To the Editor,
Nirmatrelvir/ritonavir (Paxlovid) is an oral antiviral drug that has been shown to reduce the risk of hospitalization, severe COVID‐19, and mortality among mild‐to‐moderate COVID‐19 patients who are at risk of developing severe disease. 1 , 2 Paxlovid received emergency use authorization from the Chinese National Medical Products Administration on February 31, 2022. The efficacy and safety of Paxlovid in COVID‐19 have been studied in clinical studies. 1 , 2 , 3 Further, Fangfang Sun et al. 4 recently reported that early administration of Paxlovid within 5 days since diagnosis is associated with a faster clearance of viral load and a shorter time to viral elimination compared with administration of Paxlovid beyond 5 days in high‐risk COVID‐19 patients who are immunocompromised. However, limited clinical studies have evaluated the effects of early Paxlovid use in the entire COVID‐19 inpatient. In this retrospective cohort study, we analyzed the characteristics and outcomes of patient infected with the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) Omicron variant and evaluated the effect of timing of Paxlovid treatment from patient's symptom onset on the time to viral elimination and hospital stay.
Data of 470 patients were obtained from the medical records of patients infected with the SARS‐CoV‐2 Omicron variant from April 12, 2022 to May 20, 2022. SARS‐CoV‐2 Omicron variant infection was confirmed by reverse‐transcription polymerase chain reaction assay (RT‐PCR). Inclusion criteria were mild‐to‐moderate COVID‐19 patients who have at least one comorbidity or condition associated with high risk of progression to severe COVID‐19: age ≥60 years, cigarette smoking, hypertension, diabetes, cardiovascular disease, chronic lung disease, chronic liver disease, chronic kidney disease, malignancy, immunosuppressive disease, neurological disease, and body mass index ≥30 kg/m.2 1 The exclusion criteria were as follows: contraindications to use Paxlovid, 1 severe or critical COVID‐19, transfer to another hospital, and lack of data. The diagnosis and severity of COVID‐19 was in accord with the Diagnosis and Treatment Scheme for COVID‐19 released by the National Health Commission of China (9th Edition). Viral clearance was defined as two consecutive negative results (cycle threshold ≥35 by RT‐PCR) at least 24 h apart, according to the Diagnosis and Treatment Scheme for COVID‐19 released by the National Health Commission of China (9th Edition). 5 Patients who were treated with Paxlovid were administered the drug (300 mg nirmatrelvir and 100 mg ritonavir) every 12 h for 5 days. This study was approved by the Medical Ethics Committee of Shidong Hospital, which is affiliated with the University of Shanghai for Science and Technology. The requirement for individual consent for this retrospective study was waived.
Data were shown as number (%) or median (interquartile range). Continuous variables with skewed distribution were analyzed by Kruskal−Wallis test, and categorical variables were analyzed by χ 2 test to compare the characteristics of three groups. The early Paxlovid group, late Paxlovid group, and no Paxlovid group were compared by Kruskal−Wallis test and Bonferroni post hoc correction. Statistical analyses were conducted with the IBM SPSS software (version 25.0).
Of the 470 patients, there were 209 patients did not receive treatment of Paxlovid (no Paxlovid group) and 261 patients treated with Paxlovid, among which 141 patients received Paxlovid early within 5 days of symptoms onset (early Paxlovid group) and 120 patients received Paxlovid late at 5 days or later following their symptom's onset (late Paxlovid group). The characteristics of three groups are presented in Table 1. The differences in the viral elimination time between the three groups were statistically significant (p < 0.001). Moreover, the difference in hospital stay between three groups was no significance. The median time of viral elimination was 11 versus 15 versus 16 days (early Paxlovid group vs. late Paxlovid group vs. no Paxlovid group). The viral elimination time was evidently shorter in the early Paxlovid group compared with the late Paxlovid group and no Paxlovid group, respectively (Figure 1), Kruskal−Wallis test and Bonferroni post hoc test indicated that the differences of viral elimination time in the early Paxlovid group and late Paxlovid group, the early Paxlovid group and no Paxlovid group were statistically significant, and there was no statistical difference between late Paxlovid group and no Paxlovid group in viral elimination time.
