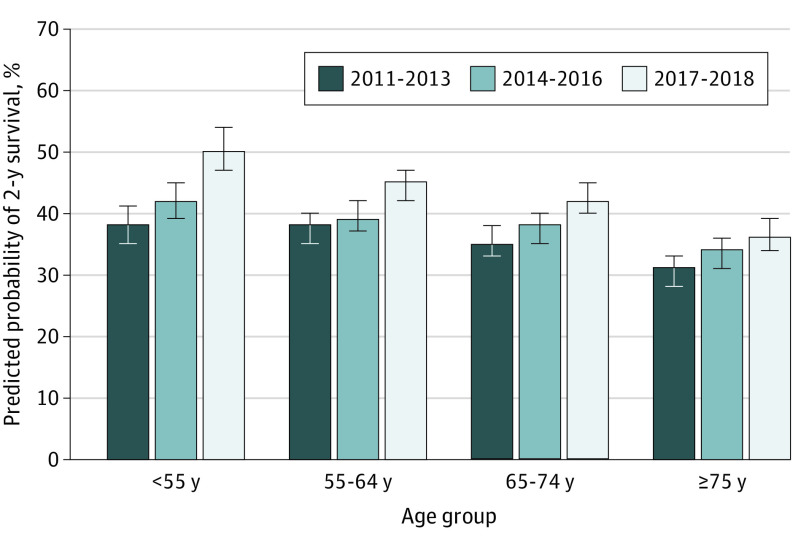This cohort study evaluates trends in survival after immune checkpoint inhibitor therapy in patients with advanced non–small cell lung cancer by age.
Key Points
Question
Has the introduction of immune checkpoint inhibitors (ICIs) changed survival in younger vs older patients with non–small cell lung cancer (NSCLC)?
Findings
In this cohort study of 53 719 adults with NSCLC, ICIs disseminated rapidly into clinical practice across all age groups after US Food and Drug Administration approval. However, there were differential implications for survival: patients younger than 55 years experienced clinically meaningful survival gains, whereas patients 75 years or older did not.
Meaning
Survival gains from novel cancer therapeutics may not be generalizable to older adults, who are often poorly represented in clinical trials.
Abstract
Importance
The introduction of immune checkpoint inhibitors (ICIs) has transformed the care of advanced non–small cell lung cancer (NSCLC). Although clinical trials suggest substantial survival benefits, it is unclear how outcomes have changed in clinical practice.
Objective
To assess temporal trends in ICI use and survival among patients with advanced NSCLC across age strata.
Design, Setting, and Participants
This cohort study was performed in approximately 280 predominantly community-based US cancer clinics and included patients aged 18 years or older who had stage IIIB, IIIC, or IV NSCLC diagnosed between January 1, 2011, and December 31, 2019, with follow-up through December 31, 2020. Data were analyzed April 1, 2021, to October 19, 2022.
Main Outcomes and Measures
Median overall survival and 2-year survival probability. The predicted probability of 2-year survival was calculated using a mixed-effects logit model adjusting for demographic and clinical characteristics.
Results
The study sample included 53 719 patients (mean [SD] age, 68.5 [9.3] years; 28 374 men [52.8%]), the majority of whom were White individuals (36 316 [67.6%]). The overall receipt of cancer-directed therapy increased from 69.0% in 2011 to 77.2% in 2019. After the first US Food and Drug Administration approval of an ICI for NSCLC, the use of ICIs increased from 4.7% in 2015 to 45.6% in 2019 (P < .001). Use of ICIs in 2019 was similar between the youngest and oldest patients (aged <55 years, 45.2% vs aged ≥75 years, 43.8%; P = .59). From 2011 to 2018, the predicted probability of 2-year survival increased from 37.7% to 50.3% among patients younger than 55 years and from 30.6% to 36.2% in patients 75 years or older (P < .001). Similarly, median survival in patients younger than 55 years increased from 11.5 months to 16.0 months during the study period, while survival among patients 75 years or older increased from 9.1 months in 2011 to 10.2 months in 2019.
Conclusions and Relevance
This cohort study found that, among patients with advanced NSCLC, the uptake of ICIs after US Food and Drug Administration approval was rapid across all age groups. However, corresponding survival gains were modest, particularly in the oldest patients.
Introduction
Lung cancer places an enormous burden on the population as the leading cause of death from cancer.1 However, the lung cancer death rate in the US has decreased by one-third over the past 2 decades,2 a trend that has largely been attributed to reductions in tobacco use.3 In the past decade, there has also been an increase in the number of treatment options for non–small cell lung cancer (NSCLC), which represents most lung cancer diagnoses.4 Since 2015, there have been over 20 new US Food and Drug Administration (FDA) approvals for NSCLC treatments, with nivolumab being approved for second-line treatment of squamous NSCLC in March 2015 and pembrolizumab being approved for first-line use in October 2016.5,6 Several new immune checkpoint inhibitors (ICIs) substantially prolong survival after a diagnosis of advanced-stage NSCLC in the clinical trial setting.7,8 Accordingly, use of newly approved treatments has been rapid—1 study found that anti–programmed cell death 1 agents were being used in most eligible patients within months after approval.9
Although these newer treatments have had comparable effectiveness between younger and older patients in clinical trials,10,11 such results may not be reflective of those in the community setting given that these patients tend to differ from those who participate in clinical trials.9,12,13,14,15 For example, 1 study of pembrolizumab as an initial treatment in patients with stage IV NSCLC found that median overall survival among patients in the nonclinical trial setting was about 16 months compared with 30 months among those in a clinical trial.16
Despite uncertainty regarding the clinical effectiveness of novel therapeutics, several studies have pointed to adoption of such therapeutics as a potential key factor in the decrease in lung cancer mortality.3,17,18,19 Notably, a recent study using Surveillance, Epidemiology, and End Results data suggested that the implementation of targeted therapies played a substantial role in improvements in lung cancer survival.20 Conversely, another study asserted that decreased lung cancer mortality may be due to earlier stage at diagnosis rather than adoption of new therapies.21 It remains unclear whether improvement in survival after a diagnosis of advanced NSCLC is similar across age groups.
