Abstract
Aims and Objectives
Long COVID is defined as the continuation of symptoms for four or more weeks after initial contraction of the virus. This review article examines the role of four select micronutrients (zinc, vitamins C, D and polyphenols) for their anti‐inflammatory and therapeutic potential to improve sleep‐related symptoms in persons with long COVID.
Background
Evidence suggests a link between long COVID and increased inflammation. There are currently no therapeutic interventions for common sleep‐related symptoms associated with long COVID. Micronutrients, due to their antioxidant and anti‐inflammatory properties, may have a role in the treatment of sleep‐related symptoms in the context of long COVID.
Design
A narrative literature review was conducted and guided by the PRISMA checklist.
Methods
All articles were screened from PubMed, ScienceDirect, NCBI or Google Scholar and were limited to human studies. The following keywords were used: ‘COVID‐19’, ‘sleep symptoms’, ‘zinc’, ‘vitamin C’, ‘vitamin D’, ‘polyphenols’ and ‘micronutrients’.
Results
There are currently no studies that examine the usage of micronutrients and its impacts on long‐term, sleep‐related symptoms post‐COVID‐19 infection. We focussed our review on prior studies that examined micronutrients in the context of sleep symptoms and inflammation, while exploring the potential for micronutrients to help improve sleep‐related symptoms associated with long COVID.
Conclusions
There is evidence to suggest that sleep‐related symptoms associated with long COVID, such as fatigue and poor sleep quality, are associated with inflammation. Zinc, vitamins C, D and polyphenols all have the potential to improve both inflammation and sleep quality to alleviate symptoms. Future research should further examine these micronutrients in the context of long COVID to improve sleep and quality of life.
Relevance to Clinical Practice
This article provides implications for clinicians to be at the forefront of research on the usage of micronutrients to improve sleep‐related symptoms in persons with long COVID.
Keywords: COVID‐19, micronutrients, polyphenols, sleep, vitamin C, vitamin D, zinc
What does this paper contribute to the wider global clinical community?
A large proportion of people infected with COVID‐19 continue to have persistent sleep‐related symptoms, which lead to a decrease in quality of life. Safe and effective treatment options to manage sleep‐related COVID symptoms are needed.
Underlying inflammation may be a mechanism of persistent sleep related symptoms of long COVID. Zinc, vitamins C, D and polyphenols have been shown in the literature to improve sleep and possess anti‐inflammatory properties. These select micronutrients have the potential to decrease inflammation and improve sleep‐related symptoms in persons with long COVID symptoms.
Nurses can be at the forefront of this research to improve sleep‐related long COVID symptoms. They can guide clinical trials to explore the potential of micronutrients to improve inflammation and sleep‐related symptoms. Nurses are in a unique position to be at the forefront of this area of this research in order to improve sleep‐related symptoms in persons with long COVID.
1. INTRODUCTION
‘Long COVID’ has been frequently used to describe the continuation of symptoms even after recovery from the SARS‐CoV‐2 virus (Mahase, 2020). The CDC defines this as symptoms lasting for more than four weeks after initial infection (Centers for Disease Control & Prevention, 2021). Although the recent development of vaccines is a grand step towards bringing this pandemic to an end, it has been reported that a considerable proportion of infected persons are still experiencing persistent symptoms, even after more than three and a half months post‐discharge (Garrigues et al., 2020). Evidence suggests that a proportion of COVID survivors will continue to have health problems, such as breathing issues, muscle fatigue, mental health problems and difficulty performing everyday tasks (Mahase, 2020). Additionally, long‐term, sleep‐related symptoms have been especially prevalent in many cases, causing chronic fatigue and worsened sleep quality (Mandal et al., 2021).
Throughout this review, we present research from primary articles on four select micronutrients, namely zinc, vitamins C, D and polyphenols. We focused on these, as they have been studied in the context of improving inflammation and/or sleep and have potential utility in the management of long COVID symptoms. Zinc is an essential mineral that is found to improve sleep and has anti‐inflammatory properties (Butters & Whitehouse, 2021; Cherasse et al., 2015). Vitamin C has been found to reduce fatigue in healthy populations (Suh et al., 2012). Vitamin D has anti‐inflammatory properties and has been shown to reduce fatigue in vitamin D deficient, but otherwise healthy populations (Nowak et al., 2016). Higher intake of certain polyphenols may be associated with improved sleep quality in otherwise healthy adults (Godos et al., 2020).
In a systematic review of the prevalence of sleep problems during the pandemic, approximately 74.8% of those with COVID‐19 have sleep‐related problems (Jahrami et al., 2021). Common sleep‐related issues may become longer term problems in COVID‐19 patients even after recovery.
There are many similarities between long COVID symptoms and chronic fatigue syndrome (CFS) (Wong & Weitzer, 2021); thus, comparing these may give insight into the pathophysiology of sleep‐related symptoms post‐COVID‐19. CFS is defined as persistent sleep difficulties with various symptoms, such as severe fatigue (Cortes Rivera et al., 2019). Long COVID symptoms have overlaps with the clinical presentation of myalgic encephalomyelitis (ME)/chronic fatigue syndrome (Wong & Weitzer, 2021). The pathophysiological mechanism of ME/CSF is not known, although it may trigger an increase in proinflammatory cytokines and diminished antioxidants (Cortes Rivera et al., 2019).
