Abstract
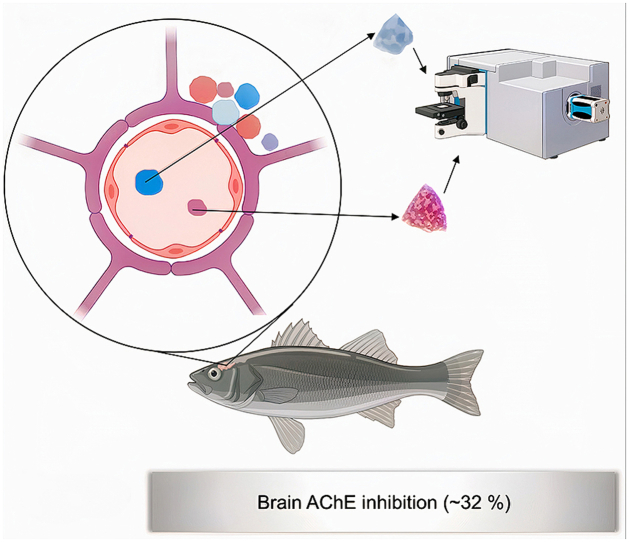
Pollution-induced neurotoxicity is of high concern. This pilot study investigated the potential relationship between the presence of microplastics (MPs) in the brain of 180 wild fish (Dicentrarchus labrax, Platichthys flesus, Mugil cephalus) from a contaminated estuary and the activity of the acetylcholinesterase (AChE) enzyme. MPs were found in 9 samples (5% of the total), all of them from D. labrax collected in the summer, which represents 45% of the samples of this species collected in that season (20). Seventeen MPs were recovered from brain samples, with sizes ranging from 8 to 96 μm. Polyacrylamide, polyacrylic acid and one biopolymer (zein) were identified by Micro-Raman spectroscopy. Fish with MPs showed lower (p ≤ 0.05) AChE activity than those where MPs were not found. These findings point to the contribution of MPs to the neurotoxicity induced by long-term exposure to pollution, stressing the need of further studies on the topic to increase ‘One Health’ protection.
Keywords: Microplastics, Brain, Neurotoxicity, Wild fish, AChE activity, One health
Graphical abstract
1. Introduction
Worldwide plastic production has been growing faster than the manufacture of any other material [1]. Compared with two decades ago, the plastic production has doubled [2] and the environmental contamination by plastics considerably increased during the SARS-CoV-2 pandemics [[3], [4], [5], [6]]. Due to their long persistence in the environment, plastics became a major pollution problem worldwide.
Among plastics, debris with size lower than 5 mm commonly designated by microplastics (MPs) are of high concern. MPs present in the wild result from the progressive break down of larger plastic fragments and from the input of particles already with size lower than 5 mm into the environment [7]. Studies have shown that MPs are worldwide distributed in the environment, being present in the atmosphere, freshwater, transitional waters and marine water, soils and sediments, and food webs [[8], [9], [10], [11], [12], [13], [14]]. Such MPs generally have other chemicals (MP-Chem), including additives added during their production and preparation for several applications, and other environmental contaminants that adsorb to their surface when they are in the environment [[15], [16], [17]]. MPs may also carry microorganisms, including multi-resistant pathogenic ones [18].
When MPs enter into the body of animals through ingestion, respiration, or other ways, some of them are internalized into the circulatory system, as shown by several studies with different species [19,20], including humans [21]. Then, they are distributed, some are likely excreted whereas others reach internal organs and tissues where they can be retained and possibly accumulated [5,[22], [23], [24]]. Laboratory studies demonstrated that very small MPs, including nanoplastics (NPs), can cross the blood-brain barrier [25] and cause neurotoxicity through inhibition of the acetylcholinesterase (AChE) enzyme [[26], [27], [28]], brain oxidative stress and damage [[29], [30], [31]], alteration of neurotransmitters’ levels and other processes as recently reviewed [32]. In fish, such effects have been related with decreased feeding [33,34], reduced swimming performance [35] and other behaviour alterations [36,37], among other effects that can reduce the individual and population fitness, with potential negative impacts on biodiversity, and human food safety and security [38]. Moreover, MPs interact with the toxicity of many other contaminants in fish often leading to a greater negative impact [29,39,40]. Furthermore, warmer water temperature and other alterations resulting from global warming influence the uptake, accumulation and toxicity of MPs [41,42], and often act synergistically with chemical toxicity in model animals, including in long-term exposures [4,43]. Therefore, there is high concern on the combined long-term effects of MPs, MP-Chem and other stressors on wild organisms and human health, including regarding neurotoxicity.
Data on the potential MP-induced neurotoxicity in wild populations of fish is still very limited [22,32,44]. The effects induced in real scenarios can be different from those obtained in the laboratory due to the influence of several factors, such as individual and population adaptation, properties of the MPs and other pollutants present, seasonal variation in the levels of MPs and environmental conditions (e.g., temperature, water volume, pH), among several others [45]. The differences may be more evident in populations living in highly dynamic ecosystems, such as contaminated estuaries of large rivers, which often have high loads of MPs and are important contributors to the marine pollution by these particles [46]. Considerable concentrations of MPs were documented in fish from estuaries located in different regions of the world, such as the La Plata River estuary, Argentina [47], the Ciliwung River estuary, Indonesia [48], the Tecolutla River estuary, Gulf of Mexico [49], and the Minho River estuary, Iberian Peninsula [5]. Further research with fish in real scenarios is needed to better understand the pollution-induced long-term neurotoxicity in fish, which is also important regarding human health and wellbeing.
The main goal of this pilot study was to investigate the potential presence of MPs in the brain of wild fish in relation to brain AChE activity in a real scenario of long-term exposure to pollution caused by a diversity of chemicals. Two null hypotheses were tested: H01 - The samples collected from fish brain do not have MPs; H02 – Brain AChE activity is not related with the presence of MPs in fish brain samples.
2. Material and methods
2.1. Alternative hypotheses, fish sampling and animal ethical issues
The alternative hypotheses to H01 and H02 were, respectively: HA1 - The samples collected from fish brain have MPs; HA2 - Brain AChE activity is related with the presence of MPs in fish brain samples.
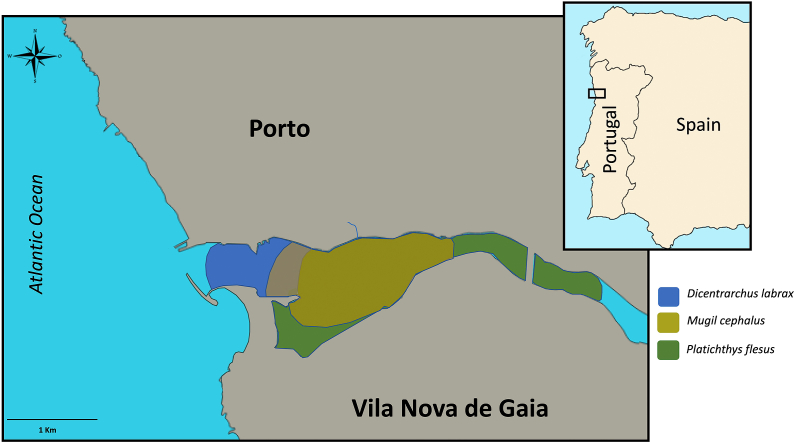
Fish were from the estuary of the Douro River, hereafter indicated as Douro estuary, which is located in the Northwest (NW) coast of Portugal and ends into the North East (NE) Atlantic Ocean (Fig. 1). This estuary was selected because the hydrological basin of the Douro River is one of the most important in the Iberian Peninsula, the Douro estuary has an extent of about 22 km, draining into the Atlantic Ocean [50], its environmental conditions variate along the year [51], and it is contaminated with a diversity of MPs [52,53], among many other environmental contaminants [[54], [55], [56]].
Fig. 1.
Localization of the Douro River estuary with the fish sampling areas. All fish were captured in the area defined by the coordinates 41° 8‘37.95″N, 8°40'29.27″W and 41° 8'39.11″N, 8°37'49.35″W. Sea bass were mainly collected in the downstream area (blue) and grey mullet and flounders in the areas indicated in yellow and green. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Three fish species were investigated: the European seabass (Dicentrarchus labrax Linnaeus, 1758), the European flounder (Platichthys flesus Linnaeus, 1758) and the flathead grey mullet (Mugil cephalus Linnaeus, 1758). They were chosen for this study because they can be found in the Douro estuary along the year, they are fished for human consumption as food, and they were used in biomonitoring studies in relation to MPs and other contamination in previous studies in estuaries [5,[57], [58], [59], [60]]. A total of 180 fish were investigated, 20 per species and per season (summer 2019, autumn 2019, and winter 2019–2020). All the fish were obtained from local fishery, were captured in the area defined by the coordinates 41° 8′37.95″N, 8°40′29.27″W and 41° 8′39.11″N, 8°37′49.35″W, and aimed at being sold for human food consumption. Shortly after their capture by fishermen, the fish corps were immediately transported intact to the laboratory to avoid contamination by MPs present in the environment, in thermally isolated boxes in cold conditions.
