Abstract
Purpose of Review
Allergen immunotherapy (AIT) is a novel treatment approach with disease-modifying and preventative benefits that are not shared with other strategies for treating allergic illnesses. It has been demonstrated to be safe and effective in children. This review provides the most recent information on AIT in children as well as any pertinent updates.
Recent Findings
Although there is not a standard way to begin AIT, there are clear indications for AIT. Each case needs to be evaluated on its own by weighing the pros and downsides. AIT has been proven to significantly improve symptoms and quality of life in children with allergic illness, reduce medication use, stop the development of new allergen sensitizations, and stop the progression of allergic rhinitis to asthma. Novel approaches are under investigation to overcome some known AIT disadvantages.
Summary
This review provides a thorough summary of the most recent research and updates on AIT in children.
Keywords: Allergic rhinitis, Allergen immunotherapy, Children, Atopy, Treatment
Introduction
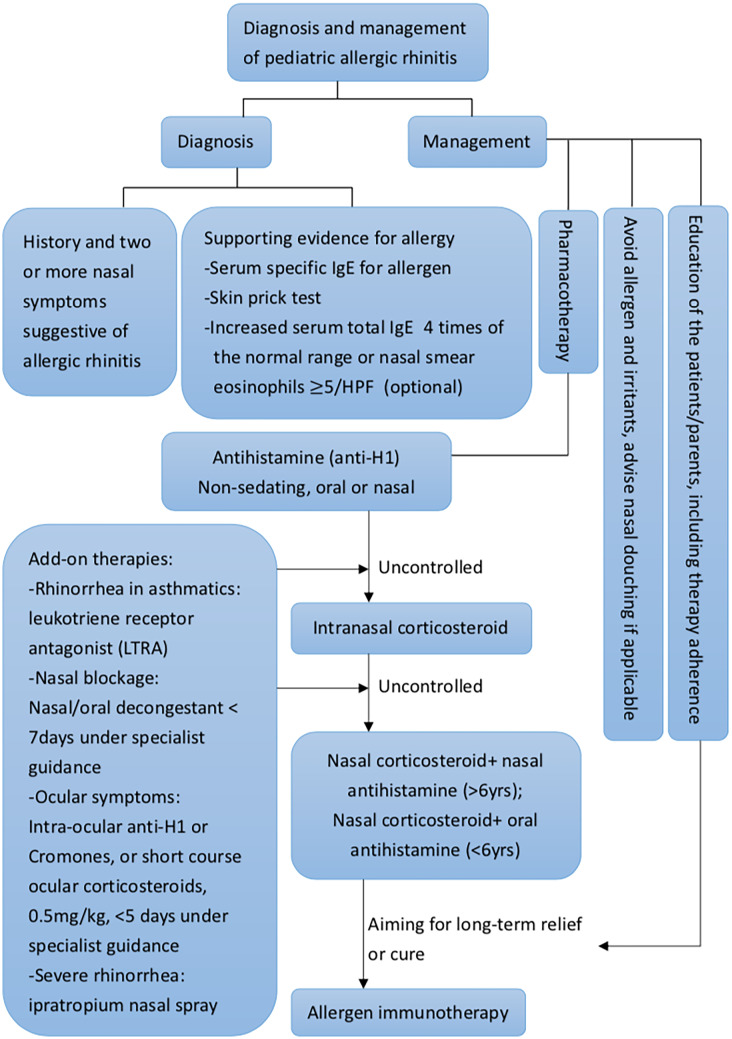
Around the world, reports of allergy disorders as allergic rhinitis, asthma, and atopic dermatitis have increased and are highly prevalent [1–4]. There are 10 to 30% of adults and up to 40% of children impacted, according to epidemiologic research [3]. Pharmacotherapy, allergy immunotherapy, and education about allergen-specific avoidance precautions are possible treatment options for these illnesses [5••, 6]. To achieve a more comprehensive approach, common clinical diagnosis and management algorithm was summarized as Fig. 1. Pharmacotherapy is usually the first step of the management for pediatric patients with allergic rhinitis. However, advantages and disadvantages exist between different treatment options. We listed the pros and cons of current treatment modalities in Table 1.
