Abstract
A statewide assessment of neonicotinoids in groundwater was conducted among a sample of public water supply wells in Iowa from October 2017 to August 2018. Samples from all the state’s major aquifer groups were initially collected from 118 wells in 69 counties. Subsets of 55 untreated samples and 45 paired pre- and post-treatment samples were then collected in summer 2018, post-planting season for primarily corn and soybeans, to assess seasonal differences and the efficacy of treatment. Samples prepared using solid phase extraction were analyzed using LC/MS/MS for six neonicotinoids: acetamiprid, clothianidin, dinotefuran, imidacloprid, thiacloprid, thiamethoxam, and a sulfoximine (i.e., sulfoxaflor). Clothianidin was the most frequently detected (34%, max: 13.4 ng/L), followed by thiamethoxam (14.4%, max: 20.6 ng/L), imidacloprid (13%, max: 2.3 ng/L), and dinotefuran (0.1%, max: 1.4 ng/L). Alluvial aquifers (unadjusted odds ratio (UOR)=14.1; 95% CI (5.4-36.9), p=<0.0001), wells with confining layers <15 m (UOR= 13.5, 95% CI (4.8-38.4), p=<0.0001), and less than 19.4 m in depth (UOR= 20.0; 95% CI (6.5-58.0), p=<0.0001) had the greatest risk for contamination. In vulnerable aquifers, neonicotinoids were detected in 62% of winter and 46% of summer samples, with winter samples over 3 times (UOR=3.2; 95% CI (1.2-8.8), p=0.02) more likely to have at least two neonicotinoids detected. In 55 public water supply systems, the median concentrations of clothianidin (p=0.6), imidacloprid (p=0.7), and thiamethoxam (p=0.7) were unchanged following treatment. These results suggest that neonicotinoid contamination may be present year-round in treated drinking water from vulnerable groundwater sources and represent a source of human exposure.
2. Introduction
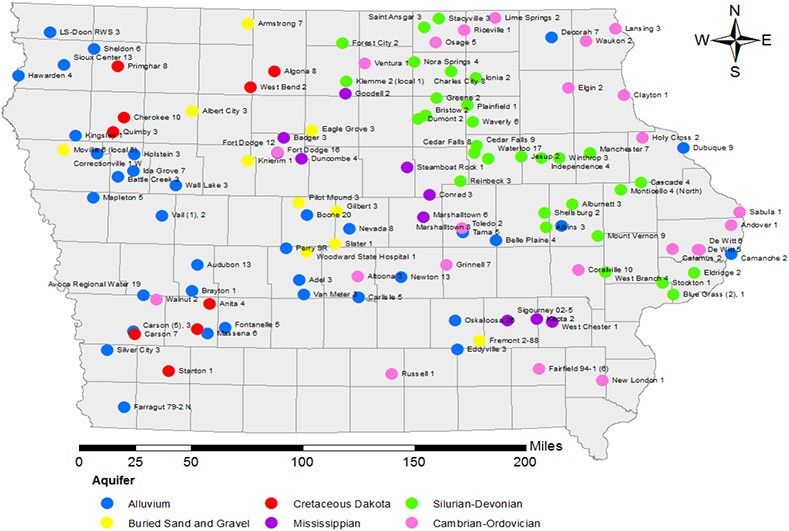
Groundwater in Iowa is the primary source of drinking water for over 75% of the population [1]. Over 2.3 million people obtain drinking water from groundwater sources, through either public water supplies (2,000,000 people) or unregulated private wells (300,000 people) [1, 2]. The state’s groundwater drinking supply generally comes from six major types of aquifers – 1) alluvial aquifers, 2) buried sand and gravel aquifers, 3) Cretaceous (Dakota) sandstone, 4) Silurian-Devonian bedrock, 5) Mississippian bedrock, and 6) Cambrian-Ordovician bedrock (Figure 1) [1]. Prior studies have found that the occurrence of contaminants, like herbicides in groundwater, was commonplace, and varied substantially among these major aquifer systems [2-10].
Figure 1:
Wells sampled during winter 2017-18 by major aquifer group (Iowa, USA).
Estimates of neonicotinoid use throughout the Midwest have shown a dramatic increase over the past twenty years [11]. Nearly 1.8 million kilograms of neonicotinoids are applied to farmland in the United States annually [12]. Neonicotinoid use has become particuarly prominent in the states of Iowa, Illinois, Indiana, Michigan, Ohio, Nebraska, Kansas, Minnesota, and Missouri [11, 13]. Neonicotinoids are used in both urban and rural environments to protect row-crops, orchards, ash trees, gardens, houseplants, and pets, from insect pests [14]. The primary neonicotinoid compounds used in the Midwest are clothianidin, imidacloprid, and thiamethoxam. Neonicotinoids have been found in groundwater sources primarily used for irrigation in agricultural areas with concentrations as high as 10,000 ng/L [15-17]. In a prior study in Iowa documented the prevalence of neonicotinoids in alluvial aquifers, with 73% of the wells having at least one detection [18]. The most frequently detected neonicotinoid was clothianidin (68%), (68%, max: 391.7 ng/L), followed by imidacloprid (43%, max: 6.7 ng/L), and thiamethoxam (3%, max: 0.2 ng/L)[18].
We are not aware of any peer-reviewed published studies that document the occurrence of neonicotinoids in public water groundwater supplies or neonicotinoid concentrations by aquifer type. This study explores groundwater contamination of neonicotinoid insecticides in a statewide assessment of wells representing every major aquifer system. Samples were tested for seven insecticides: acetamiprid, clothianidin, dinotefuran, imidacloprid, thiacloprid, thiamethoxam, and sulfoxaflor. In addition, secondary sampling was conducted to understand differences by season and by treatment, and to calculate exposure estimates from drinking water. The study is designed to broaden our understanding of the potential human exposure risks from drinking water.
