1. Chronic kidney disease–associated pruritus, although highly prevalent is underreported, associated with poorer health-related quality of life, and increased mortality.
Chronic kidney disease–associated pruritus, which we will call “pruritus” from here on, is defined as itching directly related to kidney disease, without any other comorbid condition to explain the itching.1 Dialysis Outcomes and Practice Patterns Study (DOPPS) reported that most patients (~65%) on hemodialysis (HD) experience pruruitis,2 with 37% being moderately bothered and 7% being extremely bothered.3 Pruritus can be debilitating and has been associated with decreased recovery time post sessions,3 insomnia,4 reduced quality of life,5 depression,6 missed HD sessions,7 and withdrawal from dialysis.3 It is underreported with 17% of symptomatic patients not reporting symptoms to health care providers,8 and it is underappreciated by healthcare professionals in HD units.9
2. Pruritus can be severe, risk factors are: older age, higher comorbidity, lower hemoglobin and albumin, and higher C-reactive protein.
Symptoms from pruritus can vary from mild discomfort to unremitting and refractory and can occur at any time in relation to dialysis.5 In most patients, symptoms are most noticeable at night and involve bilateral, symmetrical, large nondermatomal areas of skin.5 Compared with those without pruritus, patients with pruritus tend to be slightly older, had greater comorbidity, were more likely to dialyze with a central venous catheter, had higher C-reactive protein, and had lower hemoglobin and serum albumin levels.3 Sex, ethnicity, years on dialysis, and etiology of kidney failure have not consistently been shown to be risk factors for pruritus.5
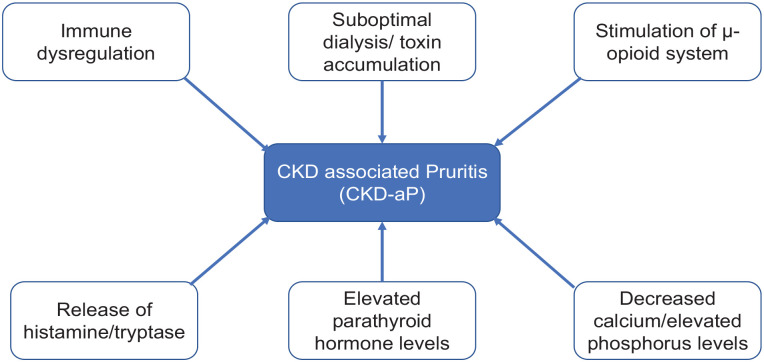
3. The pathogenesis of pruritus is unclear. There is some support for mechanisms involving stimulation of opioid receptors and immune dysregulation in a uremic milieu.
Pruritus is likley to involve cross talk between keratinocytes, immune system, and neurons in a uremic environment.10 Markers of mineral metabolism (calcium, phosphorus, parathyroid hormone)11 and dialysis efficiency (uremic toxins)11,12 have been proposed, but causality has not been determined. Opioid imbalance (overstimulation of central µ-opioid receptors (MORs), antagonism of peripheral κ-opioid receptors (KORs), or a discrepancy of stimulation and antagonism of MOR and KOR), immune system dysregulation (microinflammation,13 elevated interleukin levels),14,15 elevated levels of histamine16 and tryptase,17 peripheral neuropathy, and dry skin have been proposed as etiological and/or contributing factors (Figure 1).
Figure 1.
Risk factors and proposed mechanisms for pruritus.
CKD = chronic kidney disease.
4. Low-quality evidence exists for optimizing the hemodialysis prescription and medium cutoff (MCO) dialyzers. Consider parathyroidectomy for severe hyperparathyroidism. Kidney transplantation reverses pruritus in most patients
In a randomized controlled trial (RCT) of 22 HD patients with severe pruritus,12 increased small solute clearance (mean Kt/V, 1.28) led to a decrease in pruritus scores (12.6 ± 5.1 to 6.3 ± 3.2). In comparison, when the HD prescription was left unchanged (mean Kt/V, 1.09), pruritus scores remained unchanged (12.3 ± 4.7 to 12.7 ± 6.4).11 Ko et al18 also found a kT/V >1.5, and use of high-flux dialyzers was associated with less itching. However, other studies have not shown a similar relationship between kT/V and pruritus,19 implying that middle molecules and not small molecules play a role in pruritus.
