Abstract
Identification of the critical isthmus of the reentrant tachycardia is essential to maximize the effect of catheter ablation (CA) and to minimize the myocardial injury of CA. An 81-year-old woman presented recurrent palpitations after CA of atrial fibrillation (AF) and atrial tachycardia (AT). She had moderate aortic valve stenosis and coronary artery disease. She had received a pulmonary vein isolation, left atrial (LA) posterior wall isolation, and LA anterior linear ablation for atrial fibrillation 1 year prior. At the start of the procedure, she was in sinus rhythm. Atrial burst pacing induced an AT (230msec). High-density mapping revealed a figure-of-eight activation pattern within the LA appendage (LAA), accounting for 99% of the tachycardia cycle length. The critical isthmus was identified at the mid LAA and the local electrogram of the critical isthmus was not fractionated. A single radiofrequency application at the critical isthmus of the AT, terminated the AT. She was free from any ATs for 28 months.
Radiofrequency ablation of the localized reentrant AT was usually performed targeting long fractionated electrograms. In our case, the local electrogram at the critical isthmus was not fragmented compared with the LAA distal part. Long fractionated electrograms were recorded at a more distal part of the LAA than the common isthmus and we could avoid the potential risk of a perforation. A recent developed 3-dimensional electro-anatomical mapping system can identify the critical isthmus and allow us to select a new therapeutic strategy for a critical isthmus ablation of an AT within the LAA.
Keywords: Atrial tachycardia, Localized reentry, Left atrial appendage, Activation, High density
1. Introduction
The left atrial appendage (LAA) has been reported as one of the emerging important arrhythmogenic substrates for the treatment of atrial fibrillation (AF). A localized reentrant atrial tachycardia (AT) within the LAA after AF ablation including a pulmonary vein isolation has been reported [1]. It is important to identify the critical ablation site of the localized reentrant tachycardia with the LAA, because limited area radiofrequency ablation could avoid a risk of perforation and preserve LAA function. Here, we report a case, in which a high-density activation map of localized reentrant AT was obtained and AT was terminated by a single radiofrequency application at the mid LAA.
2. Case report
An 81-year-old woman presented recurrent palpitations after catheter ablation of AF and AT. She had moderate aortic valve stenosis and coronary artery disease. She underwent coronary artery intervention (drug eluting stents at left anterior descending artery #6–7) 2 years ago. She had received a pulmonary vein isolation, left atrial (LA) posterior wall isolation, and LA anterior linear ablation for persistent AF and AT 1 year ago. Twenty-four hours Holter monitoring revealed regular ATs of 130 bpm. Medical therapy with β-blocker was ineffective, and she underwent electrophysiology study. LA diameter was 45mm, left ventricular ejection fraction was 57% and brain natriuretic peptide was elevated to 818.0 pg/ml.
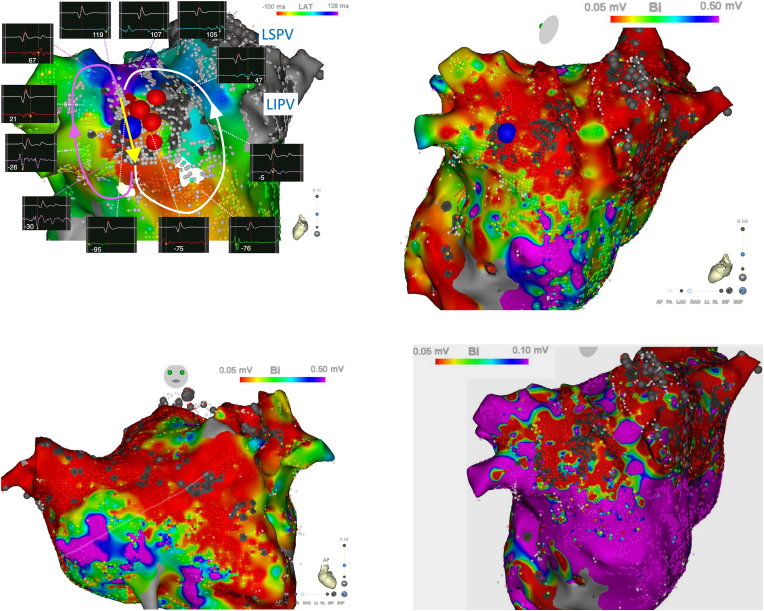
At the start of the procedure, she was in sinus rhythm. The pulmonary veins and LA posterior wall remained isolated. The LA anterior line also remained blocked. Atrial burst pacing induced an AT. The tachycardia cycle length was 230 ms. High-density mapping using a CARTO system and PentaRay® NAV catheter (Biosense Webster, Diamond Bar, CA) revealed a localized reentrant activation pattern within the LAA (Fig. 1, supplemental Movie1, 2), accounting for 99% of the tachycardia cycle length. The voltage map during AT showed severe low voltage area around the LAA (Fig. 1B, C and 1D). Entrainment pacing could not be assessed because the voltage around the LAA was too low to capture constantly.
