Abstract
Osteoarthritis (OA) has been proven as the second primary cause of pain and disability in the elderly population, impact patients both physically and mentally, as well as imposing a heavy burden on the global healthcare system. Current treatment methods, whether conservative or surgical, that aim at relieving symptoms can not delay or reverse the degenerative process in the structure. Scientists and clinicians are facing a revolution in OA treatment strategies. The emergence of exosomes brings hope for OA treatment based on pathology, which is attributed to its full potential in protecting chondrocytes from excessive death, alleviating inflammation, maintaining cartilage matrix metabolism, and regulating angiogenesis and subchondral bone remodeling. Therefore, we summarized the recent studies of exosomes in OA, aiming to comprehensively understand the functions and mechanisms of exosomes in OA treatment, which may provide direction and theoretical support for formulating therapeutic strategies in the future.
Keywords: Osteoarthritis, Exosomes, Mesenchymal stem cells, Chondrocytes, Extracellular matrix
1. Introduction
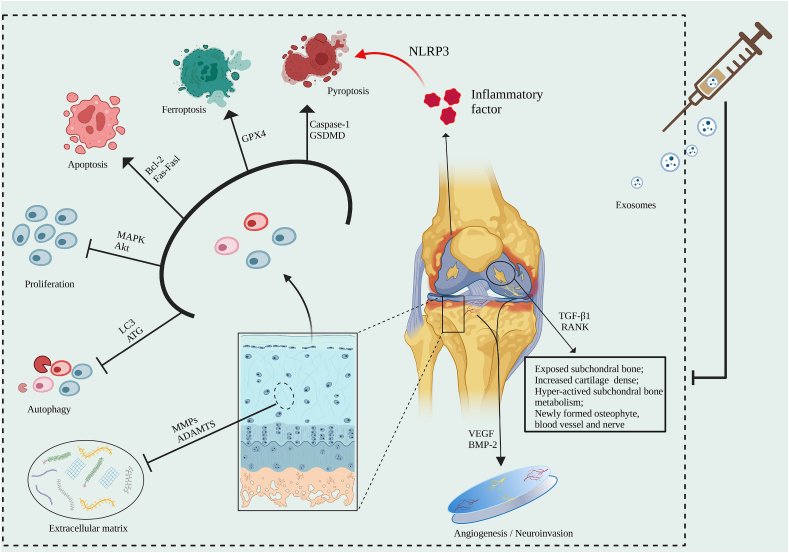
Osteoarthritis (OA), the most common skeletal system disease, is associated with the excessive joint use, obesity, and poor living habits, often leading to pain, limited mobility, and even disability. It is a health problem that cannot be ignored in middle-aged and older people [1]. Knee, shoulder, hip, elbow, wrist, ankle and even temporomandibular joint are common positions of OA occurrence. The joint consists of the articular surface, joint capsule, and joint cavity. OA progression is often characterized by articular cartilage injury, synovial inflammation, and symptomatic pain, with the consequences of severe disability, high economic burden, and poor quality of life [2]. Conservative treatment (physical and medical treatment) and surgical treatment are the main treatment management for OA nowadays. Conservative treatment is considered the first choice on the first visit, and surgery is considered when conservative treatment fails. However, physical therapy may be ineffective or even worsen OA; NSAIDs and glucocorticoids have side effects such as gastrointestinal damage and a certain dependence, while invasive surgery is often accompanied by long postoperative recovery time, high recurrence rate, and postoperative complications [3,4]. Here we must note that neither conservative nor surgical treatments, which aim to relieve symptoms, can delay or reverse the course of degenerative disease, mainly due to the unclear pathogenesis of OA. Researchers constantly disclose the potential pathological mechanism of OA to make it possible to formulate a novel strategy. Excessive chondrocytes death, extracellular matrix (ECM) degradation, synovial inflammation, intra-articular neovascularization, nerve invasion, and subchondral bone remodeling are the main pathological manifestations in the development of OA [[5], [6], [7], [8]]. Therefore, therapeutic management to inhibit these pathological processes is a potential and promising approach. In recent years, exosomes have made good progress in regenerative medicine [9]. As rising stars of tissue regeneration, exosomes have progressed in diverse diseases. Exosome products that anti-aging and promote wound healing have also been approved and marketed, which has inspired the development of therapeutic strategies for degenerative diseases such as OA [[10], [11], [12]]. Thus, we summarize the functions and mechanisms of multi-source cell-derived exosomes in OA, especially in therapy (Fig. 1), which gives us a deeper understanding of the pathomechanism of OA and provides us a wide view to find reliable treatment options for OA in the future.
Fig. 1.
In the progression of OA, excessive chondrocyte loss, synovial inflammation, extracellular matrix degradation, abnormal angiogenesis, nerve invasion, and subchondral bone remodeling are the main pathological features. All these processes lead to pain and limited movement in patients with OA. Exosomes have shown good therapeutic effects against these pathological processes (Created with BioRender.com).
1.1. Search strategy
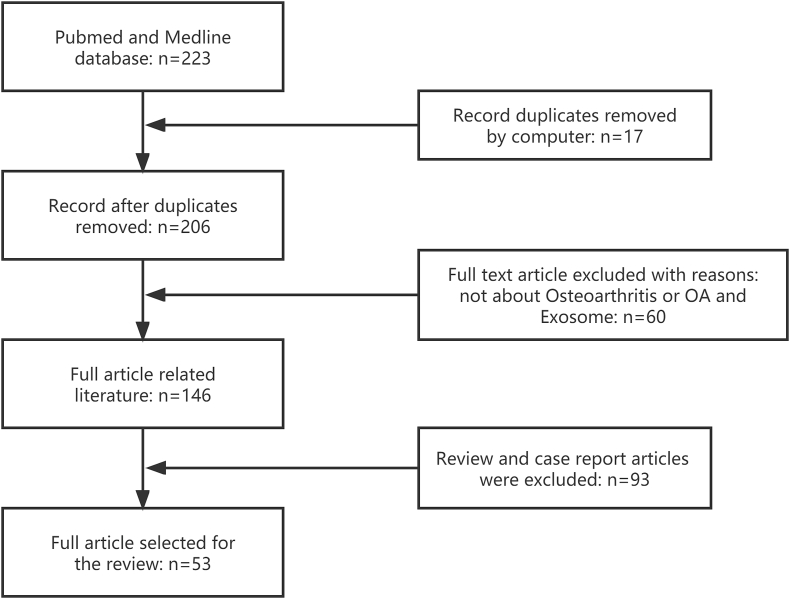
Retrieved articles were from the PubMed (MEDLINE) database (January 2017 to July 2022) by keyword (Osteoarthritis) OR (OA) AND (Exosome). A total of 223 publications were retrieved. Seventeen duplicate articles were eliminated by computer. Sixty articles unrelated to Osteoarthritis or OA and exosomes, and 93 review and case report articles were excluded. Eventually, 53 articles were included in to review (Fig. 2). This study may not include relevant articles due to our not-perfect search strategy.
Fig. 2.
Flow chart of published articles for review based on keywords (Osteoarthritis) OR (OA) AND (Exosome), as well as showing the process of inclusion and exclusion.
2. Exosomes
Exosomes were discovered as microvesicles in 1983 and were initially thought to be a way for cells to expel metabolites from the cells. Exosomes are disc-shaped vesicles with a diameter of 40–100 nm, which can be secreted by cells and play different roles in both normal and disease states. The intracellular lysosomal particle invaginates to form multivesicular bodies and fusion with the extracellular membrane and the cell membrane of multivesicular bodies, and release into the ECM in the form of exosomes [13]. Endocytosome sorting and transport complex (ESCRT), recombinant human programmed cell death 6 interacting protein (PDCD6IP), phospholipase, vacuolar sorting protein 4(Vps4), Rab GTPase activating protein (RA-gaps), sphingomyelinase and ceramide participated in production, transportation, and secretion of exosomes [[14], [15], [16]]. In most cells, ESCRT is considered the core mechanism of exosome production and release. ESCRT contains a series of cytosolic proteins (including ESCRT-0, ESCRT-Ⅰ, ESCRT-Ⅱ, and ESCRT-Ⅲ). The first three are involved in recognizing ubiquitinated membrane proteins on endosomes, while ESCRT-III is in sprouting and separating exosomal vesicles [17,18]. Various molecules, including cytokines, proteins, lipids, and non-coding RNAs, are contained in exosomes. Exosomes have been confirmed to have multiple functions through extensive studies on cell sources, distribution, contents, transport, and intercellular communication of exosomes. Among many biological functions, exosomes transfer content between different cells to participate in intercellular communication and drug delivery are considered the main functions of exosomes, which provide a novel insight into disease treatment [19,20]. Cell type determines the function of exosomes derived from it. In other words, exosomes derived from different cells are endowed with different functions. Previous studies have confirmed that exosomes are involved in immune response, inflammation, cell senescence, apoptosis, and differentiation in diverse diseases [21].
