Key Points
-
•
The median ANC among Duffy-null individuals is significantly lower than that among Duffy non-null individuals.
-
•
Nearly a quarter (23.8%) of Duffy-null individuals had an ANC lower than the reference range (<2000 cells/μL) which likely causes iatrogenic harm.
Abstract
Many people of African ancestry have lower absolute neutrophil counts (ANCs) without increased risk for infection. This is associated with the Duffy-null phenotype (nonexpression of the Duffy antigen on red blood cells), which is commonly found in those of African descent. Currently, there are no studies that compare the ANC of individuals with Duffy-null phenotype to those with Duffy non-null phenotypes within a self-identified Black population. The aim of this study was to assess the impact of Duffy status on ANCs based on complete blood counts with differential and Duffy testing in a healthy population of self-identified Black individuals at a single primary care center. This study found that 66.7% (80 of 120) of Black individuals have the Duffy-null phenotype and that there is a significant difference in ANCs between Duffy-null and Duffy non-null individuals (median, 2820 cells per μL vs 5005 cells per μL; P < .001). Additionally, 19 of 80 (23.8%) Duffy-null individuals had an ANC of <2000 cells per μL compared with no (0) Duffy non-null individuals. The Duffy-null phenotype is clinically insignificant; however, inappropriate reference ranges can propagate systemic racism. Therefore, we advocate for the development of Duffy-null–specific ANC reference ranges as well as replacing the term benign ethnic neutropenia with Duffy-nullassociated neutrophil count.
Introduction
It is a well-established fact that the absolute neutrophil count (ANC) in many individuals with African ancestry is lower than that in White individuals.1,2 Population data reveal that African Americans have an average white blood cell count that is ∼700 cells per μL lower than that of White Americans.1 Additionally, the prevalence of neutropenia (ANC <1500 cells per μL) is reported to be 4.5% among Black individuals and 0.79% among White people in the United States.3 Most Black people with lower ANC have no increased risk of infection, and this clinically insignificant reduction in ANC has been previously referred to as benign ethnic neutropenia.4,5
Lower neutrophil counts were historically linked with African ancestry, but we now know that this lower ANC is strongly associated with a single nucleotide polymorphism residing in the promoter region of the DARC gene that prevents transcription and results in the red blood cell Duffy-null phenotype [Fy(a−b−)].6,7 The Duffy antigen is postulated to be a cytokine sink that binds to inflammatory cytokines, and therefore, the null form attracts neutrophils into the periphery less readily.8 Additionally, this single nucleotide polymorphism affects hematopoiesis and results in phenotypically distinct neutrophils that readily leave the periphery.9 However, people with the Duffy-null phenotype have normal bone marrow cellularity and maturation as well as a robust response to infection.10,11 Thus, Duffy-null individuals are hypothesized to have appropriate neutrophil function and response despite lower baseline circulating neutrophils.
The geographic distribution of the Duffy-null phenotype correlates closely with the geographic ancestry typically associated with lower neutrophil counts. This is likely because the null phenotype provides a selective advantage against Plasmodium vivax.12 In fact, the Duffy-null phenotype is seen in 80% to 100% of people from Sub-Saharan Africa, but in <1% of people of Asian or Caucasian descent.13,14 Interestingly, ANC reference ranges from African countries, where the majority of the population is Duffy null, are much lower than the ANC reference range in the United States. There is greater admixture in the United States, but ∼68% of people identifying as Black or African American are expected to have the Duffy-null phenotype.15
Although studies show that (1) ANCs are lower in Black individuals at a population level; (2) Duffy-null status is closely correlated with lower ANCs; and (3) most Black individuals have the Duffy-null phenotype, to our knowledge, there are no studies that elucidate the expected difference in ANCs between those with Duffy-null vs Duffy non-null phenotypes within a Black population. It is possible that there are other genetic or environmental factors that could contribute to the lower ANC observed among those of African ancestry in addition to the impact of the Duffy-null phenotype. This study aims to assess the impact of the Duffy-null phenotype on ANCs within a healthy, self-identified Black population.
