Key Points
Question
Does CYP1A2 genotype modify the association between coffee intake and kidney dysfunction?
Findings
In this cohort study involving a 7.5-year follow-up of 1180 untreated participants with stage 1 hypertension from the Hypertension and Ambulatory Recording Venetia Study, those with a genetic variant in CYP1A2 who were slow metabolizers of caffeine were 2.7 times more likely to develop albuminuria, 2.5 times more likely to develop hyperfiltration, and 2.8 times more likely to develop hypertension with heavy coffee intake compared with low coffee intake. No associations were observed between coffee intake and albuminuria, hyperfiltration, or hypertension among fast metabolizers of caffeine.
Meaning
These findings suggest that heavy coffee intake is associated with increases in the risk of kidney dysfunction among slow metabolizers of caffeine, who genetically comprise approximately half of the population, but not among fast metabolizers of caffeine.
Abstract
Importance
Caffeine is detoxified by cytochrome P450 1A2 (CYP1A2), and genetic variation in CYP1A2 impacts the rate of caffeine clearance. Factors that may modify the association between coffee intake and kidney disease remain unclear.
Objective
To assess whether CYP1A2 genotype modifies the association between coffee intake and kidney dysfunction.
Design, Setting, and Participants
The Hypertension and Ambulatory Recording Venetia Study (HARVEST) was a prospective cohort study of individuals with stage 1 hypertension in Italy; HARVEST began on April 1, 1990, and follow-up is ongoing. The current study used data from April 1, 1990, to June 30, 2006, with follow-up of approximately 10 years. Blood pressure and biochemical data were collected monthly during the first 3 months, then every 6 months thereafter. Data were analyzed from January 2019 to March 2019. Participants were screened and recruited from general practice clinics. The present study included 1180 untreated participants aged 18 to 45 years with stage 1 hypertension; those with nephropathy, diabetes, urinary tract infection, and cardiovascular disease were excluded.
Exposures
Coffee intake and CYP1A2 genotype rs762551 were exposures analyzed over a median follow-up of 7.5 (IQR, 3.1-10.9) years.
Main Outcomes and Measures
Albuminuria (defined as an albumin level of ≥30 mg/24 h) and hyperfiltration (defined as an estimated glomerular filtration rate of ≥150 mL/min/1.73 m2) were the primary outcomes as indicators of kidney dysfunction.
Results
Among 1180 participants, genotyping, lifestyle questionnaires, and urine analysis data were obtained from 604 individuals (438 [72.5%] male) with a mean (SD) age of 33.3 (8.5) years and a mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) of 25.4 (3.4). A total of 158 participants (26.2%) consumed less than 1 cup of coffee per day, 379 (62.7%) consumed 1 to 3 cups per day, and 67 (11.1%) consumed more than 3 cups per day. Genotype frequencies for rs762551 (260 participants [43.1%] with genotype AA, 247 participants [40.8%] with genotype AC, and 97 participants [16.1%] with genotype CC) did not differ between coffee intake categories. The level of risk of developing albuminuria, hyperfiltration, and hypertension, assessed by Cox regression and survival analyses, was not associated with coffee intake in the entire group or among fast metabolizers. The risks of albuminuria (adjusted hazard ratio [aHR], 2.74; 95% CI, 1.63-4.62; P < .001), hyperfiltration (aHR, 2.11; 95% CI, 1.17-3.80; P = .01), and hypertension (aHR, 2.81; 95% CI, 1.51-5.23; P = .001) increased significantly among slow metabolizers who consumed more than 3 cups per day.
Conclusions and Relevance
In this study, the risks of albuminuria, hyperfiltration, and hypertension increased with heavy coffee intake only among those with the AC and CC genotypes of CYP1A2 at rs762551 associated with slow caffeine metabolism, suggesting that caffeine may play a role in the development of kidney disease in susceptible individuals.
This cohort study assesses whether CYP1A2 genotype modifies the association between caffeine intake and kidney dysfunction among untreated individuals in Italy aged 18 to 45 years with stage 1 hypertension.
Introduction
Preventable kidney disease is one of the leading causes of morbidity and mortality worldwide.1 Factors that may modify the association between coffee consumption and kidney disease remains unclear. Coffee is a major source of caffeine and the most widely consumed caffeinated beverage in the world. Some investigations have suggested that caffeine is associated with impaired kidney function in humans2 and adversely alters kidney tissue in animals and humans by stimulating glomerular remodeling and sclerosis,3,4 worsening hypertension5,6 and proteinuria,7 and accelerating preexisting chronic kidney failure.8 However, other studies have found that caffeine intake can slow the progression of diabetic nephropathy,9 while others have observed a lower risk of developing chronic kidney disease (CKD) with increasing coffee consumption.10 Some studies have reported an increased risk of cyst enlargement in patients with autosomal dominant polycystic kidney disease with increasing caffeine intake,11 while others have reported no consequences.12,13 Previous studies, however, have not accounted for the wide interindividual variation in metabolism of caffeine, which could modify any associations.
Caffeine is a central nervous system stimulant and the most commonly consumed psychoactive substance.14 Its mechanisms of action include inhibiting the phosphodiesterase enzyme, antagonizing adenosine receptors, and activating the ryanodine receptors with several actions on multiple organs.15 Caffeine has the potential to exert adverse effects on kidney function and structure by stimulating some of the key proliferative mechanisms involved in glomerular remodeling and sclerosis.2,3,4,5,6,7,8 In addition, because adenosine acts as a vasodilator to prevent hypoxic injury in the renal medulla,16 caffeine’s antagonistic action could induce hypoxic injury. However, data are inconclusive, with evidence ranging from coffee consumption being protective against chronic kidney disease17,18,19,20 to having no associations with5,21,22 or even accelerating kidney disease.6,11,23
More than 95% of caffeine is metabolized by cytochrome P450 1A2 (CYP1A2) and a common polymorphism in the CYP1A2 gene has been reported to be significantly associated with caffeine metabolism.24 The rs762551 variant decreases enzyme activity and inducibility.24,25 Individuals with AC and CC genotypes are considered slow metabolizers, and those with the AA genotype are considered fast metabolizers.26 This polymorphism has been found to modify the association between coffee intake and the risk of myocardial infarction,27 hypertension,28 and impaired fasting glucose29 in a dose-dependent manner. In those studies,27,28,29 an increased risk was observed in slow metabolizers with increasing cups of coffee consumed per day, whereas in fast metabolizers, increasing cups of coffee per day was either associated with a lower risk, or no association was found. Those findings might explain some of the inconsistencies observed in reports of coffee intake and kidney disease; however, previous studies examining the association between coffee and the risk of kidney disease have not examined the role of CYP1A2. The objective of the present study was to assess whether CYP1A2 genotype rs762551 modified the association between intake of caffeinated coffee and markers of kidney dysfunction.
