Abstract
Introduction
This systematic review aimed to retrieve patients diagnosed with de novo immune thrombocytopenic purpura (ITP) after COVID‐19 immunization to determine their epidemiological characteristics, clinical course, therapeutic strategies, and outcome.
Materials and Methods
We conducted the review using four major databases, comprising PubMed, Scopus, Web of Science, and the Cochrane library, until April 2022. A systematic search was performed in duplicate to access eligible articles in English. Furthermore, a manual search was applied to the chosen papers' references to enhance the search sensitivity. Data were extracted and analyzed with the SPSS 20.1 software.
Results
A total of 77 patients with de novo COVID‐19 vaccine‐associated ITP were identified from 41 studies, including 31 case reports and 10 case series. The median age of patients who developed COVID‐19 vaccine‐associated ITP was 54 years (IQR 36–72 years). The mRNA‐based COVID‐19 vaccines, including BNT16B2b2 and mRNA‐1273, were most implicated (75.4%). Those were followed by the adenovirus vector‐based vaccines, inclusive of ChAdOx1 nCoV‐19 and vAd26.COV2.S. No report was found relating ITP to other COVID‐19 vaccines. Most cases (79.2%) developed ITP after the first dose of COVID‐19 vaccination. 75% of the patients developed ITP within 12 days of vaccination, indicating a shorter lag time compared to ITP after routine childhood vaccinations. Sixty‐seven patients (87%) patients were hospitalized. The management pattern was similar to primary ITP, and systemic glucocorticoids, IVIg, or both were the basis of the treatment in most patients. Most patients achieved therapeutic goals; only two individuals required a secondary admission, and one patient who presented with intracranial hemorrhage died of the complication.
Conclusions
De novo ITP is a rare complication of COVID‐19 vaccination, and corresponding reports belong to mRNA‐based and adenovirus vector‐based vaccines, in order of frequency. This frequency pattern may be related to the scale of administration of individual vaccines and their potency in inducing autoimmunity. The more the COVID‐19 vaccine is potent to induce antigenic challenge, the shorter the lag time would be. Most patients had a benign course and responded to typical treatments of primary ITP.
Keywords: COVID‐19, idiopathic thrombocytopenic purpura, immune thrombocytopenia, platelet, SARS‐CoV‐2, vaccine
NOVELTY STATEMENT.
What is the new aspect of your work?
This study is the first systematic review on one of the rare but potentially life‐threatening adverse effects of SARS‐CoV‐2 vaccination.
What is the central finding of your work?
Clinical presentations of COVID‐19 vaccine‐associated ITP (COVID‐19 VITP) are very similar to idiopathic ITP and the mRNA‐based and adenovirus vector‐based vaccines are the most common culprits of COVID‐19 VITP, respectively.
What is (or could be) the specific clinical relevance of your work?
Most COVID‐19 VITP cases have a benign disease course and respond to treatment appropriately.
1. INTRODUCTION
Since the emergence of SARS‐CoV‐2 in 2019, COVID‐19 is still an ongoing pandemic that has devastated global health. It has resulted in an iconic international cooperative effort to produce and distribute vaccines to limit the outbreak, safeguard human lives, and avert further socio‐economic impacts. 1 , 2 , 3 , 4 COVID‐19 vaccines have shown substantial efficacy in clinical trials and real‐world data. 5
However, the safety and adverse effects of the COVID‐19 vaccines have been a major public concern, resulting in vaccine hesitancy that necessitates ongoing and comprehensive observation and research. 6 , 7 , 8 , 9 Contrary to mild and moderate local or systemic adverse effects that may occur after vaccination, intense medical consequences are uncommon. 2 , 10 , 11 Several case series, nevertheless, revealed hematological problems such as immune thrombocytopenic purpura (ITP) induced by vaccination. 8 , 12
ITP is an autoimmune disease of platelet destruction resulting in a platelet count below 100 000 per cubic millimeter and has an incidence of two to five cases per 100 000 person‐years. 13 , 14 Even today, it is quite frustrating that ITP remains a diagnosis of exclusion. 15 The most common type of ITP is the idiopathic primary condition, though it can also be found secondary to other conditions, such as systemic autoimmune disorders, medications, a variety of infections, and vaccinations. 14 , 16 The latter is termed vaccine‐associated ITP and has been reported following different types of vaccines. This phenomenon, still rare, has been diagnosed more frequently since employing programs for massive vaccination against COVID‐19. 17 Similar to primary ITP, COVID‐19 vaccine‐associated ITP can manifest with a broad range of bleeding symptoms, from petechiae to fatal bleeding. 18 , 19 Nonetheless, it remains unclear whether COVID‐19 vaccine‐associated ITP is self‐limited or persists and progresses to chronic ITP. 17
The recent literature has witnessed a surge in research on COVID‐19 vaccine‐associated ITP. Several reports or case series have shown new presentations of ITP following different COVID‐19 vaccinations. 7 , 12 , 20 However, none of the prior works provide systematic information concerning the subject.
This systematic review aimed to highlight the characteristics of COVID‐19 vaccine‐associated ITP by analyzing the reaction onset, clinical manifestations, laboratory features, and management strategies based on pooled data extracted from the literature.
2. MATERIALS AND METHOD
2.1. Protocol and registration
This systematic review was conducted, and the results were reported according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 21
2.2. Search strategy and databases
Two researchers (APM and SA) conducted an independent search on major databases, including PubMed, Scopus, Web of Science, and Cochrane library, and retrieved all eligible articles published until 13 April, 2022. The adopted search keywords were COVID‐19, coronavirus disease 2019, SARS‐CoV‐2, vaccine, vaccination, specific COVID‐19 vaccine names, AND ITP, immune thrombocytopenia, ITP, idiopathic thrombocytopenia, idiopathic thrombocytopenic purpura, petechiae, bleeding and their close word variations in addition to the related MESH terms. Table S1 represents the search strategy applied to each database. Furthermore, a manual search was performed through the selected articles' references to avoid missing relevant studies.
2.3. Eligibility criteria
The eligible articles included case reports, case series, and observational studies that reported at least one case of de novo ITP following COVID‐19 vaccination. Our search was limited to the original clinical studies published in English and did not include in vitro or animal studies. To enhance search validity, we sought to exclude reports and studies on vaccine‐induced immune thrombotic thrombocytopenia (VITT), cerebral venous sinus thrombosis (CVST), disseminated intravascular coagulation (DIC), heparin‐induced thrombocytopenia (HIT), thrombotic thrombocytopenic purpura (TTP) or inferring evidence of thrombosis, COVID‐19 infection‐induced ITP, or thrombocytopenia with any justification other than ITP. In addition, any case with evidence of previous episodes of ITP suggesting a relapse or exacerbation (depending on chronological interlude) was set aside from the final analysis.
2.4. Screening
After removing study duplicates from the initial search results, two reviewers (APM and SA) independently screened the titles and abstracts of the retrieved articles to check gross relevancy. Afterward, MGM, SA, DN, and SRA formed two separate groups and scrutinized initially selected articles' full text to ascertain eligibility. Subsequently, the two groups cross‐checked their results as a quality control measure. Disagreements were resolved in online meeting sessions held with the guidance of AB as the senior researcher.
2.5. Case duplicates removal
We exerted a careful effort across included studies to eliminate case duplicates. For that purpose, a computer‐assisted algorithm was generated. Eligible cases were extracted from included studies, and a trio of variables, including age, gender, and vaccine type, was used collectively for screening duplicates according to the algorithm. In this step, potential duplicates were indexed. Subsequently, two independent researchers (AP and MGM) reviewed the index cases for other available details such as demographic data, clinical manifestations, and applied management. Lastly, we removed verified duplicates.
2.6. Data extraction
Data extraction was initiated by generating a structured database containing articles' titles, authors, and publication dates. Then, the following detailed information was obtained for every patient: the medical and drug history, vaccine brand and dose, time lag to develop ITP, selected clinical findings, and relevant laboratory markers such as platelet count prior to vaccination, platelet count at admission, the nadir platelet count, anti‐platelet antibodies (nonspecific anti‐platelet IgG antibodies, anti‐platelet factor IV, anti GP IIb/IIIa), peripheral blood smear and bone marrow aspiration/biopsy findings, mode and setting of the treatment, length of hospital stay, and convalescent platelet count. Any patients with positive anti‐platelet factor IV in laboratory data were excluded.
2.7. Statistical analysis
Descriptive data were reported using frequency, median, mean, and standard deviation. Comparison across groups was made using Chi‐square and Kruskal–Wallis test for qualitative and quantitative data, respectively. p values less than .05 were considered statistically significant. SPSS 20.1 software was used for all analyses.