Evaluating differences in patient outcomes associated with a new treatment requires both a quantitative (“What is the survival benefit?”) and a qualitative (“Is the benefit clinically meaningful?”) assessment. Prominent organizations have issued guidance regarding the latter concern. The American Society of Clinical Oncology (ASCO) Cancer Research Committee convened working groups to define a clinically meaningful benefit for trials in 4 cancer types.22 All 4 groups selected overall survival as the primary outcome. The lung cancer group defined a meaningful improvement in survival as more than 3.25 months for patients with nonsquamous and more than 2.5 months for patients with squamous histological features. Similarly, the European Society for Medical Oncology (ESMO) published the Magnitude of Clinical Benefit Scale.23 For conditions with a baseline survival of less than 1 year, an increase in survival of 3 months or more was deemed a substantial benefit.
The aim of the study was to assess the implications of the adoption of ICIs for lung cancer care by evaluating treatment patterns and survival trends across age groups, with an emphasis on comparing the outcomes of patients diagnosed before and after ICIs became widely available. We used the ASCO and ESMO clinical benefit frameworks to guide our interpretation of whether changes in survival were clinically meaningful. In a secondary analysis, we evaluated patterns of ICI use with and without concomitant chemotherapy because evidence has suggested superiority of ICIs in combination with chemotherapy in some settings.24,25,26,27,28
Methods
Study Design and Data Sources
We conducted a retrospective, multicenter cohort study of patients with advanced (stage IIIB, IIIC, or IV) NSCLC diagnosed between January 1, 2011, and December 31, 2019, with follow-up through December 31, 2020. Our primary objective was to compare the outcomes of patients in the time periods before and after the initial FDA approval of ICIs in 2015 to assess the degree to which the change in clinical practice was associated with improved outcomes. The Yale University Institutional Review Board deemed this study exempt from review because it was considered to be non–human participant research. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
We used the nationwide Flatiron Health electronic health record–derived deidentified database. The Flatiron Health database is a longitudinal database that includes deidentified, patient-level structured and unstructured data curated via technology-enabled abstraction.29,30 During the study period, the data originated from approximately 280 cancer clinics (approximately 800 sites of care, with approximately 80% of patients coming from community oncology practices and approximately 20% from academic centers). Practices contributing data to this database are self-selected but have patients with age, sex, and race and ethnicity similar to patients with advanced NSCLC in the US, as estimated in Surveillance, Epidemiology, and End Results data from 2014.31
Cohort Selection and Construction of Variables
Patients included in the study cohort were 18 years or older with a diagnosis of advanced primary NSCLC (International Classification of Diseases, Ninth Revision diagnosis code 162.x; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes C34x or C39.9) diagnosed from 2011 to 2019 (eFigure in Supplement 1). Patients needed to have at least 2 documented clinical visits on or after January 1, 2011. Patients were required to have evidence of structured activity within the Flatiron Health network within 90 days of diagnosis of advanced NSCLC to ensure exclusion of patients who were potentially receiving treatment outside the network. Patients were excluded if they had multiple primary cancers, were missing data regarding diagnosis, or had received treatment in the context of a clinical study. We divided the sample into 3 time periods: before ICI (2011-2013), early ICI (2014-2016), and after ICI (2017-2019) FDA approval. Age was stratified into groupings of patients younger than 55 years, 55 to 64 years, 65 to 74 years, and 75 years or older. Patient race and ethnicity was included as a covariate because patient race may be associated with treatment receipt as well as outcomes, such as survival. Race and ethnicity were considered a social, rather than biological, construct. This information was collected from the electronic health record and is usually based on patient self-report on intake forms, although this varies by practice. Clinical group stage at the time of initial diagnosis was collapsed into stages I, II, III, and IV. Treatment status was ascertained according to whether a patient received a cancer-directed treatment at least once after diagnosis of advanced NSCLC. This treatment included ICIs, tyrosine kinase inhibitors, or traditional doublet or single-agent chemotherapy provided as the initial treatment regimen after diagnosis of advanced cancer, including if it was used in the off-label setting.
Statistical Analysis
Data were analyzed April 1, 2021, to October 19, 2022. The percentage of patients receiving a given class of treatment each year was calculated by age group. To study survival over time, unadjusted estimates of overall and median survival were generated using Kaplan-Meier curves and plotted by year of advanced diagnosis. Survival was calculated from the date of advanced diagnosis until death, end of observed activity in the Flatiron Health database, or end of the study period (December 31, 2019). We used log-rank tests to compare survival across cohorts of patients defined by time period (ie, before ICIs vs after ICIs). Additionally, we calculated the predicted probability of 2-year survival using a mixed-effects logit model for patients diagnosed in each of the time periods among patients diagnosed through 2018 (for whom 2 years of follow-up data were available), adjusting for demographic and clinical characteristics (sex, race and ethnicity, smoking status, geographic region, stage at initial diagnosis, insurance type, and histological subtype) and including an interaction term between time period and age stratum. The predicted probabilities were calculated over strata of age group and time period, keeping other covariates at their actual values. Cox proportional hazards regression analysis was not performed due to violation of the proportional hazards assumption on several variables, most importantly age at advanced diagnosis and year of advanced NSCLC diagnosis. In addition, we analyzed use of ICIs with or without chemotherapy across age strata among patients diagnosed in the most recent time period and assessed the predicted probability of survival for each age group using the previously described approach. All tests were 2-sided with a significance level of α = .05. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc) and Stata, version 17 (StataCorp LLC).