3. Materials and Methods
3.1. Sample Collection
Samples were collected in partnership with Iowa Department of Natural Resources’ (IDNR) Ambient Water Quality Monitoring and Assessment Program [1]. These sampled wells represented all major aquifer groups, and a wide range of depths, with vulnerabilities based on estimated confining layer thicknesses, well ages, and types of land use. Samples were collected from 118 public water supply wells in 69 Iowa counties from October 2017 to March 2018. Water samples (1-L) were collected from the well without filtering by certified water operators. Samples were packaged and shipped on ice, then refrigerated at 2-6°C in the laboratory prior to analysis.
A subset of 55 untreated samples were collected from the same public water supply wells again in summer 2018, post-planting season. These samples were collected from wells defined by IDNR as highly vulnerable to contamination from surface activities. High vulnerability wells were defined as wells with less than 15 meters (50 feet) of confining bed thickness based on past water quality assessments [2]. Intermediate and low vulnerability wells had between 15-30 meters (50-100 feet) and >30 meters (>100 feet) of confining material, respectively [2]. These repeat samples were collected to evaluate changes in neonicotinoid concentrations over time. In addition, 45 samples were collected post treatment from the water supply system distribution point. Each pair of untreated and treated samples were then compared to assess whether existing blending and treatment processes impacted neonicotinoid concentrations.
3.2. Analytical Method
All groundwater samples were concentrated using solid phase extraction following the method described previously [18]. Each sample was analyzed for six neonicotinoid compounds (acetamiprid, clothianidin, dinotefuran, imidacloprid, thiacloprid, and thiamethoxam) and sulfoxaflor, a sulfoxamine pesticide. Chemical standards for the six test analytes were obtained with a chemical purity >99% (Chem Service, West Chester, PA, USA). Isotope-labeled imidacloprid-d4 and thiamethoxam-d3 (C/D/N Isotopes, Pointe-Claire, Canada) were used as the internal standard and recovery surrogate. Samples were analyzed at the State Hygienic Laboratory at the University of Iowa (SHL) using a Linear Ion Trap Quadrupole LC/MS/MS Mass Spectrometer in positive ion monitoring mode (ESI+) mode (AB Sciex Instruments, Concord, Canada).
Calibration curves were evaluated for linear and quadratic fits. Both calibration curves had mean R-squared values greater than 99%). This fit is recommended by SHL’s neonicotinoid analysis method [19]. The calibration standards had a mean accuracy of 100% (± 6) and mean precision of 5%.
Analyte concentrations were confirmed using quality assurance and quality control methods, including replicate samples (n= 9), and field (n=4) and laboratory (n=4) blanks. Neonicotinoids were not detected in any of the field or laboratory blanks. Reported concentrations for 9 pairs of replicate samples (samples taken from the same well less than 5 minutes apart) varied by an average of 10%. A replicate for each sample was also tested using direct aqueous injection (DAI). In this study, eight samples collected during the winter of 2017-18 are reported based upon the result of DAI because surrogate recovery for the SPE sample exceeded the acceptable recovery range of 70-130% [19]. No neonicotinoids or sulfoxaflor were detected in any of these samples. Recovery of neonicotinoids in the laboratory matrix spikes had a mean of 99 ± 15%, with a precision of ±15%. Recovery of the surrogate used with the SPE samples, thiamethoxam-d3, had a mean recovery 105% (±15 SD, ± 15% RSD). No recovery correction was been made to the data reported. The enriched method detection limit (MDL) was 0.03 – 0.2 ng/L respectively.
3.3. Data Analysis
Analyst and MultiQuant software (AB Sciex, Concord, Canada) was used to process LCMS/MS data. Statistical analysis was conducted with SAS 9.4 (SAS Institute, Cary, NC). Non-detects of clothianidin, dinotefuran, imidacloprid, and thiamethoxam were set at half the limit of detection (LOD) [20]. Acetamiprid, sulfoxaflor, and thiacloprid were not detected and LCMS data did not indicate their presence below the limit of detection. Non-detects for these three insecticides were set at zero [20]. Continuous variables were evaluated for normality using the Shapiro-Wilk test. The continuous variables that were not normally distributed where then transformed using the natural log. This transformation did not improve normality. Nonparametric statistical tests were used for data interpretation to account for non-detects [21]. Data that were not normally distributed are reported with the median and a range to account for their distribution. Wilcoxon Rank-Sum Test (N=2) or Kruskal-Wallis Test (N≥3), were used to compare differences in median neonicotinoid concentrations by variables, including aquifer type, depth, well age, sampling season and treatment. Spearman's correlation analyses were used to measure the strength and direction of association between analyte concentrations and continuous variables. Well variables (i.e., well depth and well age) were categorized to evaluate concentrations differences within different categories. Categorical data were analyzed using Chi-square or Fishers Exact Test to assess relationships between categorized variables. Odds ratios were determined using logistic regression [22]. Multivariate models were not considered for this study. Odds ratios are most commonly used in epidemiological studies. In this study unadjusted odds ratios (UOR) were calculated solely to estimate the measure of association between neonicotinoid concentrations detected and covariables. The results have been used to guide additional research looking at human exposure. Two-tailed p-values were considered statistically significant if they were <0.05.
4. Results and Discussion
Neonicotinoids were detected in thirty-six percent of the wells sampled from October 2017 to March 2018. The most commonly detected neonicotinoid was clothianidin (34%, max: 13.4 ng/L), followed by thiamethoxam (14.4%, max: 20.6 ng/L), imidacloprid (13%, max: 2.3 ng/L), and dinotefuran (0.1%, max: 1.4 ng/L) (Table 1). Over 18% of the samples collected contained a single neonicotinoid, 11% contained two neonicotinoids, 6% contained three or more compounds. The highest reported neonicotinoid concentration was 20.6 (ng/L) for thiamethoxam. Acetamiprid, sulfoxaflor, and thiacloprid were not detected in any samples.
Table 1.