Methods
We performed a cross-sectional study in adults presenting for nonurgent care visits at a single primary care center. Local institutional review board approval was obtained. Race was ascertained through electronic health record demographic data. Patient lists were made by generating an electronic health record report selecting for the inclusion criteria of self-identified “Black or African American” race and presenting for nonurgent primary care. Exclusion criteria are shown in supplemental Table 1. Of 6551 individuals screened for eligibility, 1354 patients (20.7%) were excluded. Verbal consent was obtained, and samples were collected for this study if any laboratory draws were obtained at the appointment. Complete blood counts with differential were performed on a Sysmex XN-9000 analyzer (Sysmex, Lincolnshire, IL). Phenotyping for Fya and Fyb was performed by tube testing using serologic reagents from Bio-Rad (Hercules, CA) and Quotient (Newtown, PA), respectively. Genotyping was performed using the PreciseType HEA Molecular BeadChip Test (Immucor, Norcross, GA).
A power calculation performed before enrollment found that 120 individuals were needed to provide 83% power to detect a 0.50 standard deviation difference between Duffy-null and Duffy non-null groups. Of the 5197 eligible individuals, 1326 randomly selected individuals (25.5%) were contacted. One hundred ninety-seven individuals consented (14.9%), of whom 121 (61.4%) completed the study, and 1 was excluded because of a laboratory error. We speculate that the low enrollment rate is likely from recruitment during the COVID-19 pandemic, historical mistrust of the medical system, difficulty connecting with individuals through phone calls and email, and no clear personal benefit in participating. Fisher exact tests were used to assess the association between ANC <2000 cells per μL and Duffy status. A two-sided Wilcoxon rank-sum test was performed to compare age and ANC between Duffy-null and Duffy non-null groups with a preset significance level of .05, and a one-sample Wilcoxon signed-rank test was used to assess ANC reference median to our cohorts.
Results and discussion
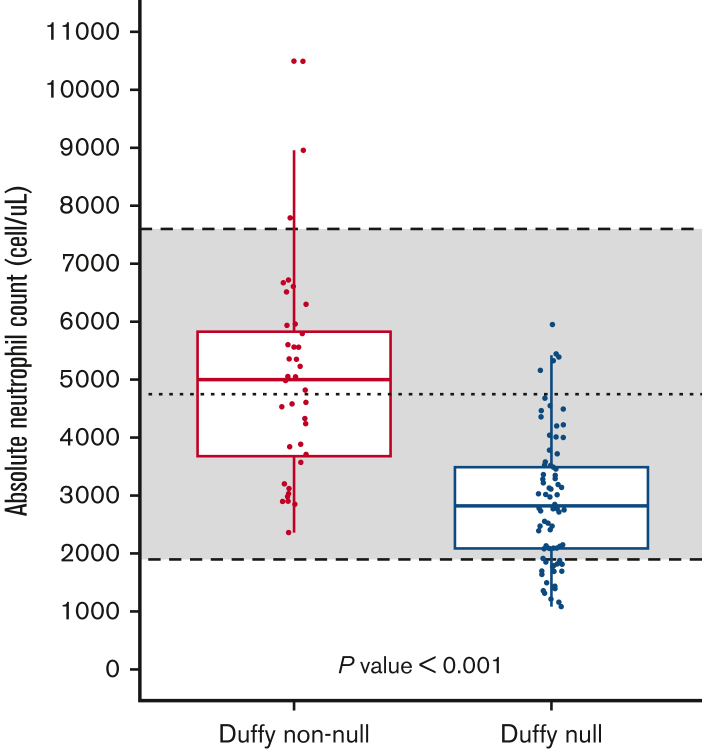
Among the 120 participants, 80 (66.7%) were Duffy-null [Fy(a−b−)], and the other 40 participants (33.3%) were Duffy non-null. Based on serologic testing, 1 participant was Fy(a+b+), whereas 39 participants (32.5%) were Fy(a−b+) or Fy(a+b−). Demographic and laboratory features by Duffy status are tabulated in Table 1. There was no significant difference between the Duffy non-null group and the reference range (P = .64), but there was a significant difference in the ANC by Duffy status (Figure 1).
Table 1.
