Dear Editor,
The COVID‐19 pandemic is still a global health crisis. Vaccination is a key method to control the pandemic. 1 As of October 1, 2022, more than 120 billion COVID‐19 vaccine doses were administered globally and more than 3 billion in China. 2 Despite the high level of vaccine coverage, limited data are available on the safety of COVID‐19 vaccination for women planning pregnancy, especially those receiving in vitro fertilization (IVF) treatment.
The commonly used COVID‐19 vaccines include inactivated virus vaccines and mRNA vaccines. 3 Regarding mRNA vaccines, three small studies have examined their potential impacts on IVF outcomes and concluded that mRNA vaccines did not influence patients' performance during IVF treatment. 4 , 5 , 6 However, limited data are available on inactivated virus vaccines. The aim of this study was to assess the associations between vaccination with inactivated vaccines and IVF and early pregnancy outcomes.
This retrospective cohort study was performed using data from a tertiary‐care hospital in Shanghai, China. Between May 1, 2021, and December 31, 2021, a total of 3443 patients who received 4846 IVF cycles at the reproductive center of the hospital were included. We excluded 811 patients with incomplete medical record, only received one dose of an inactivated vaccine before embryos transferred, or received adenoviral‐vectored vaccine. The flow chart of participants enrollment was shown in Supporting Information: Figure S1. The study protocol was approved by the hospital's Human Ethics Committee.
Vaccine administration information were obtained from each participant's vaccination record. Participants were categorized as exposure if they received at least two doses of inactivated vaccines before embryos transferred. IVF and early pregnancy outcomes were extracted from the electronic medical record system of the hospital. The primary outcome was clinical pregnancy rate (clinical pregnancy out of all embryo transfer), and the secondary outcome was clinical pregnancy loss rate.
A multivariable generalized estimating equation model based on a binomial distribution for repeat measures (repeated IVF cycles within the same woman during the studied period) was used to estimate the relative risks with 95% confidence intervals. Potential confounders including baseline demographics and cycle‐specific characteristics of the participants were adjusted in multivariable analysis. In multivariable generalized estimating equation model, the unvaccinated patients were served as reference.
After exclusion, a total of 2632 patients were included in the final analysis, of whom 781 were fully vaccinated and 1851 were unvaccinated. The baseline demographic characteristics of the vaccinated and unvaccinated participants were general similar (Supporting Information: Table S1). During the study period, the 2632 women received 3778 IVF cycles, of which 728 conducted among fully vaccinated women and 3050 among unvaccinated women. The cycle‐specific characteristics were also similar between the two groups (Supporting Information: Table S2).
The clinical pregnancy rate (47.5% vs. 47.7%, p = 0.939) was similar between the vaccinated and unvaccinated groups (Figure 1A). Similarly, the two groups were comparable in the rate of clinical pregnancy loss (12.6% vs. 11.8% p = 0.705) (Figure 1B). As one study reported that the binding and neutralizing antibodies declined over time 3 months after receiving two doses of an inactivated virus vaccine, 7 based on the time intervals from complete vaccination to embryos transferred, vaccinated group was further divided into two subgroups (≤3 and >3 months). The clinical pregnancy rate in women who had been vaccinated within 3 months and more than 3 months before embryos transferred was 45.4% and 49.0%, respectively (Figure 1A). While the clinical pregnancy loss rate in women who had been vaccinated within 3 months and more than 3 months before embryos transferred was 10.6% and 12.5%, respectively (Figure 1B). Results of multivariable analysis were shown in Table 1 and no significant association was seen between COVID‐19 vaccination and clinical pregnancy or clinical pregnancy loss.
Figure 1.
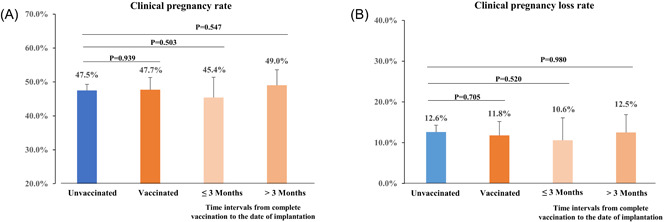
The differences in IVF and early pregnancy outcomes between vaccinated and unvaccinated groups. (A) Difference in clinical pregnancy rate; (B) Difference in clinical pregnancy loss rate. Error bars represent 95% CIs. CI, confidence interval; IVF, in vitro fertilization.