Methods
Participants
This study was conducted with participants from the Hypertension and Ambulatory Recording Venetia Study (HARVEST), a prospective longitudinal study of untreated participants aged 18 to 45 years with stage 1 hypertension.30 HARVEST was conducted in 17 hypertension units in Italy beginning on April 1, 1990, and follow-up is ongoing. The current study used data from April 1, 1990, to June 30, 2006, with follow-up of approximately 10 years. Data were analyzed from January 2019 to March 2019. Detailed study methods have been reported elsewhere.30 The present study included 1180 male and female individuals aged 18 to 45 years who took part in HARVEST30; those with nephropathy, diabetes, urinary tract infection, and cardiovascular disease were excluded. A genetic substudy included 639 participants from 4 centers (Padova, Vittorio Veneto, San Daniele del Friuli, and Trento) who agreed to participate in the genetic study.28 Data on race and ethnicity were not empirically collected for this study, but the study population has been described as being of White-European ethnicity with the majority being of Italian origin. The ethics committee of the University of Padova approved the study, and the participants gave written informed consent to participate. Further details are provided in the eAppendix in Supplement 1. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline for cohort studies.
Details about the interview, lifestyle assessment, and criteria used for participant classification according to lifestyle have been reported elsewhere.28,31,32 Clinic blood pressure (BP) was the mean of 6 readings obtained during 2 visits to the clinic performed 2 weeks apart. Body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) was considered as an index of adiposity.
Follow-up Procedures
Blood samples were obtained on an annual basis for routine measures. Clinic BP and lifestyle habits were assessed monthly during the first 3 months of follow-up, then after 6 months and every 6 months thereafter. After baseline examination, participants were given general lifestyle information about nonpharmacological measures following the suggestions of the most current guidelines on the management of hypertension.33,34,35,36 To ensure homogeneous counseling by physicians participating in the study, training in current international guidelines was provided to them throughout the study duration. Coffee intake recommendations were not provided as a part of the intervention because coffee intake was not included in any of the available guidelines.
HARVEST participants were followed up until they developed sustained hypertension requiring antihypertensive treatment according to the guidelines available at the time.33,34,35,36 When patients developed sustained hypertension, the investigators performed a final clinical assessment including biochemical tests before they exited the study and pharmaceutical treatment was initiated. Participants who did not meet the criteria for treatment continued to be followed up at 6-month intervals. Routine blood tests, including assessment of creatinine levels, were performed at yearly intervals and at the end of the study. Of 1180 participants, 683 (57.9%) developed hypertension during a median follow-up of 6.1 (IQR, 2.5-9.9) years. This proportion was similar among participants included in the genetic substudy. Among participants who remained normotensive, the last available clinical assessment was used. All data used for the present study were collected from untreated patients.
The estimated glomerular filtration rate (eGFR) was calculated from creatinine clearance, which was computed from creatinine excretion in a 24-hour urine collection and a single measurement of serum creatinine, and the data were normalized by body surface area.37 The urinary albumin level was measured by a commercially available radioimmunoassay kit (H ALB kit–double antibody; Sclavo SpA). Results were expressed as milligrams per 24 hours and were transformed logarithmically. The albumin excretion rate (AER) was available for 954 participants in the parent study. The adequacy of the 24-hour urine collections was assessed by self-report of missed or spilled collections as well as by creatinine excretion per kilogram of body weight.38 Participants were defined as normofilterers or hyperfilterers according to whether their eGFR was lower than 150 mL/min/1.73 m2 or 150 mL/min/1.73 m2 or higher, respectively.39 Albuminuria was defined as an albumin level of 30 mg/24 h or higher, and hypertension was defined as a clinic BP of 140/90 mm Hg or higher.
Coffee Consumption and Genotyping
Participants completed questionnaires about their medical history, family history of hypertension, physical activity, and dietary habits, including coffee intake, alcohol use, and cigarette smoking. Detailed methods for these procedures and their validity have been reported elsewhere.28,29,31,32 Briefly, coffee consumption was defined according to the number of caffeine-containing cups of coffee consumed per day. The caffeine content per cup was defined as 100 mg of Italian espresso coffee, which was the most abundantly consumed type of coffee by the HARVEST participants.40 Decaffeinated coffee, tea, and other caffeinated drinks were not taken into account in the present study because they were rarely consumed in these areas of Italy.41 Coffee consumption was then categorized into 3 groups: low coffee intake (<1 cup per day), moderate coffee intake (1-3 cups per day), and heavy coffee intake (>3 cups per day) based on classification used in previous analyses.31,42,43 DNA was extracted from whole blood and genotyped for the rs762551 polymorphism in CYP1A2 as previously described.28
Statistical Analysis
The present analysis included 604 of 1180 participants who had biochemical data and information on lifestyle habits and genotype available (both at baseline and final assessments) and at least 6 months of follow-up. Participants were grouped into slow (AC and CC genotypes) or fast (AA genotype) metabolizers according to CYP1A2 genotype and habitual consumption of coffee (low, moderate, or heavy). The distribution of participant characteristics was compared by genotype and across groups of coffee consumption using analysis of variance for continuous variables and χ2 testing for categorical variables.
The cumulative incidence of albuminuria, hyperfiltration, and hypertension associated with coffee consumption at baseline was calculated using Kaplan-Meier analysis for each outcome separately. The difference in albuminuria, hyperfiltration, and hypertension incidences between coffee drinking categories was assessed using the log-rank test. Coffee intake was also modeled as a time-dependent categorical variable in a Cox proportional hazards analysis adjusted for possible confounding variables derived from significant associations of univariate analyses with the respective outcome measure. The variables found to be associated with outcome on the univariate survival analysis and/or considered to have potential prognostic importance were age, sex, BMI, baseline plasma glucose level, and serum triglyceride levels. Separate models were created to examine the associations with coffee consumption among slow and fast metabolizers. Estimates of relative risk (hazard ratios [HRs]) and corresponding 95% CIs for categories of CYP1A2 genotype and coffee consumption were computed from Cox regression models that were adjusted for relevant variables in each model reported.
The threshold for statistical significance was 2-tailed P < .05. Analyses were performed using IBM SPSS Statistics software, version 27 (IBM Corporation).
