Abstract
Aim
To assess safety of COVID‐19 vaccination in paediatric patients with immune‐mediated inflammatory disease (IMID).
Methods
Subjects of 5–21 years of age with IMID who received at least one COVID‐19 vaccine completed electronic surveys after each vaccine to assess side effects within 1 week of vaccination, current medications and COVID‐19 testing after vaccination. Charts were reviewed for COVID‐19 polymerase chain reaction and IgG response to SARS‐CoV‐2 spike protein results and for disease flare during the study period.
Results
Among 190 enrolled subjects, 71% were female, with median age 17 (range 6–21) years. The most common diagnosis was juvenile idiopathic arthritis/rheumatoid arthritis (55%). 78% of subjects were taking immunosuppressive medication. At least one side effect was reported in 65% of subjects after any dose of the vaccine; with side effects in 38%, 53% and 55% of subjects after the first, second and third vaccine doses, respectively. The most common side effects were injection site pain (59%), fatigue (54%) and headache (39%). No anaphylaxis or myocarditis was reported. Three subjects (2%) experienced disease flare.
Conclusion
In our cohort of paediatric patients with IMID, observed side effects were found to be mild and disease flare rates were found to be low following COVID‐19 vaccination.
Keywords: autoimmune disease, coronavirus, immunosuppressive therapy, vaccination
Abbreviations
- Ab
antibody
- COVID‐19
coronavirus disease 2019
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IMID
immune‐mediated inflammatory disease
- JIA
juvenile idiopathic arthritis
- MIS‐C
multisystem inflammatory syndrome in children
- MRI
magnetic resonance imaging
- PCR
polymerase chain reaction
- RA
rheumatoid arthritis
- REDCap
Research Electronic Data Capture
- RF
rheumatoid factor
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SLE
systemic lupus erythematosus
- TMJ
temporomandibular joint
- TNF
tumour necrosis factor
Key Notes.
In our paediatric immune‐mediated inflammatory disease (IMID) cohort, side effects were mild following COVID‐19 vaccination.
Severe side effects, such as anaphylaxis or myocarditis, following COVID‐19 vaccination were not observed.
Our data suggest that COVID‐19 vaccination is likely safe in paediatric IMID.
1. INTRODUCTION
Patients with immune‐mediated inflammatory disease (IMID) are at increased risk for infection, likely related to both underlying immune dysregulation, as well as frequent need for immunosuppression to control disease. 1 , 2 , 3 Fortunately, vaccination has proven to be a crucial strategy to prevent or mitigate severity of disease for various infections in patients with IMID. In December 2020, the US Food and Drug Administration issued Emergency Use Authorization for Pfizer‐BioNTech and Moderna vaccines for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus responsible for the coronavirus disease 2019 (COVID‐19) pandemic. Although trials have demonstrated that both of these vaccines are generally safe and effective, initial trials excluded most paediatric patients, as well as patients who were immunocompromised due to underlying disease or medications [except for subjects with human immunodeficiency virus (HIV) infection in the Moderna trial]. 4 , 5 Subsequent studies have been reassuring, demonstrating that both Pfizer‐BioNTech and Moderna vaccines are generally safe and effective in healthy children, as well as in adults with IMID, including those with autoimmune rheumatic diseases or inflammatory bowel disease (IBD). 6 , 7 , 8 , 9 It has been noted that certain immunosuppressive therapies may be associated with lower immune response (e.g. COVID‐19 spike antibody response) to COVID‐19 vaccination in IMID patients. 10 , 11 , 12 , 13 A recent study by Syversen SW et al. 14 was performed to assess whether a three‐dose primary vaccination strategy could increase serological responses in adult IMID patients on immunosuppressive therapy and to assess adverse events in IMID patients receiving a three‐dose primary series in comparison with healthy controls receiving a two‐dose primary series. This study demonstrated COVID‐19 spike antibody levels in IMID patients after a three‐dose primary series were comparable with those in healthy controls receiving a two‐dose primary series and that a lower proportion of IMID patients receiving three doses reported adverse events compared with healthy controls receiving two doses. 14 Despite these previously available studies in adult patients with IMID, data on the safety and efficacy of COVID‐19 vaccination for children with IMID remain limited. 9 , 15 , 16 , 17
Although COVID‐19 generally causes more severe disease in adults, children remain at risk for symptomatic and severe disease and long‐term complications from COVID‐19. Overall estimated paediatric mortality from COVID‐19 is very low. 18 However, children with COVID‐19 may develop subsequent Multisystem Inflammatory Syndrome in Children (MIS‐C), a temporally related systemic hyperinflammatory syndrome following COVID‐19 infection that may result in intensive care unit admission, and rarely, mortality. 19 , 20 , 21 , 22 Risk factors for MIS‐C may include obesity as well as African American race, with further research needed. 22 Overall risk factors for admission and/or severe disease related to COVID‐19 may include immunocompromising conditions such as systemic lupus erythematosus, mixed connective tissue disease and vasculitis. 23 Additionally, children may be vulnerable to long‐term COVID‐19 manifestations, which can present with multiple chronic symptoms. 24
The risk of acute and long‐term complications from COVID‐19 infection can be mitigated by vaccination, making the study of COVID‐19 vaccine safety in paediatric patients with IMID important to protect this vulnerable population. We therefore proposed to evaluate the safety of COVID‐19 vaccination in children and young adults with IMID.
