Abstract
This study aimed to examine the efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for coronavirus disease 2019 (COVID‐19). PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar were searched to identify the relevant evidence up to November 10, 2022. The reference lists of key studies were also scanned to find additional records. The quality of the studies was evaluated using the Cochrane tools for assessing the risk of bias. The Comprehensive Meta‐Analysis software version 3.0 was employed for data analysis. Twenty‐three studies involving 314 353 patients were included in the analysis. The findings of the meta‐analysis showed a significant difference between the Paxlovid and no‐Paxlovid groups in terms of mortality rate (odds ratio [OR] = 0.25; 95% confidence interval [CI]: 0.14–0.45), hospitalization rate (OR = 0.40; 95% CI: 0.24–0.69), polymerase chain reaction negative conversion time (mean difference [MD] = −2.46; 95% CI: −4.31 to −0.61), and hospitalization or death rate (OR = 0.17; 95% CI: 0.06–0.46). However, no significant difference was observed between the two groups in terms of COVID‐19 rebound (OR = 0.84; 95% CI: 0.67–1.04), emergency department visit (OR = 0.75; 95% CI: 0.45–1.24), intensive care unit admission (OR = 0.37; 95% CI: 0.13–1.01), and adverse events (OR = 2.20; 95% CI: 0.42–11.47). The results of the present study support the efficacy and safety of Paxlovid in the treatment of patients with COVID‐19. Further research is needed to investigate the COVID‐19 rebound after Paxlovid treatment.
Keywords: COVID‐19, nirmatrelvir/ritonavir, Paxlovid, SARS‑CoV‑2
1. INTRODUCTION
Since the outbreak of coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus‐2 (SARS‑CoV‑2), various antiviral treatments have been proposed or developed to treat COVID‐19 patients. Current evidence shows the therapeutic potential of antiviral agents such as arbidol, 1 remdesivir, 2 lopinavir/ritonavir, 3 molnupiravir, 4 , 5 and nirmatrelvir/ritonavir (Paxlovid) against SARS‑CoV‑2 infection. 6 , 7 More recently, two new oral antivirals, molnupiravir and Paxlovid, have been shown to be promising treatment options for the treatment of mild to moderate COVID‐19 in patients at risk of hospitalization or progression to severe cases. 8 Paxlovid consists of two active drugs, nirmatrelvir and ritonavir, that are approved by the Food and Drug Administration (FDA) for the treatment of COVID‐19 patients. 9 Nirmatrelvir is an antiviral agent targeting the SARS‐CoV‐2 3‐chymotrypsin‐like cysteine protease enzyme, while ritonavir is a CYP3A4 inhibitor and combines with nirmatrelvir to enhance nirmatrelvir pharmacokinetics. 10 Several studies 6 , 7 , 8 , 11 have suggested that Paxlovid might be effective in COVID‐19 patients in terms of reducing mortality and hospitalization rate. However, no comprehensive meta‐analysis has been reported on the use of Paxlovid in the management of patients infected with SARS‑CoV‑2. Therefore, the present study is aimed to evaluate the efficacy and safety of Paxlovid in the treatment of COVID‐19 patients.
2. METHODS
The Preferred Reporting Items for Systematic reviews and Meta‐Analysis‐Rapid Review (PRISMA‐RR) guideline was used to prepare this research. 12
2.1. Search strategy
PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar were systematically searched to find the relevant evidence up to November 10, 2022. Moreover, the reference lists of final studies and systematic reviews were scanned to explore additional records. No language restriction was applied. The search keywords included 2019‐novel coronavirus, SARS‐CoV‐2, COVID‐19, 2019‐nCoV, nirmatrelvir/ritonavir, and Paxlovid. The following search strategy was utilized to identify the relevant records in PubMed: ((((((((Coronavirus [Title/Abstract]) OR (Coronavirus [MeSH Terms])) OR (COVID‐19 [Title/Abstract])) OR (SARS‐CoV‐2 [Title/Abstract])) OR (COVID‐19 [MeSH Terms])) OR (SARS‐CoV‐2 [MeSH Terms])) OR (2019 novel coronavirus infection [Title/Abstract])) OR (2019‐nCoV infection [Title/Abstract])) AND ((Paxlovid [Title/Abstract] OR (Nirmatrelvir/Ritonavir [Title/Abstract])).
2.2. Study selection
The studies were included in the meta‐analysis if they fulfilled the following criteria: (1) COVID‐19‐positive patients based on polymerase chain reaction (PCR) test, (2) Paxlovid as the treatment intervention, (3) any treatment intervention as control, and (4) efficacy and safety outcomes of interest.