3. RESULTS
3.1. Summary of evidence
A total of 1040 published articles were identified from four different databases: PubMed, Scopus, Web of Science, and Cochrane. Thereof, 320 articles were duplicated and removed. The remaining articles were screened for gross eligibility by evaluating titles and abstracts. As a result, 95 articles were selected. From excluded, most pertained to VITT. In the last screening step, the articles' full texts were scanned and scrutinized to determine whether they fulfilled all eligibility criteria. Accordingly, 54 articles were dismissed from the final analysis for various reasons; the most common were ITP relapse (14 articles) and inadequate data (12 articles). Eventually, we adopted 41 articles for our systematic review. Figure 1 depicts the PRISMA chart.
FIGURE 1.
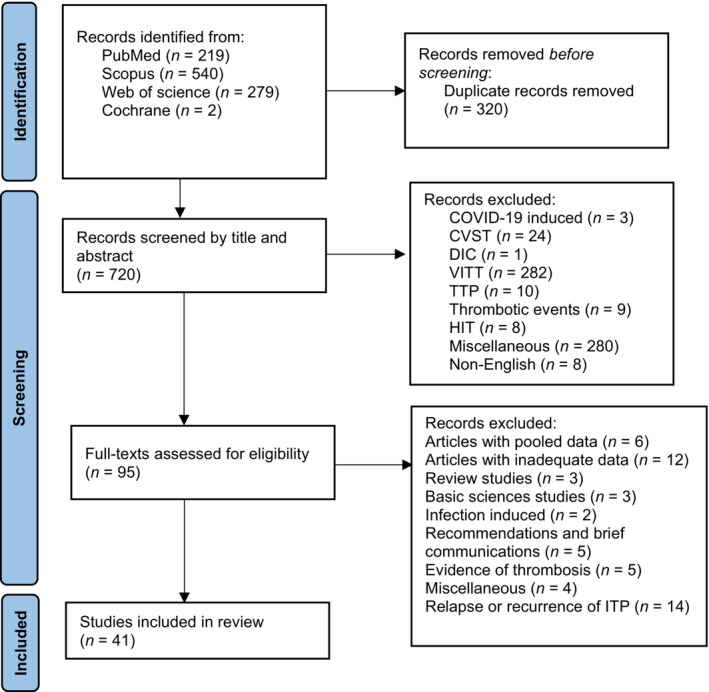
PRISMA chart
3.2. Baseline characteristics
A total of 77 patients with de novo post‐COVID‐19 vaccination ITP were identified from 41 articles, including 31 case reports and 10 case series. Females were dominant, comprising 46 (59.7%) of the entire selected cases. The median age of the study population was 54 years (IQR 36–72 years). The culprit COVID‐19 vaccine types in the study population included BNT16B2b2 mRNA vaccine (Pfizer‐BioNTech) in 35 cases (45.5%), mRNA‐1273 vaccine (Moderna) in 23 cases (29.9%), ChAdOx1 nCoV‐19 vaccine (AstraZeneca) 17 cases (22.1%), and Ad26.COV2.S vaccine (Johnson & Johnson) in two cases (2.6%). Sixty‐one patients (79.2%) were diagnosed with ITP after receiving the first vaccine dose for COVID‐19, 14 patients (18.2%) after the second dose, one patient following the booster dose (1.3%), and the dose number was not specified in another case. The first and second vaccine doses were from the same brand for patients who developed ITP after the second dose. The premorbid autoimmune disease was cited in only eight patients (10.4%). The information for each case in all included studies are represented in Tables 1 and 2 demonstrates the summary of the entire extracted cases.
TABLE 1.
Epidemiologic characteristics of included cases
| Author, year | Age/gender | Vaccine | Dose | REAC. ONSET (days) | PMH/Hematologic PMH | Clinical features | Plt on admission (×109/L) | Plt prior to vaccination (×109/L) | Plt nadir (×109/L) | Antibody profile | Treatment | Hospital admission/hospital stay (days) | Follow‐up Plt (×109/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baba, Y., 2022 42 | 90/M |
BNT16B2b2 mRNA BNT16B2b2 mRNA |
First | 7 | HTN/DLP/MI | Extensive purpura on the extremities/ICH/duodenal bleeding/impaired consciousness/pallor of palpebral conjunctiva/tarry stool | 3 | 224 | NM | Elevated platelet antibodies‐IgG/negative anti PF4 |
PSL 40 mg/day/ IVIG/TPO‐RA (EPAG)/platelet and RBC transfusion |
Yes/67 | NM |
| Sivaramakrishnan, P., 2022 17 | NM/F | ChAdOx1. | First | 30 | Previously treated pulmonary TB | Hemoptysis/menorrhagia/fever/streaked sputum | 8 | NM | NM | NM | Plt transfusion | Yes/7 | NM |
| NM/F | ChAdOx1. | Second | 11 | NM | Hemoptysis/menorrhagia | 10 | NM | NM | NM | PSL 80 mg/day | Yes/NM | NM | |
| Chong, K. M., 2022 43 | 75/F | mRNA‐1273 | First | 3 | Refractory lung adenocarcinoma | Hemoptysis | 7 | NM | NM | HBV profile compatible with a previous infection | Plt transfusion/PSL 1 mg/kg/day | Yes/5 | NM |
| Shonai, T., 2022 44 | 34/F | mRNA‐1273 | Second | 21 | NM | Generalized purpura/irregular vaginal bleeding | 11 | NM | 3 | NM |
At first: followed without treatment At first week of follow up: PSL 1 mg/kg/day TPO‐RA (EPAG) |
Yes/NM | NM |
| Chanut, M., 2022 45 | NM/NM | mRNA‐1273 | First | 7 | IgA kappa (MGUS)/obesity/HTN/hypothyroidism/corticoid‐induced glaucoma/recurrent rheumatic diseases | Extensive bruises/generalized petechiae/ICH/epistaxis | 2 | 287 | NM | NM | IVIG 1 g/kg for 2 days | Yes/2 | 310 |
| Al‐Ahmad, M., 2022 46 | 54/F | ChAdOx1. | First | 17 | NM | NM | 10 | NM | NM | NM | PSL 1 mg/kg/day/IVIG 1 g/kg | Yes/NM | NM |
| 33/F | ChAdOx1. | First | 21 | NM | NM | 3 | NM | NM | NM | PSL 1 mg/kg/day/IVIG 1 g/kg for 2 days/TPO‐RA (Romiplostim) | Yes/NM | NM | |
| 56/F | BNT16B2b2 mRNA | Second | 7 | NM | NM | 2 | NM | NM | NM | PSL 1 mg/kg/day/IVIG 1 g/kg for 2 days/TPO‐RA (EPAG) | Yes/NM | NM | |
| Battegay, R., 2021 47 | 77/M | BNT16B2b2 mRNA | First | 8 | CAD/AF/HTN/CKD | Wet petechiae | 28 | 126 | 17 |
Detectable anti‐SARS‐CoV2 spike(S1) and anti‐RBD specific IgM and IgG Anti‐S1‐IgG seroconversion confirmed in the ELISA assay at day 25 after vaccination |
Oral anticoagulant withdrawal/ oral vitamin K/PSL 1.2 mg/kg/day/IVIG 0.48 g/kg for 2 days/TPO‐RA (EPAG) |
Yes/NM | NM |
| Nakamura, T., 2022 48 | 32/F | BNT16B2b2 mRNA | Second | 5 | NM | Petechiae and purpura on the extremities/gingival and oral mucosal bleeding/wet purpura | <1 | 210 | NM | Elevated PA‐IgG//negative anti PF4 | PSL 50 mg/day | Yes/12 | NM |
| Malayala, S. V., 2021 49 | 75/F | BNT16B2b2 mRNA | Third/Booster | NM | Mixed connective tissue disease (RA + scleroderma)/HTN/osteopenia | Petechiae | 13.9 | 198 | 9 | NM | Dexamethasone 40 mg/day | Yes/NM | NM |
| Nutalapati, S., 2021 18 | 25/F | mRNA‐1273 | Second | 26 | well‐controlled bronchial asthma | Generalized scattered petechiae/extensive bruising/intermittent epistaxis/gross hematuria/hematochezia/subconjunctival hemorrhage | 1 | NL | 1 | NM |
Dexamethasone 40 mg/day/IVIG 2 g/kg for 2 days/tranexamic acid/plt transfusions Mycophenolate mofetil 1 g/TPO‐RA (Romiplostim) |
Yes/14 | 140 |
| Cooper, K. M., 2021 50 | 24/F | BNT16B2b2 mRNA | First | 10 | Dysfunctional uterine bleeding secondary to Etonogestrel implant/mild‐controlled asthma/previous allergic reaction to vaccine | Generalized petechiae/menorrhagia/wet petechiae/cutaneous and mucosal bleeding | NM | NM | 1 | NM | PSL 0.5–2.0 mg/kg/IVIG 1 g/kg | Yes/36 | NM |
| Ogai, A., 2022 51 | 73/F | mRNA‐1273 | First | 11 | HTN/DLP | Generalized petechiae/oral mucosal bleeding/melena | 2 | 230 | <1 | NM | PSL/IVIG/TPO‐RA (EPAG) | Yes/NM | NM |
