Abstract
7,8-Dihydroxyflavone (7,8-DHF) is a kind of natural flavonoid with the potential to cross the blood-brain barrier. 7,8-DHF effectively mimics the effect of brain-derived neurotrophic factor (BDNF) in the brain to selectively activate tyrosine kinase receptor B (TrkB) and downstream signaling pathways, thus playing a neuroprotective role. The preclinical effects of 7,8-DHF have been widely investigated in neuropsychiatric disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), depression, and memory impairment. Besides the effect on TrkB, 7,8-DHF could also function through fighting against oxidative stress, cooperating with estrogen receptors, or regulating intestinal flora. This review focuses on the recent experimental studies on depression, neurodegenerative diseases, and learning and memory functions. Additionally, the structural modification and preparation of 7,8-DHF were also concluded and proposed, hoping to provide a reference for the follow-up research and clinical drug development of 7,8-DHF in the field of neuropsychiatric disorders.
Keywords: 7,8-DHF; drug development; learning and memory; neuropsychiatric disorders; neuroprotective effect; TrkB agonist
1. INTRODUCTION
Flavonoids are a large class of plant secondary metabolites, and they are also common polyphenols in the human diet. Modern studies have shown that flavonoids have various pharmacological activities, such as anti-tumor, anti-inflam-matory, and antioxidant properties [1-4]. In recent years, there have been increasing reports on the neuroprotective effects of flavonoids in neuropsychiatric disorders. For example, baicalein, a flavonoid with neurobiological activity in Scutellaria baicalensis Georgi, improves Parkinson’s disease (PD)-like motor behaviors, reduces the loss of dopaminergic neurons, and inhibits the increase of proinflammatory cytokines by inhibiting NOD‐like receptor family pyrin domain containing 3 (NLRP3)/caspase-1/Gasdermin D (GSDMD) pathway [5]. The release of harmful substances from activated astrocytes can cause inflammation in neuropsychiatric disorders [6-8]. Flavonoids, apigenin and luteolin, prevent lipopolysaccharide (LPS)-induced astrocyte activation and inhibit the production of interleukin (IL)-31 and IL-33 through mitogen-activated protein kinase (MAPK), signal transducer and activator of transcription 3 (STAT3), and nuclear factor-kappa B (NF-κB) signaling pathways to play a neuroprotective role [9]. Quercetin has a flavonoid structure, which alleviates the dyskinesia of 6-hydroxydopamine (6-OHDA)-induced PD model rats, reduces neuronal death, mitochondrial damage, and the accumulation of α-synuclein (ASN) [10]. Similarly, the neuroprotective effect of flavonoids in Astragalus membranaceus on Parkinson’s disease (PD) and Alzheimer’s disease (AD) and the beneficial effects of cocoa flavanols on cognitive function and neuroplasticity also show potential application prospects [11-15]. Research results such as these have been continuously discovered, and the role of flavonoids in neuropsychiatric disorders has attracted widespread attention from researchers [16].
7,8-DHF is a natural compound found in Godmania aesculifolia, Tridax procumbens, and Primula halleri leaves [17]. It is worth noting that 7,8-DHF is also found in the whole plant of Lepisorus ussuriensis, traditional Chinese medicine for heat-clearing and detoxification [18] (Fig. 1). In 2010, Jang et al. reported that 7,8-DHF could penetrate the blood-brain barrier (BBB), simulate the BDNF effect, and selectively activate TrkB receptors, which has a strong protective effect on neurons [19]. Since then, 7,8-DHF has attracted wide attention as a high-affinity TrkB small molecule agonist and has been extensively investigated in diseases related to the BDNF-TrkB signaling pathway, including schizophrenia [20], anxiety disorders [21], gastrointestinal diseases [22], metabolic syndrome [23], cardiovascular diseases [24], Huntington’s disease (HD) [25, 26], and Down syndrome (DS) [27-29] (Fig. 2). Marco et al. gave a comprehensive overview of the experimental evidence and potential significance of 7,8-DHF in studying many diseases, which will not be repeated in this review [30]. From the current research situation, the study on the neuroprotective effect of 7,8-DHF is more comprehensive than other effects. In view of the excellent neuroprotection and the regulation of mental function, can 7,8-DHF be used as a candidate drug for the treatment of neuropsychiatric disorders? This review focuses on the recent experimental studies on the role of 7,8-DHF in depression, neurodegenerative diseases (Alzheimer's disease and Parkinson's disease), and learning and memory functions, summarizes the mechanisms of 7,8-DHF on neuropsychiatric disorders, and puts forward the prospect of its transformation from the perspective of structural modification and preparation. It is expected to provide a reference for the follow-up research and clinical drug development of 7,8-DHF in neuropsychiatric disorders.
Fig. (1).
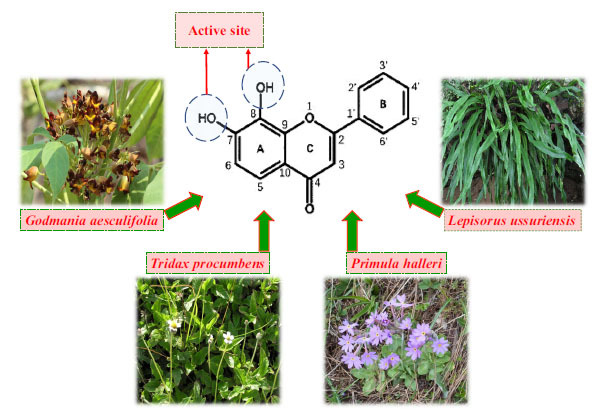
Chemical formula of 7,8-DHF, active site identification, and source plants. (7,8-DHF:7,8-Dihydroxyflavone).
Fig. (2).
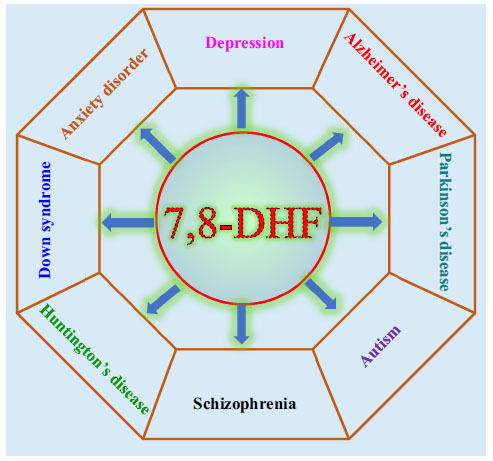
Effects of 7,8-DHF on neuropsychiatric disorders including depression, AD, PD, schizophrenia, anxiety disorder, and Huntington's disease. Abbreviations: (7,8-DHF:7,8-Dihydroxyflavone, AD: Alzheimer's disease, PD: Parkinson's disease).
2. BDNF-TRKB SIGNALING PATHWAY AND NEUROPSYCHIATRIC DISORDERS
2.1. BDNF-TrkB Signaling Pathway
BDNF was primarily isolated and purified from pig brain by German neurobiologist Barde et al. in 1982 [31]. It is one of the most important neurotrophic factors, which forms a family of exocrine proteins with neurotrophic factor, neurotrophic factor-3, and neurotrophin-4/5 [32]. BDNF is widely present in the central and peripheral nervous systems of the body, such as the hippocampus, amygdala, and cerebral cortex. The structure of the human BDNF gene is relatively complex, consisting of 11 exons, 9 of which contain functional promoters [33]. BDNF, like other members of the neurotrophin family, is synthesized in the form of precursors. After the transcription and translation of the BDNF coding gene, the 32kDa BDNF precursor (proBDNF) is initially synthesized, and then cleaved into mature BDNF of bioactive 14 kDa under the cleavage enzymes such as furin and proprotein convertase [34]. BDNF is released from the presynaptic membrane in an activity-dependent manner and binds to two receptors with completely different structures on the target cell membrane [35]. One is low-affinity P75 neurotrophin receptor (P75NTR), and the other is high-affinity TrkB, which activates downstream signaling pathways to promote axonal growth by maintaining neuronal differentiation, development, growth, and survival [36]. It also participates in the regulation of synaptic plasticity and plays a key role in the development of the nervous system [37].
TrkB, belonging to the Trk family of tyrosine kinase receptors, is a high-affinity specific receptor for BDNF and one of the most widely distributed neurotrophic receptors in the brain, which is highly enriched in the cerebral cortex, hippocampus, striatum, and brainstem [38]. TrkB is composed of a single transmembrane peptide chain. The extracellular domain consists of two cysteine-rich domains, three leucine-rich domains, and two immunoglobulin-like domains. The intracellular domain contains the tyrosine kinase domain, which is necessary for binding to BDNF with high affinity [39, 40]. There are two TrkB subtypes in the mammalian central nervous system (CNS), including full-length TrkB (TrkB-FL) and truncated TrkB (TrkB-T1). The truncated TrkB receptors lack the tyrosine kinase domain of full-length TrkB receptor cells [41, 42].
