Abstract
Background
Two related lifestyle behaviors associated with sleep disturbance are sedentary behavior and physical exercise participation. We aimed to use a population-based study to disentangle the relationships between sedentary behavior, exercise, and sleep disturbance based on blood-cell-based inflammatory biomarkers.
Methods
A total of 22,599 participants from the National Health and Nutrition Examination Survey (NHANES) were included in the analyses. Sleep disturbance was assessed according to the NHANES questionnaire. Exercise participation ansd sedentary behavior were evaluated by the global physical activity questionnaire. The inflammatory biomarkers in the examination were white blood cell (WBC) count, neutrophil count (NEU), neutrophil-to-lymphocyte ratio (NLR), and systemic immune inflammation index (SII). A complex multistage sampling design and weighted multivariable logistic regression were applied for further analysis. Mediation models were constructed to figure out the mediating role of inflammatory biomarkers.
Results
The weighted prevalence of sleep disturbance was 24.17%. Sedentary behavior and exercise were associated with sleep disturbance after full adjustment [for sedentary behavior, OR (95% CI): 1.261 (1.154, 1.377); for exercise, OR (95% CI): 0.849 (0.757, 0.953)]. In severe sedentary behavior groups, the mitigation effect of exercise on sleep disturbance was observed [OR (95% CI): 0.687 (0.551, 0.857)]. For the mechanism, strong associations were detected between inflammatory biomarkers and sleep disturbance. Mediation analysis showed that WBC, NEU, NLR, and SII mediated the statistical association between sedentary behavior and sleep disturbance with proportions (%) of 2.09, 2.27, 1.76, and 0.82, respectively.
Conclusions
Our data suggested that sedentary behavior was a risk factor for sleep disturbance. Blood-cell-based inflammatory biomarkers were an easily accessible and cost-effective strategy for identifying sleep disturbance and also significantly mediated the association between sedentary behavior and sleep disturbance. Exercise was proved to be effective in severe sedentary behavior groups to improve sleep disturbance symptoms, while the internal mechanism needed further exploration.
Keywords: population-based study, sleep disturbance, sedentary behavior, exercise, blood-cell based inflammatory biomarkers
Introduction
Sleep is essential for both physical and mental health. However, the high prevalence of sleep disturbance is becoming a serious public health issue nowadays, affecting 30%–50% of the global population (1, 2). Additionally, evidence also demonstrates that sleep disturbances are associated with approximately $3,400-5,200/person/year spent on healthcare burden (3). Furthermore, it has been suggested that sleep disturbance and bad sleep quality are associated with a number of chronic diseases including diabetes (4), hypertension (5), depression (6), and obesity (7). There is abundant evidence that lifestyle factors significantly affect the prevalence of sleep problems (8), and reducing sedentary time and doing more exercises are the necessary modifications to benefit people.
When it comes to the influence of sedentary behavior, existing literature tended to show that sedentary behavior was associated with an elevated risk of insomnia and sleep disturbances (9–11). In detail, sedentary behavior was defined by a sitting or reclining posture and low-energy expenditure, such as screen time, driving, reading, and study time, which has been regarded as an independent health risk factor in modern society (12, 13). However, the potential mechanisms underpinning these observations were not well understood. Studies found that prolonged sedentary behavior led to a high body mass index score and obesity, which might play a prominent role in the inflammation process (14, 15). The literature also detected that sedentary behavior was associated with mental and psychological problems such as anxiety and depression, which further contributed to sleep problems (16). Additionally, one of the best ways to reduce sedentary time was to spare more time for physical activity and exercise.
Contrary to sedentary behavior, regular exercise provided a wide variety of health benefits and built a fundamental base for fitness and longevity, including inducing beneficial effects to sleep quality. From the perspective of definition, physical exercise refers to any bodily movement produced by skeletal muscles that requires energy expenditure. Previous research (17) suggested that chronic regular exercise was positively related to sleep quality and psychological functioning. Current evidence also proved that a 6-month supervised exercise program was helpful for sleep respiratory disturbance index as well as sleep efficiency (18, 19). As for the mechanism, it seemed that exercise-induced cytokine response, which was predominantly an anti-inflammatory response, may explain part of the improvement of sleep (20). Although the underlying mechanisms that exercise improved the symptoms of sleep disturbance were not fully understood, previous studies demonstrated that exercise-induced multiple hormones, anti-inflammatory biomarkers, and brain neurogenesis promotion may play a role in the beneficial effect (21–23).
Building on the current findings mentioned above, it seemed that inflammatory potential played an important role in modulating lifestyle factors and sleep disturbance. Induced short-term sleep deprivation in a controlled laboratory setting has been proved to increase C-reactive protein (24). Simultaneously, there has been increasing evidence pointing to the correlation between sleep disturbance and inflammation biomarkers (25–27). There was a bidirectional relationship between sleep disturbances and physical activity status. Among them, inflammatory markers played an important role in modulating these changes. In recent years, blood-cell-based inflammatory parameters including white blood cell, neutrophil, and neutrophil-to-lymphocyte ratio have received more and more attention as they are predictive of several disorders such as blunted rest-activity rhythm (28), psychiatry problems (29), and cardiovascular anomalies (30). Nevertheless, these biomarkers only involved one or two types of immune inflammatory cells and might be difficult to comprehensively detect the inflammation status. The systemic immune inflammation index (SII), a novel and integrated inflammatory biomarker based on neutrophil, lymphocyte, and platelet counts, offered additional insight into the accurate reflection of inflammation status in multiple conditions (31, 32). However, the role of these blood-cell-based inflammatory parameters and SII in modulating sleep disturbance by sedentary behavior and physical activity remained largely unelucidated.
As noted above, research on the influence of sedentary behavior and physical exercise on sleep disturbance has focused on the biological mechanisms underlying these effects, a substantial interest in the role of novel inflammatory biomarkers. To the best of our knowledge, few studies, especially large population-based studies, explored the role of blood-cell-based inflammatory biomarkers in mediating sleep disturbance by sedentary behavior and physical exercise. This cross-sectional study aimed to i) explore the association between sedentary behavior, physical exercise, and sleep disturbance using samples from the National Health and Nutrition Examination Survey; ii) elucidate the mechanism between blood-cell-based inflammatory biomarkers and sleep disturbance; and iii) investigate whether these inflammatory biomarkers mediated the associations between sedentary behavior and physical exercise with sleep disturbance.
