Abstract
Background: Dopamine is one of the main mediators capable regulate the neuroimmune interaction and is involved in multiple sclerosis (MS) pathogenesis.
Objective: The aim of this study was to clarify the role of dopamine and its receptors in modulation of Th17-cells in MS.
Methods: 34 relapsing-remitting MS patients and 23 healthy subjects were examined. To assess the effect of dopamine on Th17-cells, CD4+ T-cells were cultured in the presence of dopamine and antagonist or agonist of D1- or D2-like dopaminergic receptors and stimulated with anti-CD3/CD28-microbeads. The levels of cytokines in supernatants were assessed by ELISA.
Results: Production of interleukin-17 (IL-17), interferon-γ (IFN-γ), granulocyte-colony stimulating factor (GM-CSF), and IL-21 by CD4+ T-cells as well as dopamine were comparable between the groups. Dopamine suppressed cytokine secretion by activated СD4+ T-cells in both groups. Blockade of D1-like dopaminergic receptor with a specific antagonist SCH23390 did not affect dopamine-mediated cytokine suppression. In contrast, blockade of D2-like dopaminergic receptor by sulpiride decreased dopamine's inhibitory effect on IL-17 secretion in both groups and GM-CSF and IL-21 production in MS patients. Blockade of D1-like dopaminergic receptor directly inhibited IL-17, IFN-γ, GM-CSF in both groups and IL-21 production in healthy subjects, while blockade of D2-like dopaminergic receptor had no effect on cytokine secretion. Finally, activation of D2-like dopaminergic receptor with a specific agonist quinpirole decreased cytokine production in both groups.
Conclusion: These data suggest an inhibitory role of dopamine on Th17-cells in MS, which could be mediated by the activation of the D2-like dopaminergic receptor.
Keywords: Dopamine, dopaminergic receptors, Th17-cells, multiple sclerosis, neuroimmunology, neuropharmacology
1. INTRODUCTION
Multiple sclerosis (MS) is an immune-mediated demyelinating and neurodegenerative disease of the central nervous system (CNS), which affects mainly young adults [1].
Recent studies showed that disturbance of neuroimmune interaction is involved in MS pathogenesis [2, 3]. Biogenic amines are direct mediators of this interaction and affect MS pathogenesis by modulating immune cell function.
Among the neurotransmitters that participated in the regulation of neuroimmune interaction in MS, dopamine attracted the most attention. In MS, dopamine mediates neuropsychological symptoms, such as fatigue, cognitive impairments, depression [3]. On the other hand, dopamine modulates both innate and adaptive immune systems' cells. In particular, macrophages, dendritic cells, monocytes, CD4+ and CD8+ T-cells express all types of dopaminergic receptors. Furthermore, some of them may produce dopamine suggesting the possible dopaminergic autoregulation [4].
It is known that Th17-cells play a crucial role in the pathogenesis of a number of autoimmune diseases, including MS [5]. Th17-cells produce pro-inflammatory cytokines such as interleukin-17 (IL-17), IL-21, granulocyte- and granulocyte-macrophage colony-stimulating factors (G-CSF and GM-CSF). Th17-cells can penetrate the blood-brain barrier and mediate neuroinflammation in MS [6, 7]. The relapse of MS is characterized by an increasing number of Th17-cells in the CSF and peripheral blood as well as IL-17 and IFN-γ production by activated PBMCs [8, 9]. According to literature data, almost all pathogenetic treatments of MS affect the Th17-cells function [10].
In this regard, the effect of dopamine on Th17-cells function in MS has been met with great interest [11]. In some previous studies, the ability of dopamine to modulate Th17-cytokine production by activated peripheral blood mononuclear cells (PBMCs) in MS was shown [11]. However, the effect of dopamine on Th17-cells function in MS is still controversial [9, 12]. In addition, the role of dopaminergic receptors in the modulation of Th17-cells has not been sufficiently investigated.
This study was aimed at clarification of the dopamine effect and involvement of its receptors in the modulation of Th17-cytokine production by CD4+ T-cells in patients with relapsing-remitting MS.