Fig. 1.
Pediatric allergic rhinitis diagnosis and management algorithm. HPF, high power field; IgE, immunoglobulin E
Table 1.
Pros and cons on treatment modalities for pediatric allergic rhinitis (AR)
| Medication | Pros | Cons |
|---|---|---|
| Oral H1 antihistamines | Non-sedating antihistamine as the first-line treatment and well tolerable | Mild fatigue, headache, nausea, dry mouth, poor drug adherence |
| Intranasal antihistamines | First or second-line treatment, effective for ocular symptoms | Concerns for patient tolerance, especially with regard to taste |
| Intranasal corticosteroids |
First or second-line treatment All nasal symptoms relief as well as ocular symptoms |
Nasal irritation, epistaxis, slow onset, some negative effects on short-term growth in children, but it is unclear for long term |
| Oral decongestant | For short-term relief of nasal obstruction | Insomnia, loss of appetite, irritability, palpitations, and increased blood pressure. Risk of toxicity in young children |
| Topical decongestant | For short-term nasal decongestion | Chronic use may carry the risk of rhinitis medicamentosa. Rebound congestion |
| Leukotriene receptor antagonist | For AR combined asthma symptoms relief |
Little effect as monotherapy for AR Cost |
| Cromones | As alternative for patient cannot tolerate intranasal corticosteroid | Nasal irritation, slow onset, frequent dosing needed |
| Ipratropium nasal spray | Adjunct to intranasal corticosteroid for the uncontrolled rhinorrhea | Nasal irritation, headache, pharyngitis, epistaxis, nasal dryness, over-dosing |
| Nasal saline douching |
Adjunct to pharmacotherapy Effective in discharge removal |
Practice and education needed, intranasal irritation, headaches, and ear pain |
| Combination: intranasal antihistamine and corticosteroid | Rapid onset, effective when monotherapy fail to control symptoms. Used as second-line therapy |
Patient intolerance, especially due to taste Cost |
For individuals with these cross-linked allergy disorders, allergen immunotherapy (AIT), which has been used as a treatment for allergic disease for more than a century, has been shown to be safe, efficient, and potentially disease-modifying. Patients with moderate to severe allergic rhinitis who do not react well to medical treatment are candidates for AIT. The hazards and benefits of each case should be carefully weighed. The use of fewer medication, a considerable improvement in symptoms and quality of life, the prevention of the emergence of new allergen sensitizations, and the prohibition of progression of allergic rhinitis to asthma are all advantages of AIT in children with allergic illness. Severe systemic allergic reactions are a rare but possible risk of AIT.
Mechanism
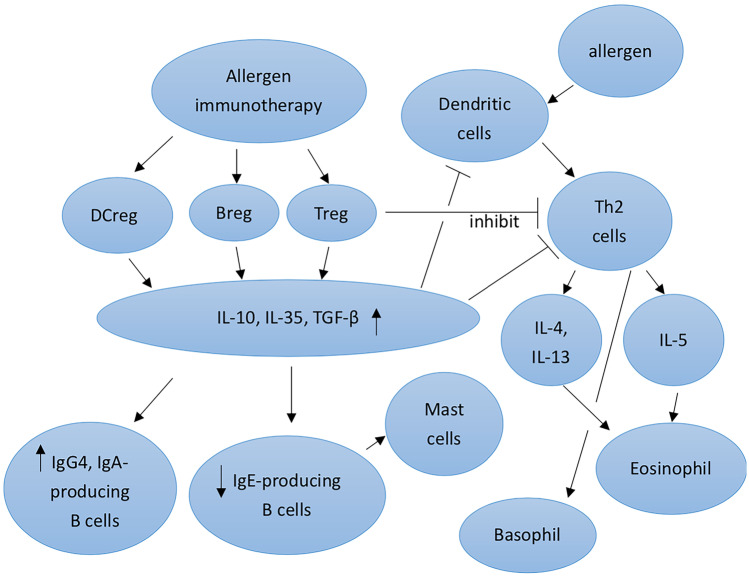
AIT normalizes allergen-specific T and B cells, controls IgE and IgG production, and modifies mast cells, basophil activation thresholds, and dendritic cell phenotypes through general processes of immunological tolerance to allergens. To decrease type 2 immune responses and allergic inflammation, the major objectives are to retain regulatory T cells (Tregs), regulatory B cells (Bregs), and several other regulatory cells [7•].
