Abstract
The development of highly efficacious positive allosteric modulators (PAMs) of α7 nicotinic acetylcholine receptors (nAChR) has proven useful in defining the ligand dependence of the conformational dynamics of α7 receptors. No such effective modulators are known to exist for the α4β2 nAChR of the brain, limiting our ability to understand the importance of desensitization for the activity profile of specific ligands. In this study, we used mutant β2 subunits that allowed the use of the α7 PAM 3a,4,5,9b-tetrahydro-4-(1-naphthalenyl)-3H-cyclopentan[c]quinoline-8-sulfonamide (TQS) to probe the desensitizing effects of nicotinic ligands on the two forms of α4β2 receptors; high sensitivity (HS) (two α4 and three β2 subunits) and low sensitivity (LS) (three α4 and two β2 subunits). A total of 28 different ligands of 8 different categories, based on activity and selectivity, were tested for their ability to induce TQS-sensitive desensitization of HS and LS α4β2 receptors. Results confirm that HS α4β2 receptor responses are strongly limited by desensitization, by at least an order of magnitude more so than the responses of LS receptors. The activation of α4β2 receptors by the smoking-cessation drugs cytisine and varenicline is strongly limited by desensitization, as is the activation of LS receptors by the HS-selective agonists 6-[5-[(2S)-2-Azetidinylmethoxy]-3-pyridinyl]-5-hexyn-1-ol dihydrochloride and 4-(5-ethoxy-3-pyridinyl)-N-methyl-(3E)-3-buten-1-amine difumarate. The evaluation of drugs previously identified as α7-selective agonists revealed varying patterns of α4β2 cross-desensitization that were predictive of the effects of these drugs on the activation of wild-type α4β2 receptors by acetylcholine, supporting the utility of TQS-sensitive receptors for the development of focused therapeutics.
SIGNIFICANCE STATEMENT
To varying degrees, ligands regulate the balance of active and desensitized states of the two forms of the primary nAChR subtypes in brain. Using mutant beta subunits, an allosteric modulator can reverse ligand-induced desensitization, revealing the differential desensitization of the receptors by specific ligands. This study shows that drugs believed to be selective for therapeutic targets may cross-desensitize other targets and that, within a class of drugs, improved specificity can be achieved by using agents that reduce such cross-desensitization.
Introduction
Any meaningful interpretation of the physiology and pharmacology of nicotinic acetylcholine receptors (nAChR) must include a consideration of the balance between activation and desensitization (Katz and Thesleff, 1957). It might be argued that the lifetime of the endogenous acetylcholine (ACh) signal at a mature neuromuscular junction is too brief for desensitization to play a large role (Land et al., 1981). However, in virtually any other context, desensitization should be considered as a factor shaping macroscopic responses (Papke, 2010). Typical in vitro approaches used to study heterologously expressed receptors rely on solution application/exchange methods that are slower than receptor desensitization rates, so drug application rates and receptor desensitization are both factors limiting the responses. Likewise, a balance between activation and desensitization must be important for the function of nAChR in the brain, where ACh is delivered by diffuse volume transmission (Descarries et al., 1997) and nicotine is delivered through relatively slow self-administration by smokers (Picciotto et al., 2008).
Most of the nAChR in vertebrate brain that bind ACh and nicotine with high affinity are pentameric complexes containing α4 and β2 subunits (Millar and Gotti, 2009). Pentamers composed of just two different subunits necessarily can vary in subunit stoichiometry, such that while two agonist binding sites are configured at α4−β2 interfaces, the fifth position can be occupied by either an α4 or a β2 subunit (Nelson et al., 2003). While some expression systems may bias receptor expression toward the α4(3)β2(2) configuration, both types are present in the brain (Fasoli et al., 2016), and chronic nicotine favors the expression of the α4(2)β2(3) configuration due to nicotine’s ability to selectively chaperone receptors of that configuration to the membrane (Nelson et al., 2003; Kuryatov et al., 2005; Srinivasan et al., 2011).
The two configurations of α4β2 nAChR differ greatly in their functional properties, with one notable difference being that receptors with the α4(2)β2(3) configuration respond to low concentrations of ACh or nicotine but saturate their responses when agonist concentrations are raised to higher levels. They have therefore come to be referred to as a high sensitivity (HS) subtype. In contrast, receptors of α4(3)β2(2) configuration, in general, generate larger currents across a wider range of concentration; these are known as the low sensitivity (LS) subtype (Nelson et al., 2003; López-Hernández et al., 2004; Eaton et al., 2014). It is the core hypothesis of this study that desensitization is the primary factor limiting the responses of HS receptors to high concentrations of agonist (Corrie et al., 2020). We will use mutant forms of the receptors that are sensitive to positive allosteric modulators (PAMs) that activate desensitized receptors to test that hypothesis. Although it should be noted that our experiments do not necessarily fulfill the criteria for the statistical testing of a null hypothesis, we show that the use of a PAM that reverses desensitization selectively increases the response of HS receptors compared with LS receptors.
It is well established that desensitization profoundly limits the ion channel function of homomeric α7 nAChR (Uteshev et al., 2002), the second most abundant nAChR in brain (Millar and Gotti, 2009). Our understanding of α7 receptor desensitization has been greatly enhanced by the discovery of the type II class of α7-selective PAMs (Grønlien et al., 2007), which destabilize one of the nonconducting states, allowing desensitized receptors to reactivate (Papke et al., 2009; Williams et al., 2011).
The α7-selectivity of PAMs like 1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)-urea (PNU-120596) and 3a,4,5,9b-tetrahydro-4-(1-naphthalenyl)-3H-cyclopentan[c]quinoline-8-sulfonamide (TQS) is due to the presence of a methionine, unique to α7 among nAChR, in the 15’ position of the pore-forming second transmembrane domain (Young et al., 2008). The transfer of that residue into β2 or β4 allows for the formation of heteromeric nAChR that are sensitive to potentiation by TQS (Stokes et al., 2019). In the present study, we used a concatamer (Zhou et al., 2003) of α4 and β2L15’M mutants, coexpressed in Xenopus oocytes with monomers of wild-type α4 or β2 subunits, to selectively form TQS-sensitive LS or HS α4β2 receptors. Using TQS to reveal the extent of α4β2 receptor desensitization, we demonstrate the great degree to which desensitization limits HS receptor responses to determine the degree to which their activities on the two α4β2 isoforms are limited by TQS-sensitive desensitization. Finally, we tested the relevance of these observation to the functional activation of wild-type α4β2 receptors by ACh in the presence of putative α7-selective agonists.
Materials and Methods
Acetylcholine chloride, atropine, choline, methyllycaconitine citrate (MLA), dihydro-b-erythrodine hydrobromide, N-(3R)-1-Azabicyclo[2.2.2]oct-3-yl-4-chlorobenzamide (PNU-282987), cytisine, arecoline, nicotine, cotinine, and other chemicals were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). TQS, 2-(3-Pyridinyl)-1-azabicyclo[3.2.2]nonane dihydrochloride (TC-1698), (3S)-Spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidine]-2’-one hydrochloride (AR-R17779), 3-[3-(3-Pyridinyl)-1,2,4-oxadiazol-5-yl]benzonitrile, varenicline, 6-[5-[(2S)-2-Azetidinylmethoxy]-3-pyridinyl]-5-hexyn-1-ol dihydrochloride (sazetidine-A), and epibatidine were purchased from Tocris (Minneapolis, MN). 4-(4-cyanophenyl)-1,1-diethylpiperazin-1-ium (pCN-diEPP) and 4-(4-carbamoylphenyl)-1,1-diethylpiperazin-1-ium (pCONH2-diEPP) were synthesized as previously reported (Quadri et al., 2016). 1,1-dimethylpiperidinium was synthesized by Kinga Chojnacka (Papke et al., 2014a). 1,4-Diazabicyclo[3.2.2]non-4-yl[5-[3-(trifluoromethyl)phenyl]-2-furanyl]methanone hydrochloride (NS6740) and desformylflustrabromine (dFBr) were provided by Ganesh Thakur (Northeastern University). (±)-nornicotine (free base) was synthesized as previously described (Swango et al., 1999), a gift from Peter Crooks. Anabaseine was synthesized by Jingyi Wang in the Nicole Horenstein laboratory (University of Florida). Other compounds were sourced as follows: methyl pyridinium chloride (n-MP) from AK Scientific (Union City CA); triethylmethylammonium chloride (triEMA) from Tokyo Chemical Industry (Portland, OR); 3-(2,4-Dimethoxybenzylidene)-anabaseine dihydrochloride (GTS-21) from Taiho Pharmaceuticals (Tokyo, Japan); (E)-N-Methyl-4-(3-pyridinyl)-3-buten-1-amine oxalate (TC-2403) and 4-(5-ethoxy-3-pyridinyl)-N-methyl-(3E)-3-buten-1-amine difumarate (TC-2559) from Targacept (Winston-Salem, NC); and 1,2,3,6-tetrahydro-2,3′-bipyridine (anatabine) from Cayman Chemical (Ann Arbor, MI).
Fresh ACh stock solutions were made in Ringer’s solution each day of experimentation. Stock solutions of TQS, PNU-282987, NS6740, pCN-diEPP, pCONH2-diEPP, and dFBr were made in DMSO and kept at −20°C and diluted in Ringer’s solution each day. Other compounds’ stock solutions were prepared in Ringer’s solution and held at 4°C and diluted in Ringer’s solution each day.
