Abstract
The role of gut microbiota in health and diseases has been receiving increased attention recently. Emerging evidence from previous studies on gut-microbiota-brain axis highlighted the importance of gut microbiota in neurological disorders. Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating disease of the central nervous system (CNS) resulting from T-cell-driven, myelin-directed autoimmunity. The dysbiosis of gut microbiota in MS patients has been reported in published research studies, indicating that gut microbiota plays an important role in the pathogenesis of MS. Gut microbiota have also been reported to influence the initiation of disease and severity of experimental autoimmune encephalomyelitis, which is the animal model of MS. However, the underlying mechanisms of gut microbiota involvement in the pathogenesis of MS remain unclear. Therefore, in this review, we summerized the potential mechanisms for gut microbiota involvement in the pathogenesis of MS, including increasing the permeability of the intestinal barrier, initiating an autoimmune response, disrupting the blood-brain barrier integrity, and contributing to chronic inflammation. The possibility for gut microbiota as a target for MS therapy has also been discussed. This review provides new insight into understanding the role of gut microbiota in neurological and inflammatory diseases.
Keywords: Gut microbiota, multiple sclerosis, neuro-inflammatory diseases, gut-microbiota-brain axis, blood-brain barrier, fecal microbiota transplantation, antibiotic treatment, probiotic microbiota
1. INTRODUCTION
The human microbiota, mainly composed of non-pathogenic bacteria, is widely distributed on the skin, in the mouth, and in the respiratory, urogenital, and gastrointestinal (GI) tracts [1, 2]. Adult human microbiota express 100 times more genes than the human genome [3, 4]. The human microbiome is involved in various physiological functions, including digestive, immune, metabolic, and even neurological functions [5-7]. The intestinal epithelium provides the largest human-microbial interface [8], making the gut microbiota the most abundant microbial ecosystem in humans (containing up to 100 trillion microorganisms belonging to more than 1000 microbial species) to support various physiological functions [3, 9]. The dysbiosis of gut microbiota has been reported to be linked with various diseases, including neurological diseases [10, 11], gastric disorders [12], diabetes [13], asthma [14], and obesity [15]. Gut microbiota could regulate the development and function of the central nervous system (CNS) through the immune and circulatory pathways, and in turn, the CNS can also influence the constitution of gut microbiota [6, 16].
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating disease of the CNS that mainly affects the white matter of the brain, spinal cord, and optic nerves [17]. It has become the most common cause of neurologic disability in young adults with a prevalence of 24 to 265 in 100000 people and an incidence of 7 to 20 per 100,000 people per year in western populations from 2001 to 2016 [18]. The occurrence of this disease is more common in females and the sex ratio is around 3:1 (female: male) [19]. MS is currently considered to be a disorder existing within a spectrum extending from a relapsing disease to a progressive disease [20]. Various mechanisms have been reported to play important roles in the initiation and development of MS, including the disruption of the blood brain barrier (BBB), T-cell-driven and myelin-directed autoimmunity, chronic inflammation, neurodegeneration caused by oligodendrocyte damage, and axonal loss [21, 22]. Because of recent advancements in understanding MS pathology, MS treatment has been further developed and mainly consisting of disease-modifying therapies administered in the early stages of the disease (including immunosuppressant, immunomodulatory, and immune reconstitution therapies) and symptomatic therapies used for different symptoms of the disease such as anticholinergics for bladder dysfunction and medication for neuropathic pain [23].
Although the etiology of MS is still not completely understood, accumulating evidence indicates that a combination of factors including environment and genetic susceptibility drive the initiation, development, and progression of MS [24-28]. Environmental factors (including viruses infection, smoking, sun exposure and diet) account for 2/3 risk of MS as monozygotic twins have only 30% concordance to develop MS [27, 28], and may act many years before clinical onset [29]. Among these factors, diet is an important element that influences the diversity and number of gut microbiota [30]. Moreover, in the past few years, increasing evidence suggests that gut microbiota participate in the pathology of MS development [31-37]. Therefore, in this manuscript, we aim to summarize the evidence of gut microbiota involvement in the pathogenesis of MS and the potential mechanism by which gut microbiota modulates the development of MS. These delineations might provide a comprehensive insight for researchers to understand the role of gut microbiota in CNS diseases.
2. THE INTERACTION BETWEEN GUT MICROBIOTA AND THE CNS
Gut microbiota has been reported to be an important contributor to the development and function of the CNS. It can modulate various neurological processes, including BBB formation, myelination, neurogenesis, and microglia maturation [38]. The bidirectional communication between the gut microbiota and the brain, called the microbiota-gut-brain axis, has also been discovered in the past few years and includes top-down modulation (brain-gut axis) and bottom-up modulation (gut-brain axis) [39, 40].
2.1. Top-down Modulation
Brain activity can modulate gut microbiota by the stress response of gut via direct neural input and the hypothalamic-pituitary-adrenal (HPA) axis [41]. The brain can regulate gut functions, including regional motility, secretion of gastric acid, mucus, bicarbonate, gut peptides, antimicrobial peptides, epithelial fluid maintenance, intestinal permeability, and mucosal immune response via parasympathetic and sympathetic nerve fibers directly or by stimulating the enteric nervous system in the submucosal and myenteric plexus of the gut indirectly. As a consequence, these changes in gut physiology can affect the microbial habitat, thereby modulating microbiota composition and activity [42]. Besides, host neuroendocrine system can communicate with gut microbiota directly via intraluminal release of host signaling molecules, including but not limited to catecholamines, 5-HT, dynorphin, and cytokines, secreted from neurons, immune cells, and enteroendocrine cells [39].
2.2. Bottom-up Modulation
In turn, gut microbiota can influence CNS through at least three pathways, including neurotransmitter, neuroendocrine, and neuroimmune signaling mechanisms [41]. First, gut microbial metabolites and hormones (including serotonin (5-HT), cholecystokinin, glucagon-like peptide-1, and peptide YY) released by enteroendocrine cells (EECs) of the gut epithelial layer can stimulate vagal afferent fibers, thus initiating bottom-up signaling [43]. Furthermore, these afferent projections can spread throughout the entire brain, involving the hypothalamic neurons and nucleus tractus solaritarius with its downstream projections [43, 44]. In addition, the gut microbiota can also independently generate neuroactive molecules (γ-aminobutyric acid, 5-HT, norepinephrine, and dopamine) or directly stimulate the release of these molecules [45, 46]. Second, the gut microbiota can produce or contribute to the production of a variety of metabolites, such as neurotransmitters, short-chain fatty acids (SCFAs), and secondary bile acids (2BAs), which could enter systemic circulation and travel to the brain to modulate the function of neurons, microglia, astrocytes, and the BBB [47-49]. Third, immunogenic endotoxins generated by the gut microbiota, such as lipopolysaccharide (LPS), can induce neuroinflammation and autoimmunity by traveling directly to the brain via blood circulation, or indirectly activate peripheral immune cells, subsequently migrating to the brain [50-52].
2.3. Dysbiosis of Gut Microbiota in MS
Several autoimmune inflammatory conditions, including inflammatory bowel disease (IBD) and rheumatoid arthritis, have been reported to be associated with the enrichment or depletion of certain gut microbiota [53-55]. MS has been reported to be associated with several gut disorders. Anorectal dysfunction, including constipation and fecal incontinence, is a common symptom of MS. Approximately, 40% of MS patients have been reported to have anorectal dysfunction [56]. In addition, Minuk et al. reported a familial concurrence of IBD and MS [57, 58]. Yacyshyn et al. and Kimura et al. also reported the coexistence of IBD and MS [59, 60]. Moreover, the incidence of demyelinating diseases, including MS and optic neuritis, has been reported to be higher in patients with IBD [61]. The reduction in the diversity of gut bacteria in IBD has been reported, even though it is not known if it initiates IBD or is a result of IBD [62, 63]. However, the dysbiosis of gut microbiota might be a bond connecting MS and gut disorders. Alterations in the gut microbiota might also participate in the pathology of MS.