The regulation of antigen-specific immune cells, including T and B cells, was assumed to be AIT’s main mechanism of action since it operates in an antigen-specific manner. Innate lymphoid cells, monocytes/macrophages, natural killer cells, and dendritic cells are examples of non-antigen-specific immune cells that may be modulated by AIT, according to recent research. The amelioration of symptoms following AIT may also be attributed to these effects [7•]. Possible mechanism of allergen immunotherapy was illustrated as Fig. 2.
Fig. 2.
Mechanism of allergen immunotherapy
Indications
Patients who exhibit allergen-specific IgE antibodies as determined by serum specific IgE laboratory testing or skin prick testing and have allergic rhinitis with or without conjunctivitis, allergen-induced asthma, or stinging insect hypersensitivity should consider AIT [8, 9]. Children with allergic rhinitis frequently acquire asthma over time since the two diseases are closely related. However, there are still a lot of unanswered questions regarding whether allergen immunotherapy for allergic rhinitis can prevent asthma. These questions concern the age groups, how to prepare allergens, how to administer AIT, and how long to treat patients [10].
Contraindications
Communication difficulties and a few medical illnesses are contraindications to AIT. A rare but potential risk of AIT is the development of severe systemic allergic reactions [11, 12]. Patients chosen for AIT should be able to verbally and physically express to the medical care team any discomforts and symptoms that might point to an adverse reaction. Starting AIT with children under the age of 5 is a topic of some discussion. Although there is a benefit to starting AIT before the age of 5 years old due to the preventative effect of AIT on the development of new aeroallergen sensitizations and the progressive march to asthma, each case to start AIT should be carefully assessed by evaluating the severity of disease and benefits/risks ratios. Because there is a higher risk of systemic reactions to AIT injections in individuals with uncontrolled labile asthma, allergen immunotherapy is not advised for these patients. According to survey studies, people with uncontrolled and/or labile asthma were more likely to die from AIT; hence, asthma control must be attained before beginning immunotherapy [13]. Medical diseases that make it more difficult for the patient to overcome the systemic allergic reaction or the subsequent treatment are also relative contraindications for AIT. Heart disease, significantly reduced lung function, and ailments needing beta-blockers and angiotensin-converting enzyme inhibitors (ACEI) are among these medical disorders. These comorbidities are present in children even if they are less common than in adults.
Route for Administration
AIT can be given sublingually or subcutaneously, and new delivery methods including intra- and epicutaneous are continuously being researched. AIT attempts to alter innate and adaptive immunologic responses to induce allergen tolerance. Induction of diverse functional regulatory cells, such as regulatory T cells (Tregs), follicular T cells (Tfr), B cells (Bregs), dendritic cells (DCregs), innate lymphoid cells (IL-10 + ILCs), and natural killer cells, is the primary mechanism of AIT for controlling type 2 inflammatory cells.
For AIT, subcutaneous delivery (SCIT) was the usual route of administration. The typical SCIT regimen for allergen extracts involves dose titration by once-weekly injection, followed by maintenance dose injections at intervals of 4 to 8 weeks, continuing for at least 3 to 5 years. By using cluster or rush protocols to help the patients reach maintenance, the build-up period can be cut short [14]. These accelerated AIT offer patients quicker relief from allergy symptoms while maintaining comparable safety to standard regimens. However, compared to typical timetables, these protocols require more time commitment initially, but they ultimately save time and money in the long term. In order to reduce the frequency of systemic allergic reactions during accelerated AIT, premedication, which typically only requires an H1 antihistamine 1 h before the treatment, is advised. In appropriately selected patients, the risk for severe systemic reactions during accelerated AIT is low, but life-threatening reactions can occur.