| Hidaka, D., 2022 40 | 53/F | BNT16B2b2 mRNA | Second | 35 | Bronchial asthma/Vogt–Koyanagi–Harada disease/Hashimoto | Evans syndrome/mild anemia/icteric skin and bulbar conjunctiva/pallor of palpebral conjunctiva/wheezing/generalized petechiae/oral mucosal bleeding | 3.9 | NL | 30 | Positive lupus coagulant/positive ANA with speckled and nucleolar pattern/positive direct and indirect coombs tests/low level of cold agglutinin | PSL 1 mg/kg | Yes/NM | NM |
| Gardellini, A., 2021 52 | 27/M | BNT16B2b2 mRNA | First | 10 | NM | Hematoma/epistaxis | 1 | NM | NM | NM |
PSL/IVIG dexamethasone |
NM/NM | NM |
| 63/M | ChAdOx1. | First | 14 | DM/HTN | Hematoma/epistaxis | 2 | NM | NM | NM | PSL | Yes/NM | NM | |
| Jasaraj, R. B., 2021 41 | 67/F | BNT16B2b2 mRNA | Second | 2 | HTN/D2M/Hypothyroidism/depression/vitamin B12 deficiency/cluster headaches | Generalized petechiae/oral mucosal bleeding/epistaxis/subconjunctival hemorrhage | 3 | NL | NM | NM | PSL/IVIG/Plt transfusion/aminocaproic acid/rituximab/TPO‐RA (EPAG) | Yes/14 days | 200 |
| Kenney, A., 2021 53 | 69/F | mRNA‐1273 | First | 7 | Hypothyroidism/primary hyperaldosteronism/osteoporosis/migraine headache | Generalized petechiae/minor bruising/gingival mucosal bleeding | 4 | NM | NM | NM | PSL/IVIG | Yes/3 days | 258 |
| Wong, J. S. Y., 2021 54 | 86/M | ChAdOx1. | First | 2 | NM | Gingival mucosal bleeding/wet petechiae/widespread ecchymoses | 4 | NM | NM | Negative anti PF4 |
Dexamethasone/IVIG /Plt transfusion/rituximab |
Yes/10 days | NL |
| 38/F | ChAdOx1. | First | 10 | NM | Generalized petechiae and purpura/oral mucosal bleeding | 3.2 | NM | NM | Negative anti PF4 | PSL/IVIG | No/− | 430 | |
| Bennett, C., 2021 55 | 32/F | mRNA‐1273 | First | 11 | Negative | Bruising/petechiae | 1 | 268 | NM | NM | PSL/IVIG/dexamethasone | Yes/3 days | 303 |
| Akiyama, H., 2021 56 | 20/F | BNT16B2b2 mRNA | NM | 12 | Negative | Generalized subcutaneous hemorrhage/oral mucosal bleeding | 16 | NL | NM | NM | PSL | Yes/nm | 153–343 |
| Koch, M., 2021 57 | 41/M | ChAdOx1. | First | 10 | Negative | Petechiae/mocusal bleeding | <1 | 189 | NM | Negative anti PF4 | PSL/IVIG | Yes/4 days | 80 |
| Hines, A., 2021 58 | 26/F | mRNA‐1273 | First | 7 | Irregular menses on OCP | Petechiae/bruising | 19 | NM | NM | NM | PSL/IVIG/dexamethasone | Yes/5 days | 213 |
| Vaira, L. A., 2022 59 | 81/M | BNT16B2b2 mRNA | Second | 3 | Stage III CKD/hypercholesterolemia | Copious bleeding through the surgical wound/massive hematoma/ecchymosis of the right cheek | 4 | 156 | NM | Negative anti PF4 | Methylprednisolone/Plt transfusion | Yes/7 days | 188 |
| Paulsen, F. O., 2021 60 | 72/M | ChAdOx1. | First | 11 | Autoimmune thyroiditis | Petechiae/epistaxis/headache | NM | NM | <5 | Negative anti PF4 | Glucocorticoid/IVIG | Yes/nm | 253 |
| 71/F | ChAdOx1. | First | 11 | Latent hyperthyroidism/nodular goiter/breast cancer/stroke | Petechiae/hyposphagma | NM | NM | <5 | Negative anti PF4 | Glucocorticoid/IVIG/TPO‐RA | Yes/nm | 71 | |
| 66/M | ChAdOx1. | First | 2 | HTN/mild thrombocytopenia | Petechiae | NM | NM | <5 | Negative anti PF4 | Corticosteroid | Yes/4 | 89 | |
| 64/F | ChAdOx1. | First | 15 | HTN/COPD/steatosis hepatitis | None | NM | NM | 6 | Negative anti PF4 | Corticosteroid | Yes/6 | 121 | |
| King, E. R., 2021 61 | 39/F | BNT16B2b2 mRNA | Second | 3 | PCOS | Petechiae | 1 | NL | NM | NM | Plt transfusion/IVIG | Yes/3 | 243 |
| Shah, S. R. A., 2021 62 | 53/M | BNT16B2b2 mRNA | Second | 8 | Crohn's disease | Episodes of high‐grade fever/diffuse myalgia/generalized petechiae | 2 | 254 | NM | NM | IVIG/Dexamethasone | Yes/4 | NM |
| Idogun, P. O., 2021 39 | 54/F | BNT16B2b2 mRNA | First | 7 | Congenital epidermal dysplasia/anxiety/CKD/HTN/mild cognitive impairment | Rash | NM | NM | NM | NM | No referral to physician | No/NM | NM |
| 54/F | BNT16B2b2 mRNA | Second | 5 | Congenital epidermal dysplasia/Anxiety/CKD/HTN/Mild cognitive impairment | Ecchymosis/petechiae/oral mucosal bleeding | <1 | NM | <1 | NM |
Dexamethasone IVIG Plt transfusion |
Yes/14 | 114 | |
| Fueyo‐Rodriguez, O., 2021 63 | 41/F | BNT16B2b2 mRNA | First | 1 | Multiple allergies/hypothyroidism | Petechiae/gingival mucosal bleeding/fever/tachycardia/nausea/malaise/headache/loose stool | 65 | NL | 38 | Elevated anti‐dsDNA |
Dexamethasone Methyl prednisolone IVIG |
Yes/5 | 629 |
| Julian, J. A., 2021 64 | 72/F | mRNA‐1273 | First | 1 | Gout/D2M/seasonal contact dermatitis | Petechiae/oral mucosal bleeding/melena/headache | 12 | NM | 1 | Positive parvovirus IgG | Methylprednisolone/IVIG/aminocaproic acid/rituximab/Plt transfusion | Yes/NM | NM |
| Welsh, K. J., 2021 7 | 56/M | BNT16B2b2 mRNA | First | 2 | Negative | Petechiae/purpura/gingival mucosal bleeding/scleral hemorrhage/ICH | <1 | NM | NM | NM | PSL/platelet and RBC transfusion/TPO‐RA (EPAG)/dexamethasone/cyclosporine/rituximab/IVIG/emergent craniotomy/splenectomy | NM/NM | NM |
| 22/M | BNT16B2b2 mRNA | First | 3 | Negative | Petechiae/Epistaxis/gingival mucosal bleeding/scleral hemorrhage/hematuria | 2 | NM | NM | NM | Dexamethasone/Plt transfusion/IVIG | Yes/NM | NM | |
| 82/F | BNT16B2b2 mRNA | First | NM | NM | Pulmonary embolism/dyspnea/MI | 3 | NM | NM | NM | NM | NM/NM | NM | |
| 59/M | BNT16B2b2 mRNA | First | NM | Negative | None | 3 | NM | NM | NM | NM | NM/NM | NM | |
| 39/F | BNT16B2b2 mRNA | First | 2 | Depression/PCOS | Petechiae/menorrhagia | 1 | NM | NM | NM | PSL/IVIG/Plt transfusion/methylprednisolone | Yes/NM | NM | |
| 80/M | BNT16B2b2 mRNA | First | 6 | HTN/DM/DLP/aortic stenosis/diverticulosis | GI bleeding | 1 | NM | NM | NM | RBC and platelet transfusion | NM/NM | NM | |
| 78/F | BNT16B2b2 mRNA | First | 6 | AF/essential tremor | Petechiae | 6 | NM | NM | NM | Dexamethasone/IVIG/Plt transfusion | Yes/NM | NM | |
| 55/F | BNT16B2b2 mRNA | First | 4 | HTN/DM/arthritis | Petechiae/gingival mucosal bleeding | 2 | NM | NM | NM | Dexamethasone/IVIG/Plt transfusion | Yes/NM | NM | |
| 43/F | mRNA‐1273 | First | 8 | GERD | Petechiae/bruising | 2 | NM | NM | NM | PSL/IVIG | Yes/NM | NM | |
| 37/M | mRNA‐1273 | First | NM | NM | NM | NM | NM | NM | NM | Plt transfusion | NM/NM | NM | |
