Abstract
Objectives
This systematic review aimed to answer the following question ‘What are the worldwide prevalence of SARS‐CoV‐2 infection and associated factors among oral health‐care workers (OHCWs) before vaccination?’
Methods
Seven databases and registers as well as three grey databases were searched for observational studies in the field. Paired reviewers independently screened studies, extracted data and assessed the methodological quality. Overall seroprevalence for SARS‐CoV‐2 infection was analysed using a random‐effect model subgrouped by professional category. Meta‐regression was used to explore whether the Human Development Index (HDI) influenced the heterogeneity of results. The associated factors were narratively evaluated, and the certainty of the evidence was assessed using the GRADE approach.
Results
Seventeen studies were included (five cohorts and twelve cross‐sectional studies), summing 73 935 participants (54 585 dentists and 19 350 dental assistants/technicians) from 14 countries. The overall estimated pooled prevalence of SARS‐CoV‐2 infection among OHCWs was 9.3% (95% CI, 5.0%–14.7%; I 2 = 100%, p < .01), being 9.5% for dentists (95% CI, 5.1%–15.0%; I 2 = 100%, p < .01) and 11.6% for dental assistants/technicians (95% CI, 1.6%–27.4%; I 2 = 99.0%, p < .01). In the meta‐regression, countries with lower HDI showed higher prevalence of SARS‐CoV‐2 infection (p = .002). Age, comorbidities, gender, ethnicity, occupation, smoking, living in areas of greater deprivation, job role and location/municipalities, income and protective measures in dental settings were associated with positive serological SARS‐CoV‐2 test, with very low certainty of evidence.
Conclusions
The SARS‐CoV‐2 virus infected 9.3% of the OHCWs evaluated worldwide before vaccination. OHCWs should be included in policy considerations, continued research, monitoring and surveillance (PROSPERO CRD42021246520).
Keywords: coronavirus infections, COVID‐19, health workforce, prevalence, SARS‐CoV‐2, Seroepidemiologic studies
1. INTRODUCTION
Globally, the new coronavirus disease 19 (COVID‐19) pandemic has produced significant numbers of infected people and deaths. In 2020, approximately 100 000 health care workers worldwide were believed to have been infected with SARS‐CoV‐2 because of the occupational risk and the scarcity of protective equipment. 1 , 2
The oral health care workers (OHCWs) are part of the frontline in the struggle against COVID‐19, being at a higher occupational risk related to the acquisition of transmissible diseases due to characteristics of professional practice. 3 The aerosol generated during dental interventions is an important source of transmission of several viruses, including SARS‐CoV‐2. 4 The viral transmission occurs through the oral, nasal and ocular mucosa contact with the droplets generated by dental aerosol, which contains saliva, subsequently exposing patients and colleagues. 5
A global discussion about occupational hazards in dental practice related to the COVID‐19 pandemic has heated up. Understanding the factors associated with the SARS‐CoV‐2 infection on OHCWs, including the risk of acquisition at different occupations, age, gender and income, is crucial. 6 Prediction of risk can inform how to protect OHCWs, such as recommendations for policymakers regarding resource allocation strategies and would also serve as an important role in the effective and rational use of personal protective equipment (PPE) in different settings. 7 The dental team has reinforced biosafety measures by improving physical barriers, reducing aerosol production and vaccinating health professionals. 5 , 8 Considering the unequal distribution of the vaccine in the world population yet, and the frequent emergence of variants of coronavirus, this data could assist in identifying high‐risk OHCWs and the reassessment of biosafety measures.
Understanding the burden of SARS‐CoV‐2 infections among OHCWs is a crucial component to inform occupational health policy and strategy. Although vaccination remains highly effective in preventing severe illness and death from SARS‐CoV‐2 infection, the vaccine is not sufficient to prevent transmission of SARS‐CoV‐2 and its variants. 9 Notwithstanding, there are no systematic reviews regarding the prevalence of SARS‐CoV‐2 infection and associated factors among OHCWs before vaccination, limiting the identification of the viral burden of prevalence to those unvaccinated individuals within the dental clinic, hospitals or the community. Even so, such data could help to understand the new infections caused by the variants currently identified and reinforce the need for vaccination by those OHCWs who have not yet been vaccinated. Further, we do not know until now which factors may be associated with the risk of SARS‐CoV‐2 by OHWCs. Limited data are available on the potential for dissemination of SARS‐CoV‐2 produced by aerosols and splashes. More recent studies do not allow definitive conclusions about saliva as a major source of aerosolized microorganisms during aerosol‐generating dental procedures. 10 , 11 , 12 , 13 Therefore, this systematic review and meta‐analysis aimed to answer the following question: ‘What are the worldwide prevalence of SARS‐CoV‐2 infection and the associated factors among OHCWs before vaccination?’
2. MATERIALS AND METHODS
2.1. Eligibility criteria and outcomes
The inclusion criteria were defined by the CoCoPop strategy (Condition, Context, Population): Condition was positive RT‐PCR for SARS‐CoV‐2 or serological diagnosis of SARS‐CoV‐2 infection and associated factors; the context was the clinical practice during the COVID‐19 pandemic; population were OHCWs (i.e. dentists, dental hygienists/technicians, dental assistants and/or dental practitioners). In addition, the study design was cross‐sectional and cohort studies.
Exclusion criteria were studies conducted among dental students or dental residents; studies that do not report the confirmed RT‐PCR or serological diagnosis of SARS‐CoV‐2 infection; clinical trials, animal/in vitro studies, abstracts, poster presentations, reviews, case reports, case series, opinion articles, correspondence, editorials and letters.
