75 mL/kg/h), and net ultrafiltrate flow rate from 0 to 40 mL/h (0 to 4 mL/kg/h).
Drug dosing and samplings
Acyclovir was intravenously administered every 8 h with a 1-h infusion of 500 mg/m2 [8]. Acyclovir powder was reconstituted with 10 mL of solvent. No dosing adjustment was empirically made upon the CKRT initiation. Serum and effluent samples were collected in heparin lithium tubes, then were immediately centrifuged (4000 g, 5 min), and were stored at − 20 °C until analysis.
Drug concentrations
Plasmatic concentrations were measured by liquid chromatography-tandem mass spectrometry assay at the Department of Pharmacology, Cochin Hospital, as previously described (9). Serum and effluent samples were obtained at − 1, 1, 2, 4, 5, 7, and 8 h after infusion (first infusion − 1, 2, 5, 7 h and second infusion 1 day apart 1, 4, and 8 h). They were obtained after 2 days of treatment with acyclovir and after 1 day of CKRT.
Pharmacokinetics
Trough concentration was measured at 3.19 and 2.65 mg/L for a target above 0.56 mg/L (or > IC50 measured for HSV). Peak concentration was measured at 28 mg/L. CKRT clearance (CLKRT) was estimated by 2 integrated pharmacometric models [9]. The first one, the post-filter specimen, is based on an adjusted blood flow rate as follows:
where Qblood adj is the adjusted blood flow rate, based on hematocrit (Hct), blood flow rate (Qblood), blood cell concentration (CRBC), and plasma concentration of acyclovir pre- and post-filter (respectively Cpl(pre) and Cpl(post)).
For the effluent specimen, the CLKRT was calculated as follows:
Here, Qeff represents the total effluent flow rate, as the sum of dialysate, pre- and post-filter replacement fluid flow rate, and fluid removal rate Qdial, QRFpre, QRFpost, and QFRR; Ceff represents the drug concentration in the effluent.
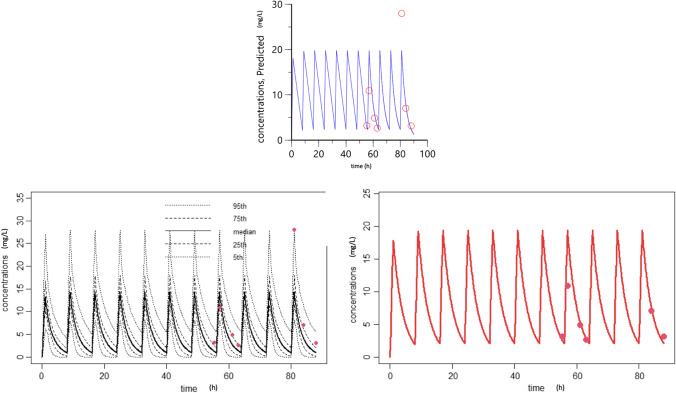
With the post-filter model, CLKRT was estimated between 19.2 and 26.3 mL/min. With the effluent model, it was estimated between 27.6 and 44.3 mL/min (Table 1). The area under a curve (AUC) was estimated with two methods: compartmental model with Phoenix™ software (one compartment model, Fig. 2) and by the Bayesian adaptation using a model for pediatric patient without dialysis, as concentrations of the patient were in the 90% prediction interval of the model (Fig. 2). The AUC was estimated respectively at 75 mg/L.h and 65 mg/L.h during 8 h. Total clearance was estimated at 52.2 mL/min. CLKRT represented 36.72 to 50.39% of total clearance, with a clearance calculated with the post-filter specimen.
Table 1.
Dosing results and kidney replacement therapy clearance
| Concentrations (mg/L) | CLKRT (1) | CLKRT (2) | Ceff/Cpl | % clearance dialysis | |||
|---|---|---|---|---|---|---|---|
| Prefilter | Postfilter | Effluent | |||||
| H1 | 28 | 20.9 | 15.6 | 19.2 | 27.6 | 0.56 | 36.72 |
| H2 | 10.93 | 8.26 | 9.34 | 19.8 | 42 | 0.85 | 38 |
| H4 | 7.03 | 5.26 | 5.34 | 21.8 | 37.9 | 0.76 | 41.66 |
| H5 | 4.87 | 3.59 | 4.37 | 24.6 | 44.1 | 0.9 | 47.09 |
| H7 | 2.65 | 2.03 | 2.17 | 26.3 | 40.3 | 0.82 | 50.39 |
| H8 | 3.15 | 2.51 | 2.8 | 21.2 | 44.3 | 0.89 | 40.53 |
CLKRT, continuous kidney replacement therapy clearance (1) post-filter specimen (2) effluent specimen; Ceff, drug concentration in effluent; Cpl, plasmatic concentration
Fig. 2.
