Abstract
Background
Treatment decisions for multiple sclerosis (MS) are influenced by many factors such as disease symptoms, comorbidities, and tolerability.
Objective
To determine how much relapsing MS patients were willing to accept the worsening of certain aspects of their MS in return for improvements in symptoms or treatment convenience.
Methods
A web-based discrete choice experiment (DCE) was conducted in patients with relapsing MS. Multinomial logit models were used to estimate relative attribute importance (RAI) and to quantify attribute trade-offs.
Results
The DCE was completed by 817 participants from the US, the UK, Poland, and Russia. The most valued attributes of MS therapy to participants were effects on physical fatigue (RAI = 22.3%), cognitive fatigue (RAI = 22.0%), relapses over 2 years (RAI = 20.7%), and MS progression (RAI = 18.4%). Participants would accept six additional relapses in 2 years and a decrease of 7 years in time to disease progression to improve either cognitive or physical fatigue from “quite a bit of difficulty” to “no difficulty.”
Conclusion
Patients strongly valued improving cognitive and physical fatigue and were willing to accept additional relapses or a shorter time to disease progression to have less fatigue. The impact of fatigue on MS patients’ quality of life should be considered in treatment decisions.
Keywords: Multiple sclerosis, fatigue, relapsing-remitting, discrete choice experiment, patient preference, disease progression, disease-modifying therapies
Introduction
Patients with multiple sclerosis (MS) experience multiple symptoms including physical and cognitive fatigue, dysesthesia, gait difficulties, vision problems, dizziness, pain, and depression.1 Fatigue is the most common MS symptom, occurring in about 80% of MS patients.1 A variety of disease-modifying therapies (DMTs) with different mechanisms of action are available to lessen disease activity and disability progression in MS.2 These DMTs include oral, injectable, and infused medications, although their efficacy is greatest in relapsing MS and less in primary and secondary progressive MS. With the large range of DMTs available, selecting the appropriate DMT for each patient has become complex.2,3 Recent European4 and US5 guidelines for MS treatment provide updated general guidance, but no specific algorithm or pathway, leading management of MS to varying widely between clinicians.2 The choice of DMT is influenced by disease factors (e.g. disease activity and course), tolerability, and the patient profile (e.g. comorbidities), as well as healthcare system factors (e.g. resources and drug availability/costs).2,3
The growing consensus is that patient input is important during the development of new treatments and in treatment decision-making.6–8 Regulatory agencies are increasingly requesting and using patient preference data to support benefit-risk assessment and treatment decisions.9–11 Patient preference data provides insight into what value patients place on the benefits, risks, and other attributes of treatments, including the route of administration, as well as the trade-offs that patients are willing to make between the different attributes. This information can be used to assess what treatment profiles are most acceptable to patients and impact the overall value of a treatment. In MS, shared decision-making between patients and clinicians can improve adherence to DMTs,12 and modeling studies suggest that incorporating patient preferences into prescribing practices may provide health benefits.13
Attribute-based stated preference studies, especially discrete choice experiments (DCEs), have been used to examine the MS patient preferences in their choice of DMT.14 Although many studies have investigated important attributes of MS DMTs, most were performed 4 or more years ago and did not consider attributes relevant to contemporary MS therapies.14 The most common attributes considered were effects on relapse and progression, side effects, and route and frequency of administration; few stated preference studies have examined other aspects of administration, treatment monitoring, MS symptoms, or quality of life.14 Importantly, little is known about how MS patient preferences are affected by fatigue, even though fatigue is considered by patients to be more disabling than pain or physical disability and is one of the main reasons for reduced quality of life in MS.15 Understanding how fatigue affects patients with MS has been complicated by heterogeneous concepts and definitions and multiple domains of MS-related fatigue (physical, cognitive, and psychosocial).16
The current study explored the importance of improving cognitive and physical fatigue, two key domains of MS-related fatigue, and other key attributes of MS treatments to patients with relapsing MS. The objective of this study was to assess the relative importance of fatigue improvement compared to the improvement of common MS treatment attributes.
Methods
Study design
This study was a web-based DCE conducted in the US, the UK, Poland, and Russia in patients with relapsing MS. The DCE survey included a training component to orient participants to the treatment attributes and the choice tasks in the DCE. They were presented with DCE choice tasks, a set of three brief screening questions to assess health literacy, a subset of five items from the Numeracy Scale to assess numeracy,17,18 a sociodemographic questionnaire, and a clinical questionnaire including the Patient Determined Disease Steps (PDDS).19 Following an interim analysis of the DCE study, Fatigue Symptom and Impact Questionnaire – Relapsing Multiple Sclerosis – Symptom (FSIQ-RMS-S), a 20-item questionnaire for assessing the severity of fatigue, was incorporated into the study to accommodate other planned analyses. The results for the FSIQ-RMS-S are presented in a companion paper.20
Attribute selection
Attribute selection was based on the clinical relevance of the current treatment options and side effects in patients with MS.21–24 Physical and cognitive fatigue (independent of disability) were the primary attributes of interest whereas immune system recovery time, monitoring visits, number of relapses in 2 years, drug interactions, and time until MS progresses are common treatment characteristics typically considered when choosing an MS therapy (Table 1). The treatment benefits of a number of relapses and time until MS progression align with typical primary and secondary efficacy endpoints in MS clinical trials.21 A primary aspect of MS treatment selection is tolerability and convenience, which drove the decision to include drug interactions and monitoring visits.