The primary outcome was the prevalence of SARS‐CoV‐2 infection among OHCWs through tests for the virus detection as RT‐PCR or the presence of antibodies, such as IgM (2019‐nCoV IgM) and IgG (2019‐nCoV IgG). The secondary outcome was associated factors with the SARS‐CoV‐2 infection.
2.2. Information sources and search strategy
The search strategy was conducted up to 28 March, 2022, on seven electronic databases and registries: Embase through OVID, Latin American and Caribbean Health Sciences Literature database (LILACS) through Virtual Health Library (BIREME), Livivo, MedLine through PubMed, Preprint server medRxiv, Scopus and Web of Science. Grey literature was searched on Google Scholar, OpenGray and ProQuest Dissertations & Theses Global (PQDT Global). There was no limit regarding publication date or language. The search strategy was designed by an experienced librarian (KML) from the Brazilian Centre for Evidence‐Based Research ‐ Federal University of Santa Catarina (COBE) and is shown in detail in Appendix S1: Table S1.
An additional search was performed to identify any articles missed, including a manual search across the reference list of included studies, the reference list of review studies previously published on the subject, and expert consult. EndNote X7 (Clarivates) was used to organize the references and remove duplicates. Also, Rayyan software 14 was used to remove duplicates.
2.3. Study selection, data extraction and quality of evidence
Paired reviewers (FVB and ENL) independently screened studies based on titles and abstracts using Rayyan software. 14 Studies that met the inclusion criteria were selected for full‐text screening. Before each screening stage, the reviewers underwent training exercises with 100 studies. A third reviewer (PP) was consulted to reach the final decision in case of any disagreement.
The same reviewers independently extracted the following data of included studies using predesigned and piloted form: authors, year of publication, country, sample size, study design, settings, age, gender, the prevalence of SARS‐CoV‐2 infection, comorbidity, serological diagnostic method of SARS‐CoV‐2 infection, testing sensibility and specificity, testing trademark, associated factors, funding source, conflict of interest and Human Development Index (HDI) of each country through the 2018 statistical update of the United Nations Development Programme. 15 HDI is measured by income per capita and by health and education indicators.
The methodologic quality of the included studies was evaluated independently by the same investigators using the Joanna Briggs Institute critical appraisal tools. Cross‐sectional and cohort studies were assessed with the appropriate checklist for each study design. 16 This tool consists of eight items for cross‐sectional design and 11 for cohort design that judge the sampling process, data analysis process and statistical methods, study settings, measurement tools and response rate. A study was considered high quality when the methods were appropriate, and it was described descriptively. Divergences rated were resolved by consensus or arbitrated by the same third investigator. If any data aforementioned were not reported, we contacted the corresponding authors by email.
2.4. Data synthesis and statistical analysis
Narrative synthesis and statistical analysis regarding primary and secondary outcomes were critically analysed by grouping and comparing data reported by the included studies.
We extracted the prevalence of events from the first time point for cohort studies and, therefore, treated all data as cross‐sectional data rather than incidence. The overall prevalence and subgroup analysis were assessed into two professional categories: dentists (dentists and/or dental practitioners) and dental assistants/hygienists. They were expressed through relative/absolute frequencies and a 95% confidence interval (CI). The I 2 test (ratio of true heterogeneity over the total variation observed) was used to calculate statistical heterogeneity. 17 The estimated crude and global prevalence were calculated by pooling study‐specific estimates using a random‐effects model due to inherent heterogeneity among different populations (Mantel–Haenszel model applied to meta‐analysis). 18 Also, the prediction interval (the measure of data dispersion around the mean effect size of different populations) was graphically represented in the meta‐analyses. 19
The meta‐analysis and meta‐regression were performed using the R program, version 3.5.2 with R Studio (R Core Team, 2018) using the meta package. 20 , 21 A meta‐regression model was used to explore whether the independent variable HDI could explain the results in the meta‐analysis for the primary outcome. Diagnosis of SARS‐CoV‐2 infection was incorporated into the model as a continuous and dependent variable (prevalence in %) and HDI was used in this study as a continuous variable to determine whether differences in HDI among countries could explain the prevalence of SARS‐CoV‐2 infection in the OHCWs model.
The secondary outcome was synthesized descriptively following the SWiM recommendation. 22 Meta‐analysis of associated factors with SARS‐CoV‐2 infection was not possible because of the high clinical and methodological heterogeneity among the included studies. Therefore, the results were pooled according to the different associated factors, and a funnel plot was not possible.
2.5. Reporting bias assessment
Single‐arm forest plots were performed considering the nature of data retrieved from included studies (prevalence). However, using funnel plots for publication bias analysis was not feasible. So, a broad literature search was conducted to prevent publication bias. Additionally, the sponsors of the included studies and the conflict of interests of the authors were evaluated.
2.6. Certainty of evidence
GRADE approach 23 for a narrative synthesis of different estimates across studies was followed to assess the certainty of the evidence of associated factors with SARS‐CoV‐2 positivity (secondary outcome). Two independent researchers (CCM and FVB), previously trained, assessed the certainty of the evidence and divergences between them were resolved by consensus. Evidence certainty can be high, moderate, low or very low. 24 Observational studies start with low certainty of evidence and can be a downgrade to one (serious) or two levels (very serious problems) due to the risk of bias, inconsistency, imprecision, indirectness or publication bias. 25
In addition, the certainty can be upgraded due to the large effect, the dose–response gradient and the investigation of plausible confounders or other biases. 23 GRADEpro (McMaster University dbEP, 2015) was used to create the Summary of Finding (SoF) Table of the narrative synthesis.
2.7. Protocol, registration and reporting
A systematic review protocol based on the PRISMA‐P guideline 26 was developed and registered in the PROSPERO database (CRD42021246520). The present study was reported according to PRISMA 27 and MOOSE guidelines 28 (Appendix S1: Figure S1).