Results
The study sample consisted of 53 719 patients (mean [SD] age, 68.5 [9.3] years; 28 374 men [52.8%] and 25 345 women [47.2%]), the majority of whom were White individuals (36 316 [67.6%]) (Table). At initial diagnosis, 61.9% of patients were diagnosed with stage IV disease. Overall, 39 272 patients (73.1%) received treatment. The percentage of patients receiving treatment increased from 69.0% in 2011 to 77.2% in 2019 (P for trend <.001; eTable 1 in Supplement 1). Since the initial FDA approval of an ICI for NSCLC in 2015, the receipt of ICIs as an initial treatment increased from 4.7% in 2015 to 45.6% in 2019 (χ2 P < .001) (eTable 2 in Supplement 1). Conversely, the use of traditional chemotherapy as an initial treatment declined from 60.2% in 2011 to 22.8% in 2019 (P for trend <.001). Receipt of tyrosine kinase inhibitors was relatively stable, ranging from 8.6% in 2011 to 8.9% in 2019 (P = .04).
Table. Baseline Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| All (n = 53 719) | Treated (n = 39 272) | |
| Sex | ||
| Female | 25 345 (47.2) | 18 526 (47.2) |
| Male | 28 374 (52.8) | 20 746 (52.8) |
| Age, mean (SD), y | 68.5 (9.3) | 67.9 (9.3) |
| Age at advanced NSCLC diagnosis | ||
| <55 y | 4436 (8.3) | 3527 (9.0) |
| 55-64 y | 12 452 (23.2) | 9658 (24.6) |
| 65-74 y | 19 050 (35.5) | 14 243 (36.3) |
| ≥75 y | 17 781 (33.1) | 11 844 (30.2) |
| Race and ethnicity | ||
| Asian | 1285 (2.4) | 1021 (2.6) |
| Black or African American | 4642 (8.6) | 3447 (8.8) |
| Hispanic or Latino | 1748 (3.3) | 1352 (3.4) |
| White | 36 316 (67.6) | 26 727 (68.1) |
| Othera | 4212 (7.8) | 3121 (7.9) |
| Missing | 5516 (10.3) | 3604 (9.2) |
| Stage at initial diagnosis | ||
| I | 2761 (5.1) | 1788 (4.6) |
| II | 2816 (5.2) | 1881 (4.8) |
| III | 11 145 (20.7) | 8544 (21.8) |
| IV | 33 253 (61.9) | 24 680 (62.8) |
| Unknown | 3744 (7.0) | 2379 (6.1) |
| Smoking status | ||
| History of smoking | ||
| Yes | 46 991 (87.5) | 34 055 (86.7) |
| No | 6146 (11.4) | 4895 (12.5) |
| Unknown | 582 (1.1) | 322 (0.8) |
| Geographic region | ||
| Northeast | 9636 (17.9) | 7200 (18.3) |
| South | 21 096 (39.3) | 15 676 (39.9) |
| Midwest | 7993 (14.9) | 5895 (15.0) |
| West | 8040 (15.0) | 6096 (15.5) |
| Missing | 6954 (12.9) | 4405 (11.2) |
| Insurance type | ||
| Medicare | 38 184 (71.1) | 27 034 (68.8) |
| Commercial | 5976 (11.1) | 4792 (12.2) |
| Other, including Medicaidb | 3085 (5.7) | 2382 (6.1) |
| Uninsured or unknown | 6474 (12.1) | 5064 (12.9) |
| Histological subtype | ||
| Squamous cell carcinoma | 13 938 (26.0) | 9991 (25.4) |
| Nonsquamous cell carcinomac | 36 881 (68.7) | 27 343 (69.6) |
| Not otherwise specified | 2900 (5.4) | 1938 (4.9) |
| First-line treatment regimen | ||
| No treatment | 14 447 (26.9) | 0 |
| Immune checkpoint | ||
| Inhibitor aloned | 4449 (8.3) | 4449 (11.3) |
| Inhibitor with chemotherapye | 4258 (7.9) | 4258 (10.8) |
| Tyrosine kinase inhibitorf | 4490 (8.4) | 4490 (11.4) |
| Other treatmentg | 26 075 (48.5) | 26 075 (66.4) |
Abbreviation: NSCLC, non–small cell lung cancer.
Other races and ethnicities include American Indian or Alaska Native, Hawaiian or Pacific Islander, and individuals of multiple races.
Other insurance types include Medicaid, other government program, other payer (unknown type), patient assistance program, and self-pay.
Examples include adenocarcinoma and large cell carcinoma.
Immune checkpoint inhibitors include pembrolizumab, nivolumab, atezolizumab, durvalumab, ipilimumab, and cemiplimab.
Also includes a small number of patients who received immune checkpoint inhibitors with tyrosine kinase inhibitors.
Tyrosine kinase inhibitors include osimertinib, erlotinib, afatinib, gefitinib, dacomitinib, mobocertinib, lazertinib, icotinib, sotarasib, adagrasib, alectinib, brigatinib, lorlatinib, ceritinib, crizotinib, entrectinib, dabrafenib, trametinib, vemurafenib, encorafenib, tepotinib, capmatinib, pralsetinib, selpercatinib, and larotrectinib.
Other treatments include chemotherapy including carboplatin, paclitaxel, and cisplatin.