2.3. Risk of bias assessment
The methodological quality of randomized clinical trial (RCT) was evaluated using the Cochrane risk of bias tool. 13 The risk of bias in nonrandomized studies of interventions (ROBINS‐I) tool was also applied to assess the quality of nonrandomized studies. 14 The above steps were conducted by two researchers.
2.4. Data extraction
The data extraction was independently conducted by two researchers using an identical extraction form to extract the following data: (1) study characteristics (first author, place, year of publication, and design), (2) patient characteristics (sample size, sex, and mean age), (3) intervention and control (sample size), (4) efficacy and safety outcomes (mortality rate, hospitalization rate, hospitalization or death rate, PCR‐negative conversion time, intensive care unit (ICU) admission, emergency department (ED) visit, and the incidence of any adverse events).
2.5. Evidence synthesis
The Comprehensive Meta‐Analysis software version 3.0 was used to compare the efficacy and safety of Paxlovid with the no‐Paxlovid group. The mean difference (MD) and odds ratio (OR) with the 95% confidence interval (CI) were taken into account to analyze the continuous and dichotomous variables, respectively. The I 2 > 50% and p < 0.1 values were also considered as high heterogeneity. The random and fixed‐effect models were used for studies with high and low heterogeneity, respectively.
3. RESULT
3.1. Search result
Figure 1 shows the identification process of the studies. After removing duplicates, a total of 312 studies were reviewed by title, abstract, and full text. Finally, 37 studies were eligible for review by full text. Twenty‐three studies 6 , 7 , 8 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 involving 314 353 patients were included in the meta‐analysis. All included studies except one 7 were retrospective. The main characteristics of included studies are presented in Table 1.
Figure 1.
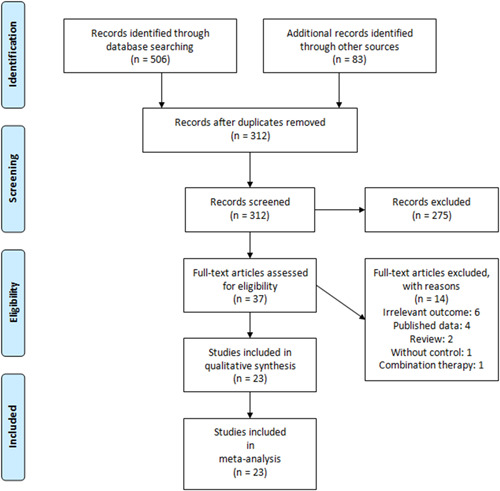
Preferred Reporting Items for Systematic reviews and Meta‐Analysis flow diagram of the included studies in the meta‐analysis
Table 1.
Characteristics of the included studies
| First author | Year | Place | Design | Sample size | Intervention | Control (s) | ||
|---|---|---|---|---|---|---|---|---|
| Total | M | F | ||||||
| Aggarwal 6 | 2022 | USA | OS | 8449 | 3518 | 4931 | Paxlovid | No treatment |
| Cai 15 | 2022 | China | OS | 104 | 52 | 52 | Paxlovid | No treatment |
| Dai 16 | 2022 | USA | OS | 36 | 15 | 21 | Paxlovid | No treatment |
| Ganatra 18 | 2022 | USA | OS | 2260 | 824 | 1436 | Paxlovid | No treatment |
| Gentile 19 | 2022 | Italy | OS | 257 | 124 | 133 | Paxlovid | Molnupiravir |
| Hammond 7 | 2022 | USA | RCT | 2246 | 1148 | 1098 | Paxlovid | Placebo |
| Hedvat 20 | 2022 | USA | OS | 103 | 43 | 60 | Paxlovid | No treatment, sotrovimab |
| Li 21 | 2022 | China | OS | 478 | 278 | 200 | Paxlovid | No treatment |
| Dryden‐Peterson 17 | 2022 | USA | OS | 30 322 | 12 356 | 17.966 | Paxlovid | No treatment |
| Qian 22 | 2022 | USA | OS | 704 | 168 | 536 | Paxlovid | Monoclonal antibody, no treatment |
