Abstract
Actinomyces spp. exhibit type 1 fimbria-mediated adhesion to salivary acidic proline-rich proteins (PRPs) and statherin ligands. Actinomyces spp. with different animal and tissue origins belong to three major adhesion types as relates to ligand specificity and type 1 fimbria genes. (i) In preferential acidic-PRP binding, strains of Actinomyces naeslundii genospecies 1 and 2 from human and monkey mouths displayed at least three ligand specificities characterized by preferential acidic-PRP binding. Slot blot DNA hybridization showed seven highly conserved type 1 fimbria genes (orf1- to -6 and fimP) in genospecies 1 and 2 strains, except that orf5 and orf3 were divergent in genospecies 1. (ii) In preferential statherin binding, oral Actinomyces viscosus strains of rat and hamster origin (and strain 19246 from a human case of actinomycosis) bound statherin preferentially. DNA hybridization and characterization of the type 1 fimbria genes from strain 19246 revealed a homologous gene cluster of four open reading frames (orfA to -C and fimP). Bioinformatics suggested sortase (orfB, orf4, and part of orf5), prepilin peptidase (orfC and orf6), fimbria subunit (fimP), and usher- and autotransporter-like (orfA and orf1 to -3) functions. Those gene regions corresponding to orf3 and orf5 were divergent, those corresponding to orf2, orf1, and fimP were moderately conserved, and those corresponding to orf4 and orf6 were highly conserved. Restriction fragment length polymorphism analyses using a fimP probe separated human and monkey and rat and hamster strains into phylogenetically different groups. (iii) In statherin-specific binding, strains of A. naeslundii genospecies 1 from septic and other human infections displayed a low-avidity binding to statherin. Only the orf4 and orf6 gene regions were highly conserved. Finally, rat saliva devoid of statherin bound bacterial strains avidly irrespective of ligand specificity, and specific antisera detected either type 1, type 2, or both types of fimbria on the investigated Actinomyces strains.
Adhesion of commensal and pathogenic bacteria to host tissue surfaces is a crucial event in colonization and infections (13, 19). Commensal bacterial species, which protect against pathogens by competing for host binding sites (47), may involve a diversity of adhesion types with multiple ecological niches (42, 45).
Actinomyces naeslundii and Actinomyces viscosus are dominant commensal Actinomyces spp. colonizing dental and mucosal surfaces of various animal hosts. They show extensive phenotypic and serologic variations (23). Human strains of A. naeslundii were recently grouped into genospecies 1 (A. naeslundii serotype I) and genospecies 2 (A. naeslundii serotypes II, III, and NV and A. viscosus serotype II) based on genetic relatedness (23). A. viscosus serotype I is the dominant species in the rat and hamster mouths. Actinomyces spp. have also been implicated in caries (34), periodontitis (24), and root canal infections (46), as well as in actinomycosis and septic infections (37).
The animal and tissue tropism of A. naeslundii and A. viscosus appears to involve a diversity of type 1 and type 2 fimbria adhesion types (8, 45, 49). Type 1 fimbriae, which mediate binding to acidic proline-rich proteins (PRPs) and statherin, are more common on A. naeslundii genospecies 2 (an early plaque colonizer) than on genospecies 1 (a late plaque colonizer) (11, 16, 17). Moreover, while type 1 fimbriae on A. naeslundii of human origin bind ProGln in acidic PRPs, type 1 fimbriae on A. viscosus of rat and hamster origin bind ThrPhe in statherin (28). Type 2 fimbriae, which mediate binding to β-linked galactose structures, are highly prevalent on both A. naeslundii genospecies 1 and 2. Type 2 fimbriae involve at least four β-linked galactose specificities with different coaggregation and biological properties (16, 17, 45).
Biogenesis, assembly, and function of type 1 fimbriae of A. naeslundii strain T14V require the FimP subunit and additional proteins encoded by a cluster of seven genes (orf1 to -6 and fimP) (52). Recently, a type 2 fimbria gene cluster containing three or four genes (ef-TU, orf977, fimA, and orf365) was found in A. naeslundii strain T14V (51) (GenBank accession no. AJ401093). Structural variations in the major type 1 (FimP) and type 2 (FimA) subunit proteins correlate with different acidic-PRP and statherin and β-linked galactose adhesion types (17, 28). Allelic replacement of the orf1 to -4 and fimP genes of type 1 fimbriae and of the orf365 and fimA genes of type 2 fimbriae abolish PRP adhesion and coaggregation by A. naeslundii strain T14V, respectively (51, 52).
Acidic PRPs and statherin are present in exocrine secretions, e.g., saliva (26) and nasal and bronchial secretions (9, 38), of different animal species, e.g., humans (18, 38), monkeys (39, 40), and rabbits (43). Acidic PRPs, but not statherin, are also present in rats (2, 33) and hamsters (31). Acidic PRPs are highly polymorphic and multifunctional proteins that may determine host susceptibility and resistance to dental caries (3, 7, 26, 44, 53). While acidic PRPs promote avid adhesion of commensal species, such as A. naeslundii (14) and Streptococcus gordonii (15), statherin promotes the adhesion of potentially invasive species, such as Porphyromonas gingivalis (1) and Candida albicans (6, 21).
The aim of the present study was to investigate the structural and functional polymorphism of type 1 fimbriae on Actinomyces spp. with specificity for acidic PRPs and statherin. We found a diversity of Actinomyces spp. with different protein ligand specificities, type 1 fimbria genes, and tropisms. Those adhesion types typical of human commensal strains bound acid PRPs preferentially, while those typical of rat and hamster hosts and human infections bound statherin preferentially.
MATERIALS AND METHODS
Actinomyces strains, typing, and culturing.
A. viscosus and A. naeslundii strains were isolated as previously described (16) or were from other sources. For A. viscosus strains, these sources were as follows: 19246, Culture Collection at the National Bacteriology Laboratory, Stockholm, Sweden; T6-1600, R28, and A828, the late M. Yeung, University of Texas Health Science Center, San Antonio, Tex.; 14476, 35452, and 35451, Culture Collection of the University of Göteborg (CCUG). A. naeslundii genospecies 2 strains M4356 and M4301 were from the late M. Yeung; A. naeslundii genospecies 1 strains 17534, ATCC 12104, 30267, 35334, and 29952 were from CCUG; A. naeslundii genospecies 2 strain PK1259 and genospecies 1 strains PK947 and PK606 were from P. Kolenbrander, National Institutes of Health, Bethesda, Md.
Strains were characterized by using the Analytical Profile Index products Rapid ID 32 strp and ID coryne (bioMérieux, Marcy l'Etoile, France), by whole-cell protein patterning by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and by whole-cell agglutination with Actinomyces-specific rabbit antisera (G. H. Bowden, University of Manitoba, Winnipeg, Canada) (16, 28, 36).
Actinomyces strains were cultured overnight on Columbia II agar base plates (Becton Dickinson Microbiology Systems, Cockeysville, Md.) supplemented with a human erythrocyte suspension (30 ml/l) at 37°C in a nitrogen atmosphere with 5% CO2 and 10% H2 or in an atmosphere with 5% CO2 or in candle jars. Bacteria were metabolically labeled with [35S]methionine (200 μCi; Amersham, Little Chalfont, United Kingdom) for adhesion experiments.
