Summary
Background
Knowledge regarding the risks associated with Zika virus (ZIKV) infections in pregnancy has relied on individual studies with relatively small sample sizes and variable risk estimates of adverse outcomes, or on surveillance or routinely collected data. Using data from the Zika Brazilian Cohorts Consortium, this study aims, to estimate the risk of adverse outcomes among offspring of women with RT-PCR-confirmed ZIKV infection during pregnancy and to explore heterogeneity between studies.
Methods
We performed an individual participant data meta-analysis of the offspring of 1548 pregnant women from 13 studies, using one and two-stage meta-analyses to estimate the absolute risks.
Findings
Of the 1548 ZIKV-exposed pregnancies, the risk of miscarriage was 0.9%, while the risk of stillbirth was 0.3%. Among the pregnancies with liveborn children, the risk of prematurity was 10,5%, the risk of low birth weight was 7.7, and the risk of small for gestational age (SGA) was 16.2%. For other abnormalities, the absolute risks were: 2.6% for microcephaly at birth or first evaluation, 4.0% for microcephaly at any time during follow-up, 7.9% for neuroimaging abnormalities, 18.7% for functional neurological abnormalities, 4.0% for ophthalmic abnormalities, 6.4% for auditory abnormalities, 0.6% for arthrogryposis, and 1.5% for dysphagia. This risk was similar in all sites studied and in different socioeconomic conditions, indicating that there are not likely to be other factors modifying this association.
Interpretation
This study based on prospectively collected data generates the most robust evidence to date on the risks of congenital ZIKV infections over the early life course. Overall, approximately one-third of liveborn children with prenatal ZIKV exposure presented with at least one abnormality compatible with congenital infection, while the risk to present with at least two abnormalities in combination was less than 1.0%.
Funding
National Council for Scientific and Technological Development - Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq); Wellcome Trust and the United Kingdom's Department for International Development; European Union's Horizon 2020 research and innovation program; Medical Research Council on behalf of the Newton Fund and Wellcome Trust; National Institutes of Health/National Institute of Allergy and Infectious Diseases; Foundation Christophe et Rodolphe Mérieux; Coordination for the improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Capes); Ministry of Health of Brazil; Brazilian Department of Science and Technology; Foundation of Research Support of the State of São Paulo (Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP); Foundation of Research Support of the State of Rio de Janeiro (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ); Foundation of Support for Research and Scientific and Technological Development of Maranhão; Evandro Chagas Institute/Brazilian Ministry of Health (Instituto Evandro Chagas/Ministério da Saúde); Foundation of Research Support of the State of Goiás (Fundação de Amparo à Pesquisa do Estado de Goiás – FAPEG); Foundation of Research Support of the State of Rio Grande do Sul (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul – FAPERGS); Foundation to Support Teaching, Research and Assistance at Hospital das Clínicas, Faculty of Medicine of Ribeirão Preto (Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto); São Paulo State Department of Health (Secretaria de Saúde do Estado de São Paulo); Support Foundation of Pernambuco Science and Technology (Fundação de Amparo à Ciência e Tecnologia de Pernambuco – FACEPE).
Keywords: Meta-analysis, Zika, Cohort, Pregnant women, Children, Adverse outcomes
Research in context.
Evidence before this study
We searched PubMed on 18 April 2022 for studies published on clinical outcomes among offspring with prenatal Zika virus exposure using keywords in English, Portuguese, Spanish, and French related to “Zika virus”, “pregnancy”, “offspring”, and “clinical outcomes” (for details of full search strategy, please see Supplementary Seearch Terms). We identified 20 systematic reviews, of which 6 included meta-analyses, and no individual participant data meta-analyses (IPD-MA). To our knowledge, three protocols for IPD-MA on the risks associated with Zika virus (ZIKV) infections during pregnancy have been published to date by the following groups: (i) the Zika Brazilian Cohorts Consortium (i.e., for the current study), (ii) the ZIKAlliance, ZikaPLAN, and ZIKAction Consortia], and (iii) the World Health Organization-led Zika Virus Individual Participant Data Consortium.
Added value of this study
This study is the first IPD-MA and the largest single investigation of adverse offspring outcomes associated with RT-PCR-confirmed ZIKV infections during pregnancy. This study advances understanding of congenital ZIKV infections by demonstrating that:
-
•
Approximately one-third of liveborn children with prenatal ZIKV exposure present with at least one abnormality compatible with congenital infection during the first years of life; however, congenital abnormalities are more likely to present in isolation than in combination.
-
•
Birth evaluations may underestimate the risk of ZIKV-related microcephaly, as approximately 1.5% of exposed children may develop microcephaly postnatally.
-
•
The risk of ZIKV-related microcephaly is relatively homogeneous across study sites and does not appear to be modified across geographic, educational, or racial/ethnic groups.
Implications of all the available evidence
There is robust evidence that children with prenatal exposure to ZIKV experience elevated risks of being born with congenital abnormalities and of developing adverse outcomes postnatally. These findings underscore the continued importance of having a multi-disciplinary health team available to prenatally exposed children born during the Zika pandemic of 2015–2017 to provide follow-up care, including support for known disabilities and diagnosis of late manifestations.
Looking forward, the potential re-emergence of ZIKV remains a concern, owing to the lack of an approved ZIKV vaccine, the growing proportion of the population that is susceptible to ZIKV, and the potential for new ZIKV strains to evolve with enhanced transmissibility and/or virulence, particularly during pregnancy. Efforts toward developing affordable and accurate ZIKV diagnostic and screening tests remain critically important. Their use for early detection of circulating ZIKV in communities would enable rapid deployment of public health measures for averting new epidemics and would thus minimize the risks of infections during pregnancy and of preventable developmental disabilities in infants.
Introduction
After an outbreak of microcephaly cases was observed in the Northeastern Brazilian state of Pernambuco in October 2015,1 researchers and subsequently, health authorities2,3 raised suspicion of a possible association between the increase in birth defects and the emergence of Zika virus (ZIKV) transmission in the region, which was first detected in May 2015.4,5 In November 2015, the Brazilian Ministry of Health declared the situation a public health emergency,1,4 and in February 2016, the World Health Organization (WHO) officially recognized the cluster of cases as a Public Health Emergency of International Concern.6
Subsequent research has demonstrated that ZIKV could be isolated from the amniotic fluid of pregnant women with fetuses with microcephaly and other brain malformations7,8 and also from malformed fetal brain tissue and spinal cord in post-mortem analyses.9, 10, 11 Other findings suggest a ZIKV tropism for progenitor neurons of the cerebral cortex, and it has been proposed that congenital infections may induce cell death, with consequent changes in brain structure.12,13 The causal link has been further corroborated by a case–control study, which showed a strong association between congenital ZIKV infection and microcephaly and ruled out other potentially teratogenic factors.14,15 Detailed case series of children with microcephaly have described a range of adverse impacts of congenital ZIKV infections,16, 17, 18 and a spectrum of birth defects and developmental disabilities are now recognized as manifestations of congenital ZIKV infections.