In the past few years, several studies have explored the differences in fecal gut microbiota between MS patients and healthy controls, revealing that gut microbial dysbiosis with both depletion and enrichment of certain gut microbiota in MS patients (Table 1). Chen et al. observed increases in Pseudomonas, Mycoplasma, Haemophilus, Blautia, and Dorea genera and decreases in Parabacteroides, Adlercreutzia, and Prevotella genera in relapsing-remitting MS (RRMS) patients [64]. Jangi et al. also reported that Methanobrevibacter and Akkermansia levels were increased, whereas the levels of Butyricimonas were decreased in RRMS patients [34]. In addition, these changes were associated with variations of expression in the genes involved in dendritic cell maturation, interferon signaling, and NF-κB signaling pathways in circulating T cells and monocytes [34]. The authors also demonstrated the influence of MS drug treatment on gut microbiota. MS patients with beta-interferon or glatiramer acetate treatment exhibited increased Prevotella and Sutterella and decreased Sarcina compared to untreated patients [34]. These results were consistent with findings from the study conducted by Castillo-Álvarez et al. [65]. Another investigation also revealed a decreased presence of Bacteroidaceae and Faecalibacterium, and an increased levels of Ruminococcus, after administration of vitamin D in RRMS patients without glatiramer acetate treatment [31]. In a Japanese cohort research study, the high proportion of several gut bacteria belonged to Clostridia clusters XIVa and IV and several Bacteroides were significantly reduced in patients with MS [32]. Tremlett et al. analyzed the relationship between gut microbiota diversity changes and relapse risk in pediatric MS, and their results showed that the depletion of Fusobacteria was associated with the risk of pediatric MS relapse [33, 66]. However, all findings discussed above were from gut microbiota in stool samples of MS patients in remission. A recent investigation analyzed the microbiota changes in small intestinal tissues from MS patients in the active phase [67]. The authors showed the ratio of Firmicutes to Bacteroidetes and presence of Streptococcus were increased, whereas the presence of the Prevotella strain was decreased in patients with active MS compared to healthy controls and MS patients in remission [67]. Moreover, the relative presence of Prevotella strains was negatively correlated with the proportion of Th17 cells in the small intestine, which is positively associated with the disease activity [67]. Although the changes of gut microbiota have been reported, it is still unclear whether these changes were the result or the cause of the disease.
Table 1.
The gut microbiota changed in MS patients and their related functions.
| Researches |
Changes of Gut
Microbiota |
Associated Functions and Pathways | Sample Sources | Sample Size |
Geographiccal
Location |
|---|---|---|---|---|---|
| Chen et al. [64] | Increased: Pseudomonas, Pedobacter, Blautia, Dorea, Mycoplana . Decreased: Adlercreutzia, Collinsella, Lactobacillus, Parabacteroides. |
Signal transduction mechanism, lipid transport and metabolism, intracellular trafficking, defense mechanisms, fatty acid biosynthesis, glycolysis, porphyrin and chlorophyll metabolism, transporters. |
Stool samples from RRMS patients with active disease or in remission (vs. healthy controls). | n = 31 in MS, n = 36 in healthy controls. | Rochester, MN, USA. |
| Jangi et al. [34] |
Increased: Methanobrevibacter, Akkermansia. Decreased: Butyricimonas. |
Inflammatory cells recruitment, human dendritic cells activation, proinflammatory pathways regulation, barrier function disruption. |
Stool samples from RRMS patients in remission (vs. healthy controls). | n = 60 in MS, n = 43 in healthy controls. | Boston, Massachusetts, USA. |
| Cantarel et al. [31] |
Increased: Akkermansia, Faecalibacterium, Coprococcus. Decreased: Moraxellaceae. |
Not mentioned. | Stool samples from RRMS patients in remission (vs. healthy controls). | n = 7 in MS, n =8 in healthy controls. | San Francisco, CA, USA. |
| Miyake et al. [32] |
Increased: Bifidobacterium, Streptococcus. Decreased: Bacteroides, Faecalibacterium, Prevotella, Anaerostipes. |
Systemic inflammation. | Stool samples from RRMS patients in remission (vs. healthy controls). | n = 20 in MS, n = 40 in healthy controls. | Tokyo, Japan. |
| Cosorich et al. [67] | Increased: Firmicutes/Bacteroidetes ratio, Streptococcus, Decreased: Prevotella. |
Th17 cell differentiation and expansion. | small intestinal mucosa from RRMS patients with active disease (vs. healthy controls and MS patients with no disease activity). | n = 19 in MS, n = 17 in healthy controls | Milan, Italy |
| Cekanaviciute et al. [74] |
Increased: Acinetobacter, Akkermansia. Decreased: Parabacteroides |
The differentiation of Treg cells and Th1 cells. | Stool samples from RRMS patients in remission (vs. healthy controls). | n = 71 in MS, n = 71 in healthy controls | San Francisco, CA, USA. New York, NY, USA. |
The influence of gut microbiota on the development of MS has also been widely explored in animal models. Mice maintained in germ-free conditions exhibited attenuated experimental autoimmune encephalomyelitis (EAE) development compared to mice housed in a non-sterile environment. Moreover, disease activity was significantly reduced in germ-free mice when EAE was induced [35, 68, 69]. The reduction of gut microbiota through oral antibiotic treatment also remarkably inhibits the development of EAE [52]. Accordingly, reconstitution with wild type Bacteroides fragiles maintained the EAE induction resistance, whereas that with Bacteroides fragiles, which is deficient in polysaccharide A (PSA) production, restored EAE susceptibility [70], suggesting the role of PSA-producing Bacteroides fragiles in the suppression of EAE initiation. In an HLA-DR3.DQ8 double transgenic mouse EAE model, Prevotella histicola also exhibited significant inhibition of EAE initiation after PLP91–110 induction [71]. Besides, Lavasani et al. reported that mixing three probiotic Lactobacillus strains (L. paracasei DSM 13434, L. plantarum DSM 15312 and DSM 15313) could suppress disease progression and reverse the clinical and histological manifestations in the EAE mice [72]. When microbiota from twins was transferred into transgenic mice that expressed a myelin autoantigen-specific T-cell receptor, gut microbiota from twins with MS showed a higher incidence to induce CNS-specific autoimmunity than microbiota from healthy twins [73]. It has also been reported that germ-free mice with microbiota transplants from MS patients displayed more severe EAE symptoms and reduced proportions of IL-10+ Tregs compared to mice with microbiota transplants from healthy controls [74]. These results indicate that the dysbiosis of gut microbiota in MS patients is an important contributor to the development of MS.
2.4. Potential Mechanisms of Gut Microbiota Involvment in MS
2.4.1. Increased Intestinal Permeability
Increased intestinal permeability in MS patients was first reported by Yacyshyn et al. in 1996 [59]. A recent investigation demonstrated that more than 70% of MS patients displayed increased intestinal permeability, which is significantly higher than healthy controls (28%) [75, 76]. In an EAE mouse model induced by adoptive transfer of autoreactive T-cells, the increase in intestinal permeability occurred before the onset of neurological symptoms and was worse when neurological symptoms were already present [77]. The destruction of the intestinal epithelial barrier might be a result of increased production of toxic metabolites and pro-inflammatory cytokines, while beneficial substances, such as SCFAs and other anti-inflammatory factors produced by gut microbiota are reduced [78]. The increase in intestinal permeability might further facilitate gut microbiota activate immune cells in the periphery (especially gut-associated lymphoid tissue, GALT) and transport of toxigenic metabolites into blood [78].
2.4.2. Connection between Gut Microbiota and the Autoimmune Response
The autoimmune response has been proven to be an essential component of the initiation and development of MS. Oligoclonal immunoglobulin bands (OCBs) due to the intrathecal secretion of immunoglobulin (Ig) by CNS antigen-driven B-cells are detectable in 95% of MS patients in their initial presentation and could be a prognostic biomarker for future development of MS [79-81]. Several Igs that are auto-reactive to various auto-antigens, including myelin components (MBP, PLP, and MOG) [82], axoglial proteins (neurofascin and contactin-2) [83, 84], and ion channels (potassium channel KIR 4.1) [85], have also been found in MS. Compared to auto-antibodies, the auto-reactive T-cells were thought to play more important roles in the pathogenesis of MS [17, 86]. They were thought to be activated in the periphery, subsequently invaded into the CNS, and initiated a series of responses against the local auto-antigenic tissues [87]. However, how and where these auto-reactive T-cells are triggered is still unclear. The gut-associated lymphoid tissue (GALT) contains a rich diversity of innate and adaptive immune cells, which can respond to both specific antigens and microbial signals, and subsequently turn GALT to a pro- or anti-inflammatory micro-environment [88]. It was also considered to be an important organ where the autoimmune T-cells were generated followed by an interaction between T-cells and the gut microbiota [87]. However, the gut microbiota involvement in the generation and activation of these auto-reactive T-cells in MS or EAE is still unknown. One important hypothesis is that the autoreactive T-cells were activated in GALT due to a molecular mimicry response to microbial components mimicking the autoantigen, such as myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG).