2. MATERIALS AND METHODS
2.1. Patients
Thirty-four patients with a documented diagnosis of relapsing-remitting MS according to McDonald criteria (modification 2017) [13] were examined. Their main demographic and clinical characteristics are shown in Table 1. All patients were subjected to a standard neurological examination with an assessment of the EDSS score [14]. All patients were examined during clinical remission without the magnetic resonance imaging (MRI) activity of the disease. All patients had been treated with glatiramer acetate for more than one year. At the time of blood sampling, all patients studied had not been treated with corticosteroid therapy or therapy with antidepressants for more than six months. All patients were non-smoking and had no mental disorders, according to the Beck Depression Inventory [15], and cognitive impairments, according to Montreal Cognitive Assessment [16]. The control group consisted of 23 healthy donors matched with patients by sex and age (Table 1).
Table 1.
Clinical and demographic characteristics of MS patients and healthy subjects. Data are medians (25th; 75th percentiles).
| Factor | MS Patients, n=34 | Healthy Subjects, n=23 |
|---|---|---|
| Age, years | 29 (25; 33) | 30 (28; 35) |
| Men/women (% women) | 12/22 (65) | 8/15 (65) |
| Duration of MS, years | 2 (2; 6) | NA* |
| EDSS score | 1.5 (1.5; 2) | NA* |
*NA - not applicable.
All patients signed the written informed consent to participate in this study. The study was approved by the ethics committee of the Pirogov Russian National Research Medical University (protocol No. 192).
2.2. CD4+ T-cells Cultures and Activation
To evaluate the function of Th17-cells, CD4+ T-cells were isolated and activated by anti-CD3/CD28-microbeads as previously described [17].
To assess the effect of dopamine on the function of Th17-cells, samples of CD4+ T-cells were cultured in the presence of dopamine (Sigma, USA) at a concentration of 10–5 M [9] for 15 min. whereafter anti-CD3/CD28-microbeads were added to the cultures.
To study the involvement of dopaminergic receptors in dopamine-mediated modulation of cytokine production, some samples of CD4+ T-cells were pre-incubated with antagonists of D1- or D2-like dopaminergic receptors (SCH23390 and sulpiride respectively) (both from Sigma, USA) at a concentration of 10–5 M [9] for 15 min, whereafter dopamine (at 10–5 M) was added to the cultures and stimulation was proceeded.
To study the direct effect of blockading or activation of the dopaminergic receptor, CD4+ T-cells were pre-incubated in the presence of D1- or D2-like receptors antagonists (SCH23390 and sulpiride respectively [at 10–5 M]) or D2-like receptor agonist (quinpirole [at 10–7 M] Tocris, Switzerland) and activated by anti-CD3/CD28-microbeads [18].
2.3. Dopamine and Homovanillic Acid Evaluation
The concentration of dopamine and its metabolite homovanillic acid (HVA) in culture supernatants were determined by high-performance liquid chromatography (HPLC) as previously described [17]. Dopamine and HVA concentrations in samples were calculated by the “internal standard” method, based on the ratio of peak areas in the standard mixture and the sample, and expressed in pmol/ml.
2.4. Cytokine Assessment
Levels of cytokine (IL-17, IFN-γ, GM-CSF, and IL-21) in the supernatants were determined by enzyme-linked immunosorbent assay ELISA (Invitrogen, USA). In all cases of ELISAs, the instructions of the kit manufacturers were followed. Data are expressed as pg/ml or as the percentage of cytokine production by stimulated CD4+ T-cells in the absence of dopamine and antagonists/agonists of dopaminergic receptors [17].
2.5. Statistical Analysis
The statistical analysis of the results was performed using Prizm 6 software. The nonparametric Mann-Whitney U test or Wilcoxon signed-rank test was used to compare the two groups. One-way ANOVA followed by Bonferroni correction was used for multiple comparisons. Differences were considered statistically significant if p<0.05.