Methods
Study population
The data analyzed in this study were from the National Health and Nutrition Examination Survey (NHANES), a comprehensive population-based survey designed for the collection of civilian population data in the United States. Since 1999, the NHANES has collected data on approximately 10,000 people in 2-year cycles, and a multistage probability sampling design was applied to derive a representative sample of the non-institutionalized household Americans.
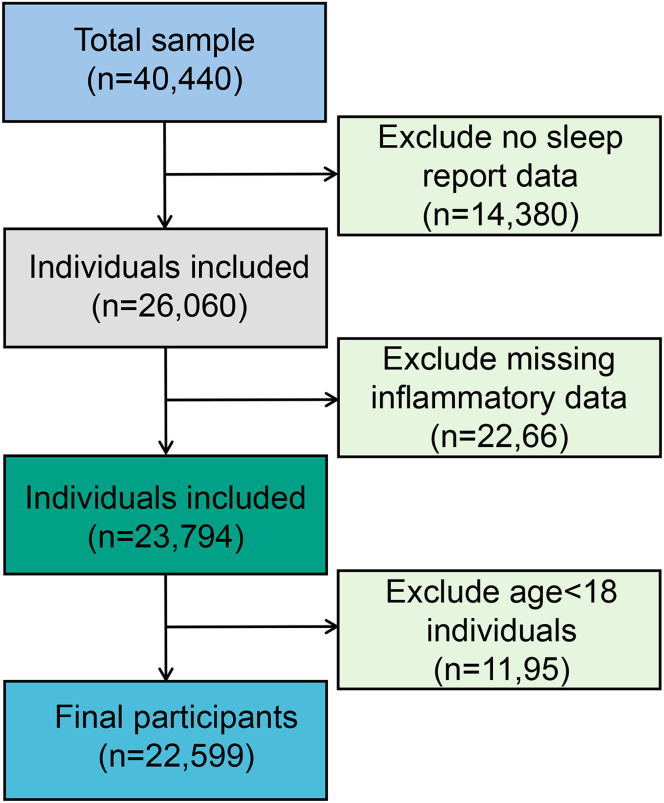
The present study population was from four cycles of “continuous NHANES” (2007/2008, 2009/2010, 2011/2012, 2013/2014). A total of 26,060 participants were included in the analysis after excluding participants with no sleep report data (n = 14,380). Subsequently, eligible participants needed to have complete data on inflammatory biomarkers. This resulted in an analytic sample of 23,794 survey participants. In addition, participants over 18 years of age were included in this study, leaving 22,599 samples for the final analysis (see Figure 1 ).
Figure 1.
Flowchart of the study design and participants excluded from the study.
Measures
Outcomes: Sleep disturbances
The outcome, sleep disturbance, was assessed by the NHANES questionnaire: “Have you ever told a doctor or other health professional that you have trouble sleeping?” The response categories of this question were “Yes,” “No,” “Refused,” and “Do not know.” For participants whose response was “Do not know” or “Refused,” their data were considered as a missing value.
Duration of sleep was assessed by two questions consistent in NHANES surveys conducted from 2007 to 2014. Sleep duration was assessed by the question “How much sleep do you usually get at night on weekdays or workdays?” The categorical range of sleep duration was distributed from 2 to 12. Referring to the recommendations of the National Sleep Foundation’s sleep duration (33), the categorical variable of sleep duration was divided into three groups: insufficient (<7 h/day), recommended (7~9 h/day), and excessive sleep time (>9 h/day).
Exposure: Exercise activity and sedentary behavior
As independent variables, the information on exercise activity and sedentary behavior was self-reported in the NHANES by the Physical Activity Questionnaire. Since the NHANES Physical Activity Questionnaire was changed after 2007, moderate and vigorous recreational activity status was used to assess exercise activity, and the unit was calculated by minute per week. The corresponding questions were as follows: “In a typical week, do you do any vigorous-intensity sports, fitness, or recreational activities that cause large increases in breathing or heart rate like running or basketball for at least 10 min continuously?” and “In a typical week, do you do any moderate-intensity sports, fitness, or recreational activities that cause a small increase in breathing or heart rate such as brisk walking, bicycling, swimming, or volleyball for at least 10 min continuously?” Additionally, when it comes to sedentary behavior, it was assessed by the question “How much time do you usually spend sitting on a typical day?” Specifically, sedentary behavior was defined as the time spent sitting at school or at home and getting to and from places, including sitting at a desk, traveling in a car or bus, reading, playing cards, watching television, or using a computer, which do not include time spent sleeping.
Referring to the World Health Organization Guidance on physical activity status (34, 35), more than 150 min per week of moderate-intensity aerobic exercise or 75 min/week of vigorous-intensity aerobic exercise or an equivalent combination of both moderate and vigorous exercise (1 min of vigorous-intensity exercise is equivalent to 2 min of moderate-intensity exercise) totaling at least 150 min per week was identified as vigorous/moderate exercise volume. Similarly, light exercise volume was defined as less than 150 min per week of moderate-intensity aerobic exercise or equivalent status. In addition, on the basis of self-reported sedentary behavior, sedentary status was categorized as follows: more than 480 min/day as severe and less than 480 min/day as mild sedentary behavior (36, 37).
Measurement of inflammatory biomarkers
According to the NHANES 2007–2014 cycle protocol, the Beckman Coulter method of counting and sizing, in combination with an automatic diluting and mixing device for sample processing, and a single beam photometer for hemoglobinometry were applied for counting blood cells from the peripheral blood samples obtained from the NHANES Mobile Examination Center (MEC).
In this study, the inflammatory biomarkers of the subjects participating in the examination were white blood cell (WBC) count, neutrophil (NEU) count, neutrophil-to-lymphocyte ratio (NLR), and SII. The unit of lymphocyte, neutrophil, and platelet counts was 109 cells/L. Referring to previous literature, the SII level was calculated as platelet count * neutrophil count/lymphocyte count (31).