| 49/F | mRNA‐1273 | First | 1 | Migraine/psoriasis | Petechiae/shortness of breath | 66 | NM | NM | NM | NM | NM/NM | NM | |
| Candelli, M., 2021 65 | 28/M | ChAdOx1. | First | 20 | Negative | Oral mucosal bleeding/petechiae/purpura/fatigue/fever/headache/trace hematuria | 4 | NM | 2 | Positive lupus anti‐coagulant | Dexamethasone | Yes/4 | 180 |
| Helms, J. M., 2021 66 | 74/M | mRNA‐1273 | First | 1 | HTN/gout/DLP/Non‐ischemic cardiomyopathy | Epistaxis/purpura/cardiomyopathy/urinary retention/constipation/encephalopathy/dysarthria/AIDP | 10 | 224 | NM | NM |
Dexamethasone/PSL /Plt transfusion/IVIG /rituximab/TPO‐RA (EPAG) plasma exchange for AIDP |
Yes/17 | NM |
| Lee, E. J., 2021 67 | 36/F | BNT16B2b2 mRNA | First | 5 | Negative | Menorrhagia/blood blisters/petechiae/epistaxis/weakness | 9 | NM | NM | NM | NM | Yes/nm | NM |
| 25/F | mRNA‐1273 | First | 10 | Anxiety | Mucosal bleeding/bruising | 3 | NM | NM | NM | Corticosteroids/IVIG | Yes/nm | 286 | |
| 26/F | mRNA‐1273 | First | 2 | Negative | Bruising | 2 | NM | NM | NM | Corticosteroids/IVIG/Plt transfusion | Yes/NM | 142 | |
| 73/M | BNT16B2b2 mRNA | First | 1 | HTN/DLP/DM/hyperthyroidism | Bruising/petechiae | 1 | NM | NM | NM | Corticosteroids/IVIG/Plt transfusion | Yes/2 | 38 | |
| 53/M | BNT16B2b2 mRNA | First | 15 | Fatty liver | None | 10 | NM | NM | NM | Plt transfusion/IVIG | Yes/1 | 47 | |
| 72/F | mRNA‐1273 | First | 1 | Gout/DM | Bruising/petechiae/blood blisters | 1 | NM | NM | NM |
Corticosteroids/IVIG/rituximab/Aminocaproic acid /vincristine/TPO‐RA (Romiplostim) |
Yes/NM | 71 | |
| 50/F | mRNA‐1273 | First | 23 | HTN | Petechiae | 2 | NM | NM | NM |
Corticosteroids IVIG Plt transfusion |
Yes/NM | 20 | |
| 36/F | mRNA‐1273 | First | 16 | Inherited thrombocytopenia | Headache/petechiae/bruising/oral ecchymosis | 3 | NM | NM | NM |
Corticosteroids IVIG |
Yes/NM | 28 | |
| NM/NM | mRNA‐1273 | First | 1 | NM | Headache/blood blisters | 29 | NM | NM | NM | Corticosteroids | Yes/NM | 45 | |
| 41/F | BNT16B2b2 mRNA | First | 3 | Neuropathy | Chest pain/rash | 11 | NM | NM | NM |
Corticosteroids IVIG |
Yes/2 | 104 | |
| 48/F | mRNA‐1273 | First | 13 | HTN/Obesity | Heavy vaginal bleeding | 1 | NL | NM | NM |
Corticosteroids IVIG Plt transfusion |
Yes/NM | 300 | |
| 38/F | mRNA‐1273 | Second | 2 | NM | Headache/myalgia/petechiae | 1 | NM | NM | Positive antiplatelet antibody |
Corticosteroids /IVIG |
Yes/NM | 60 | |
| 53/M | BNT16B2b2 mRNA | First | 7 | Crohn's disease/HTN/GERD/prediabetic stage | Petechiae/oral mucosal bleeding | 2 | NM | NM | NM |
Corticosteroids /IVIG |
Yes/NM | 65 | |
| 63/M | mRNA‐1273 | First | 11 | DM/HTN/DLP | NM | 1 | NM | NM | NM | Have not responded to typical ITP therapies | Yes/NM | 4 | |
| 36/M | mRNA‐1273 | First | 15 | Epilepsy | NM | 1 | NM | NM | NM | NM | Yes/NM | NM | |
| 39/F | BNT16B2b2 mRNA | First | 12 | NM | ICH | 36 | NM | NM | NM | Plt transfusion | Yes/NM | NM | |
| Tarawneh, O., 2021 68 | 22/M | BNT16B2b2 mRNA | First | 3 | Negative | Petechiae/gingival mucosal bleeding | 2 | 145 | NM |
Elevated Sjogren's syndrome antibody/ positive for GP IIb/IIIa and Ia/IIa platelet autoantibodies/low C4 |
Dexamethasone/IVIG /Plt transfusion |
Yes/6 | 173 |
| Pasin, F., 2022 69 | 84/M | BNT16B2b2 mRNA | First | 3 | Essential tremor/localized bladder cancer/mild CKD/paroxysmal AF | Petechiae/bruising | 3 | 220 | NM | Negative anti PF4/positive anti GP IIb/IIIa |
Apixaban Discontinued /PSL/IVIG Plt transfusion |
Yes/NM | 63 |
| Liao, P. W., 2021 70 | 79/M | ChAdOx1. | First | 7 | Ischemic stroke (2 years bedridden) | None | 2 | NL | 2 | Negative anti PF4 | Hydrocortisone IV 300 mg/day then oral PSL | Yes/12 | 114 |
| Al‐Samkari, H., 2021 71 | 71/F | Ad26.COV2.S | First | 35 | Polymyalgia rheumatica | None | 115 | 429 | 59 |
Negative anti PF4/positive for all: anti–GPIIb/IIIa anti–GPIb/IX anti–GP Ia/IIa |
No treatment | NM/− | 248 |
| Krajewski, P. K.2021 72 | 74/M | BNT16B2b2 mRNA | First | 1 | HTN | Oral and nasal mucosal bleeding/purpuraon the extremities/ecchymosis at injection site | 2 | NM | NM | NM |
Dexamethasone/ Plt transfusion |
Yes/NM | NM |
| Banerjee, S., 2021 73 | 63/F | Ad26.COV2.S | First | 14 | Cervical Cancer/total hysterectomy | Gingival and nasal mucosal bleeding | 2 | NM | NM | NM |
Plt transfusion/ PSL 60 mg/ /Dexamethasone /IVIG |
Yes/5 | 252 |
| Condorelli, A., 2021 74 | 52/M | ChAdOx1. | First | 3 | Negative | Oral mucosal bleeding/diffuse cutaneous purpura | 1 | NM | NM | Anti‐platelet Abs: ‐ | PSL | Yes/7 | 168 |
| 24/M | BNT16B2b2 mRNA | Second | 4 | CHD/heart transplant/Hodgkin lymphoma (complete remission) | None | 15 | 150 | NM | NM | PSL 1 mg/kg/day | Yes/30 | 102 | |
| 73/M | BNT16B2b2 mRNA | Second | 2 | HTN/DM/DLP/CABG/IDA | Petechiae/oral mucosal bleeding/diffuse ecchymosis | 2 | 256 | NM | Anti‐platelet Abs: ‐ |
Methylprednisolone/ IVIG 0.4 g/kg/ PSL 1 mg/kg/day |
Yes/5 | 140 | |
| Bayas, A., 2021 75 | 55/F | ChAdOx1. | First | 10 | Negative | None | 30 | NM | NM |
Negative anti PF4/elevated platelets antibodies‐IgG/ positive PSIFT and MAIPA |
Dexamethasone IV 40 mg | Yes/26 | NM |
| Kim, G., 2021 76 | 66/F | ChAdOx1. | First | 2 | Pulmonary TB | Bruising on the extremities/gingival mucosal bleeding | 4 | 213 | NM | Negative anti PF4 | Dexamethasone 40 mg/IVIG 1 g/kg for 2 days | Yes/6 | 100 |
Abbreviations: AF, atrial fibrillation; AIDP, inflammatory demyelinating polyneuropathy; Anti PF‐4, anti‐platelet factor 4; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; DLP, dyslipidemia; DM, diabetes mellitus; EPAG, eltrombopag; GERD, gastroesophageal reflux disease; HTN, hypertension; IDA, iron deficiency anemia; IVIG, intravenous immune globulin; MAIPA, monoclonal antibody‐specific immobilization of platelet antigen; MGUS, monoclonal gammopathy of undetermined significance; NL, within normal range; NM, not mentioned; OCP, oral contraceptive pills; Plt, platelets; PCOS, poly cystic ovary syndrome; PSL, prednisolone; PSIFT, platelet suspension immunofluorescence test; RA, rheumatoid arthritis; TPO‐RA, thrombopoietin receptor agonist.
TABLE 2.