| Radcliffe 23 | 2022 | USA | OS | 122 | 70 | 52 | Paxlovid | Sotrovimab, molnupiravir, mo treatment |
| Razonable 24 | 2022 | USA | OS | 3607 | 1501 | 2106 | Paxlovid | Bebtelovimab |
| Schwartz 25 | 2022 | Canada | OS | 177 545 | 65 346 | 112.199 | Paxlovid | No treatment |
| Shao 26 | 2022 | China | OS | 131 | NR | NR | Paxlovid | Lianhuaqingwen |
| Valentina 27 | 2022 | Italy | OS | 521 | 271 | 250 | Paxlovid | Sotrovimab, molnupiravir, remdesivir |
| Vora 28 | 2022 | USA | OS | 66 | 36 | 30 | Paxlovid | Sotrovimab, remdesivir |
| Wai 29 | 2022 | China | OS | 54 355 | 27 300 | 27.055 | Paxlovid | Molnupiravir, no treatment |
| Wang 30 | 2022 | USA | OS | 13 644 | 5455 | 8189 | Paxlovid | Molnupiravir |
| Wong 8 | 2022 | Hong Kong | OS | 17 614 | 8887 | 8730 | Paxlovid | Molnupiravir, no treatment |
| Yan 31 | 2022 | China | OS | 35 | 9 | 26 | Paxlovid | No treatment |
| Yip 32 | 2022 | Hong Kong | OS | 93 883 | 41 656 | 52.227 | Paxlovid | No antiviral, molnupiravir |
| Zhong 33 | 2022 | China | OS | 142 | 58 | 84 | Paxlovid | No treatment |
| Zhou 34 | 2022 | USA | OS | 13 657 | 5722 | 7935 | Paxlovid | No treatment |
Abbreviations: F, female; M, male, NR, not reported; OS, observational study; RCT, randomized clinical trial.
3.2. Risk of bias assessment
The methodological quality of included studies in the meta‐analysis was acceptable. The results of the risk of bias in the included studies are shown in Supporting Information: Tables S1 and S2.
3.3. Efficacy outcomes
3.3.1. Primary outcomes
Mortality rate
Thirteen studies 6 , 7 , 8 , 17 , 18 , 19 , 20 , 22 , 23 , 24 , 25 , 29 , 34 involving 298 913 patients were included in the meta‐analysis. The pooled estimate showed a significant difference in the mortality rate of Paxlovid‐recieving patients compared to those who not received Paxlovid (OR = 0.25; 95% CI: 0.14–0.45; p = 0.000) (Figure 2A).
Figure 2.
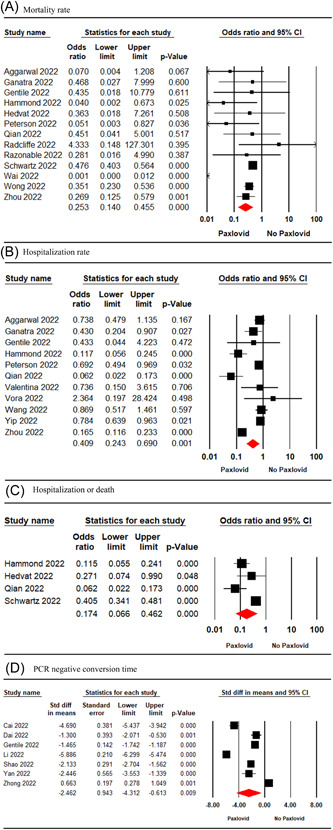
Forest plot of Paxlovid versus control for mortality rate (A), hospitalization rate (B), hospitalization or death rate (C), PCR negative conversion time (D). CI, confidence interval; PCR, polymerase chain reaction.
Hospitalization rate
The pooled estimate of 11 studies 6 , 7 , 17 , 18 , 19 , 22 , 27 , 28 , 30 , 32 , 34 involving 71 675 patients indicated a significant difference in the hospitalization rate of Paxlovid‐treated patients and the no‐Paxlovid group (OR = 0.40; 95% CI: 0.24–0.69; p = 0.001) (Figure 2B).
Hospitalization or death rate
Four studies 7 , 20 , 22 , 25 involving 180,318 patients were included in the meta‐analysis. The pooled estimate revealed a significant difference in hospitalization or death rate between the two treatment groups (OR = 0.17; 95% CI: 0.06–0.46; p = 0.000) (Figure 2C).
PCR‐negative conversion time
The pooled estimate of seven studies 15 , 16 , 19 , 21 , 26 , 31 , 33 involving 1187 patients showed a significant difference between the Paxlovid and no‐Paxlovid groups in terms of PCR‐negative conversion time (MD = −2.46; 95% CI: −4.31 to −0.61; p = 0.009) (Figure 2D).