Covariates
In this research, sociodemographic and lifestyle factors were collected. Referring to previous research (38), sociodemographic characteristics including age, sex, race/ethnicity, body mass index (BMI), marital status, education attainment, and family poverty index ratio (PIR, a ratio of family income to the poverty threshold) were adjusted. Smoking status (never, former, and current) and alcohol use status (never, moderate drinkers, heavy drinkers) were obtained from the cigarette use and alcohol use questionnaires, respectively. Smoking status was classified as never if subjects have never smoked, former if subjects have smoked at least 100 cigarettes in their lifetime, and current if subjects are smoking at the present time (39). Moderate drinkers were defined as 14 or fewer drinks/week for men or 7 or fewer drinks/week for women or 5 or fewer drinks/day in any single day during the past year for either men or women. Heavy/excessive drinkers were defined as more than 14 drinks/week for men or more than 7 drinks/week for women or 5 or more drinks/day in a single day at least once during the past year for either men or women (40).
Statistical analysis
In view of the complex multistage (strata and cluster) sampling design of the NHANES, the survey package of R 4.2.0 was used to conduct the weighted analysis. Sample weights from the MEC interviews were reweighted to merge 8 years of total survey data from the NHANES 2007 to 2014. The merged weights were represented as WT07-14 = (1/4) × WTMEC2YR07–08 + (1/4) × WTMEC2YR09–10 + (1/4) × WTMEC2YR11–12 + (1/4) × WTMEC2YR13–14.
Differences in baseline characteristics in the sleep disturbance and non-disturbance groups were compared using an independent sample t-test for continuous variables and the χ2 test for categorical variables. Multivariate logistic regression analysis was performed to examine the association between exercise, sedentary behavior, and sleep disturbance, with 95% confidence intervals (CI) and odds ratio (OR) calculated. In the crude model, no confounding factors were adjusted; in model 1, age, sex, and race/ethnicity were adjusted; in model 2, age, sex, race, body mass index, marital status, sleep duration, education attainment, poverty income ratio, smoking status, and alcohol drinking status were adjusted.
The mediation package of R software was used for mediation analysis. Through mediation analysis, we can calculate how much mediation effect needs to be generated. It was an ideal strategy to shed light on pathways and to provide statistical evidence for the mechanism analysis. In this study, direct effect represented the association between sedentary behavior, exercise, and sleep disturbance; indirect effect, i.e., the association between sedentary behavior, exercise, and sleep disturbance, was mediated by inflammatory markers; the proportion mediated indicated the percentage of the mediating effect. All analyses were performed using R (version 4.2.0, http://www.R-project.org, The R Foundation). P <0.05 indicated a significant difference.
Results
There were 22,599 participants eligible for our final analysis, and the weighted number of participants was 219,676,911. Among them, the weighted prevalence of sleep disturbance symptoms was 24.17%. The mean age of the participants was 48.01 ± 18.51 years, and 51.74% of the participants were women ( Table 1 ). More specifically, the weighted prevalence of sleep disturbance was higher in women, which took up 59.67% of all the participants. Additionally, the weighted prevalence of sleep disturbance stratified by age, sex, race, body mass index, marital status, sleep duration, education, smoking status, and alcohol drinking status was statistically significantly different (P < 0.05).
Table 1.
Weighted characteristics of the study population by sleep disturbance status.
| Variable | All participants | Non-sleep disturbance | Sleep disturbance | P-value | ||
|---|---|---|---|---|---|---|
| Age | <0.001 | |||||
| <44 | 45.80 (0.76) | 49.92 (0.87) | 34.01 (0.90) | |||
| [44, 60) | 29.68 (0.45) | 26.91 (0.48) | 37.59 (0.98) | |||
| ≥60 | 24.52 (0.53) | 23.17 (0.62) | 28.39 (0.72) | |||
| Sex | <0.001 | |||||
| Male | 48.26 (0.31) | 51.03 (0.48) | 40.33 (0.83) | |||
| Female | 51.74 (0.31) | 48.97 (0.48) | 59.67 (0.83) | |||
| Race/ethnicity | <0.001 | |||||
| Non-Hispanic White | 67.74 (1.76) | 64.82 (1.82) | 76.07 (1.62) | |||
| Non-Hispanic Black | 10.90 (0.87) | 11.27 (0.90) | 9.83 (0.85) | |||
| Mexican American | 8.63 (0.91) | 9.89 (0.98) | 5.05 (0.73) | |||
| Other race/ethnicity | 12.73 (0.78) | 14.02 (0.83) | 9.04 (0.73) | |||
| Marital status | <0.001 | |||||
| Never married | 18.13 (0.77) | 19.21 (0.86) | 15.12 (0.82) | |||
| Married/living with partner | 63.25 (0.77) | 64.53 (0.85) | 59.67 (1.04) | |||
| Widowed/divorced | 18.63 (0.37) | 16.26 (0.37) | 25.21 (0.68) | |||
| Poverty income ratio | 0.435 | |||||
| <1 | 15.90 (0.74) | 15.68 (0.77) | 16.52 (0.95) | |||
| [1, 3) | 35.71 (0.85) | 35.58 (0.89) | 36.09 (1.17) | |||
| ≥3 | 48.39 (1.23) | 48.74 (1.23) | 47.39 (1.63) | |||
| Education | 0.032 | |||||
| elow high school | 5.70 (0.31) | 5.98 (0.34) | 4.92 (0.44) | |||
| High school | 36.33 (0.93) | 36.45 (0.94) | 35.98 (1.19) | |||
| College or above | 57.97 (1.06) | 57.57 (1.08) | 59.10 (1.26) | |||
| Smokers | <0.001 | |||||
| Never smoker | 55.41 (0.75) | 58.62 (0.76) | 46.42 (1.05) | |||
| Former smoker | 24.08 (0.54) | 22.46 (0.55) | 28.59 (0.88) | |||
| Current smoker | 20.52 (0.56) | 18.92 (0.53) | 24.99 (1.02) | |||
| Alcohol drinkers | 0.027 | |||||
| Non-drinker | 34.00 (0.88) | 34.07 (0.88) | 33.80 (1.24) | |||
| Moderate alcohol use | 48.76 (0.89) | 48.15 (0.92) | 50.50 (1.34) | |||
| High alcohol use | 17.24 (0.45) | 17.78 (0.53) | 15.70 (0.69) | |||
| BMI | <0.001 | |||||
| <25 | 31.55 (0.62) | 33.07 (0.69) | 27.19 (0.83) | |||
| [25, 30) | 33.33 (0.5) | 34.03 (0.56) | 31.32 (0.85) | |||
| ≥30 | 35.12 (0.55) | 32.89 (0.66) | 41.49 (0.81) | |||
| Sleep duration | <0.001 | |||||
| Insufficient | 56.43 (0.60) | 60.42 (0.63) | 44.99 (0.90) | |||
| Recommended | 36.21 (0.58) | 31.74 (0.59) | 49.03 (0.98) | |||
| Excessive | 7.36 (0.23) | 7.84 (0.28) | 5.98 (0.41) | |||
| Sedentary behavior | <0.001 | |||||
| Mild | 63.09 (0.71) | 64.52 (0.75) | 58.99 (1.05) | |||
| Severe | 36.91 (0.71) | 35.48 (0.75) | 41.01 (1.05) | |||
| Exercise | <0.001 | |||||
| Light | 30.47 (0.75) | 28.80 (0.77) | 35.73 (1.25) | |||
| Vigorous/moderate | 69.53 (0.75) | 71.20 (0.77) | 64.27 (1.25) | |||
| WBC | 7.22 ± 0.03 | 7.14 ± 0.04 | 7.42 ± 0.05 | <0.001 | ||
| NEU | 4.30 ± 0.03 | 4.25 ± 0.03 | 4.47 ± 0.04 | <0.001 | ||
| SII | 543.51 ± 4.23 | 533.32 ± 4.25 | 572.62 ± 7.58 | <0.001 | ||
| NLR | 2.22 ± 0.02 | 2.19 ± 0.02 | 2.30 ± 0.03 | <0.001 | ||
Mean ± SE for continuous variables: P-value was calculated by the weighted linear regression model. % (SE) for categorical variables: P-value was calculated by the weighted chi-square test.