Sublingual immunotherapy (SLIT) tablets serve as another allergen immunotherapy option for clinicians. Nowadays, there are five SLIT tablets that have been licensed for the treatment of allergic rhinoconjunctivitis in North America. These tablets are directed against home dust mites, ragweed, Timothy grass, and other allergens. On the other hand, the FDA has not yet approved any SLIT drops products. In SLIT, allergens are often given daily under the tongue. Large, double-blind, placebo-controlled trials involving both patients who were monosensitized and those who were polysensitized found that SLIT tablets consistently demonstrated therapeutic efficacy [15]. Patients who are allergic to pollen during their individual pollen seasons have showed success with treatment with house dust mite SLIT tablets [15]. Efficacy studies of SLIT drops demonstrate substantial heterogeneity of treatment effect, in contrast to SLIT tablets [15, 16]. Although data are limited, studies that compared the efficacy of SLIT tablets versus pharmacotherapy generally indicated that SLIT tablets had a greater benefit than pharmacotherapy when compared with placebo, particularly for perennial allergic rhinoconjunctivitis. When compared with subcutaneous immunotherapy, the results showed that SLIT tablets were superior to subcutaneous immunotherapy in terms of safety but somewhat less superior in terms of efficacy [15]. Additionally, there is no build-up phase necessary with SLIT, and it may be done securely and successfully at home. An intricate immunological network that includes the mouth mucosa and local lymph nodes is a necessary requirement for SLIT [17]. The efficient dosing range of allergy management is another obvious distinction between SCIT and SLIT. For many allergens, SCIT employs a small effective dose range of 5 to 25 μg of allergen per injection, but SLIT needs at least 50 to 100 times more allergen than SCIT to be equally effective [18].
Direct injection of allergens into the lymphatic system is known as intra-lymphatic immunotherapy (ILIT). By reducing the number of treatment applications and the length of the therapy, attaining good compliance and quick symptom relief, and demonstrating safety, ILIT tend to increase the efficiency of AIT. Only three low allergen dosage injections into the inguinal lymph nodes under ultrasound guidance, spaced 1 month apart, are needed for ILIT. When compared to SCIT, the cumulative allergen dose can be reduced 1000-fold [19, 20]. The demand for experienced professionals for injection under ultrasound guidance, which may make this procedure less practical, is the drawback of ILIT.
A unique therapy being researched right now is epicutaneous immunotherapy(EPIT). EPIT involves applying allergens to the skin and antigen-presenting cells in the superficial layers of the skin repeatedly. Electronic spreading, ablative fractional laser, and microneedle arrays are examples of epidermal allergen powder delivery technologies [21]. In contrast to mast cells or the vasculature, epidermal Langerhans cells are the focus of EPIT, which can lessen both local and systemic side effects [22]. The following benefits have been noted for EPIT: (1) a high safety profile due to the application of the allergen into the non-vascularized epidermis and subsequent delivery of the allergen to the less-vascularized dermis, (2) increased patient convenience due to the non-invasive (needle-free) and self-administrable application method, likely improving compliance, (3) absence of additional potential irritant constituents (e.g., alum, preservatives), and (4) less expensive. Regarding patients with AR and indoor allergen sensitivity, further information is required.
Local nasal immunotherapy(LNIT) appears to be only beneficial on rhinitis symptoms, according to considerable research conducted over the past 40 years. Local nasal LNIT, however, is not well accepted by patients due to its difficulties in use and local adverse effects that must be prevented using topical nasal premedication [23]. LNIT is not advised for clinical use at this time.