Table 1.
Attributes and levels included in the discrete choice experiment.
| Attribute | Description | Levels |
|---|---|---|
| Drug interactions | Some MS medications do not interact well with
other medications that you may need to take for other
conditions. A medication may cause unexpected side effects or cancel out the effects of another medication. If a drug interacts with other drugs, you will have to be aware of what other medications you take to manage and treat any illnesses. If you need a prohibited medication, you may need to stop your MS medication. Note that drug interaction is not related to the number of drugs you need to take to treat your MS. |
None: Your MS medication works well with other medications. |
| Few: Your MS medication interacts with a few other medications. As a result, you need to remember one to two medications that are not to be combined with your MS medication. | ||
| Many: Your MS medication interacts with many (more than two) other medications. You would need to be careful and have a list of prohibited medications to check against before taking any other medication. (Reference level) | ||
| Cognitive fatigue | Having difficulty thinking clearly and not being
able to understand what you hear or read. Some parts of your brain and nerve tissue related to cognitive function are affected by MS. This is commonly referred to as ‘brain fog.’ Because people’s circumstances vary, some people need to make lifestyle changes while some do not. |
No difficulty |
| A little difficulty | ||
| Moderate difficulty | ||
| Quite a bit of difficulty (Reference level) | ||
| Physical fatigue | Having difficulty staying active throughout the
day. MS-related fatigue tends to get worse as the day goes on, is often aggravated by heat and humidity, and comes on more easily and suddenly than normal fatigue. Fatigue warning signs may include tired eyes, tired legs, whole-body tiredness, stiff shoulders, decreased energy or a lack of energy. |
No difficulty |
| A little difficulty: You have to be careful with planning daily activities in advance. | ||
| Moderate difficulty: You need a rest during the day or accomplish all tasks in the morning. | ||
| Quite a bit of difficulty: You can barely get anything done. (Reference level) | ||
| Immune system recovery time | Time your immune system takes to recover after
changing your MS medication. The faster your immune system
recovers, the better you are protected. With some MS medications, your immune system is compromised and unable to reproduce immune cells, even after changing your MS medication. This may leave you less able to fight various infections, such as respiratory infections or the common cold. Time your immune system takes to recover varies depending on MS medications; but the shorter, the better. |
1 to 2 weeks: It takes between 1 and 2 weeks for your immune system to return to normal. |
| 2 months: It takes 2 months for your immune system to return to normal. | ||
| 3 months: It takes 3 months for your immune system to return to normal. | ||
| More than 6 months: It takes at least 6 months (and up to 2 years) for your immune system to return to normal. (Reference level) | ||
| Monitoring visits | How often and for what time after starting the
treatment you will have to visit a clinic to monitor your
health for any potential side effect of the
treatment. While you do not experience any symptoms, some MS medications may affect your heart and need to be monitored. You may have to stay at a hospital from 4 to 12 hours for non-invasive laboratory and other screening tests (e.g., electrocardiogram [EKG] test) at each monitoring visit. |
Once weekly, for 1 month |
| Once every 2 weeks, for 2 months | ||
| Once every 3 weeks, for 3 months | ||
| Once every month, for 4 months (Reference level) | ||
| Number of relapses within 2 years | You may experience an MS relapse (also called an exacerbation, attack, or flare-up) within the next two years. A relapse can be characterised by signs or symptoms such as muscle weakness, tingling, double vision, or dizziness; and are due to changes in the central nervous systems due to inflammation that occurs in MS. The frequency of relapses varies between patients. | 1 relapse within 2 years |
| 2 relapses within 2 years | ||
| 3 relapses between 2 years a | ||
| 4 relapses within 2 years | ||
| 6 relapses within 2 years b (Reference level) | ||
| Time until your MS progresses | MS may lead to significant decline in different
functions by the central nervous system, such as your muscle
strength, sight, bowel and bladder control, mental
performance, sensation, balance, coordination of movements,
and ability to walk. MS treatments, in addition to reducing the frequency of relapses, also aim at delaying the time to the next level of disability. Significant worsening does not necessarily mean that the person is always seriously disabled but covers a wide range of functions from almost normal to severely impaired. |
8 years |
| 6 years | ||
| 4 years | ||
| 2 years (Reference level) |
Pilot testing
Qualitative pilot interviews (n = 20, n = 5 from each country) with participants diagnosed with MS were completed to confirm that the DCE questionnaire was understood and to assess whether the participants thought that attributes other than those included in the DCE would be important MS treatment characteristics and relevant for the DCE study. Validation of the DCE followed best practice guidelines.11,25 The survey was translated to target languages and pilot interviews were conducted in participants’ native languages. No changes were made to the DCE following the pilot interviews. Following insight gained from an interim analysis in the first 201 participants who completed the web-based DCE, levels for the number of relapses in 2 years were changed (from 1, 2, 3, and 4 to 1, 2, 4, and 6), so that the maximal acceptable relapses would fall within the range of relapses included in the survey.