The study had authorization from the “ORBEA – Orgão Responsável pelo Bem Estar Animal” of ICBAS – School of Medicine and Biomedical Sciences of the University of Porto, reference number P372/2020/ORBEA, and was carried out according the European and Portuguese principles and procedures regarding animal's ethics and experimentation. L. Guilhermino and L.R. Vieira have accreditation from the Portuguese Authority (“Direção Geral de Alimentação e Veterinária”), to coordinate studies and carry animal experimentation.
2.2. Quality assurance and quality control
Several quality assurance and quality control (QA/QC) measures were implemented during all the stages of sample collection and preparation (dissection, digestion, and filtration), MPs isolation and characterization (primary and chemical) to prevent external and cross-contamination of samples and particles as in previous studies [5,22]. Briefly, researchers used nitrile gloves and 100% cotton laboratory coats covering other clothes. The work was done in spaces with restrict access, the surface areas, materials and instruments were previously carefully cleaned and disinfected, the instruments were washed before and between tissue collection, among other measures. Dissections, preparation of samples and filtration were performed in a flow cabinet, to avoid the risk of external contamination. All the filters were previously observed under a stereo-microscope to remove any plastic debris potentially present. Blanks to control for external contamination were used in all the steps, and controls with potassium hydroxide (KOH) solution in ultra-pure water (10% v/v) were also used.
2.3. Sample collection
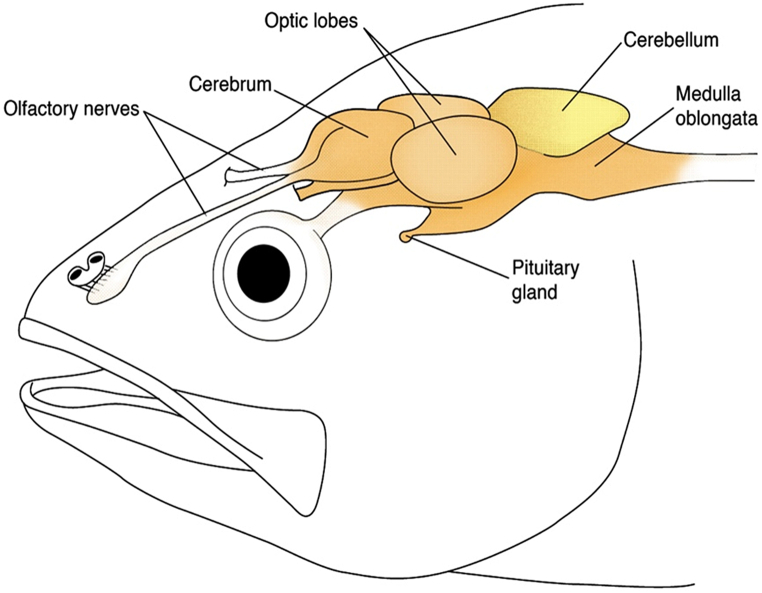
In the laboratory, the total body length (length) and the total body weight (wet weight – ww, hereafter indicated as body weight) of each fish were determined, and a basic physical examination of each fish was made. Then, each fish was carefully washed to remove external particles, and several tissue/organ samples were collected for different types of analyses to take the best advantage of animal corps. For this study, the whole brain of each fish was isolated and divided into two portions (Fig. 2): the cerebellum was isolated and prepared for AChE activity determination because this region of fish brain has high activity of this enzyme [61,62]; the remaining regions of the brain were put together for MP analyses. Samples for AChE analyses were weighted, put in cold phosphate-buffer (1:10 w/v; 0.1 M, pH = 7.2), and kept at −80 °C until further analyses. Samples for MP analyses were weighted (Kern ABS 120–4, Kern & Sohn GmbH, Germany), carefully rinsed with ultrapure water to remove any potential contamination [63], and then transferred to pre-cleaned glass vessels, which were sealed and externally covered with aluminium foil, and kept at −20 °C until further analyses.
Fig. 2.
Illustrative representation of the fish brain structure. Adapted from [64].
2.4. Microplastic isolation, visual identification and chemical characterization
The particles isolation and primary characterization were carried out as previously described [5,22,65]. In brief, each brain sample was covered with a volume of a 10% potassium hydroxide solution in ultra-pure water and incubated at 60 °C for 24 h in an oven (Drying oven EV50, Raypa, Spain) [65]. After cooling, the product was filtered through 42 mm diameter glass-microfiber filter membranes (pore size 1.2 μm, Munktell & Filtrak GmbH, Germany) in vacuum conditions. Subsequently, the filters were put in previously cleaned glass Petri dishes, which were sealed and dried at 40 °C for 24 h (Drying oven EV50, Raypa, Spain). All the particles present in the filters were observed and pictures were taken (Nikon SMZ800 Stereo Microscope with integrated camera DS-Fi1, Japan). Each particle was measured using the ImageJ software (https://imagej.nih.gov/ij/), and its shape and colour were recorded.
The chemical composition of all the particles isolated from brain samples was determined by Raman spectroscopy, a method used successfully to identify the plastic nature of particles in other studies [66,67]. The Raman spectra of the particles were recorded using a WITec confocal CRM alpha 300 R Micro-Raman spectroscopy (WITec GmbH, Ulm Germany) with an air-cooled solid state laser operating at 532 nm a CCD detector cooled to −60 °C. Different Raman settings for the same spot were used or multiple spectra at different spots on the same sample were collected [67]. Subsequent processing of the data was conducted using a WITec Project software (FIVE 5.2, WITec GmbH, Ulm, Germany). All spectra were compared to reference libraries using the search/match software TrueMatch integrated with the WITec Suite FIVE software. Spectral identification was accepted only after spectra underwent visual confirmation with matched reference spectra and a minimum Hit Quality Index (HQI) of 70%.
After characterization, in addition of being sorted by chemical composition, the particles identified as MPs were quantified by shape, colour and size. For the shape, the following types were considered based on the recommendations of the Technical Subgroup on Marine Litter (TSG-ML) for the European Marine Strategy Framework Directive document: plastic fragments, pellets, filaments/fibres, plastic films, foamed plastic, granules and styrofoam [68]. Regarding colour, the following categories were considered: red, orange, yellow, green, blue, violet, black, white and transparent [69]. Considering size (longest dimension for fibres; Feret's diameter for the other shapes) the following size classes were considered: <100 μm; 101–150 μm; 151–500 μm; 501–1500 μm; 1501–3000 μm; 3001 < 5000 μm [22].
The concentrations of MPs in brain samples were expressed as the number of MP items per fish (MPs/fish) or per weight (ww) of the analysed brain tissue (MPs/g).
2.5. Determination of acetylcholinesterase activity
The activity of AChE was selected for the present study because it is widely used as a neurotoxicity biomarker, and several MPs were found to alter the activity of this enzyme in fish, both in laboratorial [26,29,70,71] and field conditions [22,72].
The procedures for further preparation of samples and determination of AChE activity were described in detail elsewhere [73,74]. Briefly, after defrosting on ice, each cerebellum sample was kept in phosphate-buffer (0.1 M, pH = 7.2), homogenized (1:10 w/v; Ystral GmbH d-7801 homogenizer, Germany) and centrifuged for 3 min at 3,300 g and 4 °C (Sigma 3K30 centrifuge, Germany). The supernatant was carefully collected, its protein content was determined by the Bradford method [75] adapted to microplate [76], using bovine gama globulin as protein standard and absorbance read at 600 nm (Power Wave HT 340, BioTek, USA). The protein content of the samples was standardized to 0.3 mg/mL [74]. The AChE activity was determined by the Ellman technique [77] adapted to microplate [73], using acetylcholine as substrate and the absorbance read at 412 nm (Power Wave HT 340, BioTek, USA). After enzymatic determinations, the protein content of the remaining samples was determined again (as previously indicated), and used to express the enzymatic activity in nanomoles of substrate hydrolysed per minute per mg of protein (nmol/min/mg protein).
2.6. Statistical analysis
Statistical analyses were carried out in the IBM® SPSS Statistics® package, version 28.0, and the significance level was 0.05.
For each species, the data set of each biological parameter (i.e., total body weight, total body length or AChE activity) was tested for normal distribution and homogeneity of variances through the Shapiro-Wilk test and the Levene's test respectively, and the variables were transformed when necessary [78]. When normal distribution and homogeneity of variances were achieved, a one-way analysis of variance (ANOVA) was used to compare different seasons (summer, autumn and winter), followed by the Tukey's multicomparison post-hoc test when significant differences were found. When the ANOVA assumptions could not be achieved, the Kruskal-Wallis test was used, followed by pairwise comparisons (with significance values adjusted by the Bonferroni correction for multiple test) when significant differences were found.
The Student's t-test was used to compare the AChE activity of fish having MPs in the brain sample analysed with the enzymatic activity of fish where MPs were not found in the analysed brain sample. For text simplicity, these two groups of fish will be hereafter indicated as fish with MPs and fish without MPs in the brain, respectively. However, it should be noted that we cannot assure that fish with brain samples negative to MPs did not have these items in the brain because the cerebellum was used for AChE determinations and not analysed for MP content.