3. RESULTS
3.1. Study selection
A total of 5264 records were identified from the electronic databases and registers searched, reducing to 3713 after the removal of 1551 duplicate records. Titles and abstracts were screened based on the eligibility criteria. Thirty reports met the inclusion criteria, and 3683 were excluded in this phase. After full‐text reading, 16 did not meet the eligibility criteria, resulting in 14 studies included from databases and registers.
In relation to the identification of studies via other methods, 1847 records were identified, and no study was duplicated. Subsequently, 1833 studies were excluded after reading titles and abstracts, resulting in 14 studies for full‐text reading. Eleven studies did not meet the eligibility criteria; thereby, three reports were included.
The final sample was composed of 17 observational studies: five cohorts and 12 cross‐sectional studies. All these studies were included in the meta‐analysis.
Figure 1 shows the flow diagram of the results of searching, screening and study exclusion. Furthermore, the reasons for exclusions on full‐text screening are provided in Appendix S1: Table S2.
FIGURE 1.
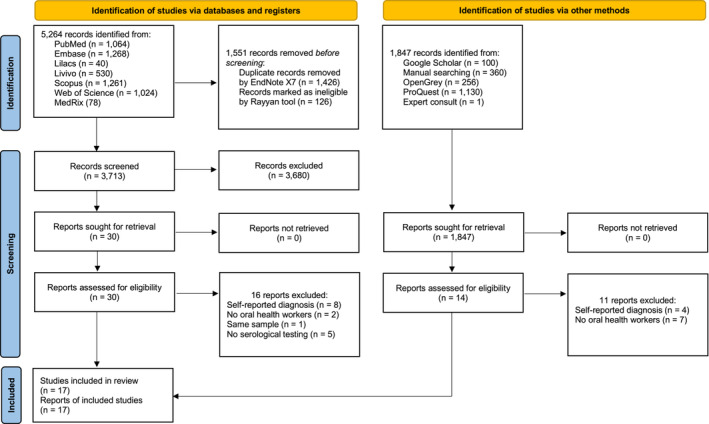
PRISMA 2020 flow diagram
3.2. Study characteristics
Appendix S1: Table S3 summarizes the main characteristics of the included studies. All articles were published in English between the years 2020 (n = 5), 2021 (n = 11) and 2022 (n = 1). This systematic review included 73 935 participants (54 585 dentists and 19 350 dental assistants/technicians) from 14 countries. Four studies were performed in Asia, 29 , 30 , 31 , 32 eight in Europe, 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 three in South America 40 , 41 , 42 and two in North America. 43 , 44 , 45 The mean age of participants ranged from 38 to 46 years, and the number of females ranged from 58% to 89%. Systemic conditions or comorbidities varied across studies. Most of the selected studies (n = 10) presented data from other health care workers (i.e. nurses, pharmacy staff, occupational therapists and others), but this systematic review only answered the question defined by the CoCoPoP strategy.
All articles provided reasonably clear descriptions of the participants, study design and settings. Regarding the testing phase, the evaluated period ranged from February to December (2020), 29 , 30 , 31 , 32 , 33 , 34 , 35 , 37 , 38 , 40 , 41 , 42 , 43 , 45 from January to April (2021) 39 , 44 and from May (2020) to January (2021). 36 Furthermore, all of them reported SARS‐CoV‐2 infection among OHCWs through accurate tests for the virus detection as RT‐PCR or the presence of antibodies, such as IgM (2019‐nCoV IgM) and IgG (2019‐nCoV IgG). However, only seven 34 , 36 , 37 , 39 , 40 , 42 , 46 and eight 30 , 34 , 36 , 37 , 39 , 40 , 42 , 46 studies provided detailed information on testing sensibility/specificity and testing trademark, respectively. Only two studies disclosed conflict of interest, 32 , 36 and eight studies presented funding by diverse companies. 30 , 36 , 37 , 39 , 40 , 41 , 46
3.3. Methodological quality
A summary of the methodological quality assessment, based on the JBI tool, is provided in Appendix S1: Table S4. Overall, cross‐sectional studies clearly reported study participants, settings and measurement of the SARS‐CoV‐2 infection diagnosis. Besides, the outcomes were measured validly and reliably for all studies. However, most studies performed inappropriate statistical analysis (n = 7) since they did not identify confounding factors (n = 5) and did not report how the strategies to deal with confounding factors were stated (n = 7).
Likewise, cohort studies described sufficient information about study participants, settings and outcomes. Nevertheless, all studies had not described the reasons for the follow‐up loss nor strategy to address incomplete follow‐up. Only two studies performed the appropriate statistical analysis, while the others did not identify confounding factors (n = 2) or strategies to deal with them (n = 3).
3.4. Meta‐analysis and meta‐regression
The overall pooled prevalence of SARS‐CoV‐2 infection in OHCWs from 17 studies was 9.3% (95% CI, 5.0%–14.7%; I 2 = 100%, p < .01) (Figure 2). Subgroup analysis showed that the prevalence of SARS‐CoV‐2 infection in dentists from 16 studies was 9.5% (95% CI, 5.1%–15.0%; I 2 = 100%, p < .01) (Figure 3). When another subgroup analysis with dental assistants and technicians was evaluated through 10 studies, the prevalence of SARS‐CoV‐2 infection was 11.6% (95% CI, 1.6%–27.4%; I 2 = 99.0%, p < .01) (Figure 4). The number of reports included differed because some studies did not report data of all professional categories.
FIGURE 2.