Molecular mimicry, where a foreign antigen shares a similar sequence or structure with self-antigens, is one of the leading mechanisms by which infectious or chemical agents might initiate or exacerbate autoimmunity [89, 90]. The gut microbiome contains 100 times more genes than the host and this encodes various products [91], meaning that their products might share certain similarities with certain antigens in humans. Gut microbiota has been proven to be associated with the initiation of several autoimmune diseases through molecular mimicry mechanisms in the past few years. For example, integrase expressed by Bacteroides has been reported to encode a low-avidity mimotope of islet-specific glucose-6-phosphatase-catalytic-subunit-related protein (IGRP206-214) [92], which is an important pancreatic β-cell autoantigen recognized by islet-associated CD8+ T-cells in type 1 diabetes (T1D) in nonobese diabetic (NOD) mice [93, 94]. AQP4 p61–80, a naturally processed immunodominant determinant of intact AQP4, also contained 10 residues (66–75) with a 90% homology to a sequence (207-106) within Clostridium perfringens adenosine triphosphate-binding cassette (ABC) transporter permease [95]. Interestingly, neuromyelitis optica, which is caused by the AQP4 auto-antibody, shares many similarities with MS. The cross-recognition of viral peptides and myelin peptides in MS has been identified [96-98], indicating molecular mimicry might be an important mechanism involved in the initiation of MS. Bacterial peptidoglycan has been found within antigen-presenting cells in the brain of MS patients and was thought to be a contributer of T- and B-cell activity during brain inflammation [99]. Hughes et al. have also revealed the cross-reaction of auto-antibodies in MS and peptides in Acinetobacter and Pseudomonas [100]. Antisera against MBP (residues 110-124) could cross-react with 4-carboxymucono- lactone decarboxylase in Acinetobacter and γ-carboxymu- conolactone decarboxylase in Pseudomonas. MOG (residues 43-57) antisera can cross-react with 3-oxoadipate-CoA-transferase subunit A in Acinetobacter [100]. These two genera of bacteria are important compositions of gut microbiota and have been reported to be increased in MS patients [64]. In a recent investigation, Planas et al. have identified guanosine diphosphate (GDP)-L-fucose synthase as an autoantigen, which is recognized by CSF-infiltrating CD4+ T cells from HLA-DRB3*-positive patients with MS [101]. In addition, these CSF-infiltrating CD4+ T cells that were reactive against the human GDP-L-fucose synthase peptide (161 - 175) also recognized two gut microbial GDP-L-fucose synthase peptides from bacterial genera (Akkermansia and Prevotella), which have been associated with MS101. Therefore, the molecular mimicry might be a potential pathway for gut microbiota participating in the initiation of autoimmune response and the development of MS.
2.4.3. The Disruption of the Blood-brain Barrier
In physiological conditions, the BBB acts as a gatekeeper to control the passage of molecules and cells between the brain parenchyma and the blood, and restrict the nonspecific influx of ions, proteins, and other substances into the CNS to ensure homeostasis in the CNS [102]. The breakdown of the BBB is associated with a variety of CNS disorders, including MS [102]. The disruption of the BBB is evident and considered to be important in the development and progression of MS [103-105]. BBB disruption is thought to occur in the early phases of MS [106], which might facilitate the migration of leukocytes into the CNS, further promoting the development of MS [107].
The impact of gut microbiota on BBB development and maintenance has been revealed by several studies in the recent years [108]. Fetal mice at E16.5 to E18.5 days of embryonic development in germ-free mice displayed an increased BBB permeability compared to fetal mice in pathogen-free mothers in the same stages of development [109]. In addition, the disruption of BBB integrity was also found in germ-free adult mice, partially accounting for the disorganized tight junctions and low expression of the transmembrane tight junction proteins, including human tight junction protein 1 (ZO1), occluding, and claudin-5 [109]. However, the transplantation of fecal microbiota from pathogen-free adult mice or administration of bacterial strains that produce SCFAs to germ-free adult mice restored the integrity of the mouse BBB [109], implying that SCFAs might be an important molecule involved in the impact of gut microbiota on BBB integrity. SCFAs, such as butyrate, acetate, and propionate are mainly produced through the fermentation of dietary fibers by gut microbiota [110]. Bacteria producing high levels of butyrate, such as Clostridium tyrobutyricum, have been reported to improve BBB integrity in germ-free mice via the upregulation of tight junction protein expression [111]. Bacterial cell wall constituents such as LPS from Gram-negative bacteria and lipoteichoic acid (LTA) from Gram-positive bacteria might also be involved in the alteration of the tight junction expression and BBB integrity through interaction with toll-like receptors (TLRs) expressed on the neurovasculature [112, 113].
In animal models of MS, mice treated with Prevotella histicola displayed reduced BBB permeability and reduced inflammatory Th1 and Th17 cells in the CNS compared to normally developed EAE mice [71]. This study indicated that Prevotella histicola might protect mice against EAE by modulating the migration of inflammatory cells to the CNS via the regulation of BBB intergrity [71]. This study demonstrated that the dysfunction of gut microbiota might be one contributor to the disruption of the BBB, which is the basis of inflammatory immune cells infiltrating into the CNS.
2.4.4. The Formation of Chronic Inflammation
Various immune cell types in both innate and adaptive systems and cytokines released by them participate in the formation of chronic inflammation in MS [114]. The infiltration of activated and CNS-specific immune cells from the periphery into the perivascular space and CNS is a classic characteristic of MS [115, 116]. The infiltrated immune cells might cause oligodendrocyte death, thus inducing demyelination and axonal damage [25, 117]. Moreover, these cells can also release various cytokines to activate vascular endothelial cells and enhance the disruption of the BBB, leading to the recruitment of additional immune cells into the CNS, increasing CNS inflammation, and promoting the progression of MS [24, 25, 118]. Among these immune cells, Th17 cells are considered to be the central effector cells in chronic MS inflammation [119-122]. They are the main source of inflammatory cytokines, including IL-17, IL-21, and IL-22 [123].
Gut microbiota plays an important role in maintaining the balance between pro- and anti-inflammatory immune responses through mediating the development of Treg cells and maturation of Th17 cells (Table 2) [69, 124]. The disruption of gut microbiota might promote the development of several inflammatory disorders, including MS [125]. Several gut microbiota genera (such as Akkermansia and Acinetobacter) increased in MS patients could induce the differentiation of Th1 and Th17 cells [74]. However, this result was drawn from an in vitro study by exposing peripheral blood mononuclear cells (PBMCs) from healthy donors to bacterial extracts from MS patients. Nevertheless, gut microbiota may not interact with PBMCs directly in vivo.
Table 2.
The pro-inflammatory and anti-inflammatory characteristics of gut microbiota changed in MS.
| - | Gut microbiota | Functions | Sample sources | References |
|---|---|---|---|---|
| Pro-inflammatory | Bacteroides fragilis | Induce the production of inflammatory cytokines, including IFN-γ, IL-1β, IL-6, TNF-α, IL-17, and IL-2. | PBMC derived from healthy individuals stimulated by certain bacteria | [147] |
| - | Ruminococcus gnavus | Metabolize sialic acids and degrade mucin to promote proinflammatory response. | Ruminococcus gnavus isolated from the fecal microbiota of a healthy human adult | [148] |
| - | Akkermansia | Promote the expansion of proinflammatory cytokines. Induce differentiation of IFN-γ-producing Th1 cells. |
Exposing PBMCs from healthy donors to bacterial extracts from MS patients. | [34, 64, 74] |
| - | Acinetobacter | Suppress the differentiation of Treg cells and induce differentiation of IFN-γ-producing Th1 cells | Exposing PBMCs from healthy donors to bacterial extracts from MS patients. | [74] |
| Anti-inflammatory | Parabacteroides | Increase the percentage of CD4+CD25+IL-10+ FoxP3+ Treg cells. | Exposing PBMCs from healthy donors to bacterial extracts from MS patients. | [74] |
| - | Prevotella histicola | Induce CD4+Foxp3+ regulatory T cells, tolerogenic dendritic cells and suppressive macrophage. Inhibit the myelin antigen-specific T cells response and decreased the level of IL-17 and IFN-γ in the periphery and CNS. |
Experimental autoimmune encephalomyelitis (EAE) in a human leukocyte antigen (HLA) class II transgenic mouse model. | [71] |
| - | - | Induce CD4+FoxP3+ Treg cells in the GALT . Reduce the level of Th1 and Th17 cells, and activation of microglia and astrocytes in CNS. |
Experimental autoimmune encephalomyelitis (EAE) in a human leukocyte antigen (HLA) class II transgenic mouse model. | [149, 150] |
| - | Bacteroides fragilis | Promote the convention of CD4+ T cells into Foxp3+ regulatory T (Treg) cells and inducing the production of IL-10 through the modulation of PSA. | Experimental autoimmune encephalomyelitis (EAE) model induced by PLP. | [70] |
| - | Lactobacillus | Induce the production of SCFAs. | An inoculum prepared from human feces | [151] |
In EAE models, antibiotic treatment profoundly enhanced the frequency of IL-10-producing Foxp3+ Treg cells but suppressed the Th17 cells response [52], indicating the modulation of gut microbiota for Th 17 and Treg cells. It has also been reported that Prevotella histicola can suppress the pro-inflammatory Th1 and Th17 cells and promote the immune suppressive cells, including Treg cells, tolerogenic dendritic cells, and suppressive macrophages, to inhibit the development of EAE [71]. Besides, the administration of Prevotella histicola inhibited the myelin antigen-specific T-cell response and decreased the level of IL-17 and IFN-γ in the periphery and CNS [71].