Obtaining high-purity exosomes is a critical step in basic research. Currently, diverse methods have been developed to isolate exosomes based on their size, shape, density, and surface protein, including hypercentrifugation, size-based ultrafiltration, immunoaffinity purification, immunomagnetic bead capture method, polyethylene glycol precipitation (precipitation), and microfluid-based separation techniques [22]. After exosome isolation, exosome identification is another crucial step before studying. Morphological characteristics and classical proteins (including CD9, 63,81, and HSP90) are the main basis for exosome identification. Scanning electron microscopy (SEM) or transmission electron microscopy (TEM), nanoparticle tracking analysis, western blotting, and flow cytometry are the most common methods for exosome identification based on the above characteristics and proteins [23].
3. Exosomes in OA
In recent years, the functions and mechanisms of extracellular vesicles, especially exosomes, have been gradually uncovered in OA. The research direction of this topic mainly focuses on two categories. On the one hand, it focuses on the diagnostic significance and biological effects of exosomes during OA. The number and contents of exosomes secreted in the synovial fluid (SF-exo) of human joints are found to be significantly different in degenerative arthritis (including OA, rheumatoid arthritis, and reactive arthritis), which may be biomarkers for disease diagnosis and prognosis [24,25]. For example, SF-exo miRNAs in OA patients differ between male and female patients [26]. And citrulline peptides have been observed in exosomes of the synovium in rheumatoid arthritis but not in patients with OA, suggesting that it may be special in different joint diseases [27]. In addition, levels of SF-exo in early and late OA were significantly higher than those in the control group, and SF-exo lncRNA PCGEM1 levels in OA were gradually increased as OA progressed [28]. Moreover, exosomes in patients with end-stage knee OA have higher levels of chemokines, which can promote inflammatory response, inhibit cartilage proliferation, and eventually lead to joint degeneration [25]. On the other hand, it focuses on the therapeutic effect of exosomes from different cell sources on OA and the formulation of potential therapeutic strategies. Chondrocytes, synovial fibroblasts, osteoblasts, and tendon cells can produce and secrete exosomes that affect the biological effects of target cells, promoting or inhibiting the pathological process in the progression of OA [29]. And this review mainly focuses on the functions of exosomes from different sources in treating OA and their possible mechanisms.
4. Protect chondrocytes from excessive death
Articular cartilage, which consists of ECM synthesized by sparsely distributed chondrocytes, is a highly specialized tissue. The imbalance of cartilage matrix anabolic and catabolism is considered to be the main pathological characteristics of OA [30]. In OA, the number of chondrocytes in articular cartilage decreases and fails to regenerate and reshape the ECM properly. Decreased chondrocyte density cannot provide nutritional support to the cartilage matrix, which is related to excessive ECM loss and calcification, the severity of which is positively correlated with cell death. In short, excessive chondrocytes apoptosis and matrix loss may lead to a vicious cycle process that eventually leads to an ongoing degenerative process [31]. In the early stages of OA, “chondrocyte cloning” occurs, in which chondrocytes expand into clusters, which is considered the evidence of cartilage metabolic activity and chondrocytes repair capacity in OA [32]. Although chondrocytes are in the active proliferation stage, the content of glycosaminoglycans in the matrix is significantly decreased compared with the normal group, manifesting that the chondrocyte's compensatory level remains low and cartilage has always been in the process of degeneration. While in the late stages, cartilage degeneration is presented with increased chondrocyte death, including decreased cell density and empty lacunae. Therefore, maintaining chondrocytes density and reducing excessive chondrocytes death can provide a stable internal environment for OA repair [33]. Exosomes play a role in inhibiting excessive chondrocyte death, promoting cell proliferation and differentiation, and activating autophagy to maintain chondrocyte density, which is closely related to chondrocytes renewal and metabolism.
4.1. Inhibit apoptosis, pyroptosis, and ferroptosis
Normal cell death keeps indispensable in maintaining human development, while the development of the disease, the balance in the process of normal cell death is broken and usually accompanied by excessive cell death [34]. There are two forms of cell death: autonomous and ordered genes-controlled programming and negative states-induced non-programmed cell death. More precisely, apoptosis means that phagocytes can clear cells without an inflammatory response [35], while pyroptosis is characterized by extravasation of cell contents accompanied by a severe inflammatory response [36]. Necrosis belongs to non-programmed cell death, which is a process of irreversible cell damage and death caused by extreme stimulation [37]. In recent years, a form of death, also genetically controlled and driven by highly iron-dependent lipid peroxidation, has been uncovered, known as ferroptosis. Microscopically, altered mitochondrial phenotype, atrophy of mitochondria, and increased membrane density were observed [38]. These diverse forms of cell death have been demonstrated in OA, and exosomes have shown great potential in inhibiting different types of chondrocyte death.
4.1.1. Exosome in apoptosis
Intraarticular injection of bone marrow mesenchymal stem cell-derived exosomes (BMSCs-exo) alleviated cartilage injury and modulated subchondral bone remodeling in a surgically induced rat model of knee OA. Further, in vitro experiments disclosed that BMSCs-derived exosomal LncRNA MEG-3 increased the synthesis of collagen type II (COL II) and inhibited the chondrocyte's senescence and apoptosis induced by IL-1β [39]. Platelet-rich plasma-derived exosomes (PRP-exo) inhibited pro-inflammatory cytokine release and chondrocytes apoptosis by activating Wnt/β-catenin signaling in IL-1β-induced OA model [40]. Exosomes derived from dental pulp stem cells (DPSCs-exo) inhibited chondrocytes apoptosis and enhanced ECM synthesis by up-regulating Bcl-2. And DPSC-exo rich in miR-140-5p worked better than DPSC treatment [41]. In addition, synovial mesenchymal stem cells derived exosomes (SMSCs-exo) rich in miR-155-5p played the same role through the Runx2 pathway in OA [42]. Another study found that SMSCs-exo rich in miR-129-5p inhibited the inflammation and chondrocytes apoptosis induced by IL-1β via inhibiting HMGB1 [43]. Exosomes derived from human umbilical cord mesenchymal stem cells (HUCMSCs-exo) rich in miR-100-5p inhibited ROS production and cell apoptosis induced by cyclic tension by directly targeting NADPH oxidase 4 (NOX4) [44]. Moreover, miR-100-5p-rich exosomes derived from infrapatellar fat pad MSCs reduced chondrocytes apoptosis and ECM degradation through mTOR in vitro. While in vivo, the exosomal miR-100-5p not only maintained cartilage homeostasis, reduced chondrocytes apoptosis, and promoted ECM synthesis but also improved the poor gait of OA mice induced by surgery [45]. It is worth noting that not all exosomes can do a good job of treating OA. Exosomes secreted by vascular endothelial cells can promote chondrocytes apoptosis and attenuate antioxidant ability by activating ROS. It presents another potential treatment strategy for OA that inhibits articular vascular endothelial cells from secreting exosomes [46].
4.1.2. Exosome in pyroptosis
There is a close relationship between pyroptosis and inflammation, which means that inflammation and pyroptosis must be considered when we develop therapeutic strategies for OA. Macrophages activate caspase-1 and release inflammasomes (such as NLRP3), resulting in an increasing concentration of proinflammatory cytokines in chondrocytes, which promotes pyroptosis and exacerbates inflammation during OA progress [47]. In addition, the secretion of metabolic enzymes such as MMP13 and ADAMTS5 is increased in response to the stimulation of released inflammatory cytokines, which promote cartilage matrix degradation, leading to ECM metabolic imbalance and cartilage destruction [48]. Pyroptosis can also lead to an increase in symptomatic pain. In the process of pyroptosis, inflammasomes in macrophages accelerate the secretion of pro-inflammatory cytokines (including IL-1 β and TNF-α), which can aggravate the pain feeling of the injured joint [49]. Under chronic inflammatory conditions, pyroptosis has been certified can lead to cartilage loss, osteochondral fissures, osteophyte formation, and synovitis, which makes the dense network of nerves around the blood vessel and sympathetic nerves extend to the synovium, ligaments, and meniscus, increases pain sensitivity and transmission of pain signals, and ultimately aggravates pain in OA patients [39].
