Abstract
While γ/δ T cells are involved in host defense and immunopathology in a variety of infectious diseases, their precise role is not yet clearly defined. In the absence of γ/δ T cells, mice die after infection with a dose of Listeria monocytogenes that is not lethal in immunologically intact animals. Morbidity might result from insufficient levels of cytokines normally produced by γ/δ T cells or conversely from an excess of cytokines due to a lack of down-regulation of the inflammatory response in the absence of γ/δ T cells. Consistent with a regulatory role, we found that systemic levels of proinflammatory cytokines (interleukin-6 [IL-6], IL-12, and gamma interferon [IFN-γ]) were significantly higher in the absence of γ/δ T cells during the innate phase of the response. Using combinations of genetically altered and immunodepleted mice, we found evidence for γ/δ T-cell-mediated regulation of IFN-γ production by multiple cell types of both lymphoid and myeloid lineages. The antigen-specific α/β T-cell response that followed the exaggerated innate response was also increased in γ/δ T-cell-deficient mice. These findings are consistent with an emerging picture from a variety of immune response models of a critical role for γ/δ T cells in down-modulation of the immune response.
A number of functions have been attributed to γ/δ T cells in the years since their discovery over a decade ago. Their strategic location at epithelial surfaces and sites of infection and their evolutionary conservation (24) suggest an important role in host defense. This is substantiated by a studies showing that resistance to a variety of pathogens is altered in the absence of γ/δ T cells (reviewed in references 9 and 24). Although the γ/δ T-cell-deficient host is usually able to survive the infection, the immune response may be qualitatively and quantitatively different. The nature of these differences and the mechanisms of regulation are not yet fully elucidated. The complexity of the system is underscored by recent reports showing that elimination of T-cell receptor (TCR) V region subsets of γ/δ T cells can change the pattern of resistance or susceptibility to infections (28, 42). V gamma subsets have differing cytokine production patterns which influence the Th1/Th2 balance of the immune response (27). Few ligands for the unique TCR of γ/δ T cells have been defined despite intense efforts. Based on their localization to epithelial tissues, their response to stress proteins, their response to proinflammatory cytokines, and their response to insults from nonbiological agents (33, 37), these cells may contribute to host defense by surveillance of tissues for signs of disturbance rather than by responding directly to microbial antigens.
The effector functions of γ/δ T cells may vary as the immune response develops. They can respond rapidly to cytokines that are up-regulated in response to infection by producing additional cytokines that contribute to an expanding immune response (19, 48). Production of gamma interferon (IFN-γ) by activated γ/δ T cells may enhance the innate response by activating macrophages (30, 41). The importance of IFN-γ in the primary response to infection with Listeria monocytogenes has been clearly established using genetically altered mice deficient for either IFN-γ (22) or its receptor (26). These mice are highly susceptible to infection with Listeria. Thus, cell populations that contribute to IFN-γ production in response to infection are critical to host defense. IFN-γ production in vitro by NK (21, 54) and γ/δ T cells (48) is induced by culture with interleukin-12 (IL-12), indicating that IL-12 is an important component of this immune response pathway. Increased susceptibility to infection with Listeria in the absence of IL-12 in vivo supports this concept (11, 53).
In addition to this early proinflammatory role, γ/δ T cells may later down-modulate the immune response as an infection is resolved. In their absence, abnormally large granulomatous responses persist after infection with L. monocytogenes (20, 36) or Mycobacterium tuberculosis (17). Similarly, the inflammatory response in the gut is exacerbated after infection with Eimeria vermiformis when γ/δ T cells are absent (45). Taken together, these finding suggest a complex regulatory role for γ/δ T cells following their initial activation either through their TCR or via cytokine receptors.
Since IL-12 is upregulated in response to Listeria infection and can induce IFN-γ production by γ/δ T cells, we proposed that the systemic increase in IFN-γ which follows Listeria infection would be reduced or delayed in the absence of γ/δ T cells. Instead, the increase in IFN-γ was both exaggerated and prolonged in γ/δ T-cell-deficient mice. A general pattern of disregulation extended to production of multiple cytokines, both in vivo and in vitro, by multiple cell types, affecting both innate and adaptive responses. This suggests that down-modulation of the immune response by γ/δ T cells may be more important than their contribution to the production of proinflammatory cytokines.
MATERIALS AND METHODS
Mice and immunizations.
Mouse strains were obtained as follows: C3HeB/FeJ (C3H), Jackson Laboratory (Bar Harbor, Maine); C57BL/6 (B6), National Cancer Institute (Frederick, Md.), C57BL/6 Tcrd (γ/δ TCR knockout [KO]) (29), Jackson Laboratory; C57BL/6 CD1−/− (CD1KO) (51), M. J. Grusby (Harvard); C57BL/6 RAG-1−/− (RAG-1 KO), Jackson Laboratory; C57BL/6 IL-12b KO (35), Hoffman-La Roche, Inc. All KO strains were maintained as breeding colonies at Emory University. Mice were housed in filter-topped microisolator cages in a specific-pathogen-free facility and were used at 8 to 16 weeks of age. All experimental procedures were approved by the Institutional Animal Care and Use Committee. L. monocytogenes wild-type strain 43251 (American Type Culture Collection, Manassas, Va.) were grown overnight in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) at 37°C with aeration and then washed three times in phosphate-buffered saline (PBS) prior to intraperitoneal (i.p.) injection. Concentrations were determined by measuring optical density, with confirmation by colony counts on brain heart infusion agar plates.
Cell preparation and culture.
Peritoneal exudate cells (PEC) were harvested by lavage with cold Hanks balanced salt solution (HBSS) containing 0.06% bovine serum albumin (BSA) and 10 U of heparin/ml. Cells were washed and resuspended for culture in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 5 × 10−5 M 2-mercaptoethanol, 0.5 mM sodium pyruvate, 10 mM HEPES buffer, 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 2 mM l-glutamine. Cells were used without further separation, or nonadherent cells were separated by vigorous washing with warm HBSS-BSA from cells which had adhered to plastic during incubation for 2 h at 37°C.
Antibodies for flow cytometry.
The following reagents were purchased from PharMingen (San Diego, Calif.): GK1.5-fluorescein isothiocyanate (FITC) (anti-CD4), 53-6.72-peridinin chlorophyll protein (PerCP) or -FITC (anti-CD8), GL3-FITC (anti-γ/δ TCR), H57-597-FITC (anti-α/β TCR), CD45R/B220-FITC, anti-NK1.1-phycoerythrin (PE), anti-CD3-PerCP or -PE, RB6-8C5-PE (anti-GR-1 or Ly-6G), XMG1.2-APC (anti-IFN-γ), JES6-5H4-APC (anti-IL-2), and MP6-XT22-APC (anti-tumor necrosis factor alpha [TNF-α]). F4/80-FITC was purchased from Caltag Laboratories (Burlingame, Calif.). Isotype controls for these antibodies were purchased as follows: hamster immunoglobulin G (IgG)-FITC, rat IgG-biotin, rat IgG-PE, rat IgG-allophycocyanin (APC), and mouse IgG-PE, PharMingen; rat IgG-FITC, Southern Biotechnology Associates (Birmingham, Ala.).
Analysis of lymphoid and myeloid populations by flow cytometry.