BDNF binds to TrkB receptors to form the BDNF-TrkB signaling pathway, which is the most important signaling pathway for BDNF to exert its biological function. When BDNF specifically binds to the extracellular domain of TrkB, it induces self-dimerization of TrkB, activates the tyrosine kinase region of the intracellular receptor, promotes the phosphorylation of tyrosine residues of the TrkB receptor, and then stimulates one or more downstream signaling pathways, including phosphatase C-γ (PLC-γ), phosphatidylinositol 3-kinase (PI3K), and MAPK cascades. These downstream signaling pathways play an important role in protecting the CNS disorders by affecting neuronal proliferation, differentiation and survival, synaptic plasticity, axon, and dendritic growth (Fig. 3) [43-46].
Fig. (3).
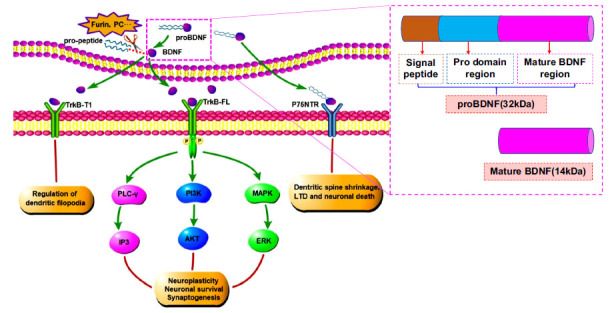
BDNF affects neuronal plasticity, survival, and nerve growth through PLC-γ, PI3K, and MAPK signaling pathways. Abbreviations: (BDNF: brain-derived neurotrophic factor, proBDNF: brain-derived neurotrophic factor precursor, PLC-γ: phosphatase C-γ, PI3K: phosphatidylinositol 3-kinase, MAPK: mitogen-activated protein kinase, AKT: protein kinase B, ERK: extracellular signal-regulated kinase, IP3: inositol 1,4,5-trisphosphate, TrkB-FL: full-length tyrosine kinase receptor B, TrkB-T1: truncated tyrosine kinase receptor B, P75NTR: P75 neurotrophin receptor).
2.2. BDNF and Neuropsychiatric Disorders
Neuropsychiatric disorders are a combination of neurological diseases and mental disorders, which refer to a series of diseases that occur in the central or peripheral nervous system and are characterized by sensory, cognitive, emotional, behavioral, and psychological disorders, including PD, AD, traumatic brain injury, depression, schizophrenia, etc. [47-50]. The occurrence of neuropsychiatric disorders causes great harm to human health, especially in the post-epidemic era. The survey indicated that the probability of developing neurological or mental disorders in the 236,000 patients diagnosed with COVID-19 within 6 months was as high as 33.62%, which affected the quality of life of the patients after recovery [51]. However, the condition of neuropsychiatric disorders is more complex, and there are many neural networks involved, and the pathogenesis is not completely clear. Modern studies have shown that neurotrophic factor (NTF) plays a very important role in the occurrence, development, and functional maintenance of the peripheral and CNS. NTF can provide nutritional support for neurons and key brain regions related to regulating emotional behaviors in the CNS. Therefore, the occurrence of many neuropsychiatric disorders is closely related to the abnormal changes of NTF [52-54].
2.2.1. BDNF is a Key Regulator of Depression
Depression is one of the most common psychiatric diseases in clinics, with a high prevalence rate [55]. It is also the primary cause of disability worldwide, which greatly burdens medical treatment and the economy [55]. The main characteristics of the patients are affective disorders, which are characterized by persistent and significant low moods. Depression has become an important factor affecting psychosocial function and daily work and life. Although the medical field has made great progress in the research on the etiology and pathogenesis of depression, there is still no ideal mechanism that can fully clarify all problems of the pathogenesis of depression [56, 57]. In 1997, the NTF hypothesis was proposed by Duman et al. as important pathogenesis of depression, i.e., the occurrence and development of depression is the impairment of neuroplasticity caused by the lack of BDNF and other neurotrophic factors [58]. Through a clinical study on 50 major depressive disorder (MDD) patients (30 of whom had suicidal tendencies), Lee et al. found that the expression level of BDNF in plasma of MDD patients was significantly lower than that of the healthy population [59]. In MDD patients, the level of BDNF in suicidal MDD patients was lower than that in non-suicidal MDD patients, and the decrease of plasma BDNF level in MDD patients was considered to be a pathogenic factor during the onset of MDD [59]. Giovanni et al. conducted a clinical study on 77 patients with depression and found that the serum BDNF level of patients with depression was lower than that of healthy people. After 2 weeks of treatment with agomelatine, serum BDNF levels were increased, which was associated with improved anhedonia in patients with depression [60]. Analysis of human postmortem samples showed that the expression level of mature BDNF in the parietal cortex of MDD patients was significantly lower than that of the normal population, while the level of proBDNF was higher than that of the normal population [61]. Animal experimental studies on depression have also found that the BDNF level of model animals is significantly lower than that of the control group. After drug treatment, the BDNF level is recovered, and the depression-like behavior is significantly improved. It also indicates that many antidepressant drugs function by mediating BDNF and regulating the downstream pathway [62-66]. Similarly, it has also been reported that infusion of BDNF into the hippocampus of a depressed animal model also produces a long-lasting antidepressant effect [67]. A study reported that heterotrimeric Gαi1 and Gαi3 proteins in the hippocampus were required for the anti-depressant effect of BDNF and the activation of downstream TrkB signal transduction. When Gαi1 and Gαi3 were knocked out, the endocytosis of TrkB on BDNF and the activation of downstream PI3K-protein kinase B (AKT)-mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK)-MAPK signaling pathways were blocked. Therefore, Gαi1 and Gαi3 proteins were down-regulated, reducing BDNF signaling pathways in the depression model. Meanwhile, PI3K and MAPK pathways, the key pathways for dendritic growth and development, were inhibited, leading to dendritic atrophy and loss of dendritic spines in the hippocampal neurons [68]. BDNF has become a potential biomarker for the diagnosis and key therapeutic target for depression (Fig. 4).
Fig. (4).
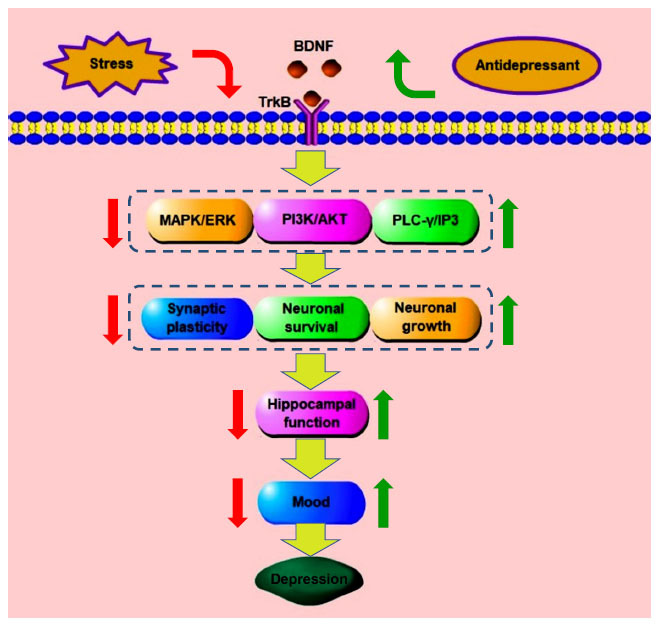
BDNF is correlated with the occurrence of depression and its antidepressant effects. Stress reduces synaptic plasticity, neuronal survival, and nerve growth through BDNF-TrkB and downstream signaling pathways, thereby affecting hippocampal function and inducing depression. The antidepressant drugs restore hippocampal function by up-regulating the expression of impaired signaling pathways and produce antidepressant effects. Abbreviations: (BDNF: brain-derived neurotrophic factor, TrkB: tyrosine kinase receptor B, PLC-γ: phosphatase C-γ, PI3K: phosphatidylinositol 3-kinase, MAPK: mitogen-activated protein kinase, AKT: protein kinase B, ERK: extracellular signal-regulated kinase, IP3: inositol 1,4,5-trisphosphate).