2. PATIENTS AND METHODS
2.1. Study design and participants
Subjects with IMID between 5 and 21 years of age were enrolled in this retrospective cohort study from May 2021 to February 2022 from paediatric rheumatology and gastroenterology clinics at a tertiary center in New York. Subjects who received a COVID‐19 vaccination, irrespective of treatment with immunosuppressive medication, were eligible for inclusion. Parents/guardians gave consent for subjects under age 18, with assent also being obtained from subjects ages 7 to 17 years. Included subjects completed an electronic survey regarding their demographic background, underlying disease, medication use, COVID‐19 infection history and side effects following COVID‐19 vaccination. Subject race and ethnicity were determined based on subject survey responses. Side effects were assessed in list form with yes/no responses and included injection site pain, fever, fatigue, headache, myalgia, gastrointestinal upset, lymph node swelling, rash, allergic reaction/anaphylaxis or other (with opportunity to free text response). Subjects starting a new immunosuppressive medication within 30 days of COVID‐19 vaccination were excluded. Our primary objective was to determine the rate of side effects after COVID‐19 vaccination in paediatric patients with IMID. Our secondary objectives were to determine if underlying diagnosis or medication use was related to risk of side effects, to determine the risk of disease flare following vaccination, to assess rates of acute COVID‐19 infection following vaccination and to assess IgG response to SARS‐CoV‐2 spike protein [hereafter referred to as COVID‐19 spike antibody (Ab)] following vaccination.
Subjects who consented to participate were emailed surveys via the Research Electronic Data Capture [REDCap® (copyright 2011 Vanderbilt University)] web application to be completed at least 2 weeks, but no more than 3 months, after each vaccine dose was received (including third dose/booster vaccines when applicable). Subjects were contacted multiple times to ensure best possible completion rates of surveys and accurate recall of any reported side effects. Electronic medical records were reviewed for enrolled subjects to record any available COVID‐19 polymerase chain reaction (PCR) test results, COVID‐19 spike Ab results and evidence of underlying IMID flare within 1 month of receiving the COVID‐19 vaccine. COVID‐19 spike Ab results from our hospital laboratory used the Roche Elecsys Anti‐SARS‐CoV‐2 immunoassay. COVID‐19 spike Ab results from other laboratories were also collected when available, with varying immunoassays used as determined by the performing laboratory. Disease flare was defined as a change in underlying disease activity requiring a change in the subject's treatment regimen, understanding that flare of disease around vaccination did not prove causality. This study was approved by our institutional review board and ethics committee (IRB# 21–0249) prior to subject recruitment.
2.2. Statistical analysis
For all aims, descriptive statistics were computed. Chi‐square or Fisher's exact tests, as appropriate, were used to assess associations between chronic disease categorisation or medication type and the outcome of side effects post‐vaccination (for doses one, two, and three). As injection site pain was a frequent side effect (affecting 59% of subjects in our cohort and also common after vaccination in the general population), this side effect was excluded from further analyses of associations between underlying subject characteristics and side effects. Statistics were computed based on cohort size for each vaccine dose (one, two, and three) to account for missing data. The exact Cochran‐Armitage test for trend was used to assess for any trend in the proportion of those who had side effects according to the number of immunosuppressive medications subjects were taking (classified as zero, one or two+). In addition to whether any immunosuppressive medication (or sum) was associated with side effects, potential combinations of medications of interest were also evaluated: specifically methotrexate + anti‐tumour necrosis factor (TNF) agents (vs. other), corticosteroids + any other medication (vs. other) and rituximab + any other medication (vs. other). The rates of side effects were also compared between subjects taking immunosuppressive medications versus those not taking immunosuppressive medications.