Table 1.
Characteristics of the overall study population
| Variables | No Paxlovid (n = 209, 44.47%) | Late Paxlovid (n = 120, 25.53%) | Early Paxlovid (n = 141, 30.00%) | p Value |
|---|---|---|---|---|
| Age (years) | 75 (67−86) | 78 (66−86) | 80 (70−88) | 0.056 |
| Time to negative conversion of viral RNA (days) | 16 (12−21) | 15 (13−19) | 11 (9−14) | <0.001 |
| Hospital stay (days) | 12 (10−16) | 12 (9−15) | 12 (10−14) | 0.241 |
| Sex | 0.776 | |||
| Female | 115 (46.0%) | 62 (24.8%) | 73 (29.2%) | |
| Male | 94 (42.7%) | 58 (26.4%) | 68 (30.9%) | |
| COVID‐19 vaccine received | 0.289 | |||
| 0 | 143 (42.3%) | 88 (26.0%) | 107 (31.7%) | |
| 1 | 66 (50.0%) | 32 (24.2%) | 34 (25.8%) | |
| Hypertension | 0.628 | |||
| No | 87 (46.0%) | 50 (26.5%) | 52 (27.5%) | |
| Yes | 122 (43.4%) | 70 (24.9%) | 89 (31.7%) | |
| Diabetes | 0.216 | |||
| No | 154 (42.9%) | 90 (25.1%) | 115 (32.0%) | |
| Yes | 55 (49.5%) | 30 (27.0%) | 26 (23.4%) | |
| Coronary artery disease | 0.176 | |||
| No | 162 (44.3%) | 100 (27.3%) | 104 (28.4%) | |
| Yes | 47 (45.2%) | 20 (19.2%) | 37 (36.6%) | |
| Cerebrovascular disease | 0382 | |||
| No | 170 (45.9%) | 94 (25.4%) | 106 (28.6%) | |
| Yes | 39 (39.0%) | 26 (26.0%) | 35 (35.0%) | |
| Chronic obstructive pulmonary disease | 0.872 | |||
| No | 192 (44.1%) | 112 (25.7%) | 131 (30.1%) | |
| Yes | 17 (48.6%) | 8 (22.9%) | 10 (28.6%) | |
| Chronic kidney disease | 0.063 | |||
| No | 195 (43.3%) | 117 (26.0%) | 138 (30.7%) | |
| Yes | 14 (70.0%) | 3 (15.0%) | 3 (15.0%) | |
| Severity status of COVID‐19 | 0.612 | |||
| Mild | 97 (42.4%) | 59 (25.8%) | 73 (31.9%) | |
| Moderate | 112 (46.5%) | 61 (25.3%) | 68 (28.2%) | |
| Fever | 0.422 | |||
| No | 163 (43.2%) | 96 (25.5%) | 118 (31.3%) | |
| Yes | 46 (49.5%) | 24 (25.8%) | 23 (24.7%) | |
| Cough | 0.125 | |||
| No | 52 (40.9%) | 28 (22.0%) | 47 (37.0%) | |
| Yes | 157 (45.8%) | 92 (26.8%) | 94 (27.4%) | |
| Sputum | 0.087 | |||
| No | 80 (41.2%) | 45 (23.2%) | 69 (35.6%) | |
| Yes | 129 (46.7%) | 75 (27.2%) | 72 (26.1%) | |
| Myalgia | 0.058 | |||
| No | 189 (43.1%) | 116 (26.4%) | 134 (30.5%) | |
| Yes | 20 (64.5%) | 4 (12.9%) | 7 (22.6%) | |
| Headache | 0.882 | |||
| No | 207 (44.5%) | 119 (25.6%) | 139 (29.9%) | |
| Yes | 2 (40.0%) | 1 (20.0%) | 2 (40.0%) | |
| Dyspnea | 0.987 | |||
| No | 195 (44.5%) | 112 (25.6%) | 131 (29.9%) | |
| Yes | 14 (43.8%) | 8 (25.0%) | 10 (31.3%) | |
| Diarrhea | 0.193 | |||
| No | 206 (44.4%) | 117 (25.2%) | 141 (30.4%) | |
| Yes | 3 (50%) | 3 (50%) | 0 (0%) | |
| Treatment with glucocorticoid | 0.591 | |||
| No | 203 (44.9%) | 115 (25.4%) | 134 (29.6%) | |
| Yes | 6 (33.3%) | 5 (27.8%) | 7 (38.9%) |
Note: Data are presented as number (%), or median (interquartile range). Continuous variables use Kruskal−Wallis test and categorical variables use χ 2 test for comparing the characteristics of three groups.