Results
Population Characteristics
Among 604 participants (438 [72.5%] male) who completed genetic testing and lifestyle questionnaires and provided urine analysis data, the mean (SD) age was 33.3 (8.5) years, and the mean (SD) BMI was 25.4 (3.4). Genotype frequencies for rs762551 (260 participants [43.1%] with genotype AA, 247 participants [40.8%] with genotype AC, and 97 participants [16.1%] with genotype CC) did not differ between coffee intake categories. Among the entire cohort, 158 participants (26.2%) had low coffee intake (<1 cup per day), 379 (62.7%) had moderate coffee intake (1-3 cups per day), and 67 (11.1%) had heavy coffee intake (>3 cups per day). Compared with participants with low coffee intake, those with moderate and heavy intake were older (mean [SD], 33.9 [8.1] years for moderate intake and 37.2 [6.8] years for heavy intake vs 30.7 [9.3] years for low intake; P < .001) and had higher BMI (mean [SD], 25.6 [3.4] for moderate intake and 26.0 [4.0] for high intake vs 24.6 [3.3] for low intake; P = .003). The proportion of those with slow metabolism genotypes (AC and CC) did not differ significantly between the coffee intake categories (58.9% for low intake, 54.9% for moderate intake, and 64.5% for heavy intake; P = .31).
Overall, 4 (6.3%) of 64 participants with hyperfiltration also had albuminuria, and 5 (16.7%) of 30 participants with albuminuria had hyperfiltration. At baseline, clinic systolic BP was lower in fast metabolizers compared with slow metabolizers (mean [SD], 144.64 [10.15] mm Hg vs 146.65 [10.62] mm Hg; P = .02). Fasting glucose at baseline was also marginally lower in fast metabolizers vs slow metabolizers (mean [SD], 91.93 [10.09] mg/dL vs 93.99 [11.60] mg/dL; P = .03). No other participant characteristics were different at baseline and follow-up according to genotype (Table44).
Table. Characteristics of Study Participants by CYP1A2 Genotype.
| Characteristic | CYP1A2 genotype, No. (%) | P value | |
|---|---|---|---|
| AA (n = 260) | AC and CC (n = 344) | ||
| Age, mean (SD), y | 32.9 (8.5) | 33.7 (8.5) | .23 |
| Sex | |||
| Female | 70 (26.9) | 96 (27.9) | .77 |
| Male | 190 (73.1) | 248 (72.1) | |
| Coffee intake, cups/d | |||
| <1 | 62 (23.8) | 96 (27.9) | .56 |
| 1-3 | 172 (66.2) | 207 (60.2) | .18 |
| >3 | 26 (10.0) | 41 (11.9) | .20 |
| Drinks alcohol | 107 (41.2) | 134 (39.0) | .47 |
| Smokes | 55 (21.2) | 62 (18.0) | .34 |
| Follow-up period, mean (SD), d | 2819 (1702) | 2631 (1600) | .16 |
| BMI, mean (SD) | |||
| Baseline | 25.3 (3.4) | 25.4 (3.5) | .77 |
| Follow-up | 26.1 (3.9) | 25.8 (3.4) | .30 |
| eGFR, mean (SD), mL/min/1.73 m2a | |||
| Baseline | 101.42 (17.06) | 101.89 (17.59) | .74 |
| Follow-up | 120.25 (29.40) | 121.50 (29.38) | .69 |
| AER, mean (SD), mg/24 h | |||
| Baseline | 12.96 (30.23) | 11.10 (22.14) | .79 |
| Follow-up | 19.03 (56.62) | 13.80 (25.16) | .57 |
| Systolic BP, mean (SD), mm Hg | |||
| Baseline clinical | 144.64 (10.15) | 146.65 (10.62) | .02 |
| Follow-up | 142.76 (12.95) | 145.66 (14.32) | .009 |
| Diastolic BP, mean (SD), mm Hg | |||
| Baseline clinical | 93.28 (5.59) | 93.60 (5.39) | .48 |
| Follow-up | 93.52 (9.59) | 94.25 (10.87) | .39 |
| Glucose, mean (SD), mg/dL | |||
| Baseline | 91.93 (10.09) | 93.99 (11.60) | .03 |
| Follow-up | 94.80 (14.20) | 94.50 (12.93) | .81 |
| HDL cholesterol, mean (SD), mg/dL | |||
| Baseline | 51.97 (14.22) | 52.07 (14.44) | .94 |
| Follow-up | 55.73 (16.31) | 53.52 (13.99) | .11 |
| Triglycerides, mean (SD), mg/dL | |||
| Baseline | 113.73 (81.35) | 114.79 (71.44) | .40 |
| Follow-up | 119.37 (90.12) | 118.98 (75.26) | .88 |
| Epinephrine, mean (SD), pg/mL | |||
| Baseline | 104.14 (18.18) | 104.85 (18.13) | .71 |
| Follow-up | 101.92 (20.58) | 103.29 (22.27) | .55 |
| Heart rate, mean (SD), beats/min | |||
| Baseline | 74.55 (10.18) | 74.97 (9.73) | .60 |
| Follow-up | 72.05 (9.74) | 71.47 (9.19) | .46 |
Abbreviations: AER, albumin excretion rate; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein.
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0555; to convert HDL cholesterol to mmol/L, multiply by 0.0259; to convert triglycerides to mmol/L, multiply by 0.0113; to convert epinephrine to pmol/L, multiply by 5.459.
Baseline eGFR was calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine equation.44
Follow-up
Participants were followed up for a median of 7.5 (IQR, 3.1-10.9) years. During the follow-up, coffee intake habits remained the same for 581 participants. Among those who reported changing their coffee intake habits, 11 increased consumption and 12 decreased consumption. Recategorizing these participants did not materially alter any of the results; therefore, all participants remained in their baseline categories. Prevalence of albuminuria did not differ according to genotype at baseline (16 participants [6.2%] who were fast metabolizers and 21 participants [6.1%] who were slow metabolizers). However, at follow-up, this proportion increased to 8.1% (28 participants) of slow metabolizers, while it remained unchanged in the fast metabolizers. Hyperfiltration was not prevalent at baseline; however, at follow-up, 16.9% of fast metabolizers (44 participants) and 18.0% of slow metabolizers (62 participants) had an eGFR higher than 150 mL/min/1.73 m2. A higher prevalence of hypertension was observed in slow metabolizers vs fast metabolizers at baseline (204 participants [59.7%] vs 122 participants [46.9%]; P = .002), but these differences were no longer statistically significant at follow-up (175 participants [50.9%] vs 117 participants [45.0%]; P = .15).