Demographic and laboratory data by the Duffy phenotype among healthy individuals identifying as Black or African American at a single primary care center
| Duffy non-null (n = 40) | Duffy-null (n = 80) | P value | |
|---|---|---|---|
| Median age (IQR), y | 50 (37-59) | 51 (41-60) | .53 |
| Median ANC (IQR), cells per μL | 5 005 (3 675-5 828) | 2820 (2088-3490) | <.001 |
| Mean ANC (range), cells per μL | 4 972 (2 360-10 500) | 2907 (1080-5950) | — |
| ANC <2000 cells per μL, n (%) | 0 (0) | 19 (23.8%) | <.001 |
| ANC <1500 cells per μL, n (%) | 0 (0) | 8 (10.0%) | — |
IQR, interquartile range.
Bold indicates statistical signficance.
Figure 1.
ANC by the Duffy phenotype in Black patients who presented for a routine primary care visit. The shaded area represents the reference range for ANC at the institution where the blood work was obtained, with the dashed line indicating the reference median. Duffy-null refers to the Fy(a−b−) phenotype, and Duffy non-null indicates Fy(a−b+), Fy(a+b−), or Fy(a+b+) phenotypes, as determined serologically. Dots show individual ANC values, whereas the box represents median ANC and interquartile range. There is a statistically significant difference in ANC between the Duffy non-null (n = 40) and the Duffy-null (n = 80) individuals (P < .001). There is no statistically significant difference between the Duffy non-null ANC median and the reference median (P = .64), but there is a significant difference between the Duffy-null ANC median and the reference median (P < .001).
This study of healthy Black individuals presenting for routine primary care shows a significant difference in the ANC by Duffy status, with nearly a quarter of Duffy-null individuals having an ANC below the lower limit of “normal” of 2000 cells per μL and 1 in 10 individuals having neutropenia (ANC <1500 cells per μL) compared with no difference from current reference interval among Duffy non-null individuals. Additionally, the median ANC of Duffy-null individuals was more than 2000 cells per uL lower than the median ANC of Duffy non-null individuals, which is a much greater difference than anticipated. It is Duffy-null status rather than the race that predicts lower ANC, and the difference in median ANC is profound.
Although Duffy-null status is clinically insignificant, ANC reference ranges that do not reflect Duffy-null individuals (the majority phenotype among Black individuals) can cause iatrogenic harm. For example, among Black individuals who underwent bone marrow biopsy for ANCs below the lower limit of normal, 97% were Duffy-null and 97% of these Duffy-null individuals had a normal biopsy result.16 In rheumatology, azathioprine discontinuation for lower ANC was shown to be higher in Black Duffy-null patients than Black Duffy non-null or White patients.17 Additionally, many clinical trials require an ANC >2000 cells per μL for enrollment, which means that 16% of the healthy individuals in this cohort would have been excluded, extrapolated to ∼6.6 million Americans potentially excluded from clinical trials. This is unacceptable and directly contributes to the lack of racial diversity in clinical trials.
Laboratory reference intervals should be critically examined to ensure all healthy individuals are accurately represented, and alternatives driven by biologic markers should be used when appropriate, such as von Willebrand factor intervals by blood type. This study emphasizes that race-specific intervals are not sufficient because ANCs of Black Duffy non-null individuals did not differ from the current reference range. Additionally, race is a social construct and a poor proxy when there is a simple test of biologic variation such as the Duffy phenotype to inform care.
Thus, we advocate for tangible actions to address this inequity. First, we propose erasing the term “benign ethnic neutropenia” from the lexicon and replacing it with “Duffy-null associated neutrophil count.” This change depathologizes a common and healthy variant as well as correlates the variant with a biologic marker rather than race or ethnicity.18 Second, these data support the pressing need for the implementation of Duffy-null–specific ANC reference ranges. Future studies should establish ANC reference ranges for Duffy-null individuals and investigate the impact of this variant on other aspects of clinical care such as administration of chemotherapeutics. These investigations would likely improve health care for ∼27 million Americans and ameliorate a significant inequity in medicine.
Conflict-of-interest disclosure: M.O.A. is a member of the scientific advisory board for Pharmacosmos A/S, AMAG, Global Blood Therapeutics, and Fulcrum Pharmaceuticals. The remaining authors declare no competing financial interests.