PEC (106/sample) were incubated for 30 min at 4°C with fluorochrome-conjugated antibodies to surface markers which define lymphoid and myeloid subsets. Cells were then washed twice with wash buffer (PBS with 3% FCS and 0.1% sodium azide) and fixed with 1% paraformaldehyde. Data from a minimum of 10,000 cells per population were collected using a FACSCalibur flow cytometer (Becton Dickinson) and analyzed using CellQuest software. Antibody conjugates used for each experiment are indicated in the figure legends.
Detection of intracellular cytokines in individual lymphocyte populations by flow cytometry.
Various stimuli were used in concentrations indicated in the figure legends to induce cytokine production. Stimuli included phorbol myristate acetate (PMA; Calbiochem, San Diego, Calif.), ionomycin (Calbiochem), murine recombinant IL-12 (rIL-12; Hoffman-La Roche, Inc.), human rIL-1 (kindly provided by Immunex Corp., Seattle, Wash.), murine recombinant TNF-α (rTNF-α) (Endogen, Cambridge, Mass.), and murine rIL-18 (PeproTech, Norwood, Mass.). For antigen-specific stimulation, PEC were cultured for 5 h with either heat-killed L. monocytogenes (HKLM; 107/ml) or with macrophages infected overnight with live Listeria. Brefeldin A (Sigma Chemical Co., St. Louis, Mo.) was added at 10 μg/ml to inhibit cytokine secretion and thereby increase the probability of its detection in the intracellular compartment (44). Listeria-infected macrophages for antigen presentation were generated as previously described (25). Briefly, thioglycolate-elicited PEC were harvested 3 to 5 days after i.p injection of 2.5 ml of thioglycolate broth. Viable Listeria cells and PEC were incubated in equal numbers (1.5 × 106 of each/ml) in 24-well tissue culture dishes for 16 to 20 h in antibiotic-free medium. Extracellular bacteria were removed from the adherent infected macrophages by washing. PEC from Listeria-immune mice were then added in medium containing antibiotics.
For analysis by flow cytometry, 106 cells were incubated for 30 min at 4°C with FITC- or PerCP-conjugated monoclonal antibodies to cell surface markers to identify individual lymphocyte populations and then washed twice with wash buffer (PBS with 3% FCS and 0.1% sodium azide). Cells were incubated for 15 min at room temperature with 50 μl of fixation medium (Fix & Perm kit; Caltag Laboratories) and then washed once. Predetermined optimal concentrations of fluorochrome-conjugated anticytokine antibodies diluted in permeabilization buffer (Fix & Perm kit) were added for 15 min at room temperature, followed by two washes. If cells were not permeabilized, staining with anticytokine antibodies was reduced to background levels (data not shown). Background fluorescence was less than 0.5% after incubation with Ig isotype controls conjugated to fluorochromes. Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson) by gating on the lymphocyte populations defined by forward scatter and side scatter parameters.
In vivo immunodepletion of lymphocyte and myeloid subsets.
Antibodies were injected i.p. to deplete specific subsets of cells as follows: 200 μg of GL3 or UC7-597 for γ/δ T cells, 150 μg of RB6-8C5 (anti-GR-1) for granulocytes, and 50 to 100 μl of anti-asialo-GM1 (Wako Pure Chemical Industries, Ltd. Richmond, Va.) for NK cells. With the exception of anti-asialo-GM1, antibodies were purified in the laboratory either by protein G affinity chromatography or by ammonium sulfate precipitation from serum-free culture supernatants from the appropriate B-cell hybridomas. Injection schedules are indicated in the figure legends. Population depletions were confirmed by flow cytometry.
Analysis of systemic cytokines or cytokines secreted in vitro by ELISA.
Systemic cytokines were measured in serum or in peritoneal fluid obtained by lavage of the peritoneal cavity with 7 ml of HBSS. Cytokines secreted in response to in vitro restimulation were measured in tissue culture supernatants either from unseparated PEC (1.5 × 106/ml in 24-well plates) or from the plastic-adherent subpopulation. Stimuli and incubation times are indicated in the figure legends. Antibody pairs used for sandwich enzyme-linked immunosorbent assays (ELISAs) for individual cytokines were as follows: R4-6A2 and XMG1.2-biotin for IFN-γ, JES6-1A12 and JES6-5H4-biotin for IL-2, MP5-20F3 and MP5-32C11-biotin for IL-6, JES-2A5 and SXC-1-biotin for IL-10, and C17.8.20 and C15.6.7-biotin for IL-12. Assays were developed using ExtrAvidin-alkaline phosphatase (Sigma) and p-nitrophenylphosphate as the substrate (Bio-Rad, Hercules, Calif.). Absorbance was read at 405 nm using a microplate reader (Bio-Tek Instruments, Inc., Winooski, Vt.). Standard curves were constructed using known amounts of recombinant murine cytokines. Sensitivity limits of ELISAs were as follows: ∼1.5 U/ml for IFN-γ and ∼25 pg/ml for IL-6, IL-10, and IL-12. Hybridoma R4-6A2 (HB-170) was purchased from the American Type Culture Collection, XMG1.2 was provided by DNAX Inc. (Palo Alto, Calif.), and C17.8.20 and C15.6.7 hybridomas were a generous gift from G. Trinchieri (Wistar Institute, Philadelphia, Pa.). Antibodies were purified from culture supernatants from these hybridomas by protein A or G chromatography or by ammonium sulfate precipitation from serum-free supernatants. Biotinylation was accomplished using standard techniques. JES6-1A12, JES6-5H4-biotin, MP5-20F3, MP5-32C11-biotin, JES-2A5, and SXC-1-biotin were purchased from PharMingen.
RESULTS
Survival is impaired in the absence of γ/δ T cells after a high dose of Listeria.
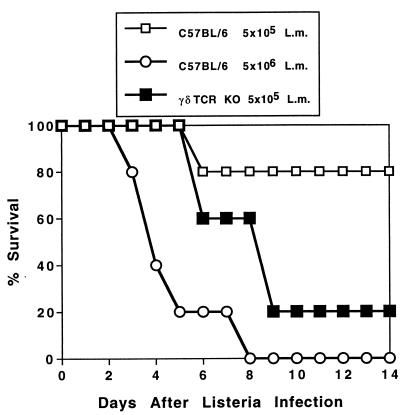
Previous studies have shown that after infection with a moderate dose (∼10% of the 50% lethal dose [LD50]) of Listeria, bacterial burden is temporarily elevated and clearance is delayed in the absence of γ/δ T cells. Eventually the bacteria are cleared, the mice survive, and antigen-specific immunity and memory responses can be demonstrated in γ/δ T-cell-deficient mice (20, 36, 49). If, however, the dose of Listeria approaches ∼80% of the LD50 for intact mice, survival is greatly reduced in the absence of γ/δ T cells (Fig. 1). γ/δ TCR KO mice died after infection with a dose of Listeria that 80% of intact C57BL/6 (B6 control) mice were able to resist. A 10-fold increase in the Listeria dose was required to kill the B6 control mice. Findings for C3HeB/FeJ mice in which γ/δ T cells were depleted by antibody injection were similar (not shown). Thus, if the initial bacterial challenge is high, a critical role for γ/δ T cells during the innate phase of the immune response is apparent. For the remainder of the experiments in this study, we injected Listeria in the range of 1 × 104 to 2 × 104 CFU/mouse. This is sufficient to generate both innate and adaptive immune responses while ensuring the survival of the mice for the duration of the experiment.
FIG. 1.
γ/δ TCR KO mice die after infection with a dose of Listeria cells that intact C57BL/6 (B6 control) mice are able to resist. Listeria cells (L.m.; 5 × 105 CFU) were injected i.p. into B6 control or γ/δ TCR KO mice (five mice/group), and survival was monitored daily. For comparison, a group of B6 control mice received a 10-fold higher dose (5 × 106 CFU). Results were confirmed in C3HeB/FeJ mice immunodepleted of γ/δ T cells (not shown).
