Abstract
Nirmatrelvir/ritonavir (NMV‐r) is an effective anti‐SARS‐CoV‐2 agent and has been recommended in the treatment of nonhospitalized patients with COVID‐19. In rare occasions, some patients experience virologic and symptomatic rebound after initial resolution, which we call COVID‐19 rebound after NMV‐r. Although COVID rebound can also occur after molnupiravir treatment or even no antiviral treatment, we have more serious concern about the rebound after NMV‐r, which remains the most effective antiviral. Due to a lack of information about its frequency, mechanism, outcomes, and management, we conducted this review to provide comprehensive and updated information to address these questions. Based on the limited evidence, the incidence of COVID‐19 rebound after NMV‐r was less than 2%, and most cases developed 5–15 days after initiating NMV‐r treatment. Almost all reported cases had mild symptoms, and the clinical condition gradually subsided without additional treatment. Overall, the clinical outcome was favorable, and only a small number of patients required emergency department visits or hospitalization. Regarding virologic rebound, culturable SARS‐CoV‐2 with possible transmission was observed, so re‐isolation may be needed.
Keywords: COVID‐19, mechanisms, nirmatrelvir/ritonavir, rebound, SARS‐CoV‐2, virological rebound
1. INTRODUCTION
Despite the implementation of nonpharmacological interventions and the development of vaccines against coronavirus disease 2019 (COVID‐19), numbers of patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) are rapidly growing. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 As of November 1, 2022, there have been 627 573 579 confirmed cases of COVID‐19 reported to the World Health Organization. 7 Although most confirmed cases had only mild COVID‐19 or asymptomatic presentations, a significant portion of patients with SARS‐CoV‐2 infections could progress to severe‐to‐critical illness or acute respiratory distress syndrome and could be associated with high morbidity and mortality. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 However, effective therapy against COVID‐19 is limited. 15 , 20 , 21 , 22 , 23 , 24 For hospitalized patients with severe‐to‐critical COVID‐19, systemic corticosteroids and interleukin‐6 blockade can help reduce mortality. 25 , 26 For patients who do not require hospitalization with supplemental oxygen, three antiviral agents, nirmatrelvir/ritonavir (NMV‐r), molnupiravir and remdesivir, and new neutralizing monoclonal antibodies can help prevent progression to severe COVID‐19. 27 , 28 , 29 Among these three antivirals, NMV‐r is associated with the lowest risk of hospitalization or death and is the first choice for outpatients with mild symptomatic COVID‐19. 30
To date, the effect of NMV‐r has been evaluated in many randomized controlled trials (RCTs) and real‐world studies. 27 , 31 , 32 , 33 , 34 Phase 2‐3 double‐blind RCTs found that the incidence of COVID‐19‐related hospitalization or death by Day 28 was lower in the NMV‐r group than in the placebo group by 6.32 percentage points (95% confidence interval [CI], −9.04 to −3.59; p < 0.001; relative risk reduction, 89.1%) among symptomatic, unvaccinated, nonhospitalized adults at high risk for progression to severe COVID‐19. 27 A comparative retrospective multinational study using the TriNetX research network and propensity score matching methods showed that the composite outcome of all‐cause emergency department (ED) visits, hospitalization, or death in 30 days occurred in 89 (7.87%) patients in the NMV‐r cohort compared to 163 (14.4%) patients in the non‐NMV‐r cohort (OR 0.5, CI 0.39–0.67; p < 0.005), consistent with a 45% relative risk reduction. 31 Najjar‐Debbiny et al., using the database of the largest health care provider in Israel showed that NMV‐r was associated with a significant decrease in the rate of severe COVID‐19 or mortality, with an adjusted HR of 0.54 (95% CI, 0.39–0.75). 33 Moreover, they found that NMV‐r seemed more effective in older patients, immunosuppressed patients, and patients with underlying neurological or cardiovascular disease (interaction p value <0.05 for all), and no significant interaction was detected between NMV‐r treatment and COVID‐19 vaccination status. 33 Even for the hospitalized patients with COVID‐19 without supplemental oxygen requirements on admission, NMV‐r remained associated with a lower risk of all‐cause mortality versus matched controls (10.28 events [7.03–14.51] vs. 26·47 events [21.34–32.46]; HR 0.34 [0.23–0.50], p < 0·0001). 32 Compared with the control, NMV‐r was associated with a lower risk of composite disease progression outcomes, including all‐cause mortality, initiation of invasive mechanical ventilation [MV], intensive care unit [ICU] admission, or the need for oxygen therapy (HR, 0·57 [0·45–0·72], p < 0·0001) (HR, 0·73 [0·54–0·97], p = 0·032). 32
Although the above findings indicated the effectiveness of NMV‐r in the improvement of clinical outcomes of COVID‐19 and supported the use of NMV‐r, some patients experienced clinical and virologic rebound after therapy completion. This phenomenon—COVID‐19 rebound after NMV‐r was defined as recurrence of COVID‐19 symptoms following successful completion of 5 days of NMV‐r therapy. Although NMV‐r therapy has been observed in several studies, 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 its frequency, mechanism, outcomes, and management remain unknown. To address these questions, we conducted a review to provide comprehensive and updated information.