Compartmental model with the Phoenix and Bayesian adaptation. On top, red circles depict the observed concentrations from the present case, and the solid line presents the time course of predicted concentration using the individual PK model derived from Phoenix software. On the left, red points represent the patient’s dosing, and black lines represent distribution for patients without hemodialysis with similar clinical characteristics. As the patient’s concentrations were in the 90% prediction interval, a Bayesian adaptation was made on the right to predict concentrations and AUC for our patient using this model
Endogenous clearance was estimated between 25.9 and 33 mL/min.
There was no important variability in CLKRT during CKRT with both methods: first method from 19.2 to 26.3 (σ2 = 7.7, σ = 2.78) and second method from 27.6 to 44.3 (σ2 = 39.1, σ = 6.3). Flow rates were not modified during the study period except the fluid removal rate without significant consequence on CLKRT.
Because of combined CKRT modalities (filtration and dialysis), we were not able to calculate separately the sieving coefficient and the saturation coefficient. Therefore, the ratio of Ceff/Cpl reflected the ability of the filter to eliminate acyclovir via both diffusion and convection phenomena; it ranged from 0.56 to 0.9.
Discussion
There is a lack of data describing the impact of CKRT on pharmacokinetics and dosing in pediatric patients. Dosing regimens are mainly derived from adult studies. In our study, very high flow rates were administrated to avoid neurotoxicity and cerebral edema associated with hyperammonemia. CKRT alters the pharmacokinetics of drugs, due to extracorporeal clearance, depending on related physicochemical properties and CVVHDF settings, mainly the replacement fluid administration mode and ultrafiltration flow rates [3].
For acyclovir, adult studies show 15% protein-binding capacity, and the elimination is mostly kidney with time-dependent killing and a half-life between 2 and 4 h [5]. A dosage of 5–7.5 mg/kg every 24 h is recommended associated with concentration monitoring during CKRT, because of the narrow therapeutic index in patients with kidney impairment [5]. PK parameters are markedly different in pediatric patients due to multiple factors such as growth, organ maturation, critical illness, and CKRT, exposing therefore to inadequate exposure when dosing is based on adult studies.
In the present case, CLKRT represented between 36.72 and 50.39% of total clearance. In adult studies, the clearance by ultrafiltration varied from 17.4 to 22.3 mL/min and reached nearly 35% of the total clearance [10]. In the present case, the effluent flow rate was remarkably high which explains the high killing level by ultrafiltration, especially as acyclovir has a low binding protein with a small molecular weight [10].
In our study, concentrations were sufficient with the usual dosing under CKRT. But concentrations were also highly above therapeutic index—target above 0.56 mg/L (or > IC50 for HSV) [11]. There was no dose adaptation after CKRT initiation because kidney function was initially preserved. Therapeutic drug monitoring suggested an increased risk of adverse effects since the peak level was higher than 25 mg/L, even though the AUC0–24 h was appropriate [12].
Unexpectedly, using the available acyclovir PK model in children without CKRT allowed us to predict well the concentrations in our patient. Nevertheless, building CKRT PK models is mandatory as the CKRT modalities differ greatly between individuals.
In our study, there was little variability in CLKRT according to time or dialysis parameters, but these were rarely modified. Usually, critically ill patients observe within-subject variability of PK because of rapid changes in body weight, circulating volumes, organ function, and binding protein levels [13]. Adult studies show intraindividual acyclovir PK variability according to CKRT methods or membrane efficiency [7, 14]. There was no important variability in our study in CLKRT with both modalities despite measurement after 2 different injections 1 day apart from each other.
Few pediatric studies have monitored acyclovir dosing during CKRT. One neonatal patient treated for disseminated HSV infection with AKI and fulminant hepatitis received 20 mg/kg/dose [6]. The trough level was 0.22 mg/L, and Cmax levels were between 18.9 and 24.5 mg/L under CKRT. Another neonatal case reported dosing during extracorporeal life support and CKRT for a similar clinical presentation: the patient was treated with continuous infusion adding acyclovir to the dialysate fluid at a concentration of 5.5 mg/L with a serum concentration between 5.3 and 8.8 mg/L [15]. Patient heterogeneity in the literature in terms of CKRT modalities, age, disease, associated therapies, and dosing prevent us from comparing them.
Our study showed important differences in CLKRT according to the measurement method: the effluent specimen was nearly twice the post-filter specimen. In the effluent compartment, we measured what is collected or what has been removed by the dialysis and not adsorbed by the membrane. A desorption phenomenon of acyclovir might explain such a difference between the two methods. In our case, the dialyzer used was made of polyarylethersulfone (PAES) membranes. The importance of this phenomenon might be explained by the important number of other treatments associated and potential competition to other molecules. This hypothesis could be investigated with complementary in vitro studies.