1.1. Aims
Sleep symptoms are highly prevalent post‐COVID‐19, yet there is insufficient research assessing the application of micronutrients as treatment. This review proposes that zinc, vitamins C, D and polyphenols could potentially improve long‐term sleep‐related symptoms, in individuals with past COVID‐19 infection.
2. METHODS
A narrative literature review guided by the PRISMA checklist (File S1) was conducted. The articles were screened from PubMed, ScienceDirect, NCBI and Google Scholar. Our searches were related to three main concepts: micronutrients, sleep‐related symptoms and long COVID. Key search terms included ‘COVID‐19’, ‘long‐term sleep symptoms’, ‘zinc’, ‘vitamin C’, ‘vitamin D’, ‘polyphenols’ and ‘micronutrients’. The general criteria for inclusion of data were current, human studies that discuss mechanisms for long COVID and micronutrient usage on sleep‐related symptoms. Each micronutrient was searched along with the keywords, ‘COVID‐19’ and/or ‘sleep’ to find the most relevant primary data studies based on our inclusion criteria.
3. RESULTS
There are no studies that examine the effects of micronutrient supplementation in, specifically, sleep‐related symptoms post‐COVID‐19. Of the existing studies examined in this review, one article discusses zinc deficiency among COVID patients, while five articles provide information on zinc to relieve sleep symptoms in other populations. Three other articles provide information on vitamin C’s potential to reduce fatigue. Two sources focus on vitamin D reducing fatigue in general populations and another two in vitamin D‐deficient COVID patients. Moreover, three sources examine polyphenol supplementation to reduce inflammation and sleep‐related symptoms in CFS patients and healthy adults. See Figure 1.
FIGURE 1.
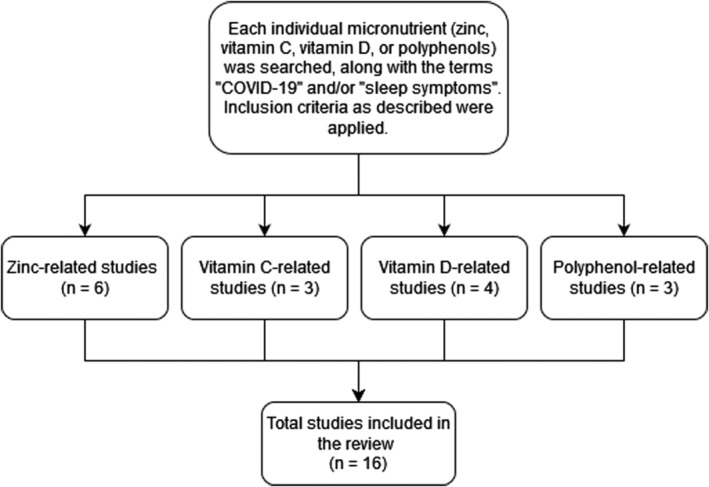
Illustrates our search process for the narrative literature review
3.1. Common lingering symptoms of COVID‐19
Approximately 67% of participants in a study by Taboada et al., 2021 reported a decreased quality of life six months after ICU discharge due to persistent symptoms (Taboada et al., 2021). As seen in Table 1, multiple published articles have identified continuous symptoms that are experienced after infection of COVID‐19.
TABLE 1.
Primary articles reporting long‐term symptoms of COVID‐19
| Author and Year | Type of study | Study population | Long‐term symptoms | Timeline of the assessment of symptoms/outcomes |
|---|---|---|---|---|
| Carfì et al. (2020) | Cross‐sectional study | 143 patients who were discharged from the hospital after recovery from COVID‐19 |
Fatigue (53.1%) Dyspnoea (43.4%) Joint pain (27.3%) Chest pain (21.7%) |
The symptoms were assessed a mean of 60.3 (SD, 13.6) days after onset of the first COVID‐19 symptom |
| Davis et al. (2021) | Cross‐sectional study | 3762 respondents with suspected or confirmed COVID‐19 infection |
Fatigue (86.7%) Decreased cognition or memory (88%) Relapses in symptoms due to physical/mental activity or stress (85.9%) |
>91% of respondents took a time to recovery that exceeded 35 weeks |
| Garrigues et al. (2020) | Cross‐sectional, study | 120 patients |
Fatigue (55%) Dyspnoea (42%) Memory Loss (34%) Problems concentrating (28%) Sleep disorders (30.8%) |
Post‐discharge persistent symptoms were assessed more than 100 days after their admission |
| Ladds et al. (2020) | Qualitative research design (Focus groups and individual interviews) | 114 participants | Nerve issues, fatigue, exhaustion, ‘brain fog’, decreased ability for daily activities | NA |
| Mandal et al. (2021) | Cross‐sectional study | 384 patients followed a median 54 days post discharge |
Fatigue (69%) Breathlessness (53%) Cough (34%) Depression (15%) 38% of chest radiographs stayed abnormal and 9% deteriorated |
4–6 weeks post‐discharge |
| Sudre et al. (2020) | Prospective observational cohort study | 4,182 incident cases of COVID‐19 were analyzed in which individuals self‐reported their symptoms |
Most commonly reported symptoms were the following: Fatigue (97.7%) and Intermittent headaches (91.2%) |
Data gathered from COVID‐19 infected persons using app from March 24‐September 2, 2022 |
| Taboada et al. (2021) | Cross‐sectional study | 91 participants previously admitted to COVID‐19 ICU |
Difficulty breathing (57%) Physical weakness (37%) Muscle pain (37%) Joint pain (29%) Moderately anxious or depressed (41.8%) Extremely anxious or depressed (4.4%) |
6 months after ICU discharge |
Other commonly reported symptoms include difficulty concentrating, brain fog, and continuous fatigue (Ladds et al., 2020). Fatigue was an especially prevalent, long‐term symptom that was self‐reported by 53.1% of 143 post‐acute care COVID patients about two months after first COVID‐19 symptom onset (Carfì et al., 2020). Fatigue was similarly reported in approximately 69% of 384 previously hospitalised patients after a median of 54 days post‐discharge (Mandal et al., 2021). Moreover, individual narrative interviews found that, in several of the interviewees, fatigue, exhaustion and ‘brain fog’ limited their daily routines, where one participant noted that ‘[their] life is just nothing like it was and it's not really the life [they] want’ (Ladds et al., 2020).