2.2.2. The Decrease in BDNF Contributes to the Development of Neurodegenerative Diseases
Neurodegenerative diseases are a type of disease that affects a wide range of populations all over the world, with rising morbidity and mortality, especially in the elderly. AD and PD are two well-known neurodegenerative diseases [69]. Patients with AD and PD have some common clinical manifestations, such as cognitive impairment, memory loss, and dyskinesia [70, 71]. It has been found that the neuropathologic changes of AD patients include atrophy of the hippocampus and basal ganglia, loss of synapses and neurons, accumulation of amyloid protein, and neurofibrillary tangles composed of hyperphosphorylated tau protein [72-74]. The main pathological features of PD patients are progressive loss of dopaminergic neurons in the compact part of Substantia Nigra and Lewy's disease caused by abnormal aggregation of ASN [75]. Although the two have different pathological characteristics, a large number of studies have shown that the expression level of BDNF in the brain of AD and PD patients is negatively correlated with the severity of the disease [76, 77]. Clinical studies have found that the expression level of serum BDNF of AD patients is significantly lower than that of healthy people [76, 77]. After drug treatment, the BDNF level has been reversed to a certain extent, and the development of AD is delayed [76, 77]. At the same time, Romain et al. also found that the expression level of BDNF decreased in the brain of AD patients, which was consistent with the expression trend of serum BDNF [78]. Jiao et al. reported that the level of BDNF in the brain of AD mice was low [79]. Restoring the expression of BDNF in AD mice blocked the loss of neurons, reduced synaptic degeneration and neurogenic damage, and had a protective effect on amyloid-beta (Aβ)-induced neurotoxicity [79]. A number of clinical studies have detected the level of BDNF in the blood of PD patients. The results showed that the level of BDNF in the blood of PD patients was significantly lower than the healthy group, which might be related to the degeneration of dopaminergic neurons in patients [80-83]. Our team also found a downward trend of BDNF expression in the animal model of PD, which was consistent with the results of clinical samples [84]. Xu et al. demonstrated that the expression of BDNF decreased, and motor dysfunction occurred in MPTP-induced PD model mice [85]. Lactoferrin (Lf) prevented the loss of dopaminergic neurons and provided neuroprotection. Its mechanism was not only related to the regulation of iron metabolism but also depended on the anti-inflammatory, anti-oxidative, and BDNF activation effects of Lf [85]. The above studies indicated that the normal and stable level of BDNF had a protective effect on neurons and delayed the occurrence of neurodegenerative diseases.
2.2.3. BDNF Participates in the Improvement of Learning and Memory
Due to the progressive loss of brain neurons and synaptic deficits in neuropsychiatric disorders, the learning and memory functions are seriously affected, including the formation of new memory and the extinction of fear memory [86]. A large number of experimental studies have shown that the decline of learning and memory is related to synaptic deficit and neuronal loss, and BDNF is a key neurotrophic factor to maintain synaptic structure [87-89]. Therefore, the key point to improving the impairment of learning and memory caused by synaptic damage or neuronal loss lies in promoting the expression of BDNF [87-89]. In our study, we found that after 14 consecutive days of curculigoside (CUR) treatment, the spatial memory was improved in the mice model of learned helplessness (LH), and extinction of fear memory was also accelerated by promoting the expression of BDNF in the hippocampus through activating the AKT-mTOR signaling pathway [90] (Fig. 5). Esmaeil et al. used LPS to replicate the learning and memory impairment model of adult male rats. After 3-week of Levisticum officinale extract (LOE) treatment, the dysfunction of learning and memory was alleviated. Mechanically, LOE improved the learning and memory likely through increasing the level of BDNF in the brain and promoting hippocampal neurogenesis [91]. Saray et al. pointed out that insufficient secretion of BDNF was associated with cognitive impairment in HD mice. Supplementation of BDNF restored synaptic deficits and reversed cognitive impairment in HD mice [92]. Relevant experimental studies on improving the expression of BDNF in the brain to recall the decreased learning and memory ability have been reported successively [93, 94]. It is also confirmed that BDNF has a protective effect on neurons and has become an important target for the treatment of memory impairment [95].
Fig. (5).
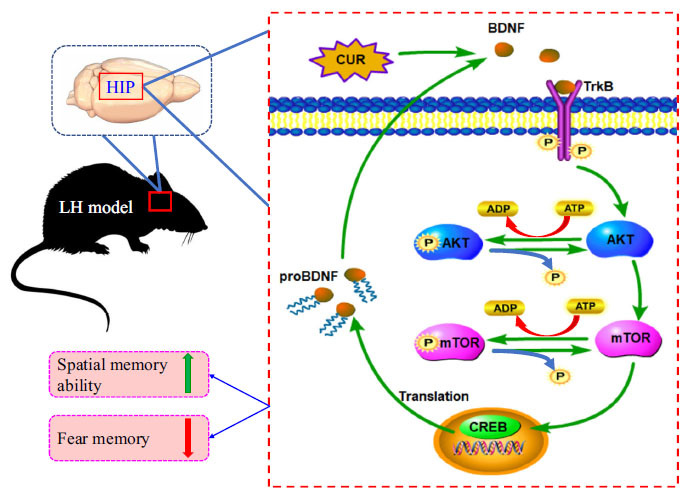
Curculigoside increases the level of BDNF, the level of p-AKT and p-mTOR in the hippocampus of the LH model, and improves the spatial memory ability of the model, and accelerates the extinction of fear memory by activating the AKT-mTOR signaling pathway. Abbreviations: (BDNF: brain-derived neurotrophic factor, proBDNF: brain-derived neurotrophic factor precursor, TrkB: tyrosine kinase receptor B, AKT: protein kinase B, mTOR: mammalian target of rapamycin, CUR: curculigoside, HIP: hippocampus, CREB: Cyclic AMP response-element binding protein, LH: learned helplessness, ATP: adenosine triphosphate, ADP: adenosine diphosphate).
3. EFFECTS OF 7,8-DHF ON NEUROPSYCHIATRIC DISORDERS AND THE MECHANISMS
Although BDNF has important therapeutic significance for neuropsychiatric disorders, exogenous BDNF is partially blocked by the BBB and is difficult to access the brain regions, which limits the therapeutic potential of BDNF [96]. Therefore, looking for BDNF mimics that can penetrate the BBB has become a key way to develop therapeutic drugs for neuropsychiatric disorders. In 2010, a study published in PNAS by Jang et al. showed that 7,8-DHF was a high-affinity TrkB small molecule agonist. After intraperitoneal injection, it could pass through the BBB and mimic BDNF to selectively activate TrkB receptors in the brain. It had a powerful protective effect on neurons [19].
3.1. 7,8-DHF and Depression
Based on the NTF hypothesis, the deficiency of BDNF can lead to neuronal damage, and 7,8-DHF, as a mimic of BDNF, can simulate the protective effect of BDNF on neurons and is an effective anti-depressant. Yukihiko et al. replicated the LH depression model in rats and injected single microinjection of the TrkB agonist 7,8-DHF into the lower edge of the medial prefrontal cortex (mPFC), dentate gyrus (DG), and hippocampal CA3 area of bilateral brain regions. The results showed that the depression-like behaviors of the LH model were improved by 7,8-DHF. This was consistent with the increase of TrkB phosphorylation in the brain by antidepressants (such as fluoxetine). It is suggested that 7,8-DHF may exert antidepressant effects by activating TrkB in the mPFC, hippocampal DG, and CA3 region [97]. Similarly, we also showed that 7,8-DHF could improve the depression-like behaviors of LH model mice. This effect might be generated through the activation of the AKT-mTOR signaling pathway and the downstream of TrkB [90]. Zhang et al. pointed out that LPS induced inflammation in mice, leading to obvious depression-like behaviors and reducing the expression of BDNF in hippocampus CA3, DG, and PFC, thereby resulting in abnormal BDNF-TrkB signaling pathway. Intraperitoneal injection of 7,8-DHF reduced the depression-like behaviors in mice, and at the same time, attenuated the decrease of p-TrkB level. It suggested that the antidepressant effect of 7,8-DHF might be related to the improvement of the abnormality of dendritic spines in the hippocampus and PFC and the regulation of BDNF expression in the related brain regions [98]. Zhang et al. investigated the antidepressant effects of 7,8-DHF in mice with chronic mild stress (CMS) model [99]. The experimental results showed that after 28 days of 7,8-DHF treatment, CMS-induced down-regulation of TrkB phosphorylation and associated synaptic proteins (PSD95 and synaptophysin), as well as BDNF in the PFC, were restored, reversing the depression-like behaviors of model mice. It is suggested that the antidepressant-like mechanism of 7,8-DHF may be through the activation of the BDNF-TrkB signaling pathway and the promotion of downstream synaptic protein expression [99]. Yao et al. found that the BDNF-TrkB was decreased in the CA3, DG, and PFC regions of nuclear factor erythroid 2-related factor 2 (Nrf2) knockout mice, which may mediate the depression-like behaviors, and a single intraperitoneal injection of 7,8-DHF could produce an antidepressant effect [100]. The mechanism may be that 7,8-DHF can stimulate TrkB in those regions and enhance the BDNF-TrkB signaling pathway [100]. Nashwa et al. combined 7,8-DHF with fluoxetine in the treatment of depression induced by chronic unpredictable mild stress (CUMS) in the perimenopausal period. The combination of the two can improve the depression-like behaviors of the model animals in the test of sugar water preference and forced swimming and regulate the PI3K/AKT/mTOR/p-ERK1/2 signaling pathway [101]. In addition, 7,8-DHF showed a good antagonistic effect on depression-like behavior in both the social defeat stress model and postoperative depression model [102, 103]. However, increased BDNF-TrkB signaling pathway in the nucleus accumbens shell was also found in the depression model induced by methamphetamine administration. Like paroxetine, 7,8-DHF did not show antidepressant effects in the initial depressive symptoms [104]. Thus, 7,8-DHF is only effective in treating depression-like behaviors associated with the reduction of the BDNF-TrkB signaling pathway.