Ethics
The study was approved by Ethical & Independent (E&I) Review Services (ref. no. 18156) and participants provided electronic consent before participating in the study.
Participants
The study enrolled adults (aged ≥ 18 years) who self-reported a diagnosis of relapsing MS (clinically isolated syndrome, relapsing MS, or secondary progressive MS with relapses). Participants were recruited through Global Perspectives, a vendor specializing in patient recruitment for online research studies, and participants were drawn from a population who had previously opted in to be contacted about research studies. Relapsing MS could include clinically isolated syndrome, relapsing-remitting MS, or secondary progressive MS. MS disease was defined as ≥ 1 patient-reported MS attack in the previous year or ≥ 2 patient-reported MS attacks in the 2 years before starting DMT. Participants had to be able to read, understand, and communicate in English (the US and the UK), Polish (Poland), or Russian (Russia) and could not have a significant cognitive impairment or acute psychopathology that would interfere with participation in the survey. Participants received a stipend for study participation.
DCE design
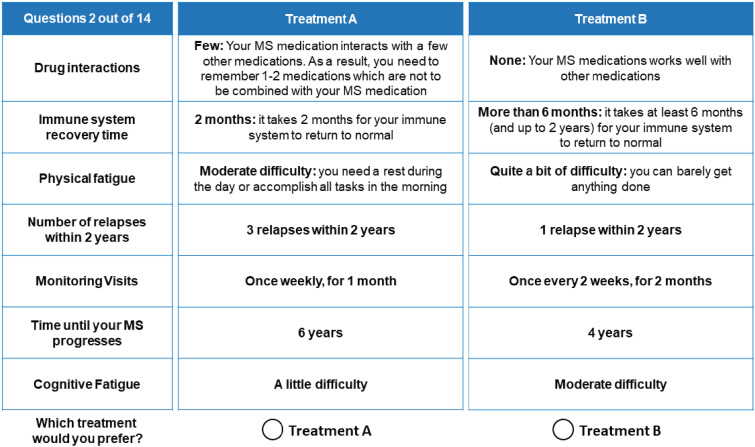
Prior to completing the experimental choice tasks, participants were presented with a brief description of the study's purpose and completed a practice choice task. In each choice task, participants were presented with two hypothetical treatments whose performance was described using the seven attributes. They were asked to indicate which profile was preferable. An example choice task is shown in Figure 1. For each participant, the survey included 12 experimental choice tasks, one repeated choice task, and a fixed-choice dominance task. The repeated question was included in a random location and was the same as the first DCE choice question. This allowed for an assessment of the internal validity or consistency of the participants’ answers. In the fixed-choice question, one treatment was dominant, performing better than the other treatment on at least one attribute, and the same as the other treatment on all other attributes. The fixed-choice question was used to assess whether participants understood the discrete choice task and were consistent in their choices.
Figure 1.
Example choice task. Participants were introduced to a vignette of a physician telling them they needed to start MS treatment that day and that the physician was reviewing different medication options with them. The choice tasks in the discrete choice experiment (DCE) included those options.
Statistical analysis
Only data from completely answered surveys were included in the analysis. StataMP 15.0 (StataCorp, College Station, TX, USA) and R version 3.6.1 (R Foundation, Vienna, Austria) were used to conduct the analyses. All statistical tests were two-sided and used a significance level of 0.05. No adjustments for multiple comparisons were performed.
Health literacy17 and numeracy18 were classified according to validated scoring rules. Health literacy was defined as inadequate if the health literacy score (score range was 0–3) was ≤ 217 and adequate if the health literacy score was > 2. Numeracy skills were defined as inadequate if the numeracy score (score range was 0–5) was ≤ 2 and adequate if the numeracy score was > 2.18 Patients were not excluded from the analysis based on their health literacy or numeracy; these measures were solely used for characterizing the sample.