Unfortunately, none of the trials comparing short daily or nocturnal HD with incenter HD evaluated pruritus as an outcome measure. We identified only 1 RCT comparing MCO dialyzer (n = 24) with high-flux dialyzer (n = 25). Patients with MCO dialyzers had better pruritus scores (1.29 ± 0.46 vs 1.64 ± 0.64, P = .034) and sleep, reflecting a relationship between efficient elimination of middle molecules and symptom relief.20
Parathyroidectomy has been found to be consistently associated with itch reduction. A prospective, uncontrolled study of 37 HD patients (22 had symptomatic pruritus) with a mean parathyroid hormone level of 156 pmol/L showed a significant decrease in visual analog scale (VAS) score before and after parathyroidectomy (5.4 ± 3.2 to 1.8 ± 1.5, P < .001).21 A couple of other pre/post parathyroidectomy studies from the mid-1960s have shown similar reduction in pruritus.22 -24
Kidney transplantation has consistently shown to provide substantial symptom relief. In a prospective study of 1298 HD patients and 521 transplant recipients, pruritus (of the 11 symptoms measured) had the greatest improvement after transplant.25
5. Consider the following therapeutic options:
A. Topical options:
Emollients with low pH and/or high water content have been found to be helpful as xerosis exists in 50% to 85% of patients and is an aggravating factor of pruritus.26 There are several over-the-counter (OTC) options for the clinicians:
a. Consider a cream containing 2.2% gamma linolenic acid (GLA): In a double-blind, placebo-controlled, crossover study (n = 17) with refractory pruritus, a cream containing 2.2% GLA significantly improved pruritus compared with a placebo.27 Gamma linolenic acid is an essential fatty acid that is metabolized to a prostaglandin precursor with known anti-inflammatory properties and is available OTC as evening primrose oil.
b. Consider a cream containing 1% pramoxine: In a randomized, double-blind, controlled comparative trial with 28 patients using 1% pramoxine lotion versus placebo, there was 61% reduction in pruritus scores in comparison with 12% in the placebo group.28 The cream is available OTC in 0.5% to 1.0% concentrations as CeraVe, Gold Bond, and Aveeno Anti-Itch cream. Both topical GLA and pramoxine show promise and should be attempted initially in the management of pruritus.
c. Topical cannabinoids can be effective, but more data is needed: A single study of 21 patients showed that topical cannabinoids were effective in reducing xerosis and pruritus and were well tolerated.29 The baseline itch intensity measured by VAS was 6.24 ± 2.19 and had improved by day 21 (1.29 ± 1.41).29 The long-term effects of cannabis use, particularly in CKD, are yet unknown, and there are no data on systemic absorption. In the absence of randomized trials, a recent Canadian review on the topic suggested caution regarding its use until further independent and controlled studies were undertaken.10
d. OTC baby oil is inexpensive and can be effective: In a study of 70 Turkish HD patients (n = 35 in the intervention vs 35 in the control group), patients who applied baby oil to the affected areas reported a positive impact on itching, quality of life, and sleep quality.30 A prospective pre-post-test study looking at chilled/unchilled baby oil showed improvement in pruritus scores and quality of life.31
B. Oral and intravenous options:
a. Consider using gabapentin/pregabalin: In an RCT involving 42 HD patients, 21 received gabapentin and 21 received pregabalin. Both medications produced a significant difference in itching intensity.32 Another study reported significant reduction in mean pruritus score before and after administration of 300 mg of oral gabapentin post-HD over 4 weeks in HD patients with pruritus (8.4 ± 0.94 vs 1.2 ± 1.8) (P = .0001), with no demonstrable side effects at that dose.33 While initially approved to treat epilepsy, use of gabapentin and pregabalin in patients with pruritus is supported by European Dermatology Guidelines and a recent Cochrane systematic review.34,35
b. Consider using a selective KOR agonist (difelikefalin): In the largest multicenter RCT in HD patients with pruritus, difelikefalin (0.5 mg/kg of dry body weight) or placebo was administered intravenously, 3 times per week post-HD for 12 weeks. In all, 378 HD patients with moderate to severe uremic pruritus (Worst Itching Intensity Numerical Rating Scale [WI-NRS] >4 points) were randomized 1:1 to a placebo. Difelikefalin significantly reduced WI-NRS compared with placebo (49.1% vs 27.9%) and led to significant improvements in quality of life.36 It has been approved by Food and Drug Administration for use in the United States and is under review at Health Canada.37
c. Consider using MOR antagonists (naltrexone): A placebo-controlled, double-blind, crossover trial (n = 15) showed administration with oral naltrexone 50 mg once a day for 1 week led to an almost complete resolution of itching with few side effects.38 However, another double-blind, placebo-controlled crossover study of 23 patients over 4 weeks failed to show a statistically significant reduction in itch scores.39 Naltrexone is approved for use in Canada only for alcohol and opiate use disorder and used as a component of an alcohol counseling program.