Summary of patients' demographics
| Variable | Count (% of total)/median (interquartile range) | |
|---|---|---|
| Age | 54 (36–72) years | |
| Gender | Female | 46 (59.7%) |
| Male | 29 (37.7%) | |
| Not mentioned | 2 (2.6%) | |
| Vaccine type | Ad26.COV2.S | 2 (2.6%) |
| BNT16B2b2 mRNA | 35 (45.5%) | |
| ChAdOx1. | 17 (22.1%) | |
| mRNA‐1273 | 23 (29.9%) | |
| Vaccine dose | First | 61 (79.2%) |
| Second | 14 (18.2%) | |
| Third/booster dose | 1 (1.3%) | |
| Not mentioned | 1 (1.3%) | |
| Positive history of autoimmune disease | 8 (10.4%) | |
| ITP disease presentation | Skin manifestation | 8 (10.4%) |
| Mucosal manifestation | 32 (41.6%) | |
| Internal organs manifestation | 17 (22.1%) | |
| Onset of ITP symptoms from vaccination day | 7 (3–12) days | |
| Platelets prior to vaccination | 220 (172.5–255) × 109/L | |
| Platelets on admission | 3 (2–10) × 109/L | |
| Platelets' nadir | 4.5 (1.25–26.75) × 109/L | |
| ITP related antibodies | Negative anti‐platelet factor 4 | 16 (20.8%) |
| Positive anti‐platelet factor 4 | 0 (0%) | |
| Positive anti‐glycoprotein IIb/IIIa | 16 (20.8%) | |
| Negative anti‐glycoprotein IIb/IIIa | 16 (20.8%) | |
| Positive non‐specific antibodies against platelets | 4 (5.1%) | |
| Management settings of patients | Outpatients | 2 (2.5%) |
| Inpatients | 67 (87%) | |
| Not Mentioned | 8 (10.5%) | |
| Hospital stay of admitted patients | 6 (4–13) days | |
| Treatment | Glucocorticoids in total | 62 (80.5%) |
| Prednisolone or methylprednisolone | 34 (44.2%) | |
| Dexamethasone | 19 (24.7%) | |
| Intravenous immunoglobulin | 47 (61%) | |
| Thrombopoietin receptor agonists in total | 12 (15.6%) | |
| Eltrombopag | 8 (10.4%) | |
| Romiplostim | 3 (3.9%) | |
| Platelet transfusion | 30 (39.0%) | |
| Follow‐up platelets | 140 (71–248) × 109/L | |
3.3. ITP lag time and manifestations
Among 71 patients with available data, the median time between vaccination and the clinical presentation of ITP was 7 days (IQR 3–12). While ITP in 75% of cases had been presented by day 12 of vaccination, a lag time from 1 to 35 days was observed. The median lag times for the four different types of vaccines were calculated separately. The figure for BNT16B2b2 mRNA vaccine was 5 days (IQR 3–7.7), for mRNA‐1273 vaccine was 7.5 days (IQR 1.75–13.5), for ChAdOx1 nCoV‐19 vaccine was 11.0 days (IQR 5–16), and for Ad26.COV2.S vaccine was 24.5 days. Kruskal–Wallis test was conducted to examine the differences on duration onset of ITP reaction according to the types of vaccines administered. A significant difference (Chi square = 10.25, p = .017, df = 3) was found among the four categories of vaccine.
At the time of diagnosis, 10 patients (13%) had no evidence of thrombocytopenic‐related bleeding, while 62 patients (80.5%) exhibited signs of bleeding, and clinical manifestations were not described in the other five cases (6.4%). The most prevalent manifestations were cutaneous involvement, encompassing petechiae, purpura, and ecchymoses, reported in 70.1% of the patients. Mucosal manifestations were seen in 46.7% of the patients. In 22.1% of the cases, internal organ involvements including hemoptysis, hematuria, scleral hemorrhage, gastrointestinal bleeding, vaginal bleeding, and cerebral hemorrhage were reported. Four individuals had intracranial hemorrhage (ICH), one of them had a fatal outcome. 22
Evans syndrome, a rare condition characterized by the coexistence of autoimmune hemolytic anemia (AIHA) and ITP, was diagnosed in one case after receiving the BNT16B2b2 mRNA vaccine.
3.4. Laboratory findings
Seventeen patients had available prior‐to‐vaccination platelet count, which ranged from 126 × 109/L to 429 × 109/L with a median of 220 × 109/L (IQR 172.5–255 × 109/L). Patients' platelet count at admission had a median of 3 × 109/L (IQR 2–10 × 109/L) and a mode of 2 × 109/L. The minimum platelet count on the initial day of admission was <1 × 109/L, and the maximum count was 66 × 109/L. The median platelet nadir count was 4.5 × 109/L (IQR 1.25–26.75 × 109/L) in 12 cases with attainable data, ranged from <1 to 59 × 109/L.
Patients underwent various tests based on managing physicians' discretion. In 36 patients (46.7%), antiplatelet antibodies were evaluated. Anti‐platelet factor‐4 antibody was negative in all 16 tested patients (20.7%). Anti‐Glycoprotein IIb/IIIa was assessed in 19 patients (24.6%), it was negative in 16 patients (20.8%) and positive in three (3.9%). In four patients (5.1%), non‐specific antibodies against platelets were detected.
Data for the peripheral blood smear (PBS) was available in 21 patients (27.2%); 20 patients had no morphologic changes in leukocytes and red blood cells. In one patient's PBS, rare schistocytes were seen, but other evidence was against the diagnosis of thrombotic thrombocytopenic purpura (TTP). Bone marrow aspiration was performed in 15 patients (19.5%), which showed megakaryocyte proliferation in nine patients (11.7%), and histopathologic findings in other reports were otherwise negligible.
3.5. Treatment and clinical outcomes
Management settings of 69 patients were procurable; of them, only two (2.5%) were managed ambulatory. For 67 patients (87%) who were hospitalized, the median length of hospital stay was 6 days (IQR 4–13 days).
Glucocorticoids, alone or in combination, were used in 62 (80.5%) patients. The most frequent corticosteroid choices were prednisolone or methylprednisolone in 34 (44.2%) and dexamethasone in 19 (24.7%) patients. The next frequent medication was intravenous immunoglobulin (IVIg), used in 47 (61%) patients. Thrombopoietin receptor agonists (TPO‐RA) were administered in 12 (15.6%) cases, with eltrombopag accounting for 8 (10.4%) and romiplostim accounting for 3 (3.9%) of circumstances. Six (7.8%) patients received rituximab as a rescue treatment, as they had not responded to a combination of treatments at least including corticosteroids and IVIg. Except for one, who had already sustained ICH and was pronounced to death, others improved.
Medications were implemented apart or in various combinations. Thirteen (16.9%) patients received corticosteroids alone, 19 (24.7%) in combination with IVIg, 4 (5.2%) in combination with platelet transfusion, and 14 (18.2%) in combination with both IVIg and platelet transfusion. One patient (1.3%) was given corticosteroids jointly with TPO‐RA and platelet transfusion. Five patients (6.5%) received corticosteroids in combination with IVIg, TPO‐RA, and platelet transfusion. One patient (1.3%) was managed by IVIg alone, and four patients (5.2%) underwent platelet transfusion as the only management modality. In two patients (2.6%), a combination of IVIg and platelet transfusion was the basis of treatment.
In summary, corticosteroids and IVIg, alone or in combination, were the most common treatment modalities. The various treatment strategies were successful in most circumstances. Nevertheless, platelet counts dropped in two patients after discharge, necessitating re‐admission.
A follow‐up platelet count was available in 39 patients, of whom two achieved normal platelet counts. The included studies adopted different targets of platelet count to define a treatment response. Platelet counts of 30 × 109/L and 50 × 109/L were the most adopted thresholds, and according to those definitions, 38 and 35 patients responded to treatment, respectively. 13 , 23
4. DISCUSSION
There is another systematic review on the COVID‐19 vaccine and its association with ITP, which covers both de novo and relapsed ITP. However, we assume induction of de novo ITP following COVID‐19 vaccination differs in pathophysiology from relapsed ITP post‐COVID‐19 vaccination, which primarily occurs due to another cause than COVID‐19 vaccination. Accordingly, this is the first study that systematically reviews the medical literature for a specific focus on de novo ITP following COVID‐19 immunization. 24 We observed that COVID‐19 vaccine‐associated ITP was more frequent in the middle‐aged population and female gender. Most reported cases developed ITP after the first dose of vaccination. Furthermore, the mRNA‐based and adenovirus vector‐based COVID‐19 vaccines were the most prevalent culprits in order of frequency.
Hemorrhagic mucocutaneous manifestations were the most common presentation, and thrombocytopenia was moderate to severe at the outset in affected patients. The vast majority of patients were managed in an in‐patient setting and received various therapeutic regimens that included either glucocorticoids, IVIg, or both. Therapeutic goals were accomplished in most patients who had available follow‐up data.
4.1. COVID‐19 vaccine‐associated ITP pathophysiology
Scant data elaborates on immunopathogenic mechanisms of vaccine‐induced thrombocytopenia.