3. Results
The mean (± standard deviation – SD) of the biological parameters determined per species and season are indicated in Table 1, as well as the results of the statistical analysis comparing different seasons. In all the species, significant differences of body weight, length and AChE activity among seasons were found (Table 1). D. labrax specimens had significantly lower body weight and higher length in the winter, and lower AChE activity in the summer than in the other seasons. P. flesus specimens had significantly lower body weight, length and AChE activity in the winter than in the other seasons. M. cephalus specimens had significantly lower body weight in the winter, higher length in the autumn, and lower AChE activity in the autumn than in the other seasons.
Table 1.
Mean and standard deviation of total body weight, body length and brain acetylcholinesterase activity in Dicentrarchus labrax, Platichthys flesus, and Mugil cephalus from the Douro estuary. Different letters indicate statistically significant differences (p ≤ 0.05) among seasons per species.
| N | Total body weight (g) | Total body length (cm) | AChE activity (nmol/min/mg protein) | |
|---|---|---|---|---|
| Dicentrarchus labrax | ||||
| Summer | 20 | 656 ± 89 a | 31 ± 4 a | 100 ± 24 a |
| Autumn | 20 | 625 ± 95 a | 35 ± 3 b | 165 ± 20 b |
| Winter | 20 | 532 ± 61 b | 36 ± 2 b | 151 ± 24 b |
| Total mean | 60 | 604 ± 97 | 34 ± 4 | 139 ± 36 |
| Comparison among seasons | 60 | H (2) = 21.000 p < 0.001 | H (2) = 20.643, p < 0.001 | F (2,57) = 46.017 p < 0.001 |
| Platichthys flesus | ||||
| Summer | 20 | 307 ± 30 a,b | 20 ± 2 a | 91 ± 29 a |
| Autumn | 20 | 337 ± 65 a | 30 ± 2 b | 96 ± 37 a |
| Winter | 20 | 290 ± 60 b | 29 ± 2 b | 51 ± 16 b |
| Total mean | 60 | 311 ± 56 | 26 ± 5 | 79 ± 35 |
| Comparison among seasons | 60 | H (2) = 41.721, p < 0.001 | F (2,57) = 4.024 p = 0.023 | F (2,57) = 25.181 p < 0.001 |
| Mugil cephalus | ||||
| Summer | 20 | 811 ± 74 a | 33 ± 4 a | 87 ± 13 a |
| Autumn | 20 | 1003 ± 511 a | 46 ± 3 b | 43 ± 17 b |
| Winter | 20 | 386 ± 78 b | 36 ± 2 a | 81 ± 25 a |
| Total mean | 60 | 733 ± 394 | 38 ± 7 | 71 ± 27 |
| Comparison among seasons | 60 | H (2) = 33.446 p < 0.001 | H (2) = 29.806 p < 0.001 | H (2) = 31.639 p < 0.001 |
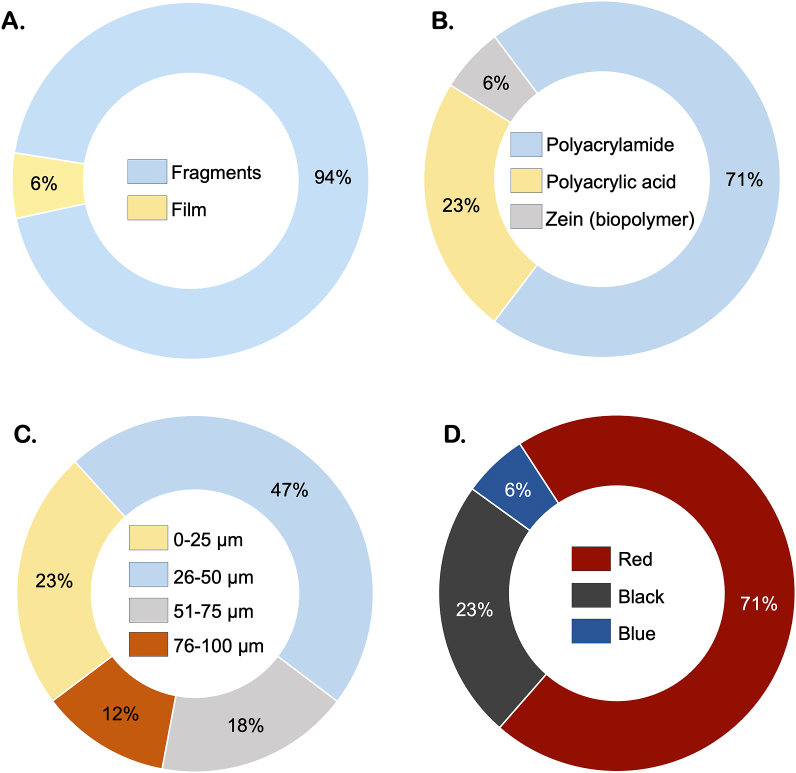
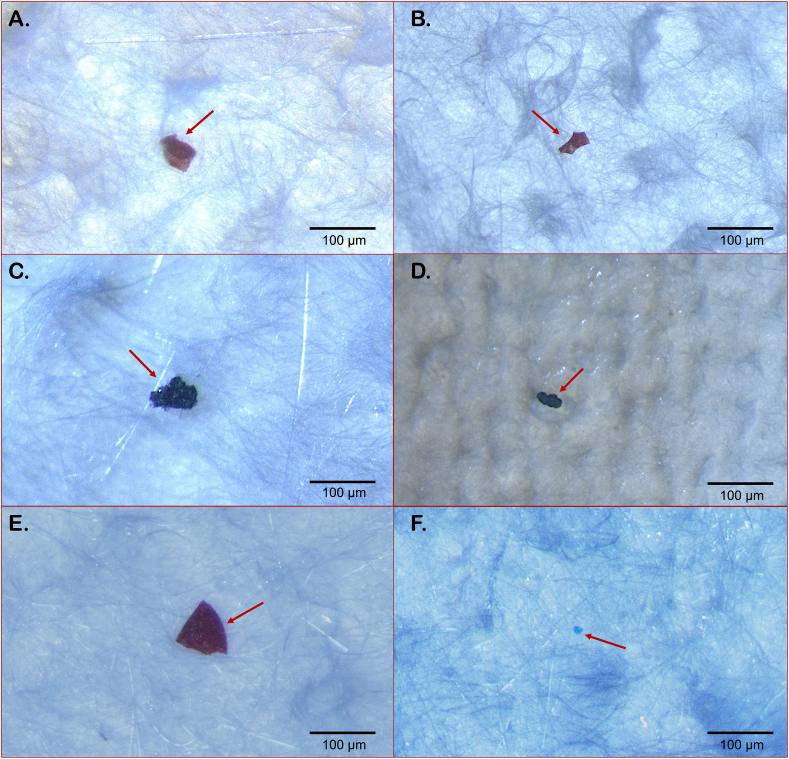
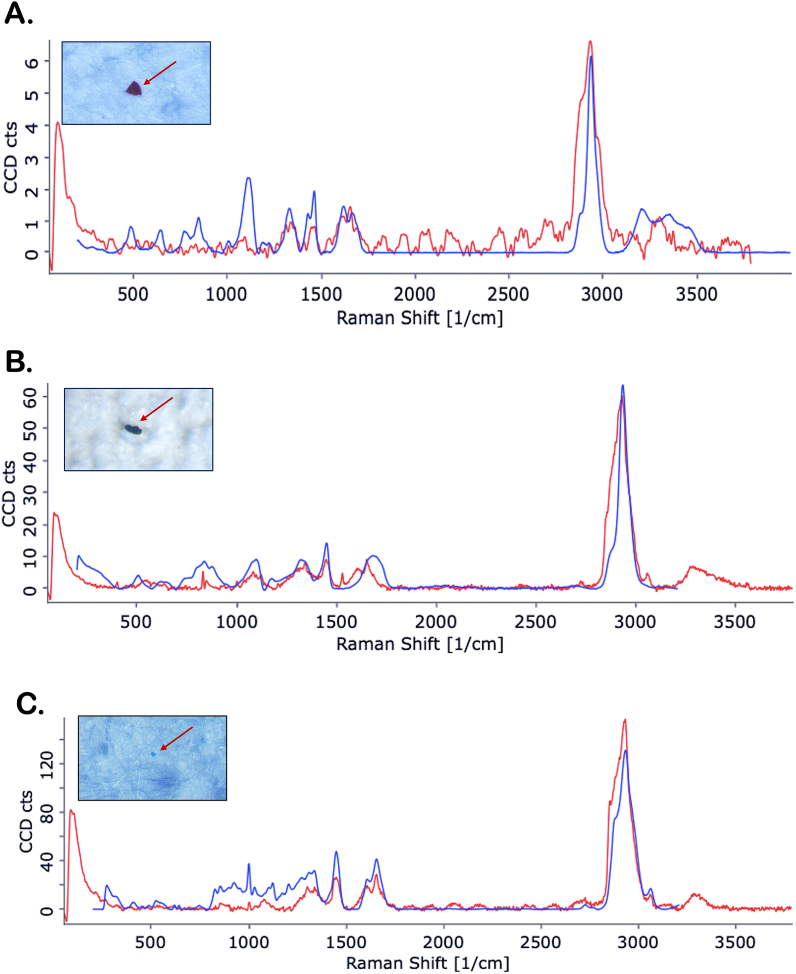
Seventeen MPs were recovered from the brain samples, 94% fragments and 6% film (Fig. 3-A), which are exemplified in Fig. 4(A-F). The size of the MPs ranged from 8 μm to 96 μm, with 70% of them having less than 50 μm (Fig. 3-B). Red, black and blue were the colours observed (Fig. 3-D). Three polymer types were identified: the superabsorbent polymers polyacrylamide (71%) and polyacrylic acid (23%), and one biopolymer (Zein) (6%) (Fig. 3-C). Representative spectra are shown in Fig. 5(A-C). Blue and green fibres were found in the blanks, however, no fibres were found in the analysed brain samples.