NF-κB, as one of the most typical inflammatory signaling pathways, has been proven to mediate the inflammatory response in various diseases. BMSCs-exo treated OA by carrying overexpressed miR-326 to degenerative chondrocytes that it played roles in inhibiting pyroptosis and inflammation and increasing aggrecan and COL II expression through targeting HDAC3 and regulating STAT1/NF-κB p65 [50]. Xing et al. found that the injectable exosome-functionalized ECM hydrogel derived from adipose mesenchymal stem cells (AMSCs) could regulate MMP to balance ECM synthesis and degradation and inhibit pyroptosis by alleviating inflammatory response in vitro, which is used to reduce nucleus pulposus cell (NPCs) pyroptosis and maintain ECM metabolic balance in intervertebral disc degeneration [51]. Another study uncovered that exosomal miR-410 derived from MSCs acted as a key modulator of pyroptosis, which inhibited LPS-induced pyroptosis of NPCs by targeting NLRP3 both in vivo and in vitro [52].
4.1.3. Exosome in ferroptosis
Highly expressed unsaturated fatty acids on cell membranes catalyze lipid peroxidation and induce ferroptosis through divalent iron or ester oxygenase, which is different from the classical form of cell death [53]. Interference of oxidative lipid defense mediated by glutathione peroxidase 4 (GPX4) is considered to be a common trigger of ferroptosis [54]. Ferroptosis has been shown to contribute to injured cell clearance processes in cardiovascular and cerebrovascular diseases and degenerative diseases, as well as accelerating tumor cells clearance [[55], [56], [57]]. A recent study disclosed that extracellular vesicles, especially exosomes, assisted the clearance of intracellular iron, which is considered an approach to mediate ferroptosis resistance [58]. Frustratingly, compared with pyroptosis and apoptosis, the research on exosomes inhibiting ferroptosis in OA remains quite limited. Most studies on the inhibition of ferroptosis by exosomes have focused on the tumor, cardiovascular and cerebrovascular diseases, and liver-related diseases [[59], [60], [61]]. In the last few years, phytochemicals and iron chelators have shown great therapeutic potential in inhibiting the ferroptosis of chondrocytes. Here we desire that the mechanism of exosomes inhibiting ferroptosis in OA will certainly be revealed in the future.
4.2. Promote proliferation and differentiation
Cells proliferate to replace damaged, aged, and dead cells in tissues and organs, which plays a role in metabolism [62]. Cell differentiation, in which homologous cells (such as mesenchymal stem cells) gradually form target cell populations, is another way to replenish insufficient or excessive cell loss. Cell differentiation has good plasticity, which means that the differentiated cells can purposefully differentiate into another type of cell again [63]. Both proliferation and differentiation promote the increase of cell number and density, which is crucial for maintaining the intracellular environment. It is undoubtedly helpful for the loss of chondrocytes in OA induced by various adverse conditions. Exosomes have been demonstrated to promote chondrocyte proliferation and differentiation into chondrocyte phenotype both in vivo and in vitro, which provides another idea for delaying OA.
BMSCs-exo significantly ameliorated IL-1β-induced inhibition of chondrocytes proliferation and migration. In addition, the up-regulation of COL II and aggrecan and down-regulation of MMP13 and ADAMTS5 were further confirmed in vivo [64]. Another study also found that increased apoptosis and inhibition of cell proliferation were reversed in the OA group after BMSCs-exo treatment. OA mice present with a limited running ability, severely damaged cartilage tissue, increased chondrocytes apoptosis and inflammation, and decreased COL II, proteoglycan, and glutamate metabolism. After BMSCs-exo injection treatment, all the negative situations were improved [65]. Both exosomes derived from synovial mesenchymal stem cell-secreted exosomes (SMMSCs-exo) and induced pluripotent stem cells (IMSCs-exo) have been shown to treat OA, but IMSCs-exo worked better than SMMSCs-exo. Similarly, IMSCs-exo did a better job of stimulating chondrocytes migration and proliferation [66]. BMSCs from congenital polydactyly tissue (pBMSCs) have shown a better differentiation trend in chondrocytes than BMSCs. Mechanically, higher BMP4 in pBMSCs regulated the chondrogenic migration, proliferation and differentiation potential, and function of MSCs. In addition, both pBMSCs-exo and BMSCs-exo injection can alleviate OA, but pBMSCs-exo showed a better therapeutic effect than BMSCs-exo [67]. HUCMSCs-exo inhibited chondrocyte apoptosis and promoted chondrocyte proliferation and migration in vitro. METTL3 was shown to be involved in the OA protection of exosomes. Further research showed that the binding of exosomal miR-1208 to METTL3 decreased the m6A level of NLRP3 mRNA and then reduced the overproduction of inflammatory cytokines [68]. Treatment with hMSCs-exo increased the main ECM composition COL II and proteoglycan and decreased the chondrocytes hypertrophy markers Runx2 and ECM degradation enzyme MMP-13. Moreover, overexpressing KLF3-AS1-MSCs exosomes ameliorated limited chondrocytes proliferation and excessive apoptosis in IL-1β pre-treated OA chondrocytes. KLF3-AS1 was confirmed to be a sponge of miR-206, which promoted the expression of GIT1 and played a protective role in OA [69].
A large number of exosomal microRNA, LncRNA, and circRNA have been certified to participate in the process of chondrocytes proliferation and differentiation, which will not be described here, but listed in Table 1.
Table 1.
The functions and mechanisms of different source exosomal miRNA, LncRNA, and circRNA in OA treatment.
| Cells type | Category/Name | Function(s) | Pathway(s) | Ref |
|---|---|---|---|---|
| Human synovial MSCs | miR-140-5p | Promote proliferation and migration; Maintain the balance of ECM. |
Wnt/YAP | [70] |
| Human MSCs | miR-92a-3p | Promote chondrocyte proliferation; Remold ECM. |
WNT5A | [71] |
| Human synovial MSCs | miR-155-5p | Promote proliferation and migration; Attenuates apoptosis; Regulate ECM secretion. |
Runx2 | [42] |
| Human synovial MSCs | circRNA3503 | Promote proliferation and migration; Attenuates apoptosis; Maintain the balance of ECM. |
miR-181c-3p/let-7b-3p | [72] |
| Human MSCs | miR-135b | Promote proliferation; Enhance cell viability. |
TGF-β1/SP1 | [73] |
| Chondrocytes | circRNA-RWD1 | Promotes cell viability and proliferation; Inhibits apoptosis, inflammation, and ECM degradation |
miR-1277/TRAF6 | [74] |
| Synovial fibroblasts | miRNA-126-3p | Promotes proliferation and migration; Inhibits apoptosis and inflammation. |
IL-1β/TNF-α | [75] |
| Bone marrow MSCs | miR-206 | Promotes proliferation and differentiation; Inhibits apoptosis and inflammation. |
Elf3 | [76] |
| Human bone MSCs | miRNA-361-5p | Promotes proliferation; Inhibits inflammation; Maintain the balance of ECM. |
DDX20/NF-κB | [77] |
| Fibroblast-like synoviocytes | Lnc-RNA H19 | Promotes proliferation; Inhibits the degradation of ECM |
miR-106b-5p/TIMP2 | [78] |
| Human MSCs | Lnc-RNA-KLF3-AS1 | Promotes proliferation; Inhibits the degradation of ECM. |
Runx2 | [79] |
| Chondrocytes | circRNA-CDK14 | Promotes proliferation; Inhibits apoptosis and ECM degradation. |
miR-1183/KLF5 | [80] |
| Fibroblast-like Synoviocytes | Lnc-RNA-PCGEM1 | Inhibits proliferation; Promotes apoptosis and ECM degradation. |
miR-142-5p/Runx2 | [81] |
| Human Urine MSCs | miR-140-5p | Promote proliferation and migration; Attenuates apoptosis; Regulate ECM secretion. |
VEGFA | [82] |
4.3. Activate autophagy
Autophagy is an important evolutionary process of substance renewal in eukaryotic cells. Autophagy is a self-eating process characterized by phagocytosis and degradation of aging and damaged organelles to achieve the renewal of organelles [83]. And it can be arisen both in physiological and pathological processes [84]. When chondrocytes are senescent, damaged, or dead under various negative conditions, whether autophagy is activated determine whether OA develops in a good or bad trend. When autophagy is activated, it will accelerate the degradation of damaged chondrocytes and promote the metabolism and renewal of chondrocytes. Exosomes have shown excellent roles in activating autophagy in various diseases and are no exception in OA. LC3 in autophagosomes is regarded as a molecular marker of autophagy, and the content of LC3-II is directly proportional to the level of autophagy. In the process of autophagy, the autophagy level is often reflected by the ratio of LC3-II/I [85]. Moreover, Beclin-1, which is involved in the formation of autophagosome membranes, is also considered to be a key regulator of autophagy [86].