3. RESULTS
3.1. Production of Cytokines, Dopamine, and HVA by CD4+ T-cells in MS Patients and Healthy Subjects
To study cytokine production by Th17-cells, CD4+ T-cells were activated by anti-CD3/CD28-microbeads, whereafter the levels of IL-17, IFN-γ, GM-CSF, and IL-21 were assessed in culture supernatants by ELISA. Although IFN-γ is mainly a product of Th1-cells, it was shown that Th17.1-cells (a subset of Th17-cells) also produce IFN-γ and play an important role in the pathogenesis of MS [19]. According to ELISA, the production of cytokine by non-activated or activated CD4+ T-cells was comparable between the groups (Table 2).
Table 2.
The production of cytokine by CD4+ T-cells (ELISA) in MS patients and healthy subjects. Data are medians (25th; 75th percentiles).*
| Cytokine | Stimulation | MS Patients, n=34 | Healthy Subjects, n=23 |
|---|---|---|---|
| IL-17, pg/ml | None | 0 (0; 7) | 3 (0; 11) |
| Anti-CD3/anti-CD28 | 700 (321; 1125) | 788 (165; 1351) | |
| IFN-γ, pg/ml | None | 6 (2; 22) | 2 (0; 11) |
| Anti-CD3/anti-CD28 | 5199 (2777; 7960) | 4557 (1590; 5315) | |
| GM-CSF, pg/ml | None | 0 (0; 4) | 4 (0; 10) |
| Anti-CD3/anti-CD28 | 493 (201; 787) | 390 (107; 874) | |
| IL-21, pg/ml | None | 0 (0; 0) | 2 (0; 15) |
| Anti-CD3/anti-CD28 | 87 (45; 162) | 118 (52; 247) |
* No statistically differences between the groups (Mann-Whitney U-test).
We also studied the ability of CD4+ T-cells to produce dopamine and HVA without or upon CD3/CD28-stimulation. We found that dopamine production by CD4+ T-cells upon CD3/CD28-stimulation was higher than unstimulated production in both groups (Table 3). There were no significant differences in spontaneous or stimulated dopamine production by CD4+ T-cells between the groups (Table 3).
Table 3.
The production of dopamine and its metabolites by CD4+ T-cells (HPLC) in MS patients and healthy subjects. Data are medians (25th; 75th percentiles).
| Factor | Stimulation | MS Patients, n=32 | Healthy Subjects, n=18 |
|---|---|---|---|
| Dopamine, pmole/ml |
None | 0.75 (0.39; 1.38) | 0.69 (0.52; 1.14) |
| Anti-CD3 / anti-CD28 | 1.11 (0.47; 2.42)* | 1.81 (1.11; 2.3)** | |
| HVA, pmole/ml |
None | 0.81 (0; 2.7) | 0 (0; 0.33) |
| Anti-CD3 / anti-CD28 | 0.54 (0; 2.3) | 0 (0; 1) |
* p<0.05 compared with unstimulated production in MS patients (Wilcoxon matched-pair test).
** p<0.01 compared with unstimulated production in healthy subjects (Wilcoxon matched-pair test).
3.2. The Role of Dopaminergic Receptors in Dopamine-mediated Modulation of Th17-cells in MS Patients and Healthy Subjects
To assess the dopamine effect on cytokine production, we stimulated CD4+ T-cells in the presence of dopamine at a concentration of 10–5 M. Dopamine reduced IL-17, IFN-γ, GM-CSF, and IL-21 production by stimulated CD4+ T-cells in both groups (Fig. 1) without affecting proliferative response (data not shown).
Fig. (1).