Fig. 3.
Characteristics of microplastics recovered from brain samples of wild fish. (A-) shape; (B) polymer composition; (C) size class; and (D) colour. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Examples of microplastics recovered from brain samples of Dicentrarchus labrax from the Douro estuary (Portugal). (A–E) fragments; (F) film.
Fig. 5.
Representative spectra of the microplastic particles polymers found in brain samples of wild fish. (A) Polyacrylamide; (B) Polyacrylic acid; (C) Zein (biopolymer).
Among the 180 fish analysed, 9 (5%) had MPs in the brain, all were D. labrax specimens collected in the summer. From the total number of D. labrax specimens (60), 15% had MPs in the brain, with a mean concentration (± standard deviation - SD) in the brain of 0.3 ± 0.8 MPs/fish (2 ± 6 items/g). Considering the samples of D. labrax collected in the summer (20), 45% had MPs in the brain, with a mean (±SD) concentration of 0.9 ± 1.1 MPs/fish (7 ± 10 MP/g).
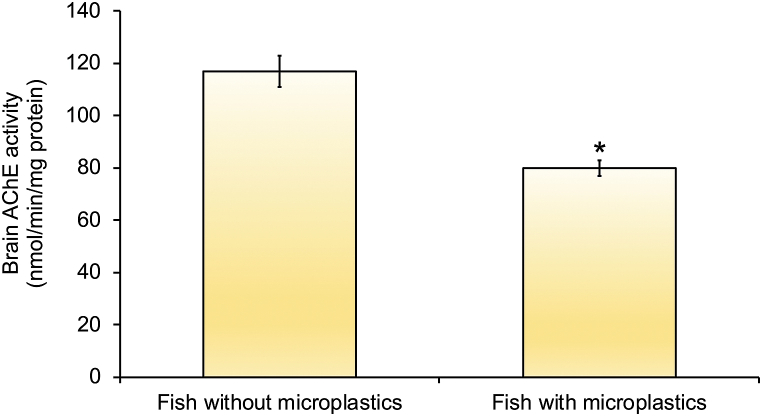
Among the specimens of D. labrax collected in the summer, significant differences in the activity of AChE between fish with and without MPs were found (t(18) = 5.383, p < 0.001). The mean (±SD) of AChE activity was lower (80 ± 8 nmol/nmol/min/mg protein) in fish with MPs in the brain than in fish where MPs were not found in the analysed samples (117 ± 19 nmol/nmol/min/mg protein) (Fig. 6).
Fig. 6.
Acetylcholinesterase activity (AChE) in brain of Dicentrarchus labrax, in groups of fish with (n = 9) and without (n = 11) microplastics in the brain captured during the summer period. Results are expressed as mean values ± standard errors for each group of fish. * indicates statistical significant differences between groups of fish with and without microplastics (Student's t-test, p ≤ 0.05).
4. Discussion
The values of AChE activity determined in the studied specimens of D. labrax and P. flesus are in the range values reported in other studies for the same species. For example, AChE activity between 100 and 140 nmol/min/mg protein in wild D. labrax specimens from the Arade Estuary, Portugal [79], between 89 and 174 nmol/min/mg protein in wild P. flesus from the Minho River estuary, Portugal [80], and between 50 and 121 nmol/min/mg protein in wild P. flesus collected in the Gulf of Gdańsk, Poland [81], were documented. The total mean of AChE activity determined in M. cephalus from the Douro estuary is lower than the mean of 45.1 ± 26.4 μmol/min/mg protein documented in wild flathead grey mullets from the Pontine Lakes [82], possibly reflecting biological and environmental differences.
Seasonal variability in fish weight and length was found, which did not have a common pattern to all the species. This is a common situation in estuarine fish and a diversity of factors can account for the differences, such as the biology of distinct species, life-cycle phase of each species, specific habitat and feeding ecology, variation of environmental conditions, such as availability of food, water temperature, concentration of contaminants, among others, and is important to take into consideration such variation in biomonitoring studies [83].
4.1. MPs were found in brain samples of fish
MPs were found in brain samples of D. labrax fish collected in the summer, leading to the refusal of H01 and acceptance of HA1. The presence of MPs in brain samples indicates that fish have uptake MPs, which entered into the blood circulation, crossed the blood-brain barrier and entered into the brain where they were accumulated or at least retained for some time. The presence of NPs and MPs in the brain was documented before in fish and crabs exposed in laboratorial conditions. In fish, particles in the brain were found in Crucian carps (Carassius carassius) exposed for 64 days to amino-modified polystyrene NPs with 53 or 180 nm [25], in Nile tilapia (Oreochromis niloticus) exposed for 14 days to polystyrene MPs with 0.1 μm [20], 0.3, 5 or 70–90 μm [70], and in zebrafish (Danio rerio) exposed for 96 h to polystyrene NPs with 100 nm [84] or after ∼7 weeks to polystyrene NPs with 70 nm [85]. In crabs, plastic particles entered and remained within the brain of the velvet swimming crab (Necora puber) after 1 h, 24 h, 7 days and 21 days post consumption of mussels contaminated with 0.5 μm polystyrene MPs [86]. Our study shows that this happens also in real scenarios, in agreement with the prior report of MPs in the brain of wild fish from the Black Sea [65].
Our results show that from all the three sampling seasons and studied species, MPs were only found in one species, in the summer period. Such difference could be driven by contrasting seasonal MP abundance and distribution patterns in the Douro estuary [52] or by differences in the feeding features and habitat preferences of the studied fish species [87]. Given these results, further research is needed to understand the factors that control and influence the presence of MP particles in the brain of wild fish populations.
The high observed proportion (70%) of small MP particles (<50 μm in size) in the brain of D. labrax specimens indicates that the smaller the particle size is, the easier it is for MPs to penetrate the blood-brain barrier. Our findings also relate to previous research highlighting that smaller MP particles are seemingly able to be retained in the brain of fish more readily than larger particles (70 − 90 μm) [70], although MPs up to 200 μm in size were also found in the brain of fish species caught in the Black Sea [65].
In addition to the size, the internalization of MPs into cells and tissues has been also related to other characteristics, such as shape, surface chemistry, softness and smoothness of the material [88]. Therefore, the results of the present pilot study showing that irregular shaped MPs, such as fragments and films, can reach the brain in fish exposed in real scenarios increases the concerns regarding the long-term exposure of wildlife and humans to a variety of MPs present in the environment.
The mechanisms allowing the MPs to cross the blood-brain barrier are not known. Several mechanisms were proposed, such as the disturbance of the tight junction of the blood-brain barrier facilitating MPs entry [89], microglial phagocytosis [90], and via retrograde transport through olfactory nerve endings [32]. Independently of the mechanisms involved, the presence of MPs in the brain of fish can cause several adverse effects, such as neuronal degeneration and necrosis, cytoplasmic vacuolation, inflammatory cell infiltration and haemorrhage [91], decreased brain mass and morphological changes in the cerebral gyri [25], alterations in neural circuits [71] and alterations of brain enzymes [70], among other adverse effects as recently reviewed [32]. Further studies need to be performed to improve our understanding about the mechanisms involved in the internalization and retention of MPs (including bioplastics) in the brain of fish and other animals, and the influence of several factors, including biological variables (e.g., species, life-cycle phase), properties of the MPs (surface chemistry, size, shape), and environmental determinants (MP concentration, temperature variation, presence of other stressors).
The polymer composition of the MPs found in the brain of fish from the Douro estuary was dominated by two acrylic polymers (polyacrylamide (PAM) and polyacrylic acid (PAA)), followed by one biopolymer (Zein). Considering that the seasonal distribution of MPs in the environment is likely more related to specific plastic types than to the overall plastic concentration [92], one can hypothesise that the predominance of acrylic polymers in the brain of D. labrax specimens in the summer can be related with the increasing demand for shipping activities during the summer period (e.g., boat tours) or with the seasonal cleaning of the boats in the boat dock near the area where the fish were captured. These hypotheses can be supported by reports indicating that the presence of acrylic polymer fragments in aquatic environments are normally generated during boat maintenance and cleaning [93,94] or in areas of intense maritime traffic and related activities [95]. In addition, previous studies from the Douro estuary provide supporting evidence of micro-sized acrylic polymers fragments presence near a boat dock/maintenance area [53]. The presence of Zein within fish brain also deserves attention, because it is a main component of bioplastics [96,97]. Products manufactured from Zein can be used to package and/or protect food stuffs [98]. The presence of Zein in fish from the Douro estuary is in agreement with the findings in commercial fish species from the Adriatic Sea [97]. These findings draw attention to the new plastic materials that are now being used, and the need of more studies on their potential adverse effects.