Association of Genotype and Coffee Intake With Albuminuria
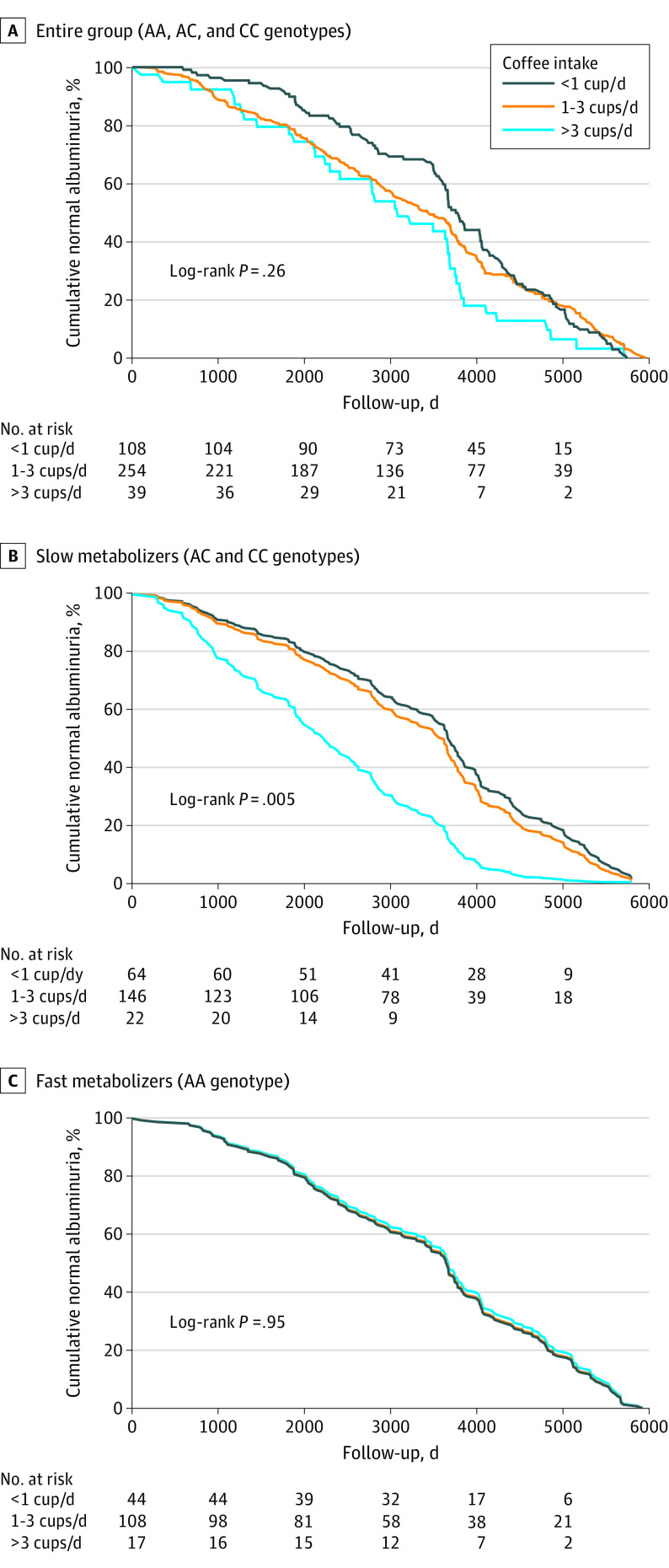
A total of 407 participants had AER records both at baseline and end of follow-up. Albuminuria (AER >30 mg/24 h) was detected in 28 participants (6.9%) by study end, and 52 participants (12.8%) had elevated AER (16-29 mg/24 h). The risk of developing albuminuria was not associated with coffee intake in the entire group (Figure 1A). However, when analyses were stratified by genotype, the risk of developing albuminuria in slow metabolizers increased significantly for heavy coffee drinkers (Cox regression model: unadjusted HR, 2.72 [95% CI, 1.64-4.50; P < .001]; model adjusted for age, sex, baseline BMI, baseline clinic systolic BP, baseline eGFR: HR, 2.74 [95% CI, 1.63-4.62; P < .001) (Figure 1B). In fast metabolizers, no association between coffee intake and albuminuria was observed (Figure 1C). Kaplan-Meier analysis of the slow metabolizers found that heavy coffee intake was associated with albuminuria 2.8 years earlier than low coffee intake.
Figure 1. Kaplan-Meier Survival Curves of the Risk of Albuminuria by Coffee Intake and CYP1A2 Genotype.
Among 407 participants. Normal albuminuria was defined as albumin level lower than 30 mg/24 h. Median follow-up was 7.5 (IQR, 3.1-10.9) years.
Association of Genotype and Coffee Intake With Hyperfiltration
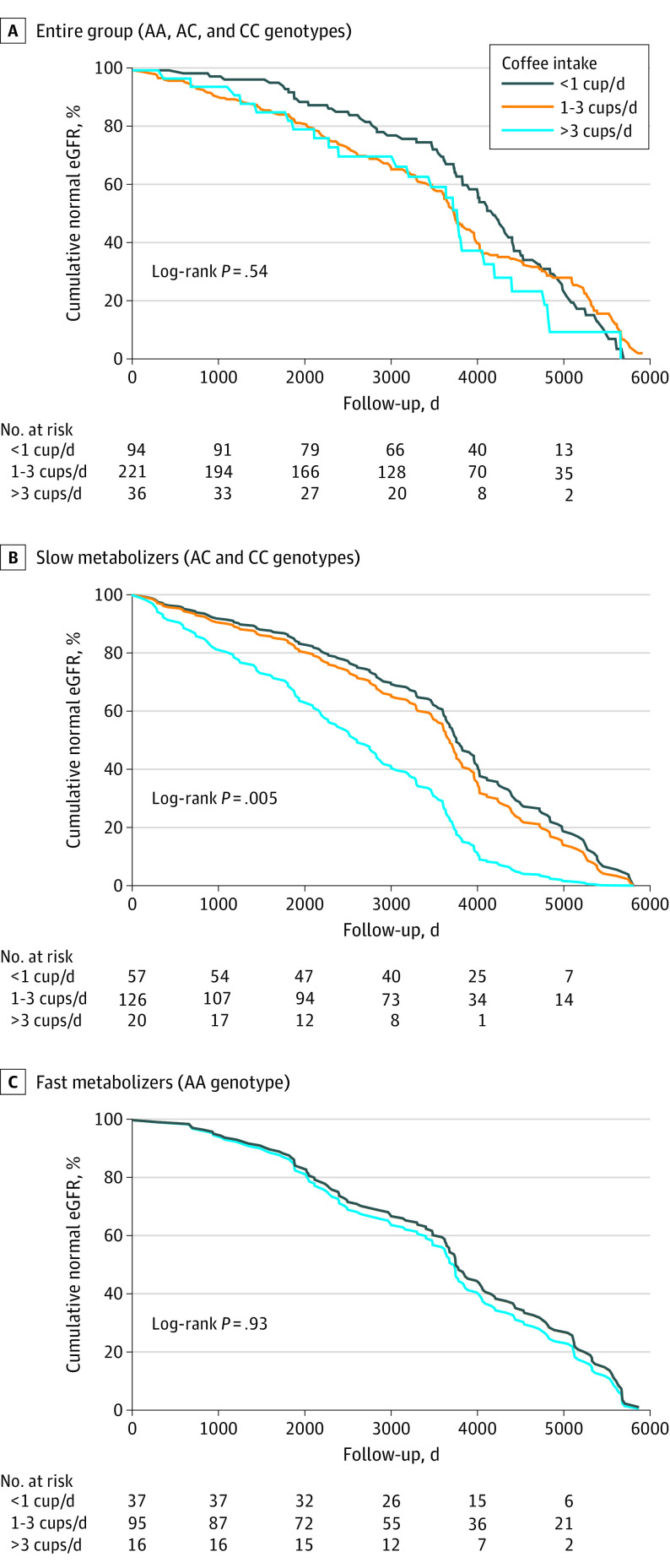
A total of 351 participants had eGFR records both at baseline and end of follow-up. Hyperfiltration (eGFR >150 mL/min/1.73 m2) was detected in 61 participants (17.4%) by study end, and 73 participants (20.8%) had an elevated eGFR (125-150 mL/min/1.73 m2). The risk of developing hyperfiltration was not associated with coffee intake in the entire group (Figure 2A). When stratified by genotype, the risk of developing hyperfiltration in slow metabolizers increased significantly with heavy coffee drinking (Cox regression model: unadjusted HR, 2.47 [95% CI, 1.41-4.34; P = .002]; model adjusted for age, sex, baseline BMI, baseline clinic systolic BP, and baseline urinary albumin: HR, 2.11 [95% CI, 1.17-3.80; P = .01]) (Figure 2B). In fast metabolizers, no association between coffee intake and hyperfiltration was observed (Figure 2C). Kaplan-Meier analysis of the slow metabolizers found that heavy coffee intake was associated with hyperfiltration 4.9 years earlier than low coffee intake.
Figure 2. Kaplan-Meier Survival Curves of the Risk of Hyperfiltration by Coffee Intake and CYP1A2 Genotype.
Among 351 participants. Normal estimated glomerular filtration rate (eGFR) was defined as 90 to 150 mL/min/1.73 m2. Median follow-up was 7.5 (IQR, 3.1-10.9) years.
Association of Genotype and Coffee Intake With Hypertension
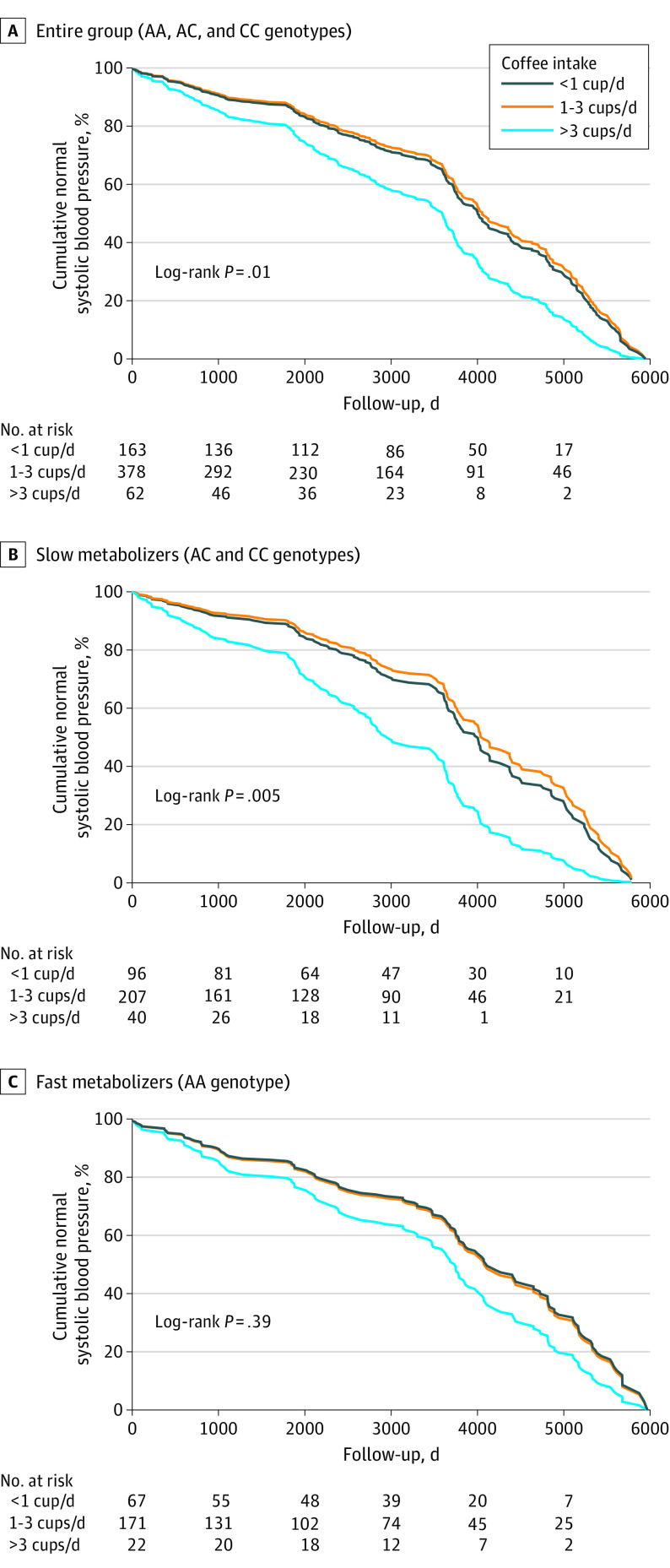
A total of 604 participants had clinic systolic and diastolic BP records both at baseline and end of follow-up. Hypertension (clinic BP >140/90 mm Hg) was detected in 292 participants (48.3%) by study end. Heavy coffee drinkers had a 60% higher risk of developing hypertension compared with nondrinkers (HR, 1.60; 95% CI, 1.08-2.37; P = .02). The risk of developing hypertension was associated with coffee intake in the entire group (Figure 3A). However, when stratified by genotype, the risk of developing hypertension in slow metabolizers increased significantly with heavy coffee drinking (Cox regression model: unadjusted HR, 2.39 [95% CI, 1.34-4.29; P = .003]; model adjusted for age, sex, baseline BMI, baseline eGFR, and urinary albumin: HR, 2.81 [95% CI, 1.51-5.23; P = .001]) (Figure 3B), while no effect of coffee intake on hypertension was observed in fast metabolizers (Figure 3C). Kaplan-Meier analysis of the slow metabolizers found that heavy coffee intake was associated with hypertension 1.5 years earlier than low coffee intake.