7,8-DHF could improve depression-like behaviors in different animal models. These experimental results not only confirm the correlation between the occurrence of depression and BDNF but also explain the mechanism of 7,8-DHF as TrkB agonist in the intervention of depression. The PFC and hippocampus are brain regions related to the occurrence of depression. When depression occurs, the expression level of BDNF in the brain region is low, and the TrkB receptor and downstream AKT-mTOR, MAPK-ERK, and other signaling pathways cannot be effectively activated, affecting the survival and plasticity of neurons, and thus inducing depression-like behaviors [87, 105]. After crossing the BBB, 7,8-DHF binds to TrkB receptors in the PFC and hippocampus, simulating BDNF to activate TrkB receptors and increasing the phosphorylation level of TrkB. At the same time, it can reverse the decreased expression of BDNF in the prefrontal cortex and hippocampus, and then regulate the BDNF-TrkB and downstream AKT-mTOR and ERK signaling pathways, promote the increase of synaptic protein expression levels, thus protecting neurons, enhancing synaptic plasticity, improving the depression-like behaviors of model animals, and playing an effective antidepressant role (Fig. 6).
Fig. (6).
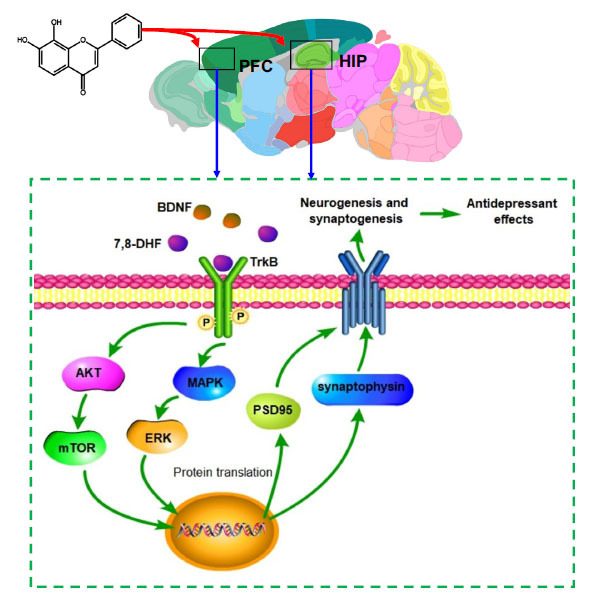
7,8-DHF crosses the blood-brain barrier, restores the abnormal BDNF-TrkB signaling pathway in the PFC and hippocampus, activates the downstream AKT-mTOR and MAPK-ERK pathway, and increases the expression of synaptic proteins (PSD95 and synaptophysin), thus exerting anti-depressive effects. Abbreviations: (HIP: hippocampus, PFC: prefrontal cortex, BDNF: brain-derived neurotrophic factor, TrkB: tyrosine kinase receptor B, AKT: protein kinase B, mTOR: mammalian target of rapamycin, MAPK: mitogen-activated protein kinase, ERK: extracellular signal-regulated kinase, 7,8-DHF:7,8-Dihydroxyflavone, PSD95: postsynaptic density 95).
3.2. 7,8-DHF and Neurodegenerative Diseases
A typical pathological change of neurodegenerative diseases is the progressive loss of neurons and synaptic deficit. Therefore, delaying the progressive loss of neurons in the brain and repairing the damaged synapses is an important idea for the treatment of neurodegenerative diseases [106]. Scopolamine (Sco) can induce learning and memory impairment and hippocampal synaptic deficit in rats, increase the accumulation of Aβ40 and Aβ42 amyloid protein in the hippocampus, decrease antioxidant capacity and inhibit AKT and ERK pathways. Chen et al. used Sco to replicate the AD model in rats and deliver 7,8-DHF for 4 weeks. The experimental results showed that 7,8-DHF could alleviate or reverse the above effects induced by Sco [107]. It is suggested that 7,8-DHF protected neurons by activating the TrkB signaling pathway [107]. Gao et al. investigated the effect of 7,8-DHF on memory function, synaptic structure, and synaptic protein expression in Tg2576 AD mice. The results showed that after treatment with 7,8-DHF for 4 weeks, the spatial memory impairment of Tg2576 AD mice was recovered, the number, density, and length of dendrites in the hippocampal neurons were increased, synaptic plasticity was restored, and hippocampal glutamate receptor subunit 1(GluA1), glutamate receptor subunit 2 (GluA2), and their phosphorylation levels were increased to regulate AMPAR. 7,8-DHF also increased the phosphorylation level of TrkB in hippocampus and then activated the phosphorylation of downstream Calcium/calmodulin-dependent protein kinase II (CaMKII), cyclic AMP response-element binding protein (CREB), AKT, and ERK1/2 [108] (Fig. 7). Nicholas et al. also found similar results in the study on the effect of 7,8-DHF on AD animal model. Their study suggested that 7,8-DHF could improve the spatial learning of animal model through TrkB signaling pathway [109, 110]. Remarkably, the dysregulation of intestinal flora in 5xFAD mice was found to be related to the C/EBPβ/AEP pathway in the intestine and amyloid protein in the brain. R13, the prodrug molecule of 7,8-DHF, could act as probiotics to inhibit the C/EBPβ/AEP pathway and reduce intestinal oxidative stress and brain amyloid protein accumulation. The therapeutic effect of R13 on AD was achieved through the direct activation of the TrkB receptor and the beneficial regulation of intestinal flora [111]. He et al. used the MPP+-induced PD monkey model and gave long-term oral treatment for 7 months. The results showed that 7,8-DHF had no toxic reaction and could reduce the loss of dopaminergic neurons in the midbrain of monkeys (a non-human primate), showing a powerful neurotrophic effect [112]. Several studies have replicated PD animal models through MPTP or rotenone to investigate the therapeutic effect of 7,8-DHF on PD-like behaviors. Behavioral data showed that 7,8-DHF could effectively alleviate the motor dysfunction of the PD animal model. Western blot and immunohistochemical staining analysis indicated that 7,8-DHF could block the loss of dopaminergic neurons in the striatum of the PD animal model. It is considered that this effect might be related to the activation of TrkB and its signaling cascade by 7,8-DHF [113-117].
Fig. (7).
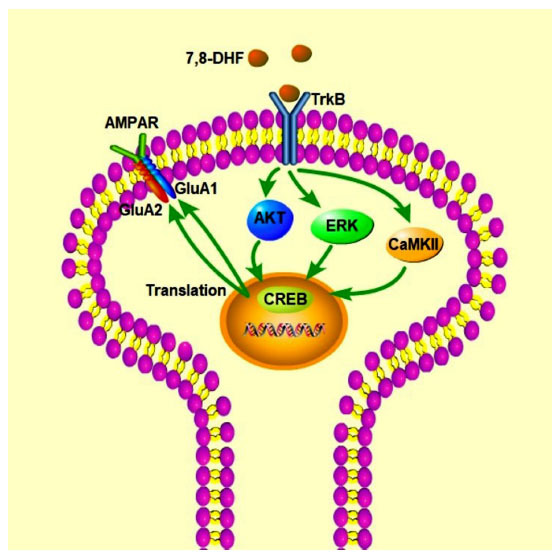
7,8-DHF can increase the levels of GluA1, GluA2 and their phosphorylation in the hippocampus to regulate AMPAR, increase the phosphorylation level of TrkB, and then activate the phosphorylation of downstream TrkB signaling molecules CaMKII, CREB, AKT, and ERK1/2 proteins, which can restore the synaptic plasticity of neurons in model animals. Abbreviations: (TrkB: tyrosine kinase receptor B, 7,8-DHF:7,8-Dihydroxyflavone, AKT: protein kinase B, ERK: extracellular signal-regulated kinase, CREB: Cyclic AMP response element-binding protein, CaMKII: Calcium/calmodulin-dependent protein kinase II, AMPAR: AMPA receptor, GluA1: glutamate receptor subunit 1, GluA2: glutamate receptor subunit 2).
The above studies indicate that the mechanism of 7,8-DHF in AD may be through the activation of the BDNF-TrkB signaling pathway in the hippocampus and the downstream expression of AKT, ERK, and CaMKII and their protein phosphorylation levels, which further activate the signaling molecule CREB and increase GluA1, GluA2, and their protein phosphorylation levels, thereby regulating AMPAR, promoting the growth and proliferation of neuronal dendrites, enhancing neuronal synaptic plasticity, and improving AD-induced memory dysfunction and neuronal damage. In addition, 7,8-DHF has the effect of antioxidation [118], which can also protect the hippocampus from oxidative stress damage and protect neurons. In addition to the aforementioned enhancement of neuronal synaptic plasticity, 7,8-DHF can also regulate the growth and survival of dopaminergic neurons in the striatum, maintain the morphological structure of neurons, prevent the progressive loss of dopaminergic neurons, and play a protective role by activating BDNF-TrkB and downstream signaling pathways, thus alleviating the motor dysfunction of PD.