3.3.2. Secondary outcomes
COVID‐19 rebound
Three studies 16 , 22 , 30 involving 13,998 patients were included in the meta‐analysis. The meta‐analysis findings showed no significant difference between the Paxlovid and no‐Paxlovid groups in terms of COVID‐19 rebound (OR = 0.84; 95% CI: 0.67–1.04, p = 0.11) (Figure 3A).
Figure 3.
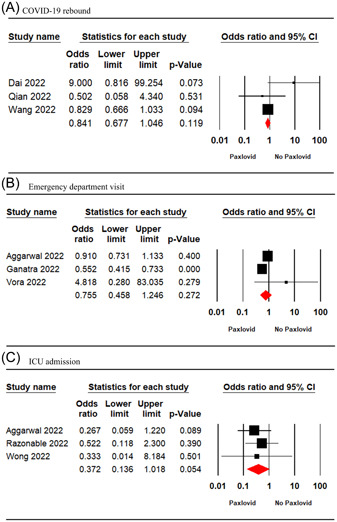
Forest plot of Paxlovid versus control for COVID‐19 rebound (A), emergency department visit (B), ICU admission (C). CI, confidence interval; COVID‐19, coronavirus disease 2019; ICU, intensive care unit.
ED visit
Three studies 6 , 18 , 28 involving 10 775 patients reported the outcome of ED visits. The result of the meta‐analysis showed no significant difference between the Paxlovid and no‐Paxlovid groups in terms of ED visit (OR = 0.75; 95% CI: 0.45–1.24; p = 0.27) (Figure 3B).
ICU admission
The pooled estimate of three studies 6 , 8 , 24 involving 13 836 patients showed no significant difference between the two treatment groups in terms of rate of ICU admission (OR = 0.37; 95% CI: 0.13–1.01; p = 0.05) (Figure 3C).
3.4. Safety outcomes
Five studies 7 , 19 , 28 , 31 , 33 involving 2143 patients reported the incidence of adverse events in patients. The pooled analysis showed no significant difference between the two treatment groups in terms of the incidence of any adverse events in patients (OR = 2.20; 95% CI: 0.42–11.47; p = 0.34) (Figure 4).
Figure 4.
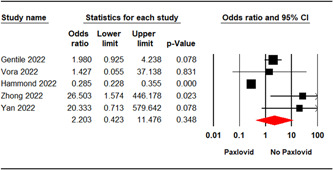
Forest plot of Paxlovid versus control for any adverse events. CI, confidence interval.
3.5. Sensitivity and subgroup analyses
A subgroup analysis was carried out for the outcomes of mortality and hospitalization rate based on the study design and sample size (Table 2). Furthermore, a sensitivity analysis was conducted to compare studies with or without propensity score matching (PSM). The sensitivity analysis revealed no significant change in mortality rate by studies with PSM (OR = 0.40; 95% CI: 0.31–0.53; p = 0.000) and studies without PSM (OR = 0.12; 95% CI: 0.22–0.74; p = 0.000). However, a significant change was detected in hospitalization rate by studies with PSM (OR = 0.51; 95% CI: 0.25–1.02; p = 0.05) and studies without PSM (OR = 0.32; 95% CI: 0.10–0.99; p = 0.49) (Table 2). Moreover, a sensitivity analysis was performed by excluding Wai's study. The result showed no significant change in mortality rate (OR = 0.44; 95% CI: 0.37–0.51; p = 0.000).
Table 2.