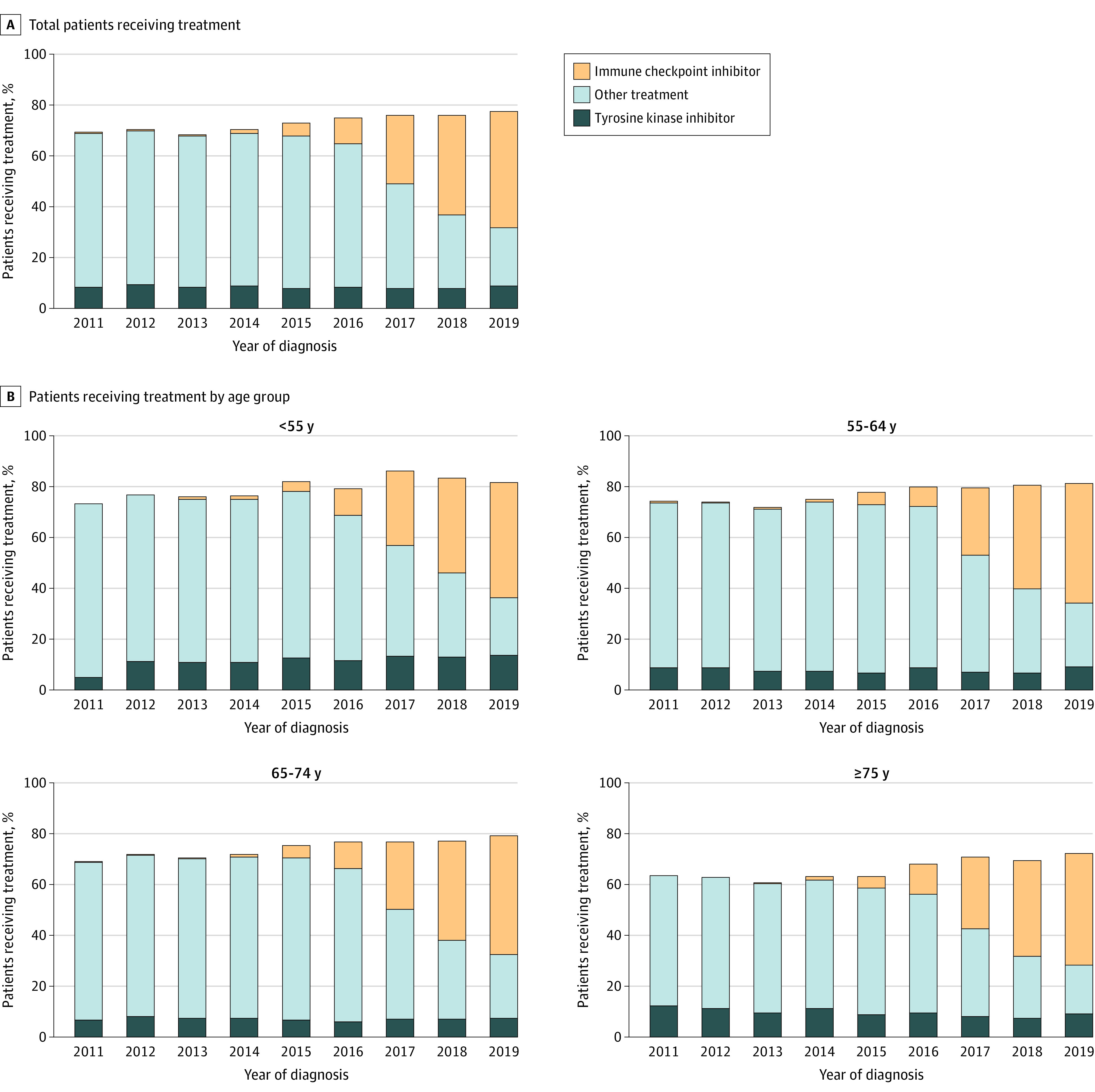
Trends in treatment receipt were similar across age groups (Figure 1). The overall treatment rate increased from 73.2% to 81.5% in patients younger than 55 years and from 63.4% to 72.3% among patients 75 years or older. Similarly, the receipt of ICIs as an initial treatment was consistent across age groups by the end of the study period, ranging from 43.8% to 46.7% of patients. Use of ICIs in 2019 was similar between the youngest and oldest patients (aged <55 years, 45.2% vs aged ≥75 years, 43.8%; P = .59).
Figure 1. Patients Receiving Treatment by Year of Diagnosis and by Age Group .
Median overall survival showed little variability from 2011 to 2015 (Figure 2A) across all age groups before ICI approval. In 2011, median survival in patients younger than 55 years was 11.5 months (95% CI, 9.1-13.0 months). By 2019, 4 years after the first approval of an ICI, median survival increased to 16.0 months (95% CI, 13.6-19.0 months) in this age group (log-rank P = .001). Among patients aged 55 to 64 years, survival increased from 12.9 months (95% CI, 11.1-13.9 months) in 2011 to 15.0 months (95% CI, 13.7-17.0 months; P = .02) in 2019. In patients aged 65 to 74 years, survival increased from 11.2 months (95% CI, 10.3-12.2 months) in 2011 to 13.3 months (95% CI, 9.6-11.0 months; P < .001) in 2019. By comparison, in patients 75 years or older, survival increased from 9.1 months (95% CI, 8.4-9.9 months) in 2011 to 10.2 months (95% CI, 9.6-11.0 months; P = .007) in 2019. In treated patients younger than 55 years, an increase in median overall survival from 14.8 months (95% CI, 13.0-16.6 months) to 19.0 months (lower 95% CI, 16.7 months; upper 95% CI, not estimated due to insufficient number of events) was observed from 2015 to 2019 (log-rank P = .01) (Figure 2B). However, among treated patients 75 years or older, median overall survival changed modestly from 12.3 (95% CI, 11.1-13.3) months in 2015 to 13.0 (95% CI, 12.0-13.9) months in 2019 (log-rank P = .02).
Figure 2. Median Overall Survival by Year of Diagnosis and Age Group.