Estimates of the risks of adverse pregnancy outcomes after ZIKV infection have relied mainly on routine surveillance data published between 2016 and 201819, 20, 21 and also on a few prospective pregnancy cohorts followed up in several regions of Latin America.22, 23, 24, 25, 26, 27 Most of these studies did not reach sufficiently large sample sizes, and because of variations in the magnitude of risk estimates for microcephaly and/or other congenital abnormalities across studies, it was suggested that the risk may vary over geography and time.28, 29, 30 Information from previously published systematic reviews is also limited, as those published so far have focused on specific outcomes and those which included meta-analyses have relied on aggregate rather than individual participant data. To date, three protocols for individual participant data meta-analysis (IPD-MA) of Zika cohort studies, including one for the current study and authored by our group (i.e., the Zika Brazilian Cohorts Consortium) have been published.31, 32, 33 Using individual-level data from the Zika Brazilian Cohorts Consortium (ZBC-Consortium), this study aims to estimate more precisely the occurrence of adverse outcomes among fetuses and infants of women with laboratory-confirmed ZIKV infection during pregnancy and to explore heterogeneity between studies.
Methods
This individual participant data meta-analysis (IPD-MA) of the ZBC-Consortium brings together and harmonizes individual-level data from 13 pregnancy cohorts from the 4 regions in Brazil affected by the ZIKV epidemic (Table 1). More detailed information about the ZBC-Consortium and the study protocol has been published previously.33
Table 1.
Women with RT-PCR-confirmed ZIKV infections during pregnancy participating in studies of the ZBC-Consortium.
| Cohort | Study population | Recruitment dates | ZIKV RT-PCR + pregnant women, n (%) |
|---|---|---|---|
| North Region | 404 (26.0%) | ||
| Belém | Pregnant women with rash | October 2015–December 2017 | 82 (5.3%) |
| Manaus | Pregnant women with rash | December 2015–January 2017 | 322 (20.7%) |
| Northeast Region | 157 (10.1%) | ||
| Campina Grande | Pregnant women with rash | November 2015–October 2016 | 33 (2.1%) |
| Recife - MERG | Pregnant women with rash | December 2015–June 2017 | 108 (7.0%) |
| Salvador | Pregnant women with rash | February 2016–November 2016 | 16 (1.0%) |
| Central-West Region | 87 (5.6%) | ||
| Tangará da Serra | Pregnant women with rash | January 2016–December 2016 | 36 (2.3%) |
| Goiânia | Pregnant women with rash | March 2017–March 2019 | 51 (3.3%) |
| Southeast Region | 900 (58.2%) | ||
| Rio de Janeiro – Ped UFRJ | Pregnant women with rash | November 2015–May 2016 | 30 (1.9%) |
| Rio de Janeiro – DMP UFRJ | Pregnant women with rash | April 2016–Present | 7 (0.4%) |
| Rio de Janeiro – IFF-Fiocruz | Pregnant women with rash | September 2015–March 2017 | 241 (15.6%) |
| São José do Rio Preto | Pregnant women with rash | February 2016–June 2016 | 57 (3.7%) |
| Jundiaí | Pregnant women with high-risk and/or rash | March 2016–August 2018 | 55 (3.6%) |
| Ribeirão Preto | Pregnant women with rash | December 2015–July 2016 | 510 (33.0%) |
| Total | 1548 (100.0%) |
Abbreviations: DMP UFRJ, Departamento de Medicina Preventiva, Universidade Federal do Rio de Janeiro; IFF-Fiocruz, Oswaldo Cruz Foundation's Fernandes Figueira Institute; MERG, Microcephaly Epidemic Research Group; PED UFRJ, Departamento de Pediatria, Universidade Federal do Rio de Janeiro.
Identification of the studies
The ZBC-Consortium leadership team contacted all research teams in Brazil that received grants for human ZIKV research from the major Brazilian funding bodies (i.e., the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Level-Education Personnel (CAPES) and the Ministry of Health) as of October 2017 and/or that participated in the Pan American Health Organization (PAHO)/WHO-supported meetings for harmonization of protocols for ZIKV-related investigations, which were held in Recife, Brazil, in March 2016; in Mexico City, Mexico, in June 2016; and in Geneva, Switzerland, in February 2017. All identified groups were asked to identify other cohort studies that were not included in the list initially raised.
Eligibility criteria
Studies were eligible for inclusion in the IPD-MA if they were based in Brazil and recruited pregnant women who underwent laboratory testing for confirmation of acute ZIKV infection prior to any detection of abnormalities in the fetus and were followed up until at least the end of the pregnancy. The main analysis described here was restricted to pregnant women who tested positive for ZIKV by reverse transcription-polymerase chain reaction (RT-PCR). No additional exclusion criteria were applied.
Harmonization and data storage
During a series of in-person meetings, the principal investigators (PIs) of the 13 participating studies compared individual cohort questionnaires and protocols to identify relevant common variables for data sharing and harmonization. Subsequently, recategorization and recoding of variables were performed by the team of each site in line with the analysis plan. The pooled databases were exported to a single platform built using the GeneXus X Ev 1. When received, databases were checked for data consistency and completeness by the ZBC-Consortium analysis team, and if discrepancies were identified, data were returned via the secure file transfer protocol to the original study team for correction.
Outcomes
For the offspring of women with RT-PCR evidence of acute ZIKV infection during pregnancy, this study evaluated the absolute risks of: miscarriage; stillbirth; preterm deliveries; low birth weight; small for gestational age; microcephaly (proportionate and disproportionate),34 skeletal anomalies: orthopedic abnormality, arthrogryposis; neuroimaging: calcifications, ventriculomegaly, cerebellum hypoplasia/atrophy, corpus callosum dysgenesis or agenesis, corpus callosum hypoplasia, diffuse cortical atrophy, mega cisterna magna, lissencephaly; neurological abnormalities: convulsive seizures, change in consciousness/behavior (i.e., alteration of at least one of the following: social smile, irritability, ability to follow with the eyes or to fix the gaze, ocular motricity, response to visual stimuli and response to auditory stimuli), localized motor deficit, abnormal tonus (i.e., involuntary state of contraction) or trophism (i.e., volume of muscle mass), deep tendon reflex and Babinski sign, abnormal visual and auditory response on clinical evaluation, and dysphagia; ophthalmic abnormalities: alterations in the optic nerve, retina, and fundus of the eye; audiological outcomes: failure in the auditory brainstem response (ABR) or otoacoustic emission (OAE) tests. Moderate microcephaly was defined as a z-score of ≤−2SD and >−3SD, for sex and gestational age (GA); severe microcephaly was defined as a z-score of ≤−3SD, for sex and GA. For the head circumference (HC) z-score calculation at birth we used the INTERGROWTH-21st curves.35 For the HC z-score after birth for children born preterm, we used the INTERGROWTH-21st curves with a correction for GA made until the completion of 64 weeks of GA.36 After this age and for children born at term, we used the WHO curves.37 To assure the same standard in the estimation of HC z-score, the z-score of all children of the ZBC-Consortium was re-calculated.