Systemic IFN-γ is higher in Listeria-infected mice in the absence of γ/δ T cells.
γ/δ T cells may play an effector role in this resistance to Listeria by producing IFN-γ in response to the increased levels of IL-12 induced by infection. We showed previously that purified peritoneal γ/δ T cells produce IFN-γ in vitro after culture with IL-12 and IL-1 (48). We have confirmed that observation using flow cytometry to simultaneously examine multiple cell populations directly ex vivo without extensive cell manipulations (not shown). γ/δ T cells also produced IFN-γ when cultured with IL-12 in combination with IL-18 or with PMA and ionomycin (see Fig. 6) but not with IL-12 alone (not shown).
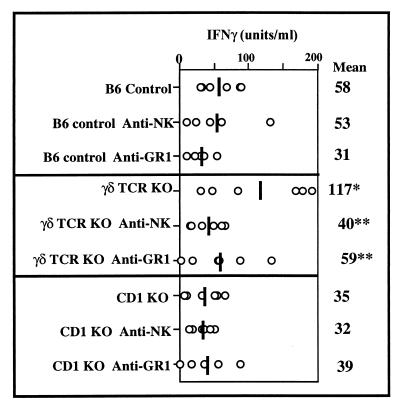
FIG. 6.
Depletion of NK cells or GR-1+ cells reduces the exaggerated increase in systemic IFN-γ in γ/δ TCR KO mice after Listeria infection but has no significant effect in either B6 control or CD1 KO mice. IFN-γ was measured by ELISA in serum samples collected from individual mice 48 h after i.p. infection with 1.4 × 104 CFU of Listeria. NK cells were depleted by i.p. injection of 50 μl of anti-asialo-GM1 24 h prior to Listeria infection. Granulocytes were depleted by i.p. injection of 150 μg of purified anti-GR-1 antibody 24 h before and after Listeria infection and with the injection of Listeria itself. The phenotype of all groups was confirmed by flow cytometry. Means of five mice/group are shown as vertical lines and as numerical values on the right. Circles, values from individual mice. Mean serum IFN-γ in γ/δ TCR KO mice was significantly different from that in B6 controls (asterisk, P < 0.05; unpaired t test). Depletion of either NK cells or bright GR-1+ cells resulted in significant differences from nondepleted mice in γ/δ TCR KO mice (double asterisk, P < 0.05) but not in B6 control or CD1 KO mice. IFN-γ was not detectable in uninfected mice.
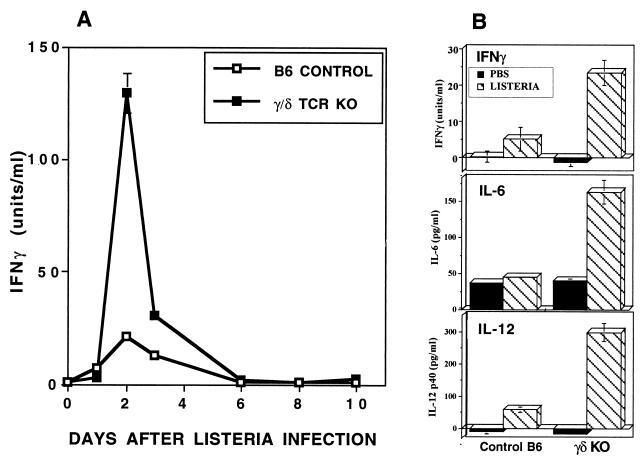
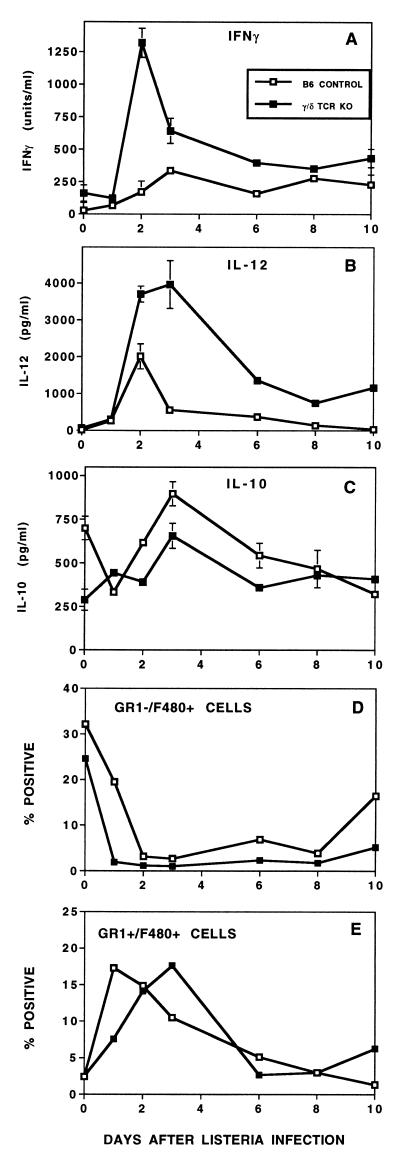
These observations led to the hypothesis that γ/δ T cells may be responsible for a significant portion of the well-documented increase in systemic IFN-γ after infection with Listeria. We tested this in γ/δ TCR KO and B6 control mice using a dose of Listeria that was below the lethal level but sufficient to induce an immune response. Paradoxically, elimination of γ/δ T cells resulted in an even greater and more-prolonged increase in serum IFN-γ, with peak levels occurring 48 h after infection (Fig. 2A). Although absolute amounts of IFN-γ varied among experiments, this pattern of elevated systemic IFN-γ was highly reproducible in γ/δ TCR KO mice (Fig. 2B; see also Fig. 3 and 6) and in mice immunodepleted of γ/δ T cells. For example, after 48 h of Listeria infection, serum IFN-γ in eight mice depleted of γ/δ T cells by injection of anti-γ/δ TCR (GL3 or UC7) averaged 133 U/ml compared to 48 U/ml in control mice (not shown). IL-6 and IL-12 were also more elevated systemically in γ/δ TCR KO mice 48 h after infection (Fig. 2B), suggesting broad disregulation of proinflammatory cytokines in the absence of γ/δ T cells. No differences in systemic levels of TNF-α and IL-10 were detectable at this point in the infectious process (not shown). Since TNF-α elevation occurs primarily during the first 6 h following infection (40), differences in TNF-α may not have been detectable at the times sampled in these experiments.
FIG. 2.
Systemic IFN-γ is higher in γ/δ TCR KO mice than in C57BL/6 control mice after infection with Listeria. (A) IFN-γ was analyzed by ELISA in serum from mice injected i.p. with 1.2 × 104 CFU of Listeria on day 0. Bacterial burden was maximal in both strains on day 2, and Listeria colonies were no longer detectable in either strain after day 6. Values are means and standard deviations from three mice/group. The increased elevation of systemic IFN-γ in the absence of γ/δ T cells was reproducible in five separate experiments using KO mice and in two experiments in which γ/δ T cells were depleted by antibody injection. (B) Exaggerated cytokine elevation in γ/δ TCR KO mice was not restricted to IFN-γ but also included IL-6 and IL-12. Cytokines in peritoneal cavity fluids harvested from three mice/group infected 48 h previously by i.p. injection of 1.8 × 104 CFU of Listeria were measured by ELISA. Since 7 ml of HBSS was injected into the peritoneal cavity to obtain the peritoneal cells and fluids, cytokines from this site were more dilute than in the serum.