2. DEFINITION
COVID‐19 rebound is characterized by a recurrence of symptoms or a new positive viral test after having tested negative. 45 , 46 Therefore, COVID‐19 rebound after NMV‐r can be defined as SARS‐CoV‐2 viral rebound or the recurrence of COVID‐19 symptoms in patients who have completed five‐day treatment with NMV‐r. Therefore, the definition can vary in different studies, where some use viral rebound, some use symptom recurrence, and some use concomitant viral and symptom rebound. 35 , 38 , 39 , 47 , 48 Some studies used the COVID‐19 related ED visit or hospitalization after complete treatment of NMV‐r. 37 Therefore, the interpretation of associated studies should be done with caution.
3. EPIDEMIOLOGY
At the beginning, the manufacturer reported to the US Food and Drug Administration of several cases of rebound in SARS‐CoV‐2 RNA levels in <2% of patients at Day 10 or 14 following NMV‐r completion. 49
Using the definition of viral load rebound—a half‐log increase in viral load on Day 10 or Day 14, the post hoc analysis of EPIC‐HR RCT reported that viral load rebound occurred in 23 of 990 patients (2.3%) in the NMV‐r group and in 17 of 980 (1.7%), and the incidence of viral load rebound was similar between the NMV‐r group and placebo group. 35 Furthermore, there were no significant differences in viral load rebound between the NMV‐r group and the placebo group in subgroup analyses of the presence of coexisting illnesses, recurrence of moderate to severe COVID‐19 symptoms, the occurrence of hospitalization or death, and baseline SARS‐CoV‐2 serologic status. 35 Based on the retrospective review of 483 high‐risk patients receiving NMV‐r for mild to moderate COVID‐19 at Mayo Clinic in Rochester by Ranganath and his colleagues, only four patients (0.8%) experienced rebound of mild symptoms at a median of 9 days (IQR, 7–14.5) after the treatment. 43 All four patients were fully vaccinated, and all resolved without hospitalization or additional COVID‐19‐directed therapy. 43 Another large series using electronic health record (EHR) data from a large integrated health care system in California reported that only 45 (0.85%) of 5,287 persons who received NMV‐r required further COVID‐19 related ED visits (n = 39) or hospitalization (n = 6) during the 5–15 days after pharmacy dispensation of a 5‐day treatment course of NMV‐r. 37 All hospitalized patients had comorbidities or were of advanced age (range = 61–104 years), and two of them had mortalities attributed to underlying disease. 37 Using the TriNetX Analytics network platform, which contains nationwide and real‐time deidentified EHRs of 93 million unique patients from 67 health care organizations, Wang et al., showed that 398 (3.53%) tested positive, 260 (2.31%) had COVID‐19 related symptoms and 50 (0.44%) were hospitalized during the 7‐day period from 8 days after the last day of NMV‐r among 11,270 patients treated with NMV‐r. 44