Heterologous Expression of nAChRs in Xenopus Laevis Oocytes
Two approaches have been developed to study HS and LS α4β2 receptors independently of each other in Xenopus oocytes. One approach has been to inject the α4 and β2 RNA at ratios that would favor the assembly of LS or HS receptors (usually 10:1, α4 to β2 for LS receptors or 1:10 α4 to β2 for HS receptors) (Zwart et al., 2008). However, this approach generates a heterogeneous population of receptors and if applied to the present study would give an unequal number of mutant β subunits in the LS and HS biased population. The alternative approach is to use linked α4−β2 subunits (Zhou et al., 2003), which permits the coexpression of the concatamer with monomeric α4 or β2 subunit to yield pure populations of defined subunit composition; furthermore, placement of L15’M mutation in the concatamer allows for LS and HS receptors to be formed with the same number of mutant subunits in both receptor types. The original publication of the concatamers (Zhou et al., 2003) provided a thorough validation of the constructs with Western blots and other analyses. The fidelity with which the concentration-response data of the receptors formed with the concatamer containing the L15’M mutation match the data obtained with the original concatamer, obtained and characterized by the Lindstrom laboratory, indicate that the mutation did not disrupt the function of the concatamers. The human nAChR clones and the original β2 − 6−α4 concatamer were obtained from Dr. J. Lindstrom (University of Pennsylvania, Philadelphia, PA). The β2 L15’M mutant in the concatamer was made as previously described (Stokes et al., 2019). Subsequent to linearization and purification of the plasmid cDNAs, cRNAs were prepared using the mMessage mMachine in vitro RNA transfection kit (Ambion, Austin, TX).
Oocytes were surgically removed from mature Xenopus laevis frogs (Nasco, Ft. Atkinson, WI) and injected with appropriate nAChR subunit cRNAs as described previously (Papke and Stokes, 2010). Frogs were maintained in the Animal Care Service facility of the University of Florida, and all procedures were approved by the University of Florida Institutional Animal Care and Use Committee (Approval #202002669). In brief, the frog was first anesthetized for 15 to 20 minutes in 1.5 L frog tank water containing 1 g of 3-aminobenzoate methanesulfonate buffered with sodium bicarbonate. The harvested oocytes were treated with 1.25 mg/ml collagenase (Worthington Biochemicals, Freehold, NJ) for 2 hours at room temperature in calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, and 12 mg/l tetracycline, pH 7.6) to remove the follicular layer. Stage V oocytes were subsequently isolated and injected with 50 nl water containing 5 ng concatamer plus 5 ng α4 or β2 nAChR subunit cRNA. Recordings were carried out 2 to 7 days after injection.
Two-Electrode Voltage Clamp Electrophysiology
Experiments were conducted at room temperature (24°C) using OpusXpress 6000A (Molecular Devices, Union City, CA) (Papke and Stokes, 2010). Both the voltage and current electrodes were filled with 3 M KCl. Oocytes were voltage-clamped at −60 mV. The oocytes were bath-perfused with Ringer’s solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, and 1 μM atropine, pH 7.2) at 4 ml/min. Drug applications were 6 seconds in duration followed by 241-second washout periods. A typical recording for each oocyte constituted two initial control applications of ACh, an application of the experimental compound applied alone, a follow-up control application of ACh, a coapplication of the test compound with 30 µM racemic TQS, and a final control application of ACh. The control ACh concentrations were 10 µM for HS receptors and 100 µM for LS receptors. The concentrations of the test compounds are provided in Table 1. All experiments began with eight oocytes voltage clamped and treated in parallel; however, some cells lost voltage clamp or otherwise failed to remain viable through the series of drug applications and were thus excluded from the analyses. Final n values are also provided in Table 1.
TABLE 1.
Test compounds concentrations and n values
| Drug | Concentration | nHS | nLS |
|---|---|---|---|
| Antagonists | |||
| MLA | 100 μM | 8 | 4 |
| DHβE | 10 μM | 8 | 7 |
| α7 silent agonists | |||
| NS6740 | 30 μM | 8 | 8 |
| n-MP | 300 μM | 7 | 8 |
| triEMA | 100 μM | 7 | 8 |
| α9 agonists | |||
| pCN diEPP | 100 μM | 8 | 7 |
| pCONH2-diEPP | 100 μM | 7 | 7 |
| diMPiPa | 100 μM | 8 | 7 |
| α4β2 modulators | |||
| NS9283b | 30 μM | 8 | 8 |
| dFBr | 30 μM | 8 | 7 |
| Non-selective agonists | |||
| Carbachol | 100 μM | 6 | 8 |
| Epibatidine | 3 μM | 8 | 6 |
| Anabaseine | 100 μM | 7 | 8 |
| Nicotine | 10 μM | 7 | 8 |
| Cotinine | 100 μM | 6 | 8 |
| Nor-nicotine | 10 μM | 5 | 6 |
| Anatabine | 100 μM | 4 | 8 |
| HS selective agonists | |||
| sazetidine-A | 30 μM | 7 | 7 |
| TC-2559 | 30 μM | 6 | 8 |
| α4β2 partial agonists | |||
| TC-2403 | 10 μM | 8 | 8 |
| cytisine | 100 μM | 6 | 6 |
| varenicline | 10 μM | 7 | 7 |
| arecoline | 100 μM | 8 | 7 |
| α7-selective agonists | |||
| GTS-21 | 100 μM | 7 | 7 |
| PNU-282987 | 10 μM | 8 | 8 |
| TC-1698 | 10 μM | 8 | 8 |
| AR-R17779 | 10 μM | 7 | 5 |
| choline | 1 mM | 7 | 8 |
a1,1-dimethylpiperidinium.
b3-[3-(3-Pyridinyl)-1,2,4-oxadiazol-5-yl]benzonitrile 3-[3-(3-Pyridinyl)-1,2,4-oxadiazol-5-yl]benzonitrile.
The responses are reported as peak current amplitudes. The average responses of the two initial ACh controls from each cell were used for normalization. Data are presented as the averages ± S.D. Statistical analyses were conducted based on T-test comparisons of the normalized peak current data or one-way ANOVA. Bonferroni corrections were applied for multiple comparisons (Aickin and Gensler, 1996). When drug responses without and with TQS were obtained from the same cells, pairwise comparisons were made. However, it was noted that, with the LS receptors, ACh control responses were inhibited by the applications of nicotine, TC-1698, varenicline, and epibatidine applied alone. Therefore, for these drugs on the LS receptors, the responses to the drugs alone and the drugs coapplied with TQS were obtained on separate sets of cells.
Data were collected at 50 Hz, filtered at 5 Hz, and analyzed by Clampfit 9.2 or 10.3 (Molecular Devices) and Excel (Microsoft, Redmond, WA). All experiments began with eight voltage-clamped oocytes set up for parallel analysis in the Opus-Xpress system. However, due to the fact that PAM-potentiated currents were sometimes very large, some cells could not be held in voltage clamp and were therefore excluded from the subsequent analyses. If more than three cells were excluded due to inadequate voltage clamp, the entire experiment was repeated. Results are expressed as means ± S.D. from at least five oocytes for each experiment or as dot plots generated by Kaleidagraph 4.5.2 (Synergy Software, Reading, PA). ANOVA and other statistical comparisons were calculated in Kaleidagraph 4.5.2. The values for the curve fits were generated using the Levenberg-Marquardt algorithm to obtain the best chi-square fit to the Hill equation using the Kaleidagraph 4.5.2 plotting program. The errors reported for the fit parameters are based on the goodness of fit.
We display multicell averages of the raw data for visual comparisons of complex responses. The averages of normalized data were calculated using an Excel (Microsoft) template for each of the 10,322 points in each of the 206.44-second traces (acquired at 50 Hz). Following subtraction of the basal holding current, data from each cell, including the ACh controls, were normalized by dividing each point by the peak of the ACh control from the same cell. The normalized data were then averaged and standard errors of the mean (SEM) for the multicell averages calculated on a point-by-point basis. The dark lines represent the average normalized currents and the shaded areas the range of the SEM. Scale bars in the figures of averaged traces reflect the scaling factor relative to the average peak current amplitude of the ACh controls used for the normalization procedures. These plots effectively illustrate the differences in peak currents, net charge, the kinetics of the responses, and the variability throughout the entire time course of the responses.
Results
The Generation of TQS-Sensitive HS and LS α4β2 nAChR
The L15’M mutation (Stokes et al., 2019) was made in the β2 subunit of the β2 − 6−α4 concatamer (Zhou et al., 2003), so that by coexpressing this concatamer with monomers of either β2 or α4, we could obtain receptors with the subunit configuration shown in Fig. 1A. These receptors show the expected differences in ACh sensitivity previously reported for wild-type HS and LS receptors (Fig. 1B). For the HS receptors, the Log ACh EC50 values were 0.431 µM (Log error = −.39) and 0.11 µM (Log error = −.1) for the wild-type and mutant receptors, respectively. For the LS receptors, the ACh Log EC50 values were 2.13 µM (Log error = 1.39) and 2.27 µM (Log error = 1.27) for the wild-type and mutant receptors, respectively.