Studies in persons with suspected and confirmed COVID‐19 have demonstrated the majority of subjects (>91%) had a time to recovery lasting over 35 weeks, with fatigue being one of the most prominent symptoms after 6 months (Davis et al., 2021). Another study showed that fatigue was reported by about 97.7% of participants with incident cases of COVID‐19, and was most commonly observed in those with symptoms persisting past 28 days (Sudre et al., 2020).
3.2. Mechanisms underlying long‐term sleep‐related symptoms post‐COVID‐19
As COVID‐19 is a new area of study, the mechanisms pertaining to the development of long‐term symptoms are still unclear. It is possible that underlying inflammation is a key mechanism in causing persistent sleep‐related symptoms. Mandal et al. (2021) studied 384 patients who were followed a median of 54 days post discharge and found that those that were discharged with elevated biomarkers, many cases had persistently elevated C‐reactive protein (CRP) and d‐dimer (Mandal et al., 2021). In fact, increased levels of CRP and d‐dimer, are seemingly characteristic of infected persons due to systemic inflammation (Yael et al., 2020). Furthermore, 34%, 53% and 69% of participants reported coughing, breathlessness and fatigue respectively, and 38% of chest radiographs stayed abnormal and 9% deteriorated (Mandal et al., 2021). Inflammation may persist even 40–60 days after initial infection in asymptomatic and moderately affected patients (Doykov et al., 2020).
Alternatively, Crook et al. (2021) present other possible mechanisms of chronic fatigue in long COVID patients. In particular, cell‐mediated immune responses and systemic inflammation have been hypothesized to cause hypometabolism in the frontal lobe and cerebellum, inducing fatigue (Crook et al., 2021). Whether this is related to long COVID remains unknown (Crook et al., 2021). It is important to note that fatigue could also be a result of psychological distress due to social isolation, fear and anxiety from the COVID‐19 lockdown (Morgul et al., 2021). Based on results from the Fatigue Assessment Scale questionnaire, Morgul et al. (2021) found that 64.1% of 3672 healthy participants in Istanbul were experiencing fatigue. Further research is necessary to clarify the mechanisms that give rise to long‐term sleep‐related COVID‐19 symptoms.
3.3. Zinc to reduce long‐term sleep‐related symptoms of COVID‐19
Zinc may dose‐dependently increase the total amount of nonrapid eye movement sleep and decrease locomotor activity (Cherasse et al., 2015). Zinc has been suggested to be a potential therapeutic for COVID‐19 itself, as it promotes antiviral activity and inhibits the production of cytokines such as tumour necrosis factor‐alpha and IL‐1beta (Butters & Whitehouse, 2021).
Saito et al. (2017) administered zinc supplementation in the form of oysters and yeast extracts, both containing rich amounts of dietary zinc. A double‐blinded experiment was conducted with 120 healthy patients who were randomly assigned to a placebo or a zinc‐rich group and measured sleep using an actigraphy sleep monitor for 12 weeks (Saito et al., 2017). The zinc‐rich food group had decreased time needed to fall asleep and improved sleep efficiency compared with the placebo group, and the group that ingested zinc‐enriched yeast and astaxanthin oil showed improved sleep onset latency (Saito et al., 2017). A nutritional survey administered to 5587 random participants in the general US population found that zinc deficiency was associated with short sleep duration of less than five hours of sleep per night, after adjustment for overall diet (Grandner et al., 2013). Moreover, when zinc sulphate capsules were administered to 54 ICU nurses, they reported improved sleep quality after one month (Gholipour Baradari et al., 2018). Significantly increasing zinc concentrations can aid in improving sleep latency and sleep efficiency (Saito et al., 2017). While these previous studies were not conducted in COVID‐19 patients, the mechanisms underlying sleep‐related issues appear to be associated with inflammation. Administering select micronutrients may help to target this pathway and improve symptoms.
Greater serum zinc concentrations are negatively associated with the severity of fatigue and depression in CFS, such that decreased zinc causes more severe CFS (Maes et al., 2006). The FibroFatigue scale was used to assess the severity of CFS symptoms, measuring items such as fatigue symptoms, muscular pain and concentration (Maes et al., 2006). It has also been noted that CFS patients had higher oxidative stress in accordance with lower serum zinc levels (Maes et al., 2006). These studies further support the potential for zinc to improve sleep‐related long COVID symptoms. It is important that future studies explore this even further, as previous articles have suggested the high likelihood of COVID patients developing CFS in the future.