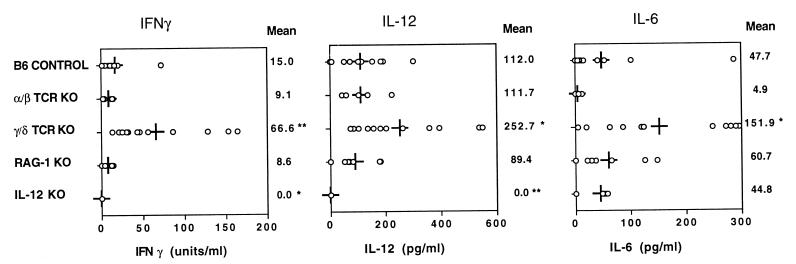
FIG. 3.
Systemic proinflammatory cytokine responses to Listeria infection in B6 control (n = 11), α/β TCR KO (n = 5), γ/δ TCR KO (n = 12), RAG-1 KO (n = 7), and IL-12 KO (n = 6) mice. Cytokines in peritoneal lavage fluid collected from individual mice 48 h after i.p. infection with 1.3 × 104 CFU of Listeria were measured by ELISA. Means for each group are shown as vertical lines and as numerical values on the right. Circles, values from individual mice. Statistically significant differences from B6 controls are indicated (asterisk, P < 0.05; double asterisk, P < 0.01; unpaired t test). These cytokines were undetectable in fluids from uninfected mice.
To examine the mechanisms by which γ/δ T cells influence cytokine levels in vivo, we evaluated systemic cytokines in peritoneal lavage fluid from a variety of genetically altered mice 48 h after Listeria infection (Fig. 3). Use of diluted peritoneal fluids rather than serum allowed for quantitation of multiple cytokines in individual mice. Patterns of change in cytokine levels in serum and peritoneal fluids were similar (not shown). In response to Listeria infection, systemic proinflammatory cytokines increased similarly in intact B6 controls, in mice lacking α/β T cells, and even in mice lacking both T cells and B cells (RAG-1 KO mice) (Fig. 3). The unique exception to this pattern was seen when mice lacked only γ/δ T cells. This suggests that γ/δ T cells may play a broad role in down-modulation of the innate inflammatory response. In IL-12 KO mice, IL-6 production in response to infection was normal but IFN-γ remained undetectable. This indicates that IL-12 is required for the increase in IFN-γ following Listeria injection.
Cellular basis of cytokine disregulation in γ/δ TCR KO mice.
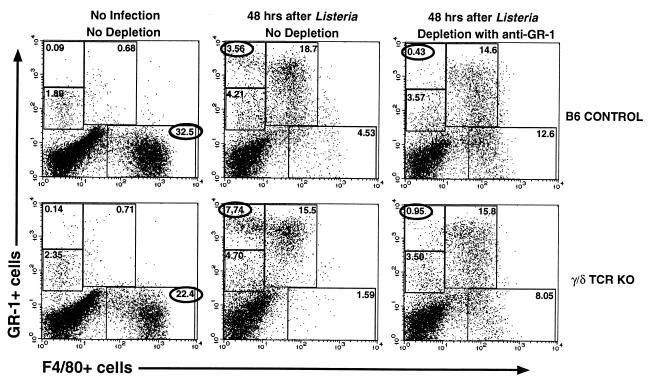
Cytokine disregulation in γ/δ TCR KO mice might be attributable to quantitative differences in numbers of cells and/or to functional differences in cytokine production by subsets of these cells. We first looked for quantitative differences in cell populations between B6 control and γ/δ TCR KO mice 24 to 48 h after infection. In multiple experiments, there were no consistent differences in lymphoid populations such as CD4+ and CD8+ α/β T cells, NK cells, and NK T cells (not shown). In contrast, the changes in peritoneal myeloid lineage populations that followed Listeria infection differed between B6 control and KO mice (Fig. 4). Cells were examined for expression of macrophage and granulocyte lineage markers using F4/80-FITC and GR-1–PE, respectively. Prior to infection, F4/80+ GR-1− cells predominated in both strains, although in lower frequency in γ/δ TCR KO mice than in B6 controls. In four experiments, these cells averaged 32 and 22% of PEC in B6 controls and in γ/δ TCR KO mice, respectively. Following infection, there was an increase in cells expressing GR-1 (Ly-6G). Some of these cells coexpressed the F4/80 marker, while few cells at this time expressed F4/80 in the absence of GR-1 (Fig. 4). General down-modulation of F4/80 expression is characteristic of macrophage activation in vivo (3). Cells expressing GR-1 but not F4/80 can be divided into dim and bright populations. Cells within the dim population also express T- and B-cell markers (not shown). The biggest difference between control and γ/δ TCR KO mice 48 h after infection was in the bright GR-1+ F4/80− population, which was consistently higher in γ/δ TCR KO mice (e.g., 7.74 versus 3.56% in Fig. 4). Immunodepletion in vivo with the anti-GR-1 antibody (RB6-8C5) resulted in a selective loss of this population (to 0.43 and 0.95%) (Fig. 4). This provided the advantage of allowing specific depletion of this classic neutrophil population while retaining the GR-1+ F4/80+ population that was more characteristic of the monocyte/macrophage lineage. This approach was used in later experiments (see Fig. 6) to determine whether the higher frequencies of bright GR-1+ F4/80− cells in γ/δ TCR KO mice are related to cytokine disregulation.
FIG. 4.
Myeloid cell populations in the peritoneal cavity in γ/δ TCR KO mice differ both before and after infection with Listeria. Cells were stained with F4/80-FITC to identify mature macrophages and GR-1–PE as a marker for mature granulocytes and examined by flow cytometry. Myeloid populations were analyzed in uninjected mice or 48 h after infection with 1.4 × 104 CFU of Listeria or 48 h after Listeria infection and depletion of granulocytes (F4/80− GR-1+ cells). To deplete granulocytes, 150 μg of purified anti-GR-1 (RB6-8C5) antibody was injected i.p. 24 h before and after Listeria infection and with the Listeria injection itself. Data are percentages of the entire peritoneal cell population included within the defined regions of the dot plots. Since the numbers of cells per mouse for control and KO mice did not differ, these percentages represent differences in cellularity between the two strains.
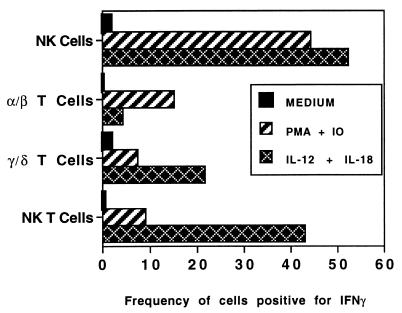
To evaluate functional differences in cytokine-producing cells that might contribute to disregulation, we targeted lymphocyte subsets that can produce IFN-γ in response to IL-12 and are therefore candidates for regulatory control by γ/δ T cells in intact mice. PEC harvested from B6 control mice 24 h after Listeria infection were incubated with IL-12 in combination with other cytokines and examined for intracellular IFN-γ expression by flow cytometry. Both NK cells and NK T cells were induced to produce IFN-γ by culture with IL-12 and IL-18 (Fig. 5) or with IL-12 plus IL-1 and TNF-α (not shown). Only a relatively small percentage of α/β T cells were stimulated to produce IFN-γ by IL-12 in combination with other cytokines (Fig. 5). Since IL-12 is upregulated after infection with Listeria, NK cells and/or NK T cells might contribute to the exaggerated IFN-γ response in γ/δ TCR KO mice. We compared IFN-γ-producing T and NK cells from B6 control and γ/δ TCR KO mice by flow cytometry but found only minor differences in either frequencies or absolute numbers of most subsets (not shown). The exception to this was that the total number of NK cells producing IFN-γ was usually higher in γ/δ TCR KO mice. This population was approximately twofold higher in KO mice after stimulation with PMA plus ionomycin (average of three experiments) and 30% higher in response to stimulation with IL-12 plus IL-18 (two experiments), suggesting that γ/δ T cells may regulate NK cell activity. Intracellular IFN-γ was detectable in less than 5% of cells in the absence of in vitro restimulation under these infection parameters.