d. Consider using a selective KOR agonist (nalfurafine) if available: A meta-analysis of 2 randomized, double blind, placebo-controlled studies on 144 HD patients showed nalfurafine administered intravenously postdialysis reduced worst itching (P = .02) and itching intensity (P = .04).40 In a study of 337 patients on HD with pruritus, participants were randomized 1:1:1 to 5.0 μg of nalfurafine, 2.5 μg of nalfurafine or placebo, and followed for 2 weeks. Both 5.0 and 2.5 μg of nalfurafine significantly improved pruritus intensity compared with placebo.41 This drug is approved for use in Japan for management of pruritus, but not in Canada.
e. Consider using a combination of KOR agonists and MOR antagonists (nalbuphine): A multicenter, randomized, double-blind, placebo-controlled trial of 373 HD patients with moderate to severe pruritus demonstrated that the group receiving nalbuphine 120 mg extended-release tablets twice a day for 8 weeks reported significantly decreased pruritus.42 Nalbuphine is approved for use in Canada for pain management and as a surgical anesthesia supplement.
f. Antihistamines are best avoided, mast cell stabilizers may help: While antihistamines are routinely prescribed in clinical practice, most studies have been unsuccessful.43 Medications such as hydroxyzine cannot be recommended as first-line use44 due to discouraging trial results and the likelihood for side effects in the elderly patients (over sedation). Orally administered mast cell stabilizers such as montelukast, cromolyn sodium, and zinc sulfate all probably reduce pruritus, but additional high-quality evidence is required before a decisive conclusion can be made.35
C. Nonpharmacological therapies:
a. Trial phototherapy (UVB radiation), if available at your institution: Eighteen HD patients with severe pruritus randomized to UVB (290-nm to 320-nm wavelength) or UVA therapy light showed a greater reduction (4-week follow-up) in the UVB therapy group (80% vs 20%).45 Gilchrest et al46 showed that side effects of narrow-band UVB radiation were less frequent and just as effective as treatment using broadband UVB. Phototherapy appears to be the most promising nonpharmacological therapy available to clinicians.
b. Acupuncture has been trialed, but more evidence is needed. A review of 3 RCTs and 3 uncontrolled observational trials found that acupuncture reduced itching in all trials. However, due to a high risk of bias in these studies, there is insufficient evidence to recommend acupuncture.47
c. While there are data on benefits of exercise in CKD patients with pruritus,48 there are no trials yet looking at intradialytic exercise on itch reduction.
Acknowledgments
Five things to know about. . . is an article type created and used by CMAJ and gratefully used by CJKHD with their permission.
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: All authors consent for publication.