In fact, COVID‐19 rebound is not unique to NMV‐r. 44 Wang et al., reported that 7‐day and 30‐day COVID‐19 rebound rates after molnupiravir treatment were 5.86% and 8.59% for COVID‐19 infection, 3.75% and 8.21% for COVID‐19 symptoms, and 0.84% and 1.39% for hospitalizations. 44 Moreover, there were no significant differences in COVID‐19 rebound risks between NMV‐r and molnupiravir in terms of infection (HR, 0.90, 95% CI, 0.73–1.11), COVID‐19 symptoms (HR, 1.03, 95% CI: 0.83–1.27), or hospitalizations (HR, 0.92, 95% CI, 0.56–1.55). 44 Finally, SARS‐CoV‐2 RNA rebound or symptom relapse can also occur in the absence of antiviral treatment. 35 , 50 The post hoc analysis of EPIC‐HR RCT reported that viral load rebound occurred in 17 of 980 (1.7%) in the placebo group. 35 Deo et al. analyzed the 568 participants enrolled in the ACTIV‐2/A5401 platform trial who received placebo and found that viral rebound, defined as a 0.5 log10 viral RNA copies/mL increase, was observed in 12% of the participants, and symptom rebound, defined as a 4‐point total symptom score increase from baseline, occurred in 27% of the participants after initial symptom improvement and in 10% of the participants after initial symptom resolution. 50
To better understand the epidemiological characteristics of COVID‐19 after the use of NMV‐r, we performed a literature review using PubMed database to search the related articles. The inclusion criteria were (1) clinical trials or retrospective studies and (2) reports with the detailed epidemiological characteristics of COVID‐19 rebound after NMV‐r. The exclusion criteria included (1) case reports; (2) in vitro or pharmacological studies; (3) conference abstracts; and (4) studies that did not report the epidemiology of COVID‐19 rebound. Table 1 summarizes the findings of the included six studies, 37 , 39 , 40 , 42 , 43 , 44 and we found that COVID‐19 rebound after NMV‐r was uncommon. The incidence of COVID‐19 rebound following NMV‐r treatment varied according to different definitions and study designs. While we used the definition of virological or symptom rebound, the estimated incidence of COVID‐19 rebound after NMV‐r ranged from 0.8% to 2.8%. 35 , 37 , 43 , 51 Recently, Wang et al. 51 also reported that the risks of COVID‐19 rebound after NMV‐r was higher in the Omicron BA.5 cohort and was significantly higher than in the propensity‐score matched BA.2.12.1 cohort (virological rebound: hazard ratio [HR], 1.32; 95% confidence interval [95% CI], 1.06–1.66, and symptom rebound: HR, 1.32; 95% CI, 1.04–1.68). Although patients with underlying conditions may opt to develop COVID rebound, immunocompetent individuals can also experience rebound. 37 , 39 , 40 , 42 , 43 , 44 Most cases had been fully vaccinated in several studies, 37 , 39 , 40 , 43 but the association between vaccination and COVID‐19 rebound was unclear. Although no additional treatment was applied for these patients, most patients had favorable outcomes, and only limited patients required ED visits or hospitalization. 37 , 39 , 40 , 42 , 43 , 44
Table 1.