Summary statistics for neonicotinoids in untreated water tested in winter 2017-18 using solid phase extraction (n=118).
| Neonicotinoid | Number of Detections |
Detection Frequency |
Mean of Detections (ng/L) |
Median of Detections (ng/L) |
Min (ng/L) |
Max (ng/L) |
MDL |
|---|---|---|---|---|---|---|---|
| Acetamiprid | ND | 0% | ND | ND | ND | ND | 0.08 |
| Clothianidin | 40 | 34% | 1.0 | < | < | 13.4 | 0.05 |
| Dinotefuran | 1 | 1% | 1.3 | < | < | 1.3 | 0.18 |
| Imidacloprid | 15 | 13% | 0.1 | < | < | 2.4 | 0.09 |
| Sulfoxaflor | ND | 0% | ND | ND | ND | ND | 0.04 |
| Thiacloprid | ND | 0% | ND | ND | ND | ND | 0.07 |
| Thiamethoxam | 17 | 15% | 0.4 | < | < | 20.6 | 0.03 |
ND = not detected. < = Less than MDL.
The study included wells from all major aquifer systems and included a wide range of depths, vulnerabilities, well ages, and dominant land uses defined by the 2016 United States National Land Cover Database (Table 2) [23]. Well depths ranged from 6 to 850 m with median well depth of 56 m. Alluvium (30%) and Silurian-Devonian (26%) aquifers were most frequently sampled. The sampled wells had an average age of 43 years with a range of 6 to 101 years. Twenty-nine percent of wells were drilled before 1960. Most of the wells sampled were classified as high vulnerability (52%) based upon IDNR’s vulnerability criteria [2]. A majority (87%) of wells were active; however, 10% of the wells were used only as stand-by wells, and three of the wells were maintained, but not used.
Table 2.
Attributes of wells tested in winter 2017-18.
| Well Attribute | Category | Count | Percentage |
|---|---|---|---|
| Aquifer Systems | Alluvium | 35 | 30% |
| Buried Sand & Gravel | 11 | 9% | |
| Cretaceous Dakota | 9 | 8% | |
| Mississippian | 10 | 8% | |
| Silurian-Devonian | 31 | 26% | |
| Cambrian-Ordovician | 22 | 19% | |
| Well Depths | <30.5 m | 38 | 32% |
| 30.5 – 152 m | 56 | 47% | |
| 153 – 850 m | 24 | 20% | |
| Confinement | Confined | 69 | 58% |
| Unconfined | 49 | 42% | |
| Status | Active | 103 | 87% |
| Not Used | 3 | 3% | |
| Standby | 12 | 10% | |
| Vulnerability based on Confining Layer Thickness | Low (>30 m) | 36 | 31% |
| Intermediate (15-30 m) | 21 | 18% | |
| High (<15 m) | 61 | 52% | |
| Year Drilled | Pre-1960 | 34 | 29% |
| 1960 - 1975 | 23 | 20% | |
| 1976 - 1989 | 31 | 26% | |
| 1990 - 2012 | 30 | 25% | |
| Dominant Land Use | 50/50 Developed/Row Crop | 1 | 1% |
| Developed | 77 | 65% | |
| Forest | 6 | 5% | |
| Grassland | 4 | 3% | |
| Row Crop | 30 | 25% |
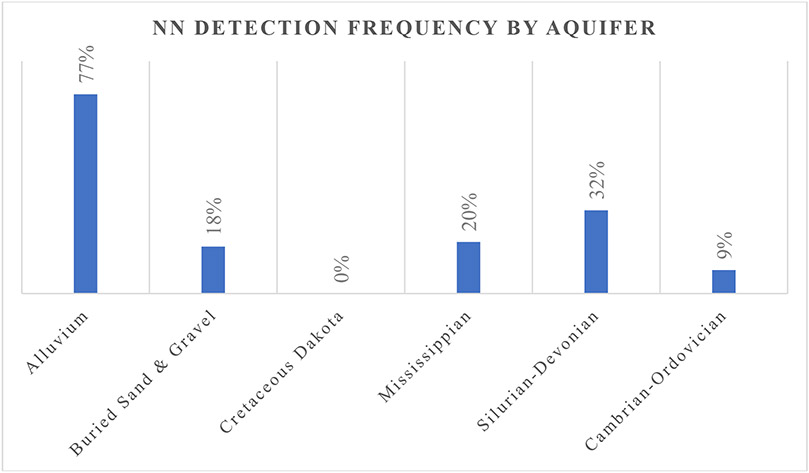
Wells from alluvial aquifers were 14 times (UOR=14.1; 95% CI (5.4-36.9), p=<0.0001) more likely to have a positive detect for neonicotinoids compared to other aquifers (Table 3). Seventy-seven percent of samples from alluvial aquifers had at least one detection compared to 19% from every other type of aquifer analyzed (Figure 2). On average alluvial wells had a total concentration of neonicotinoids 6 times greater than other aquifers (i.e., 3.6 ng/L compared to 0.6 ng/L). In alluvial aquifers, the average concentration of clothianidin, imidacloprid, and thiamethoxam from alluvial aquifers were 2.2, 0.2 and 1.1 ng/L, respectively. By comparison, other aquifers had a mean concentration of clothianidin was 0.4 ng/L and for the other neonicotinoids the concentrations were below the LOD. Using the Kruskal-Wallis Test, the median total concentration, number of detections and individual concentrations for clothianidin, imidacloprid, and thiamethoxam were found to significantly differ (p= <0.0001) between aquifers. The Cretaceous-Dakota, with depths between 35 and 188 m, was the only aquifer where no neonicotinoids were detected. This aquifer is in northwest and west-central Iowa. These sandstone deposits range from 61 to 91 meters (200 to 300 feet) in thickness and are confined by 61 to 122 meters (200 to 400 feet) of glacial till over most of their extent [24]. In addition, this study did not collect data on neonicotinoid application rates in areas near sampling sites. The study was unable to determine whether differences observed by aquifer types were correlated with differences in application of neonicotinoids around the sample sites. Overall, however, these results align with the results reported in a prior study [18], where alluvial aquifers were found to be susceptible to neonicotinoid contamination. Prior studies also reported that herbicides and herbicide-transformation products were prevalent in Iowa’s alluvial aquifers, however, this is one of the first studies to document the prevalence of neonicotinoids in this type of aquifer [2-10, 14, 25].