Data analysis
All analyses were performed using STATA, version 14 (College Station, TX, USA). Random effects meta-analyses using individual participant data were performed using both one-stage and two-stage approaches.
Multilevel mixed-effects logistic regressions (meqrlogit STATA command) with a random study-specific effect (i.e., to account for clustering of participants within studies) and a random exposure effect (i.e., to account for between-site heterogeneity in effect) were conducted to estimate the pooled odds ratios for the associations between selected exposure variables [trimester of ZIKV infection, highest level of educational attainment, skin colour (i.e., as an indicator of race/ethnicity), and region] and the outcome of microcephaly at birth or first evaluation and between the exposure of trimester of ZIKV infection and the outcome of postnatal neuroimaging abnormalities.
Two-stage random-effects meta-analyses using the metaprop38 STATA command were used to first estimate the study-specific proportions with 95% score confidence intervals and then to estimate the pooled absolute risks and 95% Wald confidence intervals. The meta-analyses conducted using metaprop were also used to explore heterogeneity by estimating I2 (i.e., the percentage of the total variation in study estimates that is due to heterogeneity), τ2 (i.e., the between-study variance), and the chi-squared test for heterogeneity.
As a sensitivity analysis to ensure the internal validity of our findings, pooled analyses of the absolute risks of adverse outcomes were also conducted as one-stage meta-analyses using (i) multilevel mixed-effects logistic regressions (melogit STATA command) with a random study-specific effect and (ii) a survey approach (svy STATA command) that accounts for clustering of participants within studies. No additional covariates were included in any of the models.
Ethics approval
All participating cohort studies had ethical approval by local ethics committees: Instituto Aggeu Magalhães/Fiocruz-PE (CAAE 53240816.4.0000.5190); Hospital Universitário Oswaldo Cruz (CAAE 52803316.8.0000.5192); Instituto Professor Joaquim Amorim Neto (CAAE 52888616.4.0000.5693); Instituto Gonçalo Moniz/Fiocruz-BA (CAAE 51889315.7.0000.0040); Fundação de Medicina Tropical do Amazonas (CAAE 60168216.2.0000.0005); Instituto Evandro Chagas (CAAE 56969516.8.0000.0019; CAAE 68067217.0.0000.0019; CAAE 29124920.6.0000.0019); Universidade Federal de Goiás (CAAE 64534017.7.0000.5083); Hospital de Clínicas de Porto Alegre (CAAE 56176616.2.1001.5327); Instituto Fernandes Figueira/Fiocruz-RJ (CAAE 52675616.0.0000.5269); Instituto Nacional de Infectologia Evandro Chagas/Fiocruz (CAAE 0026.0.009.000-07); Maternidade Escola da Universidade Federal do Rio de Janeiro (CAAE 55465616.0.0000.5275); Instituto de Puericultura e Pediatria Martagão Gesteira (CAAE 54497216.2.1001.5264); Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da USP (CAAE 56522216.0.0000.5440); Faculdade de Medicina de São José do Rio Preto (CAAE 55805516.2.0000.5415); and Faculdade de Medicina de Jundiaí (CAAE 53248616.2.0000.5412). All participating pregnant women and persons responsible for participating children provided a written informed consent form. Ethics approval for the overall meta-analysis was not required.
Role of the funding source
The funders of the study had no role in study design; data collection, data analysis, data interpretation or in the writing of the report.
Results
In the ZBC-Consortium, we performed individual participant data meta-analysis from 1548 women with RT-PCR-confirmed ZIKV infections during pregnancy, of whom 900 were from the southeast, 404 from the north, 157 from the northeast, and 87 from the central-west regions of Brazil (Tables 1 and 2). The study participant's median age was 28 (23–33, IQR) years and they were primarily multigravidae and carrying singleton pregnancies (99.2%). Among the pregnant women, 189/1200 (15.8%) had University/post-graduation education, 5.2% smoked during pregnancy and less than 3% used recreational drugs. The timing of ZIKV infections (i.e., determined by rash onset and/or positive RT-PCR test) was more frequent in the second (36.9%) and third trimesters (44.0%).
Table 2.
Characteristics of women with RT-PCR-confirmed ZIKV infections during pregnancy participating in studies of the ZBC-Consortium (N = 1548).
| Characteristic | ZIKV+ Pregnant Women (N = 1548) |
|---|---|
| Maternal age, years – median (IQR) | 28 (23–33) |
| Highest educational attainment | |
| Primary education | 437 (36.4%) |
| Secondary education | 574 (47.8%) |
| University or postgraduate | 189 (15.8%) |
| Missing education | 348 |
| Skin color | |
| Branca (White) | 364 (47.0%) |
| Parda (Mixed) | 315 (40.7%) |
| Preta (Black) | 85 (11.0%) |
| Other | 10 (1.3%) |
| Missing skin color | 774 |
| Timing of rash by trimester – n (%) | |
| First | 277 (19.1%) |
| Second | 534 (36.9%) |
| Third | 637 (44.0%) |
| Missing | 100 |
| Number of prior pregnancies – n (%) | |
| None | 97 (7.3%) |
| One | 452 (34.1%) |
| Two | 390 (29.5%) |
| Three or more | 386 (29.1%) |
| Missing | 223 |
| Number of fetuses (current pregnancy) – n (%) | |
| Single | 1528 (99.2%) |
| Twins | 13a (1.0%) |
| Missing | 7 |
| Offspring sex – n (%) | |
| Male | 785 (52.4%) |
| Female | 714 (47.6%) |
| Missing | 58b |
| Number of stillbirths in prior pregnancies – n (%) | |
| None | 458 (91.0%) |
| One | 35 (7.0%) |
| Two or more | 10 (2.0%) |
| Missing | 725 |
| Maternal comorbidities – n/N (%) | |
| Diabetes Mellitus | 70/927 (7.6%) |
| Hypertension | 114/928 (12.3%) |
| Anemia | 127/786 (16.2%) |
| Familial history of congenital malformation – n/N (%) | 9/186 (4.8%) |
| Lifestyle practices during this pregnancy – n/N (%) | |
| Smoking | 46/887 (5.2%) |
| Alcohol use | 80/645 (12.4%) |
| Illicit drug use | 23/814 (2.8%) |
Four children were not enrolled in the children cohort.
No information for 4 children born alive, 16 stillbirths and 38 miscarriages.