Bacteroides fragilis plays controversial roles in the regulation of the immune system. On the one hand, they have been reported to induce the production of inflammatory cytokines, including IFN-γ, IL-1β, IL-6, TNF-α, IL-17, and IL-2, whereas, on the other hand, they can exert an anti-inflammatory role by promoting the conversion of CD4+ T-cells into Foxp3+ regulatory T-cells (Treg) and inducing the production of IL-10 through the modulation of PSA in experimental colitis [126]. The similar modulatory role of Bacteroides fragilis on Treg cells has also been identified in the EAE model [70]. In addition, the administration of PSA alone has been reported to protect mice against EAE prophylactically and therapeutically [127]. PSA treatment could enhance the proportion of CD103+ DCs, inducing the conversion of naïve CD4+ cells into IL10-producing Treg cells in EAE mice [127]. However, the protective effect was abrogated in IL-10-deficient mice [127], indicating that IL-10 is indispensable in the protective role of PSA in EAE.
2.5. Therapeutic Potential of Gut Microbiota for MS
Since gut microbiota dysbiosis was involved in various diseases, it could be a potential target for disease therapy. Many clinical trials have investigated the effect of fecal microbiota transplantation (FMT) on several diseases, including Clostridium difficile infection, inflammatory bowel disease, irritable bowel syndrome, and hepatic encephalopathy, and have demonstrated the beneficial role of FMT on these diseases [128]. As yet, however, no randomized clinical trials (RCT) assessing the effect of FMT in MS patients have been performed. Only two case reports that included four MS patients have suggested the promising therapeutic potential of FMT on MS [129, 130]. In animal models, FMT with stool samples acquired from MS patients can elevate the disease incidence or severity in EAE [73, 74]. Several clinical trials about the effect of FMT in MS are ongoing and the results of these clinical trials will hopefully pave the way to the development of better and more effective future therapies for MS [131].
In spite of FMT, antibiotic and probiotic treatment are also widely used to modulate gut microbiota. It has been reported that germ-free mice showed reduced disease incidence in the induction of EAE and decreased disease activity when EAE was induced [35, 68, 69]. Besides, antibiotic treatment significantly altered the composition of the gut microbiota and suppressed the induction of EAE [52]. Moreover, the transplantation of several bacteria strains has also been demonstrated to inhibit the initiation of EAE or to reverse the clinical and histological manifestation of EAE [71, 72], indicating probiotic treatment might be a promising approach for MS treatment.
CONCLUSION AND PERSPECTIVES
Currently available investigations indicate that gut microbiota plays an indispensable role in MS. The alteration of gut microbiota diversity and composition in MS patients in remission or in the active phase has been identified by a number of studies. However, the results of these studies are not completely congruent, which might be a result of the differences in the disease course and ethnicity. Besides, research on the changes of gut microbiota in pre-MS patients that have not exhibited significant clinical manifestations is rare for the difficulty to define pre-MS patients. The relationship of the alteration of a certain subgroup of gut microbiota and the susceptibility of MS patients is also undiscovered and needs additional prospective clinical studies to explore this further. The resolution of these problems might promote advances in the influence of gut microbiota on MS.
In this manuscript, we proposed the potential mechanism of the gut microbiota participating in the pathology of MS (Fig. 1). The increase in intestinal barrier permeability caused by gut microbiota dysbiosis and inflammation might be the early step of gut microbiota involvement in the pathology of MS. Subsequently, certain gut microbiota products that share a similar sequence with auto-antigens in MS might be one of the contributors to initiate the autoimmune response through mimicry. Besides, several components and metabolites of the gut microbiota might enter into circulation and impact the integrity of the BBB, which plays an important role in the development of MS. The most widely investigated pathway of gut microbiota involvement in MS pathogenesis is the formation of chronic inflammation. It has been denmostrated that gut microbiota could regulate inflammation through the metabolism of sialic acids, degradation of mucin (pro-inflammatory pathway); and modulation of SCFAs, PSA, and estrogens (anti-inflammatory pathway) [132]. The dysbiosis of gut microbiota might lead to an immune imbalance, resulting in the expansion of pro-inflammatory Th1 and Th17 cells and suppression of anti-inflammatory Treg cells, finally contributing to the formation of chronic inflammation in MS.
Fig. (1).
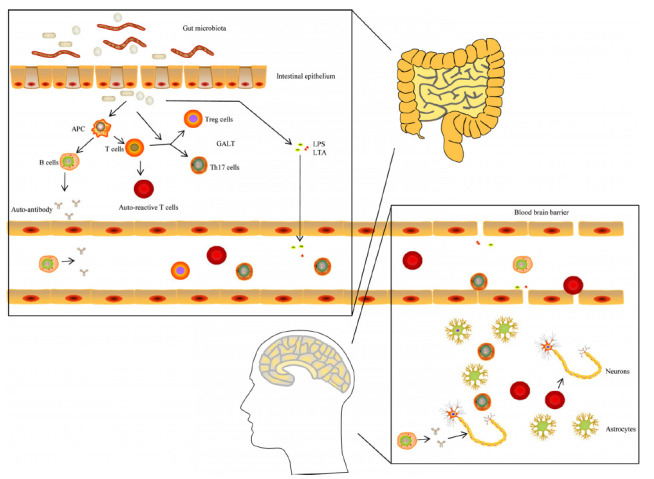
The mechanism of the gut microbiota involment in the initiation and development of MS. Gut microbiota might active the auto-reactive T cells in GALT via mimicry or/and other pathway through disrupted intestinal barrier. Besides, they might also induce the production of auto-antibodies through the stimulation of APC and the activation of B cells. The decrease of anti-inflammatory metabolites (such as SCFAs) and the enhancement of pro-inflammatory signals (such as salic acids metabolism) caused by the dysbiosis of gut microbiota could suppress the differentiation of Treg cells and promote the expansion of Th1 and Th17 cells. In addition, several toxic microbiota components and metabolites (including LPS and LTA) might enter into blood and contribute to the destruction of BBB. Subsequently, Th1, Th17 and auto-reactive T cells can infiltrate the CNS through disrupted BBB, resulting in the inflammation in CNS and the injury of myelin and neurons damage. In turn, the inflammation CNS may also recruit more inflammatory immune cells and cytokines, aggravating the CNS injury. APC, antigen presenting cell; BBB, blood brain barrier; CNS, central nervous system; GALT, gut associated lymphoid tissue; LPS, lipopolysaccharide (LPS); LTA, lipoteichoic acid; SCFAs, short-chain fatty acids.
The composition and diversity of gut microbiota were modulated by genetic [133], gender [134], age [135], and various environmental factors, including diet [30], obesity [30], smoking [136], virus [137, 138], season [139] and vitamin D [139]. Diet and obesity are the most important factors influencing the diversity and composition of gut microbiota [15, 140, 141]. What is interesting is that diet and BMI are also related to the susceptibility and disease activity of MS [142-146]. Besides, several clinical trials have reported that diet habits change might affect the development of MS [131]. These results implied that living habits might affect the initiation and development of MS via some extent of the shaping of gut microbiota. Thus, it is meaningful to explore whether the gut microbiota can be a marker in combination with other factors to predict individuals susceptible to MS and whether changing certain lifestyles can be beneficial to prevent the initiation or the relapse of MS through reshaping gut microbiota in the future. In addition, therapies targeted on gut microbiota, such as FMT and antibiotic and probiotic treatments, have been demonstrated to modulate the induction of the EAE model and severity of disease in EAE. Thus, whether the clinical use of these strategies affects the development, relapse, and treatment of MS also needs to be investigated further.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ABC
Adenosine Triphosphate-Binding Cassette
- AQP-4
Aquqporin-4
- BBB
Blood Brain Barrier
- CNS
Central Nervous System
- CRF
Corticotropin-Releasing Factor
- EAE
Experimental Autoimmune Encephalomyelitis
- EECs
Enteroendocrine Cells
- 5-HT
Serotonin
- FMT
Fecal Microbiota Transplantation
- GALT
Gut Associated Lymphoid Tissue
- GI
Gastrointestinal
- HPA axis
Hypothalamic-Pituitary-Adrenal Axis
- IBD
Inflammatory Bowel Disease
- Ig
Immunoglobulin
- IGRP
Islet-specific glucose-6-phosphatase-catalytic-subunit-related Protei
- LPS
Lipopolysaccharide
- LTA
Lipoteichoic Acid
- MBP
Myelin Basic Protein
- MHC
Major Histocompatibility Complex
- MOG
Myelin Oligodendrocyte Glycoprotein
- MS
Multiple Sclerosis
- NOD mice
Nonobese Diabetic Mice
- OCBs
Oligoclonal Immunoglobulin Bands
- PBMCs
Peripheral Blood Mononuclear Cells
- PLP
Proteolipid Protein
- PSA
Polysaccharide A
- RCTs
Random Clinical Trials
- RRMS
Relapsing-Remitting MS
- 2BAs
Secondary Bile Acids
- SCFAs
Short-Chain Fatty Acids
- TLRs
Toll-Like Receptors
- T1D
Type 1 Diabetes
- ZO1
Tight Junction Protein 1
AUTHORS’ CONTRIBUTIONS
The ideal of this manuscript was proposed by Mingqin Zhu. The manuscript was written by Xu Wang, Zhen Liang and Shengnan Wang. The figure was drawn by Xu Wang. The manuscript was revised by Jiachun Feng and Di Ma.