In one particular double‐blind, randomised study in fifteen hospitalized COVID‐19 patients, it was observed that hospitalized COVID‐19 patients showed a zinc deficiency, which was corrected when given high‐dose intravenous zinc (Patel et al., 2021). This study demonstrated that COVID‐19 patients appear to have zinc deficiency and that treatment to reverse this appears safe and feasible (Patel et al., 2021)
3.4. Antioxidants to reduce long‐term sleep‐related symptoms of COVID‐19
Many observations have led to the conclusion that COVID‐19 is associated with a substantial increase in cytokines, suggesting that infected persons are at the risk of increased systemic inflammation (Mazza et al., 2021). Thus, increased inflammation may be related to these persistent long COVID symptoms.
3.5. Vitamin C
Prior studies have examined the effect of vitamin C on fatigue (Jung et al., 2006; Ou et al., 2020; Suh et al., 2012), although none have examined this in the context of COVID‐19. As previously demonstrated by Mandal et al. (2021), long COVID is sometimes characterised by elevated CRP and d‐dimer levels. An imbalance of essential micronutrients could be an underlying cause of constant fatigue after initial infection. Recent studies have found that COVID‐19 and lung cancer have similar biological pathways that suggest a comparable relationship between the two diseases (Nan et al., 2021). Specifically, Nan et al. (2021) discovered ten similar genes involved in cellular communication, as well as common biological pathways, such as IL‐17 (pro‐inflammatory) signalling. Ou et al. (2020) also examined the use of intravenous vitamin C for a median follow‐up of 24 months to help treat lung cancer patients. While the symptoms in the control arm worsened with time, the group that was administered 1 g/kg per day of intravenous vitamin C concurrent with modulated electrohyperthermia (mEHT) reported significantly decreased patient‐reported fatigue post‐treatment (Ou et al., 2020). Additionally, Suh et al. (2012) performed a randomised clinical trial to determine whether the administration of intravenous vitamin C would reduce fatigue in healthy office workers. Not only did fatigue scores reported by the experimental group decrease after one day, but oxidative stress levels decreased on average as well, measured by the Free Oxygen Radicals Test (Suh et al., 2012). Another study explored vitamin C treatment in nineteen patients with fatigue and noted a significantly decrease in fatigue score after four weeks (Jung et al., 2006). Based on these studies, vitamin C has anti‐inflammatory properties that may potentially help to address persistent fatigue in persons post‐COVID‐19.
3.6. Vitamin D
Vitamin D is another essential micronutrient where deficiency is likely to result in impaired cognitive performance and fatigue (Nowak et al., 2016). Nowak et al. suggested that the regulation of dopamine levels by vitamin D receptors in the brain can help to combat central fatigue (Nowak et al., 2016); thus, vitamin D supplementation may be beneficial for those with fatigue associated long COVID.
Vitamin D deficiency is common in persons infected by COVID‐19, as 82% of 216 patients from Spain were deficient compared to 47.2% of the control group of 197 healthy individuals (Hernández et al., 2021). Additionally, Demir et al. found vitamin D levels were less than sufficient (<30 ng/mL) in approximately 92% of 227 persons infected with COVID‐19. Furthermore, COVID‐19 patients with sufficient (>30 ng/ml) vitamin D levels had lower risk of contracting COVID, shorter hospital stay duration, and considerably lower serum levels of CRP and d‐dimer than patients with insufficient levels (Demir et al., 2021).
Zhang et al. (2012) also determined that vitamin D inhibits the production of proinflammatory cytokines. A double‐blinded clinical trial examined the impact of vitamin D on 120 vitamin D deficient, but otherwise healthy adults with self‐reported fatigue (Nowak et al., 2016). At a follow‐up of four weeks after baseline testing, the experimental group that received 100,000 IU of vitamin D experienced improved Fatigue Assessment Scale (FAS) scores and increased serum vitamin D levels compared to placebo (Nowak et al., 2016). While not yet studied in the context of COVID‐19 patients and long‐term sleep‐related symptoms, the results demonstrate the use of vitamin D as an effective antioxidant to potentially reduce inflammation and improve fatigue symptoms.
3.7. Polyphenols
Certain food‐derived micronutrients may also mitigate the severity of COVID‐19. Polyphenols, namely curcumin, have antioxidant properties that counters inflammation (Kowluru & Kanwar, 2007). Kowluru and Kanwar, 2007 found that curcumin lowered proinflammatory marker levels and prevented increases in oxidative stress in diabetic rats (Kowluru & Kanwar, 2007). It is possible that changing diets to include more micronutrients could ultimately reduce long‐term sleep‐related symptoms. Dark chocolate is especially high in polyphenols, specifically various flavonoids which have been found to reduce mortality rates from heart disease, cancer and stroke (Sathyapalan et al., 2010). A double‐blind, randomised, clinical pilot study by Sathyapalan et al. (2010) compared the effects of high cocoa liquor/polyphenol rich chocolate with simulated iso‐calorific chocolate on fatigue and residual function in ten patients with CFS. Those consuming the 15 g of polyphenol‐rich chocolate for eight weeks demonstrated a significant improvement in their fatigue measured by the Chalder Fatigue Scale score (Sathyapalan et al., 2010). Another study reviewed the diets of 1936 adults in southern Italy and showed a relationship between higher dietary intake of certain polyphenols and better sleep quality, assessed using the Pittsburgh Sleep Quality Index (Godos et al., 2020). Subjects who consumed more foods with flavonoid subclasses (flavanones and flavones), phenolic acids (such as hydroxycinnamic acids) and consumed lignans were less likely to have inadequate sleep quality (Godos et al., 2020). It is possible that increasing certain polyphenol intake may help to alleviate sleep‐related symptoms of long COVID.