Summary of case series of COVID‐19 rebound after nirmatrelvir‐ritonavir (NMV‐r) treatment
| Case series | Source | Study population | Number of cases | Age | Male, no (%) | Comorbidity | Vaccine status | Onset after NMV‐r | ED visits, no | Hospitalization, no | Death, no |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Malden et al. 37 | A large integrated health care system in California | 5287 persons aged ≥12 years who received NMV‐r during December 31, 2021–May 26, 2022 | 45a | 63 (45–77) | 14 (31) | 35 (78%) had ≥one underlying medical condition |
0: 5 (11.1); 1: 3 (6.7); 2: 9 (20.0); 3: 27 (60.0); 4: 1 (2.2) |
5–15 days | 39 (86.7) | 6 (13.3) | 2 (4.4) |
| Epling et al. 42 | NCT04401436 | Adults age ≥18 years who either currently have COVID‐19 or have recently recovered from it | 6 | 42.5 (33–74) | 3 (50) | NA | NA | 8–14 days | 0 (0) | 0 (0) | 0 (0) |
| Wang et al. 44 |
Retrospective cohort study of electronic health records of 92 million patients from a multicentre and nationwide database in the US |
11 270 patients aged ≥18 years old who contracted COVID‐19 and took NMV‐r between 1/1/2022‐6/8/2022 | 609 | 57.9 ± 16.4 | 212 (35) |
HTN: 57.6% Cancer: 54.8% Chronic lung disease: 43.0% Obesity: 40.4% Mood disorder: 40.1% DM: 30.0% |
151 (24.8%) | 8–15 days | NA | 50 (0.44%) | NA |
| Ranganath et al. 43 | a retrospective study at Mayo Clinic in Rochester | 483 high risk patients received NMV‐r for mild‐to‐moderate SARS‐CoV‐2 infection. | 4 | 40–75 | 3 (75) |
HTN: 75% Obesity: 75% CKD: 25% DM: 25% COPD: 25% Cancer: 25% |
Full vaccinated: 4 (100) | 9 (7–14.5) | 0 (0) | 0 (0) | 0 (0) |
| Boucau et al. 39 | Postvaccination viral characteristics study cohort study | Patients treated with 5 days of NMV‐r with recurrent symptoms after initial resolution or recurrent antigen test positivity after testing negative during or after their treatment course | 7 | 31–64 | 1 (14) | Immunocompromised condition: 1 (14) | Full vaccinated: 7 (100) | NA | NA | NA | NA |
| Charness et al. 40 | Retrospective observational cohort study at Columbia University medical center | NA | 13 | 63 (29–71) | 7 (54) | Immunocompromised condition: 0 (0) | Full vaccinated: 13 (100) | 9–15 | NA | NA | 0 (0) |
Abbreviations: CKD, chronic kidney diseases; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; NA, not applicable.
COVID‐19 rebound was defined as those who required ED visit or hospitalization after NMV‐r treatment
4. MECHANISMS
Although a possible mechanism causing the recurrence of COVID‐19 symptoms after NMV‐r treatment has been proposed, such as the emergence of treatment‐resistant mutations, several in vitro studies have consistently reported that resistance mutations were not identified in patients with COVID‐19 rebound. 39 , 42 , 48 Boucau et al., evaluated seven individuals with recurrent symptoms or antigen test conversion following NMV‐r treatment and found that high viral loads (median 6.1 log10 copies/ml) were detected after rebound for a median of 17 days after initial diagnosis. 39 Although three had culturable virus for up to 16 days after initial diagnosis, no known resistance associated mutations were identified. 39 Immune responses can help eradicate replication competent viruses during NMV‐r treatment, so the impairment of immunity may contribute to COVID‐19 rebound. However, Carlin et al., demonstrated that there was no absence of neutralizing immunity in a case of COVID‐19 recrudescence after NMV‐r treatment and suggested that the absence of neutralizing antibodies was an unlikely cause of the COVID‐19 rebound. 48 One explanation for this rebound phenomenon is the resumption of SARS‐CoV‐2 viral replication following completion of therapy, triggering a secondary immune‐mediated response that manifests as recurrence of clinical symptoms. Finally, the possible explanation of COVID‐19 rebound after treatment could be due to inadequate or insufficient treatment—(1) the patients did not complete the prescribed course of treatment or developed adverse drug effects and terminated treatment, and (2) the dose was insufficient given the pharmacodynamics in that individual.