WBC, white blood cell; NEU, neutrophil; NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune inflammation index.
Three weighted logistic regression models were constructed. Table 2 shows the relationship between sedentary behavior, exercise, and sleep disturbance. In the crude model, participants with severe sedentary behavior had a higher OR = 1.264 and 95% CI 1.162-1.375 (P < 0.001) for sleep disturbance compared with those with mild sedentary behavior (model 1); however, in groups who engage in vigorous/moderate exercise, they had a relative significantly lower OR for sleep disturbance (OR = 0.728, 95% CI: 0.655-0.808, P < 0.001). Additionally, after adjusting for age, sex, and race (model 1), the trends remained the same. After further adjustment of body mass index, marital status, sleep duration, education attainment, poverty income ratio, smoking status, and alcohol drinking (model 2), the OR for comparison between different sedentary groups and different intensities of exercise was 1.261 (95% CI: 1.154-1.377, P < 0.001) and 0.849 (95% CI: 0.757-0.953, P = 0.008), respectively.
Table 2.
The associations between sedentary behavior, exercise, and sleep disturbance.
| Crude model a | Model 1 b | Model 2 c | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Mild sedentary behavior | Reference | Reference | Reference | |||
| Severe sedentary behavior | 1.264 (1.162, 1.375) | <0.001 | 1.213 (1.114, 1.320) | <0.001 | 1.261 (1.154, 1.377) | <0.001 |
| Light exercise | Reference | Reference | Reference | |||
| Vigorous/moderate exercise | 0.728 (0.655, 0.808) | <0.001 | 0.787 (0.709, 0.874) | <0.001 | 0.849 (0.757, 0.953) | 0.008 |
OR, odds ratio; CI, confidence intervals.
Crude model: no covariates were adjusted.
Model 1: age, sex, and race/ethnicity were adjusted.
Model 2: age, sex, race, body mass index, marital status, sleep duration, education attainment, poverty income ratio, smoking status, and alcohol drinking status were adjusted.
Moreover, we further investigated the associations between exercise and sleep disturbance under different levels of sedentary behavior. After adjusting for all covariates, the results showed that for the mild sedentary behavior group, the intensity of exercise activity was not significant although a negative association can be found between activity level and sleep disturbance. In the population with severe sedentary behavior, higher exercise intensity was significantly associated with a lower risk of sleep disturbance symptoms (OR = 0.687, 95% CI: 0.551-0.857, P = 0.002) ( Table 3 ), which indicated that exercise had a protective effect for sleep disorder in groups with longer sedentary status.
Table 3.
The associations between exercise and sleep disturbance by sedentary behavior.
| OR | 95% CI | P-value | |
|---|---|---|---|
| Mild sedentary behavior | |||
| Light exercise | Reference | ||
| Vigorous/moderate exercise | 0.973 | (0.861, 1.098) | 0.656 |
| Severe sedentary behavior | |||
| Light exercise | Reference | ||
| Vigorous/moderate exercise | 0.687 | (0.551, 0.857) | 0.002 |
Adjusted for age, sex, race, body mass index, marital status, sleep duration, education attainment, poverty income ratio, smoking status, and alcohol drinking status.
OR, odds ratio; CI, confidence intervals.
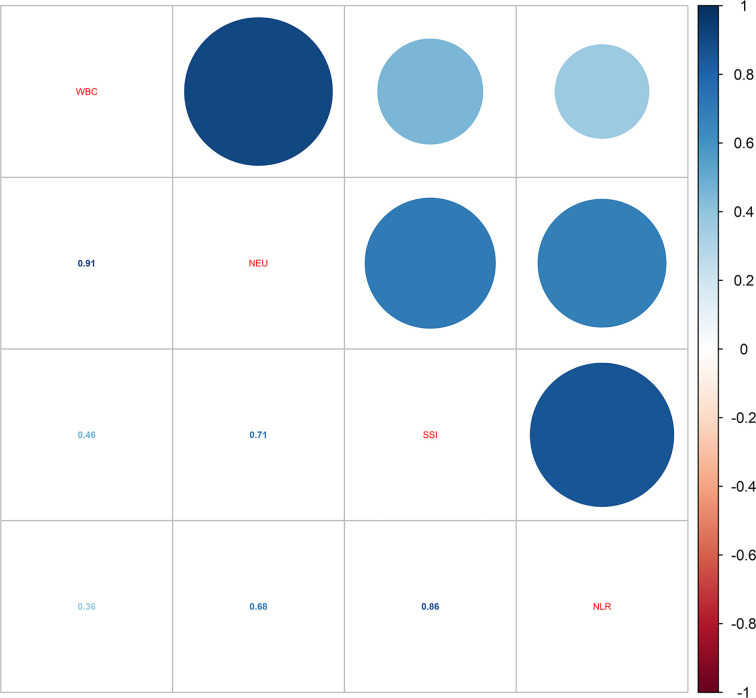
When it comes to the influence of inflammation, the correlations of the four blood inflammatory biomarkers were explored. As shown in Figure 2 , the strongest correlation was found between WBC and NEU (r-value = 0.91). Additionally, a close correlation was also identified between NLR and SII (r-value = 0.86). Table 4 demonstrates the relationship between different inflammatory markers and sleep disturbance. In the fully adjusted regression model, results showed that people with sleep disturbance tend to have a higher inflammatory tendency in all markers. A one-unit increase in WBC was associated with a 1.026 times prevalence of sleep disturbance (95% CI: 1.007-1.045, P = 0.010). There was also statistical significance found for the other three biomarkers: NEU (OR = 1.032, 95% CI: 1.005-1.059, P = 0.023), NLR (OR = 1.035, 95% CI: 0.998-1.074, P = 0.071, marginally significant), and SII (OR = 1.0001, 95% CI: 1.0000-1.0003, P = 0.041).