3.3. 7,8-DHF and Learning and Memory
The damage and pathological changes of neurons can affect learning and memory, leading to reduced cognitive function [119-121]. As mentioned earlier, BDNF is closely related to learning and memory, and 7,8-DHF, as a mimic of BDNF, has also been extensively studied in learning and memory. Bollen et al. investigated the role of 7,8-DHF performed in the process of memory consolidation in Wistar rats, wild type, and APPswe/PS1dE9 (AD model) mice. The object recognition test demonstrated that the rats injected with different doses of 7,8-DHF immediately or three hours after learning could enhance both the early and late stages of learning and memory, and it also showed an effect on the consolidation of learning and memory at lower doses. In the object localization experiment, 7,8-DHF also showed a similar improvement in spatial memory function in WT and APPswe/PS1dE9 mice [122]. Surya et al. used alcohol and a high-fat diet to induce memory dysfunction in rats. A 4-week of treatment with 7,8-DHF could alleviate the memory impairment of modeled rats. 7,8-DHF down-regulated NF-κB, inducible nitric oxide synthase (iNOS), and caspase-3, and up-regulated the expression of Nrf2, heme oxygenase-1(HO-1), and BDNF in the hippocampus. It is believed that 7,8-DHF exerts anti-oxidation, anti-inflammatory, and anti-apoptotic effects by activating the BDNF-TrkB pathway, thereby improving memory dysfunction induced by alcohol and a high-fat diet [123]. Yang et al. also conducted a similar study after inducing cognitive impairment in apolipoprotein E-knockout (ApoE-KO) mice with a high cholesterol diet, and the experimental results were consistent [124]. Zeng et al. evaluated the effect of 7,8-DHF on age-related memory impairment. Studies have shown that administration of 7,8-DHF for 4 consecutive weeks can compensate for the deficits in the fear memory in aged rats, reverse the synaptic deficits in the hippocampus, amygdala, and PFC, and activate the phosphorylation of TrkB and the downstream cascade proteins ERK, CREB, CaMKII, and glutamate receptor subunit 1(GluR1) in those regions. It is suggested that the mechanism of 7,8-DHF blocking fear memory and preventing the decline of amygdala synaptic transmission is through enhancing the expression of TrkB and downstream protein phosphorylation [125]. Yang et al. induced cognitive dysfunction in mice by irradiating the brain. After a 3-week treatment with 7,8-DHF, irradiation-induced impairment of spatial memory, episodic memory, and working memory in mice was reversed. It is believed that the mechanism of positive therapeutic effect of 7,8-DHF may be that the TrkB and downstream ERK and AKT signaling pathways in the hippocampus are activated, and the survival rate of newborn neurons after radiation is improved, and the expression of postsynaptic density 95 (PSD95), N-methyl-D-aspartate receptor subunit 2B (GluN2B), and synaptophysin increases, which protects synaptic structural integrity [126]. In addition, 7,8-DHF also showed an ideal therapeutic effect on the symptoms of reduced learning and memory in different animal models, such as BDNF gene knockout mice, aging mice, propofol-induced cognitive impairment, FMR1 knockout mice, hydraulic shock injury rats, etc. [119, 127-131]
The hippocampus is particularly important for the function of learning and memory. The physiological integrity and survival status of hippocampal neurons determine the strength of cognitive function [132]. In summary, 7,8-DHF can improve learning and memory disorders. The mechanism may be that 7,8-DHF maintains the structural integrity and information transmission function of synapses by activating the BDNF-TrkB signaling pathway and the phosphorylation levels of downstream ERK, AKT, CaMKII, and CREB cascades and increasing the expression of hippocampal synaptic proteins (such as PSD95, GluN2B, and synaptophysin) [125, 126]. In addition, 7,8-DHF has the potential to enhance the antioxidant capacity of the hippocampus and down-regulate the expression of inflammatory factors, delay the apoptosis of hippocampal neurons and increase the survival rate of newborn neurons [123]. In conclusion, the improvement of learning and memory function by 7,8-DHF is the combined effect of protecting synaptic structural integrity, anti-oxidation, anti-inflammatory, and anti-neuronal apoptosis (Fig. 8).
Fig. (8).
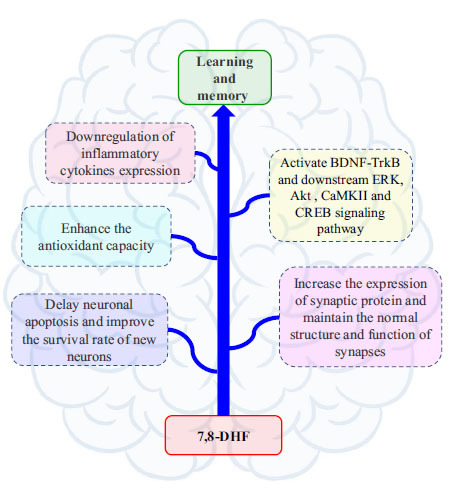
The mechanism of 7,8-DHF improving learning and memory may be through activating BDNF-TrkB and downstream signaling pathways such as ERK, AKT, CaMKII, and CREB in specific brain regions, increasing the expression of synaptic proteins in the hippocampus, maintaining normal synaptic structure and function, delaying neuronal apoptosis and improving the survival rate of newborn neurons. Meanwhile, 7,8-DHF has anti-neuroinflammation properties and increases hippocampal antioxidant capacity. Abbreviations: (7,8-DHF:7,8-Dihydroxyflavone, BDNF: brain-derived neurotrophic factor, TrkB: tyrosine kinase receptor B, AKT: protein kinase B, CREB: Cyclic AMP response-element binding protein, ERK: extracellular signal-regulated kinase, CaMKII: Calcium/calmodulin-dependent protein kinase II).
4. STRUCTURAL MODIFICATION AND DRUG DELIVERY SYSTEM
Permeability of BBB determines the biological activity of exogenous supplement, and 7,8-DHF simulates BDNF to specifically bind to TrkB receptor, activating BDNF-TrkB signaling pathway and regulating the corresponding physiological functions [133-135]. As a natural product, the study on the structure-activity relationship of 7,8-DHF shows that the phenolic hydroxyl group at the C8 position of 7,8-DHF is necessary for the binding of 7,8-DHF to TrkB receptor [19, 136]. However, compounds containing phenolic hydroxyl groups will undergo oxidation, glucuronidation, sulfation, or methylation in the liver, their chemical structure will be changed, and they are easy to be removed from the body’s circulatory system, resulting in low oral bioavailability and brain exposure [137, 138]. In order to overcome the pharmacokinetic defects of phenolic hydroxyl groups and improve the oral bioavailability and brain exposure of 7,8-DHF, structural modification and preparation design of 7,8-DHF are necessary for preclinical studies (Fig. 9).
Fig. (9).
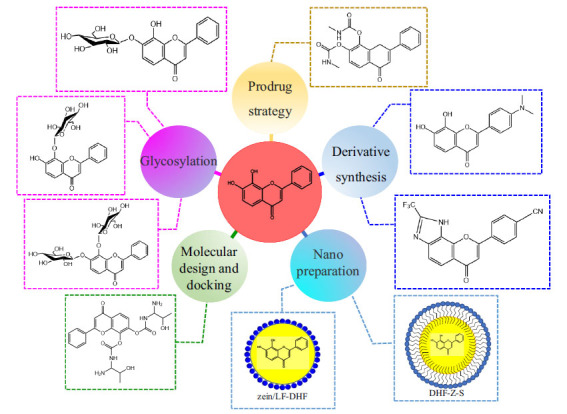
The low bioavailability of 7,8-DHF could be overcome by modifying the structure of 7,8-DHF through prodrug strategy, glycation, derivative compounds, and molecular docking methods and preparing 7,8-DHF nano-preparations. (DHF-Z-S: 7,8-DHF loaded zein-sophorolipid nanoparticles, zein/LF-DHF: 7,8-DHF loaded zein/lactoferrin composite nanoparticles).
4.1. Prodrug Strategy of 7,8-DHF
Prodrug refers to the loading of new chemical groups into the original parent drug to form a new compound, and the new compound can release the original parent drug after biotransformation, thereby exerting the drug effect. Therefore, the prodrug strategy is an effective drug design method [139, 140]. Chen et al. adopted a prodrug strategy to improve the oral bioavailability and brain exposure of 7,8-DHF. By modifying phenolic hydroxyl groups to synthesize esters or carbamates, stable compounds were obtained, and the best prodrug compound R13 was selected by the pharmacokinetic method in vitro. The follow-up experimental results further indicated that prodrug R13 could release 7,8-DHF continuously in vivo. R13 activates p-TrkB level and downstream AKT and ERK/MAPK signaling pathways and prevents synaptic dysfunction and cognitive impairment in 5xFAD mice. Compared with 4.6% oral bioavailability of 7,8-DHF, R13 can reach b10.5%. This study demonstrated that R13 could increase oral bioavailability and brain exposure and is safe, non-toxic, and has more lasting pharmacological activity [141].