Fig. 1.
TQS-sensitive HS and LS α4β2 receptors. (A) Subunit configuration of the receptors composed of α4−β2 concatamers with the β2L15’M mutation (represented by the star) in the β subunits. When coexpressed with wild-type β2 subunits, they yield HS α4(2)β2L15’M(2)β2 receptors (left). When coexpressed with wild-type α4 subunits, they yield LS α4(2)β2L15’M(2)α4 receptors (right). (B) ACh concentration-response data for wild-type (circles) and mutant (squares) receptors. Points represent the average of four to eight oocyte responses at each concentration (± S.D.), normalized to preceding control ACh responses obtained from the same cells. ACh controls were 10 µM for the HS receptors and 100 µM for the LS receptors. The Levenberg-Marquardt algorithm was used in Kaleidagraph to generate curves based on the Hill equation that best fit the data.
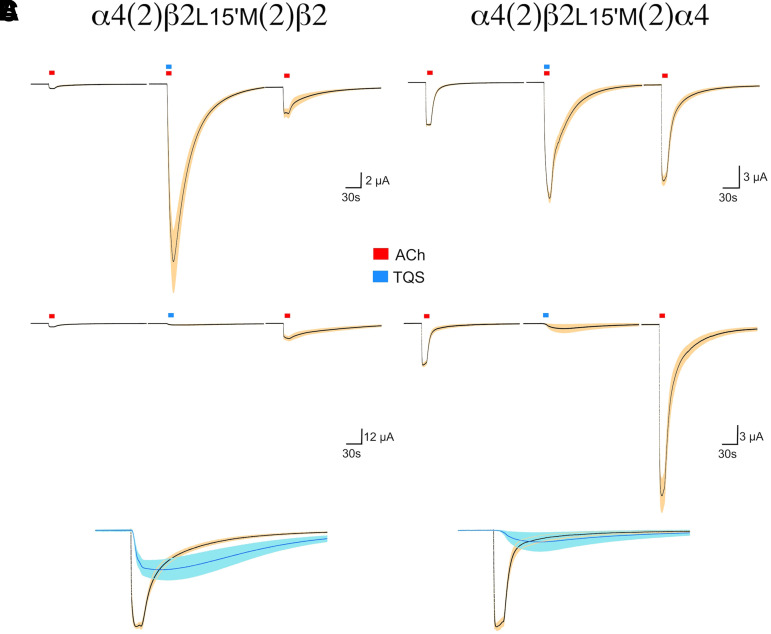
As expected, the ACh responses of oocytes expressing these constructs were strongly potentiated by coapplication of ACh with 30 µM TQS (Fig. 2A). The control ACh responses of HS receptors increased by a factor of 43 (with a standard deviation of 19.76), while the LS ACh responses were increased by a factor of only 2.546 (with a standard deviation of 0.237). We compared responses obtained with our coapplication protocol to responses obtained when TQS was preapplied for 30 seconds prior to the coapplication of ACh and TQS. Responses were essentially identical with or without preapplications (Supplemental Fig. 1).
Fig. 2.
Averaged data from HS α4(2)β2L15’M(2)β2 receptors (left) and LS α4(2)β2L15’M(2)α4 receptors (right). (A) Cells were treated with control applications of ACh (red bars) and then after washout ACh coapplied with 30 µM TQS (blue bars) and then another application of ACh. ACh controls were 10 µM for the HS receptors and 100 µM for the LS receptors. The data are the averages of seven cells for each receptor subtype. Scale bars are based on the average initial ACh controls that were used for normalization (see Materials and Methods). (B) Averaged responses of HS and LS receptors to ACh and then 30 µM TQS applied alone, followed by another ACh application. The data are the averages of eight cells for the HS configuration and seven cells for the LS configuration. (C) Superimposition of the ACh preapplication controls and the responses to TQS alone taken from the traces in (B).
As noted in the earlier work with L15’M mutants (Stokes et al., 2019), the effects of TQS persist after the washout of the drug from the bath, so that responses to ACh alone after the TQS application were also increased relative to the initial ACh control responses. This sort of priming is similar to what has been described for the TQS-related α7 ago-PAM GAT107 (Papke et al., 2014b) and was observed with the TQS-sensitive α4β2 receptors, regardless of the test compound initially coapplied with TQS (not shown). For this reason, oocytes were not used for repeated measurements following an application of TQS.
With our standard protocol involving two initial responses to ACh alone, the application of TQS alone also evoked small currents (Fig. 2B). Similar results were obtained when TQS was given prior to the ACh controls (data not shown). The responses to TQS alone were larger with the HS receptors than the LS receptors, although in both cases they were smaller than the ACh controls (Fig. 2C). This observation raised the concern that while evaluating the effects of TQS on responses to ligands expected to produce little α4β2 activation, we could only consider there to be TQS potentiation if the coapplication responses were larger than the sum of the responses to the ligand and to TQS alone.
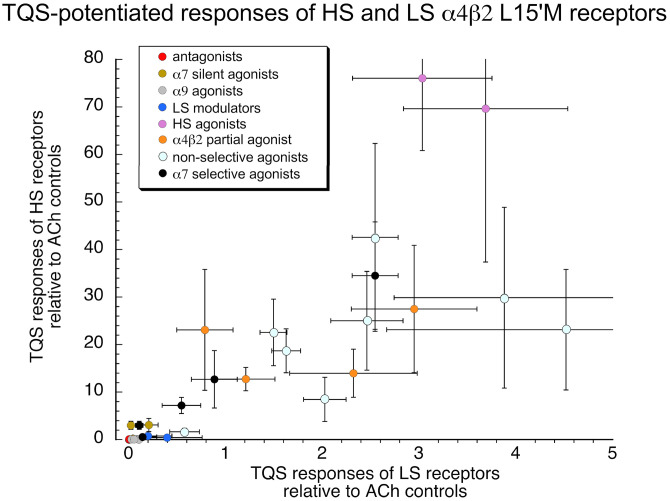
We evaluated a total of 28 drugs for their activity alone and when coapplied with TQS, and it was consistently observed that, for active compounds, regardless of the class of compound, the potentiation of HS responses was greater than that of LS receptors (P < 0.001), typically by at least an order of magnitude (Fig. 3), consistent with TQS-sensitive desensitization as a factor limiting HS receptor responses.
Fig. 3.
The TQS potentiated responses to all of the test compounds. The HS receptor responses, normalized to their ACh controls, are plotted relative to the scale on the y-axis. The LS receptor responses, normalized to their ACh controls, are plotted relative to the scale on the x-axis. All points are averages (± S.D.). The n values are provided in Table 1. The various classes of drugs are color-coded as indicated.
Dynamic Conversion of Steady-State Desensitization to PAM-Potentiated Currents
To promote a progression toward steady-state desensitization, we preapplied 30 µM nicotine to HS and LS α4β2L15’M receptors and then coapplied 30 µM nicotine and 30 µM TQS (Fig. 4A). The upper traces show the averaged responses (see Materials and Methods) of seven cells of each type, normalized to their initial ACh controls (not shown). The average peak amplitude of the HS 10 µM ACh controls was 2.47 µA (S.D. = 1.03), while the average peak amplitude of the LS 100 µM ACh controls was 15.9 µA (S.D. = 11.8). The nicotine phases of the responses are shown below the main traces, scaled as indicated. The peak of the HS nicotine response was only 330 nA (S.D. = 67 nA), while the peak of the LS nicotine response was 982 nA (S.D. = 283 nA). A comparison of the normalized responses to the TQS coapplications (Fig. 4B) would indicate a larger TQS response for the HS receptors (P < 0.01). However, comparison of the responses without normalization (Fig. 4C) is consistent with TQS selectively increasing the HS response up to the level of the LS TQS responses (see Supplemental Material for statistics). Note that both experiments were conducted on the same day with cells from the same injection set.
Fig. 4.
(A) Averaged raw data traces (n = 7 in each case) of HS (on left) and LS (on right) α4β2L15’M receptors to a 30-second preapplication of 30 µM nicotine followed by a coapplication of 30 µM nicotine and 30 µM TQS. The nicotine-only phases of the responses are shown as inserts below the main traces, scaled as indicated. (B) Peaks of TQS responses normalized to the initial ACh controls (see Supplemental Material for statistics). (C) Peaks of TQS responses measured in µAmps (see Supplemental Material for statistics).
Inactive Compounds
Since one of our goals was to probe compounds that would be equivalent to silent agonists (i.e., give currents only when coapplied with the PAM), we tested three classes of compounds that were not expected to activate α4β2 receptors when applied alone. These were the α7 silent agonists NS6740, 1-methylpyridinium (Papke et al., 2022b), and triethylmethylammonium (Papke et al., 2014a) (Supplemental Fig. 2); the α9-selective agonists pCN diEPP, pCONH2-diEPP, and 1,1-dimethylpiperidinium (Papke et al., 2022a) (Supplemental Fig. 3); and the LS α4β2 modulators, 3-[3-(3-Pyridinyl)-1,2,4-oxadiazol-5-yl]benzonitrile (Wang et al., 2015) and dFBr (Weltzin and Schulte, 2010) (Supplemental Fig. 4). As expected, none of these compounds produced activation of either α4β2 receptor when applied alone, and when coapplied with TQS using our standard protocol, none of these compounds produced responses greater than those seen to TQS applied alone.