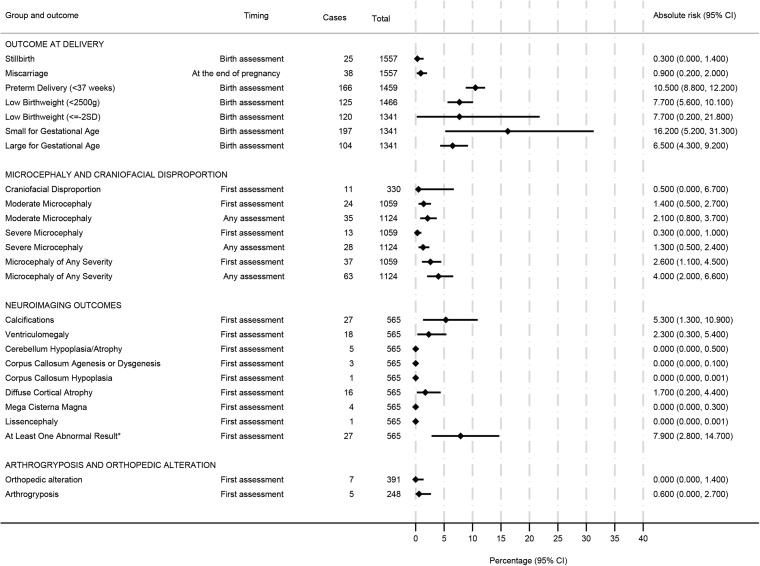
Among the 1548 ZIKV-exposed pregnancies, the risk of miscarriage was 0.9%, while the risk of stillbirth was 0.3% (Fig. 1). Among the pregnancies with liveborn children, the risk of prematurity was 10.5%, the risk of low birth weight was 7.7%, and the risk of small for gestational age (SGA) was 16.2%. The absolute risk of microcephaly at birth or at the first evaluation was 2.6% (95%-CI, 1.1–4.5), with more cases of moderate (1.4%) than severe (0.3%) while the risk of microcephaly at any time during the follow-up was 4.0% (95%-CI, 2.0–6.6). Notably, among the 37 cases of microcephaly at birth, 16 were disproportionate. Cases of disproportionate microcephaly were more frequently among children with severe microcephaly (11/13, 84.6%) than those with moderate microcephaly (5/24, 20.8%).
Fig. 1.
Absolute riska of adverse outcomes at delivery or at first evaluation of children born to women with rt-PCR-confirmed ZIKV infections during pregnancy participating in studies of the ZBC-consortium. aEstimated using a two-stage random effects meta-analyses with the metaprop STATA command.
The risk of brain imaging abnormalities was 7.9% for any abnormality, 5.3% (95%-CI, 1.3–10.9) for calcifications, 2.3% (95%-CI, 0.3–5.4) for ventriculomegaly, and 1.7% (95%-CI, 0.2–4.4) for diffuse cortical atrophy. For any of the other abnormalities the risk was below 1% (Fig. 1).
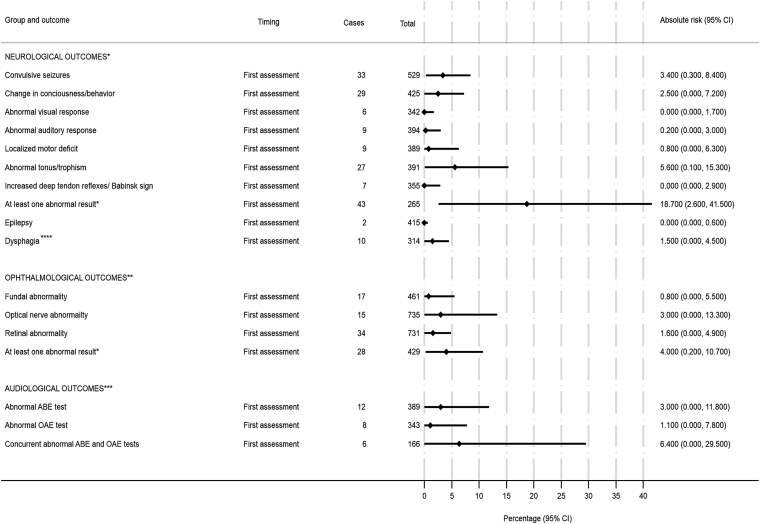
The risk of any neurological abnormality at birth or in the first evaluation (median age 11.7 months, P25–P75: 6.2–15.4) was 18.7% (95%-CI, 2.6–41.5), with 5.6% (95%-CI, 0.1–15.3) for abnormal tonus/trophism, 2.5% (95%-CI, 0.0–7.2) for altered consciousness level/behavior, and 3.4% (95%-CI, 0.3–8.4) for seizures (Fig. 2). The risk of dysphagia at first evaluation (median age 14.9 months, P25–P75:8.4–19.6) was 1.5% (95%-CI, 0.0–4.5).
Fig. 2.
Absolute riska of neurological, ophthalmological and audiological adverse outcomes at birth or at first evaluation of children born to women with rt-PCR-confirmed ZIKV infections during pregnancy participating in studies of the ZBC-consortium. aEstimated using a two-stage random effects meta-analyses with the metaprop STATA command. ∗median age of 11.7 months; ∗∗median age of 11.2 months; ∗∗∗median age of 6.5 months; ∗∗∗∗median age of 14.9 months.
The risk of ophthalmic disorders at birth or in the first evaluation (median age 11.2 months, P25–P75:4.2–19.5) was 4.0% (95%-CI, 0.2–10.7). Regarding the results of hearing screening tests at birth or in the first evaluation (median age 6.5 months, P25–P75: 0.9–13.8), the risk of concomitant failure for both exams (ABR and OAE) was 6.4% (Fig. 2).
The overall risk of having microcephaly, neuroimaging, neurological, or ophthalmic abnormalities at first evaluation was 24.7% (95%-CI, 0.10–63.6). The risk of having concurrent abnormalities was low, only 4 out of 107 children had both neuroimaging and neurological abnormalities and only 2 out of 107 had both neurological and ophthalmic abnormalities (Supplementary Table S1). Relative to infections in the third trimester, ZIKV infections during the first trimester were associated with approximately 7-times higher odds of microcephaly and 17-times higher odds of neuroimaging abnormalities (Table 3). There was no evidence of an association between microcephaly and the mother's educational level, race/ethnicity, or geographic region (Table 3).
Table 3.