Figure 3. Kaplan-Meier Survival Curves of the Risk of Hypertension by Coffee Intake and CYP1A2 Genotype.
Among 604 participants. Normal systolic blood pressure was defined as lower than 140 mm Hg. Median follow-up was 7.5 (IQR, 3.1-10.9) years.
Discussion
The findings of this cohort study revealed that consuming more than 3 cups of coffee per day was associated with increases in albuminuria, hyperfiltration, and hypertension in slow metabolizers but not fast metabolizers. A previous study38 reported no association between coffee consumption and albuminuria in the HARVEST cohort. However, once the results were analyzed according to genotype in the present study, the risk of developing albuminuria was almost 3-fold higher in heavy coffee drinkers who were slow metabolizers compared with those consuming less than 1 cup per day, while no associations were observed among fast metabolizers. According to 2 meta-analyses,20,45 coffee and caffeine may have beneficial implications for kidney outcomes20 and may be associated with decreases in all-cause mortality in those with CKD.45 However, there were several limitations in the studies included in those meta-analyses, and none of them considered individual genetic differences in caffeine metabolism.
In the present study, coffee was defined as Italian espresso, which is a homogeneous form of caffeinated beverage that corresponds to the equivalent of approximately 100 mg of caffeine per cup. In a recent meta-analysis20 that reported a protective benefit of coffee intake for kidney outcomes, coffee consumption was reported as infrequently as less than 1 cup per week to an upper limit of more than 2 cups per day. Another meta-analysis,10 which found no association between coffee intake and CKD outcomes, used binary coffee intake categories comprising those who consumed more than 1 cup of coffee per day or those who consumed less than 1 cup per day. In the present study, we observed a significant coffee-gene interaction with kidney outcomes only when comparing those consuming more than 3 cups per day with those consuming less than 1 cup per day.
In most studies, increased caffeine intake has been associated with eGFR and thus with improvements in CKD,20,21 although these associations were often inconsistent. Another important consideration missed in prior studies is acute vs long-term exposure to caffeine. Acute exposure to caffeine will likely increase glomerular filtration temporarily; however, long-term exposure might have different outcomes. Hence, the association between coffee intake and kidney dysfunction may vary according to the etiology of kidney disease, stages of kidney disease, exposure time, and genetic differences. Increased eGFR higher than 150 mL/min/1.73 m2, also called hyperfiltration, is a proposed mechanism for kidney injury in several clinical conditions.2 Certain kidney diseases may also manifest as hyperfiltration in early stages, and hyperfiltration itself is often misclassified as an indication of optimal kidney function. However, those in the highest quartile of eGFR are at significantly higher risk of death, heart failure, cardiopulmonary events,46 and subsequent kidney disease that eventually manifests as declining eGFR.39 It has been suggested that caffeine has implications for kidney function through nonselective binding to adenosine receptors, which then modulate changes in eGFR via diuresis and natriuresis.47
Hyperfiltration has been identified as a factor associated with CKD in individuals with hypertension46 and diabetes48 as well as those with prediabetes and prehypertension.48,49 In patients diagnosed with type 2 diabetes, a higher eGFR was associated with rapid kidney function decline and subsequently impaired eGFR.48 Coffee consumption has been associated with kidney hyperfiltration in several studies,2,50 but others have reported an inconsistent association.21,51 Hyperfiltration is reversible and has been suggested as an early clinical measure of kidney dysfunction and a factor associated with albuminuria.2,50, Albuminuria itself is associated with cardiovascular disease and kidney disease both in the general population52,53 and in populations with chronic illness.48,53,54,55 In a large study of patients with heart failure,56 elevated albumin excretion was an important marker of disease progression, independent of diabetes, hypertension, or serum creatinine. In the current study, albuminuria and hyperfiltration occurred independent of each other as outcome markers of kidney dysfunction in most of the study population. Only 4 of 64 participants with hyperfiltration (6.3%) also had albuminuria, and only 5 of 30 participants with albuminuria (16.7%) had hyperfiltration. Furthermore, when respective models were adjusted for urinary albumin and creatinine clearance, no material changes in the results were observed. Therefore, the association of hyperfiltration and albuminuria with lifestyle factors such as caffeine consumption may aid in risk-reduction strategies.
Since kidney disease often codevelops with other comorbidities, some studies in older adults might observe coffee being protective indirectly by being protective against other known comorbidities such as type 2 diabetes. In a large cohort of South Korean participants, coffee consumption was only protective against kidney disease in older diabetic women, and no association was found between coffee intake and kidney disease in other groups.51 In the present study of young adults with prehypertension to moderate hypertension who only received lifestyle intervention to manage hypertension, we observed an association between heavy coffee consumption and hyperfiltration that was modified by CYP1A2 genotype. Previous studies have either found an inverse association between coffee consumption and CKD outcomes19,20 or an increased risk of kidney disease with coffee intake.57 However, no previous studies have accounted for genetic differences when assessing the association between coffee intake and CKD outcomes.
Limitations
This study has several limitations. One limitation is that the parent HARVEST study was performed in 17 hypertension units in Italy, yet the present study was conducted in 4 units and did not account for clustering of participants across different units. Nevertheless, participants were from similar ethnic backgrounds, and no genetic or lifestyle differences were noted in the different groups. The observational nature of the current study could be viewed as a possible limitation, so future intervention studies that restrict caffeine intake among slow metabolizers would help provide more direct evidence to confirm these findings.
Conclusions
In the present cohort study, caffeinated coffee intake was associated with increases in the risks of albuminuria, hyperfiltration, and hypertension only among slow metabolizers of caffeine, suggesting that caffeine may play a role in the development of kidney disease in susceptible individuals. These findings have implications for DNA-based interventions, such as precision nutrition recommendations, to reduce the risk of kidney disease.