FIG. 5.
Production of IFN-γ is induced in NK, NK T, and γ/δ T cells by incubation with IL-12 and IL-18. Peritoneal cells were harvested from C57BL/6 mice 24 h after i.p. injection of 2 × 104 CFU of Listeria. Cells were incubated in medium alone or in PMA (10 ng/ml)–ionomycin (IO; 1 μM) for 3 h or with rIL-12 (5 ng/ml) and rIL-18 (50 ng/ml) for 18 h, with brefeldin A (10 μg/ml) added for the final 3 h of each condition. Cells were stained with FITC- or PE-conjugated antibodies to subset-specific surface markers and then fixed, permeabilized, and incubated with anti-IFN-γ-APC prior to analysis by flow cytometry. Frequencies of IFN-γ+ cells were calculated after gating on either NK1.1+ H57− (NK cell), NK1.1+ H57+ (NK T-cell), NK.1− H57+ (α/β T-cell), or CD3+ GL3+ (γ/δ T-cell) populations. Results are representative of three experiments. IFN-γ is also produced in these subsets of lymphocytes after incubation with IL-12 in combination with IL-1 and TNF-α (not shown). IFN-γ was not detectable in B cells under these conditions or in any population unless secretion was inhibited by brefeldin A.
To determine which of these cell populations might be important in cytokine production in vivo, we infected combinations of genetically altered and/or immunodepleted mice and monitored IFN-γ levels in serum harvested from individual mice 48 h after infection. We first compared B6 controls to γ/δ TCR KO mice and to CD1 KO mice (Fig. 6). CD1 KO mice are deficient in NK T cells because CD1 is involved in their selection (14). Serum IFN-γ was significantly increased in γ/δ TCR KO mice (P < 0.05) as in previous experiments. There was no significant difference, however, between B6 controls and CD1 KO mice. Each of these strains was also immunodepleted of either NK cells by injection of an anti-asialo-GM1 antibody or of bright GR-1+ F480− cells by injection of an anti-GR-1 antibody (Fig. 4). Depletion of NK cells decreased serum IFN-γ only in the γ/δ TCR KO mice. This was confirmed in C3H mice that were simultaneously immunodepleted of both γ/δ T cells and NK cells (not shown). Similarly, depletion of bright GR-1+ cells decreased serum IFN-γ only in γ/δ TCR KO mice. The absence of either NK, NK T, or bright GR-1+ cells had little effect on systemic IFN-γ produced in response to Listeria infection in B6 control mice. Collectively, these data support several concepts: (i) there are multiple cellular sources of IFN-γ, and elimination of only one of them has minimal effect; (ii) depletion of γ/δ T cells uniquely results in an increase in systemic IFN-γ; and (iii) γ/δ T cells may regulate NK cells and bright GR-1+ cells.
In vitro production of proinflammatory cytokines by macrophages is also increased in cells from γ/δ TCR KO mice.
We next looked for evidence of disregulation in γ/δ TCR KO mice by evaluating cytokine production in vitro by PEC harvested at various times after Listeria infection. Macrophages can be stimulated in vitro to produce a variety of proinflammatory cytokines in a non-antigen-specific manner. Their response to stimulation tends to change after infection, reflecting in vivo activation parameters (47). In addition to the well-documented production of IL-1, -6, -10, and -12 and TNF-α, macrophages have recently been shown to produce IFN-γ when cultured with IL-12 and IL-18 for 72 to 96 h (39). Using conditions known to stimulate cytokine production by these cells, we used ELISAs to quantify the production of IFN-γ, IL-12, and IL-10 by plastic-adherent PEC (Fig. 7). Although some contaminating lymphocytes remained (primarily B cells), the majority (>80%) of these adherent cells expressed the F4/80 macrophage marker. After infection, a portion of them also coexpressed the GR-1 granulocyte marker (Fig. 4 and 7E). While Listeria infection increased the ability of cells from normal mice to produce both IFN-γ (Fig. 7A) and IL-12 (Fig. 7B), a far greater and more sustained increase in cytokine production was seen in cells from γ/δ TCR KO mice. Interestingly, the pattern was reversed for IL-10 (Fig. 7C). This is consistent with a number of previous studies in which IFN-γ and IL-12 have been shown to support the development of a strong Th1 response while IL-10 has a negative influence on Th1 responses. Although functional differences were greatest 2 to 3 days after infection, frequencies of F4/80+ populations in B6 control and γ/δ TCR KO mice were similar at those times (Fig. 7D and E). This suggests that the altered cytokine responses represented functional changes rather than quantitative changes in cell populations.
FIG. 7.
Cytokine production in vitro is altered in γ/δ TCR KO mice. Plastic-adherent peritoneal cells harvested at various times from mice injected i.p. with 1.2 × 104 CFU of Listeria were restimulated in vitro, and cytokines in culture supernatants were analyzed by ELISA. IFN-γ (A) was measured after stimulation for 96 h with rIL-12 (5 ng/ml) and IL-18 (50 ng/ml). IL-12p40 (B) and IL-10 (C) were measured after incubation for 24 h with HKLM (107/ml). In previous experiments, these stimuli and incubation conditions were found to be optimal for each of these cytokines. (D and E) Frequencies of F4/80+ cell subsets as determined by flow cytometry.
The antigen-specific adaptive immune response is also increased in γ/δ TCR KO mice.