Effects of nAChR Antagonists
The α7-selective antagonist methyllycaconitine (MLA) (Turek et al., 1995) and the α4β2-selective antagonist dihydro-β-erythroidine (DHβE) (Damaj et al., 1995) were tested applied alone and in coapplication with TQS on the L15’M receptors. As expected, neither compound produced any activation when applied alone (Table 3). Interestingly, both compounds suppressed any response when coapplied with TQS using our standard protocol. Due to the larger responses of the HS receptors to TQS alone, this effect was most obvious for that isoform (P < 0.05, Supplemental Fig. 5). It may be the case that the nicotinic antagonists have selectivity for the inactive state of the receptor and inhibit the effect of TQS allosterically. Although the coapplication of TQS with the antagonists generated no responses, subsequent responses to ACh alone were primed by the coapplications (not shown).
TABLE 3.
Antagonists
| HS activation | HS TQS | LS activation | LS TQS | |
|---|---|---|---|---|
| 3a. Responses | Average ± S.D. | Average ± S.D. | Average ± S.D. | Average ± S.D. |
| MLA | 0.007 ± 0.003 | 0.016 ± 0.009# | 0.003 ± 0.003 | 0.003 ± 0.003 |
| DHβE | 0.046 ± 0.032# | 0.085 ± 0.046# | 0.004 ± 0.002 | 0.004 ± 0.002 |
| 3b. Corrected P values | ||||
| Drug versus Drug plus TQS | Drug plus TQS versus TQS alone | |||
| P value HS | P value LS | P value HS | P value LS | |
| MLA | 0.0492 | 0.1879 | 0.0053# | 1.4235 |
| DHβE | 0.0201 | 2.0367 | 0.0209# | 0.8528 |
#P < 0.05, drugs co-applied with TQS reduced response compared with TQS alone.
TQS Effects on HS ACh Responses Across a Range of ACh Concentrations
As noted earlier, it has been proposed that HS responses to high concentrations of agonist are specifically limited by desensitization. If this is the case, then it might be possible for HS receptors to continue to show progressive increases in TQS-potentiated responses in a range of ACh concentrations (i.e., > 10 µM) where applications of ACh alone show little further increase, effectively causing a rightward shift in the ACh response curve, making them more LS-like in that regard. Therefore, to determine whether the inability of HS receptors to show increased responses to higher concentrations of ACh was due to progressively larger amounts of TQS-sensitive desensitization, coapplications of TQS with ACh were conducted across a wide range of ACh concentrations (Fig. 5A). The TQS-potentiated ACh responses showed a concentration sensitivity that was similar to, or even greater than the responses to ACh alone, with an EC50 of 125 ± 22 nM for ACh plus TQS compared with 1.39 ± 0.07 µM for ACh alone. These data suggest that TQS-sensitive desensitization is a limiting factor even at the lowest ACh concentrations and not a factor especially limiting HS responses to higher concentrations of agonist.
Fig. 5.
Agonist concentration dependence of TQS-potentiated responses. (A) TQS potentiation of ACh HS receptor responses across a range of ACh concentrations. Plotted are the average peak current responses of HS receptors to coapplications of ACh and 30 µM TQS (red symbols, right y-axis) of five to eight cells (± S.D.) at each concentration, compared with the responses to ACh alone (black symbols, left y-axis, data from Figure 1B). In both cases the responses were normalized and expressed relative to the initial peak currents of the 10 µM ACh controls from the same cells. The estimated Imax for ACh alone was only 1.14 ± 0.012 the ACh controls (r = 0.999), while for the TQS-potentiated current the Imax was 82.7 ± 2.6 (r = 0.988). (B) TQS potentiation of LS receptors at two different concentrations. Circles represent the average normalized peak current responses obtained with TQS coapplied with the test compounds at the concentrations indicated in Table 1. Diamonds represent the average normalized peak current responses obtained with TQS coapplied with the test compounds at 10-fold higher concentrations than those indicated in Table 1. See Table 3 for ANOVA results. ** indicates P < 0.001 for comparisons between the low and high concentration responses.
LS Receptor Potentiation at Higher Drug Concentrations
Given that the receptors with the α4(3)β2(2) configuration are characterized as low sensitivity, the L15’M receptors with this configuration were also tested with 11 of the active compounds at 10-fold higher concentrations to determine if the effects of TQS were systematically underestimated by testing the compounds on both the HS and LS receptors at the same concentration. For 7 of the 11 compounds tested at higher concentrations (see ANOVA results Table 2), there were no statistically significant differences in the TQS-potentiated responses at the two concentrations (Fig. 5B). However, responses to sazetidine-A, nicotine, and epibatidine coapplied at the higher concentration with TQS were roughly 50% smaller (P < 0.0001) than the responses to the lower concentrations coapplied with TQS. Only responses to 1 mM arecoline coapplied with TQS were larger (P < 0.001, Table 2), by roughly a factor of 2 than when TQS was coapplied with the 10-fold lower concentration of arecoline.
TABLE 2.
Analysis of variance high versus low concentrations
Data were extracted from a larger ANOVA with Bonferroni correction for multiple comparisons and are not based on pairwise tests.
| Source | DF | SS | MS | F | p |
|---|---|---|---|---|---|
| Total | 164 | 275.44943 | 1.6795697 | ||
| A | 21 | 222.04497 | 10.57357 | 28.312629 | <.0001 |
| Error | 143 | 53.404455 | 0.37345773 | ||
| Comparison | Mean Difference | |t| | p | 95% CL | |
| Sazetidine-A low vs. high | 2.15358 | 6.8091 | <.0001 | 0.95292 to 3.3542 | |
| Nicotine low vs. high | 2.33651 | 7.6468 | <.0001 | 1.1766 to 3.4965 | |
| TC-1698 low vs. high | −0.100207 | 0.328 | 1 | −1.2601 to 1.0597 | |
| Varenicline low vs. high | −0.253913 | 0.7773 | 1 | −1.4939 to 0.98612 | |
| Epibatidine low vs. high | 2.09233 | 6.1541 | <.0001 | 0.80166 to 3.383 | |
| PNU-282987 low high | 0.0842623 | 0.2553 | 1 | −1.1686 to 1.3371 | |
| Choline low vs. high | −0.408172 | 1.3358 | 1 | −1.5681 to 0.75177 | |
| Arecoline low vs. high | −1.64859 | 5.2124 | 0.0001 | −2.8492 to -0.44794 | |
| TC-2403 low vs. high | −0.817367 | 2.5843 | 1 | −2.018 to 0.38329 | |
| Anatabine low vs. high | −0.0802815 | 0.2538 | 1 | −1.2809 to 1.1204 | |
| Anabaseine low vs. high | 0.35324 | 1.1561 | 1 | −0.8067 to 1.5132 |
Potentiation of Nonselective nAChR Agonists
We tested a selection of drugs considered relatively nonselective cholinergic agonists including nicotine, its primary metabolite cotinine (Briggs and McKenna, 1998), and its primary metabolite in brain, nor-nicotine (Crooks et al., 1995). While nor-nicotine has been shown to be a relatively potent α4β2 agonist (Papke et al., 2007), cotinine is generally thought of as primarily being a biomarker for nicotine use with very low potency as an agonist (Tan et al., 2021). We also tested the minor tobacco alkaloid anatabine (Wu et al., 2002), previously reported to be an α4β2 agonist (Alijevic et al., 2020). We also tested carbachol (Parker et al., 1998), an agonist for both nicotinic and muscarinic AChR, along with epibatidine (Badio and Daly, 1994), a toxin isolated from frogs that is among the most potent of all nicotinic agonists (Gerzanich et al., 1995), and anabaseine, an alkaloid toxin produced by Nemertine worms and Aphaenogaster ants (Wheeler et al., 1981; Kem et al., 1997).
When applied alone at the test concentration (Table 1), these compounds had varying levels of activity (Fig. 6), and when normalized to the respective ACh controls, the responses of the HS and LS receptors were not different, except in the case of epibatidine, which was more active on the LS receptors than the HS receptors (P < 0.001, see Supplemental Material for statistical analysis). The TQS effects also differed somewhat for the HS and LS receptors. TQS did not potentiate the low responses to cotinine for either subtype.
Fig. 6.
Effects of nonselective agonists. (A) Dot plot of the peak current responses of HS (left) and LS receptors (right) to the nonselective agonists when applied alone (circles), compared with the responses to drugs coapplied with 30 µM TQS, indicated by the drug name with a plus sign and plotted as half-color diamonds.
Activity and Potentiation of α4β2 Partial Agonists
Four α4β2 partial agonists, including the smoking-cessation drugs cytisine (Etter et al., 2008) and varenicline (Coe et al., 2005), as well as arecoline (Papke et al., 2015), an active agent in areca associated with betel quid addiction (Gupta and Warnakulasuriya, 2002), and TC-2403 (Papke, 2002) were tested. As expected, responses to these agents were low when applied alone, especially for the HS receptors. Normalized to their respective ACh controls, the responses of LS receptors to varenicline and TC-2403 were greater compared to those of HS receptors (P < 0.001, see Supplemental Material for ANOVA and t-tests). TQS produced potentiation (Fig. 7A) at varying levels of statistical significance for all these agents on both receptor subtypes (Supplemental Material), supporting the hypotheses that receptor desensitization is at least in part a factor that limits the efficacy of these agents for α4β2* receptors. Comparison of the data in Figs. 6 and 7A suggests that the TQS-potentiated responses of the partial agonists are roughly equivalent to those of the nonselective agonists.