Figure 2.
Correlations of the four blood inflammatory biomarkers.
Table 4.
The associations between inflammatory biomarkers and sleep disturbance.
| OR | 95% CI | P-value | |
|---|---|---|---|
| WBC | 1.026 | (1.007, 1.045) | 0.010 |
| Neutrophils | 1.032 | (1.005, 1.059) | 0.023 |
| NLR | 1.035 | (0.998, 1.074) | 0.071 |
| SII | 1.0001 | (1.0000, 1.0003) | 0.041 |
Adjusted for age, sex, race, body mass index, marital status, sleep duration, education attainment, poverty income ratio, smoking status, and alcohol drinking status.
OR, odds ratio; CI, confidence intervals.
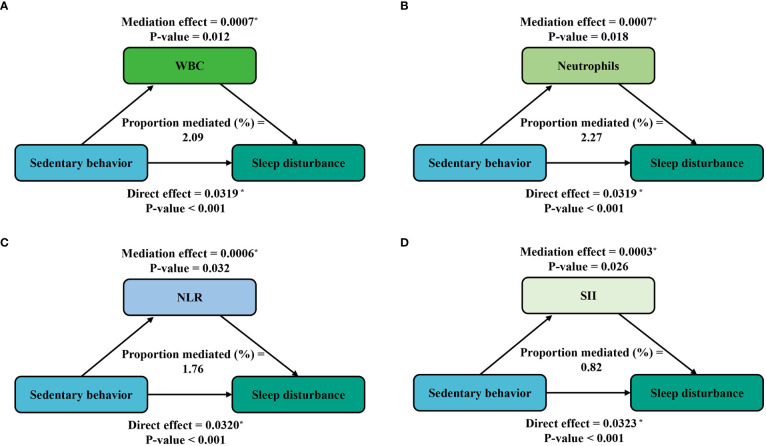
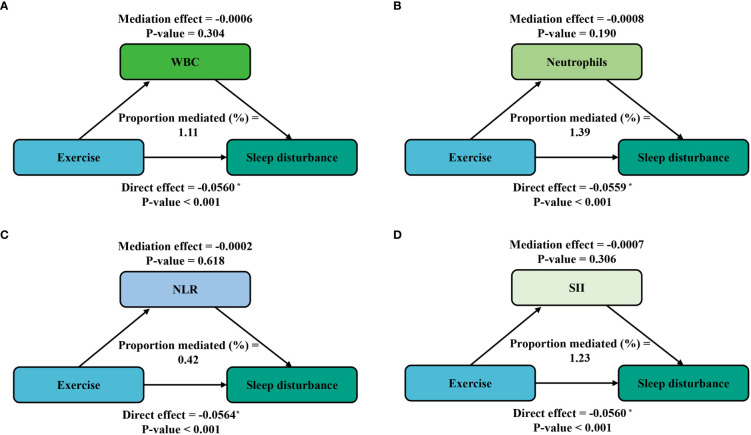
Furthermore, mediation analyses were conducted to explore the mediating effect of inflammatory markers. Figure 3 shows the mediating role of inflammatory markers in the relationship between sedentary behavior and sleep disturbance. All four inflammatory markers significantly mediated the association between sedentary behavior and sleep disturbance, with WBC, neutrophils, NLR, and SII explaining 2.09%, 2.27%, 1.76%, and 0.82% of the association, respectively (P < 0.05). Meanwhile, on the basis of the results from Table 4 , we also conducted a mediation analysis of the effect of exercise in attenuating sleep disturbance on the severe sedentary group. As shown in Figure 4 , WBC, NEU, NLR, and SII explained 1.11%, 1.39%, 0.42%, and 1.39% of the association, respectively. Although the direct effects were all significant in these four cases (P < 0.001), the mediating role of inflammatory markers tended to be non-significant.
Figure 3.
Path diagram of the mediation analysis of inflammatory biomarkers on the relationship between sedentary behavior and sleep disturbance. The graphs in (A–D) represented the mediating role of WBC, neutrophils, NLR, and SII, respectively.
Figure 4.
Path diagram of the mediation analysis of inflammatory biomarkers on the relationship between exercise and sleep disturbance in the severe sedentary groups. The graphs in (A–D) represented the mediating role of WBC, neutrophils, NLR, and SII, respectively.
Discussion
This study for the first time reported the mediation effect of blood-cell-based inflammatory biomarkers between sedentary exposure on the risk of sleep disturbance. In the present study of a nationally representative adult sample, we observed that severe sedentary behavior was associated with increased risk of sleep disturbance, while exercise can mitigate this situation to some extent. Sleep disturbance was associated with increased levels of WBC, NEU, NLR, and SII, indicating that sleep disturbance was associated with increased inflammatory states, and high blood-cell-based markers were independent risk factors for sleep disturbance. Of particular importance, there was a novel finding that all four inflammatory biomarkers significantly mediated the association between sedentary behavior and sleep disturbance. However, although a negative trend was found between exercise and sleep disturbance, the mediating role of these inflammatory biomarkers was not significant in this case.
Significant relationships were observed between inflammatory markers and sleep disturbance patients. It has been established that sleep regulated the immune system and there was evidence linking the disruption of sleep rhythm with increased inflammation (41). Assessment of blood-cell-based markers may help us predict the severity of night sleep disorder, as well as the presence of comorbidities. These blood-count-based parameters, such as WBC, NEU, and SSI, are not only inexpensive and handy tests performed in most areas but also can offer us accurate and reproducible information on systemic inflammation. In recent years, by using the novel index SII, an integrated inflammation marker developed by Hu et al. (31), we can better grasp the extensive immune and inflammatory state of the body. Previous meta-analyses have detected SII as a strong and independent predictor in patients with several malignancies (42, 43). However, there was no current study on the correlation between sleep disturbance and SII, and this population-based study further confirmed that a high level of SII can be used to reflect an elevated inflammation in sleep disturbance status.