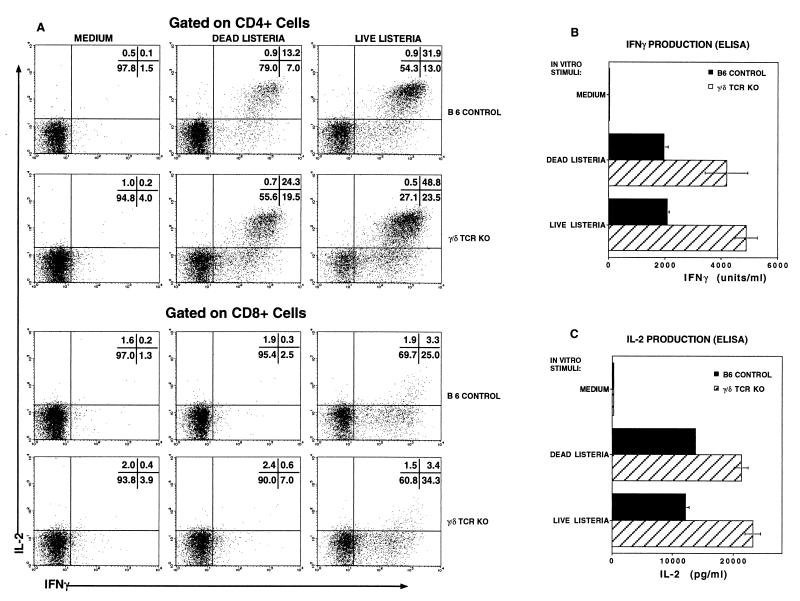
Although the disregulation described above occurred during the innate phase of the host response, we also found elevated antigen-specific adaptive responses by α/β T cells from Listeria-immune γ/δ TCR KO mice. PEC harvested 10 days after Listeria infection, when the primary adaptive immune response is at its peak, were restimulated in vitro with either killed bacteria or with macrophages infected with live Listeria. The immune response was evaluated by analyzing frequencies of cytokine-producing cells by flow cytometry and by measuring the cumulative amount of secreted cytokine by ELISA. With both measures, the response was higher in γ/δ TCR KO mice (Fig. 8). The frequency of CD4+ cells producing either IFN-γ or Il-2 or both cytokines was 1.6- to 2.2-fold higher in γ/δ TCR KO mice than in B6 controls (Fig. 8A). Although the difference was less pronounced, the frequency of antigen-specific CD8+ cells was also higher in γ/δ TCR KO mice. CD8+ cells responded to macrophages infected with live Listeria, which can lyse the endosome and enter the cytoplasm for class I processing. There was only minimal response to dead Listeria by CD8+ cells, consistent with the inability of killed bacteria to escape the endosome. The cytokine phenotype of antigen-specific CD8+ cells from both control and γ/δ TCR KO mice was primarily IFN-γ+ IL-2−. Very few CD8+ cells produced IL-2 either alone or in combination with IFN-γ. In contrast, CD4+ cells producing both IFN-γ and IL-2 predominated, although there was also a significant fraction of cells that produced IFN-γ without IL-2. Very few CD4+ cells produced IL-2 but not IFN-γ. No qualitative differences in this cytokine pattern between control and KO mice were apparent. Consistent with the increased frequency of cytokine-producing cells in KO mice, the cumulative amounts of IFN-γ and IL-2 secreted by PEC in response to culture with Listeria antigens during a 24-h period were also higher in γ/δ TCR KO mice than in controls (Fig. 8B and C). Antigen-specific IL-4 production by cells from Listeria-immune mice of either strain has not been detected either by flow cytometry or by ELISA in multiple experiments (not shown). Thus both the adaptive Th1 response and the innate proinflammatory immune response was exaggerated in γ/δ TCR KO mice.
FIG. 8.
The antigen-specific response was elevated in γ/δ TCR KO mice as evidenced both by increased frequencies of cytokine-producing cells and by increased amounts of secreted cytokine after culture with Listeria antigens. PEC were harvested and pooled (five mice/group) from B6 control and γ/δ TCR KO mice 10 days following an i.p. infection with 2.4 × 104 CFU of Listeria. They were incubated for 5 h with brefeldin A (BFA) in either medium alone or with 107 HKLM/ml or with thioglycolate-elicited peritoneal macrophages infected with live Listeria. Cells were stained with anti-CD4- or anti-CD8-PerCP and then fixed and permeabilized and stained with anti-IFN-γ–PE and anti-IL-2–APC prior to analysis by flow cytometry. Samples were gated on CD4+ or CD8+ lymphocytes (A). Numbers in the upper right corner, frequencies of cells in each quadrant of that plot. Culture supernatants harvested after 24 h from parallel cultures without BFA were analyzed by ELISA for IFN-γ (B) and IL-2 (C). Data are representative of two independent experiments.
DISCUSSION
γ/δ T cells appear to play a dual role in host defense against bacterial infection. First, γ/δ T cells produce IFN-γ in response to IL-12 combined with IL-1, IL-18, or TNF-α, all of which are upregulated after infection. Second, γ/δ T cells then appear to down-regulate this potentially destructive inflammatory process. In their absence, a broad pattern of disregulation of proinflammatory cytokines and exaggerated antigen-specific adaptive immune response is revealed.
Our results suggest that a variety of cell types contribute to the early systemic IFN-γ response to Listeria infection. With the exception of γ/δ T cells, removal of single subsets of cells that are known to produce IFN-γ in vitro had little detectable effect in vivo. For example, neither immunodepletion of NK cells nor a genetic deficiency in either α/β T cells or NK T cells significantly diminished the systemic increase in IFN-γ after infection (Fig. 3 and 6). Previous studies attempting to identify individual populations as essential for cytokine production in vivo have also produced apparent paradoxes. While NK cells may be the primary source of IFN-γ in cells from SCID mice (4) and while IFN-γ is clearly critical for clearance of Listeria infections (22, 26), depletion of NK cells in otherwise immunocompetent mice actually enhanced clearance of a moderate dose of Listeria (46, 52). This pattern was substantiated in mice that were devoid of NK cells because they lacked the common cytokine receptor γ-chain. The innate response to Listeria, including a systemic increase in IFN-γ, was unimpaired in these mice (1). Similarly the subset of T cells expressing the NK1.1 marker was first proposed as an early source of IL-4 critical for the development of a Th2 response (5, 6). Their ability to produce IFN-γ as well (2, 43) (Fig. 5) suggested that NK T cells might play a pivotal role in directing an immune response toward either a Th1 or Th2 pathway. However, mice genetically deficient in NK T cells were still able to generate a Th2 response (51) or produce systemic IFN-γ (Fig. 6) under appropriate conditions. These findings support the concept of a complex system of multiple cytokine-producing cells. Such redundancy would have obvious evolutionary advantages, assuming that a mechanism for reversal of this potentially autodestructive response is operational.
Even though elimination of individual subsets of cells usually has minimal effects, when γ/δ T cells alone were eliminated, we found measurable changes in multiple aspects of the immune response to infection. There was a consistent and prolonged increase not only in IFN-γ but also in other proinflammatory cytokines in the absence of γ/δ T cells. These systemic differences were mirrored by increased production of multiple cytokines in vitro by a variety of cells from γ/δ TCR KO mice, suggesting a broad pattern of disregulation and a lack of reversal of the inflammatory process in the absence of γ/δ T cells. This global disregulation may contribute to mortality when relatively high doses of Listeria are injected into γ/δ TCR KO mice (Fig. 1). Studies are in progress in our laboratory to determine whether death is due to “cytokine storm” or bacterial overload. Preliminary results indicate that the bacterial burden is lower in γ/δ TCR KO mice than in B6 controls at the time of death, consistent with the ability of IFN-γ to enhance bactericidal activity of activated macrophages.
Which of the multiple IFN-γ-producing cell types are regulated by γ/δ T cells? In γ/δ T-cell-deficient mice, the additional absence of either NK cells, bright GR-1+ F480−, or other T cells (in RAG-1 KO mice) dampened the exaggerated increase in systemic IFN-γ observed after infection with Listeria. Activated CD8+ T cells expressing asialo-GM1 may have been eliminated along with NK cells depleted with anti-asialo-GM1 (50). NK cells have been implicated in IFN-γ production in a variety of infections, and α/β T cells have been shown to contribute IFN-γ during the innate response to Listeria (10). Although the lymphocyte populations are probably directly contributing IFN-γ, the role of the neutrophil population is still under investigation. Neutrophils have recently been reported to prestore IL-12 (7), potentially providing a rapid source of proinflammatory cytokines at sites of infection and enhancing the IFN-γ response. Monokine production may also be regulated by γ/δ T cells as evidenced by increased production of IFN-γ and IL-12 by adherent PEC from KO mice. The importance of macrophages in vivo is difficult to assess directly because depletion is difficult and would likely result in early death after infection with Listeria.