Associations (i) between microcephaly and trimester of infection, maternal education, skin color, and geographic region and (ii) between brain imaging abnormalities and trimester of infection in children born to women with RT-PCR-confirmed ZIKV infections during pregnancy participating in studies of the ZBC-Consortium.
| Microcephaly at birth or first evaluation |
|||
|---|---|---|---|
| n/N (%) | OR (95% CI)b | p-value | |
| Timing of infection in pregnancya | |||
| First trimester | 18/169 (10.6%) | 7.52 (2.75–20.6) | <0.001 |
| Second trimester | 7/364 (1.9%) | 1.23 (0.42–3.57) | 0.706 |
| Third trimester | 7/441 (1.5%) | 1.0 | |
| Missing trimester | 5/85 | – | – |
| Highest educational attainment | |||
| Primary education | 12/362 (3.3%) | 0.80 (0.27–2.30) | 0.673 |
| Secondary education | 16/382 (4.2%) | 1.01 (0.36–2.83) | 0.978 |
| University or postgraduate | 5/121 (4.1%) | 1.0 | – |
| Missing education | 4/193 | – | – |
| Skin color | |||
| Branca (White) | 16/348 (4.6%) | 1.0 | – |
| Parda (Mixed) | 8/270 (3.0%) | 0.56 (0.22–1.42) | 0.219 |
| Preta (Black) | 4/79 (5.1%) | 1.11 (0.29–4.21) | 0.876 |
| Other | 0/8 (0%) | – | – |
| Missing skin color | 6/354 | – | – |
| Region | |||
| Northeast | 6/115 (5.2%) | 1.0 | – |
| North | 8/128 (6.2%) | 1.21 (0.41–3.60) | 0.730 |
| Central-West | 3/83 (3.6%) | 0.68 (0.17–2.81) | 0.595 |
| Southeast | 20/732 (2.7%) | 0.51 (0.20–1.29) | 0.158 |
| Brain imaging abnormalities after birth |
|||
|---|---|---|---|
| n/N (%) | OR (95% CI)b | p-value | |
| Timing of infection in pregnancya | |||
| First | 25/138 (18.1%) | 17.1 (3.82–76.6) | <0.001 |
| Second | 12/254 (4.7%) | 3.66 (0.79–17.0) | 0.098 |
| Third | 2/154 (1.3%) | 1.0 | |
Timing of infection was based on date of rash and/or positive ZIKV RT-PCR test.
Estimated using multilevel mixed effects logistic regressions allowing for within-study correlations.
In subsidiary analyses to explore heterogeneity across subgroups, in the two-stage meta-analysis, there was no evidence of heterogeneity (p for heterogeneity chiˆ2 > 0.05) for the outcomes of microcephaly at birth/first evaluation, microcephaly at any time, neuroimaging abnormalities, ophthalmic abnormalities at birth or first evaluation, and dysphagia. There was heterogeneity between studies (p for heterogeneity chiˆ2 < 0.001) for neurological abnormalities (Supplementary Table S2). For most of the variables, the I2 was <60%. The sensitivity analysis using different methods to estimate the proportions resulted in broadly consistent findings; while there were some differences in point estimates, the confidence intervals overlapped for the evaluated outcomes (Supplementary Table S2).
Discussion
This is the first individual participant data meta-analysis of cohorts of pregnant women with confirmed ZIKV infection during pregnancy ever published. This study focuses on Brazil, the epicentre of the microcephaly epidemic, and pools data from 13 cohort studies representing all Brazilian regions in which the 2015–2017 ZIKV epidemic occurred.
The absolute risk of microcephaly was 2.6% at birth or at the first evaluation and increased to 4.0% when we considered any time during follow-up. The risk of severe microcephaly was lower than that of moderate microcephaly. The overall result is consistent with other individual cohort and registry studies that demonstrated a frequency of ZIKV-associated microcephaly of less than 7%.21,22,24 Microcephaly can be detected at birth or develop postnatally in the first year of life,39 as previously documented.40,41 Our results indicate that, among the children who present with microcephaly, the fraction that is diagnosed postnatally is not negligible. The risk of post-natal ZIKV-associated microcephaly has not been documented before. This demonstrates the relevance of monitoring head growth closely in all infants with antenatal ZIKV exposure, even those born with normal head circumference.
Both proportionate and disproportionate microcephaly have been reported in infants with Congenital Zika Syndrome (CZS). In this population, the risk of disproportionate microcephaly was slightly lower than proportionate microcephaly, and it was more frequent in children with severe microcephaly. Although the observed frequencies of preterm and low birthweight births were in line with expectations for the general population, we observed a somewhat elevated risk of SGA of 16.2%, possibly a result of intra-uterine growth restriction, likely due to placental insufficiency, as suggested by Brasil et al.23
In our analysis, the risk of brain imaging abnormalities was 7.9%, which is higher than the 3.3% and 4% found in other cohorts,19,22 but similar in terms of specific findings. Intracranial calcification and ventriculomegaly were the most common findings (risks of 5.3 and 2.3%, respectively). We found severe brain imaging abnormalities (calcifications, ventriculomegaly, cortical atrophy, cortical malformations) in infants with and without microcephaly at birth. Notably, previous research has documented that children who develop postnatal microcephaly are commonly born with severe brain damage.40,41 The relatively low frequency of detection of brain imaging abnormalities alone suggests that for children who are under routine medical surveillance, further imaging tests (e.g., computerized tomography - CT or magnetic resonance imaging - MRI) beyond screening ultrasounds should not be systematically performed on all prenatally ZIKV-exposed children without a clinical indication. In addition, although there is evidence in this investigation and previous research that adverse events triggered by ZIKV can occur during any gestational period,23 our findings are consistent with several previous studies that have shown maternal infection during earlier stages of pregnancy is generally associated with more severe structural brain damage.40,42, 43, 44
We observed, at birth or at the first evaluation (median 11.7 months), a frequency of 18.7% of at least one functional neurological abnormality detected in clinical evaluations of children with and without microcephaly. The pathophysiological abnormalities observed, such as seizures, reduction of consciousness, visual and hearing impairment, as well as motor deficits, can be partially explained by structural damages in the fetal central nervous system caused by exposure to a neurotropic virus during brain development. Although the main neurotropic effect of ZIKV infection is on neuronal progenitor cells, the virus also affects cells in other stages of maturity.45 However, the discordance between the frequency of convulsive seizures (3.4%) and epilepsy (0.0%) observed in our study suggests that the early seizure disorder is probably due to a transient functional alteration rather than a permanent structural alteration. Longer follow-up of the cohorts may clarify this hypothesis.
Lesions in the retina and in the optic nerve, which are frequently associated with CZS abnormalities,42,46 were identified in 4.6% and 2.0% of the infants, respectively. Overall, we identified a risk of ophthalmic abnormalities in 4.0% of infants, less than the risk of 11.8% observed in a cohort study from Colombia47 but higher than the 2% frequency reported in another pregnancy cohort from the US48 and than the report of no eye abnormality in the cohort in the French territories in the Americas.22 The higher risk for eye abnormalities observed in our combined cohort and in the Colombia cohort are likely due to the performance of detailed fundoscopic eye evaluations by ophthalmologists during infants’ follow-up, in contrast to screening evaluations done at the time of birth.22 In the US study participants were followed through active surveillance methods and less than 15% of the children born to women with laboratory evidence of Zika virus infection during pregnancy had an ophthalmologic assessment.48 Hearing loss, described as one of the abnormalities associated with CZS, presenting as a sensorineural impairment was identified in 3.0% of included infants, using the ABR as a screening test, and in 6.4% using both ABR and OAE evaluations.49 Risk of auditory impairment was also identified in Colombia using ABR.47 Nevertheless, the risk of hearing deficits may be overestimated because it was based mainly on screening tests.