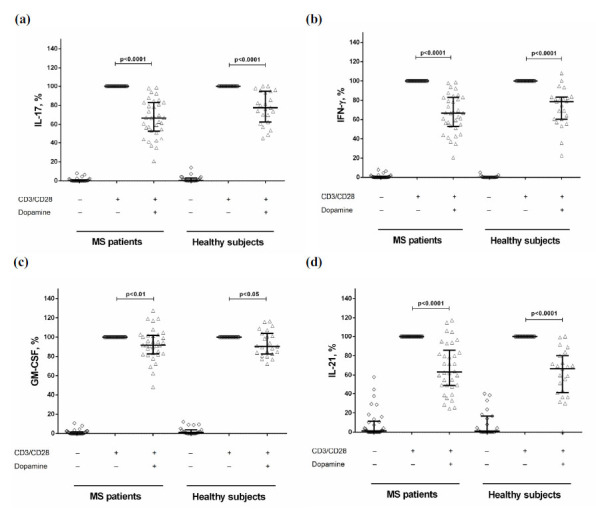
The effect of dopamine on IL-17 (a), IFN-γ (b), GM-CSF (c), and IL-21 (d) production by stimulated CD4+ T-cells in MS patients (n=34) and healthy subjects (n=23). Data are expressed as the percentage relative to anti-CD3/CD28-stimulated cytokine production in the absence of dopamine. Horizontal lines at the graphs correspond to the median, and whiskers indicate 25th and 75th percentiles. The median values were compared and the p values are indicated in the figure.
To study the dopaminergic receptors, which could mediate dopamine effect on cytokine production, CD4+ T-cells were pre-incubated with a specific antagonist of D1- or D2-like dopaminergic receptors and dopamine and activated by anti-CD3/CD28-microbeads. It was found that pre-blocking of D2-like dopaminergic receptor by sulpiride decreased dopamine-mediated IL-17 suppression in both groups (Fig. 2a and b) and GM-CSF and IL-21 production in MS patients (Fig. 2e and g). There was no influence of D1-like receptor antagonist on the inhibitory effect of dopamine on cytokine production in both groups (Fig. 2).
Fig. (2).
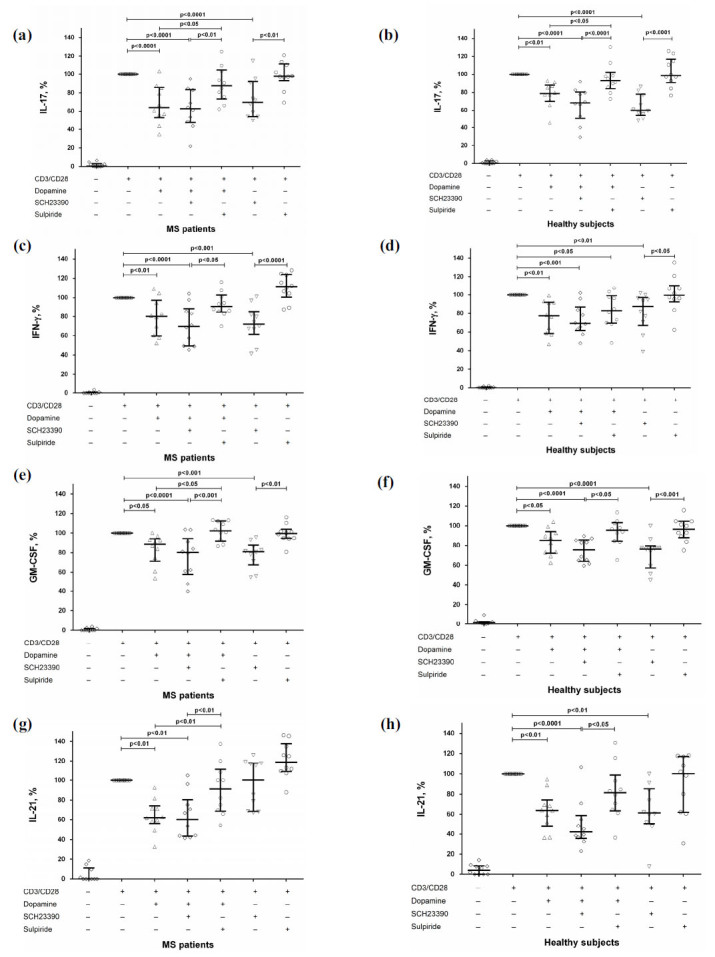
The role of dopaminergic receptors in dopamine-mediated suppression of IL-17 (a and b), IFN-γ (c and d), GM-CSF (e and f), and IL-21 (g and h) production by stimulated CD4+ T-cells in MS patients (n=10) and healthy subjects (n=10). Data are expressed as the percentage of cytokine production by activated cells in the absence of dopamine and dopaminergic receptors antagonists. Horizontal lines at the graphs correspond to the median, and whiskers indicate 25th and 75th percentiles. The median values of MS and control groups were compared, and the p values are indicated in the figure.