Evidence for an anti-inflammatory role for γ/δ T cells is accumulating from a variety of experimental models. Prolonged liver immunopathology has been reported after Listeria infection in mice lacking γ/δ T cells (20, 36). Hepatic abscesses were characterized by accumulations of macrophages and neutrophils but not excessive numbers of bacteria. Abnormal tissue pathology, associated primarily with alterations in myeloid cell populations, in lungs of γ/δ TCR KO mice infected with Mycobacterium tuberculosis (17) and in intestinal epithelia after infection with Eimeria vermiformis has also been reported (45). Lymphoid responses can also be affected. An inflammatory lymphoid response in a Listeria-induced orchitis model was exacerbated in mice lacking γ/δ T cells (38). In both this orchitis model (37) and a pulmonary injury model (33), the response by γ/δ T cells could be invoked by either pathogenic or nonpathogenic stimuli, suggesting that these cells respond to tissue insult rather than bacterial antigenic stimulation per se. The picture which begins to emerge from these studies suggests proinflammatory activation and recruitment of γ/δ T cells by tissue injury or insult. These activated γ/δ T cells likely play a role in the initial production of proinflammatory cytokines. Then, as the damage is repaired or infection is cleared, γ/δ T cells appear to contribute significantly to down-regulation of both myeloid and lymphoid inflammatory responses and avoidance of autodestructive pathology. This model is consistent with the continued presence of elevated levels of γ/δ T cells at sites of infection in both the lung (13) and peritoneal cavity (49) after pathogens have been cleared.
Several mechanisms may be operative in these regulatory events. Differences in systemic cytokines could be due either to alterations in actual numbers of cytokine-producing cells in the γ/δ TCR KO mice and/or to changes in the magnitude of proinflammatory cytokine production by one or more of the subsets of cells. Evidence that γ/δ T cells may kill a subset of activated macrophages has been presented, implying that γ/δ T cells maintain macrophage homeostasis in vivo and prevent macrophage-mediated tissue damage (18). Since γ/δ T cells can express the Fas ligand, they may also provide broad immunoregulation by selectively killing activated α/β T cells or NK cells via a Fas/Fas ligand mechanism (34, 55). Conversely γ/δ T-cell populations expressing Fas may be regulated by the same process (34). Increased neutrophil infiltration and retention in liver (20) or lung tissue (17) after infection in γ/δ TCR KO mice, similar to the increase in bright GR-1+ cells in the peritoneal cavity reported here (Fig. 4), indicate a regulatory connection between γ/δ T cells and neutrophils. The ability of subsets of γ/δ T cells to produce different types of chemokines may influence this cell trafficking during an immune response. Activated murine dendritic epidermal γ/δ T cells (8) and human peripheral blood γ/δ T cells (15) have been shown to produce macrophage inflammatory proteins α and β and lymphotactin but not macrophage chemotactic protein 1 (MCP-1). However, the MCP-1 message was greatly reduced in liver tissue from γ/δ TCR KO mice (16), suggesting that hepatic γ/δ T cells may produce or regulate production of MCP-1 at that site. Recruitment of macrophages and lymphocytes generally follows neutrophilic infiltration to inflammatory sites. Thus, impaired production of C-C chemokines and lymphotactin in the absence of γ/δ T cells may result in the accumulation of neutrophils in various tissue sites as described above.
Mice lacking γ/δ T cells are able to develop specific protective immunity to Listeria after infection with moderate amounts of bacteria (36, 49). Based on our observed increases in proinflammatory cytokines during the innate phase of the immune response in γ/δ TCR KO mice and the reported ability of inflammation per se to expand primary T cells (12), we predicted that the adaptive response would be enhanced in these mice. This was confirmed by higher frequencies of cells producing IFN-γ and/or IL-2 and greater amounts of cytokines secreted by cells from γ/δ TCR KO mice in response to restimulation in vitro with Listeria antigens (Fig. 8). The underlying cellular basis for this enhanced response by α/β T cells is under investigation. Preliminary evidence from cell mixing experiments suggests that alterations in antigen-presenting cells from γ/δ TCR KO mice may be primarily responsible for these differences. Others have also found evidence for enhanced activation of CD4+ α/β T cells from uninfected mice in which the γ/δ TCR had been down-modulated by antibody injection (31). Regulatory functions of γ/δ T cells may have far-reaching implications for fundamental immune processes such as determination of Th1/Th2 balance, tolerance development, and control of autoimmunity. Subsets of γ/δ T cells may differ in their cytokine production patterns and thereby influence whether an immune response is balanced toward Th1 or Th2 (27). In the absence of γ/δ T cells, oral tolerance does not develop normally in that α/β T-cell responses to tolerizing antigen are not diminished (32). Autoimmune diseases such as type I diabetes and the lupus-like syndrome in MRL/lpr mice are exacerbated in the absence of γ/δ T cells (reviewed in reference 23). Intriguingly, γ/δ T cells can also act as proinflammatory effectors in some autoimmune diseases such as experimental allergic encephalomyelitis, underscoring their ability to contribute both to the initiation and termination of an immune response.
The results reported here extend the growing body of information substantiating that γ/δ T cells play a broad regulatory role in immune responsiveness and homeostasis. They appear to check an immune response after the danger has passed and may maintain epithelial and tissue integrity by their anti-inflammatory activity. These early effects extend into regulation of the antigen-specific adaptive immune response of CD4+ and CD8+ cells and may have broad implications for autoimmune regulation and tolerance mechanisms.
ACKNOWLEDGMENTS
This work was supported by NIH NIAID grants RO1 AI-35285 and RO1 AI-34065.
We thank Maurice Gately of Hoffman-La Roche, Inc., for murine rIL-12, Georgio Trinchieri of the Wistar Institute for B-cell hybridomas specific for murine IL-12, DNAX, Inc., for hybridoma XMG1.2, and Michael Grusby of Harvard University for CD-1 KO mice.