4.2. Brain AChE activity is related with the presence of MPs in fish brain samples
In the summer, D. labrax specimens having MPs in the brain had significantly lower brain AChE activity than specimens of the same species where MPs were not found in the part of the brain analysed. The Douro estuary is contaminated with a diversity of MPs all through the year, and the contamination is considerable including in the summer [52]. All the specimens of D. labrax used for the statistical analyses comparing AChE activity in fish with and without MPs in brain samples were collected in the same estuary minimizing the potential influence of previous developmental conditions in different habitats, which may influence some of the MP effects [33,99]. All the fish with MPs in brain samples were collected in the same season (summer), therefore minimizing the potential influence of seasonal variability and other contaminants that may inhibit AChE activity (e.g., some metals, PAHs, organophosphate pesticides) known to be present in the Douro estuary [99,100]. Moreover, in studies carried out in laboratory conditions, AChE inhibition has been documented in several fish species exposed to MPs with a variety of sizes, chemical composition and shape. Just to give some examples, inhibition of brain AChE activity was documented in Pomatochistus microps from wild populations exposed to 1–5 μm polyethylene beds [26,33], in Argyrosomus regius exposed to 125 μm irregular low-density polyethylene [34], in Oreochromis niloticus exposed to 0.1 μm polystyrene beads [20], in Symphysodon aequifasciatus exposed to 70–88 μm polyethylene microspheres [27], in D. labrax exposed to 1–5 μm beds with unknown polymer composition [29], in Clarias gariepinu exposed to polyvinyl chloride pellets (3.0 g/kg) [28], and in Etroplus suratensis from wild populations exposed to 80.72 μm polyvinyl chloride pellets [101]. Furthermore, it is important to highlight that acrylamide, widely employed as a monomer used in the polymer industry, including to produce polyacrylamide, one of the MP types identified in the brain samples of D. labrax from the Douro estuary, can cause AChE inhibition, alterations in neurotransmitter levels among other effects in the central nervous system [[102], [103], [104], [105]]. Therefore, the lower AChE activity (∼32%) in fish with MPs in the brain than in fish with samples negative for MPs lead to the refusal of H02 and acceptance of HA2.
Brain AChE inhibition in fish with MPs points to neurotoxicity induced by long-term exposure to MPs, MP-Chem or both. These findings are in line with previous studies [32]. It may cause a diversity of effects, some of them also documented in fish exposed to MPs in controlled conditions, such as alterations in behavioral patterns, swimming performance decrease, and reduction of feeding activity [27,35,106]. Inhibition of AChE may also influence key neurodevelopmental events, including cell migration, neurite outgrowth, synaptogenesis, among others [32,91]. This highlights the importance of further research on the neurotoxic effects induced by long-term exposure to MPs and also MP-Chem.
5. Conclusions
Among the 180 wild fish (D. labrax, P. flesus, M. cephalus) analysed, 9 had MPs in brain samples (all the brain except the cerebellum that was used for AChE analyses). All the fish with MPs in brain samples were D. labrax specimens collected in the summer, accounting for 45% of the 20 seabasses collected in the summer. These findings demonstrate the ability of MPs to cross the blood barrier of fish exposed in a real scenario (Douro River estuary discharging into the NE Atlantic Ocean), likely for a considerable or long time, to a diversity of MPs and other contaminants. Seventeen MPs (16 fragments, 1 film) with sizes between 8 μm and 96 μm were recovered from the analysed brain samples. Two superabsorbent polymers and one biopolymer were identified. Compared with D. labrax specimens collected in the summer with brain samples negative for MPs, fish with MPs in brain samples had significant inhibition (by 32%) of brain AChE activity pointing to neurotoxicity induced by MPs. The findings of this pilot study carried out in a real scenario together with previous studies in the laboratorial and field conditions highlight the potential neurotoxicity that long-term exposure to MPs, MP-Chem and other pollutants may have been inducing on wild species, domestic animals and humans, and stress the urgent need of further studies on the topic using ‘One Health’ approaches.
Author contribution statement
L.G.A. Barboza: conceived and designed the experiments; performed the experiments; analysed and interpreted the data; wrote the paper; original draft of the manuscript.
X.L. Otero: contributed reagents, materials, analysis tools or data; analysed and interpreted the data; wrote the paper; drafting the article or revising it critically for important intellectual content.
E.V. Fernández: contributed reagents, materials, analysis tools or data; analysed and interpreted the data.
L.R.Vieira: performed the experiments; analysed and interpreted the data; wrote the paper; drafting the article or revising it critically for important intellectual content.
S.C. Cunha: conceived and designed the experiments; contributed reagents, materials, analysis tools or data; wrote the paper; drafting the article or revising it critically for important intellectual content.
J.O. Fernandes: conceived and designed the experiments; contributed reagents, materials, analysis tools or data; wrote the paper; drafting the article or revising it critically for important intellectual content.
L. Guilhermino: conceived and designed the experiments; analysed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper; drafting the article or revising it critically for important intellectual content.
Funding statement
This study was carried out in the scope of the project “EDCs-Seafood – Integrated Assessment of Emerging Endocrine Disruptor Contaminants in Seafood from Portuguese Estuaries” funded by Fundação para a Ciência e a Tecnologia, I.P. (FCT), Portugal, with national funds (FCT/MCTES, project reference POCI-01-0145-FEDER-028708-PTDC/ASP-PES/28708/2017), the European Regional Development Fund (ERDF) through NORTH 2020 (Northern Regional Operational Program 2014/2020). Other support was provided by the consolidation and structuring of competitive research units of the SUG IDT (ref. 2018-PG036) of the Xunta de Galicia, by the Laboratory of Ecotoxicology and Ecology (ECOTOX), Department of Population Studies of the School of Medicine and Biomedical Sciences (ICBAS) of the University of Porto, and by the Strategic Funding through national funds provided by FCT and ERDF in the framework of the programme Portugal 2020 to CIIMAR (UIDB/Multi/04423/2021 and UIDB/Multi/04423/2022) and CIMAR-LA (LA/P/0101/2021 and LA/P/0101/2022), respectively. L.G. Barboza had a fellowship from FCT through the “EDCs-Seafood” project, and has now a research contract from FCT through CIIMAR (2020.02573.CEECIND). S.C. Cunha was supported by UIDP/50006/2020 FCT/MCTES.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
References
- 1.Patel D., Mamtora D., Kamath A., Shukla A. Rogue one: a plastic story. Mar. Pollut. Bull. 2022;177 doi: 10.1016/j.marpolbul.2022.113509. [DOI] [PubMed] [Google Scholar]
- 2.OECD, Global Plastics Outlook . OECD Publishing; Paris: 2022. Economic Drivers, Environmental Impacts and Policy Options. [Google Scholar]
- 3.Canning-Clode J., Sepúlveda P., Almeida S., Monteiro J. Will COVID-19 containment and treatment measures drive shifts in marine litter pollution? Front. Mar. Sci. 2020;691 [Google Scholar]
- 4.Guilhermino L., Martins A., Cunha S., Fernandes J.O. Long-term adverse effects of microplastics on Daphnia magna reproduction and population growth rate at increased water temperature and light intensity: combined effects of stressors and interactions. Sci. Total Environ. 2021;784 doi: 10.1016/j.scitotenv.2021.147082. [DOI] [PubMed] [Google Scholar]
- 5.Guilhermino L., Martins A., Lopes C., Raimundo J., Vieira L.R., Barboza L.G.A., L.G.A., Costa J., Antunes C., Caetano M., Vale C. Microplastics in fishes from an estuary (Minho River) ending into the NE Atlantic Ocean. Mar. Pollut. Bull. 2021;173(A) doi: 10.1016/j.marpolbul.2021.113008. [DOI] [PubMed] [Google Scholar]