Activating transcription factor 4 (ATF4) has been certified to participate in bone formation and chondrocyte proliferation. Intra-articular injection of OA-exo with ATF4 overexpression can partially restore the inhibited autophagy and alleviate articular cartilage degeneration and inflammation in knee chondrocytes of OA mice. Further experiments showed that overexpressed ATF4 exosomes improved the limited autophagy and inhibited apoptosis in chondrocytes treated with TNF-α [87]. And ADMSCs-exo not only reversed the inhibition of autophagy induced by IL-1β, upregulated the expression of autophagy marker LC3B, but also significantly reduced the oxidative stress [88]. In addition, BMSCs-exo inhibited chondrocytes apoptosis and MMPs expression, which was attributed to exosomes regulating dynein-related protein 1 (Drp1) -mediated mitophagy, that is, over-expressed Drp1 down-regulated ratio of the LC3-II/I and Beclin-1 [89]. Furthermore, BMSCs-exo regulated the PI3K/AKT/mTOR signaling axis in IL-1β-induced degenerative disc disease, activated autophagy, inhibited the release of inflammatory mediators, and reduced excessive annulus fibrosus (AF) cell apoptosis [90]. There remains another voice that activated autophagy can promote exosome release in return. In intervertebral disc degeneration, the secretion of exosomes from NPCs increased after autophagy was activated, and exosomal miR-27a inhibited the excessive degradation of ECM by targeting MMP-13 [91]. With the intervention of low-intensity pulsed ultrasound, autophagy was activated in OA chondrocytes, which promoted the release of MSCs-exo, and then repaired the injured cartilage [92]. The mutually promoting and positive cycle between the secretion of exosomes and the activation of autophagy is beneficial to maintain the density of chondrocytes.
5. Inhibit extracellular matrix degradation
ECM was thought to act only as inert support, holding cells together to form tissues and organs in the past, while now it has been found to contain signaling molecules that are involved in the cell growth, migration, plasticity, and metabolism [93]. ECM's main components include water, collagens, proteoglycans, and elastic fibers. Glycoproteins and collagens combine with water to give an expansive and stretchy power to the articular cartilage to reduce friction and buffer the mechanical stress on the articular surface during exercises [94]. In the physiological state, ECM and chondrocytes feed each other, and the synthesis and degradation of ECM maintain
at a balanced level. The main pathological feature of chondrocytes is that the degradation of ECM is more than synthesis, which is manifested by an obvious decrease in collagen and proteoglycan [30]. As the major enzymes that degrade collagen and proteoglycans, matrix metalloproteinases (MMP) and A disintegrin and metalloprotease with thrombospondin motifs (ADAMTS), are in a significantly higher expression in OA [95]. Therefore, the metabolic balance of the ECM is essential for maintaining the endochondral environment of the articular cartilage.
Intraarticular injection of human embryonic stem cell-induced mesenchymal stem cells derived exosomes (ESC-induced-MSCs-exo) attenuated injured cartilage and degraded ECM in an OA rat model by increasing COL II and decreasing ADAMTS5 expression [96]. BMSCs-exo re-induced expression of COL II and aggrecan while inhibiting expression of catabolic enzymes (MMP-13, ADAMTS5), inflammatory factor iNOS, and macrophage activation, as well as protecting chondrocytes from apoptosis [97]. E2F2 is highly expressed in cartilage tissue of human traumatic OA and negatively related to miR-125a-5p. BMSCs-exo enriched with miR-125a-5p promoted the migration of chondrocytes and remodeled ECM via up-regulating COL II, aggrecan, and SOX9 and down-regulating MMP-13. Further studies in a mouse model of traumatic OA showed that exosomal miR-125a-5p could attenuate ECM degradation by regulating E2F2 [98]. Furthermore, exosomal miR-136-5p similarly attenuated ECM degradation in traumatic OA by targeting ELF3 [99]. ADMSCs-exo decreased the secretion of inflammatory mediators and MMP activity in OA treatment and significantly increased the production of anti-inflammatory cytokines IL-10 and COL II, which was closely related to NF-κB pathway and the activation of activator protein-1 [100]. Many published studies have found that exosome-related therapy (mainly exosomal microRNA, LncRNA, and circRNA) can significantly inhibit the activity of matrix-degrading enzymes and the degradation of collagen and proteoglycans in the process of promoting cartilage repair. Also, the mechanisms of more multi-source exosomes in maintaining ECM are listed in Table 1.
5.1. Alleviate synovial inflammation
Synovitis is also part of the progression of OA and is often caused by trauma, mechanical load, and the cross-talk of diet-microbiome [[101], [102], [103]]. Biomechanical injury is a widely accepted inducement of OA, which will lead to the release of inflammation-related mediators, activate different inflammatory pathways, and then trigger an inflammatory response to damage cartilage [104]. Excessive mechanical load (such as damage of the meniscus or ligaments and intraarticular fractures) are the known risk factors for OA that lead to excessive release of inflammatory mediators, further contributing to the development of synovitis [105]. Fibroblast-like synoviocytes (FLS) can secrete cytokines, growth factors, tissue inhibitors of matrix phosphatases and metalloproteinases (TIMP) that regulate the activation of macrophages, as well as catabolic pathways of chondrocytes. It is well known that the activation of macrophages can secrete pro-inflammatory factors. FLS and chondrocytes that are stimulated by pro-inflammatory factors release matrix-degrading enzymes to accelerate ECM degradation [106]. Interestingly, studies have found that the excessive ECM degradation enzymes can activate FLS and macrophages again, leading to repeated vicious cycles of inflammation and ECM degradation [107]. In addition, exosomes derived from synovial fibroblasts (SF) of OA patients promoted macrophages to produce a series of chemokines and pro-inflammatory factors, which are triggers for cartilage destruction and local inflammation within the joint [24]. IL-1β has often involved in OA synovitis as an upstream cytokine, which can promote the expression of pro-inflammatory factors and matrix-degrading enzymes (IL-6, IL-8, and MMPs) [108]. According to long-term follow-up study statistics, the severity of synovial inflammation was positively correlated with pain, and it was associated with synovial inflammation and cartilage degradation, showing that each 0.1 mm reduction in cartilage resulted in a 0.32 increase in WOMAC pain subscale score within 24 months [109]. It is well known that the inflammatory response is never present alone in OA and is often accompanied by chondrocyte apoptosis, cartilage matrix destruction, and other pathological processes. So we are not going to talk too much about the anti-inflammatory effects of exosomes because it has been verified in regulating other pathological processes.
5.2. Regulate angiogenesis and neuroinvasion
Usually, articular cartilage is avascular, similar to the intervertebral discs, probably because blood vessels are susceptible to mechanical stress, and vascularization may impair the biomechanics of the tissues [110,111]. As a result of avascular, limited repair and self-renewal capabilities make it difficult to slow down the progress of OA. Angiogenesis refers to forming a new vascular system (mainly capillary) based on the existing one. Angiogenesis plays a huge role in body growth and development, tissue regeneration and repair, and cancer progression and metastasis [112,113]. And angiogenesis promotes synovitis, cartilage destruction and degeneration, osteophyte formation, and pain, which is one of the significant factors in the formation and exacerbation of clinical symptoms of OA [7]. Neuroinvasion, which is thought to be secondary to angiogenesis, not only increases pain sensitivity but also transmits pain signals [108,114,115]. Presently, the comments on angiogenesis in the process of OA remain controversial. Specifically, on the one hand, angiogenesis offers a part nutritional supplement for injured cartilage to repair it. On the other hand, the following inflammatory response and increased pain aggravated the progression of OA.