REFERENCES
- 1.Andersson A, Dai W J, Di Santo J P, Brombacher F. Early IFN–gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol. 1998;161:5600–5606. [PubMed] [Google Scholar]
- 2.Arase H, Arase N, Nakagawa K, Good R A, Onoe K. NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307–310. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 3.Austyn J M, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft G J, Schreiber R D, Unanue E R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac A, Hunziker R D, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med. 1996;184:1285–1293. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendelac A, Matzinger P, Seder R A, Paul W E, Schwartz R H. Activation events during thymic selection. J Exp Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliss S K, Butcher B A, Denkers E Y. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–4521. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 8.Boismenu R, Feng L, Xia Y Y, Chang J C, Havran W L. Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–992. [PubMed] [Google Scholar]
- 9.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of gamma delta T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 10.Bregenholt S, Berche P, Brombacher F, Di Santo J P. Conventional alphabeta T cells are sufficient for innate and adaptive immunity against enteric Listeria monocytogenes. J Immunol. 2001;166:1871–1876. doi: 10.4049/jimmunol.166.3.1871. [DOI] [PubMed] [Google Scholar]
- 11.Brombacher F, Dorfmuller A, Magram J, Dai W J, Kohler G, Wunderlin A, Palmer-Lehmann K, Gately M K, Alber G. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int Immunol. 1999;11:325–332. doi: 10.1093/intimm/11.3.325. [DOI] [PubMed] [Google Scholar]
- 12.Busch D H, Kerksiek K M, Pamer E G. Differing roles of inflammation and antigen in T cell proliferation and memory generation. J Immunol. 2000;164:4063–4070. doi: 10.4049/jimmunol.164.8.4063. [DOI] [PubMed] [Google Scholar]
- 13.Carding S R, Allan W, Kyes S, Hayday A, Bottomly K, Doherty P C. Late dominance of the inflammatory process in murine influenza by γ/δ+ T cells. J Exp Med. 1990;172:1225–1231. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y H, Chiu N M, Mandal M, Wang N, Wang C R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 15.Cipriani B, Borsellino G, Poccia F, Placido R, Tramonti D, Bach S, Battistini L, Brosnan C F. Activation of C-C beta-chemokines in human peripheral blood gammadelta T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood. 2000;95:39–47. [PubMed] [Google Scholar]
- 16.DiTirro J, Rhoades E R, Roberts A D, Burke J M, Mukasa A, Cooper A M, Frank A A, Born W K, Orme I M. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect Immun. 1998;66:2284–2289. doi: 10.1128/iai.66.5.2284-2289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Souza C D, Cooper A M, Frank A A, Mazzaccaro R J, Bloom B R, Orme I M. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 18.Egan P J, Carding S R. Downmodulation of the inflammatory response to bacterial infection by gammadelta T cells cytotoxic for activated macrophages. J Exp Med. 2000;191:2145–2158. doi: 10.1084/jem.191.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrick D A, Schrenzel M D, Mulvania T, Hsieh B, Ferlin W G, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y X, Roark C E, Kelly K, Drevets D, Campbell P, O'Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by gamma delta T cells. J Immunol. 1994;153:3101–3115. [PubMed] [Google Scholar]
- 21.Gazzinelli R T, Hieny S, Wynn T A, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harty J T, Bevan M J. Specific immunity to Listeria monocytogenes in the absence of IFN-gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 23.Hayday A, Geng L. Gamma delta cells regulate autoimmunity. Curr Opin Immunol. 1997;9:884–889. doi: 10.1016/s0952-7915(97)80193-8. [DOI] [PubMed] [Google Scholar]
- 24.Hayday A C. Gamma delta cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 25.Hiltbold E M, Safley S A, Ziegler H K. The presentation of class I and class II epitopes of listeriolysin O is regulated by intracellular localization and by intercellular spread of Listeria monocytogenes. J Immunol. 1996;157:1163–1175. [PubMed] [Google Scholar]
- 26.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 27.Huber S A, Graveline D, Born W K, O'Brien R L. Cytokine production by Vγ+-T-cell subsets is an important factor determining CD4+-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. J Virol. 2001;75:5860–5869. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber S A, Graveline D, Newell M K, Born W K, O'Brien R L. V gamma 1+ T cells suppress and V gamma 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 29.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke A R, Hooper M L, Farr A, Tonegawa S. T cell receptor δ gene mutant mice: independent generation of α/β T cells and programmed rearrangements of γ/δ TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 30.Jones-Carson J, Vazquez-Torres A, van der Heyde H C, Warner T, Wagner R D, Balish E. Gamma delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995;1:552–557. doi: 10.1038/nm0695-552. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann S H, Blum C, Yamamoto S. Crosstalk between alpha/beta T cells and gamma/delta T cells in vivo: activation of alpha/beta T-cell responses after gamma/delta T-cell modulation with the monoclonal antibody GL3. Proc Natl Acad Sci USA. 1993;90:9620–9624. doi: 10.1073/pnas.90.20.9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke Y, Pearce K, Lake J P, Ziegler H K, Kapp J A. Gamma delta T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–3618. [PubMed] [Google Scholar]
- 33.King D P, Hyde D M, Jackson K A, Novosad D M, Ellis T N, Putney L, Stovall M Y, Van Winkle L S, Beaman B L, Ferrick D A. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- 34.Li B, Bassiri H, Rossman M D, Kramer P, Eyuboglu A F, Torres M, Sada E, Imir T, Carding S R. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. J Immunol. 1998;161:1558–1567. [PubMed] [Google Scholar]
- 35.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 36.Mombaerts P, Arnold J, Russ F, Tonegawa S, Kaufmann S H E. Different roles of α/β and γ/δ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 37.Mukasa A, Born W K, O'Brien R L. Inflammation alone evokes the response of a TCR-invariant mouse gamma delta T cell subset. J Immunol. 1999;162:4910–4913. [PubMed] [Google Scholar]
- 38.Mukasa A, Hiromatsu K, Matsuzaki G, O'Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J Immunol. 1995;155:2047–2056. [PubMed] [Google Scholar]
- 39.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakane A, Numata A, Minagawa T. Endogenous tumor necrosis factor, interleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect Immun. 1992;60:523–528. doi: 10.1128/iai.60.2.523-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura H, Emoto M, Hiromatsu K, Yamamoto S, Matsuura K, Gomi H, Ikeda T, Itohara S, Yoshikai Y. The role of gamma delta T cells in priming macrophages to produce tumor necrosis factor-alpha. Eur J Immunol. 1995;25:1465–1468. doi: 10.1002/eji.1830250551. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien R L, Yin X, Huber S A, Ikuta K, Born W K. Depletion of a gamma delta T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 43.Ogasawara K, Takeda K, Hashimoto W, Satoh M, Okuyama R, Yanai N, Obinata M, Kumagai K, Takada H, Hiraide H, Seki S. Involvement of NK1+ T cells and their IFN–gamma production in the generalized Shwartzman reaction. J Immunol. 1998;160:3522–3527. [PubMed] [Google Scholar]
- 44.Openshaw P, Murphy E E, Hosken N A, Maino V, Davis K, Murphy K, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts S J, Smith A L, West A B, Wen L, Findly R C, Owen M J, Hayday A C. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultheis R J, Kearns R J. In vivo administration of anti-asialo-GM1 antibody enhances splenic clearance of Listeria monocytogenes. Nat Immun Cell Growth Regul. 1990;9:376–386. [PubMed] [Google Scholar]
- 47.Skeen M J, Miller M A, Shinnick T M, Ziegler H K. Regulation of murine macrophage IL-12 production: activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 48.Skeen M J, Ziegler H K. Activation of γδ T cells for production of IFN–γ is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- 49.Skeen M J, Ziegler H K. Induction of murine peritoneal γ/δ T cells and their role in resistance to bacterial infection. J Exp Med. 1993;178:971–984. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slifka M K, Pagarigan R R, Whitton J L. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol. 2000;164:2009–2015. doi: 10.4049/jimmunol.164.4.2009. . (Erratum, 164:3444.) [DOI] [PubMed] [Google Scholar]
- 51.Smiley S T, Kaplan M H, Grusby M J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 52.Takada H, Matsuzaki G, Hiromatsu K, Nomoto K. Analysis of the role of natural killer cells in Listeria monocytogenes infection: relation between natural killer cells and T-cell receptor γδ T cells in the host defence mechanism at the early stage of infection. Immunology. 1994;82:106–112. [PMC free article] [PubMed] [Google Scholar]
- 53.Tripp C S, Gately M K, Hakimi J, Ling P, Unanue E R. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-γ. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 54.Tripp C S, Wolf S F, Unanue E R. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincent M S, Roessner K, Lynch D, Wilson D, Cooper S M, Tschopp J, Sigal L H, Budd R C. Apoptosis of Fashigh CD4+ synovial T cells by borrelia-reactive Fas-ligand(high) gamma delta T cells in Lyme arthritis. J Exp Med. 1996;184:2109–2117. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]