Figure 1.
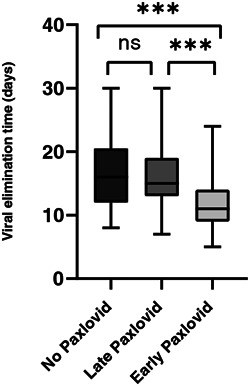
The timing to Paxlovid prescription and the duration of viral elimination. Three groups were analyzed by the Kruskal−Wallis test and Bonferroni post hoc correction, ***p < 0.001. ns, nonsignificance.
Paxlovid treatment should be initiated within 5 days after symptom onset, 6 however, limited studies have investigated the efficacy of Paxlovid based on symptom onset to Paxlovid treatment (early vs. late initiation). In this study, early administration of Paxlovid within 5 days of symptom onset significantly reduced the time to viral elimination, and administration of Paxlovid 5 days after symptom onset didn't show obvious effect on viral elimination time compared with untreated patients. These results suggested that Paxlovid works well and only works if it is given within 5 days of infection, which highlighted the importance of treatment timing of Paxlovid on high‐risk COVID‐19 patients.
AUTHOR CONTRIBUTIONS
Yu Wang, Xinbing Liu, Xubo Chen, Danyang Zhao, Wenying Xiao, and Liuliu Feng planned the study, wrote and revised the paper. Yu Wang, Xubo Chen, and Wenying Xiao collected data. Danyang Zhao worked on data revision and methodology. Yu Wang analyzed and interpreted data. All authors analyzed the data, revised the manuscript, and approved it for publication.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by The Key Discipline Construction Fund of Yangpu District (YP19ZA09) and Shanghai Sailing Program (21YF1443300).
Yu Wang and Danyang Zhao contributed equally to this work.
Contributor Information
Xubo Chen, Email: examcode@163.com.
Wenying Xiao, Email: 13917804027@163.com.
Liuliu Feng, Email: Llf20170101@usst.edu.cn.
DATA AVAILABILITY STATEMENT
Data supporting reported results will be provided on reasonable request.
REFERENCES
- 1. Hammond J, Leister‐Tebbe H, Gardner A, et al. Oral nirmatrelvir for high‐risk, nonhospitalized adults with Covid‐19. N Engl J Med 2022;386(15):1397‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Najjar‐Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in reducing severe COVID‐19 and mortality in high risk patients. Clin Infect Dis 2022: ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malden DE, Hong V, Lewin BJ, et al. Hospitalization and emergency department encounters for COVID‐19 after paxlovid treatment—California, December 2021 to May 2022. MMWR Morb Mortal Wkly Rep 2022;71(25):830‐833. [DOI] [PubMed] [Google Scholar]
- 4. Sun F, Lin Y, Wang X, Gao Y, Ye S. Paxlovid in patients who are immunocompromised and hospitalised with SARS‐CoV‐2 infection. Lancet Infect Dis 2022;22(9):1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Health Commission of the People's Republic of China . Diagnosis and treatment plan for COVID‐19 (trial version 9). Int J Epidemiol Infec Dis. 2022;49(2):73‐80. [Google Scholar]
- 6. Lamb YN. Nirmatrelvir plus ritonavir: first approval. Drugs. 2022;82(5):585‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting reported results will be provided on reasonable request.