Table 3.
Unadjusted odds ratio for neonicotinoid detections in public water supply wells.
| Odds Ratio |
95% CI | Z Statistic |
Chi-Square (p-value) |
|
|---|---|---|---|---|
| Alluvial vs Other Aquifer Types | 14.1 | 5.4 - 36.9 | 5.4 | < 0.0001 |
| High Vulnerability vs Low/intermediate Vulnerability | 17.2 | 6.0 - 49.3 | 5.3 | < 0.0001 |
| Unconfined vs confined wells | 15.1 | 6.0 - 38.2 | 5.7 | < 0.0001 |
| Confining thickness <=15 m vs >15 m 1 Detect or More | 13.5 | 4.8 – 38.4 | 4.9 | < 0.0001 |
| Confining thickness <=15 m vs >15 m 2 Detect or More | 75.8 | 4.4 – 1301.5 | 3.0 | 0.003 |
| Well depth <19.4 m vs Other Depth Quartiles with 1 or more detects | 19.4 | 6.5 - 57.9 | 5.3 | < 0.0001 |
| Well depth <19.4 m vs other depth quartiles with 2 or more detects | 35.0 | 9.4 – 129.9 | 5.3 | < 0.0001 |
| Northeast region vs other regions | 1.5 | 0.65 - 3.7 | 0.98 | 0.327 |
| Northwest region vs other regions | 2.5 | 0.92 - 7.0 | 1.8 | 0.073 |
| North Central region vs other regions | 0.22 | 0.06 - 0.8 | 2.3 | 0.021 |
| Southeast vs other regions | 0.21 | 0.05 - 0.99 | 2.0 | 0.048 |
| South Central vs other regions | 1.5 | 0.58 - 4.1 | 0.87 | 0.385 |
| Southwest vs other regions | 1.6 | 0.55 - 4.9 | 0.88 | 0.382 |
| Row crop dominant land use vs other types with at least one detect | 3.8 | 1.6 - 9.0 | 3.0 | 0.003 |
| Row crop & grassland/forest vs developed land use types with at least 1 detect | 3.6 | 1.6 - 8.1 | 3.2 | 0.002 |
| Row crop land use vs grassland/forest with at least one detect | 1.5 | 0.36 - 6.3 | 0.55 | 0.581 |
| Row crop land use vs developed with at least one detect | 4.3 | 1.75 - 10.4 | 3.2 | 0.001 |
| Row crop vs other land use for clothianidin detection | 4.5 | 1.88 - 10.8 | 3.4 | 0.001 |
| Grassland/Forest vs other land use for imidacloprid detection | 9.6 | 2.37 - 38.9 | 3.2 | 0.002 |
| Grassland/Forest vs other land use for thiamethoxam detection | 8.0 | 2.0 - 31.7 | 3.0 | 0.003 |
| Grassland/Forest vs row crop land use for imidacloprid detection | 9.0 | 1.6 - 50.3 | 2.5 | 0.012 |
| Grassland/Forest vs row crop land use for thiamethoxam detection | 4.0 | 0.87 - 18.4 | 1.8 | 0.076 |
| Row crop vs grassland/forest vs land use for clothianidin detection | 1.5 | 0.36 - 6.3 | 0.55 | 0.581 |
| Grassland/Forest vs other land use for two or more detections | 5.8 | 1.5 - 22.1 | 2.5 | 0.011 |
| Winter vs Summer CLO | 1.9 | 0.91 - 4.1 | 1.7 | 0.088 |
| Winter vs Summer IMI | 2.3 | 0.79 - 6.6 | 1.5 | 0.129 |
| Winter vs Summer THX | 2.0 | 0.76 - 5.3 | 1.4 | 0.157 |
| Winter vs Summer TD at least 1 detect | 1.9 | 0.91 – 4.2 | 1.7 | 0.087 |
| Winter vs Summer TD at least 2 or more detect | 3.2 | 1.2 – 8.8 | 2.3 | 0.022 |
Figure 2:
Frequency of neonicotinoid detection in major aquifer systems.
Detected concentrations of neonicotinoids were significantly higher (p=<0.0001) in high vulnerability wells, or wells identified as at greatest risk of contamination based upon the estimated confining layer thickness (<15 m). Prior studies in Iowa have confirmed the vulnerability of wells with less than 15 meters (50 feet) of confining materials above the source aquifer to contamination from nitrate and various pesticides [2]. High vulnerability wells were 17 times (UOR=17.2; 95% CI (6.0-49.3), p=<0.0001) more likely to have at least one detection compared to intermediate and low vulnerability wells (Table 3). Neonicotinoids were present in 58% of wells with confining layers less than 15 meters of low permeability materials. Only 9% of wells with thicker confining layers had positive detections. The mean total concentration of neonicotinoids was 2.9 ng/L in high vulnerability wells compared to 0.21 in intermediate and low vulnerability wells. Clothianidin (r=−0.5, p=<0.0001), imidacloprid (r=−0.4, p=<0.0001), and thiamethoxam (r=−0.4, p=0.0003) concentrations were also found to be inversely correlated with estimated confining layer thicknesses. Neonicotinoids were 15 times (UOR=15.1; 95% CI (6.0-38.2), p=<0.0001) more likely to be found in unconfined wells compared to those determined to be confined using static water level measurements [26] (Table 3). Average total concentrations of neonicotinoids in unconfined wells were nearly 5 times greater, at 2.9 ng/L, compared to 0.6 ng/L in confined wells. Wells with an estimated confining layer thickness less than 15 m were nearly 14 times (UOR=13.5; 95% CI (4.8-38.4), p=<0.0001) more likely to have at least one detection and 76 times (UOR=75.8; 95% CI (4.4-1301.5), p=0.003) more likely to have 2 or more neonicotinoids compared to wells with thicker confining layers. Fifty-eight percent of wells with confining layers less than 15 m had at least one detection and 32% had two or more detections. Only 9% wells with confining layers >15 m had one detection and two or more neonicotinoids were not detected.