4. DISCUSSION
Persistent COVID‐19 sleep‐related symptoms may be associated with increased inflammation. See Figure 2. There are currently no primary data studies that examine micronutrients in the context of sleep‐related symptoms, specifically in relation to COVID‐19. We have compiled prior research examining select micronutrients in disease states and the general population to show the potential for micronutrients to improve sleep‐related symptoms post‐COVID‐19 infection. Table 2 presents the 16 scholarly articles used in our review to determine how the four aforementioned micronutrients may aid in alleviating long‐term symptoms of COVID‐19 by targeting inflammation and improving sleep. Six research articles discussed the connections related to zinc intake and either inflammation and/or sleep (Cherasse et al., 2015; Gholipour Baradari et al., 2018; Grandner et al., 2013; Maes et al., 2006; Patel et al., 2021; Saito et al., 2017). Ou et al. (2020) and Suh et al. (2012) performed trials with vitamin C on lung cancer patients and healthy office workers respectively and discovered reduced fatigue associated with supplementation. Moreover, Jung et al. observed a decrease in fatigue scores after four weeks of treatment with intravenous vitamin C (Jung et al., 2006). Additionally, COVID‐19 patients with vitamin D deficiency have shown elevated d‐dimer and CRP levels (Hernández et al., 2021). Persons with adequate Vitamin D levels >30 ng/ml have significantly lower d‐dimer and CRP levels (Demir et al., 2021). Vitamin D also inhibits cytokine production (Zhang et al., 2012). Lastly, certain polyphenols may improve symptoms in persons with CSF (Sathyapalan et al., 2010) and higher intake may be associated with better sleep quality (Godos et al., 2020).
FIGURE 2.
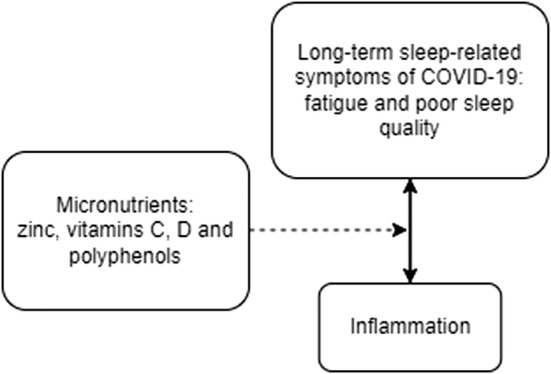
Illustrates the connection between inflammation and long‐term sleep‐related symptoms associated with COVID‐19 and the potential use of certain micronutrients to manage symptoms
TABLE 2.
Primary articles relating to COVID‐19 and micronutrients, sleep and micronutrients, or inflammation and micronutrients
| Author and Year | Micronutrient | Study design | Study population | Result | Length of follow‐up period |
|---|---|---|---|---|---|
| COVID‐related articles | |||||
| Patel et al. (2021) | Zinc | Double‐blinded, randomized controlled trial | 15 participants received IV zinc, 18 patients received placebo | COVID‐19 patients showed zinc deficiency which appears to be corrected with high dose IV zinc | Maximum of 7 days, until hospital discharge or death |
| Demir et al. (2021) | Vitamin D | Retrospective cohort study | 227 COVID‐positive patients, 260 negative | Low vitamin D levels (<30 ng/ml) in 94.27% of COVID‐positive patients. Severe vitamin deficiency (<10 ng/ml) more common in COVID‐positive participants (44%); The group of COVID‐19 positive patients with vitamin D levels >30 ng/mL showed lower CRP and d‐dimer levels | N/A |
| Hernández et al. (2021) | Vitamin D | Retrospective case–control study | 216 COVID patients (with 19 on vitamin D supplements), 197 participants in control group | 82.2% of COVID‐positive participants had vitamin D deficiency. Serum 25OHD levels were lower in hospitalized COVID‐19 patients compared with population controls | N/A |
| Sleep‐related or CSF articles | |||||
| Cherasse et al. (2015) | Zinc | Experimental study | Mice administered zinc‐containing yeast | Increased NREM sleep and decreased locomotor activity | Locomotor activity tracked each hour after yeast administration, time spent in REM, NREM, and wakefulness tracked 4 hours after yeast administration |
| Gholipour Baradari et al. (2018) | Zinc | Double‐blinded, randomised controlled trial | 54 ICU nurses; 27 receiving zinc sulphate capsules, 26 placebo | Sleep quality (measured using Pittsburgh Sleep Quality Index), sleep latency, and serum zinc concentrations improved with zinc supplementation | 1 month |
| Grandner et al. (2013) | Zinc | Observational study | 5587 participants | Less than 5 hours of sleep was associated with decreased mineral intake, after adjustment for overall diet | N/A |
| Maes et al. (2006) | Zinc | Observational study | CFS patients and normal participants | Decreased serum zinc concentrations and increased inflammatory markers/oxidative stress in CFS patients | N/A |
| Saito et al. (2017) | Zinc | Double‐blinded, randomised, controlled, parallel‐group trial | 120 healthy subjects randomly assigned placebo or zinc‐rich group | The zinc‐rich food group decreased the time needed to fall asleep and demonstrated improved sleep efficiency. The group that was given zinc‐enriched yeast and astaxanthin oil showed significantly improved sleep onset latency. | 12 weeks |
| Jung et al. (2006) | Vitamin C | Experimental study | 19 patients who reported consistent fatigue, measured fatigue scores before and after IV vitamin C treatment | Decreased fatigue scores after IV vitamin C administration | 4 weeks of treatment, then follow‐up questionnaire |