Fig. 7.
Effects of selective agonists. (A) Dot plot of the peak current responses of HS (left) and LS receptors (right) to the α4β2 partial agonists when applied alone (circles), compared with the responses to drugs coapplied with 30 µM TQS, indicated by the drug name with a plus signand plotted as half-color diamonds. (B) Dot plot of the peak current responses of HS (left) and LS receptors (right) to the HS α4β2 selective agonists when applied alone (circles), compared with the responses to drugs coapplied with 30 µM TQS, indicated by the drug name with a plus sign and plotted as half-color diamonds.
Potentiation of HS α4β2 -Selective Agonists
Sazetidine-A and TC-2559, two agents that are potent activators of HS α4β2 receptors with little or no efficacy for activating LS receptors, were tested (Fig. 7B). Sazetidine-A was actually first published as a selective α4β2 desensitizer, since it was shown to primarily desensitize receptors in an expression system that was biased toward the formation of LS-type receptors (Xiao et al., 2006) and only later shown to be an HS-selective agonist when HS receptor formation was enhanced by injection of oocytes with a 10-fold excess of β2 relative to α4 RNA (Zwart et al., 2008). The same approach was also used to demonstrate the increased efficacy of TC-2559 for HS receptors (Zwart et al., 2006). As expected, when applied alone, these agents stimulated large responses for HS receptors with very little response in the LS receptors (P < 0.0001, Supplemental Material). However, when coapplied with TQS, both compounds were strong activators of both receptor types, confirming that they are indeed subtype-selective silent agonists (Fig. 7B).
α7-Selective Agonists
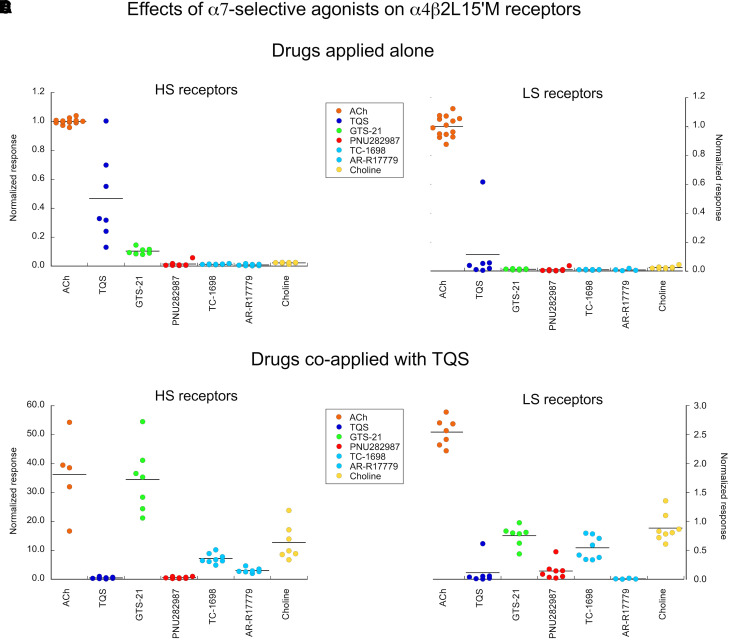
One of the most frequently sought-after goals in the preclinical developments on nicotinic drugs has been to identify drugs that will target α7 nAChR without affecting other subtypes like α4β2 receptors (Papke and Horenstein, 2021), leading to the identification of several α7-selective agonists. Among the first α7-selective agonists to be published, and one of the most widely used, is GTS-21 (DMXB) (de Fiebre et al., 1995), although even in the first publication it was noted to also antagonize α4β2 responses. Subsequently, several large pharmaceutical companies developed agents that were proposed to be more selective than GTS-21, including AR-R17779 (Levin et al., 1999), TC-1698 (Marrero et al., 2004), and PNU-282987 (Bodnar et al., 2005). The ACh precursor choline was also identified as an α7-selective agonist (Papke et al., 1996), although its low potency and ubiquitous presence in the brain and blood generally precludes its consideration as a therapeutic agent.
As expected, none of these drugs evoked much activation of the α4β2 receptors, although there were small responses of HS receptors to GTS-21 (Table 4 and Fig. 8). This activity may have been missed in earlier studies that were based on the expression of α4 and β2 injected at equal ratios in Xenopus oocytes and might have biased expression toward the LS form. In any case, these responses were smaller than responses to ACh or TQS alone (P < 0.0001, see Supplemental Material for ANOVA results) and were not larger than the responses to the other α7 agonists. When coapplied with TQS to HS receptors, GTS-21 and choline gave responses that were larger than those of TQS alone. AR-R17779 and TC-1698 gave measurable responses, but the ANOVA results did not indicate that they were statistically larger than responses to TQS alone. For the LS receptors, GTS-21 (P < 0.0001), TC-1698 (P < 0.01), and choline (P < 0.0001) coapplied with TQS gave responses larger than to TQS alone (see Supplemental Material).
TABLE 4.
α7-selective agonists
| HS activation | HS TQS | LS activation | LS TQS | |
|---|---|---|---|---|
| 4a. Responses | Average ± S.D. | Average ± S.D. | Average ± S.D. | Average ± S.D. |
| GTS-21 | 0.103 ± 0.023 | 34.514 ± 11.300 | 0.012 ± 0.004 | 0.753 ± 0.173 |
| PNU-282987 | 0.014 ± 0.018 | 0.566 ± 0.298 | 0.009 ± 0.012 | 0.142 ± 0.147 |
| TC-1698 | 0.013 ± 0.003 | 7.197 ± 1.694 | 0.009 ± 0.002 | 0.545 ± 0.199 |
| AR-R17779 | 0.008 ± 0.006 | 3.017 ± 0.847 | 0.007 ± 0.007 | 0.109 ± 0.039 |
| choline | 0.022 ± 0.005 | 12.702 ± 6.048 | 0.024 ± 0.009 | 0.885 ± 0.237 |
See Supplemental Material for ANOVA results.
Fig. 8.
Effects of α7-selective agonists (Table 4). (A) Dot plot of the peak current responses of TQS-sensitive HS receptors to the α7-selective agonists when applied alone, compared with ACh control responses and the responses to 30 µM TQS applied alone. Although GTS-21 appeared to give detectable responses, these were not statistically significant compared with the other α7-selective agonists (see Supplemental Material for ANOVA results). (B) Responses of TQS-sensitive HS α4β2 receptors to α7-selective agonists coapplied with TQS. GTS-21 and choline gave responses that were larger than those of TQS alone. AR-R17779 and TC-1698 gave measurable responses, but the ANOVA results did not indicate that they were statistically greater than TQS alone (see Supplemental Material for ANOVA results). (C) The lack of responses of TQS-sensitive LS α4β2 receptors to the α7-selective agonists when applied alone, compared with ACh control responses and the responses to 30 µM TQS applied alone. (D) Responses of TQS-sensitive LS α4β2 receptors to α7-selective agonists coapplied with TQS. GTS-21 (P < 0.0001), TC-1698 (P < 0.01), and choline (P < 0.0001) coapplied with TQS gave responses greater than to TQS alone (see Supplemental Material for ANOVA results).
Inhibition of Wild-Type α4β2 ACh Responses by α7-Selective Agonists
The data in Fig. 9 suggest that some of the compounds proposed to activate α7 receptors would be effective desensitizing antagonists of α4β2 receptors. To test this, cells expressing wild-type forms of HS and LS α4β2 receptors were pre-exposed to the commercially developed α7-selective agonists for 30 seconds, and then ACh was coapplied at the control concentration along with the α7 agonists at the test concentration. The ACh responses were compared with the control ACh responses obtained prior to the application of the α7 agonists (Fig. 9). GTS-21 preapplication evoked a small response from the LS α4β2 receptors and suppressed the ACh responses of both subtypes, with a greater effect on HS than on LS (Table 5). TC-1698 produced a nearly complete block of the ACh responses of both α4β2 subtypes, while AR-R17779 produced a 50% block of the HS responses with no effect on the LS ACh response. PNU-282987 preapplication and coapplication caused no block of either α4β2 receptor subtype (Fig. 9). These results with wild-type receptors are consistent with the TQS effects obtained on the receptors with L15’M mutant β2 subunits (Fig. 8).
Fig. 9.
Effects of α7-selective agonists on the ACh responses of wild-type HS and LS α4β2 receptors. Averaged data were prepared as described (Materials and Methods). Following the ACh control responses (red bars), the α7 agonists were preapplied for 30 seconds (colored bars). Then without washout the α7 agonists were coapplied with ACh at the control concentration (10 µM for HS and 100 µM for LS). The n values for the GTS-21 experiments were 8 for the HS receptors and 7 for the LS receptors. The n values for the TC-1698 experiments were 7 for the HS receptors and 7 for the LS receptors. The n values for the AR-R17779 experiments were 7 for the HS receptors and 4 for the LS receptors. The n values for the PNU-282987 experiments were 8 for the HS receptors and 6 for the LS receptors.
TABLE 5.