Multinomial logit (MNL) models and a heteroskedastic MNL (HMNL) were used for the DCE analysis. The HMNL model was used to validate the pooling of data from different countries. An MNL model was used to calculate the β coefficients (marginal utilities) which describe treatment preferences for attribute levels. An MNL model was also used to calculate marginal rates of substitution (MRS), which were used to quantify trade-offs participants would be willing to make (the change in one attribute they would accept for a change in another attribute). Before calculating MRS, the assumption of linearity was tested for a maximum acceptable increase in a number of relapses (MAR) within 2 years and a maximum acceptable decrease in time to MS disease progression (MAT). The relative attribute importance (RAI), which reflects the maximum proportion of the variance in the overall preferences that is accounted for by each attribute, was calculated by dividing the range of the coefficients of each attribute's levels by the sum of these ranges across all attributes. Subgroup analyses were conducted for age (≤ 40 vs. > 40 years) and gender (male vs. female). For each subgroup, an MNL model was estimated by including additional interaction effects between the participants’ observable characteristics and the attribute levels. Further details of the MNL and HMNL models are provided in the Supplemental Methods.
Results
Participant characteristics
Data collection leading to the interim analysis was conducted in January 2019 and included 201 participants (50 each in the US, Russia, and Poland and 51 in the UK). The remaining data collection (“full study”) was completed by 817 participants (350 in the US, 157 in the UK, and 155 each in Russia and Poland) between June and July 2020 (Supplemental Figure 1 and Supplemental Results). The average participant age was 41.8 years. Most were female (77%) and 58% had at least a college degree (Table 2). Participants mostly had relapsing-remitting MS (76%) and 67% reported having had a relapse within the previous 12 months. Self-reported disability was mild for 56%, moderate for 36%, and severe for 8%. The most frequently reported DMTs were dimethyl fumarate (20%) and glatiramer acetate (19%). Characteristics by country are provided in Supplemental Table 1. Most participants had adequate health literacy (n = 794, 97%) and adequate numeracy (n = 725, 89%) (Supplemental Table 2).
Table 2.
Sociodemographic and clinical characteristics of participants.
| Total | |
|---|---|
| Characteristic | (N = 817) |
| Age, mean (SD) | 41.8 (10.8) |
| Male, n (%) | 187 (23)a |
| Employment status, n (%) | |
| Employed, full-time | 316 (39) |
| Employed, part-time | 144 (18) |
| Full-time homemaker | 80 (10) |
| Student | 23 (3) |
| Retired | 73 (9) |
| Unemployed | 73 (9) |
| On disability pension | 179 (22) |
| Education level, n (%) | |
| Elementary school | 5 (1) |
| High school | 119 (15) |
| Some college | 210 (26) |
| College degree | 381 (47) |
| Postgraduate degree | 92 (11) |
| Others | 10 (1) |
| MS type, n (%) | |
| Clinically isolated syndrome | 12 (2) |
| Relapsing-remitting MS | 625 (77) |
| Secondary progressive | 98 (12) |
| Relapsing MS (exact type unspecified) | 82 (10) |
| PDDS, n (%) | |
| Normal | 146 (18) |
| Mild disability | 154 (19) |
| Moderate disability | 155 (19) |
| Gait disability | 140 (17) |
| Early cane | 103 (13) |
| Late cane | 53 (7) |
| Bilateral support | 41 (5) |
| Wheelchair/scooter | 20 (2) |
| Bedridden | 5 (1) |
| Time since the last relapse, n (%) | |
| < 12 months | 483 (59) |
| 12–24 months | 169 (21) |
| 24–36 months | 72 (9) |
| > 36 months | 93 (11) |
| Time since diagnosis (years), mean (SD) | 9.4 (8) |
| Current medication, n (%) | |
| Alemtuzumab | 45 (6) |
| Cladribine | 19 (2) |
| Corticosteroids | 38 (5) |
| Dimethyl fumarate | 164 (20) |
| Fingolimod | 85 (10) |
| Glatiramer acetate | 153 (19) |
| Interferons | 187 (23) |
| Mitoxantrone | 11 (1) |
| Natalizumab | 99 (12) |
| Ocrelizumab | 95 (12) |
| Peginterferon beta-1a | 18 (2) |
| Teriflunomide | 60 (7) |
MS: multiple sclerosis; PDDS: patient determined disease steps; SD: standard deviation.
N = 816; one participant chose not to report their gender.