In recent years, MSCs-seeded scaffold transplantation has been applied in the treatment of traumatic cartilage injury, which is to fill the defective cartilage with new tissue containing MSCs-seeded scaffold and promote its repair, possibly because of angiogenesis that promotes tissue integration [116]. Exosomes derived from human induced pluripotent stem cells (hiPSC-MSCs-exo) can promote angiogenesis and osteogenesis in a castrated rat model, thereby promoting bone regeneration and reducing osteoclast [117]. BMSCs-exo promotes osteogenesis in the treatment of femoral nonunion fracture repair in rats. In addition, capillary angiogenesis also provides essential nutritional support for fracture healing. And the regulation of BMP-2/Smad1/RUNX2 and HIF-1α/VEGF axes by BMSCs-exo may dominate this repair process [118]. Moreover, HUCMSCs-exo also promoted fracture healing that histological results showed increased angiogenesis, which was associated with HIF-1α-induced VEGF expression [119]. Indian Hedgehog (IHH) and Serpin E1 have anti-angiogenic effects. After IHH and Serpin E1 are blocked, chondrogenic differentiated BMSCs showed increased endothelial cell proliferation, as well as increasing angiogenesis, which is beneficial for articular cartilage repair [120].
Contrarily, here are some studies that present an entirely different perspective. Notochord cells (NC), as a participant in maintaining intervertebral disc development and homeostasis. NC-derived exosomes (NC-exo) transfer enriched miR-140-5p into endothelial cells, inhibit angiogenesis, and alleviate disc degeneration by regulating the Wnt/β-catenin [121]. In addition, AF-exo, the exosome derived from normal annulus fibrosus, reduced angiogenesis in degenerative discs by down-regulating classic angiogenesis-related factor VEGF [122]. Exosomes can promote angiogenesis and lead to abnormal neurogenesis in pathological processes, which is beneficial in many diseases. However, these effects in the treatment of OA still deserve further investigation. Gingival mesenchymal stem cell-derived exosomes (GMSCs-exo) hydrogel in the treatment of skin wounds in diabetic rats showed increased local angiogenesis, increased collagen expression, and inward neuronal growth, suggesting that GMSCs-exo could be a potential choice for the treatment of wounds in diabetic patients [123]. Abnormal nerve growth or invasion is detrimental to the development of OA, which can amplify and aggravate the pain experienced by OA patients. BMSCs-exo treatment repaired damaged cartilage and subchondral bone and reduced pain in a lumbar facet joint (LFJ) OA model by inhibiting abnormal nerve invasion and abnormal formation of H-type blood vessels in subchondral bone [124]. The pathogenesis of OA lacks angiogenesis regulation, and establishing a positive and negative feedback mechanism of exosomes may slow down the progression of the disease. However, the advantages and disadvantages of angiogenesis in the progression of OA are controversial, which leads to the need to be cautious in further research on the angiogenes-based treatment of OA, and whether there is a time point at which cartilage repair can be promoted without amplifying inflammation and pain remains to be debated. The regulation of angiogenesis mediated by exosomes provides different insights into the underlying mechanisms, which remain understudied. Thus, a comprehensive understanding of the mechanisms of exosomes involved in angiogenesis in OA may unlock great therapeutic potential.
6. Delay subchondral bone remodel
More and more researchers have begun to pay attention to subchondral bone injury in OA, which is usually considered to occur after cartilage injury. In the subchondral region, many tiny arteries, veins, and nerves exist. In the early stage of OA, there are microstructural changes in the subchondral bone, including microcracks, microedema, microbleeding, and subchondral bone sclerosis, and its structural remodeling can accelerate cartilage damage [125]. In the process of subchondral bone remodeling, there is an obvious vascular proliferation, which has an important influence on OA, especially in the transmission of pain sensations [126]. In the middle and late stages, subchondral bones were exposed after the complete hyaline cartilage wear. The hardened cartilage becomes quite dense, and the subchondral bone is more metabolically active but less mineralized than the underlying cancellous bone, which is known as osteosclerosis [127].
TGF-β1-modified BMSCs-exo treatment maintained the microstructure of surgery-induced OA mice and inhibited the abnormal angiogenesis of subchondral bone by inhibiting PDGF-BB, which holds a positive protective effect on pain and bone resorption [128]. Treatment with human embryonic MSCs-derived exosomes (hEMSCs-exo) showed almost complete recovery of damaged subchondral bone and cartilage. At the same time, the surface of hyaline cartilage changed from rough and uneven to smooth and regular [129]. In OA mice treated with BMSCs-exo, the bone volume of subchondral bone was increased, bone degradation was decreased, and the degeneration of subchondral bone trabecular microstructure was significantly inhibited. Histological scores showed that BMSCs-exo had an outstanding ability to repair cartilage. In addition, TRAP, as a marker reflecting subchondral osteoclasts, was also significantly decreased, which means that BMSCs-exo treatment also regulated the balance of osteoblasts and osteoclasts [39]. MiR-140-5p-enriched exosomes secreted from human urinary mesenchymal stem cells (hUMSCs-miR-140-5p-exo) were used to treat OA rats with surgically induced intra-articular structural destruction. Histology and microcomputed tomography showed improved cartilage damage and reduced subchondral bone remodeling [82]. In addition, BMSCs-exo can relieve pain in the progression of subchondral bone remodeling in lumbar joint OA via decreasing abnormal CGRP-positive nerves and H-type vessel formation through regulating the RANK-RANK-TRAF6 axis [116].
7. Preclinical and clinical application of exosomes
In the past, we have experienced various preclinical/clinical treatments for OA, and hyaluronic acid is considered a vital option for OA treatment [130]. However, a clinical study on the treatment of OA with hyaluronic acid and polynucleotide found that the VAS score and the outcome score of knee injury and OA in the polynucleotide group were significantly different from those in the hyaluronic acid group. The results supported that polynucleotides can be an effective substitute for hyaluronic acid in the treatment of symptomatic knee OA [131]. In addition, another study found that vitamin C can play a significant role in relieving inflammatory pain symptoms in the early stage of OA, significantly reducing VAS and knee social score and improving the life quality of OA patients [132]. However, their mechanism has not been clarified and only applies to early OA patients, resulting in limited patient benefits. Moreover, a large number of in vivo and in vitro experiments, and clinical trials have verified that PRP can promote the repair and regeneration of damaged or degenerated tissues (including bone) [133,134]. However, there is no denying that the results of some clinical trials on the treatment of OA by PRP are controversial [135]. Although past therapeutic strategies have potential, various shortcomings have limited their clinical application. The formulation of therapeutic strategies is constantly changing with the progress of science and technology. Therefore, there is an urgent need for new candidates MSCs and MSC-exo to enter the pre-clinical or clinical stage of treatment for OA.
BMSCs and AMSCs have shown strong cartilage repair abilities and have been applied to the clinical treatment of OA [136,137]. In addition, In phase I/II trials, HUCMSCs demonstrated greater pain relief and functional improvement in patients treated with OA compared with conventional therapies [138]. Therefore, exosome therapy based on MSCs has become the mainstream of current research. The therapeutic effect of MSCs-exo can be greatly promoted by drug pretreatment or gene/material modification. Currently, injection of hydrogel preparations containing exosomes and exosomal microRNA/LncRNA/circRNA delivery is the most common intervention choice. MSCs-exo combined with hydrogel sponges accelerated tissue repair and regeneration in animal models of cartilage injury. Zhang et al. inspired by mussels and launched alginate-dopamine, chondroitin sulfate, and regenerated silk fibroin hydrogel (AD/CS/RSF). They found that AD/CS/RSF hydrogel with encapsulated exosomes promoted BMSCs migration, proliferation, and differentiation. Even more exciting, AD/CS/RSF-exo hydrogels regenerated the defect cartilage and remodeled the ECM. Hydrogel sponges make MSCs-exo more stable in vivo and can make some changes according to the microenvironment of the joint cavity [139]. For example, increased activity of proinflammatory factors and MMPs or excessive mechanical loading allows it to control MSCs-exo release better. Genetic modifications have also shown excellent potential for the treatment of OA. However, being limited by current technology (such as exosome extraction and purification) makes it difficult to apply in clinical practice. But it has been used as a diagnostic or prognostic biomarker in many diseases [[140], [141], [142]]. PRP is currently used clinically for OA, although its efficacy is controversial. However, exosomes derived from PRP (PRP-exo) were found to be even more effective than PRP alone in cartilage protection [40].