CONSENT FOR PUBLICATION
All authors have approved the final version of this review and agree to the publication.
FUNDING
This study was supported by grants from the National Key R&D Program of China (2017YFC011304), the National Natural Science Foundation of China (NO. 31600820) and from the Science and Technology planning project of Jilin Province (NO. 20180520110JH).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley R.E., Peterson D.A., Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., Mende D.R., Li J., Xu J., Li S., Li D., Cao J., Wang B., Liang H., Zheng H., Xie Y., Tap J., Lepage P., Bertalan M., Batto J.M., Hansen T., Le Pasli-er D., Linneberg A., Nielsen H.B., Pelletier E., Renault P., Sicheritz-Ponten T., Turner K., Zhu H., Yu C., Li S., Jian M., Zhou Y., Li Y., Zhang X., Li S., Qin N., Yang H., Wang J., Brunak S., Doré J., Guarner F., Kristiansen K., Pedersen O., Parkhill J., Weissen-bach J., Bork P., Ehrlich S.D., Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson T.R., Mazmanian S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17(5):565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer K.C., Huus K.E., Finlay B.B. Microbes and the mind: emerging hallmarks of the gut microbiota-brain axis. Cell. Microbiol. 2016;18(5):632–644. doi: 10.1111/cmi.12585. [DOI] [PubMed] [Google Scholar]
- 7.Hooper L.V., Gordon J.I. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 8.Mosca A., Leclerc M., Hugot J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosys-tem? Front. Microbiol. 2016;7:455. doi: 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sender R., Fuchs S., Milo R. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Ma Q., Xing C., Long W., Wang H.Y., Liu Q., Wang R.F. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J. Neuroinflammation. 2019;16(1):53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larroya-García A., Navas-Carrillo D., Orenes-Piñero E. Impact of gut microbiota on neurological diseases: Diet composition and novel treatments. Crit. Rev. Food Sci. Nutr. 2019;59(19):3102–3116. doi: 10.1080/10408398.2018.1484340. [DOI] [PubMed] [Google Scholar]
- 12.Kostic A.D., Xavier R.J., Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., Peng Y., Zhang D., Jie Z., Wu W., Qin Y., Xue W., Li J., Han L., Lu D., Wu P., Dai Y., Sun X., Li Z., Tang A., Zhong S., Li X., Chen W., Xu R., Wang M., Feng Q., Gong M., Yu J., Zhang Y., Zhang M., Hansen T., Sanchez G., Raes J., Falony G., Okuda S., Almeida M., LeChatelier E., Renault P., Pons N., Batto J.M., Zhang Z., Chen H., Yang R., Zheng W., Li S., Yang H., Wang J., Ehrlich S.D., Nielsen R., Pedersen O., Kristiansen K., Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 14.Arrieta M.C., Stiemsma L.T., Dimitriu P.A., Thorson L., Russell S., Yurist-Doutsch S., Kuzeljevic B., Gold M.J., Britton H.M., Lefebvre D.L., Subbarao P., Mandhane P., Becker A., McNagny K.M., Sears M.R., Kollmann T., Mohn W.W., Turvey S.E., Finlay B.B. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015;7(307):307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 15.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., Egholm M., Henrissat B., Heath A.C., Knight R., Gordon J.I. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinan T.G., Cryan J.F. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. 2017;595(2):489-503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassmann H., Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 2017;133(2):223–244. doi: 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magyari M., Sorensen P.S. The changing course of multiple sclerosis: rising incidence, change in geographic distribution, disease course, and prognosis. Curr. Opin. Neurol. 2019;32(3):320–326. doi: 10.1097/WCO.0000000000000695. [DOI] [PubMed] [Google Scholar]
- 19.Orton S.M., Herrera B.M., Yee I.M., Valdar W., Ramagopalan S.V., Sadovnick A.D., Ebers G.C. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5(11):932–936. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 20.Lublin F.D., Reingold S.C., Cohen J.A., Cutter G.R., Sørensen P.S., Thompson A.J., Wolinsky J.S., Balcer L.J., Banwell B., Barkhof F., Bebo B., Jr, Calabresi P.A., Clanet M., Comi G., Fox R.J., Freedman M.S., Goodman A.D., Inglese M., Kappos L., Kieseier B.C., Lincoln J.A., Lubetzki C., Miller A.E., Montalban X., O’Connor P.W., Petkau J., Pozzilli C., Rudick R.A., Sormani M.P., Stüve O., Waubant E., Polman C.H. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg N., Smith T.W. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015;5(9):e00362. doi: 10.1002/brb3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correale J., Gaitán M.I., Ysrraelit M.C., Fiol M.P. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain. 2017;140(3):527–546. doi: 10.1093/brain/aww258. [DOI] [PubMed] [Google Scholar]
- 23.Dobson R., Giovannoni G. Multiple sclerosis - a review. Eur. J. Neurol. 2019;26(1):27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 24.Frohman E.M., Racke M.K., Raine C.S. Multiple sclerosis--the plaque and its pathogenesis. N. Engl. J. Med. 2006;354(9):942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 25.McFarland H.F., Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 2007;8(9):913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 26.Prat A., Antel J. Pathogenesis of multiple sclerosis. Curr. Opin. Neurol. 2005;18(3):225–230. doi: 10.1097/01.wco.0000169737.99040.31. [DOI] [PubMed] [Google Scholar]
- 27.Sadovnick A.D., Armstrong H., Rice G.P., Bulman D., Hashimoto L., Paty D.W., Hashimoto S.A., Warren S., Hader W., Murray T.J. A population-based study of multiple sclerosis in twins: update. Ann. Neurol. 1993;33(3):281–285. doi: 10.1002/ana.410330309. [DOI] [PubMed] [Google Scholar]
- 28.Mumford C.J., Wood N.W., Kellar-Wood H., Thorpe J.W., Miller D.H., Compston D.A. The British Isles survey of multiple sclerosis in twins. Neurology. 1994;44(1):11–15. doi: 10.1212/WNL.44.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Alfredsson L., Olsson T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019;9(4):a028944. doi: 10.1101/cshperspect.a028944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridaura V.K., Faith J.J., Rey F.E., Cheng J., Duncan A.E., Kau A.L., Griffin N.W., Lombard V., Henrissat B., Bain J.R., Muehlbauer M.J., Ilkayeva O., Semenkovich C.F., Funai K., Hayashi D.K., Lyle B.J., Martini M.C., Ursell L.K., Clemente J.C., Van Treuren W., Walters W.A., Knight R., Newgard C.B., Heath A.C., Gordon J.I. Gut microbiota from twins discordant for obesity modulate metabo-lism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantarel B.L., Waubant E., Chehoud C., Kuczynski J., DeSantis T.Z., Warrington J., Venkatesan A., Fraser C.M., Mowry E.M. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J. Investig. Med. 2015;63(5):729–734. doi: 10.1097/JIM.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyake S., Kim S., Suda W., Oshima K., Nakamura M., Matsuoka T., Chihara N., Tomita A., Sato W., Kim S.W., Morita H., Hatto-ri M., Yamamura T. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One. 2015;10(9):e0137429. doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremlett H., Fadrosh D.W., Faruqi A.A., Hart J., Roalstad S., Graves J., Lynch S., Waubant E. Gut microbiota composition and re-lapse risk in pediatric MS: A pilot study. J. Neurol. Sci. 2016;363:153–157. doi: 10.1016/j.jns.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jangi S., Gandhi R., Cox L.M., Li N., von Glehn F. Alterations of the human gut microbiome in multiple sclerosis. 2016;7:12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berer K., Mues M., Koutrolos M., Rasbi Z.A., Boziki M., Johner C., Wekerle H., Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 36.Budhram A., Parvathy S., Kremenchutzky M., Silverman M. Breaking down the gut microbiome composition in multiple sclerosis. Mult. Scler. 2017;23(5):628–636. doi: 10.1177/1352458516682105. [DOI] [PubMed] [Google Scholar]
- 37.Mirza A., Forbes J.D., Zhu F., Bernstein C.N., Van Domselaar G., Graham M., Waubant E., Tremlett H. The multiple sclerosis gut microbiota: A systematic review. Mult. Scler. Relat. Disord. 2020;37:101427. doi: 10.1016/j.msard.2019.101427. [DOI] [PubMed] [Google Scholar]
- 38.Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The central nervous system and the gut microbiome. Cell. 2016;167(4):915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durgan D.J., Lee J., McCullough L.D., Bryan R.M., Jr Examining the role of the microbiota-gut-brain axis in stroke. Stroke. 2019;50(8):2270–2277. doi: 10.1161/STROKEAHA.119.025140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burokas A., Moloney R.D., Dinan T.G., Cryan J.F. Microbiota regulation of the mammalian gut-brain axis. Adv. Appl. Microbiol. 2015;91:1–62. doi: 10.1016/bs.aambs.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 2018;6(2):133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strader A.D., Woods S.C. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128(1):175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 44.Wang F.B., Powley T.L. Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res. 2007;329(2):221–230. doi: 10.1007/s00441-007-0413-7. [DOI] [PubMed] [Google Scholar]