3.3. The Involvement of Dopaminergic Receptors in Modulation of Th17-cells in MS Patients and Healthy Subjects
Then, we investigated the direct effect of blockade of dopaminergic receptors (without subsequent dopamine addition) on cytokine production by CD4+ T-cells. We found that blockade of D1-like dopaminergic receptor suppressed IL-17, IFN-γ, and GM-CSF production in both groups (Fig. 2a-f) and IL-21 production in healthy subjects (Fig. 2h) without affecting proliferative response (data not shown). Blockade of D2-like dopaminergic receptor had no effect on cytokine production in both groups (Fig. 2).
To clarify the role of D2-like dopaminergic receptors in Th17-cells modulation, we investigated the effect of D2-like dopaminergic receptor agonist quinpirole on cytokine production by activated CD4+ T-cells. We found that activation of D2-like dopaminergic receptor reduced IL-17, IFN-γ, and GM-CSF production in both groups (Fig. 3) without affecting proliferative response (data not shown).
Fig. (3).
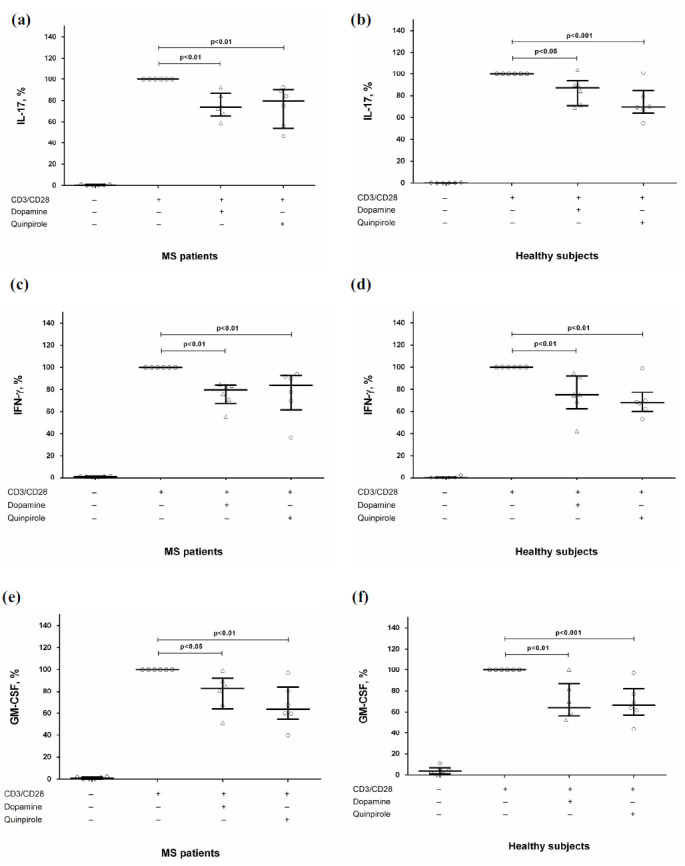
The effect of D2-like dopaminergic receptor agonist quinpirole on IL-17 (a and b), IFN-γ (c and d), and GM-CSF (e and f) production by stimulated CD4+ T-cells in MS patients (n=6) and healthy subjects (n=6). Data are expressed as the percentage of cytokine production by activated cells in the absence of dopamine and quinpirole. Horizontal lines at the graphs correspond to the median, and whiskers indicate 25th and 75th percentiles. The median values of MS and control groups were compared, and the p values are indicated in the figure.
4. DISCUSSION
The results of recent studies suggest that Th17-cells are one of the crucial therapeutic targets in MS [10]. In the present research, we did not find any difference in cytokine production by the activated CD4+ T-cells between MS patients and healthy subjects (Table 2), confirming our previous results where we showed the comparable IL-17 and IFN-γ production by PBMCs and purified CD4+ T-cells in MS patients during clinical remission and healthy subjects [20]. In addition, all MS patients had been treated with glatiramer acetate, which reduced Th17-immune response in MS [20].