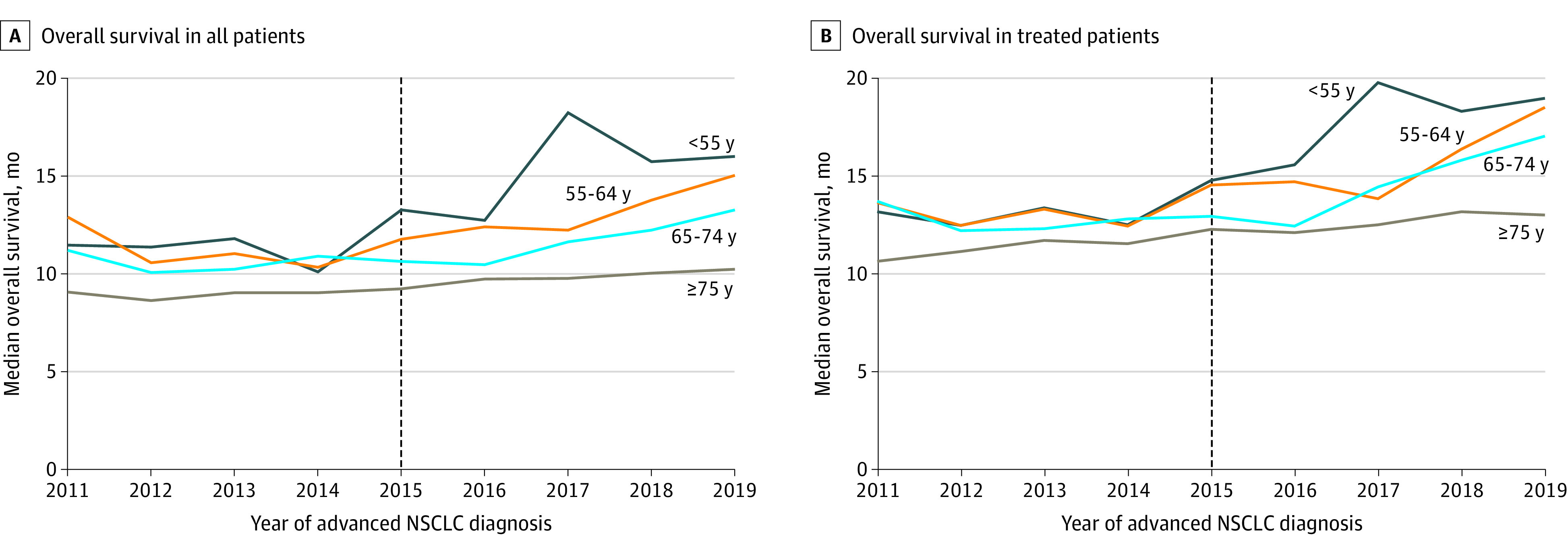
The vertical line at 2015 denotes the first approval of an immune checkpoint inhibitor by the US Food and Drug Administration. NSCLC indicates non–small cell lung cancer.
The predicted probability of 2-year survival in the youngest age group increased from 37.7% (95% CI, 34.3%-41.0%) in 2011 to 2013 to 50.3% (95% CI, 46.6%-53.9%) in 2017 to 2018 (Figure 3), a statistically significant improvement of 12.6% (95% CI, 8.8%-16.4%; P < .001). In contrast, patients 75 years or older saw a modest improvement in predicted probability of 2-year survival of 5.6% (95% CI, 3.7%-7.4%) from 30.6% (95% CI, 28.3%-33.0%) in 2011 to 2013 to 36.2% (95% CI, 33.7%-38.6%; P < .001) in 2017 to 2018 (Figure 3). This difference reflects a 7.1% higher improvement in survival for patients younger than 55 years compared with those 75 years or older (P = .001). Similarly, the formal test of interaction between year of advanced diagnosis and age group was statistically significant (P for interaction <.001). Unadjusted and adjusted associations between patient characteristics and 2-year survival are shown in eTable 3 in Supplement 1.
Figure 3. Predicted Probability of 2-Year Survival by Year of Diagnosis and Age Group .
Probability was adjusted for sex, race and ethnicity, smoking status, geographic region, stage at initial diagnosis, insurance type, and histological subtype. Error bars represent 95% CIs.
We then analyzed the use of ICI therapy with and without concomitant chemotherapy in 2017 and 2018 as a function of age (Figure 4). Treatment with an ICI alone was more common with older age, ranging from 12.7% in patients younger than 55 years to 21.4% in patients 75 years or older (P for trend <.001). Conversely, use of ICIs with concomitant chemotherapy decreased with increasing age from 20.4% in patients younger than 55 years to 11.5% in patients 75 years or older (P for trend <.001). In each of these treatment strata, age was inversely related to survival. For instance, among patients receiving an ICI alone, predicted probability of 2-year survival ranged from 51.8% (95% CI, 48.1%-55.5%) in patients younger than 55 years to 36.2% (95% CI, 33.7%-38.6%) in patients 75 years or older (P < .001 for difference). Similarly, among those treated with an ICI with concomitant chemotherapy, predicted probability of 2-year survival was 48.0% (95% CI, 44.4%-51.7%) in patients younger than 55 years compared with 34.0% (95% CI, 31.6%-36.4%) in patients 75 years or older (P < .001 for difference).
Figure 4. Treatment Patterns and Predicted Probability of 2-Year Survival by Age Group.