For all analyses, a result yielding a p‐value <0.05 was considered statistically significant. A result yielding a p‐value >0.05 was considered not significant (NS). All analyses were conducted using SAS version 9.4 (SAS Institute Inc.).
3. RESULTS
Of 358 screened subjects, 190 subjects (53%) completed at least one survey and were enrolled in the study (71% female), with a median age of 17 (interquartile range 15–19) years and overall range from 6 to 21 years. The most common diagnosis for enrolled subjects was juvenile idiopathic arthritis (JIA)/rheumatoid arthritis (RA) in 104 (55%), followed by systemic lupus erythematosus (SLE)/connective tissue disease in 36 (19%) and Crohn's disease in 26 (14%) of subjects. Screened and enrolled subject groups both shared a range of IMID diagnoses, although demographics were not directly compared between these two groups. All subjects completed surveys after vaccine dose one, 91% (173/190) completed surveys after dose two, and 48% of those who completed a survey after dose two (83/173) also completed a survey after dose three. Most subjects (88%) received the Pfizer COVID‐19 vaccine (Table 1). Immunosuppressive medications were taken by 78% of subjects. The most common immunosuppressive medications used were anti‐TNF agents in 39%, methotrexate in 22% and mycophenolate in 11% of subjects (Table 2).
TABLE 1.
Demographic and clinical characteristics of the cohort (n = 190)
| Characteristics | |
|---|---|
| Age, median (range), years | 17 (6–21) |
| Female subjects, n (%) | 135 (71) |
| Race, n (%) | |
| White or Caucasian | 109 (58) |
| Asian | 34 (18) |
| Black or African American | 14 (7.4) |
| Other, declined | 35 (18) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 41 (22) |
| IMID diagnosis, n (%) a | |
| JIA/RA/inflammatory arthritis b | 104 (55) |
| SLE/connective tissue disease c | 36 (19) |
| Crohn's disease | 26 (14) |
| Uveitis | 10 (5.3) |
| Ulcerative colitis/indeterminate colitis | 9 (4.7) |
| Vasculitis | 5 (2.6) |
| Morphea/linear scleroderma | 5 (2.6) |
| CRMO d | 3 (1.6) |
| Dermatomyositis | 2 (1.1) |
| Systemic sclerosis | 1 (0.5) |
| Undifferentiated IMID | 6 (3.2) |
| >1 IMID diagnosis | 17 (9.0) |
| Subjects on immunosuppressive med, n (%) | 148 (78) |
| Vaccine received, n (%) | |
| Pfizer | 167 (88) |
| Moderna | 20 (11) |
| Johnson & Johnson | 3 (1.6) |
IMID = immune‐mediated inflammatory disease.
JIA = juvenile idiopathic arthritis, RA = rheumatoid arthritis.
SLE = systemic lupus erythematosus.
CRMO = chronic recurrent multifocal osteomyelitis.
TABLE 2.
Immunosuppressive medication use in the cohort (n = 190)
| Immunosuppressive medications | n (%) |
|---|---|
| Anti‐TNF a | 74 (39) |
| Methotrexate | 42 (22) |
| Mycophenolate | 20 (11) |
| Corticosteroids | 17 (8.9) |
| JAK inhibitor b | 9 (4.7) |
| Abatacept | 7 (3.7) |
| Tocilizumab | 7 (3.7) |
| Vedolizumab | 5 (2.6) |
| Tacrolimus | 4 (2.1) |
| Ustekinumab | 4 (2.1) |
| Rituximab | 3 (1.6) |
| IVIg c | 3 (1.6) |
| Canakinumab | 2 (1.1) |
| Omalizumab | 2 (1.1) |
| Secukinumab | 1 (0.5) |
| Belimumab | 1 (0.5) |
| Cyclosporine | 1 (0.5) |
| Dupilumab | 1 (0.5) |
| >1 immunosuppressive medication | 48 (25) |
TNF = tumour necrosis factor.
JAK = janus kinase.
IVIg = intravenous immunoglobulin.
At least one side effect was reported in 65% of subjects after any dose of the vaccine, with side effects in 38% (72/190) of subjects after dose one, 53% (92/173) after dose two and 55% (46/83) after dose three. In the full cohort, the most common side effects were injection site pain (59%), fatigue (54%) and headache (39%) (Figure 1). All side effects were more common after vaccine doses two and three compared with after dose one (Figure 2). No anaphylaxis or myocarditis was reported. A single patient reported chest pain after both dose one and two. However, this patient did not require medical evaluation; therefore, it is unknown if this pain was simply musculoskeletal, or may have represented mild myocarditis. No cases of MIS‐C were reported in our cohort.
FIGURE 1.