Consistent with a prior study,26 we found that infant adverse outcomes were identified more frequently in isolation rather than in association with each other. Considering the four categories of microcephaly, neurological, ophthalmic, or neuroimaging abnormalities, the risk of a prenatally ZIKV-exposed liveborn child experiencing at least one of these types of abnormalities at any time during follow-up was 31.5%.
By combining individual participant data from almost all of the Brazilian pregnancy cohorts with RT-PCR confirmation of ZIKV, this study has unprecedented power for obtaining more precise estimates of the risks, including of rare events, associated with prenatal ZIKV infection. This study is also unique in that, as early as the beginning of 2016, Brazilian researchers began to meet at the study design stage to harmonize protocols and investigate a common set of key adverse outcomes. The main limitations for the interpretation of this IPD-MA are the degree of heterogeneity between individual studies and the variation in the number of children evaluated for each outcome. Heterogeneity may be explained mainly by the variation in assessment techniques and instruments and, for neurological outcomes in particular, the specific expertise of the examiner, the comprehensiveness of the neurological examination, the different construction of composite variables summarizing the neurological findings, and random error associated with small sample sizes. Despite these differences in study design, we observed that risks for the key outcome of microcephaly did not vary significantly by maternal educational level, race/ethnicity, or geographic region across the diverse Brazilian population. Similarly, the I2 statistic generally remained below 60% in the two-stage meta-analyses, suggesting only mild to moderate statistical heterogeneity in the frequency of other clinical outcomes. The variation in the number of children assessed for each outcome is not likely to have introduced selection bias in risk estimates as these evaluations were not guided by the presence of abnormalities in the individual studies. As pregnant women recruited to these studies were either those notified to the surveillance system or those attending antenatal care in public institutions, it is, however, possible that women with higher socioeconomic positions who had access to private healthcare are underrepresented in our sample. Nevertheless, any selection bias related to income is unlikely to have affected the generalizability of our results because there is no evidence that the frequency of adverse outcomes in children born to women who were infected during pregnancy is related to socioeconomic status. Furthermore, ZIKV transmission is related to socioeconomic conditions and infection occurred mainly in more deprived populations.50,51 Other sources of selection bias (e.g., refusal to participate and losses to follow-up of the pregnancies) are also unlikely to have substantially distorted our results as they occurred before the end of pregnancy (i.e., before many of the congenital manifestations could be evaluated).51 In relation to information bias, there may have been some degree of inaccuracy in the measurement of the head circumference leading to the misclassification of microcephaly, especially in moderate microcephaly. However, the consistency of the results of microcephaly at birth across studies suggests that this misclassification was not important. We did not compare the risks to those born to uninfected women as most of these cohorts did not follow pregnant women who tested negative for ZIKV infection during pregnancy.
In conclusion, this IPD-MA advances our understanding of the risks associated with ZIKV infections during pregnancy. Our findings suggest that approximately one-third of the children born to ZIKV-positive pregnant women present with at least one abnormality compatible with congenital infection; however, congenital abnormalities are more likely to present in isolation than in combination. Although abnormalities detected at birth appear to be largely permanent, our findings suggest that manifestations may evolve and present later in infant life, as the immature nervous system at birth becomes myelinated. We suggest further studies, with longer follow-up, to expand the understanding of the manifestations of the CZS: i) for children with microcephaly, in addition to the estimation of the risks of hospitalization and death at different ages, a deeper investigation by specialists using more advanced diagnostic tools may identify other complications that may either appear or become more evident later in life; ii) for children without microcephaly, the study of the risk of manifestations related to neuropsychomotor and behavioural development that may only be diagnosed as children become older, using specific tools. Notably, birth evaluations may underestimate the risk of ZIKV-related microcephaly, as approximately 1.5% of children may develop microcephaly postnatally. Finally, the risks of adverse outcomes associated with ZIKV infections during pregnancy do not appear to be modified across geographic, educational (i.e., a proxy of socioeconomic position), or racial/ethnic groups. By harmonizing and jointly analyzing individual-level data from 13 cohorts of pregnant women with ZIKV infections in Brazil, this study generates the most robust evidence to date on the risks of congenital ZIKV infections over the early life course.
Contributors
All authors contributed to the conception, design of the work, the acquisition and interpretation of data and revised the manuscript. All authors approved the submitted version and agreed to be personally accountable for the author's contributions and to ensure that questions related to the accuracy or integrity of any part of the work, are appropriately investigated, resolved, and the resolution documented in the literature.
Data sharing statement
The data that support the findings of this study are available on the Shared Data Platform, designed and protected by the Informatics Sector of the Aggeu Magalhães Institute/Fiocruz – Pernambuco, Brazil. To guarantee the privacy of all participants' personal data, access to data is restricted to the coordinators of the ZBC Consortium and, therefore, is not publicly available.
Declaration of interests
We declare no competing interests.
Acknowledgements
The “Evidence before this study” search was based on a strategy and search terms originally developed by Drs. Amber I. Raja and Leila R. Mendonça-Vieira as part of an ongoing systematic review on “Sequelae of prenatal Zika virus (ZIKV) exposure in children” (PROSPERO CRD42020185973) and was used with permission.
Funding: The Zika Brazilian Cohorts Consortium was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq) (grant number 404861/2018-0). The individual studies participating in the ZBC-Consortium were funded by: Wellcome Trust and the United Kingdom's Department for International Development (grant numbers: 205377/Z/16/Z; 201870/Z/16/Z). European Union's Horizon 2020 research and innovation programme under ZikaPLAN (grant number 734584). Wellcome Trust - Research Enrichment in Epidemic Situation (grant number 107779/Z/15/Z; with ER1505 & ER1601). Medical Research Council on behalf of the Newton Fund and Wellcome Trust (grant number MC_PC_15088). National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant number RO1/AI140718). Fondation Christophe et Rodolphe Mérieux. National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq) (grant numbers 443875/2018-9; 440573/2016-5; 441098/2016-9; 305090/2016-0; 307282/2017-1; 304476/2018-8; 465549/2014-4; 440763/2016-9; 309722/2017-9; 306708/2014-0; 440577/2016-0). Coordination for the improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Capes) (grant numbers 88881.130813/2016-01; 88887.116627/2016-01; 88887.136366/2017-00). Ministry of Health of Brazil - Emergency Response in Public Health - Zika virus and Microcephaly (Ministério da Saúde de Brasil - Resposta à Emergência em Saúde Pública – Zika vírus e Microcefalia) (grant number 837058/2016). Department of Science and Technology (Departamento de Ciência e Tecnologia - DECIT) (grant numbers 25000.072811/2016-19; 440839/2016-5). Foundation of Research Support of the State of São Paulo (Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP) (grant numbers 2016/08578-0; 2017/21688-1; 2013/21719-3; 2016/15021-1; 2015/12295-0; 2016/05115-9). Foundation of Research Support of the State of Rio de Janeiro (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ) (grant numbers E-26/201.351/2016; E-18/2015TXB; E-26/202.862/2018; E-26/010.002477/2016). Foundation of Support for Research and Scientific and Technological Development of Maranhão (Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão – FAPEMA) (grant number 008/2016). Brazilian Ministry of Health (Ministério da Saúde – MS) (grant number 929698560001160-02). Evandro Chagas Institute/Brazilian Ministry of Health (Instituto Evandro Chagas/Ministério da Saúde). Foundation of Research Support of the State of Goiás (Fundação de Amparo à Pesquisa do Estado de Goiás – FAPEG) (number grant 2017/10267000531). Foundation of Research Support of the State of Rio Grande do Sul (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul – FAPERGS) (grant number 17/2551-0000521-0). Foundation to Support Teaching, Research and Assistance at Hospital das Clínicas, Faculty of Medicine of Ribeirão Preto (Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto) and São Paulo State Department of Health (Secretaria de Saúde do Estado de São Paulo). Support Foundation of Pernambuco Science and Technology (Fundação de Amparo à Ciência e Tecnologia de Pernambuco – FACEPE) (grant numbers APQ-0172-4.01/16; APQ-0192-4.01/17; APQ-0793-4.01/17).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2022.100395.