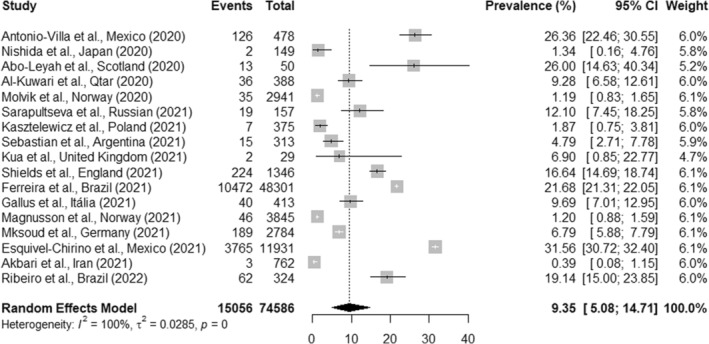
Overall pooled prevalence of SARS‐CoV‐2 infection in OHCWs
FIGURE 3.
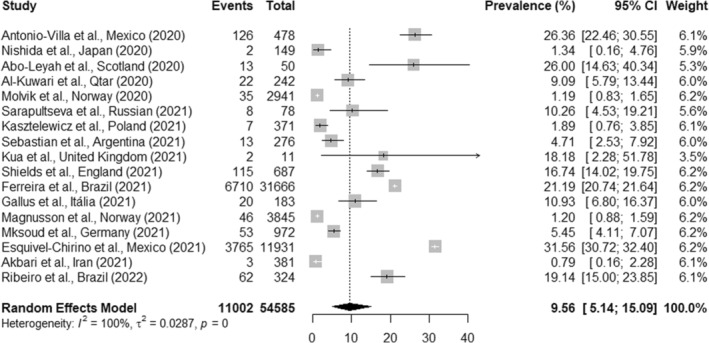
Prevalence of SARS‐CoV‐2 infection in dentists
FIGURE 4.
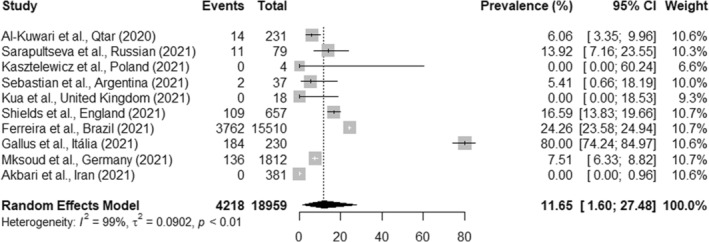
Prevalence of SARS‐CoV‐2 infection in dental assistants and technicians
The prevalence of SARS‐CoV‐2 infection in OHCWs increased with lower HDI. Meta‐regression analysis showed linear association statistically significant (p = .002, Figure 5). Moreover, I 2 was high, showing the high heterogeneity of the SARS‐CoV‐2 positivity model. Hence, the observed heterogeneity could be explained by the HDI, among other factors.
FIGURE 5.
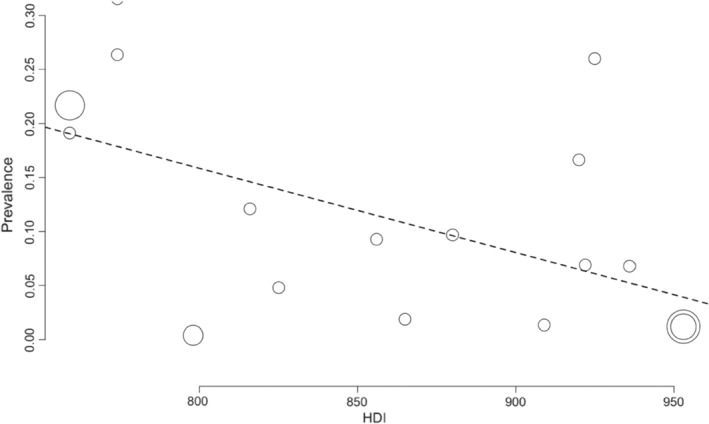
Bubble plot for meta‐regression of Human Development Index (HDI) against the prevalence of SARS‐CoV‐2 infection
3.5. Reporting biases
After evaluating the methods and results of the included studies, reporting bias was not detected. Four studies 31 , 33 , 38 , 42 did not report funding, and two studies 32 , 36 reported conflict of interest. Five studies reported receiving no funding, 29 , 34 , 35 , 43 , 44 and two studies declared industry support. 32 , 36
3.6. Narrative synthesis and certainty of evidence
The SoF Table (Appendix S1: Table S5) displays the narrative synthesis for seropositive diagnosis of SARS‐CoV‐2 infection according to the following associated factors: age groups, comorbidities/symptoms/signs, gender, ethnicity, OHCWs roles, clinical/non‐clinical occupations, smoking, living in areas of greater deprivation, job location, income, municipalities and protective measures.
Briefly, the assessment of the certainty of the evidence for the comparisons revealed a very low level of confidence for the results related to associated factors with SARS‐CoV‐2 infection. There were very serious problems due to imprecision, once the Optimal Information Size was <300 events. 45 Likewise, indirectness was an issue. Some evidence comes from studies using single tests to diagnose SARS‐CoV‐2 (e.g. either only PCR or serological tests), limiting the applicability of all testing options. Major concerns were raised regarding the criteria for inclusion in the samples since they were not clearly defined, and the statistical analysis used was not appropriate. According to the GRADE Working Group, publication bias was strongly suspected once publication bias is more likely to occur in observational studies than in RCTs, once the former ones do not need registration. 45 Finally, there were no reasons for upgrading the certainty of evidence in either analysis.