| Suh et al. (2012) | Vitamin C | Double‐blinded, randomised, controlled clinical trial | 141 healthy volunteers | IV vitamin C decreased fatigue 2 hours post‐administration and until next day | Two hours after vitamin C administration, and follow‐up one day after |
| Nowak et al. (2016) | Vitamin D | Double‐blind placebo‐controlled clinical trial | 120 vitamin D‐deficient individuals with fatigue; randomised into placebo or vitamin D supplement groups | Decreased fatigue assessment scores in vitamin D group | 4 weeks post‐vitamin D supplementation |
| Godos et al. (2020) | Polyphenols | Observational study | 1936 participants | Increased polyphenols in the diet is associated with improved sleep quality (measured using Pittsburgh Sleep Quality index) | N/A |
| Sathyapalan et al. (2010) | Polyphenols (flavonoids) | Double‐blinded, randomised, clinical pilot crossover study | Ten CFS patients | Reduced CFS symptoms associated with high‐polyphenol chocolate administration | 8 weeks of high‐polyphenol or low‐polyphenol chocolate, then 2 weeks of no treatment; another 8 weeks with other type of chocolate |
| Inflammation‐related articles | |||||
| Ou et al. (2020) | Vitamin C | Phase II clinical trial | Total of 97 lung cancer patients; 49 receiving intravenous vitamin C, 48 control | Increase in progression‐free survival and overall survival with IV vitamin C combined with modulated electrohyperthermia (mEHT) compared to best supportive care alone. Fatigue was decreased significantly between groups post treatment | Follow‐up after a median of 24 months |
| Zhang et al. (2012) | Vitamin D | Experimental study | Blood samples from healthy volunteers and mice | Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production | N/A |
| Kowluru and Kanwar (2007) | Polyphenols (curcumin) | Controlled, experimental study | Diabetic rats receiving curcumin or placebo diet | A curcumin diet is associated with decreased diabetes‐induced pro‐inflammatory marker levels | 6 weeks |
In infected persons months after initial infection of COVID‐19, long COVID causes persistent muscle pain, weakness and fatigue, and has symptoms that are similar to CFS (Wong & Weitzer, 2021). Studies show improved CFS symptoms with vitamin C (Vollbracht & Kraft, 2021) and polyphenol‐rich chocolate (Sathyapalan et al., 2010) supplementation. Thus, supplementation with these micronutrients may help to improve long‐term fatigue symptoms of COVID‐19.
To alleviate long‐term sleep‐related issues in those previously infected by SARS‐CoV‐2, vitamins C, D, zinc and certain polyphenols may serve to be beneficial. In particular, zinc is an essential mineral that has been linked to improved sleep related measures (Cherasse et al., 2015; Gholipour Baradari et al., 2018; Saito et al., 2017). Vitamins C and D may reduce fatigue (Nowak et al., 2016; Suh et al., 2012). The research discussed within this manuscript is important to consider as we take the next steps forward in improving quality of life for long COVID patients with sleep‐related symptoms. Fatigue is one of the most common persistent symptoms term after contracting COVID‐19 (Carfì et al., 2020); thus, administration of these micronutrients may serve as a safe and low‐risk treatment option.
5. CONCLUSION
It is important to consider the aforementioned select micronutrients as a low‐risk supplement treatment for those with long‐term sleep‐related symptoms due to COVID‐19. Micronutrients have the potential to target these symptoms via their effects on inflammation. Furthermore, they may improve quality of life and help to manage long‐term sleep‐related symptoms. Subsequent research should examine which micronutrients will mitigate long‐term sleep‐related symptoms. There is a need for double‐blind placebo‐controlled randomised trial designs in this area in order to more robustly examine the effect of supplementation on sleep‐related symptoms. Nurses can play an important role in conducting clinical trials that consider novel combinations of micronutrients on long‐term sleep‐related symptoms of COVID‐19 infection.
6. RELEVANCE TO CLINICAL PRACTICE
This review considers the potential use of micronutrients as a low‐risk treatment option for persistent sleep‐related symptoms of COVID‐19. Nurses are in a unique position to be at the forefront of this area of research and can lead to studies examining the impact of supplements on long‐term sleep‐related symptoms to improve the quality of care within this population.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Supplementary Material
ACKNOWLEDGEMENT
N/A.
Pak, V. M. , & Lee, J. (2022). Examining the role of micronutrients on improving long COVID sleep‐related symptoms. Journal of Clinical Nursing, 00, 1–10. 10.1111/jocn.16326
Funding information
N/A
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are openly available on PubMed, Science Direct, NCBI or Google Scholar.