Contributor Information
Ricardo Arraes de Alencar Ximenes, Email: ricardo.ximenes@ufpe.br.
Zika Brazilian Cohorts Consortium (ZBC-Consortium):
Mariana de Carvalho Leal, Maria de Fátima Pessoa Militão de Albuquerque, Jociele Malacarne, Luana Damasceno, Ana Maria Bispo de Filippis, Cleiton Silva Santos, Alan Oliveira Duarte, Pedro Fernando Vasconcelos, Mariana Borges Machado, Ana paula Antunes Pascalicchio Bertozzi, Thamirys Cosmo Gillo Fajardo, Adriana Aparecida Tiraboschi Bárbaro, Ligia Conceição Marçal Assef, Clarice Pimentel, Thalita Abreu, Jousilene de Sales Tavares, Fabiana de Oliveira Melo, Talita de Toledo Lima, Maria das Graças Nunes Brasil, Cláudia Pereira Terças-Trettel, Giovanny Vinícius de Araújo França, Camila Helena Aguiar Bôtto-Menezes, Silvana Gomes Benzecry, Jaqueline Rodrigues Robaina, and Mariana Barros Genuíno de Oliveira
Supplementary data
References
- 1.Brasil. Informe Epidemiológico no 32/2016 – Semana Epidemiológica 25/2016 (19/06/2016 A 25/06/2016) Monitoramento dos casos de microcefalia no Brasil [Internet]. Brasília. 2016. https://antigo.saude.gov.br/saude-de-a-z/microcefalia/informes-epidemiologicos Available from:
- 2.Brito C. Zika virus: a new chapter in the history of medicine. Acta Med Port. 2015;28(6):679–680. doi: 10.20344/amp.7341. [DOI] [PubMed] [Google Scholar]
- 3.Albuquerque M de FPM de, Souza WV de, Araújo T.V.B., et al. The microcephaly epidemic and Zika virus: building knowledge in epidemiology. Cad Saúde Pública. 2018;34(10) doi: 10.1590/0102-311X00069018. [DOI] [PubMed] [Google Scholar]
- 4.Campos G.S., Bandeira A.C., Sardi S.I. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanluca C., Melo V.C.A., Mosimann A.L.P., Dos Santos G.I.V., Dos Santos C.N.D.D., Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110(4):569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome [internet]. WHO. https://www.who.int/news/item/01-02-2016-who-director-general-summarizes-the-outcome-of-the-emergency-committee-regarding-clusters-of-microcephaly-and-guillain-barré-syndrome Available from:
- 7.Calvet G., Aguiar R.S., Melo A.S.O., et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16(6):653–660. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira Melo A., Malinger G., Ximenes R., Szejnfeld P., Sampaio S.A., Bispo de Filippis A. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 9.Driggers R.W., Ho C.-Y., Korhonen E.M., et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374(22):2142–2151. doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 10.Mlakar J., Korva M., Tul N., et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 11.Ramalho F.S., Yamamoto A.Y., da Silva L.L., et al. Congenital Zika virus infection induces severe spinal cord injury. Clin Infect Dis. 2017;65(4):687–690. doi: 10.1093/cid/cix374. [DOI] [PubMed] [Google Scholar]
- 12.Tang H., Hammack C., Ogden S.C., et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18(5):587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H., Saucedo-Cuevas L., Regla-Nava J.A., et al. Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell. 2016;19(5):593–598. doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Araújo T.V.B., Rodrigues L.C., Ximenes RA. de A., et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;16:1356–1363. doi: 10.1016/S1473-3099(16)30318-8. [DOI] [PubMed] [Google Scholar]
- 15.de Araújo T.V.B., Ximenes RA. de A., Miranda-Filho D. de B., et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis. 2018;18:328–336. doi: 10.1016/S1473-3099(17)30727-2. [DOI] [PubMed] [Google Scholar]
- 16.Microcephaly Epidemic Research Group Microcephaly in infants, Pernambuco state, Brazil, 2015. Emerg Infect Dis. 2016;22(6):1090–1093. doi: 10.3201/eid2206.160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miranda-Filho D.D.B., Martelli C.M.T., Ximenes R.A.D.A., et al. Initial description of the presumed congenital Zika syndrome. Public Health. 2016;106(4):598–600. doi: 10.2105/AJPH.2016.303115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Campo M., Feitosa I.M.L., Ribeiro E.M., et al. The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet A. 2017;173(4):841–857. doi: 10.1002/ajmg.a.38170. [DOI] [PubMed] [Google Scholar]
- 19.Honein M.A., Dawson A.L., Petersen E.E., et al. Birth defects among fetuses and infants of US women with evidence of possible zika virus infection during pregnancy. JAMA. 2017;317(1):59–68. doi: 10.1001/jama.2016.19006. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro-Mendoza C.K., Rice M.E., Galang R.R., et al. Pregnancy outcomes after maternal Zika virus infection during pregnancy — U.S. territories, January 1, 2016–April 25, 2017. Morb Mortal Wkly Rep. 2017;66(23):615–621. doi: 10.15585/mmwr.mm6623e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds M.R., Jones A.M., Petersen E.E., et al. Vital signs: update on Zika virus–associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure — U.S. Zika Pregnancy Registry, 2016. Morb Mortal Wkly Rep. 2017;66(13):373. doi: 10.15585/mmwr.mm6613e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoen B., Schaub B., Funk A.L., et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985–994. doi: 10.1056/NEJMoa1709481. [DOI] [PubMed] [Google Scholar]
- 23.Brasil P., Pereira Júnior J.P., Moreira M.E., et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomar L., Vouga M., Lambert V., et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: prospective cohort study in French Guiana. BMJ. 2018;363:k4431. doi: 10.1136/bmj.k4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nogueira M.L., Nery Júnior N.R.R., Estofolete C.F., et al. Adverse birth outcomes associated with Zika virus exposure during pregnancy in São José do Rio Preto, Brazil. Clin Microbiol Infect. 2018;24(6):646–652. doi: 10.1016/j.cmi.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Ximenes RA. de A., Miranda-Filho D. de B., Montarroyos U.R., et al. Zika-related adverse outcomes in a cohort of pregnant women with rash in Pernambuco, Brazil. PLoS Negl Trop Dis. 2021;15(3) doi: 10.1371/journal.pntd.0009216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coutinho C.M., Negrini S.F.B.M., Araujo D.C.A., et al. Early maternal Zika infection predicts severe neonatal neurological damage: results from the prospective natural history of Zika virus infection in gestation cohort study. BJOG. 2021;128(2):317–326. doi: 10.1111/1471-0528.16490. [DOI] [PubMed] [Google Scholar]