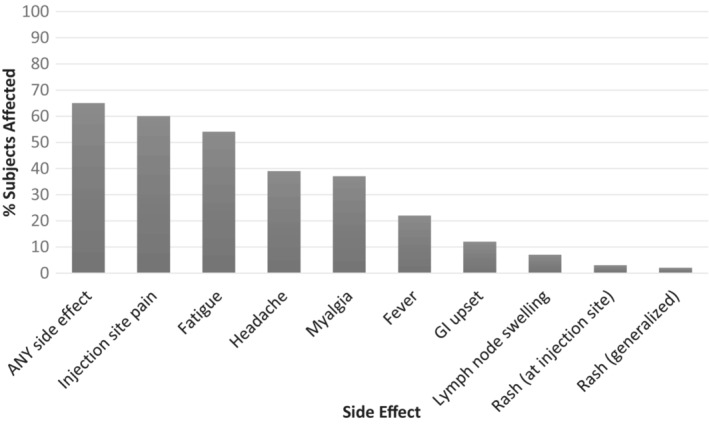
Percent of subjects with SPECIFIC side effects across ALL doses of COVID vaccination (n = 190). Across ALL doses of COVID‐19 vaccination, the most common side effects after vaccination in subjects with paediatric immune‐mediated inflammatory disease were injection site pain, fatigue and headache. GI = gastrointestinal.
FIGURE 2.
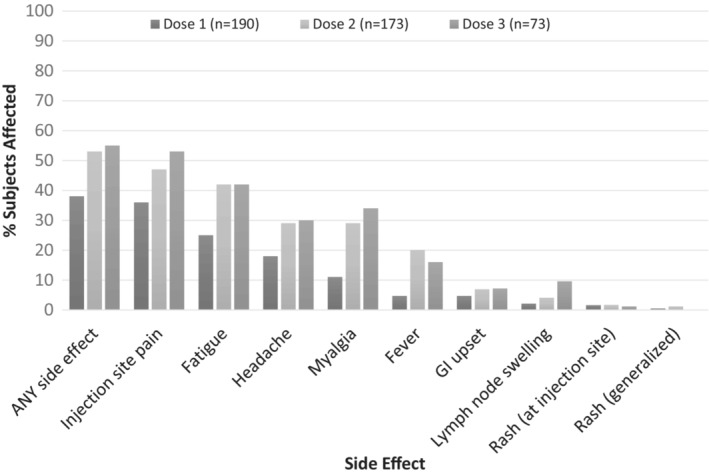
Percent of subjects with SPECIFIC side effects after EACH dose of COVID vaccination. In subjects with paediatric immune‐mediated inflammatory disease, all side effects were more common after the second and third dose of COVID‐19 vaccination compared with after the first dose of vaccination. GI = gastrointestinal.
We found no significant associations between risk of side effects and underlying IMID diagnosis, age, vaccine manufacturer, or use of most immunosuppressive medications, alone or in combination (p = NS for all). However, a few exceptions for associations between side effects and medication use were noted. There was a trend towards evidence of a decreased risk of fever after dose two noted in subjects taking any immunosuppressive medication (RR: 0.81, 95% CI: 0.64 to 1.01, p = 0.04). A decreased risk of any side effects after dose one was noted in subjects taking any corticosteroids overall (alone or in combination with another medication), as well in subjects specifically taking corticosteroids in combination with another medication (RR: 0.74, 95% CI: 0.63 to 0.87, p = 0.04; and RR: 0.69, 95% CI: 0.63 to 0.76, p = 0.007, respectively). A decreased risk of fatigue after doses one and two was noted in subjects specifically taking corticosteroids in combination with another medication (RR: 0.73, 95% CI: 0.66 to 0.79, p = 0.01; and RR: 0.59, 95% CI: 0.48 to 0.72, p = 0.005, respectively), as well as after dose two in subjects taking any corticosteroids overall (alone or in combination with another medication) (RR: 0.58, 95% CI: 0.48 to 0.71, p = 0.005).
Three subjects (2%), all with JIA, experienced disease flare within 1 month of vaccination. Of note, two out of three of these subjects also had flare in the 3–4 months preceding COVID‐19 vaccination.
Self‐reported PCR testing was surveyed among subjects after each dose of COVID‐19 vaccination but was limited in our cohort. Of the subjects who reported PCR testing, positive PCR tests were reported in 2.9% (1/34) of subjects after dose one, 3.1% (1/32) of subjects after dose two, and 27% (11/41) of subjects after dose three. No subjects reported hospitalisation for COVID‐19 infection in our cohort.