Subgroup analysis and sensitivity analysis for efficacy outcomes
| Analysis | No. of studies | Sample size | Point estimate (95% CI) | p Value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| Q value | p Value | I 2 | |||||
| Sensitivity analysis | |||||||
| Mortality rate by PSM | |||||||
| With PSM | 5 | 213 691 | 0.40 [0.31, 0.53] | 0.000 | 5.12 | 0.27 | 21.98 |
| Without PSM | 8 | 85 226 | 0.12 [0.22, 0.74] | 0.02 | 20.68 | 0.000 | 66.16 |
| Hospitalization rate by PSM | |||||||
| With PSM | 5 | 38 047 | 0.51 [0.25, 1.02] | 0.05 | 63.17 | 0.000 | 93.66 |
| Without PSM | 6 | 33 628 | 0.32 [0.10, 0.99] | 0.49 | 35.26 | 0.000 | 85.82 |
| Mortality rate (excluding Wai's 2022 study) | 12 | 250 699 | 0.44 [0.37, 0.51] | 0.000 | 12.08 | 0.35 | 8.98 |
| Subgroup analysis | |||||||
| Mortality rate by design study | |||||||
| Observational | 12 | 296 828 | 0.43 [0.37, 0.50] | 0.00 | 29.47 | 0.000 | 62.67 |
| RCT | 1 | 2085 | 0.04 [0.002, 0.67] | 0.02 | 0.00 | 1.00 | 0.00 |
| Hospitalization rate by design study | |||||||
| Observational | 10 | 69 590 | 0.56 [0.49, 0.64] | 0.000 | 83.62 | 0.000 | 89.23 |
| RCT | 1 | 2085 | 0.11 [0.05, 0.24] | 0.000 | 0.00 | 1.00 | 0.00 |
| Mortality rate by sample size | |||||||
| <1000 | 4 | 994 | 0.64 [0.15, 2.79] | 0.56 | 1.50 | 0.68 | 0.00 |
| 1000–5000 | 3 | 7472 | 0.33 [0.22, 0.50] | 0.00 | 2.23 | 0.32 | 10.68 |
| >5000 | 6 | 290 447 | 0.44 [0.38, 0.52] | 26.48 | 0.00 | 0.000 | 81.12 |
| Hospitalization rate by sample size | |||||||
| <1000 | 4 | 1221 | 0.19 [0.09, 0.42] | 0.000 | 11.77 | 0.008 | 74.52 |
| 1000–5000 | 4 | 6537 | 0.55 [0.41, 0.72] | 0.000 | 21.99 | 86.35 | 10.68 |
| >5000 | 3 | 63 917 | 0.55 [0.47, 0.64] | 0.000 | 59.87 | 0.000 | 96.66 |
Abbreviations: CI, confidence interval; PSM, propensity score matching; RCT, randomized clinical trial.
3.6. Publication bias
Neither Egger's test (p = 0.38) nor Begg's test (p = 0.06) showed evidence of publication bias for a pooled estimate of hospitalization rate. Additionally, no publication bias was detected by Egger's test (p = 0.06) and Begg test (p = 0.46) for a pooled estimate of mortality rate. The funnel plots for outcomes of mortality and hospitalization rate are shown in Supporting Information: Figures S1 and S2, respectively.
4. DISCUSSION
This study is aimed to examine the efficacy and safety of Paxlovid in the treatment of COVID‐19 patients. The effective and safe treatment options not only may reduce the mortality and hospitalization rate in COVID‐19 patients 7 but can also reduce the unprecedented pressure on the health‐care system during the COVID‐19 outbreaks. 35
The findings of the present meta‐analysis revealed that the treatment with Paxlovid is associated with a significantly lower mortality rate in COVID‐19 patients compared to the control. These findings are in line with the meta‐analysis conducted by Zheng et al., 36 in which Paxlovid reduced the death rate in COVID‐19 patients. 36 Moreover, a meta‐analysis of three new oral antivirals, molnupiravir, fluvoxamine, and Paxlovid, 37 showed that treatment with Paxlovid was associated with a significantly lower mortality rate in COVID‐19 patients compared to the placebo. Noteworthy, only one study on the efficacy of Paxlovid was included in Wen's meta‐analysis. 37 According to the present meta‐analysis, Paxlovid treatment significantly reduced the hospitalization rate in patients with COVID‐19 compared with the control. In line with these results, the meta‐analysis of seven studies 36 found a significant clinical benefit in the administration of Paxlovid to reduce the hospitalization rate in COVID‐19 patients compared with those who did not receive Paxlovid. Moreover, Wen's meta‐analysis 37 showed the efficacy of Paxlovid, molnupiravir, and fluvoxamine in reducing the hospitalization rate due to COVID‐19. The present findings also showed a significantly lower rate of hospitalization or death in COVID‐19 patients treated with Paxlovid as compared with those not receiving Paxlovid. Data showed a significantly lower hospitalization or death rate in Paxlovid‐receiving patients compared to placebo, 7 sotrovimab, 20 and no SARS‐CoV‐2‐specific treatment. 20
Some concerns have been recently raised on COVID‐19 rebound in patients treated with antiviral agents. 38 The COVID‐19 rebound is most frequently reported in patients taking nirmatrelvir/ritonavir agents. 38 However, the present study showed no significant difference between Paxlovid‐receiving patients and the no‐Paxlovid groups in terms of the incidence of COVID‐19 rebound. Zheng et al. 36 reported similar findings in their meta‐analysis in which the incidence of COVID‐19 rebound in the Paxlovid group was similar to the control group. One cohort study on Paxlovid‐treated high‐risk COVID‐19 patients showed a low rate of COVID‐19 rebound, which were mainly mild cases. 39
The pooled estimate revealed that the duration of PCR‐negative conversion time was significantly shorter in Paxlovid‐treated patients compared to nontreated patients. In comparison with molnupiravir, Paxlovid treatment led to a shorter duration of PCR‐negative conversion time in patients with mild‐to‐moderate COVID‐19. 19 The present results showed no significant advantage in the use of Paxlovid in COVID‐19 patients compared to the no‐Paxlovid group in terms of ICU admission. However, it effectively declined ED visits in COVID‐19 patients. Studies 23 , 40 , 41 , 42 , 43 , 44 , 45 demonstrate that patients treated with antiviral agents were significantly less likely to be admitted to ICU and visit the ED compared with untreated patients.