Internal validity
In the total population, 759 (93%) of participants passed the fixed choice dominance question and 630 (77%) reported the same answer for the repeated question (Supplemental Table 2). These answers indicate good validity and are aligned with other DCE studies.26
Preferences
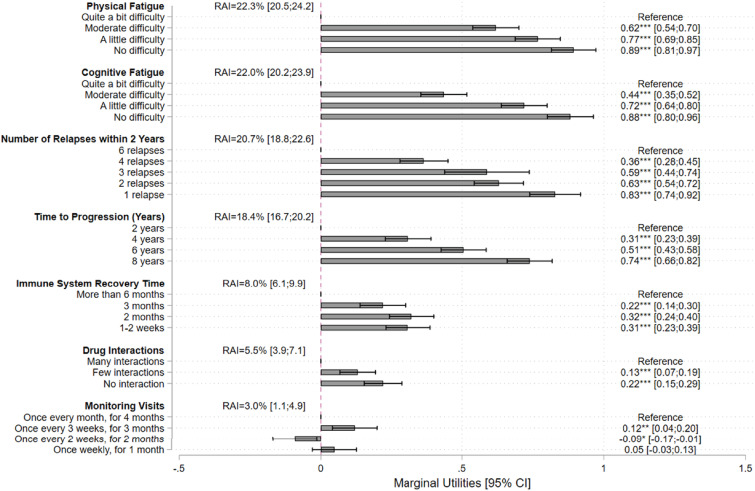
For all levels, preferences versus the reference level were significant for all attributes except “once weekly for one month” for “monitoring visits” (Figure 2 and Supplemental Tables 3 and 4), suggesting that participants valued all attributes when making their choices. In addition, all estimated preferences were in the expected direction (i.e. on average, participants preferred improvement in an attribute level) (see Supplemental Results). Physical fatigue (RAI = 22.3%), cognitive fatigue (RAI = 22.0%), number of relapses within 2 years (RAI = 20.7%), and time to MS progression (RAI = 18.4%) were the major drivers of preferences. Immune system recovery time (RAI = 8.0%), drug interactions (RAI = 5.5%), and monitoring visits (RAI = 3.0%) were the least valued attributes.
Figure 2.
Multinomial logit model results: marginal utilities and RAI.
RAI: relative attribute importance; CI: confidence interval.
Trade-offs between attributes
Table 3 shows the tradeoffs in a number of relapses and time to disease progression that participants would accept in exchange for improvements in the other attributes. To improve cognitive fatigue from the reference level “quite a bit of difficulty” to “no difficulty,” participants would be willing to accept up to 5.5 (95% CI = 4.9−6.2) additional relapses in 2 years and a decrease of up to 7.4 (6.4−8.4) years in time to MS disease progression. To improve physical fatigue from the reference level “quite a bit of difficulty” to “no difficulty,” participants would be willing to accept up to 5.6 (4.8−6.3) additional relapses in 2 years and a decrease of up to 7.4 (6.5−8.4) years in time to MS disease progression. Participants were also willing to accept up to 0.7 (0.6−0.8) additional relapses in 2 years to increase the time to MS progression by 1 year.
Table 3.
Trade-offs: marginal rates of substitution for MAR and MAT.
| Attribute | Level | MAR (relapses/ 2 years) (95% CI) | MAT (years) (95% CI) |
|---|---|---|---|
| Cognitive fatigue | Quite a bit of difficulty | Reference level | Reference level |
| Moderate difficulty | 2.7*** (2.2, 3.3) | 3.6*** (2.9, 4.4) | |
| A little difficulty | 4.5*** (3.8, 5.1) | 6.0*** (5.1, 6.9) | |
| No difficulty | 5.5*** (4.9, 6.2) | 7.4*** (6.4, 8.4) | |
| Physical fatigue | Quite a bit of difficulty | Reference level | Reference level |
| Moderate difficulty | 3.9*** (3.2, 4.5) | 5.2*** (4.3, 6.0) | |
| A little difficulty | 4.8*** (4.1, 5.5) | 6.4*** (5.5, 7.4) | |
| No difficulty | 5.6*** (4.8, 6.3) | 7.4*** (6.5, 8.4) | |
| Drug interaction | Many interactions | Reference level | Reference level |
| Few interactions | 0.8*** (0.4, 1.2) | 1.1*** (0.6, 1.6) | |
| No interactions | 1.3*** (0.9, 1.8) | 1.8*** (1.2, 2.4) | |
| Immune system recovery time | >6 months | Reference level | Reference level |
| 3 months | 1.4*** (0.9, 1.9) | 1.9*** (1.2, 2.6) | |
| 2 months | 2.1*** (1.6, 2.6) | 2.8*** (2.1, 3.5) | |
| 1 to 2 weeks | 1.9*** (1.4, 2.4) | 2.5*** (1.8, 3.2) | |
| Monitoring visits | Once every month for 4 months | Reference level | Reference level |
| Once every 3 weeks for 3 months | 0.8** (0.3, 1.3) | 1.0** (0.4, 1.7) | |
| Once every 2 weeks for 2 months | −0.5* (−1.0, −0.1) | −0.7* (−1.4, −0.1) | |
| Once weekly for 1 month | 0.3 (−0.2, 0.8) | 0.4 (−0.3, 1.0) | |
| Time to progression | An increase in 1 year | 0.7*** (0.6, 0.8) | – |
| Number of relapses in 2 years | One less relapse in 2 years | – | 1.3*** (1.2, 1.5) |
CI: confidence interval; MAR: maximum acceptable number of relapses in 2 years; MAT: maximum acceptable decrease in time to disease progression.
*p < 0.05, **p < 0.01, ***p < 0.001.
Subgroup analysis
Men placed significantly less value than women on reducing drug interactions, improving cognitive and physical fatigue from “quite a bit of difficulty” to “no difficulty,” reducing the number of relapses over 2 years, and improving time to MS disease progression (Supplemental Table 4).