A positive relationship between sedentary behavior and sleep disturbance was found in this study, which was consistent with several previous reports (44–46). Considering that sedentary behavior was characterized by waking behavior with less than an energy expenditure of 1.5 metabolic equivalents (METs) (12), excessive electronic product use and unhealthy lifestyles might aggravate this condition in modern society. Former studies have found that sedentary behavior was strongly associated with increasing levels of cytokines, which also were involved in a number of regulatory and inflammatory processes (15, 47, 48). These findings led us to propose whether there was a mediating role of inflammatory markers in the association between sedentary behavior and sleep disturbance. Strikingly, by conducting the mediation analysis, we found that all four blood-cell-based inflammatory biomarkers significantly regulated this process. Indeed, these inflammatory biomarkers provided us a clue to examine the underlying mechanism between the relationship of sedentary behavior and sleep disturbance.
Fortunately, a sedentary population suffering from sleep disturbance can be managed and improved with non-pharmacological treatment including exercise and physical activity (49–51). It was generally accepted that exercise exerted a beneficial effect on the quality of sleep. In accordance with previous findings (52–54), our research also found that moderate to vigorous physical exercise can significantly reduce the risk of sleep disturbance in this group. One population-based study reallocated 30 min of sedentary time with exercise and found that this can lead to a more favorable inflammatory profile characterized by higher adiponectin and decreased levels of complement component C3, leptin, interleukin 6 (IL-6), and WBC concentrations (55). Although our mediation analysis did not significantly detect these findings on the basis of the blood-cell inflammatory biomarkers, it complemented existing literature on the short window of potential benefits. Exercise itself was also linked to oxidative stress. Oxidative stress, a process caused by an imbalance between the production and accumulation of reactive oxygen species (ROS) in cells and tissues, may also regulate the relationship between exercise and sleep disturbance. Although a one-bout exercise can elevate ROS, systematic and regular training can prompt the adaption of organisms by increasing mitochondria biogenesis and antioxidant capacity (56). In addition, there might also be other physiological pathways. Taking melatonin as an example, being physically active rather than being sedentary can result in a shift of the onset of nocturnal melatonin and make potential alterations in sleep quality (57).
The greatest strength of our study was the use of the nationally representative NHANES population. Secondly, we adjusted for confounding factors including sociodemographic and lifestyle factors to produce more reliable results. By introducing blood-cell-based inflammatory biomarkers as mediators, this study first attempted to establish the relationship between sedentary behavior, exercise, and sleep disturbance. Importantly, realizing the magnitude and specificity of blood-cell-based inflammatory biomarkers on sleep disturbance had further health implications, considering that inflammation status appeared to be amenable to modification by reducing sedentary time and increasing physical activity. Although evidence on the relationship between physical exercise, sedentary behavior, and sleep efficiency is emerging, the mechanism is far from conclusive. Based on our works, we encouraged further studies to examine the effects of specific types of exercise and sedentary behavior on inflammation to better characterize the association with sleep disturbance.
However, the results of this research should be interpreted with caution for several limitations. Firstly, the outcome sleep disturbance was assessed by self-report in NHANES design, which tended to be imprecise compared with an objectively measured test, although the design of a large population sample and a complex multistage sampling made up for the deficiency of this result to some extent. Moreover, another limitation was that this study only analyzed the independent effects of sedentary behavior and exercise on sleep disturbance. Emerging statistical strategies like the 24-h activity model and the functional principal component model were conducive to better explore the relationship between sedentary behavior and exercise in future research (58, 59). Additionally, blood samples were not necessarily obtained temporally proximal to the survey information in NHANES settings. Last but not least, no measures of inflammatory proteins such as CRP or IL-6 were used in the analysis, and clinical conditions such as hypertension and type 2 diabetes should be further explored.
Conclusion
In conclusion, firstly, our study found that, as inexpensive and handy tests, blood-cell-based inflammatory biomarkers can be used to predict the prevalence of sleep disturbance from a national representative sample. Secondly, the mediation effect of WBC, NEU, NLR, and SII was confirmed in the association between sedentary behavior and sleep disturbance. Thirdly, we detected the mitigation role of exercise on sleep disorders in severe sedentary groups, although the mediation analysis did not examine the significant effect of the four inflammatory biomarkers included in this study. Future studies should focus on understanding the additional biology of inflammatory conditions between sedentary behavior, exercise, and sleep disturbance, testing specific interventions targeting at sleep quality through reducing sedentary time and increasing physical activity.
Data availability statement
Publicly available datasets were analyzed in this study. These data can be found here: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving human participants were reviewed and approved by the National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Study conception and design: YY, YC and WF. Data collection: YY and YC. Data analysis: YY and YC. Interpretation of results: YY, YC, WF, XL and RW. Drafting of the manuscript: YY, YC and WF. Providing valuable insight regarding the approach and organization of the manuscript: WF, XL, RW and JL. Supervision: JL and XM. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was supported by the Institute of Sports Development Research of Tsinghua University (Research on John Mo’s thought and practice of Physical Education) and China Postdoctoral Science Foundation (2022M711858).