Efficacy
It has been demonstrated that pediatric immunotherapy is both efficient and well tolerated. By reducing symptoms and medication use, SCIT and SLIT have been shown in numerous clinical trials to be helpful for allergic rhinitis and asthma. One study in children aged 5 to 10 years found that both SCIT and SLIT significantly reduced the overall score for rhinitis and asthma symptoms, the overall medical score, and skin reactivity to house dust mites when compared to pharmacotherapy [24]. Another study from 2017 showed that patients with AR who received AIT for 3 years had a considerably lower probability of developing asthma [25]. The impact persisted for up to 2 years after the end of treatment, but it was unable to draw any meaningful conclusions about whether it would last for longer. According to several studies, there might be a lower prevalence of allergy in children born to mothers who underwent AIT during pregnancy. AIT’s effectiveness is influenced by the allergen dose and length of treatment. The clinical findings revealed a significant amount of heterogenicity and responsiveness in people. The personal dose was associated to the immunological response, and the length of the treatment was related to long-term recovery after stopping it. Current practice advises doctors to stop AIT if there is no clinical response after 18 to 24 months because there are no reliable diagnostic methods or markers for identifying responder patients [26]. Each country’s extracts vary in terms of their strength, allergen dosage, allergen combinations, and adjuvants.
Safety
Although AIT is regarded as a safe treatment, it can have unfavorable side effects, including local, large local reactions (LLRs), systemic reactions, and, in rare instances, anaphylaxis. Within 30 min following injection, the majority of the severe systemic reactions will manifest. Severe systemic reactions like anaphylaxis must be promptly identified by the medical care team which is also necessary while administering injections for AIT. Because SLIT has fewer systemic adverse effects than SCIT and no fatalities have been documented, it offers a higher safety profile [27]. One prospective study that looked at the safety of AIT in children under the age of 5 reported that out of 6689 injections in 239 individuals, there was just one systemic reaction. The authors came to the conclusion that AIT is a safe treatment for children under the age of 5 [28]. AIT frequently has side effects that are localized. In a survey study of 249 individuals receiving AIT, 71% of the participants said their AIT caused a local reaction. In 96% of patients who reported local reactions, it was indicated that the local reactions would not induce them to cease AIT. Individual local reactions may not necessarily portend future systemic or local reactions [29].
Duration of AIT
Many randomized controlled trials show long-term efficacy in improving clinical and immunological change following SCIT and SLIT. When AIT was used for less than 3 years, allergy symptoms typically returned 1 year after treatment ended. In a thorough 5-year prospective controlled trial comparing 3- and 5-year HDM SCIT, it was discovered that after 3 years, both groups had significantly lower rhinitis severity scores, asthma severity scores, and visual analog scales. Additionally, both groups continued to receive the treatment benefit after 5 years [30]. For long-term clinical benefit, SCIT and SLIT should both be at least 3 years long. Numerous factors, including the inconvenience of repeated injection visits, unfavorable side effects, and expense, which are the main causes of cessation, have an impact on AIT adherence [27].
Particular Considerations
AIT has a number of drawbacks, including the prolonged duration of therapy necessary to attain better efficacy, high cost, systemic allergic reactions, and the lack of a biomarker for identifying treatment responders. To address the issues related to AIT, supplementary medicines, vaccination adjuvants, and innovative vaccine technologies are currently being researched. All are not in the same developmental stage. For instance, allergoids have not yet received US FDA approval in the USA despite being used in clinical trials in Europe. Since the effects of using biologics to minimize the systemic reaction have been minimal, the expense is not justified. In Europe, modified recombinant proteins and peptides are being developed, but thus far, their level of efficacy has been disappointing [31•]. Before being prepared for future usage or regulatory approval, all require additional research.
COVID-19 Pandemic Attack
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and AR is not a risk factor for severe disease. There is currently no immunologic or clinical proof of an interaction between AIT and SARS-CoV-2. Patients who have been diagnosed as confirmed COVID-19-positive cases should stop receiving AIT, and those who have recovered from COVID-19 and are asymptomatic can resume receiving AIT as planned. With SLIT, patients can self-treat at home rather than traveling to or staying at an allergy hospital or clinic. Regarding patients who receive AIT and contract COVID-19 infection, more information is required.