Table 1.
Adjusteda relative risks (95% confidence intervals) for incident clinical pregnancy and clinical pregnancy loss associated with vaccination
| Clinical pregnancy RR (95% CI) | Clinical pregnancy loss RR (95% CI) | |
|---|---|---|
| Unvaccinated | 1.00 (Reference) | 1.00 (Reference) |
| Vaccinatedc | 1.03 (0.87, 1.21) | 0.92 (0.64, 1.33) |
| Time intervalb (months) | ||
| ≤3c | 0.93 (0.72, 1.19) | 0.81 (0.44, 1.47) |
| >3c | 1.09 (0.89, 1.33) | 1.00 (0.65, 1.54) |
Abbreviations: CI, confidence interval; IVF, in vitro fertilization; RR, relative risk.
Models adjusted for female age, body mass index, parity, infertility type, factor of infertility, duration of infertility, previous IVF attempts, endometrial thickness, type of embryos transferred, stage of embryos transferred, and number of embryos transferred.
Time interval from complete vaccination to the date of embryos transferred.
Compared with unvaccinated participants.
This study, to our knowledge, is one of the largest to assess the impact of COVID‐19 vaccination on IVF outcomes. Consistent with three small previous studies, 4 , 5 , 6 our findings provide further evidence indicating that vaccination with inactivated COVID‐19 vaccines did not result in any measurable detrimental effects on IVF or early pregnancy outcomes.
The limitations of our study included its observational nature and the lack of antibody levels. However, no participant had ever been infected with SARS‐CoV‐2, and no participant in the unvaccinated group developed natural antibodies. Moreover, this study was conducted in a single center, which may limit the generalizability of our findings.
In summary, our findings provide safety information related to COVID‐19 vaccination for women who are receiving fertility treatment and support the recommendations of the WHO guidelines for COVID‐19 vaccination.
AUTHOR CONTRIBUTIONS
The study was designed by Liping Jin and Ai Ai. Acquisition, analysis, or interpretation of data were done by Liping Jin and Ai Ai. Drafting of the manuscript was done by Yan Zhao and Yongbo Zhao. Statistical analysis was done by Yan Zhao and Yongbo Zhao. Liping Jin and Ai Ai obtained the funding and had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the reviewing and approved the final version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
This study was supported by the Strategic Collaborative Research Program of the Ferring Institute of Reproductive Medicine, Ferring Pharmaceuticals and Chinese Academy of Sciences (FIRMSCOV02), the National Key Research and Development Program of China (2022YFC2702204), the National Natural Science Foundation of China (81730039, 82071653, 82271701, 82173533), and the Shanghai Rising‐Star Program (21QA1407300).
Yan Zhao and Yongbo Zhao contributed equally to this study.
Contributor Information
Ai Ai, Email: aiai6905@163.com.
Liping Jin, Email: jinlp01@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request from the corresponding authors.
REFERENCES
- 1. Viana J, van Dorp CH, Nunes A, et al. Controlling the pandemic during the SARS‐CoV‐2 vaccination rollout. Nat Commun. 2021;12(1):3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . WHO coronavirus disease (COVID‐19) dashboard. Accessed October 1, 2022. https://covid19.who.int/
- 3. Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576‐577. [DOI] [PubMed] [Google Scholar]
- 4. Bentov Y, Beharier O, Moav‐Zafrir A, et al. Ovarian follicular function is not altered by SARS‐CoV‐2 infection or BNT162b2 mRNA COVID‐19 vaccination. Hum Reprod. 2021;36(9):2506‐2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orvieto R, Noach‐Hirsh M, Segev‐Zahav A, Haas J, Nahum R, Aizer A. Does mRNA SARS‐CoV‐2 vaccine influence patients' performance during IVF‐ET cycle? Reprod Biol Endocrinol. 2021;19(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aharon D, Lederman M, Ghofranian A, et al. In vitro fertilization and early pregnancy outcomes after coronavirus disease 2019 (COVID‐19) vaccination. Obstet Gynecol. 2022;139(4):490‐497. [DOI] [PubMed] [Google Scholar]
- 7. Zhao W, Chen W, Li J, et al. Status of humoral and cellular immune responses within 12 months following CoronaVac vaccination against COVID‐19. mBio. 2022;13(3):e0018122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding authors.