Fig. 1.
A: A high-density activation map within the LAA. Blue tag indicated the first ablation site, at which AT was terminated. Red tags show the additional ablation sites. The lower electrogram shows the local electrogram and the upper electrogram shows the reference electrogram of the coronary sinus catheter 2–3. The number showed in the box indicated the local activation time. Window of interest was set from −100 msec to 130 msec. Auto scar setting was 0.01 mV. Scar (grey dot) point was ≦0.01 mV. B–C: Voltage map during AT. Color threshold was 0.05–0.50 mV. D: Voltage map during AT. Color threshold was 0.05–0.10mV.AT = atrial tachycardia; LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein.
An activation map around LAA showed a possibility of ① figure-of-eight atrial tachycardia, ② localized reentrant tachycardia of LAA ridge related (Fig. 1A white arrow), ③ localized reentrant tachycardia around LAA body (Fig. 1A pink arrow), and ④ focal tachycardia from LAA with conduction block. In this case, AT was reproducibly induced by the programmed stimulation and reentrant mechanism was supposed. The activation map accounted almost of all the tachycardia cycle length and red color met purple in the activation map. This is unlikely to be a focal tachycardia. However, it was difficult to determine whether this AT was figure-of-eight or not.
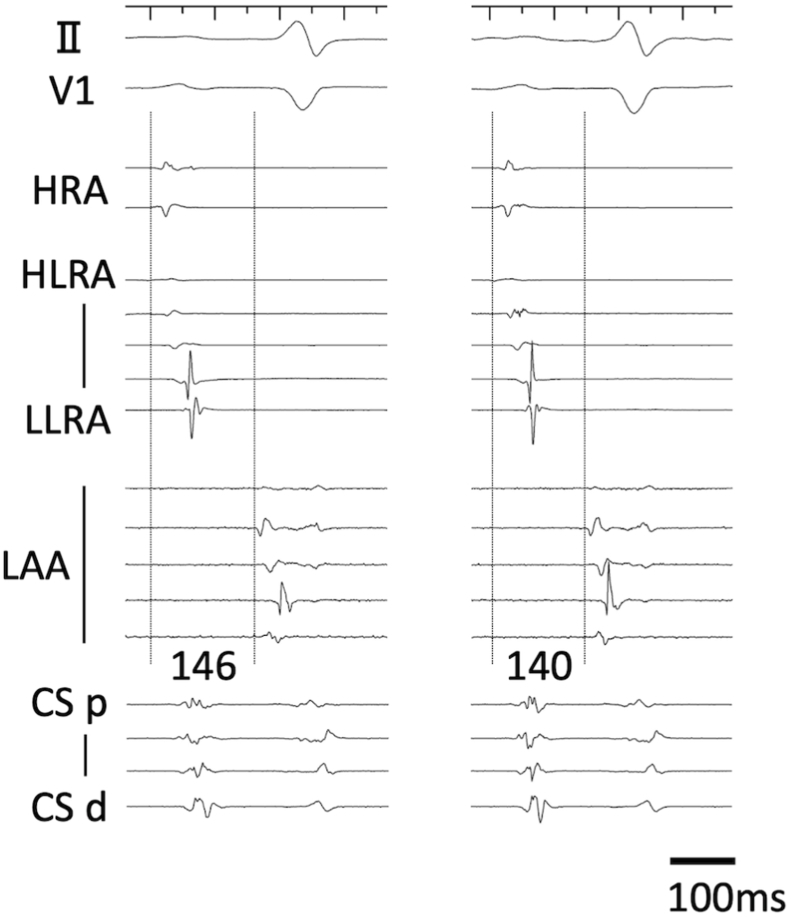
A single radiofrequency application (40W, 3g) at the common isthmus of reentrant AT (Fig. 1A, blue tag), terminated the AT in 26s using an open irrigation contact force sensing catheter (Thermocool Smart-Touch SF, Biosense Webster, Diamond Bar, CA). After a bonus application to connect two scars around the successful site, the AT could no longer be induced. LAA activation was not delayed after ablation compared with before ablation (Fig. 2). She was free from any ATs for 28 months.
Fig. 2.
Intracardiac electrograms before AT induction and after AT termination by radiofrequency ablation. Left panel: Intracardiac electrograms before AT induction. Right panel: Intracardiac electrograms after AT termination by radiofrequency ablation. LAA activation was not delayed by ablation. AT = atrial tachycardia; CS d = distal coronary sinus; CS p = proximal coronary sinus; HLRA = high lateral right atrium; HRA = high right atrium; LAA = left atrial appendage; LLRA = low lateral right atrium.