Conclusion
In practice, allergen-specific immunotherapy has been advised for the treatment of severe AR patients who do not respond to standard medication therapies. In order to reduce type 2 inflammation, AIT produces allergic immunological tolerance by increasing many regulatory cells. AIT has been demonstrated to be helpful in easing allergic symptoms, decreasing the need for medicine, lowering allergen reactivity, enhancing quality of life, and preventing the onset of asthma. However, the drawbacks of conventional SCIT include the need for many injections and clinic visits, a high cost, and systemic allergic reactions. In terms of safety, SLIT tablets outperformed SCIT, although with a little lower benefit in terms of efficacy. AIT can be administered through a variety of methods, which offers options and enhances patient compliance and safety. To increase the efficacy of AIT even more, new approaches, adjuvants, adjunctive therapies, biologicals, and novel technologies are being investigated.
Declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on PEDIATRIC OTOLARYNGOLOGY: Challenges in Pediatric Otolaryngology
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Arrais M, Lulua O, Quifica F, Rosado-Pinto J, Gama JMR, Brito M, et al. Sensitisation to aeroallergens in relation to asthma and other allergic diseases in Angolan children: a cross-sectional study. Allergol Immunopathol (Madr) 2020;48(3):281–289. doi: 10.1016/j.aller.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Christiansen ES, Kjaer HF, Eller E, Bindslev-Jensen C, Host A, Mortz CG, et al. The prevalence of atopic diseases and the patterns of sensitization in adolescence. Pediatr Allergy Immunol. 2016;27(8):847–853. doi: 10.1111/pai.12650. [DOI] [PubMed] [Google Scholar]
- 3.Okubo K, Kurono Y, Ichimura K, Enomoto T, Okamoto Y, Kawauchi H, et al. Japanese guidelines for allergic rhinitis 2020. Allergol Int. 2020;69(3):331–345. doi: 10.1016/j.alit.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Pfaar O, Togias A, Schunemann HJ, Ansotegui I, Papadopoulos NG, et al. 2019 ARIA Care pathways for allergen immunotherapy. Allergy. 2019;74(11):2087–2102. doi: 10.1111/all.13805. [DOI] [PubMed] [Google Scholar]
- 6.Wise SK, Lin SY, Toskala E, Orlandi RR, Akdis CA, Alt JA, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352. doi: 10.1002/alr.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.• Boonpiyathad T, Lao-Araya M, Chiewchalermsri C, Sangkanjanavanich S, Morita H. Allergic rhinitis: what do we know about allergen-specific immunotherapy? Front Allergy. 2021;2:747323. 10.3389/falgy.2021.747323. Epub 2022/04/08. PubMed PMID: 35387059; PubMed Central PMCID: PMCPMC8974870. This review presents an updated overview of AIT, and summarized adjunctive therapies, vaccine adjuvants, and novel vaccine technologies studied to overcome the problems associated with AIT. [DOI] [PMC free article] [PubMed]
- 8.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 9.Cox L, Esch RE, Corbett M, Hankin C, Nelson M, Plunkett G. Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes. Ann Allergy Asthma Immunol. 2011;107(4):289–299. doi: 10.1016/j.anai.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Arshad SH. Does allergen immunotherapy for allergic rhinitis prevent asthma? Ann Allergy Asthma Immunol. 2022 doi: 10.1016/j.anai.2022.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Cox LS, Sanchez-Borges M, Lockey RF. World Allergy Organization systemic allergic reaction grading system: is a modification needed? J Allergy Clin Immunol Pract. 2017;5(1):58–62.e5. doi: 10.1016/j.jaip.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Passalacqua G, Nowak-Wegrzyn A, Canonica GW. Local side effects of sublingual and oral immunotherapy. J Allergy Clin Immunol Pract. 2017;5(1):13–21. doi: 10.1016/j.jaip.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein DI, Wanner M, Borish L, Liss GM, Immunotherapy Committee AAoAA, Immunology Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J Allergy Clin Immunol. 2004;113(6):1129–1136. doi: 10.1016/j.jaci.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016;8(3):191–197. doi: 10.4168/aair.2016.8.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DuBuske L. Efficacy and safety of sublingual allergen immunotherapy. Allergy Asthma Proc. 2022;43(4):272–280. doi: 10.2500/aap.2022.43.220036. [DOI] [PubMed] [Google Scholar]