- 45.Asano Y., Hiramoto T., Nishino R., Aiba Y., Kimura T., Yoshihara K., Koga Y., Sudo N. Critical role of gut microbiota in the pro-duction of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303(11):G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 46.Barrett E. Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012;113(2):411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 47.Erny D. Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; Schwierzeck, V.; Utermöhlen, O.; Chun, E.; Garrett, W.S.; McCoy, K.D.; Diefenbach, A.; Staeheli, P.; Stecher, B.; Amit, I.; Prinz, M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J., Ling Z., Wang F., Chen W., Li H., Jin J., Zhang H., Pang M., Yu J., Liu J. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci. Lett. 2016;613:30–35. doi: 10.1016/j.neulet.2015.12.047. [DOI] [PubMed] [Google Scholar]
- 49.Sun J., Wang F., Ling Z., Yu X., Chen W., Li H., Jin J., Pang M., Zhang H., Yu J., Liu J. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 50.Singh V., Roth S., Llovera G., Sadler R., Garzetti D., Stecher B., Dichgans M., Liesz A. Microbiota dysbiosis controls the neuroin-flammatory response after stroke. J. Neurosci. 2016;36(28):7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benakis C., Brea D., Caballero S., Faraco G., Moore J., Murphy M., Sita G., Racchumi G., Ling L., Pamer E.G., Iadecola C., Anra-ther J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016;22(5):516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ochoa-Repáraz J., Mielcarz D.W., Ditrio L.E., Burroughs A.R., Foureau D.M., Haque-Begum S., Kasper L.H. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009;183(10):6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 53.Gevers D., Kugathasan S., Denson L.A., Vázquez-Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song S.J., Yassour M., Morgan X.C., Kostic A.D., Luo C., González A., McDonald D., Haberman Y., Walters T., Baker S., Rosh J., Stephens M., Hey-man M., Markowitz J., Baldassano R., Griffiths A., Sylvester F., Mack D., Kim S., Crandall W., Hyams J., Huttenhower C., Knight R., Xavier R.J. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L.G., Gratadoux J.J., Blugeon S., Bridonneau C., Furet J.P., Corthier G., Grangette C., Vasquez N., Pochart P., Trugnan G., Thomas G., Blottière H.M., Doré J., Marteau P., Seksik P., Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease pa-tients. Proc. Natl. Acad. Sci. USA. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scher J.U., Sczesnak A., Longman R.S., Segata N., Ubeda C., Bielski C., Rostron T., Cerundolo V., Pamer E.G., Abramson S.B., Huttenhower C., Littman D.R. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nusrat S., Gulick E., Levinthal D., Bielefeldt K. Anorectal dysfunction in multiple sclerosis: a systematic review. ISRN Neurol. 2012;2012:376023. doi: 10.5402/2012/376023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minuk G.Y., Lewkonia R.M. Possible familial association of multiple sclerosis and inflammatory bowel disease. N. Engl. J. Med. 1986;314(9):586. doi: 10.1056/NEJM198602273140921. [DOI] [PubMed] [Google Scholar]
- 58.Sadovnick A.D., Paty D.W., Yannakoulias G. Concurrence of multiple sclerosis and inflammatory bowel disease. N. Engl. J. Med. 1989;321(11):762–763. doi: 10.1056/NEJM198909143211115. [DOI] [PubMed] [Google Scholar]
- 59.Yacyshyn B., Meddings J., Sadowski D., Bowen-Yacyshyn M.B. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig. Dis. Sci. 1996;41(12):2493–2498. doi: 10.1007/BF02100148. [DOI] [PubMed] [Google Scholar]
- 60.Kimura K., Hunter S.F., Thollander M.S., Loftus E.V., Jr, Melton L.J., III, O’Brien P.C., Rodriguez M., Phillips S.F. Concurrence of inflammatory bowel disease and multiple sclerosis. Mayo Clin. Proc. 2000;75(8):802–806. doi: 10.4065/75.8.802. [DOI] [PubMed] [Google Scholar]
- 61.Gupta G., Gelfand J.M., Lewis J.D. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology. 2005;129(3):819–826. doi: 10.1053/j.gastro.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 62.Ott S.J., Musfeldt M., Wenderoth D.F., Hampe J., Brant O., Fölsch U.R., Timmis K.N., Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53(5):685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manichanh C., Rigottier-Gois L., Bonnaud E., Gloux K., Pelletier E., Frangeul L., Nalin R., Jarrin C., Chardon P., Marteau P., Roca J., Dore J. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J., Chia N., Kalari K.R., Yao J.Z., Novotna M., Paz Soldan M.M., Luckey D.H., Marietta E.V., Jeraldo P.R., Chen X., Weins-henker B.G., Rodriguez M., Kantarci O.H., Nelson H., Murray J.A., Mangalam A.K. Multiple sclerosis patients have a distinct gut mi-crobiota compared to healthy controls. Sci. Rep. 2016;6:28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castillo-Álvarez F., Pérez-Matute P., Oteo J.A., Marzo-Sola M.E. The influence of interferon β-1b on gut microbiota composition in patients with multiple sclerosis. Neurologia (Engl Ed) 2018:S0213-4853(18)30158-0. doi: 10.1016/j.nrleng.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Tremlett H., Fadrosh D.W., Faruqi A.A., Zhu F., Hart J., Roalstad S., Graves J., Lynch S., Waubant E. Gut microbiota in early pediat-ric multiple sclerosis: a case-control study. Eur. J. Neurol. 2016;23(8):1308–1321. doi: 10.1111/ene.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cosorich I., Dalla-Costa G., Sorini C., Ferrarese R., Messina M.J., Dolpady J., Radice E., Mariani A., Testoni P.A., Canducci F., Comi G., Martinelli V., Falcone M. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 2017;3(7):e1700492–e1700492. doi: 10.1126/sciadv.1700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goverman J., Woods A., Larson L., Weiner L.P., Hood L., Zaller D.M. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72(4):551–560. doi: 10.1016/0092-8674(93)90074-Z. [DOI] [PubMed] [Google Scholar]
- 69.Lee Y.K., Menezes J.S., Umesaki Y., Mazmanian S.K. Proinflammatory T-cell responses to gut microbiota promote experimental auto-immune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl. 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ochoa-Repáraz J., Mielcarz D.W., Ditrio L.E., Burroughs A.R., Begum-Haque S., Dasgupta S., Kasper D.L., Kasper L.H. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 2010;185(7):4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 71.Mangalam A., Shahi S.K., Luckey D., Karau M., Marietta E., Luo N., Choung R.S., Ju J., Sompallae R., Gibson-Corley K., Patel R., Rodriguez M., David C., Taneja V., Murray J. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 2017;20(6):1269–1277. doi: 10.1016/j.celrep.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lavasani S., Dzhambazov B., Nouri M., Fåk F., Buske S., Molin G., Thorlacius H., Alenfall J., Jeppsson B., Weström B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010;5(2):e9009. doi: 10.1371/journal.pone.0009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berer K., Gerdes L.A., Cekanaviciute E., Jia X., Xiao L., Xia Z., Liu C., Klotz L., Stauffer U., Baranzini S.E., Kümpfel T., Hohlfeld R., Krishnamoorthy G., Wekerle H. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA. 2017;114(40):10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cekanaviciute E., Yoo B.B., Runia T.F. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. 2017;114(40):10713-10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buscarinu M.C., Cerasoli B., Annibali V., Policano C., Lionetto L., Capi M., Mechelli R., Romano S., Fornasiero A., Mattei G., Pi-ras E., Angelini D.F., Battistini L., Simmaco M., Umeton R., Salvetti M., Ristori G. Altered intestinal permeability in patients with re-lapsing-remitting multiple sclerosis: A pilot study. Mult. Scler. 2017;23(3):442–446. doi: 10.1177/1352458516652498. [DOI] [PubMed] [Google Scholar]
- 76.Buscarinu M.C., Romano S., Mechelli R., Pizzolato Umeton R., Ferraldeschi M., Fornasiero A., Reniè R., Cerasoli B., Morena E., Romano C., Loizzo N.D., Umeton R., Salvetti M., Ristori G. Intestinal permeability in relapsing-remitting multiple sclerosis. Neurotherapeutics. 2018;15(1):68–74. doi: 10.1007/s13311-017-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nouri M., Bredberg A., Weström B., Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune en-cephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One. 2014;9(9):e106335. doi: 10.1371/journal.pone.0106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Welcome M.O. Gut microbiota disorder, gut epithelial and blood-brain barrier dysfunctions in etiopathogenesis of dementia: molecular mechanisms and signaling pathways. Neuromolecular Med. 2019;21(3):205–226. doi: 10.1007/s12017-019-08547-5. [DOI] [PubMed] [Google Scholar]