Preapplications of α7 agonists to wild-type receptors
| Drug | HS | n | LS | n | HS vs. LS | HS vs. ACh | LS versus ACh |
|---|---|---|---|---|---|---|---|
| GTS-21 | 0.064 ± 0.038 | 8 | 0.263 ± 0.122 | 7 | P < 0.05 | P < 0.01 | P < 0.05 |
| TC-1698 | 0.012 ± 0.011 | 6 | 0.042 ± 0.028 | 7 | N.S. | P < 0.01 | P < 0.05 |
| AR-R17779 | 0.518 ± 0.02 | 7 | 1.002 ± 0.039 | 4 | P < 0.0001 | P < 0.01 | N.S. |
| PNU-282987 | 0.800 ± 0.074 | 8 | 0.973 ± 0.137 | 6 | N.S. | P < 0.05 | N.S. |
Discussion
The results support the hypothesis that the responses of HS α4β2 receptors are strongly limited by desensitization, even at low agonist concentrations. They also show that the desensitization is not specifically a factor limiting the response of the HS receptors to high agonist concentrations. The survey of the several classes of ligands identified some types of compounds, like the α7 silent agonist and the α9 agonists, that appear to be free of α4β2 activating or desensitizing effects. They also indicate that desensitization is a factor limiting the efficacy of α4β2 partial agonists like cytisine and varenicline and that selective desensitization of LS receptors may tune the efficacy of agents like sazetidine-A and TC-2559 so that they are functionally HS receptor selective agonists and functionally LS receptor desensitizers.
The desensitization of nAChR is a complex and multiphasic process (Feltz and Trautmann, 1982; Simasko et al., 1986; Boyd, 1987; Sine and Steinbach, 1987; Forman and Miller, 1988; Dilger and Liu, 1992; Quick and Lester, 2002; Lester, 2004; Papke et al., 2009), and the effect of type II PAMs on α7 receptors is selective for only some form(s) of desensitization (Williams et al., 2011). Although the effects of some type II PAMs can be quite large, they do not reverse all the receptor desensitization or affect all receptors equally. Indeed, the effects of the α7 PAM PNU-120596 are to enormously increase the activation of a small fraction of receptors while the majority of receptors remain in desensitized states (Williams et al., 2011; Andersen et al., 2016). The single-channel effects of the ago-PAM 4BP-TQS (GAT107) on α7 receptors are similar to those of PNU-120596, (Pałczyńska et al., 2012; Quadri et al., 2019), while the effects of TQS on α7 ACh responses are somewhat less (Pałczyńska et al., 2012). However, the basic mechanisms of α7 desensitization are fundamentally different from those of α4β2 receptors, so likewise the TQS effects on the TQS-sensitive α4β2 receptors might be very different on the molecular level from the effects on α7 receptors. While α7 receptors show no activation at all with high agonist concentrations (Williams et al., 2011), α4β2 receptors can smolder (Campling et al., 2013), occasionally opening under predominantly desensitizing conditions.
While TQS is considered strictly a PAM for α7, since we observed it to activate the TQS-sensitive α4β2 receptors (particularly the HS receptors) when applied alone, it might be classified as a weak ago-PAM or allosteric agonist for these receptors, behaving like the TQS analog (+)4BP-TQS (GAT107) on α7 receptors. By definition, “ago-PAMs” potentiate the responses evoked by agonists but also produce activation on their own and may also prime the potentiation of subsequent agonist application. The direct allosteric activation of HS α4β2L15’M receptors by TQS was blocked by 10 µM of the α4β2-selective antagonist DHβE as well as by 100 µM MLA, a concentration at which the drug is no longer selective for α7 (Buisson et al., 1996). While the allosteric activation of α7 by GAT107 can be blocked by 10 µM of the α7 selective antagonist MLA (Papke et al., 2014b), it is insensitive to 100 µM DHβE (R.L. Papke, unpublished manuscript; data not shown). It seems unlikely that TQS itself is a suitable ligand for the ACh binding sites, since it lacks a positively charged nitrogen common to most nicotinic agonists, so it is possible that, especially for the HS receptors, there was an incomplete washout of ACh from the previous control application of ACh and that residual ACh facilitated the effects of TQS when nominally applied alone. Alternatively, TQS may actually function as a weak ago-PAM for these receptors, and occupancy of the ACh sites by the competitive antagonists might be sufficient to inhibit the allosteric activation by TQS.
The therapeutic development of α7-selective agonists for indications such as schizophrenia (Hajós and Rogers, 2010; Haydar and Dunlop, 2010; Cannon et al., 2013; Walling et al., 2016) or Alzheimer’s disease (Chen et al., 2006; D’Andrea and Nagele, 2006; Leiser et al., 2009) is largely predicated on the assumption that these drugs will not impair the normal functions of α4β2 receptors in the brain. This is a particular concern in the case of Alzheimer’s disease, since evidence suggests that α4β2 receptor function is specifically impaired in this patient population (Court et al., 2001; Gotti et al., 2006). The results with the α7-selective agonists tested indicate that, depending on the specific agent, a number of differing profiles of α7 agonism and α4β2 antagonism may be available, with PNU-282987 being the least likely to affect α4β2 function. As noted earlier, GTS-21 was the first synthetic α7-selective agonist identified, and since 1994 it has been cited in 248 PubMed publications, including 41 since 2019. PNU-282987, has been cited in 202 PubMed publications since it was first reported in 2005, and it is also in current use, with 39 citations since 2019. The α7 agonists TC-1698 and AR-R17779 are far less commonly used, with only 3 and 50 total PubMed citations, respectively. Two studies related to CAP activity reported comparable effects with GTS-21 and PNU-282987 (Yuan et al., 2021; Zhou et al., 2021). However, it is important to note that, from an electrophysiological perspective, these compounds have very different activity profiles for α7 receptors. PNU-282987 is relatively potent and nearly a full agonist (Hajós et al., 2005), while GTS-21 has lower potency and efficacy and additionally produces residual α7-receptor desensitization (Papke et al., 2009). Interestingly, the α7 desensitizing activity of GTS-21 may be important for its CAP activity (Thomsen and Mikkelsen, 2012; Horenstein and Papke, 2017). While these two agents may have similar CAP activity, it is likely that they would have distinctly different profiles in the brain, where cholinergic activity is associated with dynamically balanced function of α4β2* and α7 receptors, with GTS-21 capable of decreasing α4β2 function as well as working on α7 and PNU-282987 affecting α7 exclusively.
The perspective of nAChR as mediators of fast synaptic transmission, which is their function at neuromuscular junctions and autonomic ganglia, relegates desensitization to the background as perhaps nothing more than a safety valve to prevent overstimulation. However, as modulators of neurotransmission in the brain, often as presynaptic receptors on neurons that release other neurotransmitters, desensitization must be accounted for in the cholinergic control of brain function. Consideration of desensitization is even more important when considering the effects of self-administered nicotine, especially after a smoker’s very first cigarette puff of the day (Picciotto et al., 2008). However, does desensitization just move receptors to the sidelines, or is it possible that desensitized receptors serve other functions, independent of ion channel activity? Recent studies of α7 nAChR, especially in the context of the CAP mediated by α7, and possibly α9α10, receptors in immune cells (Tracey, 2007; Rosas-Ballina and Tracey, 2009) have suggested that the nonconducting (i.e., desensitized) conformations of those receptors function as metabotropic receptors regulating intracellular signal transduction and the release of pro- and anti-inflammatory cytokines (de Jonge and Ulloa, 2007; King et al., 2017; Kabbani and Nichols, 2018; King et al., 2018). This has led to the proposed development of weak α7 agonists like GTS-21 (Kong et al., 2018; Wang et al., 2020) and even silent agonists (; Richter et al., 2016; Horenstein and Papke, 2017; Bagdas et al., 2018; Godin et al., 2020; Papke and Horenstein, 2021) for the treatment of inflammatory and neuropathic pain.
Additionally, an exclusive focus on the ion channel activity of nAChR largely ignores potential functions for the variable, and often large intracellular domains of these receptors (Stokes et al., 2015), and interestingly the intracellular domain of α4 is the largest of any nAChR subunit, by a factor of 3 or more. Although not so well studied as α7 in this regard, there have been reports that α4-containing nAChR also play roles in the regulation of inflammatory and neuropathic pain (Nordman et al., 2014; Acharya et al., 2020) and that these effects are correlated with receptor desensitization (Zhang et al., 2012).
In conclusion, the use of TQS-sensitive receptors provides a way to probe the ligand dependence of the conformational equilibrium of the two primary forms of the brain’s α4β2 receptors and may prove useful for the development of more focused therapeutics.
Acknowledgments
Oocyte recordings were conducted by Lu Wenchi Corrie.
Abbreviations
- ACh
acetylcholine
- HS
high sensitivity
- LS
low sensitivity
- MLA
methyllycaconitine citrate
- nAChR
nicotinic acetylcholine receptor
- PAM
positive allosteric modulators
Authorship Contributions
Participated in research design: Papke.
Performed data analysis: Papke, Stokes.
Wrote or contributed to the writing of the manuscript: Papke, Stokes.
Footnotes
This work was support by National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM57481].