Before introducing the COVID‐19 vaccine, ITP had been known as a rare consequence of various vaccinations. There are reports of vaccine‐associated ITP for live measles–mumps–rubella (MMR), varicella, inactivated hepatitis B, diphtheria‐tetanus–acellular pertussis (DTaP), pneumococcus, hemophilus influenza B, varicella zoster virus (VZV), human papillomavirus (HPV) and polio vaccines. Amongst them, MMR is the most well‐known vaccine that can induce ITP, and its cause‐effect relationship has been discussed elsewhere. 25 , 26
The etiology of vaccine‐associated ITP is perceived immune‐related as antibodies can be detected on platelets in roughly 80% of cases following administration of non‐COVID‐19 vaccines. 7 , 27 The most accepted hypothesis for platelet autoantibody production after vaccine inoculation is molecular mimicry and cross‐reactions between the vaccine antigens and the human molecules. That results in the activation of autoreactive B or T lymphocytes, emergence of antiplatelet antibodies, epitope spreading, polyclonal immune reaction, and the ultimate expression of ITP. 27 , 28 Our results demonstrated that very few patients tested positive for ITP autoantibodies; we presume this finding ascribes to the very low sensitivity of platelet autoantibody test in ITP patients, and only a few numbers of patients were tested for specific ITP autoantibodies which were from various case reports and probably diverse laboratories. 29
In addition, COVID‐19 infection‐associated ITP is well‐known. 30 , 31 Thus, it would not be surprising that ITP risk after COVID‐19 immunization increases through a similar mechanism as microbial infections induce antiplatelet autoantibodies with the same antigen utilized in COVID‐19 vaccine production (e.g., Spike protein). 26
The presence of adjuvants in the COVID‐19 vaccine could be another trigger of immune cross‐reactivity, termed autoimmune/inflammatory syndrome induced by adjuvants; however, there is no investigation to prove this hypothesis in COVID‐19 vaccines and ITP has been linked to different COVID‐19 vaccines. 15 , 16 , 28
4.2. COVID‐19 vaccine‐associated ITP versus common forms of ITP (unrelated to the COVID‐19 vaccines)
4.2.1. Epidemiologic characteristics
ITP is defined as “Newly diagnosed” within 3 months from diagnosis 32 ; given this definition, all of the patients retrieved in this study fell in this duration, thereby we could consider COVID‐19 vaccine‐associated ITP a sort of newly diagnosed ITP. Interestingly, the time lag between vaccination and ITP presentation was relatively short, and most cases presented within the first week of vaccination. In comparison, this lag time is significantly longer for MMR vaccine‐related ITP, ranging from 11 to 38 days with a median of 25 days. 33
We postulate two hypotheses to justify this observation: Firstly, implicated COVID‐19 vaccines that followed ITP in our study were either mRNA or adenoviral based vector vaccines. Both vaccines employ the recipient's cells to reproduce selected epitopes of the COVID‐19 spike protein. Thus the mechanisms of antigen production in these novel vaccines are incredibly robust. 34 Data supports that COVID‐19 vaccines with novel technology are very potent in inducing favorable neutralizing antibodies against COVID‐19. We hypothesize that this may also contribute to an early surge of auto‐reactive antibodies against platelets, causing a shorter lag time for ITP presentation. On the other hand, MMR, an attenuated live virus vaccine, induces a more smooth immune reaction and a later onset ITP. Furthermore, our results showed that among COVID‐19 vaccines, ITP lag time was even shorter in mRNA than in adenovirus‐based vaccines. This observation is again in concert with the current understanding of the potency order of available vaccine choices. 34
Secondly, the typical recipient of the COVID‐19 vaccine is much older than the very young children who receive the MMR vaccine. We postulate that previous exposure to coronaviruses in adults may have already modulated their immune response to vaccination against COVID‐19.
The level of thrombocytopenia in COVID‐19 vaccine‐associated ITP is similar to non‐vaccine‐associated ITP, and a platelet count below 20 × 109/L was present in 60 (77%) cases. Indeed, in our study, the median of the lowest (nadir) platelet number was 3 × 109/L. In contrast, MMR vaccine‐related ITP is associated with milder thrombocytopenia, and a platelet count of more than 20 × 109/L is usual. 27
The median age of our patients was 54. This figure may not necessarily suggest a particular age susceptibility but may correlate with age‐related vaccination policies in the population. Notably, reports show that ITP‐associated with COVID‐19 infection has a comparable age distribution with the post‐vaccinal type, and 70% of patients had an age above 50. 30 , 31
Finally, one of the most dreaded complications of ITP is ICH. Hato et al. observed that in the setting of primary ITP, age above 60 and platelet count below 10 × 109/L are two important risk factors for ICH. Noteworthy, the same risk factors were present in our cases who complicated with ICH. 35
4.2.2. Clinical presentation and disease course
Except for 10 patients who were asymptomatic at the time of diagnosis, all other cases in our review presented with cutaneous, mucosal, and internal bleeding. These manifestations are typical for thrombocytopenic purpura and present in primary ITP, ITP secondary to COVID‐19 infection, MMR vaccine‐related ITP, drug‐induced ITP, and ITP after wild virus infections or helicobacter pylori.
Most COVID‐19 vaccine‐associated ITP cases had a benign course, and severe or life‐threatening bleeding was rare. Only four patients sustained ICH, all had received mRNA‐based vaccines and one led to a fatal outcome despite aggressive immunosuppressive therapy and craniotomy. Like COVID‐19 infection‐induced ITP, EVANS syndrome was also observed in one case. 27 , 30 , 36 , 37
Regarding the constitutional symptoms, we noticed a higher prevalence of fever, headache and a lower occurrence of fatigue, compared to primary ITP. 36 Lastly, while no mortality has ever been reported for MMR vaccine‐associated ITP, we had a single case of fatality in our data. Of notice, there are also rare reports of fatal outcomes in patients diagnosed with ITP secondary to COVID‐19 infection. 15
4.2.3. Therapeutic strategy and response
Our series showed that the general treatment pattern in COVID‐19 vaccine‐associated ITP was analogous to primary ITP. Most patients were hospitalized and received systemic corticosteroids or IVIg, alone or in combination. A minority underwent other treatments, including a TPO‐RA drug.
From the advent of TPO‐RA drugs, a paradigm shift is proceeding in the management of ITP, and the center of focus is moving from immunosuppression to improving health‐related quality of life in the patients. Accordingly, these drugs are making their way into guidelines on ITP management, especially for ITP duration of more than 3 months or refractory cases. Moreover, there has been a recent trend of employing that class of drugs in the acute management of ITP patients, though still, it is an unapproved indication. 13 , 15 , 23 Among our series, 12 (15.6%) of COVID‐19 vaccine‐associated ITP patients received either romiplostim or eltrombopag in combination with other measures. Platelet transfusion, plasmapheresis, rituximab, and tranexamic acid were reserved for rescue treatment or add‐on therapy for the most severe cases.
Except for one patient who died of complications of ICH, no other mortality case has been found, and in most cases, the convalescent platelet count was available, which was in the acceptable range. These findings are in line with the Perricone et al. study that claimed most cases of vaccine‐associated ITP are mild and treatment‐responsive. 38
Since we had excluded all cases of COVID‐19 vaccine‐associated ITP if had any prior clinical episode of ITP, we cannot comment on the risk of ITP relapse in case of repeat vaccination. Nevertheless, we noticed three cases in our series who developed purpuric rash after the first dose of the BNT16B2b2 mRNA vaccine, progressed to full‐blown thrombocytopenic syndrome following the second vaccine dose. Therefore, despite the appearance of purpura earlier, ITP was diagnosed after the second dose. That limited observation is not surprising from the pathophysiological perspective and may suggest against revaccination by the same brand if thrombocytopenia had already developed after a prior vaccine dose. 39 , 40 , 41
4.3. Study limitations
There were some important limitations to this study. First, we only retrieved articles published in the English language in full text. We may have overlooked the inherent differences in population susceptibilities and unequal availability of individual types of COVID‐19 vaccines to nations based on local policies.
Second, we could only reach out to ITP cases secondary to four COVID‐19 vaccine brands. The lack of data regarding the other brands may be related to under‐reporting, the scale of vaccine administration, or their lower chance of inducing ITP.
Third, this was a systematic review based on published cases. Since many patients with ITP can be asymptomatic, the true incidence of significant thrombocytopenia after COVID‐19 vaccination is unknown. In our series, only 10 patients (13%) were asymptomatic at the time of diagnosis, a figure that we believe is an underestimation.
Fourth, because the COVID‐19 vaccination programs set a priority for the elderly population and patients with underlying comorbidities, it is probable that our sample is not representative of the whole population. This fact can affect the demographic aspects of our study. For instance, the results may underestimate the susceptibility of the young and shift the age of the population at risk to a higher range.
Finally, we made every effort to exclude articles with dubious implications of COVID‐19 vaccine‐associated ITP. However, because of inadequate data in some circumstances, we were not able to verify the diagnosis independently in a few cases and therefore relied on the authors' discrimination.