Hydroxyapatite assay.
Binding of [35S]methionine-labeled bacteria (5 × 108 cells/ml) to salivary protein PRP-1 and coating of hydroxyapatite beads (5 mg) (Fluka, Chemie AG, Buchs, Switzerland) with saliva were done as previously described (7, 14). Inhibition experiments with ArgGlyArgProGln and lactose were performed with 108 bacterial cells/ml and 4 mg of hydroxyapatite beads.
Saliva sampling.
Parotid saliva was collected from healthy human subjects (7). Whole rat saliva was collected from Sprague-Dawley rats (body weight, 400 to 425 g). The rats were anesthetized, placed in a forward supine position, and stimulated by pilocarpine or pilocarpine and isoproterenol (5 mg/kg of body weight for each) followed by collection of whole saliva for 15 min. The saliva samples were kept on ice (22). The saliva-mediated adhesion patterns were similar irrespective of the type of saliva stimulation.
Acidic PRPs and statherin.
Acidic PRPs and statherin were isolated from human parotid saliva as previously described (28).
Vectors and bacterial strains for DNA library construction.
Phagemid vector λTriplEx and Escherichia coli strain XL1-Blue (endA1 gyrA96 hsdR17 lac negative recA1 relA1 supE44 thi-1 [F′ laclqZ ΔM15 proAB Tn10]) were used to generate a genomic DNA library from A. viscosus strain 19246. E. coli strain BM25.8 (supE44 thi Δ[lac-proAB] [F′ traD36 proAB+ laclqZ ΔM15] λimm434 (Kanr)P1 (Camr) hsdR(rk12− mk12−]) was used for converting the λTriplEx vector to a plasmid pTriplEx vector (Clontech, Palo Alto, Calif.).
Genomic DNA library construction, screening, and sequencing.
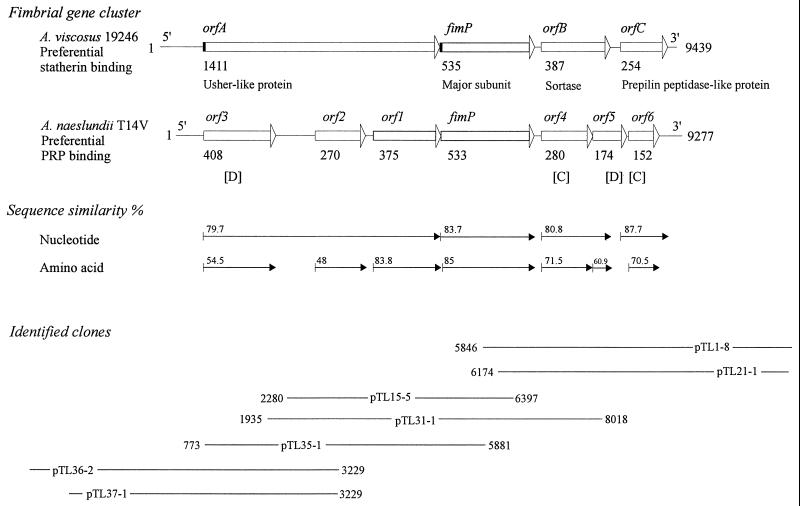
Genomic DNA was isolated from A. viscosus strain 19246 as previously described (17), partially digested with MboI, ligated to an EcoRI adapter, gel purified, and size fractionated. The 4- to 8-kb DNA fragments were ligated to the λTriplEx vector to construct a DNA library with a titer of 6.2 × 105 PFU/ml and an average insert size of 5.5 kb (Clontech). The library was amplified in E. coli XL1-Blue and screened by plaque DNA hybridization using a fimP-specific probe (λTriplEx library user manual; Clontech). Positive clones were converted from phagemid λTriplEx to plasmid pTriplEx in E. coli BM25.8 according to Clontech's instructions. Plasmid DNA was isolated by the JETstar kit (Genomed Inc.), and insert size was determined by EcoRI digestion. Clones with proper insert sizes were sequenced using vector primers (Clontech) and the BigDye terminator cycle sequencing ready reaction kit (Perkin-Elmer). Clones with overlapping sequences were subjected to further sequencing by primer walking. The sequences were established from at least two clones and from both strands. In this way, clones pTL1-8, pTL21-1, pTL15-5, pTL31-1, and pTL35-1 were identified and characterized (see Fig. 2). Clones pTL36-2 and pTL37-1 were identified using a probe derived from the 5′ end of orfA in clone pTL35-1. The nucleotide sequences were then assembled into a 9,439-bp type 1 fimbria-related gene cluster.
FIG. 2.
Type 1 fimbria genes in A. viscosus strain 19246 and A. naeslundii strain T14V with preferential statherin and acidic-PRP binding, respectively. Bars with arrowheads, ORFs and direction of transcription; black boxes, signal peptides. The numbers of amino acids in the deduced proteins are given below each bar. The most conserved (C) and diverse (D) genes among Actinomyces strains with different ligand specificities are indicated. Plasmids (clones) used for sequencing and assembly of the A. viscosus strain 19246 type 1 fimbria gene cluster are indicated (the corresponding base positions in the type 1 gene cluster of strain T14V are given).
Bioinformatics.
Nucleotide and amino acid sequence analyses were performed using the Sequence Analysis Software Package, version 9.1 (Genetics Computer Group, University of Wisconsin, Madison); bioinformatics tools available at National Center for Biotechnology Information website http://www.ncbi.nlm.nih.gov/, European Bioinformatics Institute website http://www.ebi.ac.uk/index.html, and Expert Protein Analysis System website http://www.expasy.ch/; and the FramePlot, version 2.3, program (4, 20).
Slot blot DNA hybridization.
Slot blot DNA hybridization was performed under high-stringency conditions (hybridization and stringency washing at 80°C) as previously described (28). The degree of hybridization was scored from 0 to 6 by comparison with a standardized scale based on densitometric measures (GS-700 imaging densitometer and Molecular Analyst software; Bio-Rad, Hercules, Calif.) in which 0 = <0.01, 1 = 0.01 to <0.04, 2 = 0.04 to <0.10, 3 = 0.10 to <0.16, 4 = 0.16 to <0.22, 5 = 0.22 to <0.27, and 6 = ≥0.27 (17).
Restriction fragment length polymorphism (RFLP).
Actinomyces chromosomal DNA (5 or 8 μg) was digested separately with BamHI, PvuII, BssHII, AccI, StuI, or SalI and separated on 0.7% agarose gels. The restriction fragment length variations were detected by a fimP gene probe designed from A. naeslundii strain T14V by Southern blot hybridization as previously described (28). The hybridization data matrix was processed into an Actinomyces phylogenetic tree by the PAUP3.1.1 package (phylogenetic analysis using parsimony; Sinauer Associates) with a bootstrap value of 1,000.
DNA probes and primers.
DNA probes were generated and labeled with digoxigenin by PCR as previously described (17).
The following primer sequences were used to generate fimP- and orfA-specific probes from plasmids containing strain 19246 DNA for library screening: a 822-bp fimP gene probe (forward, 5′-ACCCTCTCCGGTGTGGACAA-3′; reverse, 5′-IGGIGCYTTIGTYTCIAC-3′) and a 470-bp orfA probe (5′-AAGATGCGCCATGTCAACC-3′ and 5′-TGACCGTTGTTCACGAATCC-3′).