The production of dopamine and HVA by non-activated or activated with anti-CD3/CD28-microbeads was also comparable between the groups (Table 3), consistent with data from Cosentino et al., who showed similar dopamine production by phytohemagglutinin (PHA)-activated PBMCS in patients with inactive MS and healthy subjects, and decreased dopamine production in patients with active MS [21]. Another study showed the same dopamine production by resting T-cells obtained from relapsing-remitting MS patients in clinical remission and healthy subjects [22]. It is notable that stimulated dopamine production was significantly higher compared to spontaneous production in both groups (Table 3), consistent with data obtained from non-activated or PHA-activated PBMCS and suggested the involvement of dopamine in the modulation of activated T-cells [23]. It should be noted that we have excluded cofactors related to dopaminergic system disturbance: all MS patients who participated in the present study had no psycho-emotional or cognitive impairments and were not treated with interferon-β, which could aggravate depression and change catecholamines production by T-cells [24].
We also found that dopamine suppressed Th17-cytokine production by activating CD4+ T-cells in both groups (Fig. 1). The inhibitory effect of dopamine on Th17-cells and dendritic cells induced Th17-immune response in demyelinating CNS diseases was previously reported [11]. However, these studies were conducted in EAE or PBMCs of MS patients. Here we showed the inhibitory effect of dopamine on cytokine secretion by purified CD4+ T-cells obtained from relapsing-remitting MS patients, reaffirming the anti-inflammatory role of dopamine in MS. It is important to note that this effect is achieved using relatively high concentration (10–5 M), while at lower concentrations, dopamine may enhance IL-17 and IL-21 production by activated PBMCs or CD4+ T-cells in relapsing-remitting MS patients [12]. Although the dopamine concentration used in the present study was much higher than we measured in culture supernatants, in the distinct regions of brain tissue, dopamine level may achieve 10–3 M [25]. In addition, at this concentration, dopamine did not affect proliferative response. Thus, we showed the clear suppressive effect of dopamine on Th17-cells.
To study the role of dopaminergic receptors in Th17-cells modulation, we affected these receptors by specific antagonists or agonists of D1-like or D2-like dopaminergic receptors in the presence or absence of dopamine and stimulated with anti-CD3/CD28-microbeads. First, we confirmed the involvement of D2-like dopaminergic receptors in dopamine-mediated cytokine suppression by activated Th17-cells in MS. Thus, the antagonist of D2-like dopaminergic receptor sulpiride reduced dopamine-mediated IL-17, GM-CSF, and IL-21 suppression in MS patients (Fig. 2), which corresponds to the effect of sulpiride on the inhibitory dopamine impact on IL-17 production by activated PBMCs in patients with relapsing-remitting MS [9]. Conversely, blockade of D1-like dopaminergic receptors did not affect dopamine-mediated suppression of cytokine production (Fig. 2).
Then, we investigated the direct effect of D1- and D2-like dopaminergic receptors targeting on Th17-cells function. We found that blockade D1-dopaminergic receptor suppressed IL-17, IFN-γ, GM-CSF in both groups and IL-21 production in healthy subjects, while blockade of D2-like dopaminergic receptor had no effect on cytokine secretion in both groups (Fig. 2). The inhibitory effect of D1-like receptor blocking on Th17-immune response was previously reported by Nakano et al., who showed that SCH23390 suppressed dendritic cells induced IL-17 production [26]. In the present research, we found that D1-like dopaminergic receptor antagonists could affect Th17-immune response by directly affecting T-cells.
Finally, in line with these data, the agonist of D2-like dopaminergic receptor quinpirole decreased IL-17, IFN-γ, and GM-CSF production by activated CD4+ T-cells in both groups (Fig. 3), suggesting the role of D2-like dopaminergic receptor activation in Th17-cells suppression.