5. CONCLUSION
In essence, our review provided a comprehensive, descriptive compendium of the reported cases of COVID‐19 vaccine‐associated ITP and highlighted the clinical features and real‐world therapeutic interventions in this potentially life‐threatening complication of SARS‐CoV‐2 immunization. We found that clinical manifestations of COVID‐19 vaccine‐associated ITP were very similar to primary ITP. The disease usually responded to high‐dose corticosteroids, IVIg, or both, and fatal complications were rare.
Interestingly, we showed that the lag time for the ITP presentation after vaccination against COVID‐19 is shorter than the gap observed in ITP manifestation after routine vaccinations in childhood. The more the COVID‐19 vaccine is robust to induce antigenic challenge, the shorter the lag time would be. We also revealed that most cases of post‐vaccinal ITP were related to potent COVID‐19 vaccine brands, including BNT16B2b2 mRNA and mRNA‐1273 vaccine. We postulate that this observation might be related to the broad scale of administration of those vaccines in developed countries where vaccine surveillance, case finding, and case reports are typically more reliable. Still, we could not exclude the notion that potent vaccines are more prone to induce cross‐immunity.
It merits consideration that further large‐scale prospective studies are required to establish the features and risk factors of COVID‐19 vaccine‐associated ITP. Furthermore, we need basic research to explore the causality role of the COVID‐19 vaccines in developing ITP via investigating epitope similarities between platelets and vaccine‐driven antigens. The possible disparity between the brands of COVID‐19 vaccines to induce ITP needs further investigation and may help manufacturing vaccines with less chance of induction of autoimmunity.
AUTHOR CONTRIBUTIONS
Ali Bidari, Sara Asgarian, and Milad Gholizadeh Mesgarha were responsible for conceptualization. Arash Pour Mohammad performed systematic search and analysis, tabulated data and prepared figure. Sara Asgarian, Delaram Naderi, Milad Gholizadeh Mesgarha, Shiva Rahimipour Anaraki, Mahya Naderkhani carried out study screening and data extraction. All authors participated in composing the inital draft, finalizing the manuscript, applying revisions, and approved of the final version.
FUNDING INFORMATION
No Funding was received for this study.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Appendix S1: Supporting Information
Table S1: Search syntax across four databases
Table S2: Epidemiologic characteristics of relapsed ITP post‐COVID‐19 vaccination cases
Bidari A, Asgarian S, Pour Mohammad A, et al. Immune thrombocytopenic purpura secondary to COVID‐19 vaccination: A systematic review. Eur J Haematol. 2022;1‐19. doi: 10.1111/ejh.13917
Ali Bidari and Sara Asgarian contributed equally to this study (Co‐first authors).
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Waxman JG, Makov‐Assif M, Reis BY, et al. Comparing COVID‐19‐related hospitalization rates among individuals with infection‐induced and vaccine‐induced immunity in Israel. Nat Commun. 2022;13(1):2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gubernot D, Jazwa A, Niu M, et al. U.S. population‐based background incidence rates of medical conditions for use in safety assessment of COVID‐19 vaccines. Vaccine. 2021;39(28):3666‐3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moradians V, Shateri Amiri B, Bahadorizadeh L, Gholizadeh Mesgarha M, Sadeghi S. Concurrent COVID‐19 and pneumocystis carinii pneumonia in a patient subsequently found to have underlying hairy cell leukemia. Radiol Case Rep. 2022;17(9):3238‐3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bidari A, Hassanzadeh M, Naderkhani M, et al. Predictors of critical COVID‐19 in an Iranian population: age and disabilities play a special role. Med J Islam Repub Iran. 2021;35:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post‐vaccination SARS‐CoV‐2 infection in UK users of the COVID symptom study app: a prospective, community‐based, nested, case‐control study. Lancet Infect Dis. 2022;22(1):43‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodeghiero F, Cantoni S, Carli G, et al. Practical recommendations for the management of patients with ITP during the COVID‐19 pandemic. Mediterr J Hematol Infect Dis. 2021;13(1):e2021032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID‐19 vaccines reported to the vaccine adverse event reporting system (VAERS). Vaccine. 2021;39(25):3329‐3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sing CW, Tang CTL, Chui CSL, et al. COVID‐19 vaccines and risks of hematological abnormalities: nested case‐control and self‐controlled case series study. Am J Hematol. 2022;97(4):470‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharif N, Alzahrani KJ, Ahmed SN, Dey SK. Efficacy, immunogenicity and safety of COVID‐19 vaccines: a systematic review and meta‐analysis. Front Immunol. 2021;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seirafianpour F, Pourriyahi H, Gholizadeh Mesgarha M, Pour Mohammad A, Shaka Z, Goodarzi A. A systematic review on mucocutaneous presentations after COVID‐19 vaccination and expert recommendations about vaccination of important immune‐mediated dermatologic disorders. Dermatol Ther. 2022;35(6):e15461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hajsadeghi S, Gholizadeh Mesgarha M, Saberi Shahrbabaki E, Pishgahi M, Ebadi Fard Azar A, Pour MA. Constrictive pericarditis following inactivated virus COVID‐19 vaccine: a case report with review of the literature. Radiol Case Rep. 2022;17(10):3774‐3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee E‐J, Beltrami‐Moreira M, Al‐Samkari H, et al. SARS‐CoV‐2 vaccination and ITP in patients with de novo or preexisting ITP. Blood. 2022;139(10):1564‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829‐3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381(10):945‐955. [DOI] [PubMed] [Google Scholar]
- 15. Provan D, Semple JW. Recent advances in the mechanisms and treatment of immune thrombocytopenia. EBioMedicine. 2022;76:103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. David P, Dotan A, Mahroum N, Shoenfeld Y. Immune thrombocytopenic purpura (itp) triggered by covid‐19 infection and vaccination. Isr Med Assoc J. 2021;23(7):378‐380. [PubMed] [Google Scholar]
- 17. Sivaramakrishnan P, Mishra M. Vaccination‐associated immune thrombocytopenia possibly due to ChAdOx1 nCoV‐19 (Covishield) coronavirus vaccine. BMJ Case Rep. 2022;15(3):e249237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nutalapati S, Gupta G, Hildebrandt GC. Rapid response to mycophenolate mofetil in combination with romiplostim in a case of severe refractory immune thrombocytopenia post COVID‐19 vaccination. Clin Case Rep. 2021;9(11):e05035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mashayekhi F, Seirafianpour F, Pour Mohammad A, Goodarzi A. Severe and life‐threatening COVID‐19‐related mucocutaneous eruptions: a systematic review. Int J Clin Pract. 2021;75(12):e14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chou S‐C, Chang Y‐C, Liao C‐K, et al. New presentations and exacerbations of immune thrombocytopenia after coronavirus disease 2019 vaccinations: the Taiwan experience. Platelets. 2022;33(4):531‐535. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodeghiero F, Michel M, Gernsheimer T, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the international working group. Blood. 2013;121(14):2596‐2606. [DOI] [PubMed] [Google Scholar]
- 23. Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780‐3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saluja P, Amisha F, Gautam N, Goraya H. A systematic review of reported cases of immune thrombocytopenia after COVID‐19 vaccination. Vaccines. 2022;10(9):1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009;46(1):S2‐S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yokomichi H, Tanaka‐Taya K, Koshida R, et al. Immune thrombocytopenic purpura risk by live, inactivated and simultaneous vaccinations among Japanese adults, children and infants: a matched case‐control study. Int J Hematol. 2020;112(1):105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cecinati V, Principi N, Brescia L, Giordano P, Esposito S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Hum Vaccines Immunother. 2013;9(5):1158‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malayala SV, Mohan G, Vasireddy D, Atluri P. Purpuric rash and thrombocytopenia after the mRNA‐1273 (Moderna) COVID‐19 vaccine. Cureus. 2021;13(3):e14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vrbensky JR, Moore JE, Arnold DM, Smith JW, Kelton JG, Nazy I. The sensitivity and specificity of platelet autoantibody testing in immune thrombocytopenia: a systematic review and meta‐analysis of a diagnostic test. J Thromb Haemost. 2019;17(5):787‐794. [DOI] [PubMed] [Google Scholar]
- 30. Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID‐19: a systematic review. SN Compr Clin Med. 2020;2(11):2048‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bahadorizadeh L, Emamikhah M, Pour Mohammad A, Gholizadeh MM. Simultaneous occurrence of cerebral venous sinus thrombosis and immune thrombocytopenic purpura in a patient with a history of COVID‐19 infection. Neurol Ther. 2022;11(1):491‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386‐2393. [DOI] [PubMed] [Google Scholar]