Concentrations of neonicotinoids and total number of detections were also found to be significantly different by well depth (p=<0.01). A significant inverse correlation observed for clothianidin (r=−0.5, p=<0.0001), imidacloprid (r=−0.3, p=<0.0001), and thiamethoxam (r=−0.4, p=<0.0001) concentrations, total concentration (r=−0.6, p=<0.0001), and total number of detections (r=−0.6, p=<0.0001). Wells with depths less than 19.4 m had at least one detection in 83% of samples and two or more detections in 50% of samples. Wells with depths from 19.5 to 850 m had at least one detection in only 20% of samples. Overall, the shallower wells were nearly 20 times (UOR=19.4; 95% CI (6.5-58.0), p=<0.0001) more likely to have at least one detection and 35 times (UOR=35.0; 95% CI (9.4-129.9), p=<0.0001) more likely to have two or more detections (Table 3). The average total concentration was 3.9 ng/L in shallower wells, more than five times greater than that in deeper wells. No significant difference was observed between neonicotinoid concentrations and number of detections with elevation, status or well age.
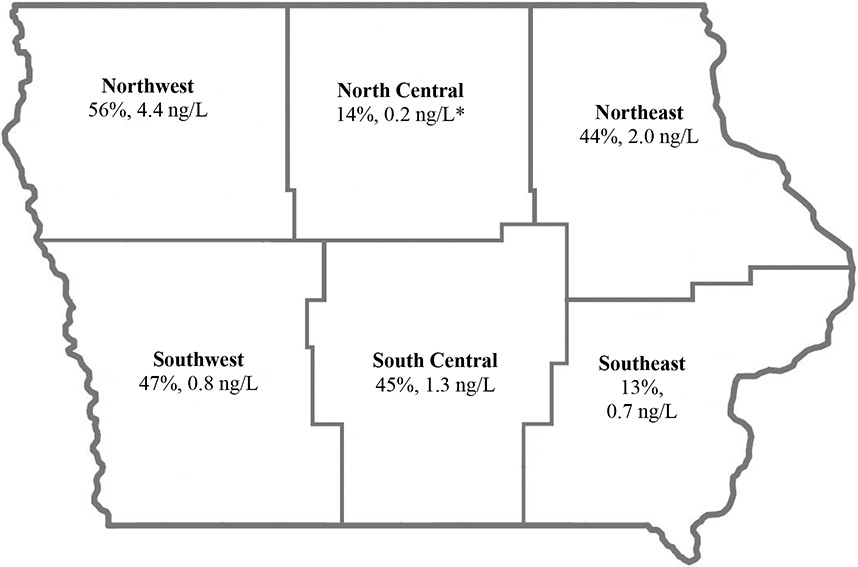
The study also assessed differences in concentrations by county and field office and landform regions. Sample sites were grouped by IDNR’s six regional field offices [27]. IDNR has six field offices, designated as northeast (FO1), north central (FO2), northwest (FO3), southwest (FO4), south central (FO5) and southeast Iowa (FO6) (Figure 3). Median concentrations of clothianidin (p=0.01) and thiamethoxam (p=0.01), total concentration (p=0.01), and number of detects per well (p=0.01) were each significantly different by region. The northwest and northeast regions had the highest detection frequency and mean total concentration at 56% (mean: 4.4 ng/L) and 44% (mean: 2.0 ng/L), although these regions were not statistically different from the entire state. North Central (UOR=0.2; 95% CI (0.1-0.8), p=0.02) and Southeast (UOR=0.2; 95% CI (0.1-1.0), p=0.05) regions were significantly different, and were nearly 5 times less likely to have a single detection per well than other regions in the state (Table 3). Differences in median neonicotinoid concentrations and detection frequencies were not found based upon the different landform areas in Iowa.
Figure 3:
Detection frequency and mean concentration of neonicotinoids by IDNR field office region (Iowa, USA).
Sampling locations were selected from IDNR’s ambient groundwater monitoring network based upon a history of sampling, spatial representation, vulnerability, and target aquifers and a willingness of water operators to test for neonicotinoids. The sample size for this study was not large enough to be statistically representative of all wells within a specific geographic area. For this reason, interactions between region and other variables, such as aquifer type and depths were not assessed. The regional analysis was primarily conducted to identify potential areas of vulnerability for future research.
The United States National Land Cover Database (2016) was used to assess linkages between neonicotinoid usage and land uses [23]. Non-parametric Spearman’s rho analyses were conducted for the percentage of each of the 15 individual land use classes within the 2-year capture zone of each well. In addition, Spearman’s rho analyses were conducted for percentages of five land use groupings, which include water, developed and barren, forest, grass/pasture, and row crops (Table 4). Statistically significant positive correlations were observed between clothianidin, imidacloprid, and thiamethoxam with water, forest, grassland and pasture, and row crop and between clothianidin and percent row crop. An inverse correlation was also found between these three neonicotinoids and developed and barren land use.
Table 4.