4.2. Synthesis of 7,8-DHF Derivatives
In the past two decades, about 1/3 of the drugs approved by the Food and Drug Administration (FDA) are derived from natural products or their derivatives [142]. Synthesizing natural product derivatives is also a way to develop innovative drugs. Liu et al. synthesized 4’-Dimethylamino-7,8-dihydroxyflavone (4’-DMA-7,8-DHF), a derivative of 7,8-DHF that has stronger TrkB activation activity than 7,8-DHF, and could activate TrkB receptor and downstream AKT signaling, thus exerting antidepressant effects. However, the addition of dimethylamino group at 4’ positions on the B ring of 7,8-DHF will accelerate the excretion of 4’-DMA-7,8-DHF and reduce its oral availability [138]. As 7,8-DHF can specifically bind to the extracellular domain (ECD) of TrkB receptor, Chen et al. used pharmacochemical method to synthesize a 7,8-DHF derivative, a compound called CF3CN, which can bind closely to the LCM/CC2 motif of TrkB ECD. Compared with the lead compound, the oral bioavailability and in vivo half-life of CF3CN are optimized. In addition, CF3CN can activate TrkB and downstream signaling pathways in the brain of 5xFAD mice, inhibit delta-secretase through the AKT phosphorylation pathway, and have a dose-dependent reduction effect on Aβ42. It also improves the learning and memory dysfunction of 5xFAD mice [143]. Ramesh et al. obtained glycoside derivatives by in vitro glycosylation method to couple 7,8-DHF with glucose, including 7-O-β-D-glucoxy-8-hydroxyflavone, 7-hydroxy-8-O-β-D-glucoxyflavone, and 7,8-di-O-β-D-glucoxyflavone. The obtained derivatives were used as ligands to dock with BACE1. The docking results show that 7,8-DHF glycoside derivatives may be effective inhibitors of BACE1 and can be used in the treatment of neurodegenerative diseases such as AD [144].
4.3. Molecular Design and Docking
In drug design, computer simulation can reduce the number of target compounds to be screened and accelerate the process of drug design. Molecular docking is a structure-based drug design method, which monitors the interaction between small-molecule ligands and biological macromolecule receptors to predict the binding pattern and affinity, and plays an important role in the development of natural drugs [145, 146]. Thangavel et al. connected 7,8-DHF with 9-Fluorenylmethoxycarbonyl (Fmoc) by molecular design and obtained 40 kinds of 7,8-DHF amino acid esters and 7,8-DHF carbamate derivatives. The obtained 7,8-DHF derivative was used as a potential lead therapeutic drug for PD to dock with ASN. 8q (7,8-DHF carbamate derivative) showed the highest docking fraction, indicating that this molecule had the strongest binding force to ASN. At the same time, the pharmacokinetics and toxicity of 8q, 8s, 8p, 8c, 8n, and 8h were predicted by molecular dynamics. It is found that the 8q molecule has better pharmacological activity than L-DOPA, suggesting that the 8q molecule may be a potential drug to inhibit ASN aggregation, but the conclusion of simulation needs to be verified by further experiments [147]. It is undeniable that computer-aided molecular design and molecular docking technology simplify the tedious screening process of 7,8-DHF drugs and provide effective help for follow-up pharmacological experiments.
4.4. 7,8-DHF Delivery System
Due to the low oral bioavailability of 7,8-DHF, the majority of experimental animal studies on 7,8-DHF were conducted by intraperitoneal injection. Taking into account the long clinical treatment cycle for AD, depression, and other neuropsychiatric disorders, oral medication is undoubtedly the best way of administration for the treatment of chronic diseases. Different oral formulation technologies have a certain impact on the bioavailability of drugs, and appropriate formulation technologies can maximize the therapeutic effects of drug molecules. Reasonable oral preparation design can provide a great convenience for future clinical research on 7,8-DHF. Chen et al. studied the oral drug delivery system of 7,8-DHF. 7,8-DHF loaded zein-sophorolipid nanoparticles (DHF-Z-S) were obtained by the anti-solvent coprecipitation (ASCP) method [148], and 7,8-DHF loaded zein/lactoferrin composite nanoparticles (zein/LF-DHF) were obtained by the anti-solvent precipitation (ASP) method [149]. The results demonstrated that the storage stability and in vitro bioavailability of the two nanoparticles were much improved. This may be an effective way to improve the oral bioavailability of 7,8-DHF, but the pharmacokinetic parameters in vivo need to be further investigated. With the development of pharmaceutical technology, new oral drug delivery technologies continue to emerge. Drug delivery technologies such as nanocrystals [150], lipid-based formulations [151], and supersaturated solid dispersions [152] have been applied in drug research and development, gradually solving the problems of poor oral absorption and low bioavailability of insoluble small molecular drugs. These new technologies may be of great help in the design of oral formulations of 7,8-DHF.
5. DEVELOPMENT POTENTIAL AND CHALLENGES COEXIST
Since 7,8-DHF was found to have TrkB agonistic activity in 2010, the research literature related to the TrkB signaling pathway has extensively increased, especially in the treatment of neuroprotection and neuropsychiatric disorders [153]. As 7,8-DHF has a definite neuronal protective effect, studies on 7,8-DHF are no longer limited to common neuropsychiatric disorders such as depression, AD, and PD, and even appear in the studies on schizophrenia, HD, and DS. For example, early intervention by 7,8-DHF may reduce the risk of schizophrenia in the juvenile offspring of poly(I: C)-treated mice [20, 154-156] and also cognitive and synaptic plasticity deficits in a rat model of schizophrenia [157]. In addition, several studies are conducted on the effect of 7,8-DHF on fear memory extinction. Due to the difficulty of fear, memory extinction is a typical feature of post-traumatic stress disorder (PTSD); however, currently, there is a lack of effective treatment drugs for PTSD [158-160]. Studies have shown that 7,8-DHF can improve the fear memory deficit or accelerate the extinction of fear memory, which indicates that 7,8-DHF is likely to be a candidate drug for the treatment of PTSD [161-165].
The reasons why 7,8-DHF has attracted attention in the study of neuropsychiatric disorders are as follows: (1) it specifically binds to TrkB receptors and activates TrkB and downstream signaling pathways; (2) Exogenous administration of 7,8-DHF could reach the brain through the BBB and produce the effect of simulating BDNF, which made up for the poor pharmacokinetics of exogenous supply of BDNF; (3) It has the advantages of non-toxicity or low toxicity and high potency; (4) It has an ameliorative effect on a variety of neuropsychiatric disorders. This suggests that 7,8-DHF has great potential in neuroprotective and psychoactive drug development. However, by summarizing relevant experimental studies, it is found that the development of 7,8-DHF still has some limitations, which may hinder the progress of 7,8-DHF toward clinical drug development.
5.1. The Mechanism of 7,8-DHF Needs Further Study
The mechanism of 7,8-DHF on neuropsychiatric disorders is mainly focused on the role of TrkB agonists, while as flavonoids, 7,8-DHF has antioxidant [166] and anti-inflam-matory properties of flavonoids [167]. Modern studies have shown that the excessive free radicals produced by oxidative stress and the weakened ability to scavenge free radicals are closely related to the occurrence of AD [168-170]. Ansab et al. found that 7,8-DHF played a neuroprotective effect on cognitive function in sporadic Alzheimer's disease (SAD) rat model by improving oxidative stress, mitochondrial dysfunction, and insulin resistance [171]. Based on the results of in vivo and in vitro studies, Sahabuddin et al. demonstrated that 7,8-DHF could reverse the neuronal death induced by 3-nitropropionic acid (3-NP), improve the integrity of neuronal mitochondria, and promote neuronal survival [172]. Li et al. found that R13, the prodrug of 7,8-DHF, also had a similar effect, promoting mitochondrial biosynthesis and improving mitochondrial dysfunction by activating AMP-activated protein kinase (AMPK)/ Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)/ Nuclear respiratory factor 1(NRF1)/Transcription factor A, mitochondrial (TFAM) signaling pathway [173]. Fang et al. pointed out that 7,8-DHF can attenuate oxidative stress injury of the optic nerve and retinal ganglion cells induced by chronic intermittent hypoxia, and its mechanism may be through the activation of the BDNF/TrkB signaling pathway [174]. DHF-BAHPC, a flavonoid derivative synthesized by Thangavel et al., has strong antioxidant activity and can scavenge free radicals 1, 1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), ferric reducing antioxidant power (FRAP), and H2O2 in zebrafish [175]. Glutamate is an important excitatory neurotransmitter in CNS, but excessive glutamate can lead to neuronal dysfunction and degeneration, which is associated with the occurrence of neurodegenerative diseases, such as PD and AD [176-178]. Chen et al. demonstrated that 7,8-DHF can play a neuroprotective role against glutamate-induced toxicity in HT-22 cells through its antioxidant activity, despite the absence of TrkB receptors in HT-22 cells [179]. According to those studies, we speculate that there is a strong correlation between the neuroprotective effects of 7,8-DHF, the effects of antioxidant stress, the improvement of mitochondrial disorders, and the activation of TrkB receptors. Unfortunately, the available evidence is insufficient to reveal this association. In addition, Zhao et al. found that 7,8-DHF-induced estrogen receptor α (ERα) and TrkB activation in female mice are interdependent, and they have a synergistic effect on the activation of energy metabolism-related pathways, including ERK, AKT, and uncoupling protein 1 (UCP1), which provides a mechanism for 7,8-DHF to alleviate metabolic syndrome in female mice [180]. In the sex-dependent study, Priyanka et al. pointed out that 7,8-DHF showed potential harmful effects on the metabolic effects of male mice fed with a high-fat diet, while beneficial effects on the metabolism in females, suggesting that the change of intestinal flora is the key to produce sex-dependent effects [181]. Therefore, the neuroprotective effect of 7,8-DHF and its therapeutic effect on neuropsychiatric disorders might also be related to the activation of ERα and the regulation of intestinal flora.