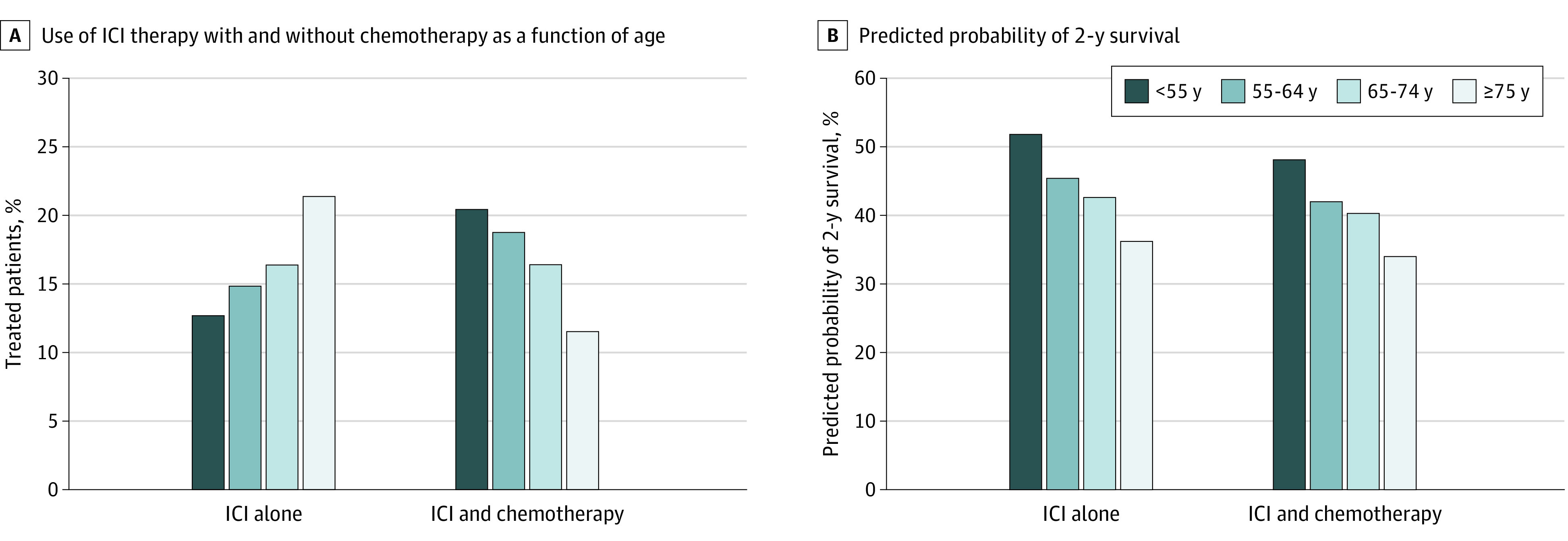
The patients included were receiving an immune checkpoint inhibitor (ICI) in 2017 and 2018.
Discussion
In this large cohort of patients with advanced NSCLC, we found that the population-level increases in survival after ICI implementation varied substantially by age despite similarly rapid and substantial uptake of ICIs across all ages. Among younger patients, median survival increased on the order of 4 to 5 months. This change meets the ASCO and ESMO threshold for a clinically meaningful benefit, suggesting that the adoption of ICIs has been associated with meaningful benefit for younger patients with NSCLC. Conversely, survival improvements were less impressive in the oldest age group, with survival improving by approximately 1 month, failing to meet either ASCO or ESMO criteria for clinically meaningful survival benefit. Given that nearly half of newly diagnosed older patients were receiving ICIs by the end of the study period, this suggests that the substantial change in NSCLC treatment patterns has had minimal implications for the survival of older patients. However, the implications for cancer-related symptoms and quality of life (QOL) were not evaluated in the present study.
As the median age at diagnosis of lung cancer is 70 years, examining outcomes in this older patient population is crucial to understanding the true impact of new treatments. The initial single-agent ICI trials27,32,33 evaluating atezolizumab, pembrolizumab, and nivolumab vs standard of care did not examine outcomes specifically among patients older than 75 years. However, current guidelines are not age specific but stage specific. The present study shows that the oldest patients did not achieve similar survival gains compared with patients in these clinical trials, highlighting the continued need to improve older adult accrual in cancer clinical trials.
Although the adoption of new therapies for lung cancer confers survival benefit in younger patients, it also raises challenges. Treatment with ICIs can be associated with substantial financial burden. Most patients with NSCLC are eligible for Medicare, and almost all have Medicare Part A and B coverage.34 In 2019, nivolumab (Opdivo) and pembrolizumab (Keytruda) were the 2 top drugs in terms of Medicare Part B spending. However, patients were also responsible for cost-sharing liability in excess of $9000 for these medications.35 Unfortunately, the harms of high drug costs on QOL, termed financial toxicity, can lead to substantial distress, gaps in treatment, and discontinuation of cancer therapy altogether.36,37 Although prior studies have suggested that ICIs may be cost-effective, such analyses have used survival from trial data to establish effectiveness. In older adults in the present study, the ASCO and ESMO criteria for effectiveness were not met; the implication is that in this population, the cost-effectiveness of ICIs may be overestimated. However, we have not examined other meaningful outcomes, including treatment burden, QOL, and symptom severity, that may be relevant in this population.38,39
The current National Comprehensive Cancer Network guidelines recommend use of ICIs with concomitant chemotherapy for patients with advanced NSCLC without actionable mutations and 1% to 49% programmed cell death 1 ligand 1 (PD-L1) expression.40 Findings of the present study suggest that actual practice varies by age in that older patients were less likely to receive concomitant therapy. This finding may be a reflection of the better tolerability of ICIs compared with chemotherapy, especially in older patients, as the likelihood of toxic side effects with chemotherapy is higher.41 Our results also suggest that, in clinical practice, patient outcomes may support these age-related differences in use of ICIs. However, the differential use of ICIs with concomitant chemotherapy across age groups is unlikely to account for age-related survival differences, as there was an inverse association between age and survival among those receiving ICIs regardless of whether they received concomitant chemotherapy or ICIs alone.
Limitations
This study has a number of limitations. First, important factors such as treatment outside the Flatiron Health database, baseline performance status, comorbidity, and cause of death could not be reliably captured in the Flatiron Health data. Second, the Flatiron Health data are drawn largely from community-based oncology clinics and thus may not be generalizable to academic centers.29,42 Third, the reason 1 treatment regimen was selected over another could not be discerned from the data. Unmeasured confounding is a common limitation of observational studies. Our focus on comparing outcomes across 2 time periods—before and after widespread adoption of ICIs—mitigates concerns about patient-level confounding, as patient characteristics, such as comorbidity or performance status, are unlikely to change over such a short period.43 Fourth, it is important to note that approximately 50% of cases of advanced NSCLC express more than 1% PD-L1, and PD-L1 expression is associated with greater benefit from ICIs.44,45,46 Regular testing of PD-L1 expression started only partway through the study period and was therefore not included as a variable here. Although more than half of the patients in our sample were receiving ICIs by the end of the study period, it is possible that better alignment of patients with PD-L1 expression greater than 1% and those receiving ICIs would confer greater benefit. Furthermore, the different adverse effect profile of ICIs may also offer benefit to the older population regardless of the less substantial survival differences.