Participants aged ≥ 40 years placed significantly more value than those aged < 40 years on having fewer drug interactions, improved cognitive and physical fatigue, having more frequent monitoring, and increased time to MS disease progression (Supplemental Table 5). Participants with a diagnosis of MS for ≥ 10 years placed significantly more value than those with a diagnosis of MS for < 10 years on improving cognitive and physical fatigue from “quite a bit of difficulty” to “no difficulty,” a faster immune system recovery time, reducing the number of relapses within 2 years, and increasing time to MS disease progression (Supplemental Table 6). No evident differences were found in preferences between countries (data not shown).
Discussion
Studies on treatment preferences of patients with MS have commonly included effects on relapse and progression, side effects, and route and frequency of administration.14 Few have examined other aspects of administration, monitoring, and MS symptoms like fatigue, or quality of life. Other preference studies in patients with MS have assessed a number of relapses, drug interactions, monitoring requirements, and time to disease progression as attributes.14 To our knowledge, this is the first study to measure the willingness of MS patients to accept the worsening of their MS (from additional relapses and time to disease progression) if their fatigue could be improved. This study showed that, when choosing a treatment, patients with relapsing MS placed the highest value on four key attributes, cognitive and physical fatigue, number of relapses in 2 years, and time to disease progression. Study participants were willing to accept more relapses and decreased time to disease progression to reduce cognitive and physical fatigue. Cognitive and physical fatigue were especially valued by patients who had been diagnosed with MS for at least 10 years.
Fatigue is a very common initial and on-going progressive symptom in MS and can be very troublesome to patients.27 Fatigue in MS can be characterized as exhaustion and a lack of energy that can be aggravated by physical activity and other MS symptoms.15 Fatigue can have many associated symptoms, including mood disruption (such as depression and anxiety) and sleep disruption. The etiology of fatigue in MS is unknown but may involve changes in neurotransmission and circadian rhythms caused by the immune system or central nervous system damage.15 The importance of fatigue to patients with MS is illustrated through this study where participants were willing to make substantial trade-offs in time to disease progression and relapses to reduce physical and cognitive fatigue. This finding emphasizes the importance of considering fatigue in patient-centered benefit–risk assessment and shared decision-making between patients and physicians when selecting treatments. Some shared decision-making tools are available to physicians treating MS, including the Considering Risks and Benefits in Multiple Sclerosis Treatment Selection (CRIMSON) project decision aid.28
Participants were willing to accept additional relapses or a shorter time to disease progression to reduce the number of drug interactions, shorten immune system recovery time, and reduce the frequency of monitoring visits, although these were less important to men than to women and to those who had been diagnosed for at least 10 years than to those who had been diagnosed more recently.
We did not detect differences in preferences between participants from different countries. Given our attributes are related mostly to health-related treatment outcomes and not to health service provision, we did not expect major differences in patient preferences across countries. Additionally, the lack of observed differences could be due to the limited sample size per country as the study was not powered for cross-country comparisons.
The study had some limitations. The attributes were selected to reflect the research objectives, and although we probed for missing attributes in the pilot interviews, there may be other attributes relevant to patients’ treatment choice that may have been elicited with additional qualitative interviews. Participants self-reported their diagnosis which may have led to misreporting if patients were uncertain about the exact subtype of their relapsing form of MS. Another limitation is that the results may not be directly transferrable to populations with different demographics than those of the four participating countries. Moreover, the participants were recruited from an online panel and therefore could be less representative of the general MS population. However, the study populations had similar sociodemographic and clinical characteristics that appear representative of the US and European MS populations.29–31 Furthermore, a common limitation of web-based stated preference surveys is that participants may not pay sufficient attention to the presented medical information or choice tasks. However, in this study, the participants’ responses displayed good internal validity that was consistent with other health-related DCE data.26 In addition, the sample demonstrated adequate levels of health literacy and numeracy in line with other preference studies.32, 33 Additionally, we chose to examine the trade-offs participants would make to reduce fatigue, separate from considerations of disability, which we recognize is also important to patients with MS. However, while patients with MS may rank bothersome symptoms differently, fatigue is known to have a profound impact on their quality of life and work productivity, which warrants its own analysis.
To better understand fatigue in MS, patient-reported outcome measures such as the recently developed FSIQ-RMS-S34 could be incorporated. Recently, the FSIQ-RMS-S was used in the OPTIMUM trial, a randomized phase 3 clinical trial that compared oral ponesimod to teriflunomide in patients with relapsing multiple sclerosis. In a companion paper, we assess how the present DCE results apply to the changes in fatigue and annualized relapse rates seen in OPTIMUM.20 Future development of other therapies for MS should measure their effects on fatigue using FSIQ-RMS-S or other appropriate instruments gave the importance of fatigue to MS patients.