4. DISCUSSION
Our findings confirm that 9.3% of the OHCWs worldwide had been infected by the SARS‐CoV‐2 virus between February 2020 and April 2021. All the studies included in this systematic review presented data on the prevalence of SARS‐CoV‐2 infection among OHCWs before the vaccination. It means that these studies have precious information because they estimated the prevalence of SARS‐CoV‐2 infection in those unvaccinated individuals, and therefore cannot be repeated. We were additionally able to identify associated factors with SARS‐CoV‐2 infection among OHCWs. Based on the narrative synthesis, the certainty that age, comorbidities, gender, ethnicity, occupation, smoking, deprivation, job role and location/municipalities, income and aspirating system in dental settings were associated with positive serological SARS‐CoV‐2 test was very low. The results of our meta‐regression suggest that countries with lower HDI presented higher prevalence of SARS‐CoV‐2 infection, which may partially explain the high heterogeneity observed among studies.
A meta‐analysis showed that HCW estimated prevalence of SARS‐CoV‐2 infection in 2020 through PCR test and antibodies detection was 11% (95% CI, 7%–15%) and 7% (95% CI, 4%–11%), respectively. 47 The pooled prevalence among OHCWs in our study was 9.3% (95% CI, 5.0%–14.7%), and it was similar to the HCW in the same period. Limited and biased testing may influence estimates of SARS‐CoV‐2 infection prevalence. Underestimates of the number of SARS‐CoV‐2 infections worthy of attention. With the lack of testing globally, most testing policies recommended that physicians prioritize testing for hospitalized patients, who tend to have moderate to severe symptoms, while a portion of the population remains untested. Furthermore, low‐income countries were most impacted by the testing rates. 48 In the US, the total number of SARS‐CoV‐2 infections by 18 April, 2020, was estimated to be 6 454 951 (19 per 1000), an estimate nine times higher than the 721 245 confirmed cases (2 per 1000) reported during this period. 49 As in the present study, there were previous attempts to explain heterogeneity in the magnitude of prevalence of SARS‐CoV‐2 infection through meta‐regression, although heterogeneity is expected in meta‐analyses involving prevalence data. Rocha et al. 50 found that countries with a higher socioeconomic vulnerability had larger SARS‐CoV‐2 infections. We could confirm such findings, and beyond that we suspected that the lower prevalence among dentists may be due to their high socioeconomic position, although the studies included in this systematic review failed to assess where exposure occurred. Also, unexplained heterogeneity might refer to the HDI and individual factors that OHCWs faced during the pandemic. The current review revealed that several factors such as age, comorbidities, gender, ethnicity, occupation, smoking, deprivation, job role and location/municipalities, income and aspirating system in dental settings were associated with infection by the SARS‐CoV‐2 test, yet with very low certainty of evidence. OHCWs should be included in policy considerations to ensure that vaccination and testing policies are strengthened, as well as monitoring, surveillance, continued research, and other aspects of public health measures during the COVID‐19 pandemic. Further studies are required to confirm associated factors with direct SARS‐CoV‐2 infection among OHCWs.
The studies analysed the prevalence of SARS‐CoV‐2 infection through different diagnosis methods, like accurate tests for virus detection or the presence of antibodies; however, the sensitivity and specificity may vary by test. 51 Ten studies reported the diagnosis of SARS‐CoV‐2 infection by RT‐PCR (reverse transcription‐polymerase chain reaction), considered the gold standard. It identifies the virus through the viral RNA in the nasal oropharyngeal mucosa swab. Ten studies identified antibodies through ELISA (Enzyme‐Linked Immunosorbent Assay) or rapid test (immunoassay). The antibody detection occurs between the seventh and eleventh days after exposure to the virus in a blood sample. Just seven studies described the sensitivity and specificity of the tests utilized, which varies between 83%–98.6% and 91%–99.9%, respectively. 52 It is important to emphasize the fact that antibodies wane after a relatively short period (a few months), and the virus is detectable (through PCR) only during a specific window of time (days or weeks). So, the actual prevalence can be modified according to the test used by the studies.
Besides, SARS‐CoV‐2 infection prevalence and mortality rate among the general population vary across the countries, and even different regions of the same country or city, at different periods of the year and month because of the transmission dynamics of the disease and systemic inequities. 53 We included studies from 14 countries and three continents which presented distinct epidemiologic populational scenarios since the initial period of the pandemic. Worldwide prevalence of SARS‐CoV‐2 infection likely reflects differences in community transmission based on behaviour, health system assistance, local resources, and the environment, besides important social and economic aspects. It is unknown whether the infections of OHCWs may be different from those of the general population considering that the primary studies included in this systematic review did not include data from the general population. However, indirect comparisons with other studies may suggest a higher risk attributed to OHCWs. In Brazil, for instance, the prevalence of SARS‐CoV‐2 infection in the same period studied was estimated about 15% in the general population, 54 meanwhile the study by Ferreira et al. 41 included in our systematic review indicated the prevalence of 22% for OHCWs. However, none of the studies included in this study examined at the potential source of infection among OHCWs, limiting the possibility of assessing the impact of occupational versus community‐acquired infection. In addition, geographic and population differences regarding SARS‐CoV‐2 infection prevalence are evident, and certain groups such as people who live in crowding areas and front‐line services workers are disproportionately affected. 55 Also, prevalence reflects differences in testing policy. Countries such as South Korea and China tested massively, so they will indeed find more cases, including asymptomatic. However, Latin American countries, for example, did not have tests enough, even for symptomatic cases demand.