The following primer sequences were used to generate orf1- to -6- and fimP-specific full-length probes for slot blot hybridization (and RFLP) from plasmids pMY261A-100 and pMY1113 containing type 1 fimbria genes from A. naeslundii strain T14V (kindly provided by the late M. Yeung) (52): orf1 probe, 5′-TCACGATGCAGATGACCTTC-3′ and 5′-TTGAGGGAGTGCATTGCTGT-3′; orf2 probe, 5′-ACTCCCTGAGCTACACCTG-3′ and 5′-GAGGTGAAGGTGCCATCAC-3′; orf3 probe, 5′-TCCCAGTCATCACGTCGCCC-3′ and 5′-GTACTCGTTGTGCCAGAC-3′; orf4 probe, 5′-CTACTCCCATCACTTCGTGACC-3′ and 5′-CTTCATCCAGGTCTGCAT-3′; orf5 probe, 5′-ATGCAGACCTGGATGAAG-3′ and 5′-GGTGTGGGTGAACACGAAC-3′; orf6 probe, 5′-CGGTCGTCCTGGTGGTGAC-3′ and 5′-AAGCGCTGAAGAGCTGCCA-3′; fimP probe, 5′-ACAGCAATGCACTCCCTCAA-3′ and 5′-TGCTTGGCAACGTGACGGC-3′.
Antisera.
Synthetic peptides were generated from the deduced amino acid sequences of FimP (DRLDKRIKKEALTPV) and Orf1 (TGKDSDTRPDHDVAC) proteins from A. naeslundii T14V (Innovagen AB, Lund, Sweden). The synthetic peptides were used for immunization of rabbits and generation of FimP- and Orf1-specific antisera (Agri Sera AB, Umeå, Sweden). The type 2:1 fimbria-specific antiserum R70-3 was kindly provided by J. O. Cisar, National Institutes of Health.
Immunofluorescence staining.
Immunofluorescence staining of whole bacterial cells was performed essentially as described previously (32, 41). Briefly, whole cells (109 cells/ml) were applied to an objective slide (Novakemi AB), incubated separately with FimP (1:200) and type 2:1 (1:800) antisera (and the corresponding presera dilutions) in 10 mM phosphate-buffered saline, pH 7.2–0.05% Tween 20, washed, and incubated with a secondary goat anti-rabbit immunoglobulin G (IgG)-fluorescein isothiocyanate conjugate (Sigma). Fluorescence signals were detected using a microscope (model DMRBE; Leica AB, Stockholm, Sweden) and scored 0 to 4; scores 0 and 1 indicate no distinguishable cells and bare fluorescence and cells without a distinguishable cell envelope but with faint fluorescence, respectively; scores 2, 3, and 4 indicate cells with a dark central region and a well-defined cell envelope with greenish fluorescence with moderate, high, and very high intensity, respectively, after subtraction of the presera reactivity.
Western blots of Actinomyces cell sonicates.
Actinomyces cells from a 3-day culture were harvested, washed, and suspended in sterile water to a concentration of 2 × 1010 cells/ml and sonicated four times for 15 s each using a B-30 Sonifier cell disruptor (Optilab-BO Philip Instrumentation AB, Stockholm, Sweden). Proteins were then precipitated with acetone, dissolved in SDS sample buffer (0.0625 M Tris, 10% glycerol, 2% SDS, 5 mM dithiothreitol, 0.01% pyronin), boiled, and frozen and thawed twice. After electrophoresis on 4 to 20% Tris-glycine gels (Bio-Rad) in electrophoresis buffer (0.1% SDS, 25 mM 2-amino-2-[hydroxymethyl]-1,3-propanediol, 192 mM glycine), pH 8.3, at 15 mA for 1.5 h, the proteins were transferred to polyvinylidene difluoride Immobilon-P transfer membranes with a pore size of 0.45 μm (Millipore Corporation, Bedford, Mass.). Membranes were incubated with FimP- and Orf1-specific antisera and presera (all diluted 1:800), washed, and incubated with secondary horseradish peroxidase-conjugated goat anti-rabbit IgG antisera (1:2,000; P0448; Dako). Antibody binding was detected using Supersignal West Pico chemiluminescent substrate (no. 34080; Pierce, Rockford, Ill.) and Cronex medical X-ray film 4 (Sterling Diagnostic Imaging, Inc.).
Nucleotide sequence accession number.
The GenBank accession number for the type 1 fimbrial gene cluster from A. viscosus ATCC 19246 is AF106034.
RESULTS
Acidic PRP and statherin ligand specificities among commensal and potentially pathogenic Actinomyces strains.
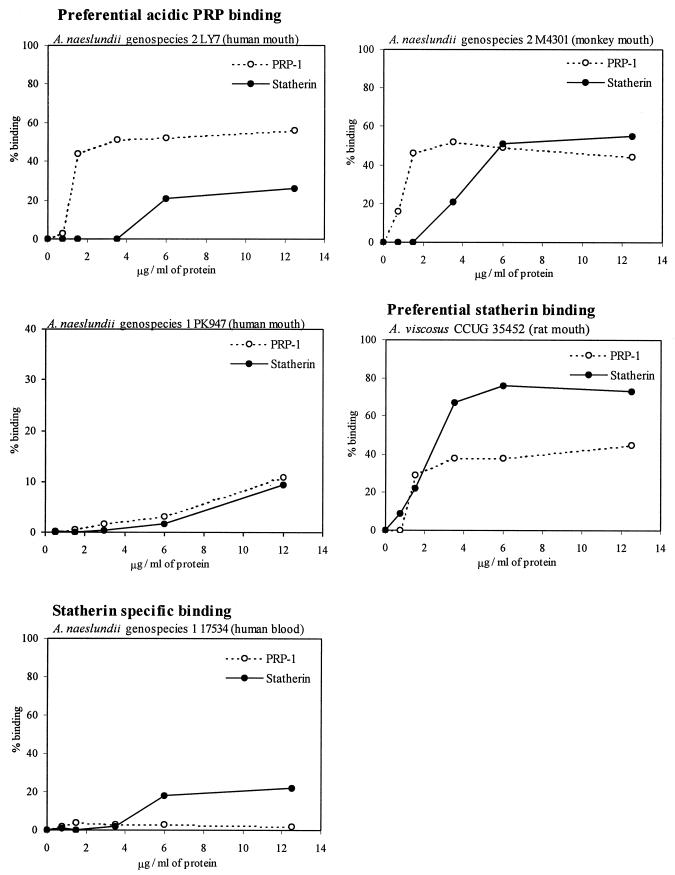
A panel of Actinomyces strains with different animal and tissue tropisms were screened for binding to acidic-PRP-1- and statherin-coated hydroxyapatite beads (Table 1). The following protein ligand specificities were detected. (i) In preferential acidic-PRP binding, oral strains of A. naeslundii genospecies 2 of human (n = 11) and monkey (n = 2) origin bound avidly to acidic PRPs but deviated in their relative, although weak, capacities for binding to statherin and oral strains of A. naeslundii genospecies 1 bound either avidly (n = 2) or weakly (n = 4) to acidic PRPs. (ii) In preferential statherin binding, A. viscosus strains from rat or hamster plaque (n = 6) and a case of human actinomycosis bound statherin avidly and preferentially. (iii) In statherin-specific binding, strains of A. naeslundii genospecies 1 from septic and other human infections displayed low-avidity binding only to statherin (n = 6). The different ligand specificities were confirmed by determining the binding of representative strains to acidic PRP-1 and statherin at various concentrations used to coat hydroxyapatite surfaces (Fig. 1).