Correlation between neonicotinoid concentrations and percent of land use types within the 2-year capture zone of each well.
| CLO | DIN | IMI | THX | |||||
|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | |
| Percent Water | 0.19 | 0.04 | 0.18 | 0.047 | 0.20 | 0.032 | 0.20 | 0.03 |
| Percent Developed and Barren | −0.43 | <.0001 | −0.14 | 0.146 | −0.19 | 0.036 | −0.31 | 0.001 |
| Percent Forest | 0.37 | <.0001 | 0.17 | 0.068 | 0.23 | 0.011 | 0.23 | 0.01 |
| Percent Grassland and Pasture | 0.33 | 0.000 | 0.13 | 0.159 | 0.24 | 0.010 | 0.21 | 0.03 |
| Percent Row Crop | 0.31 | 0.001 | −0.02 | 0.825 | 0.03 | 0.790 | 0.15 | 0.11 |
Each well was further assigned a dominant land use, which describes the primary type of land cover around each well, reduced to three categories: forest/grassland, row crop and developed. Median concentrations of neonicotinoids were significantly different by dominant land use type for clothianidin, imidacloprid, thiamethoxam, total neonicotinoid concentration and total detection (p= 0.004). Neonicotinoids were 3.8 times (UOR=3.8; 95% CI (1.6-9.0), p=0.003) more likely to be detected near row crops than any other dominant land cover. The insecticides were 1.5 to 4 times more likely to be detected near row crops than grassland/forest (UOR=1.5; 95% CI (0.4-6.3), p=0.6) or developed land (UOR=4.3; 95% CI (1.7-10.4), p=0.001) land, respectively. This was largely driven by clothianidin, which was detected in 60% of wells near row crops. Imidacloprid and thiamethoxam were 10 (UOR 95% CI: 2.4-38.9, p=0.002) and 8 (UOR 95% CI: 2.0-31.7, p=0.003) times more likely to be detected near forest/grassland compared to other land use. The odds of detecting two or more neonicotinoids were 6 times (UOR=5.8; 95% CI (1.5-22.1), p=0.01) higher for forest/grassland than any other land use type (Table 3). Clothianidin, imidacloprid, and thiamethoxam were detected in 50% of the samples with mean concentrations ranging from 0.4 – 2.2 ng/L.
Neonicotinoid insecticides are commonly applied in the spring and summer with corn and soybeans in Iowa, and elevated concentrations have been observed in surface waters during the summer months [28]. To capture potential variability in wells more likely to undergo seasonal variations, 55 high vulnerability wells were resampled between June and August 2018, following planting season. These repeat samples were paired and compared to their corresponding winter samples to evaluate changes in neonicotinoid concentrations over time. Wilcoxon Rank-Sum Test showed that there was no statistical difference between median concentrations of clothianidin (p=0.1), imidacloprid (p=0.2), and thiamethoxam (p=0.3) between summer and winter samples, but the mean concentrations for imidacloprid and thiamethoxam were twice as high during the summer months. Alternatively, clothianidin appeared to decrease by two-fold from winter to summer. Degradation of thiamethoxam to clothianidin between summer and winter months may partially explain the higher concentrations of clothianidin during the winter months [29, 30].
Changes in planting treated seeds and precipitation are not believed to account for any differences in concentrations between winter and summer samples. USDA estimated that total corn acreage was 5.3 million hectares in 2018 compared 5.4 million in hectares in 2017 [31, 32]. There was no change in total planted soybean acreage, which was 4 million hectares [31, 32]. From 2017 to 2018, Iowa experienced higher average precipitation (30 cm), of which 17 cm more fell during May – August 2018 [33]. Thiamethoxam concentrations from summer 2018 were positively correlated with winter thiamethoxam concentrations (p=0.04). No correlation was observed between either the winter or summer samples with precipitation. Other factors may also account for this difference, especially considering study-sampling period took place following two separate planting seasons. More research is needed to understand the temporal relationship between neonicotinoid contaminants in groundwater.
A significant difference was observed in the number of detects based upon season (p=0.04). Clothianidin, imidacloprid, and thiamethoxam were detected more often during the winter where they were found in 58%, 22%, and 25% of well samples, compared to 42%, 11%, and 15% in the summer. The odds of at least one detection per well was 1.9 times (UOR=1.9; 95% CI (0.91-4.2), p=0.1) higher and for two or more detects 3.2 times (UOR=3.2; 95% CI (1.2-8.8), p=0.02) higher during the winter compared to summer.
During the summer sampling period 45 pairs of raw, untreated well water and treated, finished water samples were collected. Concentrations for each pair were compared using the non-parametric Wilcoxon Rank-Sum Test to assess the impact of treatment processes. It should be noted that the term “treatment” here includes blending water from multiple wells, which is often done to dilute contaminants. The median concentrations of clothianidin (p=0.6), imidacloprid (p=0.7), and thiamethoxam (p=0.7) were not different between untreated and treated samples. The number of detections per sample also did not vary significantly (p=0.6). Total finished water concentrations were an average of 1.3 ng/L lower than the corresponding untreated sample, but generally the insecticides were still present in finished water samples. This is consistent with an earlier study that showed that common treatment techniques might not be effective at removing these compounds [34, 35]. Clothianidin, imidacloprid, and thiamethoxam were detected in 38%, 11%, and 9% of untreated samples; compared to 36%, 9%, and 9% of treated samples, respectively (Table 6). These differences in paired samples ranged from 0.03-0.6 ng/L. These differences could be due instrument variability or may also be the result of mixing of water from several wells prior to treatment. Changes in concentrations, between pre- and post-treatment, could be associated with additional contamination or dilution by these other sources. Information on the specific treatment process used by each water supply system were not collected. A comparison of specific treatment processes was not evaluated during this study. Further research is needed to better understand the efficacy of different types of treatment processes in removing neonicotinoids.
Table 6.
Summary statistics for summer 2018 neonicotinoids in untreated and treated water samples (n=45).
| Neonicotinoid | Number of Detections |
Detection Frequency |
Mean of Detections (ng/L) |
Median of Detections (ng/L) |
Min (ng/L) |
Max (ng/L) |
MDL |
|---|---|---|---|---|---|---|---|
| Treated samples | |||||||
| Clothianidin | 16 | 36% | 0.3 | < | < | 4.2 | 0.05 |
| Imidacloprid | 4 | 9% | 0.1 | < | < | 0.6 | 0.09 |
| Thiamethoxam | 4 | 9% | 0.3 | < | < | 12.5 | 0.03 |
| Untreated samples | |||||||
| Clothianidin | 17 | 38% | 0.4 | < | < | 4.4 | |
| Imidacloprid | 5 | 11% | 0.4 | < | < | 11.8 | |
| Thiamethoxam | 4 | 9% | 1.2 | < | < | 48.4 | |
ND = not detected. < = Less than MDL.