In conclusion, this study showed that patients with relapsing MS preferred to reduce physical and cognitive fatigue the most over other treatment attributes and that patients were willing to make substantial trade-offs to lessen fatigue symptoms. The information from the current study should help to inform benefit–risk assessment of different treatments, especially regarding fatigue, and support shared decision-making about treatments between patients and their physicians.
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173221150370 for Treatment preferences in relation to fatigue of patients with relapsing multiple sclerosis: A discrete choice experiment by Tommi Tervonen, Robert J Fox, Anne Brooks, Tatiana Sidorenko, Neli Boyanova and Bennett Levitan, Brian Hennessy, Andrea Phillips-Beyer in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Abbreviations
- DCE
discrete choice experiment
- DMT
disease-modifying treatment
- HMNL
heteroskedastic multinomial logit model
- MAR
maximal acceptable increase in number of relapses
- MAT
maximal acceptable decrease in time to MS disease progression
- MNL
multinomial logit model
- MRS
marginal rates of substitution
- MS
multiple sclerosis
- PDDS
patient determined disease steps
- RAI
relative attribute importance.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TT and AB were employees of Evidera, who were paid by Actelion Pharmaceuticals Ltd, now a Janssen Pharmaceutical Company of Johnson & Johnson, for work on this study. TS is employee of Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical company of Johnson & Johnson, and may hold stock in Johnson & Johnson. NB and BH are employees of Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical company of Johnson & Johnson. BL is an employee of Janssen Research and Development, LLC. BL, BH, and NB are stockholders in Johnson & Johnson and have a portfolio that at times includes other pharmaceutical and healthcare-related companies. APB is a director of Innovus Consulting Ltd and holds a stock portfolio that at times includes pharmaceutical and healthcare-related companies. RF has received personal consulting fees from AB Science, Biogen, Celgene, EMD Serono, Genentech, Genzyme, Greenwich Biosciences, Immunic, Janssen, Novartis, Sanofi, and TG Therapeutics. RF has served on advisory committees for AB Science, Biogen, Genzyme, Immunic, Janssen, Novartis, Sanofi, and TG Therapeutics, and received a clinical trial contract and research grant funding from Biogen, Novartis, and Sanofi.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Actelion Pharmaceuticals Ltd, which is now a Janssen Pharmaceutical Company of Johnson & Johnson.
Research ethics and patient consent: The DCE study was approved by the institutional review board (IRB) Ethical & Independent (E&I) Review Services (Ref No. 18156).
Research data: Data are available from the corresponding author upon reasonable request.
Third-party submissions: This manuscript was submitted by Evidera staff on behalf of the authors.
Writing assistance: Writing assistance was provided by Holly Richendrfer, Stephen Gilliver, and Phillip Leventhal of Evidera, and was paid for by Actelion Pharmaceuticals Ltd, which is now a Janssen Pharmaceutical Company of Johnson & Johnson.
ORCID iDs: Tommi Tervonen https://orcid.org/0000-0001-7303-500X
Robert J Fox https://orcid.org/0000-0002-4263-3717
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tommi Tervonen, Kielo Research, Zug, Switzerland and Evidera, London, UK.
Robert J Fox, Mellen Center for Multiple Sclerosis, Neurological Institute, Cleveland Clinic, Cleveland, OH, USA.
Anne Brooks, Evidera, Bethesda, MD, USA.
Bennett Levitan, Janssen Research & Development, Titusville, NJ, USA.
Brian Hennessy, Actelion Pharmaceuticals, Part of Janssen Pharmaceutical Companies, Allschwil, Switzerland.
Andrea Phillips-Beyer, Innovus Consulting Ltd, London, UK.
References
- 1.National MS Society. MS Symptoms, https://www.nationalmssociety.org/Symptoms-Diagnosis/MS-Symptoms (2020, accessed July 14, 2021).
- 2.Linker RA, Chan A. Navigating choice in multiple sclerosis management. Neurological Research and Practice 2019; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghezzi A. European and American guidelines for multiple sclerosis treatment. Neurol Ther 2018; 7: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 2018; 24: 96–120. [DOI] [PubMed] [Google Scholar]
- 5.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology 2018; 90: 777 −788. [DOI] [PubMed] [Google Scholar]
- 6.van Overbeeke E, Vanbinst I, Jimenez-Moreno AC, et al. Patient Centricity in Patient Preference Studies: The Patient Perspective. Front Med (Lausanne) 2020; 7. DOI: 10.3389/fmed.2020.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whichello C, Bywall KS, Mauer J, et al. An overview of critical decision-points in the medical product lifecycle: where to include patient preference information in the decision-making process? Health Policy 2020; 124: 1325–1332 [DOI] [PubMed] [Google Scholar]
- 8.van Overbeeke E, Whichello C, Janssens R, et al. Factors and situations influencing the value of patient preference studies along the medical product lifecycle: a literature review. Drug Discov Today 2019; 24: 57–68 [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims, http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf (2009, accessed July 30, 2021). [DOI] [PMC free article] [PubMed]
- 10.European Medicines Agency. Reinforce patient relevance in evidence generation: https://www.ema.europa.eu/en/documents/presentation/presentation-ema-regulatory-science-2025-reinforce-patient-relevance-evidence-generation_en.pdf. 2019.