- 6.Peng Y., Wu P., Schartup A.T., Zhang Y. Plastic waste release caused by COVID-19 and its fate in the global ocean. Proc. Natl. Acad. Sci. U.S.A. 2021;23(47) doi: 10.1073/pnas.2111530118. 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrady A.L. The plastic in microplastics: a review. Mar. Pollut. Bull. 2017;119:12–22. doi: 10.1016/j.marpolbul.2017.01.082. [DOI] [PubMed] [Google Scholar]
- 8.Carbery M., O'Connor W., Palanisami T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018;115:400–409. doi: 10.1016/j.envint.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Perumal K., Muthuramalingam S. Global sources, abundance, size, and distribution of microplastics in marine sediments - a critical review. Estuar. Coast Shelf Sci. 2021;264 [Google Scholar]
- 10.Sfriso A.A., Tomio Y., Juhmani A.S., Sfriso A., Munari C., Mistri M. Macrophytes: a temporary sink for microplastics in transitional water systems. Water. 2021;13(21):3032. [Google Scholar]
- 11.Wang Z., Zhang Y., Kang S., Yang L., Shi H., Tripathee L., Gao T. Research progresses of microplastic pollution in freshwater systems. Sci. Total Environ. 2021;795 doi: 10.1016/j.scitotenv.2021.148888. [DOI] [PubMed] [Google Scholar]
- 12.Bohdan K. Estimating global marine surface microplastic abundance: systematic literature review. Sci. Total Environ. 2022;832 doi: 10.1016/j.scitotenv.2022.155064. [DOI] [PubMed] [Google Scholar]
- 13.Dissanayake P.D., Kim S., Sarkar B., Oleszczuk P., Sang M.K., Ok Y.S. Effects of microplastics on the terrestrial environment: a critical review. Environ. Res. 2022;209 doi: 10.1016/j.envres.2022.112734. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S., Zhang Z., Chen L., Cui Q., Cui Y., Song D., Fang L. Review on migration, transformation and ecological impacts of microplastics in soil. App. Soil Ecol. 2022;176 [Google Scholar]
- 15.Rochman C.M., Brookson C., Bikker J., Djuric N., Earn A., Bucci K., Hung C. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019;38:703–711. doi: 10.1002/etc.4371. [DOI] [PubMed] [Google Scholar]
- 16.Beiras R., Verdejo E., Campoy-Lopez P., Vidal-Linan L. Aquatic toxicity of chemically defined microplastics can be explained by functional additives. J. Hazard Mater. 2021;406 doi: 10.1016/j.jhazmat.2020.124338. [DOI] [PubMed] [Google Scholar]
- 17.Wiesinger H., Wang Z., Hellweg S. Deep dive into plastic monomers, additives, and processing aids. Environ. Sci. Technol. 2021;55(13):9339–9351. doi: 10.1021/acs.est.1c00976. [DOI] [PubMed] [Google Scholar]
- 18.Kaur K., Reddy S., Barathe P., Oak U., Shriram V., Kumar V. Microplastic-associated pathogens and antimicrobial resistance in environment. Chemosphere. 2022;291(2) doi: 10.1016/j.chemosphere.2021.133005. [DOI] [PubMed] [Google Scholar]
- 19.Browne M.A., Dissanayake A., Galloway T.S., Lowe D.M., Thompson R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.) Environ. Sci. Technol. 2008;42(13):5026–5031. doi: 10.1021/es800249a. [DOI] [PubMed] [Google Scholar]
- 20.Ding J., Zhang S., Razanajatovo R.M., Zou H., Zhu W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus) Environ. Pollut. 2018;238:1–9. doi: 10.1016/j.envpol.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Leslie H.A., Van Velzen M.J., Brandsma S.H., Vethaak A.D., Garcia-Vallejo J.J., Lamoree M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- 22.Barboza L.G.A., Lopes C., Oliveira P., Bessa F., Otero V., Henriques B., Raimundo J., Caetano M., Vale C., Guilhermino L. L, Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2019.134625. [DOI] [PubMed] [Google Scholar]
- 23.McIlwraith H.K., Kim J., Helm P., Bhavsar S.P., Metzger J.S., Rochman C.M. Evidence of microplastic translocation in WildCaught fish and implications for microplastic accumulation dynamics in food webs. Environ. Sci. Technol. 2021;55(18):12372–12382. doi: 10.1021/acs.est.1c02922. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadi A., Moore F., Keshavarzi B., Soltani N., Sorooshian A. Potentially toxic elements and microplastics in muscle tissues of different marine species from the Persian Gulf: levels, associated risks, and trophic transfer. Mar. Pollut. Bull. 2022;175 doi: 10.1016/j.marpolbul.2021.113283. [DOI] [PubMed] [Google Scholar]
- 25.Mattsson K., Johnson E.V., Malmendal A., Linse S., Hansson L.A., Cedervall T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017;7:1–7. doi: 10.1038/s41598-017-10813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira M., Ribeiro A., Hylland K., Guilhermino L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae) Ecol. Indicat. 2013;34:641–647. [Google Scholar]
- 27.Wen B., Zhang N., Jin S.R., Chen Z.Z., Xu Z. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquat. Toxicol. 2018;195:67–76. doi: 10.1016/j.aquatox.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Iheanacho S.C., Odo G.E. Neurotoxicity, oxidative stress biomarkers and haematological responses in African catfish (Clarias gariepinus) exposed to polyvinyl chloride microparticles. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2020;232 doi: 10.1016/j.cbpc.2020.108741. [DOI] [PubMed] [Google Scholar]
- 29.Barboza L.G.A., Vieira L.R., Branco V., Figueiredo N., Carvalho F., Carvalho C., Guilhermino L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758) Aquat. Toxicol. 2018;195:49–57. doi: 10.1016/j.aquatox.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Hamed M., Soliman H.A., Osman A.G., Sayed A.E.-D.H. Antioxidants and molecular damage in Nile Tilapia (Oreochromis niloticus) after exposure to microplastics. Environ. Sci. Pollut. Res. 2020;27:1–8. doi: 10.1007/s11356-020-07898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usman S., Abdull Razis A.F., Shaari K., A Amal M.N., Saad M.Z., Isa N.M., Nazarudin M.F. Polystyrene microplastics exposure: an insight into multiple organ histological alterations, oxidative stress and neurotoxicity in Javanese medaka fish (Oryzias javanicus Bleeker, 1854) Int. J. Environ. Res. Publ. Health. 2021;18(18):9449. doi: 10.3390/ijerph18189449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prüst M., Meijer J., Westerink R.H.S. The plastic brain: neurotoxicity of micro- and nanoplastics, Part. Fibre Toxicol. 2020;17:24. doi: 10.1186/s12989-020-00358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luis L.G., Ferreira P., Fonte E., Oliveira M., Guilhermino L. Does the presence of microplastics influence the acute toxicity of chromium (VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat. Toxicol. 2015;164:163–174. doi: 10.1016/j.aquatox.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Campos D., Rodrigues A.C., Rocha R.J., Martins R., Candeias-Mendes A., Castanho A.S., Patrício Silva A.L. Are microplastics impairing marine fish larviculture? Preliminary results with Argyrosomus regius. Water. 2021;13(1):104. [Google Scholar]
- 35.L.G.A., Barboza, Vieira L.R., Guilhermino L. Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): changes in behavioural responses and reduction of swimming velocity and resistance time. Environ. Pollut. 2018;236:1014–1019. doi: 10.1016/j.envpol.2017.12.082. [DOI] [PubMed] [Google Scholar]
- 36.McCormick M.I., Chivers D.P., Ferrari M.C., Blandford M.I., Nanninga G.B., Richardson C.…Allan B.J. Microplastic exposure interacts with habitat degradation to affect behaviour and survival of juvenile fish in the field. Proc. R. Soc. B: Biol. Sci. 2020;287(1937) doi: 10.1098/rspb.2020.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y., Li W., Xiang L., Mi X., Duan M., Wu C. Fish personality affects their exposure to microplastics. Ecotoxicol. Environ. Saf. 2022;233 doi: 10.1016/j.ecoenv.2022.113301. [DOI] [PubMed] [Google Scholar]
- 38.Barboza L.G.A., Vethaak A.D., Lavorante B.R., Lundebye A.K., Guilhermino A.L. Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018;133:336–348. doi: 10.1016/j.marpolbul.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Roda J.F.B., Lauer M.M., Risso W.E., dos Reis Martinez C.B. Microplastics and copper effects on the neotropical teleost Prochilodus lineatus: is there any interaction? Comp. Biochem. Physiol. Part A Mol. Integr. 2020;242 doi: 10.1016/j.cbpa.2020.110659. [DOI] [PubMed] [Google Scholar]
- 40.Takai Y., Tokusumi H., Sato M., Inoue D., Chen K., Takamura T., Oshima Y. Combined effect of diazepam and polystyrene microplastics on the social behavior of medaka (Oryzias latipes) Chemosphere. 2022;299 doi: 10.1016/j.chemosphere.2022.134403. [DOI] [PubMed] [Google Scholar]
- 41.Bertucci J.I., Bellas J. Combined effect of microplastics and global warming factors on early growth and development of the sea urchin (Paracentrotus lividus) Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146888. [DOI] [PubMed] [Google Scholar]