- 33. Miller E, Waight P, Farrington CP, Andrews N, Stowe J, Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child. 2001;84(3):227‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine‐induced protection against COVID‐19 in humans. Nat Rev Immunol. 2021;21(8):475‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hato T, Shimada N, Kurata Y, et al. Risk factors for skin, mucosal, and organ bleeding in adults with primary ITP: a nationwide study in Japan. Blood Adv. 2020;4(8):1648‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh A, Uzun G, Bakchoul T. Primary immune thrombocytopenia: novel insights into pathophysiology and disease management. J Clin Med. 2021;10(4):789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pour Mohammad A, Mashayekhi F, Seirafianpour F, Gholizadeh Mesgarha M, Goodarzi A. COVID‐19 and COVID‐19 vaccine‐related dermatological reactions: an interesting case series with a narrative review of the potential critical and non‐critical mucocutaneous adverse effects related to virus, therapy, and the vaccination. Clin Case Rep. 2022;10(4):e05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perricone C, Ceccarelli F, Nesher G, et al. Immune thrombocytopenic purpura (ITP) associated with vaccinations: a review of reported cases. Immunol Res. 2014;60(2–3):226‐235. [DOI] [PubMed] [Google Scholar]
- 39. Idogun PO, Ward MC, Teklie Y, Wiese‐Rometsch W, Baker J. Newly diagnosed idiopathic thrombocytopenia post COVID‐19 vaccine administration. Cureus. 2021;13(5):e14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hidaka D, Ogasawara R, Sugimura S, et al. New‐onset evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID‐19 vaccination. Int J Hematol. 2022;115(3):424‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jasaraj RB, Shrestha DB, Gaire S, Kassem M. Immune thrombocytopenic purpura following Pfizer‐BioNTech COVID‐19 vaccine in an elderly female. Cureus. 2021;13(8):e16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baba Y, Sakai H, Kabasawa N, Harada H. Successful treatment of immune thrombocytopenic purpura with intracranial hemorrhaging and duodenal bleeding following SARS‐CoV‐2 vaccination. Intern Med. 2022;61:1891‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chong KM, Yang CY, Lin CC, Lien WC. Severe immune thrombocytopenia following COVID‐19 vaccination (Moderna) and immune checkpoint inhibitor: a case report. Am J Emerg Med. 2022;56:395.e1‐395.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shonai T, Kimura F, Watanabe J. Severe immune thrombocytopenia after COVID‐19 vaccination: two case reports and a literature review. Intern Med. 2022;61:1581‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chanut M, Jaidi R, Kohn M, et al. Successful mRNA SARS‐Cov‐2 vaccine rechallenge after a first episode of immune thrombocytopenic purpura. Platelets. 2022;33:652‐653. [DOI] [PubMed] [Google Scholar]
- 46. Al‐Ahmad M, Al Rasheed M, Shalaby N, Rodriguez‐Bouza T, Altourah L. Immune thrombocytopenia (ITP): relapse versus de novo after COVID‐19 vaccination. Clin Appl Thromb Hemost. 2022;28:10760296211073920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Battegay R, Istampoulouoglou I, Holbro A, et al. Immune thrombocytopenia associated with COVID‐19 mRNA vaccine tozinameran––a clinical case and global pharmacovigilance data. Swiss Med Wkly. 2021;151:w30084. [DOI] [PubMed] [Google Scholar]
- 48. Nakamura T, Morodomi Y, Kanaji S, Okamura T, Nagafuji K, Kanaji T. Detection of anti‐GPIbα autoantibodies in a case of immune thrombocytopenia following COVID‐19 vaccination. Thromb Res. 2022;209:80‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malayala SV, Papudesi BN, Sharma R, Vusqa UT, Raza A. A case of idiopathic thrombocytopenic purpura after booster dose of BNT162b2 (Pfizer‐Biontech) COVID‐19 vaccine. Cureus. 2021;13(10):e18985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cooper KM, Switzer B. Severe immune thrombocytopenic purpura after SARS‐CoV‐2 vaccine. Arch Clin Cases. 2021;8(2):31‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ogai A, Yoshida R, Yuasa C, Chin K, Fujimaki K, Nakajima H. Acute immune thrombocytopenia following SARS‐CoV‐2 vaccination in chronic ITP patients and a healthy individual. Int J Hematol. 2022;115(2):293‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gardellini A, Guidotti F, Maino E, Steffanoni S, Zancanella M, Turrini M. Severe immune thrombocytopenia after COVID‐19 vaccination: report of four cases and review of the literature. Blood Cells Mol Dis. 2021;92:102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kenney A, Adhikari A. Immune thrombocytopenia in a 68‐year‐old woman after COVID‐19 vaccination. Clin Case Rep. 2021;9(8):e04689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wong JSY, Kang JH, Maw KZ. Acute immune thrombocytopenic purpura post first dose of COVID‐19 vaccination. Postgrad Med J. 2022;98(e2):e129‐e130. [DOI] [PubMed] [Google Scholar]
- 55. Bennett C, Chambers LM, Son J, Goje O. Newly diagnosed immune thrombocytopenia in a pregnant patient after coronavirus disease 2019 vaccination. J Obstet Gynaecol Res. 2021;47(11):4077‐4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Akiyama H, Kakiuchi S, Rikitake J, et al. Immune thrombocytopenia associated with Pfizer‐BioNTech's BNT162b2 mRNA COVID‐19 vaccine. IDCases. 2021;25:e01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Koch M, Fuld S, Middeke JM, Fantana J, von Bonin S, Beyer‐Westendorf J. Secondary immune thrombocytopenia (ITP) associated with ChAdOx1 Covid‐19 vaccination––a case report. TH Open. 2021;5(3):e315‐e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hines A, Shen JG, Olazagasti C, Shams S. Immune thrombocytopenic purpura and acute liver injury after COVID‐19 vaccine. BMJ Case Rep. 2021;14(7):e242678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vaira LA, Podda L, Doneddu P, Careddu MG, Fozza C, De Riu G. Secondary thrombocytopenia after SARS‐CoV‐2 vaccine: report of a case of hemorrhage and hematoma after minor oral surgery. J Stomatol Oral Maxillofac Surg. 2022;123(2):95‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Paulsen FO, Schaefers C, Langer F, et al. Immune thrombocytopenic purpura after vaccination with COVID‐19 vaccine (ChAdOx1 nCov‐19). Blood. 2021;138(11):996‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. King ER, Towner E. A case of immune thrombocytopenia after BNT162b2 mRNA COVID‐19 vaccination. Am J Case Rep. 2021;22:e931478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shah SRA, Dolkar S, Mathew J, Vishnu P. COVID‐19 vaccination associated severe immune thrombocytopenia. Exp Hematol Oncol. 2021;10(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fueyo‐Rodriguez O, Valente‐Acosta B, Jimenez‐Soto R, et al. Secondary immune thrombocytopenia supposedly attributable to COVID‐19 vaccination. BMJ Case Rep. 2021;14(5):e242220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Julian JA, Mathern DR, Fernando D. Idiopathic thrombocytopenic purpura and the Moderna Covid‐19 vaccine. Ann Emerg Med. 2021;77(6):654‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Candelli M, Rossi E, Valletta F, De Stefano V, Franceschi F. Immune thrombocytopenic purpura after SARS‐CoV‐2 vaccine. Br J Haematol. 2021;194(3):547‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Helms JM, Ansteatt KT, Roberts JC, et al. Severe, refractory immune thrombocytopenia occurring after SARS‐CoV‐2 vaccine. J Blood Med. 2021;12:221‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS‐CoV‐2 vaccination. Am J Hematol. 2021;96(5):534‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tarawneh O, Tarawneh H. Immune thrombocytopenia in a 22‐year‐old post Covid‐19 vaccine. Am J Hematol. 2021;96(5):E133‐E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pasin F, Calabrese A, Pelagatti L. Immune thrombocytopenia following COVID‐19 mRNA vaccine: casuality or causality? Intern Emerg Med. 2022;17(1):295‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liao PW, Teng CLJ, Chou CW. Immune thrombocytopenia induced by the chimpanzee adenovirus‐vectored vaccine against SARS‐CoV‐2 infection. Vaccine. 2021;9(12):1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Al‐Samkari H, Leaf RK, Goodarzi K. Transient thrombocytopenia with glycoprotein‐specific platelet autoantibodies after ad26.cov2.s vaccination: a case report. Ann Intern Med. 2021;174(11):1632‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Krajewski PK, Szepietowski JC. Immune thrombocytopenic purpura associated with COVID‐19 Pfizer‐BioNTech BNT16B2b2 mRNA vaccine. J Eur Acad Dermatol Venereol. 2021;35(10):e626‐e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Banerjee S, Sandhu M, Tonzi E, Tambe A, Gambhir HS. Immune‐mediated thrombocytopenia associated with Ad26.COV2.S (Janssen; Johnson & Johnson) vaccine. Am J Ther. 2021;28(5):E604‐E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Condorelli A, Markovic U, Sciortino R, Di Giorgio MA, Nicolosi D, Giuffrida G. Immune thrombocytopenic purpura cases following COVID‐19 vaccination. Mediterr J Hematol. Infect Dis. 2021;13(1):e2021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. Lancet. 2021;397(10285):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim G, Choi EJ, Park HS, Lee JH, Lee JH, Lee KH. A case report of immune thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. J Korean Med Sci. 2021;36(43):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Table S1: Search syntax across four databases
Table S2: Epidemiologic characteristics of relapsed ITP post‐COVID‐19 vaccination cases
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