REFERENCES
- Butters, D. , & Whitehouse, M. (2021). COVID‐19 and nutriceutical therapies, especially using zinc to supplement antimicrobials. Inflammopharmacology, 29(1), 101–105. 10.1007/s10787-020-00774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì, A. , Bernabei, R. , & Landi, F. (2020). Persistent symptoms in patients after acute COVID‐19. JAMA, 324(6), 603–605. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2021). Post‐COVID Conditions. Retrieved from https://www.cdc.gov/coronavirus/2019‐ncov/long‐term‐effects.html [Google Scholar]
- Cherasse, Y. , Saito, H. , Nagata, N. , Aritake, K. , Lazarus, M. , & Urade, Y. (2015). Zinc‐containing yeast extract promotes nonrapid eye movement sleep in mice. Molecular Nutrition & Food Research, 59(10), 2087–2093. 10.1002/mnfr.201500082 [DOI] [PubMed] [Google Scholar]
- Cortes Rivera, M. , Mastronardi, C. , Silva‐Aldana, C. T. , Arcos‐Burgos, M. , & Lidbury, B. A. (2019). Myalgic encephalomyelitis/chronic fatigue syndrome. A Comprehensive Review. Diagnostics (Basel), 9(3), 91 10.3390/diagnostics9030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook, H. , Raza, S. , Nowell, J. , Young, M. , & Edison, P. (2021). Long covid—mechanisms, risk factors, and management. BMJ, 374, n1648 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- Davis, H. E. , Assaf, G. S. , McCorkell, L. , Wei, H. , Low, R. J. , Re'em, Y. , Redfield, S. , Austin, J. P. , & Akrami, A. (2021). Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. SSRN Electronic Journal, 10.2139/ssrn.3820561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir, M. , Demir, F. , & Aygun, H. (2021). Vitamin D deficiency is associated with COVID‐19 positivity and severity of the disease. Journal of Medical Virology, 93(5), 2992–2999. 10.1002/jmv.26832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doykov, I. , Hällqvist, J. , Gilmour, K. C. , Grandjean, L. , Mills, K. , & Heywood, W. E. (2020). ‘The long tail of Covid‐19’ ‐ The detection of a prolonged inflammatory response after a SARS‐CoV‐2 infection in asymptomatic and mildly affected patients. F1000Research, 9, 1349. 10.12688/f1000research.27287.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigues, E. , Janvier, P. , Kherabi, Y. , Le Bot, A. , Hamon, A. , Gouze, H. , Doucet, L. , Berkani, S. , Oliosi, E. , Mallart, E. , Corre, F. , Zarrouk, V. , Moyer, J.‐D. , Galy, A. , Honsel, V. , Fantin, B. , & Nguyen, Y. (2020). Post‐discharge persistent symptoms and health‐related quality of life after hospitalization for COVID‐19. Journal of Infection, 81(6), e4–e6. 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour Baradari, A. , Alipour, A. , Mahdavi, A. , Sharifi, H. , Nouraei, S. M. , & Emami Zeydi, A. (2018). The effect of zinc supplementation on sleep quality of ICU nurses: a double blinded randomized controlled trial. Workplace Health & Safety, 66(4), 191–200. 10.1177/2165079917734880 [DOI] [PubMed] [Google Scholar]
- Godos, J. , Ferri, R. , Castellano, S. , Angelino, D. , Mena, P. , Del Rio, D. , Caraci, F. , Galvano, F. , & Grosso, G. (2020). Specific dietary (Poly)phenols are associated with sleep quality in a cohort of Italian adults. Nutrients, 12(5), 1226. 10.3390/nu12051226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner, M. A. , Jackson, N. , Gerstner, J. R. , & Knutson, K. L. (2013). Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite, 64, 71–80. 10.1016/j.appet.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández, J. L. , Nan, D. , Fernandez‐Ayala, M. , García‐Unzueta, M. , Hernández‐Hernández, M. A. , López‐Hoyos, M. , Muñoz‐Cacho, P. , Olmos, J. M. , Gutiérrez‐Cuadra, M. , Ruiz‐Cubillán, J. J. , Crespo, J. , & Martínez‐Taboada, V. M. (2021). Vitamin D status in hospitalized patients with SARS‐CoV‐2 infection. Journal of Clinical Endocrinology and Metabolism, 106(3), e1343–e1353. 10.1210/clinem/dgaa733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrami, H. , BaHammam, A. S. , Bragazzi, N. L. , Saif, Z. , Faris, M. , & Vitiello, M. V. (2021). Sleep problems during the COVID‐19 pandemic by population: a systematic review and meta‐analysis. Journal of Clinical Sleep Medicine, 17(2), 299–313. 10.5664/jcsm.8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, G. C. , Yeom, C. H. , Cho, B. , & Choi, J. S. (2006). The effect of intravenous vitamin C in people with fatigue. Journal of the Korean Academy of Family Medicine, 27(5), 391–395. [Google Scholar]
- Kowluru, R. A. , & Kanwar, M. (2007). Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutrition & Metabolism, 4(1), 8. 10.1186/1743-7075-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladds, E. , Rushforth, A. , Wieringa, S. , Taylor, S. , Rayner, C. , Husain, L. , & Greenhalgh, T. (2020). Persistent symptoms after Covid‐19: qualitative study of 114 "long Covid" patients and draft quality principles for services. BMC Health Services Research, 20(1), 1144. 10.1186/s12913-020-06001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes, M. , Mihaylova, I. , & De Ruyter, M. (2006). Lower serum zinc in Chronic Fatigue Syndrome (CFS): relationships to immune dysfunctions and relevance for the oxidative stress status in CFS. Journal of Affective Disorders, 90(2–3), 141–147. 10.1016/j.jad.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Mahase, E. (2020). Covid‐19: What do we know about “long covid”? BMJ, 370, m2815 10.1136/bmj.m2815 [DOI] [PubMed] [Google Scholar]