The molecular mechanisms underlying the influence of D2-like dopaminergic receptor activation on Th17-cells are unclear. Huang et al. reported that activation of D2-like dopaminergic receptor by quinpirole decreased intracellular cAMP content and reduced the phosphorylated cAMP-response element-binding (CREB) level in T-cells [27]. Simultaneously, quinpirole increases the expression of Th2- and Treg-specific transcription factors (GATA-3 and Foxp3 respectively) and IL-10 mRNA expression in Con A-activated mice lymphocytes but decreases the expression of Th1- and Th17-specific transcription factors (T-bet and ROR-γt respectively) and IFN-γ and IL-17 mRNA. These effects of quinpirole are abolished by the D2-like dopaminergic receptor antagonist haloperidol [27]. These data suggest that D2-like dopaminergic receptors modulate the cAMP-protein kinase A (PKA)-CREB pathway and modulate Th17-cells function. However, more studies are needed.
CONCLUSION
Taken together, the results of present and other studies suggest the anti-inflammatory role of dopamine in MS that could be mediated by the modulation of Th17-immune response via D2-like dopaminergic receptor.
ACKNOWLEDGEMENTS
Declared none.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committee of the Pirogov Russian National Research Medical University (protocol No. 192).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All human procedures followed were in accordance with the Helsinki Declaration.
CONSENT FOR PUBLICATION
All patients signed the written informed consent to participate in this study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This study was supported by a grant from the Russian Science Foundation (project No. 19-75-00075).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Boyko A., Melnikov M. Prevalence and incidence of multiple sclerosis in russian federation: 30 years of studies. Brain Sci. 2020;10(5):305. doi: 10.3390/brainsci10050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melnikov M., Rogovskii V., Boyko A., Pashenkov M. The influence of biogenic amines on Th17-mediated immune response in multi-ple sclerosis. Mult. Scler. Relat. Disord. 2018;21:19–23. doi: 10.1016/j.msard.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Carandini T., Cercignani M., Galimberti D., Scarpini E., Bozzali M. The distinct roles of monoamines in multiple sclerosis: a bridge between the immune and nervous systems? Brain Behav Immun. 2021;1 doi: 10.1016/j.bbi.2021.02.030. S0889-1591(21)00095-7. [DOI] [PubMed] [Google Scholar]
- 4.Hodo T.W., de Aquino M.T.P., Shimamoto A., Shanker A. Critical neurotransmitters in the neuroimmune network. Front. Immunol. 1869;2020(21):11. doi: 10.3389/fimmu.2020.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milovanovic J., Arsenijevic A., Stojanovic B. Interleukin-17 in chronic inflammatory neurological diseases. Front. Immunol. 2020;11:947. doi: 10.3389/fimmu.2020.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kebir H., Kreymborg K., Ifergan I., Dodelet-Devillers A., Cayrol R., Bernard M., Giuliani F., Arbour N., Becher B., Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovett-Racke A.E., Yang Y., Racke M.K. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim. Biophys. Acta. 2011;1812(2):246–251. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brucklacher-Waldert V., Stuerner K., Kolster M., Wolthausen J., Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132(Pt 12):3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 9.Melnikov M., Belousova O., Murugin V. Pashenkov, М.; Boyко, A. The role of dopamine in modulation of Th-17 immune response in multiple sclerosis. J. Neuroimmunol. 2016;292(292):97–101. doi: 10.1016/j.jneuroim.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Moser T., Akgün K., Proschmann U., Sellner J., Ziemssen T. The role of TH17 cells in multiple sclerosis: Therapeutic implications. Autoimmun. Rev. 2020;19(10):102647. doi: 10.1016/j.autrev.2020.102647. [DOI] [PubMed] [Google Scholar]