- 16.Creticos PS, Esch RE, Couroux P, Gentile D, D’Angelo P, Whitlow B, et al. Randomized, double-blind, placebo-controlled trial of standardized ragweed sublingual-liquid immunotherapy for allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2014;133(3):751–758. doi: 10.1016/j.jaci.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Passalacqua G, Compalati E, Canonica GW. Sublingual immunotherapy: clinical indications in the WAO-SLIT Position Paper. World Allergy Organ J. 2010;3(7):216–219. doi: 10.1097/WOX.0b013e3181e8d19c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calderon MA, Simons FE, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67(3):302–311. doi: 10.1111/j.1398-9995.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 19.Senti G, Freiburghaus AU, Larenas-Linnemann D, Hoffmann HJ, Patterson AM, Klimek L, et al. Intralymphatic immunotherapy: update and unmet needs. Int Arch Allergy Immunol. 2019;178(2):141–149. doi: 10.1159/000493647. [DOI] [PubMed] [Google Scholar]
- 20.von Moos S, Kundig TM, Senti G. Novel administration routes for allergen-specific immunotherapy: a review of intralymphatic and epicutaneous allergen-specific immunotherapy. Immunol Allergy Clin North Am. 2011;31(2):391–406. doi: 10.1016/j.iac.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Kong Y, Wu MX. Innovative systems to deliver allergen powder for epicutaneous immunotherapy. Front Immunol. 2021;12:647954. doi: 10.3389/fimmu.2021.647954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheurer S, Toda M. Epicutaneous immunotherapy. Allergol Immunopathol (Madr) 2017;45(Suppl 1):25–29. doi: 10.1016/j.aller.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Walker SM, Durham SR, Till SJ, Roberts G, Corrigan CJ, Leech SC, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41(9):1177–1200. doi: 10.1111/j.1365-2222.2011.03794.x. [DOI] [PubMed] [Google Scholar]
- 24.Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010;40(6):922–932. doi: 10.1111/j.1365-2222.2009.03448.x. [DOI] [PubMed] [Google Scholar]
- 25.Kristiansen M, Dhami S, Netuveli G, Halken S, Muraro A, Roberts G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28(1):18–29. doi: 10.1111/pai.12661. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E, et al. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61(Suppl 82):1–20. doi: 10.1111/j.1398-9995.2006.01219_1.x. [DOI] [PubMed] [Google Scholar]
- 27.Tankersley M, Han JK, Nolte H. Clinical aspects of sublingual immunotherapy tablets and drops. Ann Allergy Asthma Immunol. 2020;124(6):573–582. doi: 10.1016/j.anai.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Perez NR, Moreno MDJA. Safety of immunotherapy and skin tests with allergens in children younger than five years. Rev Alerg Mex. 2006;53(2):47–51. [PubMed] [Google Scholar]
- 29.Coop CA, Tankersley MS. Patient perceptions regarding local reactions from allergen immunotherapy injections. Ann Allergy Asthma Immunol. 2008;101(1):96–100. doi: 10.1016/S1081-1206(10)60841-1. [DOI] [PubMed] [Google Scholar]
- 30.Tabar AI, Arroabarren E, Echechipia S, Garcia BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. J Allergy Clin Immunol. 2011;127(1):57–63. doi: 10.1016/j.jaci.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 31.Nelson HS. Future directions in allergen immunotherapy. Allergy Asthma Proc. 2022;43(4):356–362. doi: 10.2500/aap.2022.43.210098. [DOI] [PubMed] [Google Scholar]