Availability of Data and Materials: As this is a brief - 5 point review on Pruritis, all materials are referenced.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bhanu Prasad  https://orcid.org/0000-0002-1139-4821
https://orcid.org/0000-0002-1139-4821
References
- 1. Ständer S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the international forum for the study of itch. Acta Derm Venereol. 2007;87(4):291-294. [DOI] [PubMed] [Google Scholar]
- 2. Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12(12):2000-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sukul N, Karaboyas A, Csomor PA, et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med. 2021;3(1):42-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495-3505. [DOI] [PubMed] [Google Scholar]
- 5. Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(8):1410-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopes GB, Nogueira FC, de Souza MR, et al. Assessment of the psychological burden associated with pruritus in hemodialysis patients using the kidney disease quality of life short form. Qual Life Res. 2012;21(4):603-612. [DOI] [PubMed] [Google Scholar]
- 7. Sukul N, Speyer E, Tu C, et al. Pruritus and patient reported outcomes in non-dialysis CKD. Clin J Am Soc Nephrol. 2019;14(5):673-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aresi G, Rayner HC, Hassan L, et al. Reasons for underreporting of uremic pruritus in people with chronic kidney disease: a qualitative study. J Pain Symptom Manage. 2019;58(4):578-586.e2. [DOI] [PubMed] [Google Scholar]
- 9. Weisbord SD, Fried LF, Mor MK, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2(5):960-967. [DOI] [PubMed] [Google Scholar]
- 10. Martin CE, Clotet-Freixas S, Farragher JF, Hundemer GL. Have we just scratched the surface? a narrative review of uremic pruritus in 2020. Can J Kidney Health Dis. 2020;7:2054358120954024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626-1632. [DOI] [PubMed] [Google Scholar]
- 12. Hiroshige K, Kabashima N, Takasugi M, Kuroiwa A. Optimal dialysis improves uremic pruritus. Am J Kidney Dis. 1995;25(3):413-419. [DOI] [PubMed] [Google Scholar]
- 13. Kimmel M, Alscher DM, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant. 2006;21(3):749-755. [DOI] [PubMed] [Google Scholar]
- 14. Ko MJ, Peng YS, Chen HY, et al. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J Am Acad Dermatol. 2014;71(6):1151-1159.e1. [DOI] [PubMed] [Google Scholar]
- 15. Oweis AO, Al-Qarqaz F, Bodoor K, et al. Elevated interleukin 31 serum levels in hemodialysis patients are associated with uremic pruritus. Cytokine. 2021;138:155369. [DOI] [PubMed] [Google Scholar]
- 16. Francos GC, Kauh YC, Gittlen SD, et al. Elevated plasma histamine in chronic uremia. Effects of ketotifen on pruritus. Int J Dermatol. 1991;30(12):884-889. [DOI] [PubMed] [Google Scholar]
- 17. Dugas-Breit S, Schöpf P, Dugas M, Schiffl H, Ruëff F, Przybilla B. Baseline serum levels of mast cell tryptase are raised in hemodialysis patients and associated with severity of pruritus. J Dtsch Dermatol Ges. 2005;3(5):343-347. [DOI] [PubMed] [Google Scholar]
- 18. Ko MJ, Wu HY, Chen HY, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS One. 2013;8(8):e71404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duque MI, Thevarajah S, Chan YH, Tuttle AB, Freedman BI, Yosipovitch G. Uremic pruritus is associated with higher kt/V and serum calcium concentration. Clin Nephrol. 2006;66(3):184-191. [DOI] [PubMed] [Google Scholar]
- 20. Lim JH, Park Y, Yook JM, et al. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci Rep. 2020;10(1):7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chou FF, Ho JC, Huang SC, Sheen-Chen SM. A study on pruritus after parathyroidectomy for secondary hyperparathyroidism. J Am Coll Surg. 2000;190(1):65-70. [DOI] [PubMed] [Google Scholar]
- 22. Massry SG, Popovtzer MM, Coburn JW, Makoff DL, Maxwell MH, Kleeman CR. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. Disappearance of itching after subtotal parathyroidectomy. N Engl J Med. 1968;279(13):697-700. [DOI] [PubMed] [Google Scholar]
- 23. Kleeman CR, Massry SG, Popovtzer MM, Makoff DL, Maxwell MH, Coburn JW. The disappearance of intractable pruritus after parathyroidectomy in uremic patients with secondary hyperparathyroidism. Trans Assoc Am Physicians. 1968;81:203-212. [PubMed] [Google Scholar]
- 24. Hampers CL, Katz AI, Wilson RE, Merrill JP. Disappearance of “uremic” itching after subtotal parathyroidectomy. N Engl J Med. 1968;279(13):695-697. [DOI] [PubMed] [Google Scholar]