- 11.Food and Drug Administration. Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. 2016.
- 12.Ben-Zacharia A, Adamson M, Boyd A, et al. Impact of shared decision making on disease-modifying drug adherence in multiple sclerosis. Int J MS Care 2018; 20: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Eijndhoven E, Brauer M, Kee R, et al. Modeling the impact of patient treatment preference on health outcomes in relapsing-remitting multiple sclerosis. J Med Econ 2020; 23: 474–483. [DOI] [PubMed] [Google Scholar]
- 14.Webb EJD, Meads D, Eskyte I, et al. A systematic review of discrete-choice experiments and conjoint analysis studies in people with multiple sclerosis. Patient 2018; 11: 391–402. [DOI] [PubMed] [Google Scholar]
- 15.Braley TJ, Chervin RD. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep 2010; 33: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwid SR, Covington M, Segal BM, et al. Fatigue in multiple sclerosis: current understanding and future directions. J Rehabil Res Dev 2002; 39: 211–224. [PubMed] [Google Scholar]
- 17.Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008; 23: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fransen MP, Van Schaik TM, Twickler TB, et al. Applicability of internationally available health literacy measures in the Netherlands. J Health Commun 2011; 16: 134–149. [DOI] [PubMed] [Google Scholar]
- 19.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology 1995; 45: 251–255. [DOI] [PubMed] [Google Scholar]
- 20.Fox RJ, Scherz T, Boyanova N, et al. The relevance of fatigue to relapse rate in multiple sclerosis: applying patient preference data to the OPTIMUM trial. Mult Scler J 2022; in press. Published online 22 December 2022. doi.org/10.1177/13524585221140270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappos L, Fox RJ, Burcklen M, et al. Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study: a randomized clinical trial. JAMA Neurol 2021; 78: 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutebi A, Warholak TL, Hines LE, et al. Assessing patients’ information needs regarding drug-drug interactions. J Am Pharm Assoc 2013; 53: 39–45. [DOI] [PubMed] [Google Scholar]
- 23.Abd-Elfattah HM, Abdelazeim FH, Elshennawy S. Physical and cognitive consequences of fatigue: a review. J Adv Res 2015; 6: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amtmann D, Bamer AM, Kim J, et al. People with multiple sclerosis report significantly worse symptoms and health related quality of life than the US general population as measured by PROMIS and NeuroQoL outcome measures. Disabil Health J 2018; 11: 99–107. [DOI] [PubMed] [Google Scholar]
- 25.Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health 2011; 14: 403−413. 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Tervonen T, Schmidt-Ott T, Marsh K, et al. Assessing rationality in discrete choice experiments in health: an investigation into the use of dominance tests. Value Health 2018; 21: 1192–1197. [DOI] [PubMed] [Google Scholar]
- 27.Fox RJ, Bacon TE, Chamot E, et al. Prevalence of multiple sclerosis symptoms across lifespan: data from the NARCOMS registry. Neurodegener Dis Manag 2015; 5: 3–10. [DOI] [PubMed] [Google Scholar]
- 28.The Considering Risks and Benefits in Multiple Sclerosis Treatment Selection (CRIMSON) Project Decision Aid. Treatment, Decision Making and Relapsing Remitting Multiple Sclerosis. Available at: https://relapsingmsdecisions.com/. (Accessed October 2022).
- 29.Public Health England. Research and analysis. Multiple sclerosis: prevalence, incidence and smoking status - data briefing, https://www.gov.uk/government/publications/multiple-sclerosis-prevalence-incidence-and-smoking-status/multiple-sclerosis-prevalence-incidence-and-smoking-status-data-briefing (2020, accessed 17 July 2020).
- 30.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 2019; 92: e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinto CA, Chua GN, Bridges JFP, et al. Comparing patient preferences for antithrombotic treatment during the acute and chronic phases of myocardial infarction: a discrete-choice experiment. Patient 2021; 15(2): 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tervonen T, Hawken N, Hanania NA, et al. Maintenance inhaler therapy preferences of patients with asthma or chronic obstructive pulmonary disease: a discrete choice experiment. Thorax 2020; 75: 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudgens S, Schuler R, Stokes J, et al. Development and validation of the FSIQ-RMS: a new patient-reported questionnaire to assess symptoms and impacts of fatigue in relapsing multiple sclerosis. Value Health 2019; 22: 453−466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173221150370 for Treatment preferences in relation to fatigue of patients with relapsing multiple sclerosis: A discrete choice experiment by Tommi Tervonen, Robert J Fox, Anne Brooks, Tatiana Sidorenko, Neli Boyanova and Bennett Levitan, Brian Hennessy, Andrea Phillips-Beyer in Multiple Sclerosis Journal – Experimental, Translational and Clinical