Acknowledgments
Authorship
Contribution: L.E.M. designed the study, performed research, analyzed data, and wrote the paper; C.M.S. and M.A.O. performed research and wrote the paper; H.S.P., K.J., R.S.-W., and R.Y.F. performed research; S.R. and D.N. analyzed data; and R.M.K. and M.O.A. designed the study and wrote the paper.
Footnotes
Original data are available on request from Maureen O. Achebe (machebe@bwh.harvard.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Lim EM, Cembrowski G, Cembrowski M, Clarke G. Race-specific WBC and neutrophil count reference intervals. Int J Lab Hematol. 2010;32(6 Pt 2):590–597. doi: 10.1111/j.1751-553X.2010.01223.x. [DOI] [PubMed] [Google Scholar]
- 2.Shoenfeld Y, Alkan ML, Asaly A, Carmeli Y, Katz M. Benign familial leukopenia and neutropenia in different ethnic groups. Eur J Haematol. 1988;41(3):273–277. doi: 10.1111/j.1600-0609.1988.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146(7):486–492. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 4.Legge SE, Christensen RH, Petersen L, et al. The Duffy-null genotype and risk of infection. Hum Mol Genet. 2020;29(20):3341–3349. doi: 10.1093/hmg/ddaa208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atallah-Yunes SA, Ready A, Newburger PE. Benign ethnic neutropenia. Blood Rev. 2019;37:100586. doi: 10.1016/j.blre.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afenyi-Annan A, Ashley-Koch A, Telen MJ. Duffy (Fy), DARC, and neutropenia among women from the United States, Europe and the Caribbean. Br J Haematol. 2009;145(2):266–267. doi: 10.1111/j.1365-2141.2009.07588.x. [DOI] [PubMed] [Google Scholar]
- 7.Barreto M, Lipay ME, Santos LD, et al. Duffy phenotyping and FY∗B-67T/C genotyping as screening test for benign constitutional neutropenia. Hematol Transfus Cell Ther. 2021;43(4):489–493. doi: 10.1016/j.htct.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rappoport N, Simon AJ, Amariglio N, Rechavi G. The Duffy antigen receptor for chemokines, ACKR1,- ‘Jeanne DARC’ of benign neutropenia. Br J Haematol. 2019;184(4):497–507. doi: 10.1111/bjh.15730. [DOI] [PubMed] [Google Scholar]
- 9.Duchene J, Novitzky-Basso I, Thiriot A, et al. Atypical chemokine receptor 1 on nucleated erythroid cells regulates hematopoiesis. Nat Immunol. 2017;18(7):753–761. doi: 10.1038/ni.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason BA, Lessin L, Schechter GP. Marrow granulocyte reserves in Black Americans. Hydrocortisone-induced granulocytosis in the “benign” neutropenia of the black. Am J Med. 1979;67(2):201–205. doi: 10.1016/0002-9343(79)90391-7. [DOI] [PubMed] [Google Scholar]
- 11.Vasu S, Leitman SF, Tisdale JF, et al. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood. 2008;112(5):2092–2100. doi: 10.1182/blood-2008-03-143677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langhi DM, Jr., Bordin JO. Duffy blood group and malaria. Hematology. 2006;11(5):389–398. doi: 10.1080/10245330500469841. [DOI] [PubMed] [Google Scholar]
- 13.Howes RE, Patil AP, Piel FB, et al. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266. doi: 10.1038/ncomms1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reich D, Nalls MA, Kao WH, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 2009;5(1):e1000360. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean L. In: Blood groups and red cell antigens. (US) NCfBI, editor. National Center for Biotechnology Information (US); Bethesda, MD: 2005. Chapter 9, The Duffy blood group.https://www.ncbi.nlm.nih.gov/books/NBK2271/ [Internet] [Google Scholar]
- 16.Van Driest SL, Abul-Husn NS, Glessner JT, et al. Association between a common, benign genotype and unnecessary bone marrow biopsies among African American patients. JAMA Intern Med. 2021;181(8):1100–1105. doi: 10.1001/jamainternmed.2021.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson AL, Daniel LL, Jackson E, et al. Race, genotype, and azathioprine discontinuation. Ann Intern Med. 2022;175(8):1092–1099. doi: 10.7326/M21-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merz LE, Achebe M. When non-Whiteness becomes a condition. Blood. 2021;137(1):13–15. doi: 10.1182/blood.2020008600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.