- 25. Taylor K, Chu NM, Chen X, et al. Kidney disease symptoms before and after kidney transplantation. Clin J Am Soc Nephrol. 2021;16(7):1083-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szepietowski JC, Reich A, Schwartz RA. Uraemic xerosis. Nephrol Dial Transplant. 2004;19(11):2709-2712. [DOI] [PubMed] [Google Scholar]
- 27. Chen YC, Chiu WT, Wu MS. Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. Am J Kidney Dis. 2006;48(1):69-76. [DOI] [PubMed] [Google Scholar]
- 28. Young TA, Patel TS, Camacho F, et al. A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. J Dermatolog Treat. 2009;20(2):76-81. [DOI] [PubMed] [Google Scholar]
- 29. Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerol Croat. 2005;13(2):97-103. [PubMed] [Google Scholar]
- 30. Karadag E, Kilic SP, Karatay G, Metin O. Effect of baby oil on pruritus, sleep quality, and quality of life in hemodialysis patients: pretest-post-test model with control groups. Jpn J Nurs Sci. 2014;11(3):180-189. [DOI] [PubMed] [Google Scholar]
- 31. Lin TC, Lai YH, Guo SE, et al. Baby oil therapy for uremic pruritus in haemodialysis patients. J Clin Nurs. 2012;21(1-2):139-148. [DOI] [PubMed] [Google Scholar]
- 32. Ravindran A, Kunnath RP, Sunny A, Vimal B. Comparison of safety and efficacy of pregabalin versus gabapentin for the treatment of uremic pruritus in patients with chronic kidney disease on maintenance haemodialysis. Indian J Palliat Care. 2020;26(3):281-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19(12):3137-3139. [DOI] [PubMed] [Google Scholar]
- 34. Xander C, Meerpohl JJ, Galandi D, et al. Pharmacological interventions for pruritus in adult palliative care patients. Cochrane Database Syst Rev. 2016;11:CD008320. [DOI] [PubMed] [Google Scholar]
- 35. Hercz D, Jiang SH, Webster AC. Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst Rev. 2020;12:CD011393. doi: 10.1002/14651858.CD011393.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F, Investigators K-T. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222-232. [DOI] [PubMed] [Google Scholar]
- 37. Government of Canada. Drug and health product submissions under review (SUR): new drug submissions under review. Date unknown. https://www.canada.ca/en/health-canada/services/drug-health-product-review-approval/submissions-under-review/new-drug-submissions-under-review.html. Accessed December 26, 2022.
- 38. Peer G, Kivity S, Agami O, et al. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet. 1996;348(9041):1552-1554. [DOI] [PubMed] [Google Scholar]
- 39. Pauli-Magnus C, Mikus G, Alscher DM, et al. Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. J Am Soc Nephrol. 2000;11(3):514-519. [DOI] [PubMed] [Google Scholar]
- 40. Wikström B, Gellert R, Ladefoged SD, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16(12):3742-3747. [DOI] [PubMed] [Google Scholar]
- 41. Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25(4):1251-1257. [DOI] [PubMed] [Google Scholar]
- 42. Mathur VS, Kumar J, Crawford PW, Hait H, Sciascia T; TR02 Study Investigators. A multicenter, randomized, double-blind, placebo-controlled trial of nalbuphine ER tablets for uremic pruritus. Am J Nephrol. 2017;46(6):450-458. [DOI] [PubMed] [Google Scholar]
- 43. Russo GE, Spaziani M, Guidotti C, et al. [Pruritus in chronic uremic patients in periodic hemodialysis. Treatment with terfenadine (an antagonist of histamine H1 receptors)]. Minerva Urol Nefrol. 1986;38(4):443-447. [PubMed] [Google Scholar]
- 44. Verduzco HA, Shirazian S. CKD-Associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep. 2020;5(9):1387-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gilchrest BA, Rowe JW, Brown RS, Steinman TI, Arndt KA. Relief of uremic pruritus with ultraviolet phototherapy. N Engl J Med. 1977;297(3):136-138. [DOI] [PubMed] [Google Scholar]
- 46. Gilchrest BA, Rowe JW, Brown RS, Steinman TI, Arndt KA. Ultraviolet phototherapy of uremic pruritus. Long-term results and possible mechanism of action. Ann Intern Med. 1979;91(1):17-21. [DOI] [PubMed] [Google Scholar]
- 47. Kim KH, Lee MS, Choi SM. Acupuncture for treating uremic pruritus in patients with end-stage renal disease: a systematic review. J Pain Symptom Manage. 2010;40(1):117-125. [DOI] [PubMed] [Google Scholar]
- 48. Wilkinson TJ, Watson EL, Gould DW, et al. Twelve weeks of supervised exercise improves self-reported symptom burden and fatigue in chronic kidney disease: a secondary analysis of the “ExTra CKD” trial. Clin Kidney J. 2019;12(1):113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]