The pooled estimate of included studies showed that the incidence of adverse events was similar in both Paxlovid and no‐Paxlovid groups. Similar to our results in the present study, a meta‐analysis found no significant difference between the Paxlovid and control groups. 36 A recently published RCT on nonhospitalized adults at high risk of progression to COVID‐19 7 showed less frequency of Grade 3 or 4 adverse events, serious adverse events, and adverse events leading to discontinuation in the Paxlovid group compared to the placebo group. Furthermore, the data of 183 041 patients with COVID‐19 showed no significant difference between the Paxlovid and no antiviral treatments in terms of higher risk of abnormal liver enzymes or DILI. 46 The present result was also similar to the meta‐analysis of adverse events associated with oral antiviral molnupiravir in terms of the incidence of adverse events in COVID‐19 patients compared to the control. 47
The present study has some important limitations. First, most studies included in the meta‐analysis are retrospective, making them prone to bias and confounding. However, some studies used PSM to reduce selection bias and confounding. Second, various types of interventions were used as the control group, which can affect the reported effect size. Third, we could not perform the subgroup meta‐analysis based on some variables such as COVID‐19 vaccination status due to insufficient data from these studies. Finally, few studies reported the adverse events and COVID‐19 rebound that can affect the effect size.
5. CONCLUSION
The findings of the present meta‐analysis showed the efficacy of Paxlovid in reducing mortality rate, hospitalization rate, hospitalization or death rate, and PCR‐negative conversion time in COVID‐19 patients compared to the no‐Paxlovid group. However, it was not effective in terms of ED visits and ICU admission. In terms of safety, the incidence of adverse events in Paxlovid‐receiving was similar to those not receiving Paxlovid. Further research is needed to investigate the COVID‐19 rebound after Paxlovid treatment.
AUTHOR CONTRIBUTIONS
Conceptualization and project administration: Bahman Amani and Behnam Amani. Literature searching: Behnam Amani and Bahman Amani. Data extraction and quality assessment: Bahman Amani and Behnam Amani. Data Analysis: Bahman Amani and Behnam Amani. Writing – original draft: Behnam Amani. Writing – review and editing: Bahman Amani and Behnam Amani.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary information.
Amani B, Amani B. Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID‐19: a rapid review and meta‐analysis. J Med Virol. 2023;95:e28441. 10.1002/jmv.28441
DATA AVAILABILITY STATEMENT
Data are available online for the included studies. 6 , 7 , 8 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34
REFERENCES
- 1. Amani B, Amani B, Zareei S, Zareei M. Efficacy and safety of arbidol (umifenovir) in patients with COVID‐19: a systematic review and meta‐analysis. Immun Inflamm Dis. 2021;9(4):1197‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. Efficacy and safety of remdesivir in hospitalized Covid‐19 patients: systematic review and meta‐analysis including network meta‐analysis. Rev Med Virol. 2021;31(4):e2187. [DOI] [PubMed] [Google Scholar]
- 3. Amani B, Khanijahani A, Amani B, Hashemi P. Lopinavir/ritonavir for COVID‐19: a systematic review and meta‐analysis. J Pharm Pharm Sci. 2021;24:246‐257. [DOI] [PubMed] [Google Scholar]
- 4. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid‐19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong CKH, Au ICH, Lau KT, Lau EHY, Cowling BJ, Leung GM. Real‐world effectiveness of molnupiravir and nirmatrelvir/ritonavir against mortality, hospitalization, and in‐hospital outcomes among community‐dwelling, ambulatory COVID‐19 patients during the BA. 2.2 wave in Hong Kong: an observational study. Lancet. 2022;400(10359):1213‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aggarwal NR, Molina K, Beaty L, Bennett TD, Carlson N, Ginde AA. Real‐world use of nirmatrelvir–ritonavir in COVID‐19 outpatients during the emergence of omicron variants BA.2/BA2.12.1. medRxiv. 2022. 10.1101/2022.09.12.22279866 [DOI]