Studies examining the occupational role and risk of SARS‐CoV‐2 infection have shown conflicting results. At the onset of the first wave of SARS‐CoV‐2 infection, some studies have reported high rates of positivity among health care personnel. 56 , 57 , 58 , 59 , 60 , 61 Although OHWCs are in close contact with patients, there is also potential for greater awareness and training for appropriate PPE use among them. Parallel or inverse trends in infection rates in OHWCs and the general public may signify community transmission as a major source of infection, especially in the presence of peculiar socioeconomic and cultural factors in the community level. Studies suggested that household contacts may play a significant role in SARS‐CoV‐2 infection in OHCWs because of the rapid circulation of the virus at the community. Likewise, epidemiological studies have shown that community and intra‐family transmission are the main reasons for SARS‐CoV‐2 spread. 62 , 63 , 64 To the best of our knowledge, the exact contribution of community exposures and occupational health risks leading to SARS‐CoV‐2 infection among OHCWs in health settings has yet to be determined, due to the difficulty in actually assessing where exposure occurred. The transmission of SARS‐CoV‐2 happens through contact with respiratory droplets from an infected individual by closer contact. 65 In dental settings, aerosol transmission can also occur in specific situations where procedures that generate aerosols are realized. In our systematic review, the number of protective measures, use of rubber dam, type of aspirating system and the presence of HEPA filters utilization seem to affect the prevalence of SARS‐CoV‐2 infection across dental clinics with very low certainty of evidence. 30 , 39 A recent systematic review found that SARS‐CoV‐2 RNA can be detected in the air in various health care settings and can also be detected in community settings, sometimes at low concentrations. In this context, using the personnel protective equipment by OHCWs is essential to prevent contamination. 66
There are some strengths and potential limitations of the evidence included in this review. First, the studies included showed high heterogeneity and did not represent all continents, making comparison difficult and not permitting a worldwide vision. Second, most studies used a non‐probabilistic sample, which introduces a selection bias. Third, the accuracy of the tests was not reported in all studies, thus we cannot estimate the real prevalence of SARS‐CoV‐2 infection. Fourth, the potential source of virus contamination was not studied, limiting the possibility of differentiation between occupational and community transmission. Last, most of them did not report the level of adherence to preventive measures, modifications in the clinical routine and ambient and personnel protective equipment use, such as respiratory masks, face shields and others. Just two studies provided information about protective measures in dental settings as protection against SARS‐CoV‐2 transmission. 30 , 39 Regarding the review processes' limitations, it was not possible to stratify dental assistants into various professional categories, or the dentist's speciality. Regarding strengths, our systematic review included only studies with a confirmed diagnosis of SARS‐CoV‐2 infection based on the detection of IgM (2019‐nCoV IgM) and IgG (2019‐nCoV IgG) or/and RT‐PCR for SARS‐CoV‐2 according to standards of the WHO (2020), which not might have led to recall bias. Additionally, we included just studies that tested the OHCWs, excluding studies that self‐reported diagnoses. Moreover, we also obtained unpublished data from identified studies, which should reduce publication bias. Therefore, our findings mainly represent the prevalence of SARS‐CoV‐2 infection before introducing vaccination.
5. CONCLUSION
The pooled data from the primary studies revealed that 9.3% of the OHCWs worldwide had SARS‐CoV‐2 infection before vaccination. Subgroup analysis regarding the professional category showed that the prevalence of SARS‐CoV‐2 infection in dentists was 9.5% and dental assistants/technicians was 11.6%. Countries with lower HDI showed higher prevalence of SARS‐CoV‐2 infection. Age, comorbidities, gender, ethnicity, occupation, smoking, deprivation, job role and location/municipalities, income and protective measures were associated with positive serological SARS‐CoV‐2 test, with very low certainty of evidence. Further studies are needed to evaluate changes in prevalence in the medium and long term, considering the types of virus variants, and the types of vaccines administered in OHCWs.
The present findings should bring the attention of OHCWs and health policymakers to the hidden burden of the SARS‐CoV‐2 infection. Robust surveillance strategies and vaccination measures to prevent or limit SARS‐CoV‐2 transmission in oral health facilities should be stringently reinforced.
FUNDING INFORMATION
Patrícia Pauletto is supported by CAPES (Cooredination for the Improvement of Higher Education Personnel), Ministry of Education, Brazil. Graziela De Luca Canto is supported by CNPQ.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
The authors acknowledge the support provided by librarian Karyn Munik Lehmkuhl for the assistance and critical analysis of our search strategy. The authors declared no potential conflicts of interest concerning the research, authorship and/or publication of this article. PP is supported by CAPES (Coordination for the Improvement of Higher Education Personnel), Ministry of Education, Brazil. GLC is supported by CNPQ. FVB contributed to the conception, design, data action, analysis and interpretation, drafted and critically revised the manuscript. ENL contributed to the design, data acquisition, analysis, drafted and critically revised the manuscript. PP contributed to the design, data action, when necessary, analysis and critically revised the manuscript. CCM contributed to the design, data acquisition (GRADE approach), analysis and critically revising of the manuscript. CS and CM contributed to the design, analysis, and critically revised the manuscript. GLC coordinated our research and contributed to the design, analysis and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Bitencourt FV, Lia EN, Pauletto P, et al. Prevalence of SARS‐CoV‐2 infection among oral health care workers worldwide: A meta‐analysis. Community Dent Oral Epidemiol. 2022;00:1‐11. doi: 10.1111/cdoe.12827
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request
REFERENCES
- 1. International Council of Nurses . (2020). ICN confirms 1,500 nurses have died from COVID‐19 in 44 countries and estimates that healthcare worker COVID‐19 fatalities worldwide could be more than 20,000. 28 October 2020 1(1):1–2.
- 2. The Lancet . Covid‐19: protecting health‐care workers. Lancet. 2020;395(10228):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nardone M, Cordone A, Petti S. Occupational COVID‐19 risk to dental staff working in a public dental unit in the outbreak epicenter. Oral Dis. 2020;28(1):878‐890. [DOI] [PubMed] [Google Scholar]
- 4. Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019‐NCOV and controls in dental practice. Int J Oral Sci. 2020;12(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fini MB. What dentists need to know about covid‐19. Oral Oncol. 2020;105(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dzinamarira T, Mhango M, Dzobo M, et al. Risk factors for COVID‐19 among healthcare workers. A protocol for a systematic review and meta‐analysis. PLoS One. 2021;16(5):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. WHO . 2020. Rational use of personal protective equipment (ppe) for coronavirus disease (covid‐19): interim guidance, 19 march 2020. World Health Organization, 1(1):1–7.