Neither author has an actual or perceived conflict of interest with the contents of this article.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Acharya S, Kundu D, Choi HJ, Kim KM (2020) Metabotropic signaling cascade involved in α4β2 nicotinic acetylcholine receptor-mediated PKCβII activation. Biochim Biophys Acta Mol Cell Res 1867:118721. [DOI] [PubMed] [Google Scholar]
- Aickin M, Gensler H (1996) Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health 86:726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alijevic O, McHugh D, Rufener L, Mazurov A, Hoeng J, Peitsch M (2020) An electrophysiological characterization of naturally occurring tobacco alkaloids and their action on human α4β2 and α7 nicotinic acetylcholine receptors. Phytochemistry 170:112187. [DOI] [PubMed] [Google Scholar]
- Andersen ND, Nielsen BE, Corradi J, Tolosa MF, Feuerbach D, Arias HR, Bouzat C (2016) Exploring the positive allosteric modulation of human α7 nicotinic receptors from a single-channel perspective. Neuropharmacology 107:189–200. [DOI] [PubMed] [Google Scholar]
- Badio B, Daly JW (1994) Epibatidine, a potent analgetic and nicotinic agonist. Mol Pharmacol 45:563–569. [PubMed] [Google Scholar]
- Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI (2018) New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: a focus on α7 nAChRs. Curr Neuropharmacol 16:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar ALCortes-Burgos LACook KKDinh DMGroppi VEHajós MHigdon NRHoffmann WEHurst RSMyers JK, et al. (2005) Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors. J Med Chem 48:905–908. [DOI] [PubMed] [Google Scholar]
- Boyd ND (1987) Two distinct kinetic phases of desensitization of acetylcholine receptors of clonal rat PC12 cells. J Physiol 389:45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CA, McKenna DG (1998) Activation and inhibition of the human alpha7 nicotinic acetylcholine receptor by agonists. Neuropharmacology 37:1095–1102. [DOI] [PubMed] [Google Scholar]
- Buisson B, Gopalakrishnan M, Arneric SP, Sullivan JP, Bertrand D (1996) Human alpha4beta2 neuronal nicotinic acetylcholine receptor in HEK 293 cells: a patch-clamp study. J Neurosci 16:7880–7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campling BG, Kuryatov A, Lindstrom J (2013) Acute activation, desensitization and smoldering activation of human acetylcholine receptors. PLoS One 8:e79653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CE, Puri V, Vivian JA, Egbertson MS, Eddins D, Uslaner JM (2013) The nicotinic α7 receptor agonist GTS-21 improves cognitive performance in ketamine impaired rhesus monkeys. Neuropharmacology 64:191–196. [DOI] [PubMed] [Google Scholar]
- Chen L, Yamada K, Nabeshima T, Sokabe M (2006) Alpha7 nicotinic acetylcholine receptor as a target to rescue deficit in hippocampal LTP induction in beta-amyloid infused rats. Neuropharmacology 50:254–268. [DOI] [PubMed] [Google Scholar]
- Coe JWBrooks PRVetelino MGWirtz MCArnold EPHuang JSands SBDavis TILebel LAFox CB, et al. (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477. [DOI] [PubMed] [Google Scholar]
- Corrie LW, Stokes C, Wilkerson JL, Carroll FI, McMahon LR, Papke RL (2020) Nicotinic acetylcholine receptor accessory subunits determine the activity profile of epibatidine derivatives. Mol Pharmacol 98:328–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E (2001) Nicotinic receptor abnormalities in Alzheimer’s disease. Biol Psychiatry 49:175–184. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Li M, Dwoskin LP (1995) Determination of nicotine metabolites in rat brain after peripheral radiolabeled nicotine administration: detection of nornicotine. Drug Metab Dispos 23:1175–1177. [PubMed] [Google Scholar]
- D’Andrea MR, Nagele RG (2006) Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer’s disease pyramidal neurons. Curr Pharm Des 12:677–684. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Welch SP, Martin BR (1995) In vivo pharmacological effects of dihydro-beta-erythroidine, a nicotinic antagonist, in mice. Psychopharmacology (Berl) 117:67–73. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, Meyer EM, Zoltewicz J, Henry JC, Muraskin S, Kem WR, Papke RL (1995) Characterization of a family of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative (DMAC) is a selective agonist at neuronal nicotinic a7/[125I]a-bungarotoxin receptor subtypes. Mol Pharm 47:164–171. [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151:915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M (1997) Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol 53:603–625. [DOI] [PubMed] [Google Scholar]
- Dilger JP, Liu Y (1992) Desensitization of acetylcholine receptors in BC3H-1 cells. Pflugers Arch 420:479–485. [DOI] [PubMed] [Google Scholar]
- Eaton JB, Lucero LM, Stratton H, Chang Y, Cooper JF, Lindstrom JM, Lukas RJ, Whiteaker P (2014) The unique α4+/-α4 agonist binding site in (α4)3(β2)2 subtype nicotinic acetylcholine receptors permits differential agonist desensitization pharmacology versus the (α4)2(β2)3 subtype. J Pharmacol Exp Ther 348:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM (2008) Cytisine for smoking cessation: a research agenda. Drug Alcohol Depend 92:3–8. [DOI] [PubMed] [Google Scholar]
- Fasoli F, Moretti M, Zoli M, Pistillo F, Crespi A, Clementi F, Mc Clure-Begley T, Marks MJ, Gotti C (2016) In vivo chronic nicotine exposure differentially and reversibly affects upregulation and stoichiometry of α4β2 nicotinic receptors in cortex and thalamus. Neuropharmacology 108:324–331. [DOI] [PubMed] [Google Scholar]
- Feltz A, Trautmann A (1982) Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol 322:257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SA, Miller KW (1988) High acetylcholine concentrations cause rapid inactivation before fast desensitization in nicotinic acetylcholine receptors from Torpedo. Biophys J 54:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, Lindstrom J (1995) Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharmacol 48:774–782. [PubMed] [Google Scholar]
- Godin JRRoy PQuadri MBagdas DToma WNarendrula-Kotha RKishta OADamaj MIHorenstein NAPapke RL, et al. (2020) A silent agonist of α7 nicotinic acetylcholine receptors modulates inflammation ex vivo and attenuates EAE. Brain Behav Immun 87:286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti CMoretti MBohr IZiabreva IVailati SLonghi RRiganti LGaimarri AMcKeith IGPerry RH, et al. (2006) Selective nicotinic acetylcholine receptor subunit deficits identified in Alzheimer’s disease, Parkinson’s disease and dementia with Lewy bodies by immunoprecipitation. Neurobiol Dis 23:481–489. [DOI] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J (2007) Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72:715–724. [DOI] [PubMed] [Google Scholar]
- Gupta PC, Warnakulasuriya S (2002) Global epidemiology of areca nut usage. Addict Biol 7:77–83. [DOI] [PubMed] [Google Scholar]
- Hajós M, Hurst RS, Hoffmann WE, Krause M, Wall TM, Higdon NR, Groppi VE (2005) The selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J Pharmacol Exp Ther 312:1213–1222. [DOI] [PubMed] [Google Scholar]
- Hajós M, Rogers BN (2010) Targeting alpha7 nicotinic acetylcholine receptors in the treatment of schizophrenia. Curr Pharm Des 16:538–554. [DOI] [PubMed] [Google Scholar]
- Haydar SN, Dunlop J (2010) Neuronal nicotinic acetylcholine receptors—targets for the development of drugs to treat cognitive impairment associated with schizophrenia and Alzheimer’s disease. Curr Top Med Chem 10:144–152. [DOI] [PubMed] [Google Scholar]
- Horenstein NA, Papke RL (2017) Anti-inflammatory silent agonists. ACS Med Chem Lett 8:989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N, Nichols RA (2018) Beyond the channel: metabotropic signaling by nicotinic receptors. Trends Pharmacol Sci 39:354–366. [DOI] [PubMed] [Google Scholar]
- Katz B, Thesleff S (1957) A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol 138:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kem WR, Mahnir VM, Papke RL, Lingle CJ (1997) Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors. J Pharmacol Exp Ther 283:979–992. [PubMed] [Google Scholar]
- King JR, Gillevet TC, Kabbani N (2017) A G protein-coupled α7 nicotinic receptor regulates signaling and TNF-α release in microglia. FEBS Open Bio 7:1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Ullah A, Bak E, Jafri MS, Kabbani N (2018) Ionotropic and metabotropic mechanisms of allosteric modulation of α7 nicotinic receptor intracellular calcium. Mol Pharmacol 93:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong WKang KGao YLiu HMeng XCao YYang SLiu WZhang JYu K, et al. (2018) GTS-21 protected against LPS-induced sepsis myocardial injury in mice through α7nAChR. Inflammation 41:1073–1083. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J (2005) Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol 68:1839–1851. [DOI] [PubMed] [Google Scholar]
- Land BR, Salpeter EE, Salpeter MM (1981) Kinetic parameters for acetylcholine interaction in intact neuromuscular junction. Proc Natl Acad Sci USA 78:7200–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SC, Bowlby MR, Comery TA, Dunlop J (2009) A cog in cognition: how the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther 122:302–311. [DOI] [PubMed] [Google Scholar]
- Lester RA (2004) Activation and desensitization of heteromeric neuronal nicotinic receptors: implications for non-synaptic transmission. Bioorg Med Chem Lett 14:1897–1900. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Blosser J, Gordon J (1999) AR-R17779, and alpha7 nicotinic agonist, improves learning and memory in rats. Behav Pharmacol 10:675–680. [DOI] [PubMed] [Google Scholar]