TABLE 1.
Ligand specificity, fimbria genes, and tropism of Actinomyces spp.
| Species | Straing | Adhesion type | Binding toa:
|
DNA hybridizationb for:
|
Fluorescence signal scorec with:
|
Origind (reference or sourcef) | Coagg.e group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRP-1 | Statherin | orf3 | orf2 | orf1 | fimP | orf4 | orf5 | orf6 | FimP | Type 2:1 antisera | |||||
| Preferential PRP binding | |||||||||||||||
| A. naeslundii genospecies 2 | Strains (n = 9) | PRP ≫ statherin | 43.2 ± 14.8 | 7.4 ± 13.6 | 5 | 6 | 5 | 6 | 6 | 3 | 6 | 3 | 0 | Human mouth (16) | |
| LY7 | PRP ≫ statherin | 56.4 | 11.9 | 5 | 6 | 4 | 6 | 6 | 3 | 6 | 3 | 0 | Human mouth (16) | F | |
| T14V | PRP ≫ statherin | 53.4 | 24.5 | 6 | 6 | 6 | 6 | 6 | 4 | 6 | 4 | 0 | Human mouth (16) | A | |
| M4356 | PRP > statherin | 53.0 | 31.0 | 6 | 6 | 6 | 6 | 6 | 4 | 6 | 2 | 0 | Monkey mouth (50) | ||
| M4301 | PRP > statherin | 38.8 | 28.6 | 6 | 6 | 6 | 6 | 6 | 5 | 6 | 2 | 4 | Monkey mouth (50) | ||
| A. naeslundii genospecies 1 | Pn-19-N | PRP ≫ statherin | 68.1 | 7.5 | 6 | 6 | 6 | 6 | 6 | 0 | 6 | 3 | 3 | Human mouth (16) | |
| Pn-6-N | PRP ≫ statherin | 62.5 | 6.9 | 3 | 5 | 5 | 4 | 6 | 0 | 5 | 2 | 2 | Human mouth (16) | ||
| Strains (n = 4) | Low avidity to PRP | 13.3 ± 7.8 | 0.5 ± 0.5 | 3 | 5 | 5 | 5 | 6 | 1 | 6 | 0 | 4 | Human mouth (16) | C/D | |
| Preferential statherin binding | |||||||||||||||
| A. viscosus | Strains (n = 6) | Statherin ≫ PRP | 23.7 ± 16.2 | 55.2 ± 6.5 | 0 | 4 | 4 | 3 | 6 | 1 | 6 | 3 | 2 | Rat or hamster mouth (50) | |
| ATCC 19246 | Statherin ≫ PRP | 28.0 | 59.7 | 0 | 4 | 4 | 3 | 5 | 0 | 5 | 2 | 2 | Human actinomycosis (ATCC) | ||
| Statherin-specific binding | |||||||||||||||
| A. naeslundii genospecies 1 | Strains (n = 5) | Low avidity to statherin | 3.7 ± 1.5 | 26.3 ± 7.7 | 1 | 0 | 0 | 0 | 5 | 0 | 5 | 0 | 4 | Human blood, synovial fluid (CCUG) | |
| ATCC 12104 | Low avidity to statherin | 4.0 | 25.7 | 1 | 0 | 0 | 1 | 6 | 0 | 6 | 0 | 4 | Human sinus (ATCC) | B | |
Adhesion of bacteria to a fixed concentrations (5 to 6 μg/ml) of PRP-1 and statherin used to coat hydroxyapatite beads. Statherin-specific binding was assayed at 25 μg of proteins/ml due to no binding at the low protein amount. The numbers of adhering bacteria are given as percentages of added bacteria. Mean values ± standard deviations are given.
Slot blot DNA hybridization under high-stringency conditions with full-length DNA probes designed from the type 1 fimbria genes (orf1 to 6 and fimP) in A. naeslundii T14V.
Immunofluorescence staining of whole bacterial cells with FimP and type 2:1 fimbria-specific antisera. Scores 0 and 1, no or weak staining, respectively; scores 2, 3, and 4, moderate-, high-, and very high-intensity staining, respectively (see Materials and Methods).
The sources of the strains are given in Materials and Methods.
Coaggregation (coagg.) groups A, B, C or D, and F represent the coaggregation properties found in Actinomyces isolates from the human mouth.
ATCC, American Type Culture Collection.
Strains, individual strains grouped together based on virtually identical reactivity patterns.
FIG. 1.
Binding of Actinomyces strains to PRP-1 and statherin used to coat hydroxyapatite beads.
Type 1 fimbria genes among Actinomyces strains with different ligand specificities and tropisms.
To investigate the type 1 fimbria genes involved in generating different Actinomyces ligand specificities, bacterial DNA was hybridized with DNA probes specific to the type 1 fimbria genes (orf1 to -6 and fimP) of A. naeslundii strain T14V with preferential acidic-PRP binding (Table 1). Three major gene patterns, on the basis of ligand specificity, were detected: (i) A. naeslundii genospecies 1 and 2 strains with preferential acidic-PRP binding hybridized strongly with all DNA probes, except that genospecies 1 strains virtually lacked hybridization with orf5 and hybridized moderately with orf3; (ii) A. viscosus strains with preferential statherin binding hybridized strongly with the orf4- and orf6-specific probes, moderately with the orf2, orf1, and fimP probes, and weakly, if at all, with the orf3 and orf5 probes; and (iii) the human A. naeslundii septic strains with statherin-specific binding hybridized strongly only with the orf4 and orf6 probes. Thus, the orf4 and orf6 gene regions are highly conserved among Actinomyces spp. with different ligand specificities and tropisms.
Type 1 fimbria genes in A. viscosus strain 19246 with preferential statherin binding.
To further investigate the type 1 fimbria genes related to preferential statherin binding, we cloned and sequenced a fimP-containing 9,439-bp gene cluster from A. viscosus strain 19246 (Fig. 2; GenBank accession no. AF106034). The 9,439-bp fragment contained four open reading frames (ORFs) (orfA, fimP, orfB, and orfC) and displayed an overall 81.3% nucleotide sequence identity to the type 1 fimbrial gene cluster (orf1 to -6 and fimP) from A. naeslundii strain T14V, with preferential acidic-PRP binding. The predicted orfA, fimP, orfB, and orfC were homologous to orf3-1, fimP, orf4-5, and orf6, respectively, and had a high G+C content (>60%), typical of the genus Actinomyces.