- 79.Obermeier B., Mentele R., Malotka J., Kellermann J., Kümpfel T., Wekerle H., Lottspeich F., Hohlfeld R., Dornmair K. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat. Med. 2008;14(6):688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]
- 80.Tintoré M., Rovira A., Brieva L., Grivé E., Jardí R., Borrás C., Montalban X. Isolated demyelinating syndromes: comparison of CSF oligoclonal bands and different MR imaging criteria to predict conversion to CDMS. Mult. Scler. 2001;7(6):359–363. doi: 10.1177/135245850100700603. [DOI] [PubMed] [Google Scholar]
- 81.Cameron E.M., Spencer S., Lazarini J., Harp C.T., Ward E.S., Burgoon M., Owens G.P., Racke M.K., Bennett J.L., Frohman E.M., Monson N.L. Potential of a unique antibody gene signature to predict conversion to clinically definite multiple sclerosis. J. Neuroimmunol. 2009;213(1-2):123–130. doi: 10.1016/j.jneuroim.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Connor K.C., Appel H., Bregoli L., Call M.E., Catz I., Chan J.A., Moore N.H., Warren K.G., Wong S.J., Hafler D.A., Wucherpfen-nig K.W. Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. J. Immunol. 2005;175(3):1974–1982. doi: 10.4049/jimmunol.175.3.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yan W., Nguyen T., Yuki N., Ji Q., Yiannikas C., Pollard J.D., Mathey E.K. Antibodies to neurofascin exacerbate adoptive transfer experimental autoimmune neuritis. J. Neuroimmunol. 2014;277(1-2):13–17. doi: 10.1016/j.jneuroim.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 84.Derfuss T., Parikh K., Velhin S., Braun M., Mathey E., Krumbholz M., Kümpfel T., Moldenhauer A., Rader C., Sonderegger P., Pöllmann W., Tiefenthaller C., Bauer J., Lassmann H., Wekerle H., Karagogeos D., Hohlfeld R., Linington C., Meinl E. Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc. Natl. Acad. Sci. USA. 2009;106(20):8302–8307. doi: 10.1073/pnas.0901496106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Srivastava R., Aslam M., Kalluri S.R., Schirmer L., Buck D., Tackenberg B., Rothhammer V., Chan A., Gold R., Berthele A., Ben-nett J.L., Korn T., Hemmer B. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N. Engl. J. Med. 2012;367(2):115–123. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kinzel S., Lehmann-Horn K., Torke S., Häusler D., Winkler A., Stadelmann C., Payne N., Feldmann L., Saiz A., Reindl M., Lalive P.H., Bernard C.C., Brück W., Weber M.S. Myelin-reactive antibodies initiate T cell-mediated CNS autoimmune disease by opsonization of endogenous antigen. Acta Neuropathol. 2016;132(1):43–58. doi: 10.1007/s00401-016-1559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wekerle H. Brain autoimmunity and intestinal microbiota: 100 trillion game changers. Trends Immunol. 2017;38(7):483–497. doi: 10.1016/j.it.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 88.Lebeer S., Vanderleyden J., De Keersmaecker S.C. Host interactions of probiotic bacterial surface molecules: comparison with commen-sals and pathogens. Nat. Rev. Microbiol. 2010;8(3):171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 89.Cusick M.F., Libbey J.E., Fujinami R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 2012;42(1):102–111. doi: 10.1007/s12016-011-8294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rojas M., Restrepo-Jiménez P., Monsalve D.M., Pacheco Y., Acosta-Ampudia Y., Ramírez-Santana C., Leung P.S.C., Ansari A.A., Gershwin M.E., Anaya J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018;95:100–123. doi: 10.1016/j.jaut.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 91.Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M., Thomas L.V., Zoetendal E.G., Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hebbandi Nanjundappa R., Ronchi F., Wang J., Clemente-Casares X., Yamanouchi J., Sokke Umeshappa C., Yang Y., Blanco J. Bas-solas-Molina, H.; Salas, A.; Khan, H.; Slattery, R.M.; Wyss, M.; Mooser, C.; Macpherson, A.J.; Sycuro, L.K.; Serra, P.; McKay, D.M.; McCoy, K.D.; Santamaria, P. A gut microbial mimic that hijacks diabetogenic autoreactivity to suppress colitis. Cell. 2017;171(3):655–667.e17. doi: 10.1016/j.cell.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 93.Anderson B., Park B.J., Verdaguer J., Amrani A., Santamaria P. Prevalent CD8(+) T cell response against one peptide/MHC complex in autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 1999;96(16):9311–9316. doi: 10.1073/pnas.96.16.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lieberman S.M., Evans A.M., Han B., Takaki T., Vinnitskaya Y., Caldwell J.A., Serreze D.V., Shabanowitz J., Hunt D.F., Nathen-son S.G., Santamaria P., DiLorenzo T.P. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 2003;100(14):8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Varrin-Doyer M., Spencer C.M., Schulze-Topphoff U., Nelson P.A., Stroud R.M., Cree B.A., Zamvil S.S. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann. Neurol. 2012;72(1):53–64. doi: 10.1002/ana.23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin R., Gran B., Zhao Y., Markovic-Plese S., Bielekova B., Marques A., Sung M.H., Hemmer B., Simon R., McFarland H.F., Pinilla C. Molecular mimicry and antigen-specific T cell responses in multiple sclerosis and chronic CNS Lyme disease. J. Autoimmun. 2001;16(3):187–192. doi: 10.1006/jaut.2000.0501. [DOI] [PubMed] [Google Scholar]
- 97.Wucherpfennig K.W., Strominger J.L. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80(5):695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Markovic-Plese S., Hemmer B., Zhao Y., Simon R., Pinilla C., Martin R. High level of cross-reactivity in influenza virus hemaggluti-nin-specific CD4+ T-cell response: implications for the initiation of autoimmune response in multiple sclerosis. J. Neuroimmunol. 2005;169(1-2):31–38. doi: 10.1016/j.jneuroim.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 99.Schrijver I.A., van Meurs M., Melief M.J., Wim Ang C., Buljevac D., Ravid R., Hazenberg M.P., Laman J.D. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain. 2001;124(Pt 8):1544–1554. doi: 10.1093/brain/124.8.1544. [DOI] [PubMed] [Google Scholar]
- 100.Hughes L.E., Smith P.A., Bonell S., Natt R.S., Wilson C., Rashid T., Amor S., Thompson E.J., Croker J., Ebringer A. Cross-reactivity between related sequences found in Acinetobacter sp., Pseudomonas aeruginosa, myelin basic protein and myelin oligodendro-cyte glycoprotein in multiple sclerosis. J. Neuroimmunol. 2003;144(1-2):105–115. doi: 10.1016/S0165-5728(03)00274-1. [DOI] [PubMed] [Google Scholar]
- 101.Planas R., Santos R., Tomas-Ojer P. GDP-l-fucose synthase is a CD4(+) T cell-specific autoantigen in DRB3*02:02 patients with multi-ple sclerosis. Sci. Transl. Med. 2018;10(462):eaat4301. doi: 10.1126/scitranslmed.aat4301. [DOI] [PubMed] [Google Scholar]
- 102.Gloor S.M., Wachtel M., Bolliger M.F., Ishihara H., Landmann R., Frei K. Molecular and cellular permeability control at the blood-brain barrier. Brain Res. Brain Res. Rev. 2001;36(2-3):258–264. doi: 10.1016/S0165-0173(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 103.Alvarez J.I., Cayrol R., Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim. Biophys. Acta. 2011;1812(2):252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 104.Erdő F.; Denes, L.; de Lange, E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J. Cereb. Blood Flow Metab. 2017;37(1):4–24. doi: 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X., Jiao W., Lin M., Lu C., Liu C., Wang Y., Ma D., Wang X., Yin P., Feng J., Zhu J., Zhu M. Resolution of inflammation in neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 2019;27:34–41. doi: 10.1016/j.msard.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 106.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 107.Agrawal S.M., Yong V.W. Immunopathogenesis of multiple sclerosis. Int. Rev. Neurobiol. 2007;79:99–126. doi: 10.1016/S0074-7742(07)79005-0. [DOI] [PubMed] [Google Scholar]