5. Conclusions
In this assessment of public water supply wells, neonicotinoid contamination was detected in 77% of alluvial aquifers. Clothianidin was the most frequently detected neonicotinoid (34%, max: 13.4 ng/L), followed by thiamethoxam (14.4%, max: 20.6 ng/L), imidacloprid (13%, max: 2.3 ng/L), and dinotefuran (0.1%, max: 1.4 ng/L). Occurrence of the insecticides was significantly different among major aquifer types with at least one neonicotinoid detected in 77% of Alluvium, 32% of the Silurian-Devonian, 20% of the Mississippian, 18% of the Buried Sand and Gravel, 9% of the Cambrian-Ordovician and 0% of Cretaceous Dakota aquifer systems. Neonicotinoids were associated with row cropland use (UOR=3.8; 95% CI (1.6-9.0), p=0.003), confining layer thickness less than 15 m (UOR=14.0; 95% CI (4.8-38.4), p=<0.0001), and well depths less than 19.4 m (UOR=20.0; 95% CI (6.5-58.0), p=<0.0001). In addition, median neonicotinoid concentrations did not vary by season or treatment, suggesting the potential for long-term contamination in vulnerable aquifers and chronic exposure risk. The odds of detecting two or more neonicotinoids in a high vulnerability well sample were 3.2 times (UOR=3.2; 95% CI (1.2-8.8), p=0.02) higher during the winter compared to summer. These results suggest that neonicotinoid contamination may be present year-round in treated drinking water from vulnerable groundwater sources and may represent a source of human exposure. Further research should be conducted to measure actual exposure from vulnerable aquifers to better quantity the risk of from neonicotinoid exposure via drinking water.
Table 5.
Summary statistics for paired winter and summer untreated well water samples (n=55).
| Neonicotinoid | Number of Detections |
Detection Frequency |
Mean of Detections (ng/L) |
Median of Detections (ng/L) |
Min (ng/L) |
Max (ng/L) |
|---|---|---|---|---|---|---|
| Winter | ||||||
| Clothianidin | 32 | 58% | 1.9 | 0.2 | < | 13.4 |
| Imidacloprid | 12 | 22% | 0.2 | < | < | 2.4 |
| Thiamethoxam | 14 | 25% | 0.6 | < | < | 20.6 |
| Summer | ||||||
| Clothianidin | 23 | 42% | 0.9 | < | < | 12.8 |
| Imidacloprid | 6 | 11% | 0.3 | < | < | 11.8 |
| Thiamethoxam | 8 | 15% | 1.4 | < | < | 48.4 |
ND = not detected. < = Less than MDL.
Acknowledgements
Funding for this research was supported by University of Iowa’s Center for Health Effects of Environmental Contamination, Iowa Institute of Public Health Research and Policy, NIOSH funded Heartland Center for Occupational Health and Safety (Training Grant No. T42OH008491), NIEHS funded Environmental Health Sciences Research Center (Grant No. P30 ES005605), and the State Hygienic Laboratory at the University of Iowa. Researchers would also like to thank the Iowa Department of Natural Resources for including our sample bottles with their FY18 ambient groundwater monitoring sampling program.
The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Iowa DNR
Footnotes
Conflicts of Interest
There are no conflicts of interest to declare.
8. References
- 1.Iowa Department of Natural Resources. Groundwater Monitoring. 2018; Available from: http://www.iowadnr.gov/Environmental-Protection/Water-Quality/Water-Monitoring/Groundwater. [Google Scholar]
- 2.Hruby Claire E; Libra Robert D; Fields Chad L; Kolpin Dana W; Hubbard Laura E; Borchardt Mark R; Spencer Susan K; Wichman Michael D; Hall Nancy and Schueller Michael D. 2013. Survey of Iowa groundwater and evaluation of public well vulnerability classifications for contaminants of emerging concern. 2015; Available from: https://pubs.er.usgs.gov/publication/70155240. [Google Scholar]
- 3.Kolpin Dana W; Barbash Jack E and Gilliom Robert J, Occurrence of pesticides in shallow groundwater of the United States: Initial results from the National Water-Quality Assessment Program. Environmental Science & Technology, 1998. 32(5): p. 558–566. [Google Scholar]
- 4.Kolpin Dana W; Kalkhoff Stephen J; Goolsby Donald A; Sneck-Fahrer Debra A and Thurman E Michael, Occurrence of selected herbicides and herbicide degradation products in Iowa's ground water, 1995. Groundwater, 1997. 35(4): p. 679–688. [Google Scholar]
- 5.Detroy Mark G; Hunt Pamela K and Holub Maureen A. Ground-water-quality-monitoring program in Iowa: Nitrate and pesticides in shallow aquifers. 1988. 88–4123]; Available from: 10.3133/wri884123. [DOI] [Google Scholar]
- 6.Weyer Peter J; Smith Brian J; Feng Zhen-Fang; Kantamneni Jiji R and Riley David G, Comparison of nitrate levels in raw water and finished water from historical monitoring data on Iowa municipal drinking water supplies. Environmental monitoring and assessment, 2006. 116(1-3): p. 81–90. [DOI] [PubMed] [Google Scholar]
- 7.Kross Burton C and Hallberg George R. The Iowa state-wide rural well-water survey water-quality data: Initial analysis. 1990; Available from: https://ir.uiowa.edu/cgi/viewcontent.cgi?article=1018&context=igs_tis. [Google Scholar]
- 8.Kross Burton C; Hallberg George R; Bruner D Roger; Cherryholmes Keith and Johnson J Kent, The nitrate contamination of private well water in Iowa. American Journal of Public Health, 1993. 83(2): p. 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libra Robert D. The Iowa state-wide rural well-water survey: June 1991, repeat sampling of the 10% subset. 1993; Available from: https://ir.uiowa.edu/cgi/viewcontent.cgi?article=1025&context=igs_tis. [Google Scholar]