Abbreviations
NHANES, National Health and Nutrition Examination Survey; WBC, white blood cell; NEU, neutrophil; NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune inflammation index; OR, odds ratio; CI, confidence interval.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Ford ES, Wheaton AG, Cunningham TJ, Giles WH, Chapman DP, Croft JB. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: Findings from the national ambulatory medical care survey 1999-2010. Sleep (2014) 37:1283–93. doi: 10.5665/sleep.3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leger D, Poursain B, Neubauer D, Uchiyama M. An international survey of sleeping problems in the general population. Curr Med Res Opin (2008) 24:307–17. doi: 10.1185/030079907x253771 [DOI] [PubMed] [Google Scholar]
- 3. Hui SK, Grandner MA. Trouble sleeping associated with lower work performance and greater health care costs: Longitudinal data from Kansas state employee wellness program. J Occup Environ Med (2015) 57:1031–8. doi: 10.1097/JOM.0000000000000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep (2007) 30:1667–73. doi: 10.1093/sleep/30.12.1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American heart association. Circulation (2016) 134:e367–86. doi: 10.1161/CIR.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adamo D, Ruoppo E, Leuci S, Aria M, Amato M, Mignogna MD. Sleep disturbances, anxiety and depression in patients with oral lichen planus: A case-control study. J Eur Acad Dermatol Venereol (2015) 29:291–7. doi: 10.1111/jdv.12525 [DOI] [PubMed] [Google Scholar]
- 7. Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obes (Silver Spring) (2008) 16:643–53. doi: 10.1038/oby.2007.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaleelullah RA, Nagarajan PP. Cultivating lifestyle transformations in obstructive sleep apnea. Cureus (2021) 13:e12927. doi: 10.7759/cureus.12927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomee S, Harenstam A, Hagberg M. Computer use and stress, sleep disturbances, and symptoms of depression among young adults–a prospective cohort study. BMC Psychiatry (2012) 12:176. doi: 10.1186/1471-244X-12-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vallance JK, Buman MP, Stevinson C, Lynch BM. Associations of overall sedentary time and screen time with sleep outcomes. Am J Health Behav (2015) 39:62–7. doi: 10.5993/AJHB.39.1.7 [DOI] [PubMed] [Google Scholar]
- 11. Levenson JC, Shensa A, Sidani JE, Colditz JB, Primack BA. The association between social media use and sleep disturbance among young adults. Prev Med (2016) 85:36–41. doi: 10.1016/j.ypmed.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sedentary Behaviour Research Network . Sedentary behaviour research, n. letter to the editor: Standardized use of the terms "sedentary" and "sedentary behaviours". Appl Physiol Nutr Metab (2012) 37:540–2. doi: 10.1139/h2012-024 [DOI] [PubMed] [Google Scholar]
- 13. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: The population health science of sedentary behavior. Exerc Sport Sci Rev (2010) 38:105–13. doi: 10.1097/JES.0b013e3181e373a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yates T, Khunti K, Wilmot EG, Brady E, Webb D, Srinivasan B, et al. Self-reported sitting time and markers of inflammation, insulin resistance, and adiposity. Am J Prev Med (2012) 42:1–7. doi: 10.1016/j.amepre.2011.09.022 [DOI] [PubMed] [Google Scholar]
- 15. Allison MA, Jensky NE, Marshall SJ, Bertoni AG, Cushman M. Sedentary behavior and adiposity-associated inflammation: The multi-ethnic study of atherosclerosis. Am J Prev Med (2012) 42:8–13. doi: 10.1016/j.amepre.2011.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng Q, Zhang QL, Du Y, Ye YL, He QQ. Associations of physical activity, screen time with depression, anxiety and sleep quality among Chinese college freshmen. PloS One (2014) 9:e100914. doi: 10.1371/journal.pone.0100914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brand S, Gerber M, Beck J, Hatzinger M, Pühse U, Holsboer-Trachsler E. High exercise levels are related to favorable sleep patterns and psychological functioning in adolescents: A comparison of athletes and controls. J Adolesc Health (2010) 46:133–41. doi: 10.1016/j.jadohealth.2009.06.018 [DOI] [PubMed] [Google Scholar]
- 18. Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online (2000) 3:121–9. [PubMed] [Google Scholar]
- 19. Netzer N, Lormes W, Giebelhaus V, Halle M, Keul J, Matthys H, et al. [Physical training of patients with sleep apnea]. Pneumologie (1997) 51 Suppl 3:779–82. [PubMed] [Google Scholar]
- 20. Abd El-Kader SM, Al-Jiffri OH. Aerobic exercise modulates cytokine profile and sleep quality in elderly. Afr Health Sci (2019) 19:2198–207. doi: 10.4314/ahs.v19i2.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. You Y, Wang D, Wang Y, Li Z, Ma X. A bird's-eye view of exercise intervention in treating depression among teenagers in the last 20 years: A bibliometric study and visualization analysis. Front Psychiatry (2021) 12:661108. doi: 10.3389/fpsyt.2021.661108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. You Y, Li W, Liu J, Li X, Fu Y, Ma X. Bibliometric review to explore emerging high-intensity interval training in health promotion: A new century picture. Front Public Health (2021) 9:697633. doi: 10.3389/fpubh.2021.697633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. You Y, Wang D, Liu J, Chen Y, Ma X, Li W. Physical exercise in the context of air pollution: An emerging research topic. Front Physiol (2022) 13:784705. doi: 10.3389/fphys.2022.784705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on c-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol (2004) 43:678–83. doi: 10.1016/j.jacc.2003.07.050 [DOI] [PubMed] [Google Scholar]
- 25. Irwin MR. Why sleep is important for health: A psychoneuroimmunology perspective. Annu Rev Psychol (2015) 66:143–72. doi: 10.1146/annurev-psych-010213-115205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry (2016) 80:40–52. doi: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep (2009) 32:200–4. doi: 10.1093/sleep/32.2.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Y, Su S, McCall WV, Wang X. Blunted rest-activity rhythm is associated with increased white blood-cell-based inflammatory markers in adults: An analysis from NHANES 2011-2014. Chronobiol Int (2022) 39:895–902. doi: 10.1080/07420528.2022.2048663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Velasco Á, Rodríguez-Revuelta J, Olié E, Abad I, Fernández-Peláez A, Cazals A, et al. Neutrophil-to-lymphocyte ratio: A potential new peripheral biomarker of suicidal behavior. Eur Psychiatry (2020) 63:e14. doi: 10.1192/j.eurpsy.2019.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun H, Que J, Peng Y, Ye H, Xiang H, Han Y, et al. The neutrophil-lymphocyte ratio: A promising predictor of mortality in coronary care unit patients - a cohort study. Int Immunopharmacol (2019) 74:105692. doi: 10.1016/j.intimp.2019.105692 [DOI] [PubMed] [Google Scholar]