Sequence analysis of ORFs encoding type 1 fimbria with preferential statherin binding.
orfA contained a putative ribosome-binding site 10 bp upstream of an ATG start codon and showed a 79.7% sequence identity to orf3, orf2, and orf1 (Fig. 2). orfA encoded a hydrophilic 1,411-residue protein with a putative N-terminal signal peptide (residues 1 to 45) and a cell wall-anchoring LPLSG domain (residues 1371 to 1375) followed by a transmembrane helix. The C-terminal region of OrfA displayed a 39 to 41% sequence similarity to regions of the FimP subunit protein. OrfA showed a 35 to 40% sequence similarity to (i) usher-like proteins, (ii) autotransporters (IgA protease and pullulanase), (iii) extracellular organelle assembly proteins (K88 minor fimbrial subunit and major pilin protein), and (iv) glycosidases (muramidase, neuraminidase, and pullulanase).
fimP encoded a 535-residue FimP subunit protein with an N-terminal signal peptide, a hydrophilic protein core with four cysteine residues, and a C-terminal LPLTG domain followed by a transmembrane domain (28). FimP displayed a 35 to 40% sequence similarity to the type 2 fimbria FimA subunit protein, Streptococcus dysgalactiae M-like and IgG binding proteins, Neisseria meningitidis transferrin binding protein, and Haemophilus influenzae adhesin.
orfB displayed a putative ribosome-binding site 12 bp upstream of a GTG start codon and 80.8% sequence homology to the corresponding regions of orf4 and two-thirds of the 5′ end of orf5. A 177-bp segment corresponding to the 3′ end of orf5 was deleted in orfB. The 387-residue OrfB protein contained two transmembrane domains (residues 52 to 74 and 301 to 323) and an Arg-Gly-Asp (RGD) tripeptide (residues 30 to 32). OrfB had a 42% sequence similarity to Orf365 of type 2 fimbriae and a 182-residue region with an LITC motif (residues 256 to 259) and 39% sequence similarity to Staphylococcus aureus sortase (30, 48).
orfC displayed a putative ribosome-binding site 6 bp upstream of an ATG start codon and 87.7% sequence homology to orf6 and a 0.3-kb fragment downstream to orf6. The 254-residue OrfC protein displayed similarities to integral membrane proteins, and its N-terminal 139 residues resembled a PAP2 (type 2 phosphatidic acid phosphatase) domain, a sequence motif shared by bacterial and mammalian acid phosphatases. OrfC displayed 40% sequence similarity to Pseudomonas putida type 4 prepilin-like protein leader peptide-processing enzyme and Mycobacterium lipoprotein signal peptidase.
Antiserum detection of predicted ORFs.
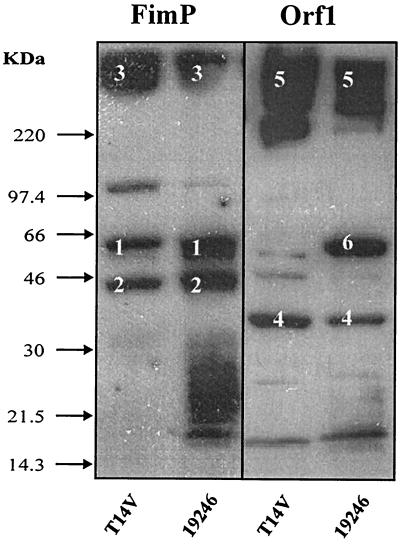
To detect the presence of predicted ORFs and posttranslational processing of the type 1 fimbrial proteins, whole-cell sonicates from A. viscosus strain 19246 and A. naeslundii strain T14V were analyzed in Western blots using FimP- and Orf1 (C-terminal OrfA domain)-specific antisera (Fig. 3). The FimP antiserum detected high-molecular-weight (hmw) components and 65- and 45-kDa proteins in both strains, indicating polymerized (hmw), native (65-kDa) and truncated (45-kDa) FimP proteins, respectively. The Orf1 antiserum detected hmw components in both strains, suggesting the presence of Orf1 (and OrfA) in polymerized fimbriae. In addition, the Orf1 antiserum detected a 39-kDa protein in both strains, indicating proteolytic processing of OrfA to a Orf1-like 39-kDa protein in strain 19246, as well as a 65-kDa protein in strain 19246, indicating larger OrfA fragments.
FIG. 3.
Detection of FimP- and Orf1-like proteins in A. viscosus strain 19246 and A. naeslundii strain T14V by specific antisera. Whole-cell proteins were separated by SDS-PAGE, blotted to membranes, and reacted with FimP- and Orf1-specific antisera. Marked proteins are native (1), truncated (2), and polymerized (3) FimP and native (4) and polymerized (5) Orf1 in strain T14V and truncated (4 and 6) and polymerized (5) OrfA in strain 19246. Arrows, molecular weight markers.
Type 1 and type 2 fimbria patterns on Actinomyces spp. with different ligand specificities.
Immunofluorescence staining with type 1 (FimP) and type 2:1 fimbria-specific antisera showed that the investigated strains carried either type 1, type 2, or both types of fimbriae on their surfaces (Table 1). The type 2:1 antisera detected type 2 fimbriae on all strains except for A. naeslundii genospecies 2 strains that harbor a structurally different FimA subunit protein (17). The type 1 (FimP) antisera detected type 1 fimbriae on all strains with high-avidity binding to acidic PRP or statherin but not on those strains with low-avidity binding to statherin or acidic PRPs. Thus, FimP and type 2:1 antisera may detect some, but not all, heterogeneity in ligand binding mediated by structural variations in type 1 and type 2 fimbriae.
Actinomyces strains with preferential acidic-PRP and statherin binding represent phylogenetically different lineages.
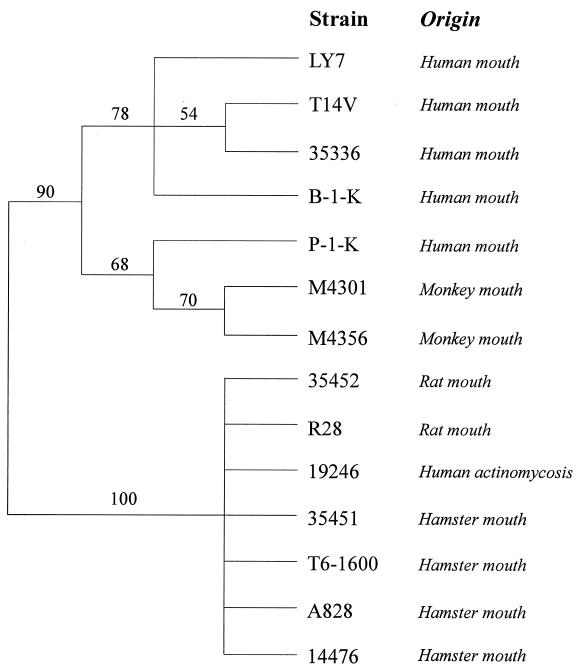
To investigate the evolutionary relatedness of strains with different ligand specificities and tropisms, Actinomyces strains were subjected to RFLP analysis with a fimP gene probe designed from strain T14V (Fig. 4). The RFLP dendrogram revealed clustering of oral human and monkey strains with preferential acidic-PRP binding, while rat and hamster strains (and strain 19246 from a human case of actinomycosis) with preferential statherin binding clustered together.
FIG. 4.
Clustering of oral strains of Actinomyces strains of human or monkey and rat or hamster origin by RFLP analyses using fimP as a probe. Confidence probability values are shown at major branches.