5.2. Insufficient Clinical Research
There is a lack in the clinical trial regarding 7,8-DHF and its derivative. It has been mentioned in the literature that R13, a prodrug molecule 7,8-DHF, has entered phase 1 clinical indications for treating AD [111]. In view of the powerful therapeutic potential of 7,8-DHF in animal models, it is reasonable to believe that 7,8-DHF or its derivatives have a bright future in clinical applications. So far, in addition to rodents, 7,8-DHF has been tested from the perspective of pharmacokinetic [182] and PD model studies in monkeys [112], showing no obvious toxic effects and high drug safety. 7,8-DHF is beneficial for the improvement of neuropsychiatric disorders in the laboratory, but how to transform 7,8-DHF in neuropsychiatric disorders treatment is still a difficult challenge. As mentioned above, we believe that studies on 7,8-DHF in depression, AD, and PD have more experimental data reported than other neuropsychiatric disorders. Therefore, considering the above three diseases as the breakthrough point, we should supplement and improve the relevant pharmaceutical preclinical research data, promote the clinical research on the effect of 7,8-DHF on depression, AD, and PD, and carry out the clinical research on other neuropsychiatric disorders accordingly. Metformin was developed from the first-line treatment of type 2 diabetes [183] to a multipotent drug with anti-tumor [184], depression [185, 186], and cardiovascular protection [187]. We can learn from this drug development paradigm, refer to the clinical research ideas of R13 in the treatment of AD, and speed up the pace of clinical research on depression and PD.
6. FUTURE RESEARCH DIRECTIONS OF 7,8-DHF
Based on the current research status of 7,8-DHF, we believe that the next research direction of 7,8-DHF should be carried out from the following aspects to improve the survival rate of 7,8-DHF in future clinical trials. First, the chemical structure of 7,8-DHF needs to be optimized. Optimization of the chemical structure of drugs is still one of the main methods in the research and development of natural drugs, which can not only solve the problems of half-life and solubility of metabolic stability of drugs but also improve the bioavailability of drugs [188]. Although the chemical structure modification of 7,8-DHF has also been studied experimentally, there are few reports on drug-like molecules. Second, oral preparations of 7,8-DHF should be largely carried out. In vitro study has characterized the transepithelial transport mechanism of 7,8-DHF in human intestinal Caco-2 cells [189]. In addition to nanoparticles, various pharmaceutical technologies, such as lipid preparations, have been applied to improve gastrointestinal absorption of 7,8-DHF and reduce the first-pass effect in the liver to improve the probability of more targeted drugs reaching the brain and improve oral bioavailability and brain exposure. Finally, supplemental preclinical studies on the optimal treatment timing of 7,8-DHF are conducive to obtaining the optimal clinical effect of treating the disease. The time window for the treatment of chronic neuropsychiatric disorders has a great influence on the prognosis, and it is generally believed that early intervention has a positive effect on the treatment of diseases [190-192]. Studies have shown that 7,8-DHF has a good intervention effect on the early stage of AD animal models [193]. The therapeutic effects of 7,8-DHF on Ts65Dn mice were different in different periods, which showed that the short-term treatment was insufficient to produce long-term effects in the neonatal period, and the dose of recovery of memory function in the adolescent period was insufficient to improve the symptoms of memory deficiency in the adult period [194]. Therefore, the research on the optimal treatment timing of 7,8-DHF has a positive significance for the development of the drug.
CONCLUSION
In conclusion, 7,8-DHF can simulate BDNF to activate BDNF-TrkB and downstream signaling pathways, thereby playing a role in neuronal protection, neuronal regeneration, and synaptic plasticity regulation. Based on the medicinal potential and value of 7,8-DHF, it is likely to be successfully developed as a natural drug for the treatment of neuropsychiatric disorders and widely used in clinical practice in the future. However, prior to this, pharmaceutical research on 7,8-DHF still needs to be further systematically discussed.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This research was supported by the National Natural Science Foundation of China (81673716), Anhui Natural Science Foundation (1808085J15), University Excellent Top Talent Cultivation Foundation of Anhui Province (gxgnfx2020089), and Key Research and Development Plan of Anhui Province (202104j07020004).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Xiao J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017;57(9):1874–1905. doi: 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
- 2.Yi Y.S. Regulatory roles of flavonoids on inflammasome activation during inflammatory responses. Mol. Nutr. Food Res. 2018;62(13):e1800147. doi: 10.1002/mnfr.201800147. [DOI] [PubMed] [Google Scholar]
- 3.Masad R.J., Haneefa S.M., Mohamed Y.A., Al-Sbiei A., Bashir G., Fernandez-Cabezudo M.J., Al-Ramadi B.K. The Immunomodulato-ry effects of honey and associated flavonoids in cancer. Nutrients. 2021;13(4):1269. doi: 10.3390/nu13041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang J.S., Choi I.W., Han M.H., Kim G.Y., Hong S.H., Park C., Hwang H.J., Kim C.M., Kim B.W., Choi Y.H. The cytoprotective effects of 7,8-dihydroxyflavone against oxidative stress are mediated by the upregulation of Nrf2-dependent HO-1 expression through the activation of the PI3K/Akt and ERK pathways in C2C12 myoblasts. Int. J. Mol. Med. 2015;36(2):501–510. doi: 10.3892/ijmm.2015.2256. [DOI] [PubMed] [Google Scholar]
- 5.Rui W., Li S., Xiao H., Xiao M., Shi J. Baicalein attenuates neuroinflammation by inhibiting NLRP3/caspase-1/GSDMD pathway in MPTP induced mice model of Parkinson’s disease. Int. J. Neuropsychopharmacol. 2020;23(11):762–773. doi: 10.1093/ijnp/pyaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z., Song Z., Shen F., Xie P., Wang J., Zhu A.S., Zhu G. Ginsenoside Rg1 prevents PTSD-like behaviors in mice through pro-moting synaptic proteins, reducing Kir4.1 and TNF-α in the hippocampus. Mol. Neurobiol. 2021;58(4):1550–1563. doi: 10.1007/s12035-020-02213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Z., Shen F., Zhang Z., Wu S., Zhu G. Calpain inhibition ameliorates depression-like behaviors by reducing inflammation and promoting synaptic protein expression in the hippocampus. Neuropharmacology. 2020;174:108175. doi: 10.1016/j.neuropharm.2020.108175. [DOI] [PubMed] [Google Scholar]
- 8.Shen F., Song Z., Xie P., Li L., Wang B., Peng D., Zhu G. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 2021;275:114164. doi: 10.1016/j.jep.2021.114164. [DOI] [PubMed] [Google Scholar]
- 9.Che D.N., Cho B.O., Kim J.S., Shin J.Y., Kang H.J., Jang S.I. Luteolin and apigenin attenuate LPS-induced astrocyte activation and cytokine production by targeting MAPK, STAT3, and NF-κB signaling pathways. Inflammation. 2020;43(5):1716–1728. doi: 10.1007/s10753-020-01245-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang W.W., Han R., He H.J., Li J., Chen S.Y., Gu Y., Xie C. Administration of quercetin improves mitochondria quality control and protects the neurons in 6-OHDA-lesioned Parkinson’s disease models. Aging (Albany NY) 2021;13(8):11738–11751. doi: 10.18632/aging.202868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa I.M., Lima F.O.V., Fernandes L.C.B., Norrara B., Neta F.I., Alves R.D., Cavalcanti J.R.L.P., Lucena E.E.S., Cavalcante J.S., Rego A.C.M., Filho I.A., Queiroz D.B., Freire M.A.M., Guzen F.P., Astragaloside I.V. Astragaloside IV supplementation promotes a neuroprotective effect in experimental models of neurological disorders: A systematic review. Curr. Neuropharmacol. 2019;17(7):648–665. doi: 10.2174/1570159X16666180911123341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Zhuang Y., Tian Y., Thomas G.N., Ying M., Tomlinson B. Study of the effects of total flavonoids of Astragalus on athero-sclerosis formation and potential mechanisms. Oxid. Med. Cell. Longev. 2012;2012:282383. doi: 10.1155/2012/282383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bratkov V.M., Shkondrov A.M., Zdraveva P.K., Krasteva I.N. Flavonoids from the genus Astragalus: Phytochemistry and biological activity. Pharmacogn. Rev. 2016;10(19):11–32. doi: 10.4103/0973-7847.176550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer J.P. Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc. Nutr. Soc. 2008;67(2):238–252. doi: 10.1017/S0029665108007088. [DOI] [PubMed] [Google Scholar]