8. Challenges and prospects
Exosomes can not be phagocytosed by mononuclear macrophages and can pass through the vascular barrier and ECM unimpeded due to their small size. Exosome that is derived from the human body remains a better compatibility between living things and lower immune response compared with synthetic liposomes and other nano or particle drug delivery methods in vitro theoretically [143]. The ways or pathways of exosomes entering cells are diverse. Endocytosis is considered to be one of the main ways that exosomes participate in communication between cells. Studies have found that enhanced exosome endocytosis can promote drug internalization and facilitate consecutive exosome drug delivery, which is the theoretical source of engineering exosome [144]. In addition, excellent homing ability and easy penetration of biological barriers (such as the blood-brain barrier) are highlights of exosome drug delivery, which are determined by the biological characteristics of exosomes themselves. However, original exosomes keep a lower targeting ability and faster drug clearance rate in vivo, which leads to poor therapeutic efficacy [145]. Therefore, the inherent advantages of exosomes make them promising delivery vehicles for targeted drugs (including genes, phytochemicals, or other drugs) after modification or pre-treatment [146,147]. To amplify the advantages of exosomes and attenuate their disadvantages, engineered exosomes have been developed to overcome the obstacles, such as exosomal miRNA or LncRNA, exosomal berberine, exosomal hydrogel, etc.
Exosome drug loading or modification has been segmented into pre-secretion and post-secretion. In the pre-secretion drug loading method, parental cells are usually co-incubated with drugs (generally transfection reagents are required) to make the drugs enter the cytoplasm, and the drugs in the cytoplasm are sorted into exosomes actively or passively. Then the drug-loaded exosomes can be obtained by appropriate extraction methods. This method only involves treating and modifying cells and almost no treatment of the extracted exosomes. Its advantage is that the integrity and functionality of exosomes are well preserved, while its disadvantage is that the drug loading efficiency is relatively low. The post-secretion drug loading method is the most commonly used exosome loading strategy, which usually requires the isolation and purification of exosomes before loading drugs into them. The advantages are that this method is relatively simple and drug loading efficiency is relatively high. However, the drug-loading process may compromise the integrity of exosomes, and additional purification steps are required to remove uncoated drugs [148]. Engineered exosomes exhibit key properties that make them attractive as therapeutic drugs, particularly their ability to target multiple therapeutic payloads, favorable safety profile, and potential for low immunogenicity.
However, various limitations have hindered the development of engineered exosomes. Firstly, the quality control of the upstream step dramatically affects the quality of exosomes, including the selection of parental cells and large-scale culture. The activity, homing ability, immunogenicity, and carcinogenicity of exosomes should be considered in selecting parental cells [[149], [150], [151]]. Secondly, although there are many methods for exosome extraction, there needs to be a more economical and efficient process, especially the lack of standardized operation specifications to ensure the production, purity, and function of exosomes with good reproducibility. Thirdly, exosome storage is still a tricky problem, as exosomes stored at 4 °C will lead to agglomeration and damage the structure. Although −80 °C is a better temperature, it increases the burden on transportation and costs [152]. At last, the functions and safety of exosomes in vivo are still controversial. Safety should be fully considered when exosomes are used as drug delivery carriers due to their biological activities. For example, MSCs-exo therapy has the potential to directly or indirectly induce tumors or accelerate the progression of existing tumors due to its anti-inflammatory and immunosuppressive effects [138,153]. When exosomes perform certain operations that may damage the membrane, such as loading drugs, the direction of membrane proteins may be changed, leading to immune recognition and then triggering a series of negative effects [137].
Another important point to note is that the routes of exosome administration are diverse, including intravenous, subcutaneous, intraperitoneal, intratumoral, intranasal, and oral administration. In the in vivo study of exosome treatment of OA, intra-articular drug injection is the preferred method of administration. However, few studies have confirmed or elucidated the drug utilization efficiency and metabolic efficiency of intra-articular injections. In essence, the route of administration is primarily responsible for the drug efficacy, and different routes of administration will determine the bio-distribution and clearance rate of drugs in the body. Oral administration is the first choice of drug administration route in clinical practice, but the complex gastrointestinal environment makes exosome drugs difficult to absorb through the intestinal wall, which is a great challenge for oral exosome products. Efforts have been made to explore functional microvesicles or nano delivery systems to improve drug absorption and utilization rate of oral routes for many years. It has been found that milk exosomes can be engineered as effective genes and drug carriers for oral administration. Milk exosome drugs, with remarkable evolutive potential and plastic characteristics, allow them possible to be absorbed as intact particles during gastrointestinal activities and protect them from waste by the bad gastrointestinal environment [154].
Whether it is used as a biomarker for the early diagnosis and prognosis assessment of OA or the treatment of OA, the significance of exosomes in OA is worthy of further discussion. Given the excellent therapeutic potential of exosomes, which can not only relieve symptoms but also delay or even reverse the degenerative progression of OA, although there are many obstacles to the clinical practice of exosomes in OA treatment, it is still worth expecting. Therefore, it is necessary to optimize exosome treatment strategies to improve their therapeutic efficacy on OA. With the advancement of technology, the exosome-based treatment strategy will be applied to OA patients in the future.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
The authors gratefully acknowledge the support for this study from the National Natural Science Foundation of China (Nos. 82072196, 82102350, and 82002058).
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
References
- 1.Glyn-Jones S., et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Litwic A., et al. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramoff B., Caldera F.E. Osteoarthritis: pathology, diagnosis, and treatment options. Med. Clin. 2020;104(2):293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Arden N.K., et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 2021;17(1):59–66. doi: 10.1038/s41584-020-00523-9. [DOI] [PubMed] [Google Scholar]
- 5.Salucci S., Falcieri E., Battistelli M. Chondrocyte death involvement in osteoarthritis. Cell Tissue Res. 2022;389(2):159–170. doi: 10.1007/s00441-022-03639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahmati M., et al. Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res. Rev. 2017;40:20–30. doi: 10.1016/j.arr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Mapp P.I., Walsh D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012;8(7):390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Lopez E., et al. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022;18(5):258–275. doi: 10.1038/s41584-022-00749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hade M.D., Suire C.N., Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10(8) doi: 10.3390/cells10081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Sowayan B., Alammari F., Alshareeda A. Preparing the bone tissue regeneration ground by exosomes: from diagnosis to therapy. Molecules. 2020;25(18) doi: 10.3390/molecules25184205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamerkar S., et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;(6478):367. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 14.Larios J., et al. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020;219(3) doi: 10.1083/jcb.201904113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrowski M., et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. sup pp 1-13. [DOI] [PubMed] [Google Scholar]
- 16.Trajkovic K., et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 17.Vietri M., Radulovic M., Stenmark H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020;21(1):25–42. doi: 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 18.Juan T., Furthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 2018;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y., et al. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Contr. Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pegtel D.M., Gould S.J. Exosomes. Ann. Rev. Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 22.Xu W.M., et al. Research development on exosome separation technology. J. Membr. Biol. 2022 doi: 10.1007/s00232-022-00260-y. [DOI] [PubMed] [Google Scholar]
- 23.Gurunathan S., et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4) doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domenis R., et al. vol. 2017. Mediators Inflamm; 2017. (Characterization of the Proinflammatory Profile of Synovial Fluid-Derived Exosomes of Patients with Osteoarthritis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao K., et al. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod. Rheumatol. 2020;30(4):758–764. doi: 10.1080/14397595.2019.1651445. [DOI] [PubMed] [Google Scholar]
- 26.Kolhe R., et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 2017;7(1):2029. doi: 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skriner K., et al. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006;54(12):3809–3814. doi: 10.1002/art.22276. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y., Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 2018;42(12):2865–2872. doi: 10.1007/s00264-018-4093-6. [DOI] [PubMed] [Google Scholar]
- 29.Ni Z., et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Z., et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120555. [DOI] [PubMed] [Google Scholar]