5. CLINICAL MANIFESTATIONS
Patients who developed COVID‐19 rebound did not differ in age or race from those without rebound, but there were more women and fewer Hispanics, significantly more comorbidities, organ transplants and immunosuppressant usage and more tobacco smokers. 44 The EHR documented COVID‐19 vaccination rate was higher in patients with COVID‐19 rebound than in those without suggesting that vaccination is not a major contributor to COVID‐19 rebound. 44
The presentations of COVID‐19 rebound are protean, including cough, fever, palpitation, rhinorrhea, myalgia, congestion, sneezing, fatigue headache, pharyngitis, sore throat, nasal congestion, diarrhea, and dyspnea. 35 , 40 , 43 , 47 , 48 The medium duration of rebound symptoms was 4 days (range: 3–10 days). 40 Most symptoms due to COVID‐19 rebound after NMV‐r would be mild; however, the symptoms could rarely be worse than the initial episode. In one small series involving six patients who experienced COVID‐19 rebound after NMV‐r, 4 patients reported milder symptoms than for their initial illness, 1 reported worse symptoms, and 1 reported similar symptoms. 42 Although most reported cases had mild symptoms that spontaneously resolved without additional treatment, severe complications have rarely been reported. 47 Birabaharan et al., described an unusual presentation of a 63‐year‐old man with type 2 diabetes mellitus who received four doses of COVID‐19 vaccines, had laboratory confirmed COVID‐19 and received NMV‐r within 2 days of symptom onset. On Day 4, he had complete resolution and returned to his baseline health. However, a recurrence of cough with dyspnea on exertion developed on Day 8, when SARS‐CoV‐2 RT‒PCR was positive and his SpO2 was only 91%. He was admitted, and bilateral pulmonary emboli were detected by computed tomography. Although anticoagulant was given, he still had dyspnea and infrequent cough four weeks after discharge. 47
6. VIROLOGICAL REBOUND
The virological and inflammatory response of COVID‐19 rebound after NMV‐r has been evaluated in several studies. 35 , 39 , 42 , 48 Carlin et al., demonstrated that high viral shedding (cycle threshold = 21.7) and culturable virus were found 5 days after the NMV‐r course. 48 Further sequence analysis of the isolate showed no amino acid differences in any coding region, including ORF1a and spike protein, compared to the BA.2 reference. 48 Similarly, Boucau et al., showed that a detectable viral load was identified for a median of 12 days (range 9–15) after completion of NMV‐r. 39 Among seven individuals with virologic rebound, viral cultures were positive in 3 individuals, and the cultures were positive until 5, and 11 days after completion of the course of NMV‐r. 39
7. INFLAMMATORY RESPONSES
Epling et al., demonstrated that the median C‐reactive protein (CRP) level was lower at the time of rebound than during acute COVID‐19, whereas neutrophil and lymphocyte counts and SARS‐CoV‐2 PCR Ct values were similar across groups with low or undetectable serum nucleocapsid antigen levels during rebound. 42 In contrast, high levels of SARS‐CoV‐2 anti‐spike immunoglobulin G (IgG) antibodies were found, and anti‐nucleocapsid IgG and Omicron‐specific neutralizing antibodies increased in the patients with rebound. 42 Moreover, robust SARS‐CoV‐2–specific T‐cell responses were observed, which were higher in rebound than in early acute COVID‐19 patients. 42
8. MANAGEMENT
According to the recommendations of the CDC and NIH, there is currently no evidence that additional treatment for COVID‐19 is needed for COVID‐19 rebound. At present, the most appropriate management should be close and continued monitoring of patients with recurrence of symptoms after completion of a treatment course of NMV‐r. Previous studies found evidence of a high viral load after NMV‐r therapy for COVID‐19. 39 , 40 , 48 Moreover, culturable viruses can be found among some individuals with recurrent clinical disease, and culturable viruses can be present for up to 2 weeks after the completion of therapy. 39 , 40 , 48 Moreover, possible transmission of infection during COVID‐19 rebound has been described. 40 All these findings of a higher viral load, ease of viral culture, and possible viral transmission suggest that patients are likely to be contagious during the rebound period. 39 , 40 Therefore, the CDC advises people with COVID‐19 rebound to follow the CDC's guidance on isolation and take precautions to prevent further transmission. 46 These patients can end their reisolation period after 5 full days, if fever has resolved for 24 h and symptoms are improving. 46 In addition, the patients should wear a mask for a total of 10 days after rebound symptoms started. 46
Based on the current evidence, the recurrence of COVID‐19 symptoms following the use of NMV‐r has not been associated with progression to severe COVID‐19. Therefore, concerns about the recurrence of symptoms should not be a reason to avoid using ritonavir‐boosted nirmatrelvir. 45 , 46 Although insufficient drug exposure by individual pharmacokinetics or insufficient duration could be the cause of COVID‐19 rebound after treatment, longer treatment courses of NMV‐r are not approved by the current emergent use authorization, and there are insufficient data on the efficacy of administering a second course. 45 , 46 Further study is needed to determine whether a longer treatment duration is indicated in this clinical entity.