- 7. Hammond J, Leister‐Tebbe H, Gardner A, et al. Oral nirmatrelvir for high‐risk, nonhospitalized adults with Covid‐19. N Engl J Med. 2022;386(15):1397‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real‐world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalised patients with COVID‐19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA. 2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22:1681‐1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Najjar‐Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in reducing severe COVID‐19 and mortality in high risk patients. Clin Infect Dis. 2022:ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tene L, Chodick G, Fallach N, Ansari W, Distelman‐Menachem T, Maor Y. Describing COVID‐19 patients during the first two months of Paxlovid (nirmatrelvir/ritonavir) large HMO in Israel. medRxiv. 2022. 10.1101/2022.05.02.22274586 [DOI]
- 11. Yip CF, Lui GC, Man Lai MS, et al. Impact of the use of oral antiviral agents on the risk of hospitalisation in community COVID‐19 patients. Clin Infect Dis. 2022:ciac687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stevens A, Garritty C, Hersi M, Moher D. Developing PRISMA‐RR, a reporting guideline for rapid reviews of primary studies (Protocol). The Equator Network. 2018. https://www.equator-network.org/wp-content/uploads/2018/02/PRISMA-RR-protocol.pdf
- 13. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai H, Yan J, Wang J, Che X, Mou S. Efficacy of Paxlovid in patients with acute kidney injury who developed COVID‐19. J Infect. 2022;85:702‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai EY, Lee KA, Nathanson AB, et al. Viral kinetics of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) omicron infection in mRNA‐vaccinated individuals treated and not treated with nirmatrelvir–ritonavir. medRxiv. 2022. 10.1101/2022.08.04.22278378 [DOI]
- 17. Dryden‐Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID‐19 and hospitalization in a large US health system. medRxiv. 2022. 10.1101/2022.06.14.22276393 [DOI]
- 18. Ganatra S, Dani SS, Ahmad J, et al. Oral nirmatrelvir and ritonavir in non‐hospitalized vaccinated patients with Covid‐19. Clin Infect Dis. 2022:ciac673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gentile I, Scotto R, Moriello NS, et al. Nirmatrelvir/ritonavir and molnuipiravir in the treatment of mild/moderate COVID‐19: results of a real‐life study. Vaccines. 2022;10(10):1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hedvat J, Lange NW, Salerno DM, et al. COVID‐19 therapeutics and outcomes among solid organ transplant recipients during the Omicron BA. 1 era. Am J Transplant. 2022;22:2682‐2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li H, Gao M, You H, et al. Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS‐CoV‐2 RT‐PCR negative conversion rates among high‐risk patients with COVID‐19. Clin Infect Dis. 2022:ciac600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qian G, Wang X, Patel NJ, et al. Outcomes with and without outpatient SARS‐CoV‐2 treatment for patients with COVID‐19 and systemic autoimmune rheumatic diseases: a retrospective cohort study medRxiv. 2022. 10.1101/2022.10.27.22281629 [DOI] [PMC free article] [PubMed]
- 23. Radcliffe C, Palacios CF, Azar MM, Cohen E, Malinis M. Real‐world experience with available, outpatient COVID‐19 therapies in solid organ transplant recipients during the Omicron surge. Am J Transplant. 2022;22:2458‐2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Razonable RR, O'Horo JC, Hanson SN, et al. Comparable outcomes for bebtelovimab and ritonavir‐boosted nirmatrelvir treatment in high‐risk patients with coronavirus disease‐2019 during severe acute respiratory syndrome coronavirus 2 BA.2 Omicron epoch. J Infect Dis. 2022;226:1683‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwartz KL, Wang J, Tadrous M, et al. Real‐world effectiveness of nirmatrelvir/ritonavir use for COVID‐19: a population‐based cohort study in Ontario, Canada. medRxiv. 2022. 10.1101/2022.11.03.22281881 [DOI]
- 26. Shao J, Fan R, Hu J, et al. Clinical progression and outcome of hospitalized patients infected with SARS‐CoV‐2 omicron variant in Shanghai, China. Vaccines. 2022;10(9):1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valentina M, Alessandro CL, Francesca C, et al. Viral load decrease in SARS‐CoV‐2 BA. 1 and BA. 2 Omicron sublineages infection after treatment with monoclonal antibodies and direct antiviral agents. J Med Virol. 2022;95 (1):e28186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vora SB, Englund JA, Trehan I, et al. Monoclonal antibody and antiviral therapy for treatment of mild‐to‐moderate COVID‐19 in pediatric patients. Pediatr Infect Dis J. 2022;42(1):32‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wai AK‐C, Chan CY, Cheung AW‐L, et al. Association of molnupiravir and nirmatrelvir–ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high‐risk patients with mild to moderate COVID‐19. Lancet Reg Health West Pac. 2023;30:100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R. COVID‐19 rebound after Paxlovid and Molnupiravir during January–June 2022. medRxiv. 10.1101/2022.06.21.22276724 [DOI]