- 28.Jaenisch T., Rosenberger K., Brito C., Brady O., Brasil P., Marques E. Risk of microcephaly after Zika virus infection in Brazil, 2015 to 2016. Bull World Health Organ. 2017;95(3):191–198. doi: 10.2471/BLT.16.178608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuevas E.L., Tong V.T., Rozo N., et al. Preliminary report of microcephaly potentially associated with Zika virus infection during pregnancy - Colombia, January-November 2016. MMWR Morb Mortal Wkly Rep. 2016;65(49):1409–1413. doi: 10.15585/mmwr.mm6549e1. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira W.K., de França G.V.A., Carmo E.H., Duncan B.B., Kuchenbecker R. de S., Schmidt M.I. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet. 2017;390(10097):861–870. doi: 10.1016/S0140-6736(17)31368-5. [DOI] [PubMed] [Google Scholar]
- 31.Ades A.E., Brickley E.B., Alexander N., et al. Zika virus infection in pregnancy: a protocol for the joint analysis of the prospective cohort studies of the ZIKAlliance, ZikaPLAN and ZIKAction consortia. BMJ Open. 2020;10(12) doi: 10.1136/bmjopen-2019-035307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilder-Smith A., Wei Y., De Araújo T.V.B., et al. Understanding the relation between Zika virus infection during pregnancy and adverse fetal, infant and child outcomes: a protocol for a systematic review and individual participant data meta-analysis of longitudinal studies of pregnant women and their infants and children. BMJ Open. 2019;9(6) doi: 10.1136/bmjopen-2018-026092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alecrim M. das GC., de Amorim M.M.R., de Araújo T.V.B., et al. Zika Brazilian Cohorts (ZBC) Consortium: protocol for an individual participant data meta-analysis of congenital Zika syndrome after maternal exposure during pregnancy. Viruses. 2021;13(4):687. doi: 10.3390/v13040687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dine M.S., Gartside P.S., Glueck C.J., Rheins L., Greene G., Khoury P. Relationship of head circumference to length in the first 400 days of life: a mnemonic. Pediatrics. 1981;67(4):506–508. [PubMed] [Google Scholar]
- 35.Villar J., Ismail L.C., Victora C.G., et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet. 2014;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 36.Villar J., Giuliani F., Bhutta Z.A., et al. Postnatal growth standards for preterm infants: the preterm postnatal follow-up study of the INTERGROWTH-21st project. Lancet Glob Health. 2015;3(11):e681–e691. doi: 10.1016/S2214-109X(15)00163-1. [DOI] [PubMed] [Google Scholar]
- 37.WHO . World Health Organization; Geneva: 2007. Child Growth Standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development; p. 237. [Google Scholar]
- 38.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):1–10. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen-Saines K., Brasil P., Kerin T., et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med. 2019;25(8):1213–1217. doi: 10.1038/s41591-019-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van der Linden V., Pessoa A., Dobyns W., et al. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth — Brazil. MMWR Morb Mortal Wkly Rep. 2016;65(47):1343–1348. doi: 10.15585/mmwr.mm6547e2. [DOI] [PubMed] [Google Scholar]
- 41.Cavalcante TB, Ribeiro MRC, Sousa P da S, et al Congenital Zika syndrome: growth, clinical, and motor development outcomes up to 36 months of age and differences according to microcephaly at birth. Int J Infect Dis. 105:309–408 [DOI] [PubMed]
- 42.Zin A.A., Tsui I., Rossetto J., et al. Screening criteria for ophthalmic manifestations of congenital Zika virus infection. JAMA Pediatr. 2017;171(9):847–854. doi: 10.1001/jamapediatrics.2017.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ventura C.V., Maia M., Ventura B.V., et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol. 2016;79(1):1–3. doi: 10.5935/0004-2749.20160002. [DOI] [PubMed] [Google Scholar]
- 44.Adachi K., Romero T., Nielsen-Saines K., et al. Early clinical infancy outcomes for microcephaly and/or small for gestational age Zika-exposed infants. Clin Infect Dis. 2020;70(12):2672. doi: 10.1093/cid/ciz704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costello A., Dua T., Duran P., et al. Defining the syndrome associated with congenital Zika virus infection. Bull World Health Organ. 2016;94 doi: 10.2471/BLT.16.176990. 406–406A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsui I., Moreira M.E.L., Rossetto J.D., et al. Eye findings in infants with suspected or confirmed antenatal Zika virus exposure. Pediatrics. 2018;142(4) doi: 10.1542/peds.2018-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calle-Giraldo J.P., Rojas C.A., Hurtado I.C., et al. Outcomes of congenital Zika virus infection during an outbreak in Valle del Cauca, Colombia. Pediatr Infect Dis J. 2019;38(7):735–740. doi: 10.1097/INF.0000000000002307. [DOI] [PubMed] [Google Scholar]
- 48.Rice M.E., Galang R.R., Roth N.M., et al. Vital Signs: zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital zika virus infection — U.S. territories and freely associated states, 2018. Morb Mortal Wkly Rep. 2018;67(31):858–867. doi: 10.15585/mmwr.mm6731e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leal M.C., Muniz L.F., Ferreira T.S.A., et al. Hearing loss in infants with microcephaly and evidence of congenital Zika virus infection — Brazil, November 2015–May 2016. Morb Mortal Wkly Rep. 2016;65(34):917–919. doi: 10.15585/mmwr.mm6534e3. [DOI] [PubMed] [Google Scholar]
- 50.Power G.M., Vaughan A.M., Qiao L., et al. Socioeconomic risk markers of arthropod-borne virus (arbovirus) infections: a systematic literature review and meta-analysis. BMJ Glob Health. 2022;7(4) doi: 10.1136/bmjgh-2021-007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lobkowicz L., Power G.M., Souza W.V., et al. Neighbourhood-level income and Zika virus infection during pregnancy in Recife, Pernambuco, Brazil: an ecological perspective, 2015–2017. BMJ Glob Health. 2021;6(12) doi: 10.1136/bmjgh-2021-006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.