9. CONCLUSIONS
A 5‐day course of NMV‐r is an effective and safe agent for the treatment of patients with COVID‐19. Rarely, symptom recurrence after initial resolution and virologic rebound after negative testing could develop in some patients after complete treatment with NMV‐r (Figure 1). Almost all reported cases had mild symptoms, and the clinical outcome was favorable. Only a small number of patients required ED visits or hospitalization. Based on the current evidence, there is no need for additional treatment; however, reisolation may be needed.
Figure 1.
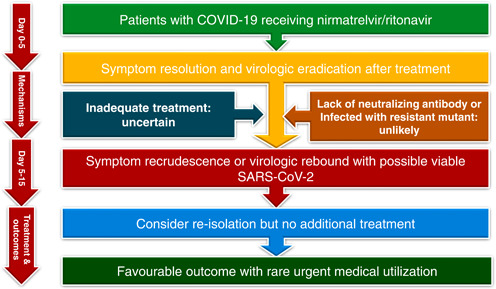
Summary of coronavirus disease 2019 (COVID‐19) rebound following nirmatrelvir/ritonavir treatment
AUTHOR CONTRIBUTIONS
Chih‐Cheng Lai: Acquisition of information; interpretation of data; drafting of the article; final approval of the version to be submitted. Po‐Ren Hsueh: Acquisition of information; interpretation of data; drafting of the article; and final approval of the version to be submitted.
CONFLICT OF INTEREST
No conflict of interest declared
Lai C‐C, Hsueh P‐R. Coronavirus disease 2019 rebounds following nirmatrelvir/ritonavir treatment. J Med Virol. 2023;95:e28430. 10.1002/jmv.28430
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Rahmani K, Shavaleh R, Forouhi M, et al. The effectiveness of COVID‐19 vaccines in reducing the incidence, hospitalization, and mortality from COVID‐19: a systematic review and meta‐analysis. Front Public Health. 2022;10:873596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai CC, Chen IT, Chao CM, Lee PI, Ko WC, Hsueh PR. COVID‐19 vaccines: concerns beyond protective efficacy and safety. Expert Rev Vaccines. 2021;20:1013‐1025. [DOI] [PubMed] [Google Scholar]
- 3. Bestetti RB, Furlan‐Daniel R, Couto LB. Nonpharmaceutical public health interventions to curb the COVID‐19 pandemic: a narrative review. J Infect Dev Ctries. 2022;16:583‐591. [DOI] [PubMed] [Google Scholar]
- 4. Shao W, Zhang W, Fang X, Yu D, Wang X. Challenges of sars‐cov‐2 omicron variant and appropriate countermeasures. J Microbiol Immunol Infect. 2022;55:387‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan WP, Yao MS, Lin MF, Chang HC, Kosik RO, Lee WS. Management and infection control practices in a Taiwanese radiology department during the COVID‐19 outbreak. J Microbiol Immunol Infect. 2021;54:349‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su WL, Hung PP, Lin CP, et al. Masks and closed‐loop ventilators prevent environmental contamination by COVID‐19 patients in negative‐pressure environments. J Microbiol Immunol Infect. 2021;54:81‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Accessed November 2, 2022. https://covid19.who.int/
- 8. Huang JH, Chang HT, Liao CH, Chiu KM. Rapid response of a medical center upon the surge of COVID‐19 epidemic in Taiwan. J Microbiol Immunol Infect. 2022;55:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang CP, Tsai CS, Su PL, Huang TH, Ko WC, Lee NY. Respiratory etiological surveillance among quarantined patients with suspected lower respiratory tract infection at a medical center in Southern Taiwan during COVID‐19 pandemic. J Microbiol Immunol Infect. 2022;55:428‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su SY, Lee WC. Monitoring the peaks of multiwave COVID‐19 outbreaks. J Microbiol Immunol Infect. 2022;55:350‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alves JG, Ferreira Lima TP. International air traffic and COVID‐19 geographical incidence in Brazil. J Microbiol Immunol Infect. 2021;54:125‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): facts and myths. J Microbiol Immunol Infect. 2020;53:404‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen WC, Lai YC, Lin CH, et al. First COVID‐19 mortality case in Taiwan with bacterial co‐infection by national surveillance of critically ill patients with influenza‐negative pneumonia. J Microbiol Immunol Infect. 2020;53:652‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID‐19. J Microbiol Immunol Infect. 