Conclusion
This study reports the case of a 21-month-old female treated with acyclovir under the hypothesis of acute liver failure induced by HSV infection and requiring CKRT for severe hyperammonemia. This is to our knowledge the first case describing acyclovir PK under CKRT in a pediatric patient other than neonatal cases. No dose adaptation was made after CKRT initiation in our study, and though concentration was efficient, the levels were high above target, and lower dosing should be investigated.
Acknowledgements
We thank the patient’s parents, the physicians, and the nurses involved in the care of this patient.
Author contribution
Drs. Collignon, Oualha, Hirt, and de Marcellus designed and conceptualized the report, acquired and interpreted patient data, drafted the manuscript for intellectual content, and reviewed and revised the manuscript. Drs. Neuranter and Heilbronner acquired and interpreted patient data and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Consent to participate
Consent was obtained from the patient’s parents for this study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robinson AM, Karvellas CJ, Dionne JC, Featherstone R, et al. Continuous renal replacement therapy and transplant-free survival in acute liver failure: protocol for a systematic review and meta-analysis. Syst Rev. 2020;9:143. doi: 10.1186/s13643-020-01405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagstaff AJ, Faulds D, Goa KL. Aciclovir: a reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:153–205. doi: 10.2165/00003495-199447010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Li X, Xia Y, Chu Y, Zhong H, Li J, et al. Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front Pharmacol. 2020;11:1–16. doi: 10.3389/fphar.2020.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King DH. History, pharmacokinetics, and pharmacology of acyclovir. J Am Acad Dermatol. 1988;18:176–179. doi: 10.1016/S0190-9622(88)70022-5. [DOI] [PubMed] [Google Scholar]
- 5.Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis. 2005;41:1159–1166. doi: 10.1086/444500. [DOI] [PubMed] [Google Scholar]
- 6.Funaki T, Miyata I, Shoji K, Enomoto Y, et al. Therapeutic drug monitoring in neonatal HSV infection on continuous renal replacement therapy. Pediatrics. 2015;136:e270–274. doi: 10.1542/peds.2014-3380. [DOI] [PubMed] [Google Scholar]
- 7.Bleyzac N, Barou P, Massenavette B, Contamin B, et al. Assessment of acyclovir intraindividual pharmacokinetic variability during continuous hemofiltration, continuous hemodiafiltration, and continuous hemodialysis. Ther Drug Monit. 1999;21:520–525. doi: 10.1097/00007691-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg I, Kimberlin DW. Acyclovir dosing and acute kidney injury: deviations and direction. J Pediatr. 2015;166:1341–1344. doi: 10.1016/j.jpeds.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 9.Broeker A, Vossen MG, Thalhammer F, Wallis SC, et al. An integrated dialysis pharmacometric (IDP) model to evaluate the pharmacokinetics in patients undergoing renal replacement therapy. Pharm Res. 2020;37:96. doi: 10.1007/s11095-020-02832-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulieu R, Bastien O, Gaillard S, Flamens C. Pharmacokinetics of acyclovir in patients undergoing continuous venovenous hemodialysis. Ther Drug Monit. 1997;19:701–704. doi: 10.1097/00007691-199712000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Abdalla S, Briand C, Oualha M, Bendavid M, et al. Population pharmacokinetics of intravenous and oral acyclovir and oral valacyclovir in pediatric population to optimize dosing regimens. Antimicrob Agents Chemother. 2020;64:1–10. doi: 10.1128/AAC.01426-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bean B, Aeppli D. Adverse effects of high-dose intravenous acyclovir in ambulatory patients with acute herpes zoster. J Infect Dis. 1985;151:362–365. doi: 10.1093/infdis/151.2.362. [DOI] [PubMed] [Google Scholar]
- 13.de Cacqueray N, Boujaafar S, Bille E, Moulin F, et al. Therapeutic drug monitoring of antibiotics in critically ill children: an observational study in a pediatric intensive care unit. Ther Drug Monit. 2022;44:319–327. doi: 10.1097/FTD.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 14.Khajehdehi P, Jamal JA, Bastani B. Removal of acyclovir during continuous veno-venous hemodialysis and hemodiafiltration with high-efficiency membranes. Clin Nephrol. 2000;54:351–355. [PubMed] [Google Scholar]
- 15.Cies JJ, Moore WS, Miller K, Small C, et al. Therapeutic drug monitoring of continuous-infusion acylovir for disseminated herpes simplex virus infection in a neonate receiving concurrent extracorporeal life support and continuous renal replacement therapy. Pharmacotherapy. 2015;35:229–233. doi: 10.1002/phar.1526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.