- 108.Michel L., Prat A. One more role for the gut: microbiota and blood brain barrier. Ann. Transl. Med. 2016;4(1):15. doi: 10.3978/j.issn.2305-5839.2015.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., Gulyás B., Halldin C., Hultenby K., Nilsson H., Hebert H., Volpe B.T., Diamond B., Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001;81(3):1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 111.Braniste V., Al-Asmakh M., Kowal C. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6(263):263ra158-263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boveri M., Kinsner A., Berezowski V., Lenfant A.M., Draing C., Cecchelli R., Dehouck M.P., Hartung T., Prieto P., Bal-Price A. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood-brain barrier disruption through glia activation: role of pro-inflammatory cytokines and nitric oxide. Neuroscience. 2006;137(4):1193–1209. doi: 10.1016/j.neuroscience.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 113.Banks W.A., Gray A.M., Erickson M.A., Salameh T.S., Damodarasamy M., Sheibani N., Meabon J.S., Wing E.E., Morofuji Y., Cook D.G., Reed M.J. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroin-flammation, and elements of the neurovascular unit. J. Neuroinflammation. 2015;12:223–223. doi: 10.1186/s12974-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nicol B., Salou M., Laplaud D-A., Wekerle H. The autoimmune concept of multiple sclerosis. Presse Med. 2015;44(4 Pt 2):e103–e112. doi: 10.1016/j.lpm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 115.Lassmann H. Multiple sclerosis pathology: evolution of pathogenetic concepts. Brain Pathol. 2005;15(3):217–222. doi: 10.1111/j.1750-3639.2005.tb00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lucchinetti C., Brück W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. Heterogeneity of multiple sclerosis lesions: implica-tions for the pathogenesis of demyelination. Ann. Neurol. 2000;47(6):707–717. doi: 10.1002/1531-8249(200006)47:6<707:AID-ANA3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 117.Dutta R., Trapp B.D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(22) Suppl. 3:S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- 118.Lassmann H., Brück W., Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol. Med. 2001;7(3):115–121. doi: 10.1016/S1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- 119.Brucklacher-Waldert V., Stuerner K., Kolster M., Wolthausen J., Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132(Pt 12):3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 120.Matusevicius D., Kivisäkk P., He B., Kostulas N., Ozenci V., Fredrikson S., Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 1999;5(2):101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 121.Durelli L., Conti L., Clerico M., Boselli D., Contessa G., Ripellino P., Ferrero B., Eid P., Novelli F. T-helper 17 cells expand in mul-tiple sclerosis and are inhibited by interferon-beta. Ann. Neurol. 2009;65(5):499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 122.Tzartos J.S., Friese M.A., Craner M.J., Palace J., Newcombe J., Esiri M.M., Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172(1):146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stockinger B., Veldhoen M., Martin B. Th17 T cells: linking innate and adaptive immunity. Semin. Immunol. 2007;19(6):353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 124.Atarashi K., Tanoue T., Oshima K., Suda W., Nagano Y., Nishikawa H., Fukuda S., Saito T., Narushima S., Hase K., Kim S., Fritz J.V., Wilmes P., Ueha S., Matsushima K., Ohno H., Olle B., Sakaguchi S., Taniguchi T., Morita H., Hattori M., Honda K. Treg in-duction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 125.Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 126.Round J.L., Mazmanian S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107(27):12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ochoa-Repáraz J., Mielcarz D.W., Wang Y., Begum-Haque S., Dasgupta S., Kasper D.L., Kasper L.H. A polysaccharide from the hu-man commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 128.Ooijevaar R.E., Terveer E.M., Verspaget H.W., Kuijper E.J., Keller J.J. Clinical application and potential of fecal microbiota transplanta-tion. Annu. Rev. Med. 2019;70:335–351. doi: 10.1146/annurev-med-111717-122956. [DOI] [PubMed] [Google Scholar]
- 129.Borody T., Leis S., Campbell J., Torres M., Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS): 942. Am. J. Gastroenterol. 2011;106:S352. doi: 10.14309/00000434-201110002-00942. [DOI] [Google Scholar]
- 130.Makkawi S., Camara-Lemarroy C., Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol. Neuroimmunol. Neuroinflam. 2018;5(4):e459. doi: 10.1212/NXI.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schepici G., Silvestro S., Bramanti P., Mazzon E. The Gut Microbiota in Multiple Sclerosis: An Overview of Clinical Trials. Cell Transplant. 2019;28(12):1507–1527. doi: 10.1177/0963689719873890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shahi S.K., Freedman S.N., Mangalam A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes. 2017;8(6):607–615. doi: 10.1080/19490976.2017.1349041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J.T., Spector T.D., Clark A.G., Ley R.E. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Markle J.G., Frank D.N., Mortin-Toth S., Robertson C.E., Feazel L.M., Rolle-Kampczyk U., von Bergen M., McCoy K.D., Macpher-son A.J., Danska J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 135.Hollister E.B., Riehle K., Luna R.A., Weidler E.M., Rubio-Gonzales M., Mistretta T.A., Raza S., Doddapaneni H.V., Metcalf G.A., Muzny D.M., Gibbs R.A., Petrosino J.F., Shulman R.J., Versalovic J. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3:36. doi: 10.1186/s40168-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Biedermann L., Zeitz J., Mwinyi J., Sutter-Minder E., Rehman A., Ott S.J., Steurer-Stey C., Frei A., Frei P., Scharl M., Loessner M.J., Vavricka S.R., Fried M., Schreiber S., Schuppler M., Rogler G. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8(3):e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Norman J.M., Handley S.A., Baldridge M.T., Droit L., Liu C.Y., Keller B.C., Kambal A., Monaco C.L., Zhao G., Fleshner P., Stap-penbeck T.S., McGovern D.P., Keshavarzian A., Mutlu E.A., Sauk J., Gevers D., Xavier R.J., Wang D., Parkes M., Virgin H.W. Dis-ease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160(3):447–460. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kernbauer E., Ding Y., Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;516(7529):94–98. doi: 10.1038/nature13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Davenport E.R., Mizrahi-Man O., Michelini K., Barreiro L.B., Ober C., Gilad Y. Seasonal variation in human gut microbiome compo-sition. PLoS One. 2014;9(3):e90731. doi: 10.1371/journal.pone.0090731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., Biddinger S.B., Dutton R.J., Turnbaugh P.J. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., Almeida M., Arumugam M., Batto J.M., Kennedy S., Leonard P., Li J., Burgdorf K., Grarup N., Jørgensen T., Brandslund I., Nielsen H.B., Juncker A.S., Bertalan M., Levenez F., Pons N., Ras-mussen S., Sunagawa S., Tap J., Tims S., Zoetendal E.G., Brunak S., Clément K., Doré J., Kleerebezem M., Kristiansen K., Renault P., Sicheritz-Ponten T., de Vos W.M., Zucker J.D., Raes J., Hansen T., Bork P., Wang J., Ehrlich S.D., Pedersen O. Richness of hu-man gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 142.Piccio L., Stark J.L., Cross A.H. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 2008;84(4):940–948. doi: 10.1189/jlb.0208133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kleinewietfeld M., Manzel A., Titze J., Kvakan H., Yosef N., Linker R.A., Muller D.N., Hafler D.A. Sodium chloride drives autoim-mune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Munger K.L., Bentzen J., Laursen B., Stenager E., Koch-Henriksen N., Sørensen T.I., Baker J.L. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult. Scler. 2013;19(10):1323–1329. doi: 10.1177/1352458513483889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Langer-Gould A., Brara S.M., Beaber B.E., Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolat-ed syndrome. Neurology. 2013;80(6):548–552. doi: 10.1212/WNL.0b013e31828154f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Esposito S., Bonavita S., Sparaco M., Gallo A., Tedeschi G. The role of diet in multiple sclerosis: A review. Nutr. Neurosci. 2018;21(6):377–390. doi: 10.1080/1028415X.2017.1303016. [DOI] [PubMed] [Google Scholar]
- 147.Schirmer M., Smeekens S.P., Vlamakis H., Jaeger M., Oosting M., Franzosa E.A., Ter Horst R., Jansen T., Jacobs L., Bonder M.J., Kurilshikov A., Fu J., Joosten L.A.B., Zhernakova A., Huttenhower C., Wijmenga C., Netea M.G., Xavier R.J. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(4):1125–1136.e8. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crost E.H., Tailford L.E., Le Gall G., Fons M., Henrissat B., Juge N. Utilisation of mucin glycans by the human gut symbiont Rumino-coccus gnavus is strain-dependent. PLoS One. 2013;8(10):e76341. doi: 10.1371/journal.pone.0076341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shahi S.K., Jensen S.N., Murra A.C., Tang N., Guo H., Gibson-Corley K.N., Zhang J., Karandikar N.J., Murray J.A., Mangalam A.K. Human Commensal Prevotella histicola Ameliorates Disease as Effectively as Interferon-Beta in the Experimental Autoimmune Encepha-lomyelitis. Front. Immunol. 2020;11:578648. doi: 10.3389/fimmu.2020.578648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shahi S.K., Freedman S.N., Murra A.C., Zarei K., Sompallae R., Gibson-Corley K.N., Karandikar N.J., Murray J.A., Mangalam A.K. Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Front. Immunol. 2019;10:462. doi: 10.3389/fimmu.2019.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sivieri K., Morales M.L., Adorno M.A., Sakamoto I.K., Saad S.M., Rossi E.A. Lactobacillus acidophilus CRL 1014 improved “gut health” in the SHIME reactor. BMC Gastroenterol. 2013;13:100. doi: 10.1186/1471-230X-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]