- 31. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 32. Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med (2017) 15:221. doi: 10.1186/s12967-017-1326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep foundation's sleep time duration recommendations: Methodology and results summary. Sleep Health (2015) 1:40–3. doi: 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 34. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pratt M. What's new in the 2020 world health organization guidelines on physical activity and sedentary behavior? J Sport Health Sci (2021) 10:288–9. doi: 10.1016/j.jshs.2021.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu H, Deng K, Lin Z, Huang Z, Gong X, Tan J, et al. The effects of physical activity and sedentary behavior in the associations between cardiovascular diseases and depression: A four-way decomposition. J Affect Disord (2020) 275:194–201. doi: 10.1016/j.jad.2020.07.017 [DOI] [PubMed] [Google Scholar]
- 37. Huang B, Huang Z, Tan J, Xu H, Deng K, Cheng J, et al. The mediating and interacting role of physical activity and sedentary behavior between diabetes and depression in people with obesity in united states. J Diabetes Complications (2021) 35:107764. doi: 10.1016/j.jdiacomp.2020.107764 [DOI] [PubMed] [Google Scholar]
- 38. You Y, Chen Y, Yin J, Zhang Z, Zhang K, Zhou J, et al. Relationship between leisure-time physical activity and depressive symptoms under different levels of dietary inflammatory index. Front Nutr (2022) 9:983511. doi: 10.3389/fnut.2022.983511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun WJ, Xu L, Chan WM, Lam TH, Schooling CM. Are depressive symptoms associated with cardiovascular mortality among older Chinese: A cohort study of 64,000 people in Hong Kong? Am J Geriatr Psychiatry (2013) 21:1107–15. doi: 10.1016/j.jagp.2013.01.048 [DOI] [PubMed] [Google Scholar]
- 40. Taylor AL, Denniston MM, Klevens RM, McKnight-Eily LR, Jiles RB. Association of hepatitis c virus with alcohol use among U.S. adults: NHANES 2003-2010. Am J Prev Med (2016) 51:206–15. doi: 10.1016/j.amepre.2016.02.033 [DOI] [PubMed] [Google Scholar]
- 41. Carter SJ, Durrington HJ, Gibbs JE, Blaikley J, Loudon AS, Ray DW, et al. A matter of time: Study of circadian clocks and their role in inflammation. J Leukoc Biol (2016) 99:549–60. doi: 10.1189/jlb.3RU1015-451R [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y, Chen B, Wang L, Wang R, Yang X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: A meta-analysis. Med (Baltimore) (2019) 98:e13788. doi: 10.1097/MD.0000000000013788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget (2017) 8:75381–8. doi: 10.18632/oncotarget.18856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang Y, Shin JC, Li D, An R. Sedentary behavior and sleep problems: A systematic review and meta-analysis. Int J Behav Med (2017) 24:481–92. doi: 10.1007/s12529-016-9609-0 [DOI] [PubMed] [Google Scholar]
- 45. Costigan SA, Barnett L, Plotnikoff RC, Lubans DR. The health indicators associated with screen-based sedentary behavior among adolescent girls: A systematic review. J Adolesc Health (2013) 52:382–92. doi: 10.1016/j.jadohealth.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 46. Wu X, Tao S, Zhang Y, Zhang S, Tao F. Low physical activity and high screen time can increase the risks of mental health problems and poor sleep quality among Chinese college students. PloS One (2015) 10:e0119607. doi: 10.1371/journal.pone.0119607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parsons TJ, Sartini C, Welsh P, Sattar N, Ash S, Lennon LT, et al. Physical activity, sedentary behavior, and inflammatory and hemostatic markers in men. Med Sci Sports Exerc (2017) 49:459–65. doi: 10.1249/MSS.0000000000001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garcia-Hermoso A, Ramirez-Velez R, Alfonso-Rosa RM, Del Pozo Cruz B. Cardiorespiratory fitness, physical activity, sedentary behavior, and circulating white blood cells in US youth. Scand J Med Sci Sports (2021) 31:439–45. doi: 10.1111/sms.13845 [DOI] [PubMed] [Google Scholar]
- 49. Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Tai chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: A randomized controlled trial. J Natl Cancer Inst Monogr (2014) 2014:295–301. doi: 10.1093/jncimonographs/lgu028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, et al. Cognitive behavioral therapy vs. tai chi for late life insomnia and inflammatory risk: A randomized controlled comparative efficacy trial. Sleep (2014) 37:1543–52. doi: 10.5665/sleep.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. You Y, Min L, Tang M, Chen Y, Ma X. Bibliometric evaluation of global tai chi research from 1980-2020. Int J Environ Res Public Health (2021) 18(11):6150. doi: 10.3390/ijerph18116150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stavrou VT, Astara K, Tourlakopoulos KN, Papayianni E, Boutlas S, Vavougios GD, et al. Obstructive sleep apnea syndrome: The effect of acute and chronic responses of exercise. Front Med (Lausanne) (2021) 8:806924. doi: 10.3389/fmed.2021.806924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rayward AT, et al. Efficacy of an m-health physical activity and sleep intervention to improve sleep quality in middle-aged adults: The refresh study randomized controlled trial. Ann Behav Med (2020) 54:470–83. doi: 10.1093/abm/kaz064 [DOI] [PubMed] [Google Scholar]
- 54. Inoue S, Yorifuji T, Sugiyama M, Ohta T, Ishikawa-Takata K, Doi H. Does habitual physical activity prevent insomnia? a cross-sectional and longitudinal study of elderly Japanese. J Aging Phys Act (2013) 21:119–39. doi: 10.1123/japa.21.2.119 [DOI] [PubMed] [Google Scholar]
- 55. Phillips CM, Dillon CB, Perry IJ. Does replacing sedentary behaviour with light or moderate to vigorous physical activity modulate inflammatory status in adults? Int J Behav Nutr Phys Act (2017) 14:138. doi: 10.1186/s12966-017-0594-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kawamura T, Muraoka I. Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants (Basel) (2018) 7(9):119. doi: 10.3390/antiox7090119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aoyama S, Shibata S. The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front Neurosci (2017) 11:63. doi: 10.3389/fnins.2017.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuzik N, Poitras VJ, Tremblay MS, Lee EY, Hunter S, Carson V. Systematic review of the relationships between combinations of movement behaviours and health indicators in the early years (0-4 years). BMC Public Health (2017) 17:849. doi: 10.1186/s12889-017-4851-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xiao Q, Lu J, Zeitzer JM, Matthews CE, Saint-Maurice PF, Bauer C. Rest-activity profiles among U.S. adults in a nationally representative sample: A functional principal component analysis. Int J Behav Nutr Phys Act (2022) 19:32. doi: 10.1186/s12966-022-01274-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://www.cdc.gov/nchs/nhanes/.