- 42.Chang M., Zhang C., Li M., Dong J., Li C., Stoks R. Warming, temperature fluctuations and thermal evolution change the effects of microplastics at an environmentally relevant concentration. Environ. Pollut. 2022;292 doi: 10.1016/j.envpol.2021.118363. [DOI] [PubMed] [Google Scholar]
- 43.Martins A., da Silva D.D., Silva R., Carvalho F., Guilhermino L. Long-term effects of lithium and lithium microplastic mixtures on the model species Daphnia magna: toxicological interactions and implications to ‘One Health. Sci. Total Environ. 2022;838(1) doi: 10.1016/j.scitotenv.2022.155934. [DOI] [PubMed] [Google Scholar]
- 44.Sangkham S., Faikhaw O., Munkong N., Sakunkoo P., Arunlertaree C., Tiwari A. A review on microplastics and nanoplastics in the environment: their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022;181 doi: 10.1016/j.marpolbul.2022.113832. [DOI] [PubMed] [Google Scholar]
- 45.Talbot R., Chang H. Microplastics in freshwater: a global review of factors affecting spatial and temporal variations. Environ. Pollut. 2022;292(2022) doi: 10.1016/j.envpol.2021.118393. [DOI] [PubMed] [Google Scholar]
- 46.Lebreton L.C.M., van der Zwet J., Damsteeg J.-W., Slat B., Andrady A., Reisser J. J., River plastic emissions to the world's oceans. Nat. Commun. 2017;8 doi: 10.1038/ncomms15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pazos R.S., Maiztegui T., Colautti D.C., Paracampo A.H., Gómez N. Microplastics in gut contents of coastal freshwater fish from Río de la Plata estuary. Mar. Pollut. Bull. 2017;122(1–2):85–90. doi: 10.1016/j.marpolbul.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Cordova M.R., Riani E., Shiomoto A. Microplastics ingestion by blue panchax fish (Aplocheilus sp.) from Ciliwung Estuary, Jakarta, Indonesia. Mar. Pollut. Bull. 2020;161 doi: 10.1016/j.marpolbul.2020.111763. [DOI] [PubMed] [Google Scholar]
- 49.Sánchez-Hernández L.J., Ramírez-Romero P., Rodríguez-González F., Ramos-Sánchez V.H., Montes R.A.M., Rubio H.R.P., Jonathan M.P. Seasonal evidences of microplastics in environmental matrices of a tourist dominated urban estuary in Gulf of Mexico, Mexico. Chemosphere. 2021;277 doi: 10.1016/j.chemosphere.2021.130261. [DOI] [PubMed] [Google Scholar]
- 50.Vieira M.E., Bordalo A.A. The Douro estuary (Portugal): a mesotidal salt wedge. Oceanol. Acta. 2020;23(5):585–594. [Google Scholar]
- 51.Azevedo I.C., Duarte P.M., Bordalo A.A. Understanding spatial and temporal dynamics of key environmental characteristics in a mesotidal Atlantic estuary (Douro, NW Portugal) Estuar. Coast Shelf Sci. 2008;76(3):620–633. [Google Scholar]
- 52.Rodrigues S.M., Almeida C.M.R., Silva D., Cunha J., Antunes C., Freitas V., Ramos S. S. Microplastic contamination in an urban estuary: abundance and distribution of microplastics and fish larvae in the Douro estuary. Sci. Total Environ. 2019;659:1071–1081. doi: 10.1016/j.scitotenv.2018.12.273. [DOI] [PubMed] [Google Scholar]
- 53.Prata J.C., Godoy V., da Costa J.P., Calero M., Martín-Lara M.A., Duarte A.C., Rocha-Santos T. Microplastics and fibers from three areas under different anthropogenic pressures in Douro river. Sci. Total Environ. 2021;776 doi: 10.1016/j.scitotenv.2021.145999. [DOI] [PubMed] [Google Scholar]
- 54.Madureira T.V., Barreiro J.C., Rocha M.J., Rocha E., Cass Q.B., Tiritan M.E. Spatiotemporal distribution of pharmaceuticals in the Douro River estuary (Portugal) Sci. Total Environ. 2010;408(22):5513–5520. doi: 10.1016/j.scitotenv.2010.07.069. [DOI] [PubMed] [Google Scholar]
- 55.Iglesias I., Almeida C.M.R., Teixeira C., Mucha A.P., Magalhães A., Bio A., Bastos L. Linking contaminant distribution to hydrodynamic patterns in an urban estuary: the Douro estuary test case. Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.135792. [DOI] [PubMed] [Google Scholar]
- 56.Cunha S.C., Ferreira R., Marmelo I., Vieira L., Anacleto P., Fernandes .J.O. Occurrence and seasonal variation of several endocrine disruptor compounds (pesticides, bisphenols, musks and UV-filters) in water and sediments from the estuaries of Tagus and Douro Rivers (NE Atlantic Ocean coast) Sci. Total Environ. 2022;838(2) doi: 10.1016/j.scitotenv.2022.155814. [DOI] [PubMed] [Google Scholar]
- 57.McGoran A.R., Clark P.F., Morritt D.J.E.P. Presence of microplastic in the digestive tracts of European flounder, Platichthys flesus, and European smelt, Osmerus eperlanus, from the River Thames. Environ. Pollut. 2017;220:744–751. doi: 10.1016/j.envpol.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 58.Bessa F., Barría P., Neto J.M., Frias J.P., Otero V., Sobral P., Marques J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018;128:575–584. doi: 10.1016/j.marpolbul.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C., Wang S., Pan Z., Sun D., Xie S., Zhou A., Zou J. Occurrence and distribution of microplastics in commercial fishes from estuarine areas of Guangdong, South China. Chemosphere. 2020;260 doi: 10.1016/j.chemosphere.2020.127656. [DOI] [PubMed] [Google Scholar]
- 60.Kılıç E., Yücel N. Microplastic occurrence in the gastrointestinal tract and gill of bioindicator fish species in the northeastern Mediterranean. Mar. Pollut. Bull. 2022;177 doi: 10.1016/j.marpolbul.2022.113556. [DOI] [PubMed] [Google Scholar]
- 61.Owasoyo J.O., Iramain C.A. Acetylcholinesterase: regional activity in the central nervous system of the rainbow lizard, Agama agama. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 1980;65(1):71–72. doi: 10.1016/0306-4492(80)90048-9. [DOI] [PubMed] [Google Scholar]
- 62.Maler L., Collins M., Mathieson W.B. The distribution of acetylcholinesterase and choline acetyl transferase in the cerebellum and posterior lateral line lobe of weakly electric fish (Gymnotidae) Brain Res. 1981;226:320–325. doi: 10.1016/0006-8993(81)91106-9. [DOI] [PubMed] [Google Scholar]
- 63.Horvatits T., Tamminga M., Liu B., Sebode M., Carambia A., Fischer L., Püschel K., Huber S., Fischer E.K. Microplastics detected in cirrhotic liver tissue. EBioMedicine. 2022;82 doi: 10.1016/j.ebiom.2022.104147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seraphin K.D., Philippoff J., Pottenger J.M., Kaupp L., Lurie M.H., Lin D., Baumgartner E., Exploring our fluid earth. Honolulu, HI: curriculum research & development group and Hawai‘i Sea Grant (2015). Available at: https://manoa.hawaii.edu/exploringourfluidearth/behavior-and-sensory-systems-fish.
- 65.Atamanalp M., Köktürk M., Uçar A., Duyar H.A., Özdemir S., Parlak V., Esenbuğa N., Alak G. Microplastics in tissues (brain, gill, muscle and gastrointestinal) of Mullus barbatus and Alosa immaculata. Arch. Environ. Contam. Toxicol. 2021;81(3):460–469. doi: 10.1007/s00244-021-00885-5. [DOI] [PubMed] [Google Scholar]
- 66.Käppler A., Fischer D., Oberbeckmann S., Schernewski G., Labrenz M., Eichhorn K.J., Voit B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016;408(29):8377–8391. doi: 10.1007/s00216-016-9956-3. [DOI] [PubMed] [Google Scholar]
- 67.Sobhani Z., Al Amin M., Naidu R., Megharaj M., Fang C. Identification and visualisation of microplastics by Raman mapping. Anal. Chim. Acta. 2019;1077:191–199. doi: 10.1016/j.aca.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 68.MSFD Technical Subgroup on Marine Litter Report. Galgani F., Hanke G., Werner S., Oosterbaan L., Nilsson P., Liebezeit G. Guidance on Monitoring of Marine Litter in European Seas, European Commission, Joint Research Centre. MSFD Technical Subgroup on Marine Litter. TSG-ML); Luxembourg: 2013. [Google Scholar]
- 69.Lusher A.L., Bråte I.L.N., Munno K., Hurley R.R. Is it or isn't it: the importance of visual classification in microplastic characterization. Appl. Spectrosc. 2020;74(9):1139–1153. doi: 10.1177/0003702820930733. [DOI] [PubMed] [Google Scholar]
- 70.Ding J., Huang Y., Liu S., Zhang S., Zou H., Wang Z., Zhu W., Geng J. Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: are larger plastic particles more harmless? J. Hazard Mater. 2020;396 doi: 10.1016/j.jhazmat.2020.122693. [DOI] [PubMed] [Google Scholar]
- 71.Santos D., Luzio A., Félix L., Cabecinha E., Bellas J., Monteiro S.M. Microplastics and copper induce apoptosis, alter neurocircuits, and cause behavioral changes in zebrafish (Danio rerio) brain. Ecotoxicol. Environ. Saf. 2022;242 doi: 10.1016/j.ecoenv.2022.113926. [DOI] [PubMed] [Google Scholar]
- 72.Zitouni N., Cappello T., Missawi O., Boughattas I., De Marco G., Belbekhouche S., Banni M. Metabolomic disorders unveil hepatotoxicity of environmental microplastics in wild fish Serranus scriba (Linnaeus 1758) Sci. Total Environ. 2022;10(838) doi: 10.1016/j.scitotenv.2022.155872. [DOI] [PubMed] [Google Scholar]