- 31.Loeser R.F. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17(8):971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varela-Eirin M., et al. Cartilage regeneration and ageing: targeting cellular plasticity in osteoarthritis. Ageing Res. Rev. 2018;42:56–71. doi: 10.1016/j.arr.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Hwang H.S., Kim H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2015;16(11):26035–26054. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tower J. Programmed cell death in aging. Ageing Res. Rev. 2015;23(Pt A):90–100. doi: 10.1016/j.arr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vicar T., et al. The quantitative-phase dynamics of apoptosis and lytic cell death. Sci. Rep. 2020;10(1):1566. doi: 10.1038/s41598-020-58474-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Khoury M.K., et al. Necroptosis in the pathophysiology of disease. Am. J. Pathol. 2020;190(2):272–285. doi: 10.1016/j.ajpath.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu Y., et al. The application of ferroptosis in diseases. Pharmacol. Res. 2020;159 doi: 10.1016/j.phrs.2020.104919. [DOI] [PubMed] [Google Scholar]
- 39.Jin Y., et al. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell Mol. Med. 2021;25(19):9281–9294. doi: 10.1111/jcmm.16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X., et al. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/beta-catenin signaling pathway. J. Orthop. Surg. Res. 2019;14(1):470. doi: 10.1186/s13018-019-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin T., et al. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR-140-5p-overexpressing human dental pulp stem cells. Int. J. Mol. Med. 2021;47(3) doi: 10.3892/ijmm.2020.4840. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z., et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 2021;37(1):85–96. doi: 10.1007/s10565-020-09559-9. [DOI] [PubMed] [Google Scholar]
- 43.Qiu M., Liu D., Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1beta induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269 doi: 10.1016/j.lfs.2020.118987. [DOI] [PubMed] [Google Scholar]
- 44.Li X., et al. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol. Int. 2021;45(10):2096–2106. doi: 10.1002/cbin.11657. [DOI] [PubMed] [Google Scholar]
- 45.Wu J., et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Yang R.Z., et al. Vascular endothelial cell-secreted exosomes facilitate osteoarthritis pathogenesis by promoting chondrocyte apoptosis. Aging (Albany NY) 2021;13(3):4647–4662. doi: 10.18632/aging.202506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An S., et al. Pyroptosis plays a role in osteoarthritis. Aging Dis. 2020;11(5):1146–1157. doi: 10.14336/AD.2019.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia S., et al. Mechanical stimulation protects against chondrocyte pyroptosis through irisin-induced suppression of PI3K/akt/NF-kappaB signal pathway in osteoarthritis. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.797855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai H., et al. Activation of adenosine A3 receptor attenuates progression of osteoarthritis through inhibiting the NLRP3/caspase-1/GSDMD induced signalling. J. Cell Mol. Med. 2022;26(15):4230–4243. doi: 10.1111/jcmm.17438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu H., Xu B. BMSC-derived exosomes ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via delivering miR-326 targeting HDAC3 and STAT1//NF-kappaB p65 to chondrocytes. Mediat. Inflamm. 2021;2021 doi: 10.1155/2021/9972805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing H., et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J. Nanobiotechnol. 2021;19(1):264. doi: 10.1186/s12951-021-00991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J., et al. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J. Cell Mol. Med. 2020;24(20):11742–11754. doi: 10.1111/jcmm.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang W.S., et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2016;113(34):E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73(11–12):2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y., et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26(11):2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan H., Pratte J., Giardina C. Ferroptosis and its potential as a therapeutic target. Biochem. Pharmacol. 2021;186 doi: 10.1016/j.bcp.2021.114486. [DOI] [PubMed] [Google Scholar]
- 57.Mou Y., et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J. Hematol. Oncol. 2019;12(1):34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown C.W., et al. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev. Cell. 2019;51(5):575–586 e4. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dai J., et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct. Targeted Ther. 2020;5(1):145. doi: 10.1038/s41392-020-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song Y., et al. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol. Toxicol. 2021;37(1):51–64. doi: 10.1007/s10565-020-09530-8. [DOI] [PubMed] [Google Scholar]
- 61.Lin F., et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 2022;13(3):271. doi: 10.1038/s41419-022-04708-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu L., et al. The cell cycle in stem cell proliferation, pluripotency and differentiation. Nat. Cell Biol. 2019;21(9):1060–1067. doi: 10.1038/s41556-019-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coffman J.A. Cell cycle development. Dev. Cell. 2004;6(3):321–327. doi: 10.1016/s1534-5807(04)00067-x. [DOI] [PubMed] [Google Scholar]
- 64.He L., et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020;11(1):276. doi: 10.1186/s13287-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang K., et al. Mesenchymal stem cell-derived exosomes modulate chondrocyte glutamine metabolism to alleviate osteoarthritis progression. Mediat. Inflamm. 2021;2021 doi: 10.1155/2021/2979124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y., et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017;8(1):64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou X., et al. BMSC-derived exosomes from congenital polydactyly tissue alleviate osteoarthritis by promoting chondrocyte proliferation. Cell Death Dis. 2020;6(1):142. doi: 10.1038/s41420-020-00374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou H., et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res. Ther. 2022;13(1):322. doi: 10.1186/s13287-022-03005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y., et al. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17(21–22):2411–2422. doi: 10.1080/15384101.2018.1526603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tao S.C., et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mao G., et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018;9(1):247. doi: 10.1186/s13287-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tao S.C., et al. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021;6(12):4455–4469. doi: 10.1016/j.bioactmat.2021.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang R., Xu B., Xu H. TGF-beta1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle. 2018;17(24):2756–2765. doi: 10.1080/15384101.2018.1556063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo Z., et al. Exosomal circ-BRWD1 contributes to osteoarthritis development through the modulation of miR-1277/TRAF6 axis. Arthritis Res. Ther. 2021;23(1):159. doi: 10.1186/s13075-021-02541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y., et al. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Dis. 2021;7(1):37. doi: 10.1038/s41420-021-00418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Y., et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 promotes osteoblast proliferation and differentiation in osteoarthritis by reducing Elf3. J. Cell Mol. Med. 2021;25(16):7734–7745. doi: 10.1111/jcmm.16654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao Y., et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-kappaB signaling pathway. Bioorg. Chem. 2021;113 doi: 10.1016/j.bioorg.2021.104978. [DOI] [PubMed] [Google Scholar]
- 78.Tan F., Wang D., Yuan Z. The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 Axis. Inflammation. 2020;43(4):1498–1509. doi: 10.1007/s10753-020-01227-8. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y., et al. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 2018;475(22):3629–3638. doi: 10.1042/BCJ20180675. [DOI] [PubMed] [Google Scholar]
- 80.Lai X., Song Y., Tian J. CircCDK14 ameliorates interleukin-1beta-induced chondrocyte damage by the miR-1183/KLF5 pathway in osteoarthritis. Autoimmunity. 2022;55(6):408–417. doi: 10.1080/08916934.2022.2081843. [DOI] [PubMed] [Google Scholar]
- 81.Zeng G., et al. Fibroblast-like synoviocytes-derived exosomal PCGEM1 accelerates IL-1beta-induced apoptosis and cartilage matrix degradation by miR-142-5p/RUNX2 in chondrocytes. Immunol. Invest. 2022;51(5):1284–1301. doi: 10.1080/08820139.2021.1936010. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y., et al. Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am. J. Sports Med. 2022;50(4):1088–1105. doi: 10.1177/03635465221073991. [DOI] [PubMed] [Google Scholar]
- 83.Kim K.H., Lee M.S. Autophagy--a key player in cellular and body metabolism. Nat. Rev. Endocrinol. 2014;10(6):322–337. doi: 10.1038/nrendo.2014.35. [DOI] [PubMed] [Google Scholar]
- 84.Mizushima N., Levine B. Autophagy in human diseases. N. Engl. J. Med. 2020;383(16):1564–1576. doi: 10.1056/NEJMra2022774. [DOI] [PubMed] [Google Scholar]
- 85.Mizushima N., Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 86.Kang R., et al. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y., et al. ATF4-modified serum exosomes derived from osteoarthritic mice inhibit osteoarthritis by inducing autophagy. IUBMB Life. 2021;73(1):146–158. doi: 10.1002/iub.2414. [DOI] [PubMed] [Google Scholar]
- 88.Guillen M.I., et al. Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J. Orthop. Translat. 2021;30:61–69. doi: 10.1016/j.jot.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang S., et al. Bone marrow mesenchymal stem cell-derived exosomes inhibit chondrocyte apoptosis and the expression of MMPs by regulating Drp1-mediated mitophagy. Acta Histochem. 2021;123(8) doi: 10.1016/j.acthis.2021.151796. [DOI] [PubMed] [Google Scholar]
- 90.Li Z.Q., et al. Human bone marrow mesenchymal stem cell-derived exosomes attenuate IL-1beta-induced annulus fibrosus cell damage. Am. J. Med. Sci. 2020;360(6):693–700. doi: 10.1016/j.amjms.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Q.C., et al. Autophagy-activated nucleus pulposus cells deliver exosomal miR-27a to prevent extracellular matrix degradation by targeting MMP-13. J. Orthop. Res. 2021;39(9):1921–1932. doi: 10.1002/jor.24880. [DOI] [PubMed] [Google Scholar]