Data were collected on COVID‐19 spike Ab titers when available. COVID‐19 spike Ab titers were evaluated in 104 subjects (55%) after two doses of the COVID‐19 vaccine, although timing between vaccination and spike Ab titre sampling varied between patients. A significantly greater proportion of subjects on immunosuppressive medications were tested for COVID‐19 spike Ab response (63%, 93/148 subjects) in comparison with those not on immunosuppressive medication (26%, 11/42 subjects) (p < 0.0001). Of those who had a COVID‐19 spike Ab test, 3/104 subjects (3%) had a negative COVID‐19 spike Ab after vaccination; all were treated with rituximab.
We also compared COVID‐19 spike Ab titers of subjects on immunosuppressive medication who had spike Ab levels checked in our local hospital lab using the Roche Elecsys Anti‐SARS‐CoV‐2 immunoassay. We defined high titre response as ≥250 U/ml, intermediate titre response as 200–250 U/ml, and low titre response as <200 U/ml. Of our subjects on immunosuppressive medications with positive spike Ab titers, 73 (85%) had a high titre response, 2 (2.3%) had an intermediate titre response, and 11 (12.7%) had a low titre response. Subjects with a low titre response were treated with a variety of immunosuppressive medications: mycophenolate alone; anti‐TNF agents alone; combination therapy with methotrexate and anti‐TNF agents; and combination therapy with prednisone, belimumab and methotrexate. All subjects not on immunosuppressive therapy who had COVID‐19 spike Ab testing performed had a titre ≥250 U/ml. However, this included only 11 subjects, as most subjects not on immunosuppressive therapy did not have COVID‐19 spike Ab testing routinely performed.
4. DISCUSSION
Our study demonstrates that the COVID‐19 vaccine is safe in paediatric patients with IMID. Reported side effects were generally mild and disease flare was rare. When available, COVID‐19 spike Ab titers following vaccination were found to be robust in many subjects but were affected by immunosuppressive medication regimens in some.
Prior limited studies in paediatric patients with IMID have sought to evaluate the safety of the COVID‐19 vaccine in this population. One study in childhood‐onset rheumatic disease included 159 subjects, ages 14–19 years, and demonstrated only mild side effects after COVID‐19 vaccination. 9 Another study in paediatric autoimmune inflammatory rheumatic disease included subjects ages 12–21 years and enrolled 91 patients and 40 healthy controls. This prior study demonstrated a good safety profile of the BNT162b2 COVID‐19 vaccine, with 96.7% of patients having mild or no side effects and no change in disease activity. Of note, three patients developed acute symptoms following COVID‐19 vaccination, two of whom required hospitalisation. 15 In comparison, in our cohort, side effects following COVID‐19 vaccination were mild and no subjects were hospitalised or required emergency department assessment following COVID‐19 vaccination. Additionally, our cohort included younger subjects than these prior studies, with subjects ranging in age from 6 to 21 years.
The most common post‐vaccination side effects from our study of children with IMID were similar to those in healthy children. Frenck and colleagues evaluated the safety of the two‐dose Pfizer vaccine in 1131 adolescents aged 12–15 years and 537 subjects aged 16–25 years. 25 Subjects were healthy or had stable pre‐existing disease (including hepatitis B, hepatitis C or HIV infection), with exclusion of subjects with immunocompromise. The most common side effects reported were pain at injection site (79%–86% of subjects), fatigue (60%–66%) and headache (55%–65%), without serious adverse events such as thromboses, hypersensitivity adverse events or vaccine‐related anaphylaxis. 25 Interestingly, the rates of these side effects were generally lower in our study: pain at injection site in 59%, fatigue in 54% and headache in 39%. Although MIS‐C has been very rarely reported after vaccination previously in other studies, 26 we had no cases of MIS‐C following vaccination in our cohort.
We found no significant associations between risk of side effects and most underlying characteristics of our subjects. However, our finding of a possible decreased risk of fever and other side effects in subjects treated with corticosteroids and other immunosuppressive medications may potentially be explained by the fact that these medications blunt immune responses overall. This may then lead to a decreased risk of fever and other side effects experienced after COVID‐19 vaccination. A lower rate of some side effects following COVID vaccination has also been reported previously in adult IMID patients taking immunosuppressive medications in comparison with healthy controls. 14 Of note, our assessment of associations between risk of side effects and underlying subject characteristics was somewhat limited due to small numbers. Additionally, adjustment for multiple comparisons was not performed in our analysis, given the exploratory nature of our study. Due to the number of inferential tests performed, a type I error, whereby a significant result was found simply by chance, is possible.