Conclusions
The present study builds on prior work in important ways.47,48 We found a marked disconnect across age groups between the rate of adoption of ICIs and the clinical impact of this change. Several implications can be generated from these findings. First, deliberate efforts must be made to include the older population in future clinical trials. Second, future work should incorporate a comprehensive geriatric assessment as part of standard practice before initiating treatment and collect patient-reported outcomes afterward.49 This can assist in making treatment decisions more patient centered and substantially decrease treatment-related toxic effects.50 Our study focused on survival; however, it is also critical to assess temporal trends in QOL, including physical and financial burden. In addition, the static pricing of cancer medications across ages, despite substantial variation in effectiveness by age group, means Medicare receives far less value with respect to survival benefit for its cancer treatment spending than it could under a value-based care model. In this case, Medicare is likely paying for therapies that do not meet criteria for clinically meaningful survival benefits, while other outcomes remain unexplored. In aggregate, the findings of the present study underscore the importance of generating evidence that can inform decision-making for older patients, their physicians, and payers who are increasingly calling for value-based pricing.
eTable 1. Baseline Characteristics Stratified by Age at Advanced Diagnosis and Year of Advanced Diagnosis
eTable 2. Initial Treatment Class by Year of Advanced Diagnosis Among All Treated Patients (% by Year)
eTable 3. Unadjusted and Adjusted Association Between Patient Characteristics and Two-Year Survival
eFigure. Cohort Formation
Data Sharing Statement
References
- 1.American Cancer Society . Cancer facts & figures: 2019. 2019. Accessed September 8, 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf
- 2.Centers for Disease Control and Prevention . U.S. cancer statistics data visualizations tool. Reviewed October 20, 2022. Accessed July 27, 2022. https://www.cdc.gov/cancer/uscs/dataviz/index.htm
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975-2017. National Cancer Institute Surveillance, Epidemiology, and End Results Program. April 15, 2020. Accessed September 8, 2020. https://seer.cancer.gov/archive/csr/1975_2017/
- 5.U.S. Food and Drug Administration . FDA approves atezolizumab as adjuvant treatment for non-small cell lung cancer. Updated October 15, 2021. Accessed July 27, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-adjuvant-treatment-non-small-cell-lung-cancer
- 6.Lung Cancer Research Foundation . FDA approvals in lung cancer treatment. Updated May 2022. Accessed July 27, 2022. https://www.lungcancerresearchfoundation.org/research/why-research/treatment-advances/
- 7.Vickers AD, Winfree KB, Cuyun Carter G, et al. Relative efficacy of interventions in the treatment of second-line non-small cell lung cancer: a systematic review and network meta-analysis. BMC Cancer. 2019;19(1):353. doi: 10.1186/s12885-019-5569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vestergaard HH, Christensen MR, Lassen UN. A systematic review of targeted agents for non-small cell lung cancer. Acta Oncol. 2018;57(2):176-186. doi: 10.1080/0284186X.2017.1404634 [DOI] [PubMed] [Google Scholar]
- 9.O’Connor JM, Fessele KL, Steiner J, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4(8):e180798. doi: 10.1001/jamaoncol.2018.0798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elias R, Giobbie-Hurder A, McCleary NJ, Ott P, Hodi FS, Rahma O. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer. 2018;6(1):26. doi: 10.1186/s40425-018-0336-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasherman L, Siu DHW, Lee KWC, et al. Efficacy of immune checkpoint inhibitors in older adults with advanced stage cancers: a meta-analysis. J Geriatr Oncol. 2020;11(3):508-514. doi: 10.1016/j.jgo.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 12.Green AK, Curry M, Trivedi N, Bach PB, Mailankody S. Assessment of outcomes associated with the use of newly approved oncology drugs in Medicare beneficiaries. JAMA Netw Open. 2021;4(2):e210030. doi: 10.1001/jamanetworkopen.2021.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnog JB, Samson MJ, Gans ROB, Duits AJ. An urgent call to raise the bar in oncology. Br J Cancer. 2021;125(11):1477-1485. doi: 10.1038/s41416-021-01495-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kehl KL, Greenwald S, Chamoun NG, Manberg PJ, Schrag D. Association between first-line immune checkpoint inhibition and survival for Medicare-insured patients with advanced non-small cell lung cancer. JAMA Netw Open. 2021;4(5):e2111113. doi: 10.1001/jamanetworkopen.2021.11113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyle JM, Hegarty G, Frampton C, et al. Real-world outcomes associated with new cancer medicines approved by the Food and Drug Administration and European Medicines Agency: a retrospective cohort study. Eur J Cancer. 2021;155:136-144. doi: 10.1016/j.ejca.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer-van der Welle CM, Verschueren MV, Tonn M, et al. ; Santeon NSCLC Study Group . Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci Rep. 2021;11(1):6306. doi: 10.1038/s41598-021-85696-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramagopalan S, Leahy TP, Ray J, Wilkinson S, Sammon CJ, Subbiah V. Association between improvements in survival of metastatic NSCLC patients and targeted- and immuno-therapy. J Clin Oncol. 2021;39(suppl 15):9090. doi: 10.1200/JCO.2021.39.15_suppl.9090 [DOI] [Google Scholar]
- 18.Qu J, Mei Q, Chen L, Zhou J. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives. Cancer Immunol Immunother. 2021;70(3):619-631. doi: 10.1007/s00262-020-02735-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction—evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18(3):135-151. doi: 10.1038/s41571-020-00432-6 [DOI] [PubMed] [Google Scholar]