- López-Hernández GY, Sánchez-Padilla J, Ortiz-Acevedo A, Lizardi-Ortiz J, Salas-Vincenty J, Rojas LV, Lasalde-Dominicci JA (2004) Nicotine-induced up-regulation and desensitization of alpha4beta2 neuronal nicotinic receptors depend on subunit ratio. J Biol Chem 279:38007–38015. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Papke RL, Bhatti BS, Shaw S, Bencherif M (2004) The neuroprotective effect of 2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane (TC-1698), a novel alpha7 ligand, is prevented through angiotensin II activation of a tyrosine phosphatase. J Pharmacol Exp Ther 309:16–27. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56:237–246. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J (2003) Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 63:332–341. [DOI] [PubMed] [Google Scholar]
- Nordman JC, Muldoon P, Clark S, Damaj MI, Kabbani N (2014) The α4 nicotinic receptor promotes CD4+ T-cell proliferation and a helper T-cell immune response. Mol Pharmacol 85:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pałczyńska MM, Jindrichova M, Gibb AJ, Millar NS (2012) Activation of α7 nicotinic receptors by orthosteric and allosteric agonists: influence on single-channel kinetics and conductance. Mol Pharmacol 82:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL (2002) Enhanced inhibition of a mutant neuronal nicotinic acetylcholine receptor by agonists: protection of function by (E)-N-methyl-4-(3-pyridinyl)-3-butene-1-amine (TC-2403). J Pharmacol Exp Ther 301:765–773. [DOI] [PubMed] [Google Scholar]
- Papke RL (2010) Tricks of perspective: insights and limitations to the study of macroscopic currents for the analysis of nAChR activation and desensitization. J Mol Neurosci 40:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Andleeb H, Stokes C, Quadri M, Horenstein NA (2022a) Selective agonists and antagonists of α9 versus α7 nicotinic acetylcholine receptors. ACS Chem Neurosci 13:624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P (1996) An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett 213:201–204. [DOI] [PubMed] [Google Scholar]
- Papke RL, Chojnacka K, Horenstein NA (2014a) The minimal pharmacophore for silent agonism of alpha7 nAChR. J Pharmacol Exp Ther 350:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA (2007) The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem 101:160–167. [DOI] [PubMed] [Google Scholar]
- Papke RL, Horenstein NA (2021) Therapeutic targeting of alpha7 nicotinic acetylcholine receptors. Pharmacol Rev 73:1118–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Horenstein NA, Kulkarni AR, Stokes C, Corrie LW, Maeng CY, Thakur GA (2014b) The activity of GAT107, an allosteric activator and positive modulator of α7 nicotinic acetylcholine receptors (nAChR), is regulated by aromatic amino acids that span the subunit interface. J Biol Chem 289:4515–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Horenstein NA, Stokes C (2015) Nicotinic activity of arecoline, the psychoactive element of “betel nuts,” suggests a basis for habitual use and anti-inflammatory activity. PLoS One 10:e0140907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Karaffa M, Horenstein NA, Stokes C (2022b) Coffee and cigarettes: modulation of high and low sensitivity α4β2 nicotinic acetylcholine receptors by n-MP, a biomarker of coffee consumption. Neuropharmacology 216:109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA (2009) Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther 329:791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Stokes C (2010) Working with OpusXpress: methods for high volume oocyte experiments. Methods 51:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MJ, Beck A, Luetje CW (1998) Neuronal nicotinic receptor beta2 and beta4 subunits confer large differences in agonist binding affinity. Mol Pharmacol 54:1132–1139. [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008) It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84:329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Garai S, Thakur GA, Stokes C, Gulsevin A, Horenstein NA, Papke RL (2019) Macroscopic and microscopic activation of alpha7 nicotinic acetylcholine receptors by the structurally unrelated ago-PAMs B-973B and GAT107. Mol Pharmacol 95:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Papke RL, Horenstein NA (2016) Dissection of N,N-diethyl-N’-phenylpiperazines as α7 nicotinic receptor silent agonists. Bioorg Med Chem 24:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Lester RA (2002) Desensitization of neuronal nicotinic receptors. J Neurobiol 53:457–478. [DOI] [PubMed] [Google Scholar]
- Richter KMathes VFronius MAlthaus MHecker AKrasteva-Christ GPadberg WHone AJMcIntosh JMZakrzewicz A, et al. (2016) Phosphocholine—an agonist of metabotropic but not of ionotropic functions of α9-containing nicotinic acetylcholine receptors. Sci Rep 6:28660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ (2009) Cholinergic control of inflammation. J Intern Med 265:663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simasko SM, Soares JR, Weiland GA (1986) Two components of carbamylcholine-induced loss of nicotinic acetylcholine receptor function in the neuronal cell line PC12. Mol Pharmacol 30:6–12. [PubMed] [Google Scholar]
- Sine SM, Steinbach JH (1987) Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol 385:325–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, Lester HA (2011) Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol 137:59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C, Garai S, Kulkarni AR, Cantwell LN, Noviello CM, Hibbs RE, Horenstein NA, Abboud KA, Thakur GA, Papke RL (2019) Heteromeric neuronal nicotinic acetylcholine receptors with mutant β subunits acquire sensitivity to α7-selective positive allosteric modulators. J Pharmacol Exp Ther 370:252–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C, Treinin M, Papke RL (2015) Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol Sci 36:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swango JH, Bhatti BS, Qureshi MM, Crooks PA(1999) A novel enantioselective synthesis of (S)-(?)- and (R)-(+)-nornicotine via alkylation of a chiral 2-hydroxy-3-pinanone ketimine template. Chirality 11:316–318. [DOI] [PubMed] [Google Scholar]
- Tan X, Vrana K, Ding ZM (2021) Cotinine: pharmacologically active metabolite of nicotine and neural mechanisms for its actions. Front Behav Neurosci 15:758252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MS, Mikkelsen JD (2012) The α7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-α release from microglia. J Neuroimmunol 251:65–72. [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek JW, Kang CH, Campbell JE, Arneric SP, Sullivan JP (1995) A sensitive technique for the detection of the alpha 7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J Neurosci Methods 61:113–118. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL (2002) Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res 948:33–46. [DOI] [PubMed] [Google Scholar]
- Walling DMarder SRKane JFleischhacker WWKeefe RSHosford DADvergsten CSegreti ACBeaver JSToler SM, et al. (2016) Phase 2 trial of an alpha-7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull 42:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cai D, Chen Z, Wang Y (2020) GTS-21 promotes α7 nAChR to alleviate intestinal ischemia-reperfusion-induced apoptosis and inflammation of enterocytes. Med Sci Monit 26:e921618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kuryatov A, Sriram A, Jin Z, Kamenecka TM, Kenny PJ, Lindstrom J (2015) An accessory agonist binding site promotes activation of α4β2* nicotinic acetylcholine receptors. J Biol Chem 290:13907–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin MM, Schulte MK (2010) Pharmacological characterization of the allosteric modulator desformylflustrabromine and its interaction with alpha4beta2 neuronal nicotinic acetylcholine receptor orthosteric ligands. J Pharmacol Exp Ther 334:917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JW, Olubajo O, Storm CB, Duffield RM (1981) Anabaseine: venom alkaloid of aphaenogaster ants. Science 211:1051–1052. [DOI] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL (2011) Investigation of the molecular mechanism of the alpha7 nAChR positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol Pharmacol 80:1013–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Ashley DL, Watson CH (2002) Determination of nicotine and other minor alkaloids in international cigarettes by solid-phase microextraction and gas chromatography/mass spectrometry. Anal Chem 74:4878–4884. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ (2006) Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol 70:1454–1460. [DOI] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS (2008) Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci USA 105:14686–14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan FJiang LLi QSokulsky LWanyan YWang LLiu XZhou LTay HLZhang G, et al. (2021) A selective α7 nicotinic acetylcholine receptor agonist, PNU-282987, attenuates ILC2s activation and alternaria-induced airway inflammation. Front Immunol 11:598165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JXiao YDJordan KGHammond PSVan Dyke KMMazurov AASpeake JDLippiello PMJames JWLetchworth SR, et al. (2012) Analgesic effects mediated by neuronal nicotinic acetylcholine receptor agonists: correlation with desensitization of α4β2* receptors. Eur J Pharm Sci 47:813–823. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zheng LF, Zhang XL, Wang ZY, Yao YS, Xiu XL, Liu CZ, Zhang Y, Feng XY, Zhu JX (2021) Activation of α7nAChR protects against gastric inflammation and dysmotility in Parkinson’s disease rats. Front Pharmacol 12:793374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J (2003) Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci 23:9004–9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Broad LM, Xi Q, Lee M, Moroni M, Bermudez I, Sher E (2006) 5-I A-85380 and TC-2559 differentially activate heterologously expressed alpha4beta2 nicotinic receptors. Eur J Pharmacol 539:10–17. [DOI] [PubMed] [Google Scholar]
- Zwart RCarbone ALMoroni MBermudez IMogg AJFolly EABroad LMWilliams ACZhang DDing C, et al. (2008) Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol 73:1838–1843. [DOI] [PubMed] [Google Scholar]