3. Discussion
Micro-reentrant ATs around LAA were recently reported in the older generations [1]. Liu et al. reported 51 LAA ATs in 45 patients (mean age 65 ± 10 years). In their report, 50 out of 51 ATs were localized reentry or micro-reentry and only 1 was a focal triggered AT, which was originating from the mid LAA. Other reentrant ATs were located at the LAA anterior ostium or the LAA ridge between the left pulmonary vein. In our case, critical isthmus was located at the mid LAA. To the best of our knowledge, this is the first to demonstrate a high-density activation map of localized reentrant AT, eliminated by radiofrequency ablation at the mid LAA.
The elimination of a localized reentrant AT after an LA substrate ablation has been reported to be effective in persistent AF patients [2]. However, the detailed activation pattern within the LAA has not been clarified. Ablation targeting long fractionated electrograms is usually performed [2]. Characteristics of localized reentry were reported using high-resolution mapping [3], and fractionated potential were reported to be possibly caused by pivot point and collision, not always caused by slow conduction corridors. In our case, long fractionated electrograms were recorded at a more distal part of the LAA than the critical isthmus that we targeted. The local electrogram at the critical isthmus was shown in Fig. 1A as the site which was annotated −95 ms and not fractionated. The distal part of the LAA is considered to be thinner than the proximal part and has trabeculations with pectinate muscles, and ablation at the distal part of the LAA has the potential risk of a perforation.
Treatment of reentrant tachycardias is performed targeting the critical isthmus using activation and entrainment mapping. Takigawa et al. reported the importance to detect common isthmus to terminate dual circuit ATs using ultra-high density mapping [4]. In their report, ablation at the common isthmus was related to direct termination. On the other hand, ablation at the non-common isthmus resulted in the ATs conversion. In our case, the LAA voltage was severely impaired, we should consider a minimal ablation around LAA. Moreover, the activation into LAA was only from LA lateral after LA anterior linear ablation. Activation along LAA ridge was important enough to be preserved. From these insights, we determined to perform ablation at the common isthmus detected by the activation map. The first radiofrequency application terminated the AT in 26 seconds, which was not considered to be immediate. There were 2 possible explanations. First, the ablation site was in the LAA. To avoid perforation, we were very careful not to have high contact force more than 5g, which might cause the long duration to terminate the tachycardia. Second, the 1st radiofrequency application site was only adjacent, and we made a lesion connecting the two scars as bonus applications. After that, we confirmed non-inducibility of ATs. It was possible that the 1st application was at the adjacent site and the following was at the critical isthmus. Even though, the area between two scars were considered to be the critical isthmus.
Although an electrical isolation of the LAA as an additional strategy of pulmonary vein isolation has gained widespread attention, an increased risk of thromboembolic events should be considered. A reduced LAA flow and high CHA2DS2-VASc score were reported as predictors of thrombus formation and thromboembolic events. Therefore, early intervention with an LAA closure device should be considered after an LAA isolation [5]. A limited ablation area at the critical isthmus would preserve the LAA muscle and function, which may prevent an increased risk of thromboembolic events.
4. Conclusions
High density mapping was useful to identify the critical ablation site of the localized reentrant tachycardia with the LAA.
Funding sources
This case report did not receive any specific funding.
Declaration of competing interest
All authors have no conflict of interest.
Acknowledgment of grant support
none.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ipej.2022.09.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Liu C.F., Daian F., Goyal R., et al. The left atrial appendage ostium: hotspots for localized Re-entry. JACC Clin Electrophysiol. 2021;7:333–342. doi: 10.1016/j.jacep.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Hocini M., Shah A.J., Nault I., et al. Localized reentry within the left atrial appendage: arrhythmogenic role in patients undergoing ablation of persistent atrial fibrillation. Heart Rhythm. 2011;8:1853–1861. doi: 10.1016/j.hrthm.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frontera A., Mahajan R., Dallet C., et al. Characterizing localized reentry with high-resolution mapping: evidence for multiple slow conducting isthmuses within the circuit. Heart Rhythm. 2019;16:679–685. doi: 10.1016/j.hrthm.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Takigawa M., Derval N., Martin C.A., et al. A simple mechanism underlying the behavior of reentrant atrial tachycardia during ablation. Heart Rhythm. 2019;16:553–561. doi: 10.1016/j.hrthm.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Fink T., Vogler J., Heeger C.H., et al. Impact of left atrial appendage closure on LAA thrombus formation and thromboembolism after LAA isolation. JACC Clin Electrophysiol. 2020;6:1687–1697. doi: 10.1016/j.jacep.2020.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.