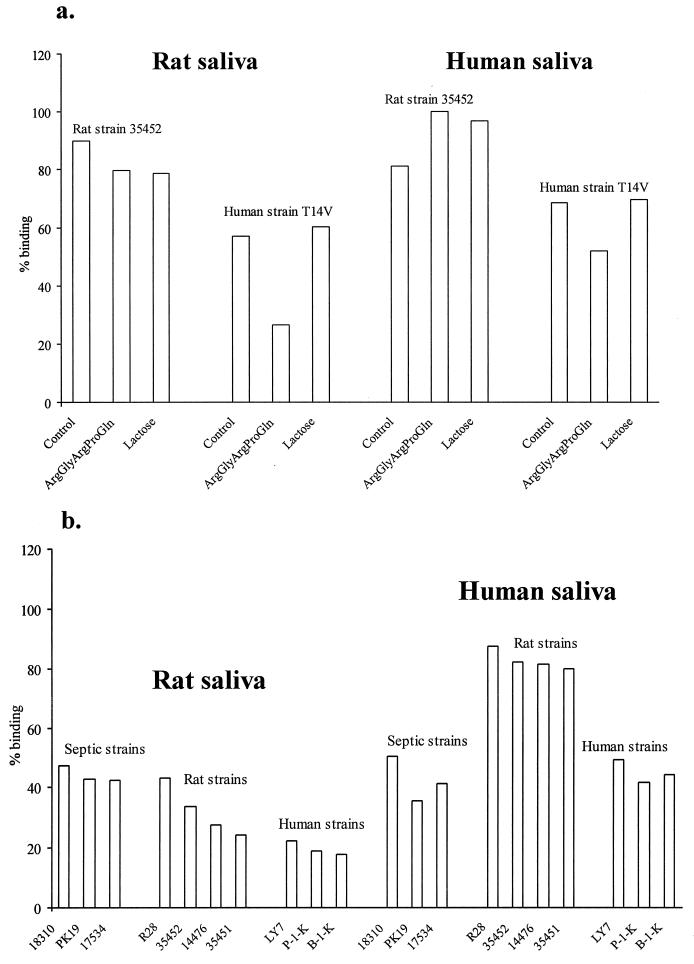
Rat and human saliva exhibit high Actinomyces binding irrespective of ligand specificity.
Both A. naeslundii strain T14V (human mouth) and A. viscosus strain 35452 (rat mouth) bound avidly to rat and human saliva (Fig. 5a). ArgGlyArgProGln, a type 1 fimbria inhibitor (27), but not lactose, a type 2 fimbria inhibitor, partially blocked the binding of strain T14V to rat and human saliva, indicating type 1 fimbria-mediated binding properties. Neither of the two inhibitors blocked the binding of strain 35452, confirming its variant type 1 fimbria binding specificity.
FIG. 5.
Adhesion of Actinomyces strains to rat and human saliva used to coat hydroxyapatite beads. (a) Adhesion without (control) and with addition of the type 1 fimbria inhibitor (ArgGlyArgProGln) and type 2 fimbria inhibitor (lactose); (b) adhesion of Actinomyces strains from the rat and human mouths and from septic infections in humans.
We next investigated the capacity of rat and human saliva to bind septic rat or hamster and human Actinomyces strains with variant ligand specificities (Fig. 5b). Both rat and human saliva displayed high binding capacity of Actinomyces strains irrespective of ligand specificity. Human saliva even bound oral strains of rat or hamster origin better than those of human origin. Statherin was detected in human, but not in rat, saliva using statherin-specific antisera in Western blot experiments (data not shown).
DISCUSSION
This paper shows a diversity of acidic-PRP and statherin adhesion types among commensal and potentially pathogenic Actinomyces strains. Those adhesion types typical of human commensal strains bound acidic PRPs preferentially, while those typical of rat and hamster hosts and human infections bound statherin preferentially. Binding to statherin (originally detected in A. viscosus strain 19246 from a human case of cervicofacial actinomycosis [12]) may be involved in human infections. A. naeslundii 12104, isolated from a human case of sinusitis (10), and potentially invasive Porphyromonas gingivalis and Candida albicans, bind also to statherin. Moreover, statherin blocks adhesion of C. albicans to epithelial cells (21). In human saliva, statherin may therefore protect against potentially invasive strains recognizing statherin-mimicking epithelial cell surface ligands. The avid adhesion of statherin-binding strains to rat saliva despite its lack of statherin and the weak adhesion of human septic strains to statherin argue also for an involvement of yet-uncharacterized statherin-mimicking molecules.
The present findings also suggest a correlation between ligand specificity and presence of type 1 fimbria genes. (i) While A. naeslundii genospecies 2 strains with preferential acidic-PRP binding harbored highly conserved type 1 fimbria genes (orf1 to -6 and fimP), genospecies 1 strains displayed divergent orf5 and orf3 genes. (ii) Strains of A. viscosus with preferential statherin binding seemed to carry a homologous gene cluster of four genes (orfA, fimP, orfB, and orfC). Additional arguments for two major evolutionary lines of type 1 fimbriae came from RFLP analyses based on fimP, clustering human and monkey and rat and hamster strains into phylogenetically different groups. (iii) The human septic (and 12104) strains with statherin-specific binding exhibited only conserved orf4 and orf6 genes. The conserved orf4 and orf6 gene regions may encode intracellular sortase-, usher-, or chaperone-like functions, while the divergent orf5 and orf3 gene regions may encode surface-localized functions subject to selective external pressures.
The present findings may indicate a diversity of A. naeslundii and A. viscosus subpopulations that have evolved specific ligand binding properties to fit different ecological niches. Notably, A. naeslundii and A. viscosus harbor taxonomically distinct subpopulations with huge phenotypic and serologic variation (23). Moreover, reference strains of Actinomyces coaggregation groups A, B, C or D, and F, which exhibit type 2 fimbria-mediated coaggregations with different streptococci (25), belonged to different acidic-PRP and statherin adhesion types. Accordingly, the type 1 (FimP) and type 2 (FimA) antisera detected either type 1, type 2, or both fimbriae on all investigated strains. In this respect, it is noteworthy that studies with E. coli type 1 fimbriae suggested that variations in ligand specificity may transform commensal phenotypes to pathogenic ones by changing their ecological niches (42).
The present findings provide some information on the biogenesis and assembly of adhesive proteins in gram-positive bacteria. The conserved orfB gene encodes a sortase homolog, as evidenced by its LITC motif and 39% sequence similarity to the S. aureus sortase (a transpeptidase anchoring LPXTG-containing proteins to the cell walls of gram-positive bacteria) (30, 48). Many gram-positive bacteria contain sortase homologs (35), and inactivation of sortase in S. aureus and Streptococcus gordonii prevented bacterial adhesion and infections (5, 29). Furthermore, inactivation of orf365, a homolog of orfB encoding sortase, abolished type 2 fimbria polymerization and FimA surface localization in Actinomyces (51), suggesting that the OrfB sortase guides the LPXTG-containing OrfA and FimP proteins during type 1 fimbria biogenesis and assembly.