- 11.Levite M., Marino F., Cosentino M. Dopamine, T cells and multiple sclerosis (MS). J. Neural Transm. (Vienna) 2017;124(5):525–542. doi: 10.1007/s00702-016-1640-4. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira T.B., Barros P.O., Teixeira B., Cassano T., Centurião N., Kasahara T.M., Hygino J., Vasconcelos C.C., Filho H.A., Al-varenga R., Wing A.C., Andrade R.M., Andrade A.F., Bento C.A. Dopamine favors expansion of glucocorticoid-resistant IL-17-producing T cells in multiple sclerosis. Brain Behav. Immun. 2014;41:182–190. doi: 10.1016/j.bbi.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., Fujihara K., Galetta S.L., Hartung H.P., Kappos L., Lublin F.D., Marrie R.A., Miller A.E., Miller D.H., Montalban X., Mowry E.M., Sorensen P.S., Tintoré M., Traboulsee A.L., Trojano M., Uitdehaag B.M.J., Vukusic S., Waubant E., Weinshenker B.G., Reingold S.C., Cohen J.A. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 15.Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 17.Sviridova A., Rogovskii V., Kudrin V., Pashenkov M., Boyko A., Melnikov M. The role of 5-HT2B-receptors in fluoxetine-mediated modulation of Th17- and Th1-cells in multiple sclerosis. J. Neuroimmunol. 2021;356:577608. doi: 10.1016/j.jneuroim.2021.577608. [DOI] [PubMed] [Google Scholar]
- 18.Besser M.J., Ganor Y., Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. J. Neuroimmunol. 2005;169(1-2):161–171. doi: 10.1016/j.jneuroim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 19.van Langelaar J., van der Vuurst de Vries R.M., Janssen M. T helper 17.1 cells associate with multiple sclerosis disease activity: per-spectives for early intervention. Brain. 2018;141(5):1334–1349. doi: 10.1093/brain/awy069. [DOI] [PubMed] [Google Scholar]
- 20.Melnikov M., Sharanova S., Sviridova A. The influence of glatiramer acetate on Th17-immune response in multiple sclerosis. PLoS One. 2020;15(10):e0240305. doi: 10.1371/journal.pone.0240305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosentino M., Zaffaroni M., Marino F., Bombelli R., Ferrari M., Rasini E., Lecchini S., Ghezzi A., Frigo G. Catecholamine produc-tion and tyrosine hydroxylase expression in peripheral blood mononuclear cells from multiple sclerosis patients: effect of cell stimulation and possible relevance for activation-induced apoptosis. J. Neuroimmunol. 2002;133(1-2):233–240. doi: 10.1016/S0165-5728(02)00372-7. [DOI] [PubMed] [Google Scholar]
- 22.Rajda C., Bencsik K., Vécsei L.L., Bergquist J. Catecholamine levels in peripheral blood lymphocytes from multiple sclerosis patients. J. Neuroimmunol. 2002;124(1-2):93–100. doi: 10.1016/S0165-5728(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 23.Cosentino M., Marino F., Bombelli R., Ferrari M., Rasini E., Lecchini S., Frigo G. Stimulation with phytohaemagglutinin induces the synthesis of catecholamines in human peripheral blood mononuclear cells: role of protein kinase C and contribution of intracellular calci-um. J. Neuroimmunol. 2002;125(1-2):125–133. doi: 10.1016/S0165-5728(02)00019-X. [DOI] [PubMed] [Google Scholar]
- 24.Zaffaroni M., Marino F., Bombelli R., Rasini E., Monti M., Ferrari M., Ghezzi A., Comi G., Lecchini S., Cosentino M. Therapy with interferon-beta modulates endogenous catecholamines in lymphocytes of patients with multiple sclerosis. Exp. Neurol. 2008;214(2):315–321. doi: 10.1016/j.expneurol.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Matt S.M., Gaskill P.J. Where is dopamine and how do immune cells see it? Dopamine-mediated immune cell function in health and disease. J. Neuroimmune Pharmacol. 2020;15(1):114–164. doi: 10.1007/s11481-019-09851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano K., Higashi T., Hashimoto K., Takagi R., Tanaka Y., Matsushita S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem. Biophys. Res. Commun. 2008;373(2):286–291. doi: 10.1016/j.bbrc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y., Chen C.C., Wang T.T., Qiu Y.H., Peng Y.P. Dopamine receptors modulate T lymphocytes via inhibition of cAMP-CREB signaling pathway. Neuroendocrinol. Lett. 2016;37(7):491–500. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