In our study, we found a very low rate of underlying disease flare in the immediate time frame following vaccination. As our cohort included subjects with multiple IMID diagnoses, disease flare was broadly defined as a change in underlying disease activity requiring a change in the subject's treatment regimen. Only three subjects (2%), all with JIA, required a change in maintenance medications due to increased disease activity following COVID‐19 vaccination, consistent with available reports. 27 , 28 , 29 Two out of three of our subjects with disease flare following vaccination also had flare in the 3–4 months preceding COVID‐19 vaccination, suggesting ongoing or subsequent flare symptoms may or may not have been related to COVID‐19 vaccination. None of the IBD patients in our cohort were noted to experience a disease flare post‐COVID‐19 vaccination. In a prior observational study evaluating 228 children with various rheumatic diseases including 75 with JIA, no disease flares were identified. 27 Additionally, a focused study of 21 patients with JIA on anti‐TNF medication found no flares within 3 months following COVID‐19 vaccination. 28 A larger dataset exists evaluating flares in IBD patients. In the PREVENT‐COVID study, a prospective observational cohort study evaluating COVID‐19 vaccine‐related adverse events in 3316 IBD patients, a 2% IBD flare rate was identified within 30 days of vaccination. 29 Prior studies have however shown a risk for IMID disease flare either at the time of SARS‐CoV‐2 infection or following infection. 30 , 31
Self‐reported PCR testing was surveyed among subjects after each dose of COVID‐19 vaccination, but was limited in our cohort and therefore difficult to interpret. The higher rate of positive PCR tests noted after dose three was likely the result of these data being collected during the time frame of the Omicron subvariant surge of infections in December 2021 and January 2022.
Several immunosuppressed subjects in our cohort demonstrated a suboptimal COVID‐19 spike Ab response after receiving two doses of the COVID‐19 vaccine. The three subjects in our cohort treated with rituximab all had a negative spike Ab serological response following vaccination. One of these subjects had granulomatosis with polyangiitis (GPA) and was treated with rituximab alone, while one with SLE and one with overlap connective tissue disease were on a combination of rituximab, mycophenolate, and corticosteroids. In addition, 11 (13%) immunosuppressed subjects who had a spike Ab titre evaluated following two doses of the COVID‐19 vaccine demonstrated a response of ≤200 U/ml. These suboptimal Ab responses raise concern for inadequate immunogenicity of the vaccine in these subjects, although spike Ab titre is not a complete measure of the immune response to vaccination and therefore does not necessarily predict protection against COVID‐19 infection. Our subjects with a spike Ab titre ≤200 U/ml included SLE subjects treated with mycophenolate alone or a combination of prednisone, belimumab, and methotrexate; as well as JIA subjects treated with anti‐TNF agents alone or a combination of anti‐TNF agents and methotrexate. A prior systematic review of adult patients with IMID showed seroconversion following two doses of the COVID‐19 vaccine was attenuated (<70%) in patients treated with anti‐CD20 therapy (rituximab) or anti‐cytotoxic T lymphocyte–associated antigen (CTLA‐4) therapy (abatacept) but was generally adequate (>90%) in patients treated with many anti‐cytokine agents. Seroconversion was mildly reduced (70%–90%) in patients treated with other immunosuppressive medications such as corticosteroids, mycophenolate mofetil and others. 12 Another prior study of neutralising Ab response to COVID‐19 vaccination in adolescents and young adults with childhood‐onset rheumatic diseases showed an overall robust neutralising Ab response in most patients, but some attenuation of response in SLE patients treated with mycophenolate and in JIA patients treated with methotrexate. 9 Our findings, in addition to this previously available data, support continued COVID vaccine booster doses for immunocompromised patients in the hopes that for many patients this may provide more adequate protection.