It is likely that the prepilin peptidase-like OrfC protein cleaves off the signal peptides from FimP and OrfA during their secretion and that FimP and OrfA interact with the OrfB sortase. OrfA, corresponding to Orf3, Orf2, and Orf1, displays sequence similarities to ushers, autotransporters, and extracellular organelle assembly proteins and contains a C-terminal FimP-homologous region. Hypothetically, OrfA could provide a platform for fimbria assembly. The C-terminal FimP-homologous region of OrfA (Orf1-like region) could be cleaved off from OrfA and integrated into polymerized fimbriae, as suggested by the observation that the Orf1-specific antisera detected truncated Orf1-like proteins and polymerized fimbriae in strain 19246. Integrated in fimbriae, OrfA-derived proteins, either the Orf1-like C-terminal or the Orf3-like N-terminal domains, could possess adhesive properties. Notably, the orf3 gene region correlated with binding specificity. Finally, the fimP and fimA gene structures also correlate with binding specificity (17, 28), and therefore the structural subunit proteins are alternative adhesin candidates.
In conclusion, fimbria-mediated adhesion of Actinomyces spp. may provide a powerful model to (i) characterize proteins involved in biogenesis and assembly of adhesive organelles in gram-positive bacteria and (ii) understand the role of commensal bacteria and adhesion types in health and chronic infectious diseases.
ACKNOWLEDGMENTS
This study was supported by grants from the Swedish Medical Research Council (10906), the Swedish Dental Society, and the County Council of Västerbotten.
Xiao Ru Wang, National Institute for Working Life, Sweden, is acknowledged for assistance with the RFLP computer analyses.
REFERENCES
- 1.Amano A, Sojar H T, Lee J Y, Sharma A, Levine M J, Genco R J. Salivary receptors for recombinant fimbrillin of Porphyromonas gingivalis. Infect Immun. 1994;62:3372–3380. doi: 10.1128/iai.62.8.3372-3380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedi G S, Bedi S K. Purification and characterization of rat parotid glycosylated, basic and acidic proline-rich proteins. Prep Biochem. 1995;25:119–132. doi: 10.1080/10826069508010115. [DOI] [PubMed] [Google Scholar]
- 3.Bennick A. Salivary proline-rich proteins. Mol Cell Biochem. 1982;45:83–99. doi: 10.1007/BF00223503. [DOI] [PubMed] [Google Scholar]
- 4.Bibb M J, Findlay P R, Johnson M W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984;30:157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- 5.Bolken T, Franke C, Jones K, Zeller G, Jones C, Dutton E, Hruby D. Inactivation of the srtA gene in Streptococcus gordonii inhibits cell wall anchoring of surface proteins and decreases in vitro and in vivo adhesion. Infect Immun. 2001;69:75–80. doi: 10.1128/IAI.69.1.75-80.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon R D, Nand A K, Jenkinson H F. Adherence of Candida albicans to human salivary components adsorbed to hydroxylapatite. Microbiology. 1995;141:213–219. doi: 10.1099/00221287-141-1-213. [DOI] [PubMed] [Google Scholar]
- 7.Carlen A, Bratt P, Stenudd C, Olsson J, Strömberg N. Agglutinin and acidic proline-rich protein receptor patterns may modulate bacterial adherence and colonization on tooth surfaces. J Dent Res. 1998;77:81–90. doi: 10.1177/00220345980770011301. [DOI] [PubMed] [Google Scholar]
- 8.Cisar J O, Vatter A E, Clark W B, Curl S H, Hurst Calderone S, Sandberg A L. Mutants of Actinomyces viscosus T14V lacking type 1, type 2, or both types of fimbriae. Infect Immun. 1988;56:2984–2989. doi: 10.1128/iai.56.11.2984-2989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole A M, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67:3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman R, Georg L, Rozzell A. Actinomyces naeslundii as an agent of human actinomycosis. Appl Microbiol. 1969;18:420–426. doi: 10.1128/am.18.3.420-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellen R P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976;14:1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerencser M, Slack J. Identification of human strains of Actinomyces viscosus. Appl Microbiol. 1969;18:80–87. doi: 10.1128/am.18.1.80-87.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbons R J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989;68:750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons R J, Hay D I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988;56:439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbons R J, Hay D I, Schlesinger D H. Delineation of a segment of adsorbed salivary acidic proline-rich proteins which promotes adhesion of Streptococcus gordonii to apatitic surfaces. Infect Immun. 1991;59:2948–2954. doi: 10.1128/iai.59.9.2948-2954.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallberg K, Hammarström K-J, Falsen E, Dahlen G, Gibbons R, Hay D I, Strömberg N. Actinomyces naeslundii genospecies 1 and 2 express different binding specificities to N-acetyl-β-D-galactosamine, whereas Actinomyces odontolyticus expresses a different binding specificity in colonizing the human mouth. Oral Microbiol Immunol. 1998;13:327–336. doi: 10.1111/j.1399-302x.1998.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 17.Hallberg K, Holm C, Öhman U, Strömberg N. Actinomyces naeslundii displays variant fimP and fimA fimbrial subunit genes corresponding to different types of acidic proline-rich protein and beta-linked galactosamine binding specificity. Infect Immun. 1998;66:4403–4410. doi: 10.1128/iai.66.9.4403-4410.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay D I, Bennick A, Schlesinger D H, Minaguchi K, Madapallimattam G, Schluckebier S K. The primary structures of six human salivary acidic proline-rich proteins (PRP-1, PRP-2, PRP-3, PRP-4, PIF-s and PIF-f) Biochem J. 1988;255:15–21. doi: 10.1042/bj2550015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hultgren S J, Abraham S, Caparon M, Falk P, de St. Geme J W, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa J, Hotta K. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol Lett. 1999;174:251–253. doi: 10.1111/j.1574-6968.1999.tb13576.x. [DOI] [PubMed] [Google Scholar]
- 21.Johansson I, Bratt P, Hay D I, Schluckebier S, Strömberg N. Adhesion of Candida albicans, but not Candida krusei, to salivary statherin and mimicking host molecules. Oral Microbiol Immunol. 2000;15:112–118. doi: 10.1034/j.1399-302x.2000.150207.x. [DOI] [PubMed] [Google Scholar]
- 22.Johansson I, Linder J, Bratt P. Comparison of saliva secretion rate and composition in the rat using a pentobarbital or a neuroleptanalgesic type of anaesthesia. Caries Res. 1989;23:75–77. doi: 10.1159/000261160. [DOI] [PubMed] [Google Scholar]
- 23.Johnson J L, Moore L V, Kaneko B, Moore W E. Actinomyces georgiae sp. nov., Actinomyces gerencseriae sp. nov., designation of two genospecies of Actinomyces naeslundii, and inclusion of A. naeslundii serotypes II and III and Actinomyces viscosus serotype II in A. naeslundii genospecies 2. Int J Syst Bacteriol. 1990;40:273–286. doi: 10.1099/00207713-40-3-273. [DOI] [PubMed] [Google Scholar]