eAppendix. Participants, Baseline Procedures, Kidney and Adrenal Function, and Data Analysis
Data Sharing Statement
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305. doi: 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 2.Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant. 2012;27(5):1708-1714. doi: 10.1093/ndt/gfs037 [DOI] [PubMed] [Google Scholar]
- 3.Singh VP, Singh N, Jaggi AS. A review on renal toxicity profile of common abusive drugs. Korean J Physiol Pharmacol. 2013;17(4):347-357. doi: 10.4196/kjpp.2013.17.4.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tofovic SP, Salah EM, Jackson EK, Melhem M. Early renal injury induced by caffeine consumption in obese, diabetic ZSF1 rats. Ren Fail. 2007;29(7):891-902. doi: 10.1080/08860220701569846 [DOI] [PubMed] [Google Scholar]
- 5.Tanner GA, Tanner JA. Chronic caffeine consumption exacerbates hypertension in rats with polycystic kidney disease. Am J Kidney Dis. 2001;38(5):1089-1095. doi:10.1053/ajkd.2001.28614 [DOI] [PubMed] [Google Scholar]
- 6.Tofovic SP, Jackson EK. Effects of long-term caffeine consumption on renal function in spontaneously hypertensive heart failure prone rats. J Cardiovasc Pharmacol. 1999;33(3):360-366. doi: 10.1097/00005344-199903000-00003 [DOI] [PubMed] [Google Scholar]
- 7.Tofovic SP, Rominski BR, Bastacky S, Jackso EK, Kost CK Jr. Caffeine augments proteinuria in puromycin-aminonucleoside nephrotic rats. Ren Fail. 2000;22(2):159-179. doi: 10.1081/jdi-100100861 [DOI] [PubMed] [Google Scholar]
- 8.Tofovic SP, Kost CK Jr, Jackson EK, Bastacky SI. Long-term caffeine consumption exacerbates renal failure in obese, diabetic, ZSF1 (fa-facp) rats. Kidney Int. 2002;61(4):1433-1444. doi: 10.1046/j.1523-1755.2002.00278.x [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Shindo D, Suzuki R, Shirataki Y, Waki H. Combined long-term caffeine intake and exercise inhibits the development of diabetic nephropathy in OLETF rats. J Appl Physiol (1985). 2017;122(5):1321-1328. doi: 10.1152/japplphysiol.00278.2016 [DOI] [PubMed] [Google Scholar]
- 10.Wijarnpreecha K, Thongprayoon C, Thamcharoen N, Panjawatanan P, Cheungpasitporn W. Association of coffee consumption and chronic kidney disease: a meta-analysis. Int J Clin Pract. 2017;71(1):e12919. doi: 10.1111/ijcp.12919 [DOI] [PubMed] [Google Scholar]
- 11.Belibi FA, Wallace DP, Yamaguchi T, Christensen M, Reif G, Grantham JJ. The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002;13(11):2723-2729. doi: 10.1097/01.asn.0000025282.48298.7b [DOI] [PubMed] [Google Scholar]
- 12.Girardat-Rotar L, Puhan MA, Braun J, Serra AL. Long-term effect of coffee consumption on autosomal dominant polycystic kidneys disease progression: results from the Suisse ADPKD, a prospective longitudinal cohort study. J Nephrol. 2018;31(1):87-94. doi: 10.1007/s40620-017-0396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vendramini LC, Nishiura JL, Baxmann AC, Heilberg IP. Caffeine intake by patients with autosomal dominant polycystic kidney disease. Braz J Med Biol Res. 2012;45(9):834-840. doi: 10.1590/s0100-879x2012007500120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyes CM, Cornelis MC. Caffeine in the diet: country-level consumption and guidelines. Nutrients. 2018;10(11):1772. doi: 10.3390/nu10111772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34(1):119-129. doi: 10.1016/0278-6915(95)00093-3 [DOI] [PubMed] [Google Scholar]
- 16.Vallon V, Osswald H. Adenosine receptors and the kidney. Handb Exp Pharmacol. 2009;(193):443-470. doi: 10.1007/978-3-540-89615-9_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu EA, Selvin E, Grams ME, Steffen LM, Coresh J, Rebholz CM. Coffee consumption and incident kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2018;72(2):214-222. doi: 10.1053/j.ajkd.2018.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy OJ, Pirastu N, Poole R, et al. Coffee consumption and kidney function: a mendelian randomization study. Am J Kidney Dis. 2020;75(5):753-761. doi: 10.1053/j.ajkd.2019.08.025 [DOI] [PubMed] [Google Scholar]
- 19.Jhee JH, Nam KH, An SY, et al. Effects of coffee intake on incident chronic kidney disease: a community-based prospective cohort study. Am J Med. 2018;131(12):1482-1490. doi: 10.1016/j.amjmed.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 20.Kanbay M, Siriopol D, Copur S, et al. Effect of coffee consumption on renal outcome: a systematic review and meta-analysis of clinical studies. J Ren Nutr. 2021;31(1):5-20. doi: 10.1053/j.jrn.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Herber-Gast GCM, van Essen H, Verschuren WM, et al. Coffee and tea consumption in relation to estimated glomerular filtration rate: results from the population-based longitudinal Doetinchem Cohort Study. Am J Clin Nutr. 2016;103(5):1370-1377. doi: 10.3945/ajcn.115.112755 [DOI] [PubMed] [Google Scholar]
- 22.Miyatake N, Shikata K, Makino H, Numata T. The relation between estimated glomerular filtration rate (eGFR) and coffee consumption in the Japanese. Health. 2011;03(9):549-552. [Google Scholar]
- 23.Meca R, Balbo BE, Ormanji MS, et al. Caffeine accelerates cystic kidney disease in a Pkd1-deficient mouse model. Cell Physiol Biochem. 2019;52(5):1061-1074. doi: 10.33594/000000072 [DOI] [PubMed] [Google Scholar]
- 24.Sachse C, Brockmöller J, Bauer S, Roots I. Functional significance of a C→A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47(4):445-449. doi: 10.1046/j.1365-2125.1999.00898.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4(4):287-291. [Google Scholar]
- 26.Cornelis MC, Kacprowski T, Menni C, et al. ; Swiss Kidney Project on Genes in Hypertension (SKIPOGH) Team . Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet. 2016;25(24):5472-5482. doi: 10.1093/hmg/ddw334 [DOI] [PubMed] [Google Scholar]
- 27.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006;295(10):1135-1141.doi: 10.1001/jama.295.10.1135 [DOI] [PubMed] [Google Scholar]
- 28.Palatini P, Ceolotto G, Ragazzo F, et al. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J Hypertens. 2009;27(8):1594-1601. doi: 10.1097/HJH.0b013e32832ba850 [DOI] [PubMed] [Google Scholar]
- 29.Palatini P, Benetti E, Mos L, et al. Association of coffee consumption and CYP1A2 polymorphism with risk of impaired fasting glucose in hypertensive patients. Eur J Epidemiol. 2015;30(3):209-217. doi: 10.1007/s10654-015-9990-z [DOI] [PubMed] [Google Scholar]