- Mandal, S. , Barnett, J. , Brill, S. E. , Brown, J. S. , Denneny, E. K. , Hare, S. S. , Heightman, M. , Hillman, T. E. , Jacob, J. , Jarvis, H. C. , Lipman, M. C. I. , Naidu, S. B. , Nair, A. , Porter, J. C. , Tomlinson, G. S. , & Hurst, J. R. (2021). ‘Long‐COVID’: a cross‐sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID‐19. Thorax, 76(4), 396–398. 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza, M. G. , Palladini, M. , De Lorenzo, R. , Magnaghi, C. , Poletti, S. , Furlan, R. , Ciceri, F. , Rovere‐Querini, P. , & Benedetti, F. (2021). Persistent psychopathology and neurocognitive impairment in COVID‐19 survivors: Effect of inflammatory biomarkers at three‐month follow‐up. Brain, Behavior, and Immunity, 94, 138–147. 10.1016/j.bbi.2021.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgul, E. , Bener, A. , Atak, M. , Akyel, S. , Aktaş, S. , Bhugra, D. , Ventriglio, A. , & Jordan, T. R. (2021). COVID‐19 pandemic and psychological fatigue in Turkey. International Journal of Social Psychiatry, 67(2), 128–135. 10.1177/0020764020941889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan, K. S. , Karuppanan, K. , & Kumar, S. (2021). Identification of common key genes and pathways between Covid‐19 and lung cancer by using protein‐protein interaction network analysis. Cold Spring Harbor Laboratory. Retrieved from 10.1101/2021.02.16.431364 [DOI] [Google Scholar]
- Nowak, A. , Boesch, L. , Andres, E. , Battegay, E. , Hornemann, T. , Schmid, C. , Bischoff‐Ferrari, H. A. , Suter, P. M. , & Krayenbuehl, P.‐A. (2016). Effect of vitamin D3 on self‐perceived fatigue: A double‐blind randomized placebo‐controlled trial. Medicine, 95(52), e5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, J. , Zhu, X. , Chen, P. , Du, Y. , Lu, Y. , Peng, X. , Bao, S. , Wang, J. , Zhang, X. , Zhang, T. , & Pang, C. L. K. (2020). A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non‐small‐cell lung cancer. Journal of Advanced Research, 24, 175–182. 10.1016/j.jare.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, O. , Chinni, V. , El‐Khoury, J. , Perera, M. , Neto, A. S. , McDonald, C. , See, E. , Jones, D. , Bolton, D. , Bellomo, R. , & Trubiano, J. & Bellomo, R. (2021). A pilot double‐blind safety and feasibility randomised controlled trial of high‐dose intravenous zinc in hospitalised COVID‐19 patients. Journal of Medical Virology. 93(5), 3261‐3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, H. , Cherasse, Y. , Suzuki, R. , Mitarai, M. , Ueda, F. , & Urade, Y. (2017). Zinc‐rich oysters as well as zinc‐yeast‐ and astaxanthin‐enriched food improved sleep efficiency and sleep onset in a randomized controlled trial of healthy individuals. Molecular Nutrition & Food Research, 61(5), 1600882 10.1002/mnfr.201600882 [DOI] [PubMed] [Google Scholar]
- Sathyapalan, T. , Beckett, S. , Rigby, A. S. , Mellor, D. D. , & Atkin, S. L. (2010). High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutrition Journal, 9(1), 55. 10.1186/1475-2891-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre, C. H. , Murray, B. , Varsavsky, T. , Graham, M. S. , Penfold, R. S. , Bowyer, R. C. , Pujol, J. C. , Klaser, K. , Antonelli, M. , Canas, L. S. , Molteni, E. , Marc Modat, M. , Cardoso, J. , May, A. , Ganesh, S. , Davies, R. , Nguyen, L. H. , Drew, D. A. , Astley, C. M. , … Steves, C. J. (2020). Attributes and predictors of Long‐COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. MedRxiv. [Google Scholar]
- Suh, S. Y. , Bae, W. K. , Ahn, H. Y. , Choi, S. E. , Jung, G. C. , & Yeom, C. H. (2012). Intravenous vitamin C administration reduces fatigue in office workers: a double‐blind randomized controlled trial. Nutrition Journal, 11, 7. 10.1186/1475-2891-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboada, M. , Moreno, E. , Cariñena, A. , Rey, T. , Pita‐Romero, R. , Leal, S. , Sanduende, Y. , Rodríguez, A. , Nieto, C. , Vilas, E. , Ochoa, M. , Cid, M. , & Seoane‐Pillado, T. (2021). Quality of life, functional status, and persistent symptoms after intensive care of COVID‐19 patients. British Journal of Anaesthesia, 126(3), e110–e113. 10.1016/j.bja.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbracht, C. , & Kraft, K. (2021). Feasibility of Vitamin C in the treatment of post viral fatigue with focus on long COVID, based on a systematic review of IV Vitamin C on fatigue. Nutrients, 13(4), 1154. 10.3390/nu13041154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, T. L. , & Weitzer, D. J. (2021). Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)‐A systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas), 57(5), 418 10.3390/medicina57050418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yael, B. , Leonid, G. , Nadav, S. , Irina, G. , & Dan, J. (2020). D‐dimer and C‐reactive protein blood levels over time used to predict pulmonary embolism in two COVID‐19 patients. European Journal of Case Reports in Internal Medicine, 7(6): 001725. 10.12890/2020_001725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Leung, D. Y. , Richers, B. N. , Liu, Y. , Remigio, L. K. , Riches, D. W. , & Goleva, E. (2012). Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase‐1. The Journal of Immunology, 188(5), 2127–2135. 10.4049/jimmunol.1102412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that supports the findings of this study are openly available on PubMed, Science Direct, NCBI or Google Scholar.