- 24.Jordan H V. Pathogenicity of Actinomyces species. In: Genco R J, Mergenhagen S E, editors. Host-parasite interactions in periodontal disease. Washington, D.C.: American Society for Microbiology; 1982. pp. 169–178. [Google Scholar]
- 25.Kolenbrander P E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- 26.Lamkin M S, Oppenheim F G. Structural features of salivary function. Crit Rev Oral Biol Med. 1993;4:251–259. doi: 10.1177/10454411930040030101. [DOI] [PubMed] [Google Scholar]
- 27.Li T, Bratt P, Jonsson A P, Ryberg M, Johansson I, Griffiths W J, Bergman T, Strömberg N. Possible release of an ArgGlyArgProGln pentapeptide with innate immunity properties from acidic proline-rich proteins by proteolytic activity in commensal Streptococcus and Actinomyces species. Infect Immun. 2000;68:5425–5429. doi: 10.1128/iai.68.9.5425-5429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li T, Johansson I, Hay D I, Strömberg N. Strains of Actinomyces naeslundii and Actinomyces viscosus exhibit structural variant fimbrial subunit proteins and bind to different peptide motifs in salivary proteins. Infect Immun. 1999;67:2053–2059. doi: 10.1128/iai.67.5.2053-2059.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazmanian S K, Liu G, Jensen E R, Lenoy E, Schneewind O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci USA. 2000;97:5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazmanian S K, Liu G, Ton That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 31.Mehansho H, Ann D K, Butler L G, Rogler J, Carlson D M. Induction of proline-rich proteins in hamster salivary glands by isoproterenol treatment and an unusual growth inhibition by tannins. J Biol Chem. 1987;262:12344–12350. [PubMed] [Google Scholar]
- 32.Mouton C, Hammond P G, Slots J, Reed M J, Genco R J. Identification of Bacteroides gingivalis by fluorescent antibody staining. Ann Microbiol (Paris) 1981;132b:69–83. [PubMed] [Google Scholar]
- 33.Muenzer J, Bildstein C, Gleason M, Carlson D M. Purification of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979;254:5623–5628. [PubMed] [Google Scholar]
- 34.Nyvad B, Kilian M. Microflora associated with experimental root surface caries in humans. Infect Immun. 1990;58:1628–1633. doi: 10.1128/iai.58.6.1628-1633.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pallen M, Lam A, Antonio M, Dunbar K. An embarrassment of sortases—a richness of substrates? Trends Microbiol. 2001;9:97–101. doi: 10.1016/s0966-842x(01)01956-4. [DOI] [PubMed] [Google Scholar]
- 36.Putnins E E, Bowden G H. Antigenic relationships among oral Actinomyces isolates, Actinomyces naeslundii genospecies 1 and 2, Actinomyces howellii, Actinomyces denticolens, and Actinomyces slackii. J Dent Res. 1993;72:1374–1385. doi: 10.1177/00220345930720100601. [DOI] [PubMed] [Google Scholar]
- 37.Raymond A, Smego J, Foglia G. Actinomycosis. Clin Infect Dis. 1998;26:1255–1263. doi: 10.1086/516337. [DOI] [PubMed] [Google Scholar]
- 38.Sabatini L M, Warner T F, Saitoh E, Azen E A. Tissue distribution of RNAs for cystatins, histatins, statherin, and proline-rich salivary proteins in humans and macaques. J Dent Res. 1989;68:1138–1145. doi: 10.1177/00220345890680070101. [DOI] [PubMed] [Google Scholar]
- 39.Schlesinger D H, Hay D I. Primary structure of the active tryptic fragments of human and monkey salivary anionic proline-rich proteins. Int J Pept Protein Res. 1981;17:34–41. doi: 10.1111/j.1399-3011.1981.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 40.Schlesinger D H, Hay D I, Levine M J. Complete primary structure of statherin, a potent inhibitor of calcium phosphate precipitation, from the saliva of the monkey, Macaca arctoides. Int J Pept Protein Res. 1989;34:374–380. doi: 10.1111/j.1399-3011.1989.tb00705.x. [DOI] [PubMed] [Google Scholar]
- 41.Slots J, Hafstrom C, Rosling B, Dahlen G. Detection of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in subgingival smears by the indirect fluorescent-antibody technique. J Periodontal Res. 1985;20:613–620. doi: 10.1111/j.1600-0765.1985.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 42.Sokurenko E V, Chesnokova V, Dykhuizen D E, Ofek I, Wu X R, Krogfelt K A, Struve C, Schembri M A, Hasty D L. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci USA. 1998;95:8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spielman A I, Bernstein A, Hay D I, Blum M, Bennick A. Purification and characterization of a rabbit salivary protein, a potent inhibitor of crystal growth of calcium phosphate salts. Arch Oral Biol. 1991;36:55–63. doi: 10.1016/0003-9969(91)90054-x. [DOI] [PubMed] [Google Scholar]
- 44.Stenudd C, Nordlund Å, Ryberg M, Johansson I, Källestål C, Strömberg N. Adhesion molecules for bacterial colonization may determine host susceptibility and resistance to dental caries. Caries Res. 1998;32:268. . (Abstract.) [Google Scholar]
- 45.Strömberg N, Ahlfors S, Boren T, Bratt P, Hallberg K, Hammarström K J, Holm C, Johansson I, Jarvholm M, Kihlberg J, Li T, Ryberg M, Zand G. Anti-adhesion and diagnostic strategies for oro-intestinal bacterial pathogens. Adv Exp Med Biol. 1996;408:9–24. doi: 10.1007/978-1-4613-0415-9_2. [DOI] [PubMed] [Google Scholar]
- 46.Sundqvist G, Reuterving C O. Isolation of Actinomyces israelii from periapical lesion. J Endod. 1980;6:602–606. doi: 10.1016/S0099-2399(80)80021-5. [DOI] [PubMed] [Google Scholar]
- 47.Tannock G V. Normal microflora. An introduction to microbes inhabiting the human body. London, United Kingdom: Chapman and Hall; 1995. [Google Scholar]
- 48.Ton That H, Liu G, Mazmanian S K, Faull K F, Schneewind O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc Natl Acad Sci USA. 1999;96:12424–12429. doi: 10.1073/pnas.96.22.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittaker C J, Klier C M, Kolenbrander P E. Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol. 1996;50:513–552. doi: 10.1146/annurev.micro.50.1.513. [DOI] [PubMed] [Google Scholar]
- 50.Yeung M K. Conservation of an Actinomyces viscosus T14V type 1 fimbrial subunit homolog among divergent groups of Actinomyces spp. Infect Immun. 1992;60:1047–1054. doi: 10.1128/iai.60.3.1047-1054.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeung M K, Donkersloot J A, Cisar J O, Ragsdale P A. Identification of a gene involved in assembly of Actinomyces naeslundii T14V type 2 fimbriae. Infect Immun. 1998;66:1482–1491. doi: 10.1128/iai.66.4.1482-1491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeung M K, Ragsdale P A. Synthesis and function of Actinomyces naeslundii T14V type 1 fimbriae require the expression of additional fimbria-associated genes. Infect Immun. 1997;65:2629–2639. doi: 10.1128/iai.65.7.2629-2639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu P L, Bixler D, Goodman P A, Azen E A, Karn R C. Human parotid proline-rich proteins: correlation of genetic polymorphisms to dental caries. Genet Epidemiol. 1986;3:147–152. doi: 10.1002/gepi.1370030302. [DOI] [PubMed] [Google Scholar]