- 10.State Hygienic Laboratory at the University Of Iowa. Iowa Well Survey - 2017 Summary Report. 2018; Available from: http://www.shl.uiowa.edu/env/privatewell/iawatersurvey.pdf. [Google Scholar]
- 11.U.S. Geological Survey. Pesticide National Synthesis Project, Estimated Annual Agricultural Pesticide Use. 2018. October 31, 2016; Available from: https://water.usgs.gov/nawqa/pnsp/usage/maps/index.php. [Google Scholar]
- 12.Han Wenchao; Tian Ying and Shen Xiaoming, Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere, 2018. 192: p. 59–65. [DOI] [PubMed] [Google Scholar]
- 13.Myers Clayton and Hill Elizabeth, Benefits of neonicotinoid seed treatments to soybean production. US Environmental Protection Agency, Washington, DC, USA, 2014. [Google Scholar]
- 14.Thompson Darrin A; Lehmler Hans-Joachim; Kolpin Dana W; Hladik Michelle L; Vargo John D; Schilling Keith E; Lefevre Gregory H; Peeples Tonya L; Poch Matthew C and Laduca Lauren E, A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environmental Science: Processes & Impacts, 2020. 22(6): p. 1315–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Perre Chloe; Murphy Tracye M. and Lydy Michael J., Fate and effects of clothianidin in fields using conservation practices. Environmental Toxicology and Chemistry, 2015. 34(2): p. 258–65. [DOI] [PubMed] [Google Scholar]
- 16.Huseth Anders S. and Groves Russell L., Environmental Fate of Soil Applied Neonicotinoid Insecticides in an Irrigated Potato Agroecosystem. PloS One, 2014. 9(5): p. e97081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford Benjamin Z; Huseth Anders S and Groves Russell L, Widespread detections of neonicotinoid contaminants in central Wisconsin groundwater. PloS One, 2018. 13(10): p.e0201753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson Darrin A; Kolpin Dana W; Hladik Michelle L; Barnes Kimberlee K; Vargo John D and Field R William, Prevalence of Neonicotinoids and Sulfoxaflor in Alluvial Aquifers in a High Corn and Soybean Producing Region of the Midwestern United States. Science of the Total Environment, 2021: p. 146762. [Google Scholar]
- 19.Vargo John, Determination of Pesticides in Water, Standard Operating Procedure. 2015, State Hygienic Laboratory at The University of Iowa: Iowa City, IA. [Google Scholar]
- 20.U.S. Environmental Protection Agency. Regional Guidance on Handling Chemical Concentration Data Near the Detection Limit in Risk Assessments. 2017; Available from: https://www.epa.gov/risk/regional-guidance-handling-chemical-concentration-data-near-detection-limit-risk-assessments. [Google Scholar]
- 21.Helsel Dennis R and Hirsch Robert M, Statistical methods in water resources. Vol. 49. 1992: Elsevier. [Google Scholar]
- 22.Szumilas Magdalena, Explaining odds ratios. Journal of the Canadian academy of child and adolescent psychiatry, 2010. 19(3): p. 227. [PMC free article] [PubMed] [Google Scholar]
- 23.Multi-Resolution Land Characteristics (Mrlc) Consortium. National Land Cover Database (NLCD) 2016. 2019; Available from: https://www.mrlc.gov/data. [Google Scholar]
- 24.Iowa Geological Survey. Dakota Aquifer. 2017. September 29, 2020]; Available from: https://www.iihr.uiowa.edu/igs/dakota-aquifer/. [Google Scholar]
- 25.Kolpin Dana W; Thurman E. Michael and Linhart S. Michael, Finding minimal concentrations of herbicides in ground water? Try looking for the degradates. 1999. [DOI] [PubMed] [Google Scholar]
- 26.Iowa Department of Natural Resources, Iowa DNR Well Program Definitions. 2018. [Google Scholar]
- 27.Iowa Department of Natural Resources. Environmental Field Offices. n.d.; Available from: https://www.iowadnr.gov/fieldoffice. [Google Scholar]
- 28.Hladik Michelle L.; Main Anson R. and Goulson Dave, Environmental risks and challenges associated with neonicotinoid insecticides. 2018, ACS Publications. [DOI] [PubMed] [Google Scholar]
- 29.Hilton Martin J; Jarvis Tim D and Ricketts Dean C, The degradation rate of thiamethoxam in European field studies. Pest Management Science, 2016. 72(2): p. 388–397. [DOI] [PubMed] [Google Scholar]
- 30.Tomizawa Motohiro and Casida John E., Neonicotinoid insecticide toxicology: mechanisms of selective action. Annual Review of Pharmacology and Toxicology, 2005. 45: p. 247–68. [DOI] [PubMed] [Google Scholar]
- 31.United States Department Of Agriculture, Iowa Ag News - 2017 Crop Production. 2018. [Google Scholar]
- 32.United States Department Of Agriculture, Iowa Ag News – 2018 Crop Production. 2019. [Google Scholar]
- 33.Iowa State University - Iowa Environmental Mesonet. 2017-18 Iowa ASOS Precipitation Report. 2020; Available from: https://mesonet.agron.iastate.edu/ASOS/reports/mon_prec.php?year=2018. [Google Scholar]
- 34.Klarich Kathryn L.; Pflug Nicholas C.; Dewald Eden M.; Hladik Michelle L.; Kolpin Dana W.; Cwiertny David M. and Lefevre Gregory H., Occurrence of Neonicotinoid Insecticides in Finished Drinking Water and Fate during Drinking Water Treatment. Environmental Science and Technology Letters, 2017. 4(5): p. 168–173. [Google Scholar]
- 35.Klarich Wong Kathryn L; Webb Danielle T; Nagorzanski Matthew R; Kolpin Dana W; Hladik Michelle L; Cwiertny David M and Lefevre Gregory H, Chlorinated byproducts of neonicotinoids and their metabolites: An unrecognized human exposure potential? Environmental Science and Technology Letters, 2019. 6(2): p. 98–105. [Google Scholar]