Our study had some limitations, including its retrospective design. Although our findings of no anaphylaxis, myocarditis or MIS‐C following vaccination were reassuring, our study was likely not powered to detect these rare potential side effects. Additionally, some reports suggest a higher risk of myocarditis after the Moderna vaccine compared with the Pfizer COVID vaccine, 32 and very few subjects in our cohort received the Moderna vaccine. Many of our specific IMID diagnoses included small numbers of subjects, making assessment for association between underlying diagnosis and risk of side effects or disease flare after vaccination difficult. In our assessment of disease flare following vaccination, flare was broadly defined as a change in underlying disease activity requiring a change in the subject's treatment regimen. This broad definition of disease flare was necessary in this cohort, as disease flare measures specific to each underlying diagnosis included were not routinely captured in chart review. Validated measures of disease activity [such as the Juvenile Arthritis Disease Activity Score (JADAS) in JIA] were not collected routinely at all visits for all patients, and therefore could not be used in this cohort as a measure of disease activity. We are therefore unable to make a clear conclusion on the risk of disease flare following COVID vaccination, as flares of disease are unpredictable and can be influenced by a multitude of external factors, making a comparison with baseline flare rates impossible. Additionally, COVID‐19 PCR and spike Ab titre results were collected from chart review and not available for all subjects. Limited availability of COVID‐19 PCR testing makes any assessment of vaccine efficacy in our paediatric IMID subject cohort difficult. A significantly greater proportion of subjects on immunosuppressive medications were tested for COVID‐19 spike Ab responses in comparison with subjects not on immunosuppressive medications, leading to possible selection bias in assessment of these data. Reported COVID‐19 spike Ab titers were also measured at varying intervals after vaccination between different subjects, making results difficult to interpret. Lastly, COVID‐19 PCR and spike Ab titers performed outside of our hospital system were not routinely available for our review.
Our study aimed to evaluate the safety of COVID‐19 vaccination in children and young adults with IMID. In our cohort of paediatric patients with IMID, observed side effects were found to be mild and the risk of disease flare was found to be low following COVID‐19 vaccination. Taken together, these data suggest that the COVID‐19 vaccine is likely safe in children and young adults with IMID, but further investigation should be performed to validate these findings.
CONFLICTS OF INTEREST
No financial support was used for this study, and the authors report no conflicts of interest.
ACKNOWLEDGEMENTS
This work was presented as an oral abstract at the Paediatric Academic Society 2022 Meeting (abstract ID 1168721). We thank the patients for their participation in this study. We thank Dr. Zanab Mian; Dr. Lydia Thomas; Dr. Matthew Eremita and Nina Skaria, NP, RN; for their contributions in recruitment of subjects. All these individuals are affiliated with the Division of Paediatric Rheumatology, Cohen Children's Medical Center of NY, New Hyde Park, NY.
Sahn B, Lu Y, Hui‐Yuen JS, Fishbein J, Gottlieb BS, Eberhard BA, et al. The safety of COVID‐19 vaccination in immunocompromised children and young adults with immune‐mediated inflammatory disease. Acta Paediatr. 2023;00:1–8. 10.1111/apa.16652
Benjamin Sahn and Ying Lu contributed equally to this work.
DATA AVAILABILITY STATEMENT
Individual participant data that underlie the results reported in this article, after deidentification, will be shared as required.
REFERENCES
- 1. Viget N, Vernier‐Massouille G, Salmon‐Ceron D, Yazdanpanah Y, Colombel JF. Opportunistic infections in patients with inflammatory bowel disease: prevention and diagnosis. Gut. 2008;57(4):549‐558. [DOI] [PubMed] [Google Scholar]
- 2. Sundbaum JK, Arkema EV, Bruchfeld J, Jonsson J, Askling J, Baecklund E. Tuberculosis in biologic‐naïve patients with rheumatoid arthritis: risk factors and tuberculosis characteristics. J Rheumatol. 2021;48(8):1243‐1250. [DOI] [PubMed] [Google Scholar]
- 3. Pego‐Reigosa JM, Nicholson L, Pooley N, et al. The risk of infections in adult patients with systemic lupus erythematosus: systematic review and meta‐analysis. Rheumatology (Oxford). 2021;60(1):60‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baden LR, el Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinte L, Negoi F, Ionescu GD, et al. COVID‐19 vaccine does not increase the risk of disease flare‐ups among patients with autoimmune and immune‐mediated diseases. J Pers Med. 2021;11(12):1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hadi YB, Thakkar S, Shah‐Khan SM, Hutson W, Sarwari A, Singh S. COVID‐19 vaccination is safe and effective in patients with inflammatory bowel disease: analysis of a large multi‐institutional research network in the United States. Gastroenterology. 2021;161(4):1336‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Botwin GJ, Li D, Figueiredo J, et al. Adverse events after SARS‐CoV‐2 mRNA vaccination among patients with inflammatory bowel disease. Am J Gastroenterol. 2021;116(8):1746‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeo JG, Chia WN, Teh KL, et al. Robust neutralising antibody response to SARS‐CoV‐2 mRNA vaccination in adolescents and young adults with childhood onset rheumatic diseases. Rheumatology (Oxford). 2022;61(11):4472‐4481. doi: 10.1093/rheumatology/keac105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330‐1338. [DOI] [PubMed] [Google Scholar]