- 31. Yan G, Zhou J, Zhu H, et al. The feasibility, safety, and efficacy of Paxlovid treatment in SARS‐CoV‐2‐infected children aged 6–14 years: a cohort study. Ann Transl Med. 2022;10(11):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yip TCF, Lui GCY, Lai MSM, et al. Impact of the use of oral antiviral agents on the risk of hospitalization in community coronavirus disease 2019 patients (COVID‐19). Clin Infect Dis. 2022:ciac687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhong W, Jiang X, Yang X, et al. The efficacy of Paxlovid in elderly patients infected with SARS‐CoV‐2 omicron variants: results of a non‐randomized clinical trial. Front Med. 2022;9:980002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou X, Kelly SP, Liang C, et al. Real‐world effectiveness of nirmatrelvir/ritonavir in preventing hospitalization among patients with COVID‐19 at high risk for severe disease in the United States: a Nationwide Population‐Based Cohort Study. medRxiv. 2022. 10.1101/2022.09.13.22279908 [DOI]
- 35. Streinu‐Cercel A, Săndulescu O, Preotescu L‐L, et al. Efficacy and safety of Regdanvimab (CT‐P59): a phase 2/3 randomized, double‐blind, placebo‐controlled trial in outpatients with mild‐to‐moderate coronavirus disease 2019. Open Forum Infect Dis. 2022;9(9):ofac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng Q, Ma P, Wang M, et al. Efficacy and safety of Paxlovid for COVID‐19: a meta‐analysis. J Infect. 2022;86(1):66‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID‐19: a meta‐analysis. Ann Med. 2022;54(1):516‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parums DV. Rebound COVID‐19 and cessation of antiviral treatment for SARS‐CoV‐2 with Paxlovid and molnupiravir. Med Sci Monit. 2022;28:e938532‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ranganath N, O'Horo JC, Challener DW, et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease 2019 (COVID‐19) in high‐risk persons. Clin Infect Dis. 2022:ciac481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aggarwal N, Beatty L, Bennett TD, et al. Real‐world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID‐19 outpatients. J Infect Dis. 2022;226(12):2129‐2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Al‐Obaidi MM, Gungor AB, Nematollahi S, et al. Effectiveness of casirivimab–imdevimab monoclonal antibody treatment among high‐risk patients with severe acute respiratory syndrome coronavirus 2 B. 1.617. 2 (Delta Variant) infection. Open Forum Infect Dis. 2022;9(7):ofac186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lamour D, Vafadari N, Clayton LM, et al. The treatment of COVID‐19 with monoclonal antibody therapy: patient‐reported outcomes. Cureus. 2022;14(9):e29247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin WT, Hung SH, Lai CC, Wang CY, Chen CH. The impact of neutralizing monoclonal antibodies on the outcomes of COVID‐19 outpatients: a systematic review and meta‐analysis of randomized controlled trials. J Med Virol. 2022;94(5):2222‐2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ong SWX, Ren D, Lee PH, et al. Real‐world use of sotrovimab for pre‐emptive treatment in high‐risk hospitalized COVID‐19 patients: an observational cross‐sectional study. Antibiotics. 2022;11(3):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piccicacco N, Zeitler K, Montero J, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high‐risk outpatients. Open Forum Infect Dis. 2021;8(7):ofab292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong GL‐H, Hui VW‐K, Yip TC‐F, Lui GC‐Y, Hui DS‐C, Wong VW‐S. Minimal risk of drug‐induced liver injury with molnupiravir and ritonavir‐boosted nirmatrelvir. Gastroenterology. 2022;164(1):151‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Amani B, Zareei S, Amani B. Rapid review and meta‐analysis of adverse events associated with molnupiravir in patients with COVID‐19. Br J Clin Pharmacol. 2022;88(10):4403‐4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
Data are available online for the included studies. 6 , 7 , 8 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34