- 73.Guilhermino L., Lopes M.C., Carvalho A.P., Soares A.M. Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphnia magna. Chemosphere. 1996;32(4):727–738. doi: 10.1016/0045-6535(95)00360-6. [DOI] [PubMed] [Google Scholar]
- 74.Vieira L.R., Gravato C., Soares A.M.V.M., Morgado F., Guilhermino L. Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: linking biomarkers to behaviour. Chemosphere. 2009;76(10):1416–1427. doi: 10.1016/j.chemosphere.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 75.M Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 76.Frasco M.F., Guilhermino L. Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulata, Fish. Physiol. Biochem. 2002;26(2):149–156. [Google Scholar]
- 77.G.L. Ellman K.D., Courtney V., Andres, Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 78.Zar J.H. fourth ed. Prentice Hall, Inc.; New Jersey: 1999. Biostatistical Analysis. [Google Scholar]
- 79.Fernandes D., Porte C., Bebianno M.J. Chemical residues and biochemical responses in wild and cultured European sea bass (Dicentrarchus labrax L.) Environ. Res. 2007;103(2):247–256. doi: 10.1016/j.envres.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 80.Capela R., Raimundo J., Santos M.M., Caetano M., Micaelo C., Vale C., Reis-Henriques M.A. The use of biomarkers as integrative tools for transitional water bodies monitoring in the Water Framework Directive context: a holistic approach in Minho river transitional waters. Sci. Total Environ. 2016;539:85–96. doi: 10.1016/j.scitotenv.2015.08.113. [DOI] [PubMed] [Google Scholar]
- 81.Kopecka J., Lehtonen K.K., Baršienė J., Broeg K., Vuorinen P.J., Gercken J., Pempkowiak J. Measurements of biomarker levels in flounder (Platichthys flesus) and blue mussel (Mytilus trossulus) from the Gulf of Gdańsk (southern Baltic) Mar. Pollut. Bull. 2006;53(8–9):406–421. doi: 10.1016/j.marpolbul.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 82.Casini S., Aurigi S., Fossi M.C., Monaci F., Focardi S. Mediterranean Ecosystems. Springer; Milano: 2001. Evaluation of environmental quality for the management of brackish wetlands: use of bioindicators and biomarkers in the pontine Lakes; pp. 65–70. [Google Scholar]
- 83.Whitfield A.K., Able K.W., Blaber S.J., Elliott M. Blackwell; Oxford, UK: 2022. Fish and Fisheries in Estuaries: A Global Perspective. [Google Scholar]
- 84.Sulukan E., Baran A., Senol O., Yildirim S., Mavi A., Ceyhun H.A., Ceyhun S.B. The synergic toxicity of temperature increases and nanopolystrene on zebrafish brain implies that global warming may worsen the current risk based on plastic debris. Sci. Total Environ. 2022;808 doi: 10.1016/j.scitotenv.2021.152092. [DOI] [PubMed] [Google Scholar]
- 85.Sarasamma S., Audira G., Siregar P., Malhotra N., Lai Y.H., Hsiao C.D. Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: throwing up alarms of wide spread health risk of exposure. Int. J. Mol. Sci. 2020;21(4):1410. doi: 10.3390/ijms21041410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crooks N., Parker H., Pernetta A.P. Brain food? Trophic transfer and tissue retention of microplastics by the velvet swimming crab (Necora puber) J. Exp. Mar. Biol. 2019;519 [Google Scholar]
- 87.Soe K.K., Hajisamae S., Sompongchaiyakul P., Towatana P., Pradit S. Feeding habits and the occurrence of anthropogenic debris in the stomach content of marine fish from Pattani Bay, Gulf of Thailand. Biology. 2022;11(2):331. doi: 10.3390/biology11020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wieland S., Balmes A., Bender J., Kitzinger J., Meyer J.F., Ramsperger A., Kress .H. From properties to toxicity: comparing microplastics to other airborne microparticles. J. Hazard Mater. 2022;438 doi: 10.1016/j.jhazmat.2021.128151. [DOI] [PubMed] [Google Scholar]
- 89.Shan S., Zhang Y., Zhao H., Zeng T., Zhao X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere. 2022;298 doi: 10.1016/j.chemosphere.2022.134261. [DOI] [PubMed] [Google Scholar]
- 90.Kwon W., Kim D., Kim H.Y., Jeong S.W., Lee S.G., Kim H.C.…Choi S.K. Microglial phagocytosis of polystyrene microplastics results in imune alteration and apoptosis in vitro and in vivo. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.150817. [DOI] [PubMed] [Google Scholar]
- 91.Umamaheswari S., Priyadarshinee S., Bhattacharjee M., Kadirvelu K., Ramesh M. Exposure to polystyrene microplastics induced gene modulated biological responses in zebrafish (Danio rerio) Chemosphere. 2021;281 doi: 10.1016/j.chemosphere.2020.128592. [DOI] [PubMed] [Google Scholar]
- 92.Prata J.C., Reis V., Paço A., Martins P., Cruz A., da Costa J.P.…Rocha-Santos T. Effects of spatial and seasonal factors on the characteristics and carbonyl index of (micro) plastics in a sandy beach in Aveiro, Portugal. Sci. Total Environ. 2020;709 doi: 10.1016/j.scitotenv.2019.135892. [DOI] [PubMed] [Google Scholar]
- 93.Galafassi S., Nizzetto L., Volta P. Plastic sources: a survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci. Total Environ. 2019;693 doi: 10.1016/j.scitotenv.2019.07.305. [DOI] [PubMed] [Google Scholar]
- 94.Pinho I., Amezcua F., Rivera J.M., Green-Ruiz C., Piñón-Colin T.J., Wakida F. First report of plastic contamination in batoids: plastic ingestion by Haller's Round Ray (Urobatis halleri) in the Gulf of California. Environ. Res. 2022;211 doi: 10.1016/j.envres.2022.113077. [DOI] [PubMed] [Google Scholar]
- 95.Song Y.K., Hong S.H., Jang M., Kang J.H., Kwon O.Y., Han G.M., Shim W.J. Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ. Sci. Technol. 2014;48(16):9014–9021. doi: 10.1021/es501757s. [DOI] [PubMed] [Google Scholar]
- 96.Lawton J.W. Zein: a history of processing and use. Cereal Chem. 2002;79(1):1–18. [Google Scholar]
- 97.Corami F., Rosso B., Sfriso A.A., Gambaro A., Mistri M., Munari C., Barbante C. Additives, plasticizers, small microplastics (< 100 μm), and other microlitter components in the gastrointestinal tract of commercial teleost fish: method of extraction, purification, quantification, and characterization using Micro-FTIR. Mar. Pollut. Bull. 2022;177 doi: 10.1016/j.marpolbul.2022.113477. [DOI] [PubMed] [Google Scholar]
- 98.Taylor J., Anyango J.O., Taylor J.R. Developments in the science of zein, kafirin, and gluten protein bioplastic materials. Cereal Chem. 2013;90(4):344–357. [Google Scholar]
- 99.Gravato C., Guimarães L., Santos J., Faria M., Alves A., Guilhermino L. Comparative study about the effects of pollution on glass and yellow eels (Anguilla anguilla) from the estuaries of Minho, Lima and Douro Rivers (NW Portugal) Ecotoxicol. Environ. Saf. 2010;73(4):524–533. doi: 10.1016/j.ecoenv.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 100.Cruzeiro C., Amaral S., Rocha E., Rocha M.J. Determination of 54 pesticides in waters of the Iberian Douro River estuary and risk assessment of environmentally relevant mixtures using theoretical approaches and Artemia salina and Daphnia magna bioassays. Ecotoxicol. Environ. Saf. 2017;145:126–134. doi: 10.1016/j.ecoenv.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 101.Vijayaraghavan G., Neethu K.V., Aneesh B.P., Suresh A., Saranya K.S., Nandan S.B., Sharma K.V. Evaluation of toxicological impacts of polyvinyl chloride (PVC) microplastics on fish, Etroplus suratensis (bloch, 1790), Cochin estuary, India, toxicol. Environ. Health Sci. 2022;14:131–140. [Google Scholar]
- 102.McCollister D.D., Oyen F., Rowe V.K. Toxicology of acrylamide. Toxicol. Appl. Pharmacol. 1964;6(2):172–181. doi: 10.1016/0041-008x(64)90103-6. [DOI] [PubMed] [Google Scholar]
- 103.Faria M., Ziv T., Gómez-Canela C., Ben-Lulu S., Prats E., K.A., Raldúa D. Acrylamide acute neurotoxicity in adult zebrafish. Sci. Rep. 2018;8(1):1–14. doi: 10.1038/s41598-018-26343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gopika C.M., Sumi N., Chitra K.C.I. Acute neurotoxicity effect of acrylamide in Oreochromis niloticus (Linnaeus, 1758) Int. J. Fish. Aquat. Res. 2018;3(1):1–8. [Google Scholar]
- 105.Kopańska M., Łagowska A., Kuduk B., Banaś-Ząbczyk A. Acrylamide neurotoxicity as a possible factor responsible for inflammation in the cholinergic nervous system. Int. J. Mol. Sci. 2022;23(4):2030. doi: 10.3390/ijms23042030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Sá L.C., Luis L.G., Guilhermino L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015;196:359–362. doi: 10.1016/j.envpol.2014.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.