- 92.Xia P., et al. Low-intensity pulsed ultrasound enhances the efficacy of bone marrow-derived MSCs in osteoarthritis cartilage repair by regulating autophagy-mediated exosome release. Cartilage. 2022;13(2) doi: 10.1177/19476035221093060. 19476035221093060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Theocharis A.D., et al. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 94.Khoshgoftar M., Torzilli P.A., Maher S.A. Influence of the pericellular and extracellular matrix structural properties on chondrocyte mechanics. J. Orthop. Res. 2018;36(2):721–729. doi: 10.1002/jor.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malemud C.J. Inhibition of MMPs and ADAM/ADAMTS. Biochem. Pharmacol. 2019;165:33–40. doi: 10.1016/j.bcp.2019.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y., et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017;8(1):189. doi: 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cosenza S., et al. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xia Q., et al. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered. 2021;12(2):11225–11238. doi: 10.1080/21655979.2021.1995580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen X., et al. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 2020;22(1):256. doi: 10.1186/s13075-020-02325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tofino-Vian M., et al. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell. Physiol. Biochem. 2018;47(1):11–25. doi: 10.1159/000489739. [DOI] [PubMed] [Google Scholar]
- 101.Liao L., et al. Acute synovitis after trauma precedes and is associated with osteoarthritis onset and progression. Int. J. Biol. Sci. 2020;16(6):970–980. doi: 10.7150/ijbs.39015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010;6(11):625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 103.Yu X.H., et al. The causal role of gut microbiota in development of osteoarthritis. Osteoarthritis Cartilage. 2021;29(12):1741–1750. doi: 10.1016/j.joca.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 104.Hodgkinson T., et al. Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 2022;18(2):67–84. doi: 10.1038/s41584-021-00724-w. [DOI] [PubMed] [Google Scholar]
- 105.Scanzello C.R., Goldring S.R. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han D., et al. The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: an update. J. Cell Mol. Med. 2020;24(17):9518–9532. doi: 10.1111/jcmm.15669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kapoor M., et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 108.Wojdasiewicz P., Poniatowski L.A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vincent T.L. Mechanoflammation in osteoarthritis pathogenesis. Semin. Arthritis Rheum. 2019;49(3S):S36–S38. doi: 10.1016/j.semarthrit.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y., Shah K.M., Luo J. Strategies for articular cartilage repair and regeneration. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.770655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jahr H., Matta C., Mobasheri A. Physicochemical and biomechanical stimuli in cell-based articular cartilage repair. Curr. Rheumatol. Rep. 2015;17(3):22. doi: 10.1007/s11926-014-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Veith A.P., et al. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019;146:97–125. doi: 10.1016/j.addr.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Viallard C., Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 114.Suri S., Walsh D.A. Osteochondral alterations in osteoarthritis. Bone. 2012;51(2):204–211. doi: 10.1016/j.bone.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 115.Ashraf S., et al. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann. Rheum. Dis. 2011;70(3):523–529. doi: 10.1136/ard.2010.137844. [DOI] [PubMed] [Google Scholar]
- 116.Bornes T.D., Adesida A.B., Jomha N.M. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: a comprehensive review. Arthritis Res. Ther. 2014;16(5):432. doi: 10.1186/s13075-014-0432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qi X., et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016;12(7):836–849. doi: 10.7150/ijbs.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang L., et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020;11(1):38. doi: 10.1186/s13287-020-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y., et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2) doi: 10.1111/cpr.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nossin Y., et al. Angiogenic potential of tissue engineered cartilage from human mesenchymal stem cells is modulated by Indian hedgehog and Serpin E1. Front. Bioeng. Biotechnol. 2020;8:327. doi: 10.3389/fbioe.2020.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun Z., et al. Notochordal-cell-derived exosomes induced by compressive load inhibit angiogenesis via the miR-140-5p/Wnt/beta-Catenin Axis. Mol. Ther. Nucleic Acids. 2020;22:1092–1106. doi: 10.1016/j.omtn.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun Z., et al. AF cell derived exosomes regulate endothelial cell migration and inflammation: implications for vascularization in intervertebral disc degeneration. Life Sci. 2021;265 doi: 10.1016/j.lfs.2020.118778. [DOI] [PubMed] [Google Scholar]
- 123.Shi Q., et al. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front. Physiol. 2017;8:904. doi: 10.3389/fphys.2017.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J., et al. BMSCs-derived exosomes ameliorate pain via abrogation of aberrant nerve invasion in subchondral bone in lumbar facet joint osteoarthritis. J. Orthop. Res. 2020;38(3):670–679. doi: 10.1002/jor.24497. [DOI] [PubMed] [Google Scholar]
- 125.Hu W., et al. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 2021;80(4):413–422. doi: 10.1136/annrheumdis-2020-218089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu Y., et al. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9(1):20. doi: 10.1038/s41413-021-00147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hugle T., Geurts J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology. 2017;56(9):1461–1471. doi: 10.1093/rheumatology/kew389. [DOI] [PubMed] [Google Scholar]
- 128.Wang R., Xu B. TGFbeta1-modified MSC-derived exosome attenuates osteoarthritis by inhibiting PDGF-BB secretion and H-type vessel activity in the subchondral bone. Acta Histochem. 2022;124(7) doi: 10.1016/j.acthis.2022.151933. [DOI] [PubMed] [Google Scholar]
- 129.Zhang S., et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 130.Jones I.A., et al. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019;15(2):77–90. doi: 10.1038/s41584-018-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meccariello L. 2013. INTRAARTICULAR KNEE JOINT INJECTION: HYALURONIC ACID VS POLYNUCLEOTIDES. [Google Scholar]
- 132.Ripani U., et al. Vitamin C may help to reduce the knee's arthritic symptoms. Outcomes assessment of nutriceutical therapy. Med. Arch. 2019;73(3):173–177. doi: 10.5455/medarh.2019.73.173-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Andia I., Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013;9(12):721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 134.Rollo G., et al. Platet Rich Plasma or Hyperbaric Oxygen Therapy as callus accellerator in aseptic tibial non union. Evaluate of outcomes. Acta Biomed. 2020;91(4):e2020188. doi: 10.23750/abm.v91i4.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bennell K.L., et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. 2021;326(20):2021–2030. doi: 10.1001/jama.2021.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kong L., et al. Role of mesenchymal stem cells in osteoarthritis treatment. J. Orthop. Translat. 2017;9:89–103. doi: 10.1016/j.jot.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Bari C., Roelofs A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 2018;40:74–80. doi: 10.1016/j.coph.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 138.Matas J., et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl. Med. 2019;8(3):215–224. doi: 10.1002/sctm.18-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang F.X., et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121169. [DOI] [PubMed] [Google Scholar]
- 140.Mori M.A., et al. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metabol. 2019;30(4):656–673. doi: 10.1016/j.cmet.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.He Y., et al. Exosomal microRNA: a novel biomarker for breast cancer. Biomarkers Med. 2018;12(2):177–188. doi: 10.2217/bmm-2017-0305. [DOI] [PubMed] [Google Scholar]
- 142.Zhou L., et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer. 2021;20(1):57. doi: 10.1186/s12943-021-01352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Teng F., Fussenegger M. Shedding light on extracellular vesicle biogenesis and bioengineering. Adv. Sci. 2020;8(1) doi: 10.1002/advs.202003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gonda A., et al. Internalization of exosomes through receptor-mediated endocytosis. Mol. Cancer Res. 2019;17(2):337–347. doi: 10.1158/1541-7786.MCR-18-0891. [DOI] [PubMed] [Google Scholar]
- 145.Gutierrez-Millan C., et al. Advances in exosomes-based drug delivery systems. Macromol. Biosci. 2021;21(1):e2000269. doi: 10.1002/mabi.202000269. [DOI] [PubMed] [Google Scholar]
- 146.Duan L., et al. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13(3):1387–1397. doi: 10.1039/d0nr07622h. [DOI] [PubMed] [Google Scholar]
- 147.Xi X.M., Xia S.J., Lu R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021;76(2):61–67. doi: 10.1691/ph.2021.0128. [DOI] [PubMed] [Google Scholar]
- 148.Kimiz-Gebologlu I., Oncel S.S. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Contr. Release. 2022;347:533–543. doi: 10.1016/j.jconrel.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 149.Sun Y., et al. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res. Ther. 2021;12(1):561. doi: 10.1186/s13287-021-02629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Seo N., Akiyoshi K., Shiku H. Exosome-mediated regulation of tumor immunology. Cancer Sci. 2018;109(10):2998–3004. doi: 10.1111/cas.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang S., et al. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J. Hematol. Oncol. 2018;11(1):82. doi: 10.1186/s13045-018-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang Y., et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang C., et al. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol. Cancer. 2021;20(1):117. doi: 10.1186/s12943-021-01411-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhong J., et al. High-quality milk exosomes as oral drug delivery system. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.