2021;54:12‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pérez‐Alba E, Nuzzolo‐Shihadeh L, Aguirre‐García GM, et al. Baricitinib plus dexamethasone compared to dexamethasone for the treatment of severe COVID‐19 pneumonia: a retrospective analysis. J Microbiol Immunol Infect. 2021;54:787‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan SY, Tsai YF, Yen MY, et al. Out‐of‐hospital cardiac arrest and in‐hospital mortality among COVID‐19 patients: a population‐based retrospective cohort study. J Microbiol Immunol Infect. 2022;55:1044‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chao CM, Lai CC, Yu WL. COVID‐19 associated mucormycosis—an emerging threat. J Microbiol Immunol Infect. 2022;55:183‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai CC, Wu CJ, Lee YC, Liu WL. COVID‐19 associated with concomitant mucormycosis and aspergillosis. J Microbiol Immunol Infect. 2022;55:353‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai CC, Yu WL. COVID‐19 associated with pulmonary aspergillosis: a literature review. J Microbiol Immunol Infect. 2021;54:46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lai CC, Chao CM, Hsueh PR. Clinical efficacy of antiviral agents against coronavirus disease 2019: a systematic review of randomized controlled trials. J Microbiol Immunol Infect. 2021;54:767‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang CJ, Wei YJ, Chang HL, et al. Remdesivir use in the coronavirus disease 2019 pandemic: a mini‐review. J Microbiol Immunol Infect. 2021;54:27‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devaux CA, Camoin‐Jau L, Mege JL, Raoult D. Can hydroxychloroquine be protective against COVID‐19‐associated thrombotic events? J Microbiol Immunol Infect. 2021;54:37‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lo CL, Syue LS, Ko WC. Oral dexamethasone for COVID‐19 patients at the initial recognition of hypoxia: can an early dose herald a better outcome? J Microbiol Immunol Infect. 2022;55:170‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mori N, Katayama M, Nukaga S. Triple therapy with hydroxychloroquine, azithromycin, and ciclesonide for COVID‐19 pneumonia. J Microbiol Immunol Infect. 2021;54:109‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Domingo P, Mur I, Mateo GM, et al. Association between administration of Il‐6 antagonists and mortality among patients hospitalized for COVID‐19: a meta‐analysis. JAMA. 2021;326:499‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324:1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hammond J, Leister‐Tebbe H, Gardner A, et al. Oral nirmatrelvir for high‐risk, nonhospitalized adults with COVID‐19. N Engl J Med. 2022;386:1397‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID‐19 in nonhospitalized patients. N Engl J Med. 2022;386:509‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID‐19 in outpatients. N Engl J Med. 2022;386:305‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lai CC, Wang YH, Chen KH, Chen CH, Wang CY. The clinical efficacy and safety of anti‐viral agents for non‐hospitalized patients with COVID‐19: a systematic review and network meta‐analysis of randomized controlled trials. Viruses. 2022;14:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ganatra S, Dani SS, Ahmad J, et al. Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with coronavirus disease 2019 (COVID‐19). Clin Infect Dis. 2022;386:1397‐1408. 10.1093/cid/ciac673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real‐world effectiveness of early molnupiravir or nirmatrelvir‐ritonavir in hospitalised patients with COVID‐19 without supplemental oxygen requirement on admission during Hong Kong's omicron ba.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22:1681‐1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Najjar‐Debbiny R, Gronich N, Weber G, et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high‐risk patients. Clin Infect Dis. 2022:ciac443. 