- 8. Mattos FF, Pordeus IA. COVID‐19: a new turning point for dental practice. Braz Oral Res. 2020;34(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 9. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS‐CoV‐2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2022;22(2):183‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allison JR, Currie CC, Edwards DC, et al. Evaluating aerosol and splatter following dental procedures: addressing new challenges for oral health care and rehabilitation. J Oral Rehabil. 2021;48(1):61‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holliday R, Allison JR, Currie C, et al. Evaluating contaminated dental aerosol and splatter in an open plan clinic environment: implications for the COVID‐19 pandemic. J Dent. 2021;105(1):103‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar PS, Subramanian K. Demystifying the mist: sources of microbial bioload in dental aerosols. J Periodontol. 2020;91(9):1113‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meethill AP, Saraswat S, Chaudhary PP, Dabdoub SM, Kumar PS. Sources of SARS‐CoV‐2 and other microorganisms in dental aerosols. J Dent Res. 2021;100(8):817‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. UNDP (United Nations Development Programme) . 2018. Human development indices and indicators: 2018 statistical update. New York, 1(1):1–123.
- 16. Institute JB . 2017. The joanna briggs institute critical appraisal tools for use in JBI systematic reviews critical appraisal checklist for analytical cross sectional studies. 1(1):1–7.
- 17. Deeks JJ, Higgins JPT, Altman DG. (editors)Chapter 10: Analysing data and undertaking meta‐analyses. In: JPT H, Thomas J, Chandler J, et al., eds. (editors)Cochrane Handbook for Systematic Reviews of Interventions version 6.2.(updated February 2021). Cochrane; 2021. 1(1):241–290. Available from: www.training.cochrane.org/handbook [Google Scholar]
- 18. Deeks JJ, Higgins JP, Altman DG, Group CSM . Analysing data and undertaking meta‐analyses. Cochrane Handbook for Systematic Reviews of Interventions. 2019;1(1):241‐284. [Google Scholar]
- 19. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Basics of meta‐analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5‐18. [DOI] [PubMed] [Google Scholar]
- 20. Schwarzer G. 2016. Package ‘Meta’: General Package for Meta‐Analysis. 1(1):1–238.
- 21. Schwarzer G, Carpenter JR, Rücker G. Meta‐Analysis with r (Use R!). Vol 1. Springer International Publishing; 2015:1, 1‐264. [Google Scholar]
- 22. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta‐analysis (swim) in systematic reviews: reporting guideline. BMJ. 2020a;16(368):68‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. 2017;22(3):85‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atkins D, Briss PA, Eccles M, et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res. 2005;5(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balshem H, Helfand M, Schünemann HJ, et al. Grade guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401‐406. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Page MJ, McKenzie JE, Bossuyt PM, et al. Mapping of reporting guidance for systematic reviews and meta‐analyses generated a comprehensive item bank for future reporting guidelines. J Clin Epidemiol. 2020;118(1):60‐68. [DOI] [PubMed] [Google Scholar]
- 28. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 29. Al Kuwari M, AbdelMalik M, Al Nuaimi A, AbdelMajeed J, Romaihi H. 2021. Epidemiology of COVID‐19 infection amongst workers in primary healthcare in qatar medRxiv [preprint]. 1(1):1–9. [Google Scholar]
- 30. Sarapultseva M, Hu D, Sarapultsev A. SARS‐COV‐2 seropositivity among dental staff and the role of aspirating systems. JDR Clin Trans Res. 2021;6(2):132‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akbari N, Salehiniya H, Abbaszadeh H. The prevalence of COVID‐19 in dentists and dental assistants. J Biostat Epidemiol. 2021;7(2):174‐184. [Google Scholar]
- 32. Abo‐Leyah H, Gallant S, Cassidy D, et al. Seroprevalence of SARS‐COV‐2 antibodies in scottish healthcare workers. MedRxiv. [preprint]. 2020;1(1):1‐13. [Google Scholar]
- 33. Molvik M, Danielsen AS, Grøsland M, Telle KE, Kacelnik O, Eriksen‐Volle H‐M. Sars‐cov‐2 in health and care staff in Norway, 2020. Tidsskr Nor Laegeforen. 2021;141(3):1‐12. [DOI] [PubMed] [Google Scholar]
- 34. Kasztelewicz B, Janiszewska K, Burzyńska J, Szydłowska E, Migdał M, Dzierżanowska‐Fangrat K. Prevalence of igg antibodies against SARS‐COV‐2 among healthcare workers in a tertiary pediatric hospital in Poland. PLoS One. 2021;16(4):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kua J, Patel R, Nurmi E, et al. Healthcare COVID: a national cross‐sectional observational study identifying risk factors for developing suspected or confirmed covid‐19 in UK healthcare workers. PeerJ. 2021;9(4):1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shields A, Faustini S, Kristunas C, et al. COVID‐19: Seroprevalence and vaccine responses in UK dental care professionals. J Dent Res. 2021;100(11):1220‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gallus S, Paroni L, Re D, et al. SARS‐COV‐2 infection among the dental staff from lombardy region, Italy. Int J Environ Res Public Health. 2021;18(7):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Magnusson K, Nygård K, Methi F, Vold L, Telle K. Occupational risk of COVID‐19 in the first versus second epidemic wave in Norway, 2020. Euro Surveill. 2021;26(40):2001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mksoud M, Ittermann T, Holtfreter B, et al. Prevalence of SARS‐CoV‐2 IgG antibodies among dental teams in Germany. Clin Oral Investig. 2022;26(5):3965‐3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sebastian P, Jorge P, Ariel G, et al. Assesment of SARS‐COV‐2 infection‐in dentists and supporting staff at a university dental hospital in Argentina. J Oral Biol Craniofac Res. 2021;11(2):169‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferreira RC, Gomes VE, da Rocha NB, et al. COVID‐19 morbidity among oral health professionals in Brazil. Int Dent J. 2021;72(2):223‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ribeiro JAM, Farias SJS, Souza TAC, Stefani CM, Lima AA, Lia EN. SARS‐CoV‐2 infection among Brazilian dentists: a seroprevalence study. Braz Oral Res. 2022;14(36):1‐13. [DOI] [PubMed] [Google Scholar]