- 30.Palatini P, Mormino P, Canali C, et al. Factors affecting ambulatory blood pressure reproducibility. results of the HARVEST trial. Hypertension and Ambulatory Recording Venetia Study. Hypertension. 1994;23(2):211-216. doi: 10.1161/01.hyp.23.2.211 [DOI] [PubMed] [Google Scholar]
- 31.Palatini P, Dorigatti F, Santonastaso M, et al. Association between coffee consumption and risk of hypertension. Ann Med. 2007;39(7):545-553. doi: 10.1080/07853890701491018 [DOI] [PubMed] [Google Scholar]
- 32.Sartori M, Semplicini A, Siffert W, et al. G-protein β3-subunit gene 825T allele and hypertension: a longitudinal study in young grade I hypertensives. Hypertension. 2003;42(5):909-914. doi: 10.1161/01.HYP.0000097600.58083.EE [DOI] [PubMed] [Google Scholar]
- 33.British Hypertension Society Working Party. Treating mild hypertension. report of the British Hypertension Society working party. BMJ. 1989;298(6675):694-698. doi: 10.1136/bmj.298.6675.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sever P, Beevers G, Bulpitt C, et al. Management guidelines in essential hypertension: report of the second working party of the British Hypertension Society. BMJ. 1993;306(6883):983-987. doi: 10.1136/bmj.306.6883.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guidelines Subcommittee. 1999 World Health Organization–International Society of Hypertension guidelines for the management of hypertension. J Hypertens. 1999;17(2):151-183. [PubMed] [Google Scholar]
- 36.European Society of Hypertension–European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21(6):1011-1053. doi: 10.1097/00004872-2003060000-00001 [DOI] [PubMed] [Google Scholar]
- 37.National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2)(suppl 1):S1-S266. [PubMed] [Google Scholar]
- 38.Palatini P, Canali C, Graniero GR, et al. Relationship of plasma renin activity with caffeine intake and physical training in mild hypertensive men. HARVEST Study Group. Eur J Epidemiol. 1996;12(5):485-491. doi: 10.1007/BF00144001 [DOI] [PubMed] [Google Scholar]
- 39.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8(5):293-300. doi: 10.1038/nrneph.2012.19 [DOI] [PubMed] [Google Scholar]
- 40.Casiglia E, Paleari CD, Petucco S, et al. Haemodynamic effects of coffee and purified caffeine in normal volunteers: a placebo-controlled clinical study. J Hum Hypertens. 1992;6(2):95-99. [PubMed] [Google Scholar]
- 41.Palatini P, Mormino P, Mos L, et al. ; HARVEST Study Group . Microalbuminuria, renal function and development of sustained hypertension: a longitudinal study in the early stage of hypertension. J Hypertens. 2005;23(1):175-182. doi: 10.1097/00004872-200501000-00028 [DOI] [PubMed] [Google Scholar]
- 42.Park KY, Kim HJ, Ahn HS, et al. Effects of coffee consumption on serum uric acid: systematic review and meta-analysis. Semin Arthritis Rheum. 2016;45(5):580-586. doi: 10.1016/j.semarthrit.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 43.Palatini P, Graniero GR, Canali C, et al. Relationship between albumin excretion rate, ambulatory blood pressure and left ventricular hypertrophy in mild hypertension. J Hypertens. 1995;13(12 Pt 2):1796-1800. [PubMed] [Google Scholar]
- 44.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622-627. doi: 10.1053/j.ajkd.2010.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bigotte Vieira M, Magriço R, Viegas Dias C, Leitão L, Neves JS. Caffeine consumption and mortality in chronic kidney disease: a nationally representative analysis. Nephrol Dial Transplant. 2019;34(6):974-980. [DOI] [PubMed] [Google Scholar]
- 46.Altay S, Onat A, Özpamuk-Karadeniz F, Karadeniz Y, Kemaloğlu-Öz T, Can G. Renal “hyperfiltrators” are at elevated risk of death and chronic diseases. BMC Nephrol. 2014;15:160. doi: 10.1186/1471-2369-15-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marx B, Scuvée É, Scuvée-Moreau J, Seutin V, Jouret F. Mechanisms of caffeine-induced diuresis (article in French). Med Sci (Paris). 2016;32(5):485-490. doi: 10.1051/medsci/20163205015 [DOI] [PubMed] [Google Scholar]
- 48.Ninomiya T, Perkovic V, de Galan BE, et al. ; ADVANCE Collaborative Group . Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813-1821. doi: 10.1681/ASN.2008121270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin CH, Chang YC, Chuang LM. Early detection of diabetic kidney disease: present limitations and future perspectives. World J Diabetes. 2016;7(14):290-301. doi: 10.4239/wjd.v7.i14.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palatini P, Dorigatti F, Saladini F, et al. Factors associated with glomerular hyperfiltration in the early stage of hypertension. Am J Hypertens. 2012;25(9):1011-1016. doi: 10.1038/ajh.2012.73 [DOI] [PubMed] [Google Scholar]
- 51.Kim BH, Park YS, Noh HM, Sung JS, Lee JK. Association between coffee consumption and renal impairment in Korean women with and without diabetes: analysis of the Fourth Korea National Health and Nutrition Examination Survey in 2008. Korean J Fam Med. 2013;34(4):265-271. doi: 10.4082/kjfm.2013.34.4.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melsom T, Stefansson V, Schei J, et al. Association of increasing GFR with change in albuminuria in the general population. Clin J Am Soc Nephrol. 2016;11(12):2186-2194. doi: 10.2215/CJN.04940516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palatini P, Mos L, Ballerini P, et al. ; HARVEST Investigators . Relationship between GFR and albuminuria in stage 1 hypertension. Clin J Am Soc Nephrol. 2013;8(1):59-66. doi: 10.2215/CJN.03470412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stehouwer CDA, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17(8):2106-2111. doi: 10.1681/ASN.2005121288 [DOI] [PubMed] [Google Scholar]
- 55.Berton G, Cordiano R, Mbaso S, De Toni R, Mormino P, Palatini P. Prognostic significance of hypertension and albuminuria for early mortality after acute myocardial infarction. J Hypertens. 1998;16(4):525-530. doi: 10.1097/00004872-199816040-00014 [DOI] [PubMed] [Google Scholar]
- 56.Masson S, Latini R, Milani V, et al. ; GISSI-HF Investigators . Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI-Heart Failure trial. Circ Heart Fail. 2010;3(1):65-72. doi: 10.1161/CIRCHEARTFAILURE.109.881805 [DOI] [PubMed] [Google Scholar]
- 57.Díaz-López A, Paz-Graniel I, Ruiz V, et al. Consumption of caffeinated beverages and kidney function decline in an elderly Mediterranean population with metabolic syndrome. Sci Rep. 2021;11(1):8719. doi: 10.1038/s41598-021-88028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Participants, Baseline Procedures, Kidney and Adrenal Function, and Data Analysis
Data Sharing Statement