10.1093/cid/ciac443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nene RV, Navarro MR, Tomaszewski CA, Lafree A. Experience using paxlovid for patients with coronavirus disease 2019 in a resource‐limited emergency department. Ann Emerg Med. 2022;80:382‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anderson AS, Caubel P, Rusnak JM. Nirmatrelvir‐ritonavir and viral load rebound in COVID‐19. N Engl J Med. 2022;387:1047‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coulson JM, Adams A, Gray LA, Evans A. COVID‐19 “rebound” associated with nirmatrelvir/ritonavir pre‐hospital therapy. J Infect. 2022;85:436‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malden DE, Hong V, Lewin BJ, et al. Hospitalization and emergency department encounters for COVID‐19 after paxlovid treatment— California, December 2021‐May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:830‐833. [DOI] [PubMed] [Google Scholar]
- 38. Antonelli G, Focosi D, Turriziani O, et al. Virological and clinical rebounds of COVID‐19 soon after nirmatrelvir/ritonavir discontinuation. Clin Microbiol Infect. 2022;28:1657‐1658. 10.1016/j.cmi.2022.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boucau J, Uddin R, Marino C, et al. Characterization of virologic rebound following nirmatrelvir‐ritonavir treatment for coronavirus disease 2019 (COVID‐19). Clin Infect Dis. 2022:ciac512. 10.1093/cid/ciac512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Charness ME, Gupta K, Stack G, et al. Rebound of sars‐cov‐2 infection after nirmatrelvir‐ritonavir treatment. N Engl J Med. 2022;387:1045‐1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai EY, Lee KA, Nathanson AB, et al. Viral kinetics of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) omicron infection in mRNA‐vaccinated individuals treated and not treated with nirmatrelvir‐ritonavir. medRxiv. 2022:2022.08.04.22278378. 10.1101/2022.08.04.22278378 [DOI] [Google Scholar]
- 42. Epling BP, Rocco JM, Boswell KL, et al. Clinical, virologic, and immunologic evaluation of symptomatic coronavirus disease 2019 rebound following nirmatrelvir/ritonavir treatment. Clin Infect Dis. 2022:ciac663. 10.1093/cid/ciac663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ranganath N, O'Horo JC, Challener DW, et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease 2019 (COVID‐19) in High‐Risk persons. Clin Infect Dis. 2022:ciac481. 10.1093/cid/ciac481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R. COVID‐19 rebound after paxlovid and molnupiravir during January‐June 2022. medRxiv. 2022:2022.06.21.22276724. 10.1101/2022.06.21.22276724 [DOI] [Google Scholar]
- 45. National Institutes of Health . Accessed October 7, 2022. https://www.COVID19treatmentguidelines.NHI.gov/
- 46. Centers for Disease Control and Prevention . COVID‐19 rebound after paxlovid treatment. Accessed October 11, 2022. https://emergency.Cdc.Gov/han/2022/han00467.asp
- 47. Birabaharan M, Martin TCS. Acute pulmonary emboli following rebound phenomenon after nirmatrelvir/ritonavir treatment for COVID‐19. Am J Emerg Med. 2022;61:235.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carlin AF, Clark AE, Chaillon A, et al. Virologic and immunologic characterization of coronavirus disease 2019 recrudescence after nirmatrelvir/ritonavir treatment. Clin Infect Dis. 2022:ciac496. 10.1093/cid/ciac496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. U.S. Food and Drug Administration . Paxlovid emergent use authorization. 2021. Accessed on October 8, 2022. https://www.Fda.Gov/media/155050/download
- 50. Deo R, Choudhary MC, Moser C, et al. Viral and symptom rebound in untreated COVID‐19 infection. medRxiv. 2022:2022.08.01.22278278. 10.1101/2022.08.01.22278278 [DOI] [Google Scholar]
- 51. Wang L, Volkow ND, Davis PB, et al. COVID‐19 rebound after paxlovid treatment during Omicron BA.5 vs BA.2.12.1 subvariant predominance period. medRxiv. 2022:2022.08.04.22278450. 10.1101/2022.08.04.22278450 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