- 43. Antonio‐Villa NE, Bello‐Chavolla OY, Vargas‐Vázquez A, Fermín‐Martínez CA, Márquez‐Salinas A, Bahena‐López JP. Health‐care workers with covid‐19 living in Mexico City: clinical characterization and related outcomes. Clin Infect Dis. 2020;73(1):191‐198. [Google Scholar]
- 44. Esquivel‐Chirino C, Valero‐Princet Y, Gaitán‐Cepeda LA, et al. The effects of COVID‐19 on healthcare workers and non‐healthcare workers in Mexico: 14 months into the pandemic. Medicina (Kaunas). 2021;57(12):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence ‐imprecision. J Clin Epidemiol. 2011;64(1):1283‐1293. [DOI] [PubMed] [Google Scholar]
- 46. Nishida T, Iwahashi H, Yamauchi K, et al. Seroprevalence of SARS‐COV‐2 antibodies among 925 staff members in an urban hospital accepting COVID‐19 patients in Osaka prefecture, Japan: a cross‐sectional study. Medicine. 2021;100(25):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gómez‐Ochoa SA, Franco OH, Rojas LZ, et al. COVID‐19 in health‐care workers: a living systematic review and meta‐analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021;190(1):161‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marziali ME, Hogg RS, Oduwole OA, Card KG. Predictors of COVID‐19 testing rates: a cross‐country comparison. Int J Infect Dis. 2021;104(1):370‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS‐CoV‐2 infection in the United States. Nat Commun. 2020;11(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rocha R, Atun R, Massuda A, et al. Effect of socioeconomic inequalities and vulnerabilities on health‐system preparedness and response to COVID‐19 in Brazil: a comprehensive analysis. Lancet Glob Health. 2021;9(6):782‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Böger B, Fachi MM, Vilhena RO, Cobre AF, Tonin FS, Pontarolo R. Systematic review with meta‐analysis of the accuracy of diagnostic tests for COVID‐19. Am J Infect Control. 2021;49(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ejazi SA, Ghosh S, Ali N. Antibody detection assays for COVID‐19 diagnosis: an early overview. Immunol Cell Biol. 2021;99(1):21‐33. [DOI] [PubMed] [Google Scholar]
- 53. Li J, Huang DQ, Zou B, et al. Epidemiology of COVID‐19: a systematic review and meta‐analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93(3):1449‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Figueiredo EA, Polli DA, Andrade BB. Estimated prevalence of COVID‐19 in Brazil with probabilistic bias correction. Cad Saude Publica. 2021;37(9):1‐12. [DOI] [PubMed] [Google Scholar]
- 55. Bobrovitz N, Arora RK, Cao C, et al. Global seroprevalence of SARS‐COV‐2 antibodies: a systematic review and meta‐analysis. PLoS One. 2021;16(6):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kahlert CR, Persi R, Gusewell S, et al. Non‐occupational and occupational factors associated with specific SARS‐CoV‐2 antibodies among hospital workers—a multicentre cross‐sectional study. Clin Microbiol Infect. 2021;27(9):1336‐1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schneider S, Piening B, Nouri‐Pasovsky PA, Kruger AC, Gastmeier P, Aghdassi SJS. SARS‐Coronavirus‐2 cases in healthcare workers may not regularly originate from patient care: lessons from a university hospital on the underestimated risk of healthcare worker to healthcare worker transmission. Antimicrob Resist Infect Control. 2020;9(1):180‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baker JM, Nelson KN, Overton E, et al. Quantification of occupational and community risk factors for SARS‐CoV‐2 seropositivity among health care workers in a large US health care system. Ann Intern Med. 2021;174(5):649‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS‐CoV‐2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(3):270‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chun‐Wai Wong R, Lee MK, Kit‐Hang Siu G, et al. Healthcare workers acquired COVID‐19 disease from patients? An investigation by phylogenomics. J Hosp Infect. 2021;115(1):59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carlsten C, Gulati M, Hines S, et al. COVID‐19 as an occupational disease. Am J Ind Med. 2021;64(4):227‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Garcia‐Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS‐COV‐2 among health care workers in a large spanish reference hospital. Nat Commun. 2020;11(1):3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kluytmans M, Buiting A, Pas S, et al. SARS‐COV‐2 infection in 86 healthcare workers in two Dutch hospitals in march 2020. MedRxiv. [preprint]. 2020;1(1):1‐13. [Google Scholar]
- 64. Sikora K, Barwick I, Hamilton C. Serological prevalence of antibodies to SARS‐COV‐2 amongst cancer Centre staff. Medrxiv. [preprint]. 2020;1(1):1‐12. [Google Scholar]
- 65. Khan M, Adil SF, Alkhathlan HZ, et al. Covid‐19: a global challenge with old history, epidemiology and progress so far. Molecules. 2021;26(1):1‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Heneghan C, Spencer EA, Brassey J, et al. SARS‐COV‐2 and the role of airborne transmission: a systematic review. F1000Research. 2021;10(232):1‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request