- 11. Fagni F, Simon D, Tascilar K, et al. COVID‐19 and immune‐mediated inflammatory diseases: effect of disease and treatment on COVID‐19 outcomes and vaccine responses. Lancet Rheumatol. 2021;3(10):e724‐e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jena A, Mishra S, Deepak P, et al. Response to SARS‐CoV‐2 vaccination in immune mediated inflammatory diseases: systematic review and meta‐analysis. Autoimmun Rev. 2022;21(1):102927. doi: 10.1016/j.autrev.2021.102927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kennedy NA, Lin S, Goodhand JR, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV‐19 SARS‐CoV‐2 vaccines in patients with IBD. Gut. 2021;70(10):1884‐1893. [DOI] [PubMed] [Google Scholar]
- 14. Syversen SW, Jyssum I, Tveter AT, et al. Immunogenicity and safety of a three‐dose SARS‐CoV‐2 vaccination strategy in patients with immune‐mediated inflammatory diseases on immunosuppressive therapy. RMD Open. 2022;8(2):e002417. doi: 10.1136/rmdopen-2022-002417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heshin‐Bekenstein M, Ziv A, Toplak N, et al. Safety and immunogenicity of BNT162b2 mRNA COVID‐19 vaccine in adolescents with rheumatic diseases treated with immunomodulatory medications. Rheumatology (Oxford). 2022;61(11):4263‐4272. doi: 10.1093/rheumatology/keac103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dailey J, Kozhaya L, Dogan M, et al. Antibody responses to SARS‐CoV‐2 after infection or vaccination in children and young adults with inflammatory bowel disease. Inflamm Bowel Dis. 2022;28(7):1019‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spencer EA, Klang E, Dolinger M, Pittman N, Dubinsky MC. Seroconversion following SARS‐CoV‐2 infection or vaccination in pediatric IBD patients. Inflamm Bowel Dis. 2021;27(11):1862‐1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liguoro I, Pilotto C, Bonanni M, et al. SARS‐COV‐2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kornitzer J, Johnson J, Yang M, et al. A systematic review of characteristics associated with COVID‐19 in children with typical presentation and with multisystem inflammatory syndrome. Int J Environ Res Public Health. 2021;18(16):8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory syndrome in children—initial therapy and outcomes. N Engl J Med. 2021;385:23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kearsley‐Fleet L, Lawson‐Tovey S, Costello RE, et al. Outcomes of COVID‐19 infection among children and young people with pre‐existing rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:872‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buonsenso D, Munblit D, de Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208‐2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid‐19 vaccine in adolescents. N Engl J Med. 2021;385(3):239‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yousaf AR, Cortese MM, Taylor AW, et al. Reported cases of multisystem inflammatory syndrome in children aged 12–20 years in the USA who received a COVID‐19 vaccine, December, 2020, through august, 2021: a surveillance investigation. Lancet Child Adolesc Health. 2022;6(5):303‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arslanoglu Aydin E, Baglan E, Bagrul I, Tuncez S, Ozdel S, Bulbul M. Safety of COVID‐19 vaccines and disease flares after vaccines in children with rheumatic disease. Postgrad Med. 2022;134(6):616‐621. doi: 10.1080/00325481.2022.2074700 [DOI] [PubMed] [Google Scholar]
- 28. Dimopoulou D, Spyridis N, Vartzelis G, Tsolia MN, Maritsi DN. Safety and tolerability of the COVID‐19 messenger RNA vaccine in adolescents with juvenile idiopathic arthritis treated with tumor necrosis factor inhibitors. Arthritis Rheumatol. 2022;74(2):365‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weaver KN, Zhang X, Dai X, et al. Impact of SARS‐CoV‐2 vaccination on inflammatory bowel disease activity and development of vaccine‐related adverse events: results from PREVENT‐COVID. Inflamm Bowel Dis. 2021;28:1497‐1505. doi: 10.1093/ibd/izab302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hugle B, Krumrey‐Langkammerer M, Haas JP. Infection with SARS‐CoV‐2 causes flares in patients with juvenile idiopathic arthritis in remission or inactive disease on medication. Pediatr Rheumatol Online J. 2021;19(1):163. doi: 10.1186/s12969-021-00653-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hadi Y, Dulai PS, Kupec J, et al. Incidence, outcomes, and impact of COVID‐19 on inflammatory bowel disease: propensity matched research network analysis. Aliment Pharmacol Ther. 2022;55(2):191‐200. [DOI] [PubMed] [Google Scholar]
- 32. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA‐based COVID‐19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331‐340. doi: 10.1001/jama.2021.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data that underlie the results reported in this article, after deidentification, will be shared as required.