- 20.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649. doi: 10.1056/NEJMoa1916623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flores R, Patel P, Alpert N, Pyenson B, Taioli E. Association of stage shift and population mortality among patients with non-small cell lung cancer. JAMA Netw Open. 2021;4(12):e2137508. doi: 10.1001/jamanetworkopen.2021.37508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis LM, Bernstein DS, Voest EE, et al. American Society of Clinical Oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol. 2014;32(12):1277-1280. doi: 10.1200/JCO.2013.53.8009 [DOI] [PubMed] [Google Scholar]
- 23.Cherny NI, Dafni U, Bogaerts J, et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28(10):2340-2366. doi: 10.1093/annonc/mdx310 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Han H, Zhang F, et al. Immune checkpoint inhibitors alone vs immune checkpoint inhibitors—combined chemotherapy for NSCLC patients with high PD-L1 expression: a network meta-analysis. Br J Cancer. 2022;127(5):948-956. doi: 10.1038/s41416-022-01832-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mok TSK, Wu YL, Kudaba I, et al. ; KEYNOTE-042 Investigators . Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi: 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 26.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase iii trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924-3933. doi: 10.1200/JCO.2017.74.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 29.Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. Preprint posted online May 30, 2020. doi: 10.1101/2020.03.16.20037143 [DOI]
- 30.Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv. Preprint posted online January 13, 2020. doi: 10.48550/arXiv.2001.09765 [DOI]
- 31.SEER*Stat software, version 8.3.4. National Cancer Institute. Accessed September 8, 2020. https://seer.cancer.gov/seerstat/
- 32.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537-546. doi: 10.1200/JCO.18.00149 [DOI] [PubMed] [Google Scholar]
- 33.Carbone DP, Reck M, Paz-Ares L, et al. ; CheckMate 026 Investigators . First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. doi: 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheehan DF, Criss SD, Chen Y, et al. Lung cancer costs by treatment strategy and phase of care among patients enrolled in Medicare. Cancer Med. 2019;8(1):94-103. doi: 10.1002/cam4.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cubanski J, Sroczynski N, Neuman T. Medicare Part B drugs: cost implications for beneficiaries in traditional Medicare and Medicare Advantage. Kaiser Family Foundation. March 15, 2022. Accessed July 19, 2022. https://www.kff.org/medicare/issue-brief/medicare-part-b-drugs-cost-implications-for-beneficiaries-in-traditional-medicare-and-medicare-advantage/
- 36.Olszewski AJ, Dusetzina SB, Eaton CB, Davidoff AJ, Trivedi AN. Subsidies for oral chemotherapy and use of immunomodulatory drugs among Medicare beneficiaries with myeloma. J Clin Oncol. 2017;35(29):3306-3314. doi: 10.1200/JCO.2017.72.2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell AP. We should treat financial toxicity with curative, rather than palliative, intent. JCO Oncol Pract. 2022;18(2):95-96. doi: 10.1200/OP.21.00540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riaz F, Gan G, Li F, et al. Adoption of immune checkpoint inhibitors and patterns of care at the end of life. JCO Oncol Pract. 2020;16(11):e1355-e1370. doi: 10.1200/OP.20.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Presley CJ, Gomes F, Burd CE, Kanesvaran R, Wong ML. Immunotherapy in older adults with cancer. J Clin Oncol. 2021;39(19):2115-2127. doi: 10.1200/JCO.21.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497-530. doi: 10.6004/jnccn.2022.0025 [DOI] [PubMed] [Google Scholar]
- 41.Magee DE, Hird AE, Klaassen Z, et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann Oncol. 2020;31(1):50-60. doi: 10.1016/j.annonc.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 42.Ma X, Bellomo L, Magee K, et al. Characterization of a real-world response variable and comparison with RECIST-based response rates from clinical trials in advanced NSCLC. Adv Ther. 2021;38(4):1843-1859. doi: 10.1007/s12325-021-01659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parrinello CM, Seidl-Rathkopf K, Bourla AB, Nussbaum N, Carson KR, Abernethy AP. Comparison of structured versus abstracted comorbidities using oncology EHR data from cancer patients in the Flatiron Health Network. Paper presented at: International Society for Pharmacoeconomics and Outcomes (ISPOR); May 2018; Baltimore, MD. [Google Scholar]
- 44.Dietel M, Savelov N, Salanova R, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer. 2019;134:174-179. doi: 10.1016/j.lungcan.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal C, Rodriguez Abreu D, Felip E, et al. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, −010, and −024. Paper presented at: European Society of Medical Oncology Congress; October 7-11, 2016; Copenhagen, Denmark. [Google Scholar]
- 46.Skov BG, Rørvig SB, Jensen THL, Skov T. The prevalence of programmed death ligand-1 (PD-L1) expression in non-small cell lung cancer in an unselected, consecutive population. Mod Pathol. 2020;33(1):109-117. doi: 10.1038/s41379-019-0339-0 [DOI] [PubMed] [Google Scholar]
- 47.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 48.Sedrak MS, Freedman RA, Cohen HJ, et al. ; Cancer and Aging Research Group (CARG) . Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71(1):78-92. doi: 10.3322/caac.21638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dotan E, Walter LC, Browner IS, et al. NCCN Guidelines insights: older adult oncology, version 1.2021. J Natl Compr Canc Netw. 2021;19(9):1006-1019. doi: 10.6004/jnccn.2021.0043 [DOI] [PubMed] [Google Scholar]
- 50.Presley CJ, Mohamed MR, Culakova E, et al. A geriatric assessment intervention to reduce treatment toxicity among older adults with advanced lung cancer: a subgroup analysis from a cluster randomized controlled trial. Front Oncol. 2022;12:835582. doi: 10.3389/fonc.2022.835582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics Stratified by Age at Advanced Diagnosis and Year of Advanced Diagnosis
eTable 2. Initial Treatment Class by Year of Advanced Diagnosis Among All Treated Patients (% by Year)
eTable 3. Unadjusted and Adjusted Association Between Patient Characteristics and Two-Year Survival
eFigure. Cohort Formation
Data Sharing Statement