- 15.Martín M.A., Goya L., de Pascual-Teresa S. Effect of cocoa and cocoa products on cognitive performance in young adults. Nutrients. 2020;12(12):3691. doi: 10.3390/nu12123691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayaz M., Sadiq A., Junaid M., Ullah F., Ovais M., Ullah I., Ahmed J., Shahid M. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci. 2019;11:155. doi: 10.3389/fnagi.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du X., Hill R.A. 7,8-Dihydroxyflavone as a pro-neurotrophic treatment for neurodevelopmental disorders. Neurochem. Int. 2015;89:170–180. doi: 10.1016/j.neuint.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Luo J., Zhou W., Cao S., Jin M., Zhang C., Jin X., Cui J., Li G. A new biflavonoid from the whole herb of Lepisorus ussuriensis. Nat. Prod. Res. 2016;30(13):1470–1476. doi: 10.1080/14786419.2015.1110702. [DOI] [PubMed] [Google Scholar]
- 19.Jang S.W., Liu X., Yepes M., Shepherd K.R., Miller G.W., Liu Y., Wilson W.D., Xiao G., Blanchi B., Sun Y.E., Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaehne E.J., Chong E.M.S., Sbisa A., Gillespie B., Hill R., Gogos A., van den Buuse M. TrkB agonist 7,8-dihydroxyflavone reverses an induced prepulse inhibition deficit selectively in maternal immune activation offspring: implications for schizophrenia. Behav. Pharmacol. 2021;32(5):404–412. doi: 10.1097/FBP.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 21.Wang N., Liu X., Li X.T., Li X.X., Ma W., Xu Y.M., Liu Y., Gao Q., Yang T., Wang H., Peng Y., Zhu X.F., Guan Y.Z. 7,8-Dihydroxyflavone alleviates anxiety-like behavior induced by chronic alcohol exposure in mice involving tropomyosin-related kinase B in the amygdala. Mol. Neurobiol. 2021;58(1):92–105. doi: 10.1007/s12035-020-02111-0. [DOI] [PubMed] [Google Scholar]
- 22.Ma L., Qu Z., Xu L., Han L., Han Q., He J., Luan X., Wang B., Sun Y., He B. 7,8-Dihydroxyflavone enhanced colonic cholinergic contraction and relieved loperamide-induced constipation in rats. Dig. Dis. Sci. 2021;66(12):4251–4262. doi: 10.1007/s10620-020-06817-y. [DOI] [PubMed] [Google Scholar]
- 23.Wood J., Tse M.C.L., Yang X., Brobst D., Liu Z., Pang B.P.S., Chan W.S., Zaw A.M., Chow B.K.C., Ye K., Lee C.W., Chan C.B. BDNF mimetic alleviates body weight gain in obese mice by enhancing mitochondrial biogenesis in skeletal muscle. Metabolism. 2018;87:113–122. doi: 10.1016/j.metabol.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z., Wang S.P., Shao Q., Li P.F., Sun Y., Luo L.Z., Yan X.Q., Fan Z.Y., Hu J., Zhao J., Hang P.Z., Du Z.M. Brain-derived neurotrophic factor mimetic, 7,8-dihydroxyflavone, protects against myocardial ischemia by rebalancing optic atrophy 1 processing. Free Radic. Biol. Med. 2019;145:187–197. doi: 10.1016/j.freeradbiomed.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Jiang M., Peng Q., Liu X., Jin J., Hou Z., Zhang J., Mori S., Ross C.A., Ye K., Duan W. Small-molecule TrkB receptor agonists im-prove motor function and extend survival in a mouse model of Huntington’s disease. Hum. Mol. Genet. 2013;22(12):2462–2470. doi: 10.1093/hmg/ddt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Díaz Barriga G., Giralt A., Anglada-Huguet M., Gaja-Capdevila N., Orlandi J.G., Soriano J., Canals J.M., Alberch J. 7,8-dihydroxyflavone ameliorates cognitive and motor deficits in a Huntington’s disease mouse model through specific activation of the PLCγ1 pathway. Hum. Mol. Genet. 2017;26(16):3144–3160. doi: 10.1093/hmg/ddx198. [DOI] [PubMed] [Google Scholar]
- 27.Stagni F., Uguagliati B., Emili M., Giacomini A., Bartesaghi R., Guidi S. The flavonoid 7,8-DHF fosters prenatal brain proliferation potency in a mouse model of Down syndrome. Sci. Rep. 2021;11(1):6300. doi: 10.1038/s41598-021-85284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parrini M., Ghezzi D., Deidda G., Medrihan L., Castroflorio E., Alberti M., Baldelli P., Cancedda L., Contestabile A. Aerobic exer-cise and a BDNF-mimetic therapy rescue learning and memory in a mouse model of Down syndrome. Sci. Rep. 2017;7(1):16825. doi: 10.1038/s41598-017-17201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stagni F., Giacomini A., Guidi S., Emili M., Uguagliati B., Salvalai M.E., Bortolotto V., Grilli M., Rimondini R., Bartesaghi R. A flavonoid agonist of the TrkB receptor for BDNF improves hippocampal neurogenesis and hippocampus-dependent memory in the Ts65Dn mouse model of DS. Exp Neurol. 2017;298(Pt A):79–96. doi: 10.1016/j.expneurol.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Emili M., Guidi S., Uguagliati B., Giacomini A., Bartesaghi R., Stagni F. Treatment with the flavonoid 7,8-Dihydroxyflavone: A prom-ising strategy for a constellation of body and brain disorders. Crit. Rev. Food Sci. Nutr. 2020:1–38. doi: 10.1080/10408398.2020.1810625. [DOI] [PubMed] [Google Scholar]
- 31.Barde Y.A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocco M.L., Soligo M., Manni L., Aloe L. Nerve growth factor: early studies and recent clinical trials. Curr. Neuropharmacol. 2018;16(10):1455–1465. doi: 10.2174/1570159X16666180412092859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruunsild P., Kazantseva A., Aid T., Palm K., Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90(3):397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lessmann V., Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 2009;65(1):11–22. doi: 10.1016/j.neures.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Numakawa T., Odaka H. Brain-derived neurotrophic factor signaling in the pathophysiology of Alzheimer’s disease: Beneficial effects of flavonoids for neuroprotection. Int. J. Mol. Sci. 2021;22(11):5719. doi: 10.3390/ijms22115719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hempstead B.L. Deciphering proneurotrophin actions. Handb. Exp. Pharmacol. 2014;220:17–32. doi: 10.1007/978-3-642-45106-5_2. [DOI] [PubMed] [Google Scholar]
- 37.Song M., Martinowich K., Lee F.S. BDNF at the synapse: why location matters. Mol. Psychiatry. 2017;22(10):1370–1375. doi: 10.1038/mp.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoilov P., Castren E., Stamm S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem. Biophys. Res. Commun. 2002;290(3):1054–1065. doi: 10.1006/bbrc.2001.6301. [DOI] [PubMed] [Google Scholar]
- 39.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cocco E., Scaltriti M., Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018;15(12):731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Middlemas D.S., Lindberg R.A., Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol. Cell. Biol. 1991;11(1):143–153. doi: 10.1128/MCB.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Squinto S.P., Stitt T.N., Aldrich T.H., Davis S., Bianco S.M., Radziejewski C., Glass D.J., Masiakowski P., Furth M.E., Valenzuela D.M. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65(5):885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- 43.Harward S.C., Hedrick N.G., Hall C.E., Parra-Bueno P., Milner T.A., Pan E., Laviv T., Hempstead B.L., Yasuda R., McNamara J.O. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016;538(7623):99–103. doi: 10.1038/nature19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minichiello L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009;10(12):850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- 46.Numakawa T., Suzuki S., Kumamaru E., Adachi N., Richards M., Kunugi H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010;25(2):237–258. doi: 10.14670/hh-25.237. [DOI] [PubMed] [Google Scholar]
- 47.Birnbaum R., Weinberger D.R. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017;18(12):727–740. doi: 10.1038/nrn.2017.125. [DOI] [PubMed] [Google Scholar]
- 48.Wolinsky D., Drake K., Bostwick J. Diagnosis and management of neuropsychiatric symptoms in Alzheimer’s disease. Curr. Psychiatry Rep. 2018;20(12):117. doi: 10.1007/s11920-018-0978-8. [DOI] [PubMed] [Google Scholar]
- 49.Nagy A., Schrag A. Neuropsychiatric aspects of Parkinson’s disease. J. Neural Transm. (Vienna) 2019;126(7):889–896. doi: 10.1007/s00702-019-02019-7. [DOI] [PubMed] [Google Scholar]
- 50.Cummings J., Ritter A., Rothenberg K. Advances in management of neuropsychiatric syndromes in neurodegenerative diseases. Curr. Psychiatry Rep. 2019;21(8):79. doi: 10.1007/s11920-019-1058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park H., Poo M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 53.Autry A.E., Monteggia L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012;64(2):238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariga A., Mitre M., Chao M.V. Consequences of brain-derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiol. Dis. 2017;97(Pt B):73–79. doi: 10.1016/j.nbd.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beurel E., Toups M., Nemeroff C.B. The Bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malhi G.S., Mann J.J. Depression. Lancet. 2018;392(10161):2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 57.Park L.T., Zarate C.A., Jr Depression in the Primary Care Setting. N. Engl. J. Med. 2019;380(6):559–568. doi: 10.1056/NEJMcp1712493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duman R.S., Heninger G.R., Nestler E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 59.Lee B.H., Park Y.M., Hwang J.A., Kim Y.K. Variable alterations in plasma erythropoietin and brain-derived neurotrophic factor levels in patients with major depressive disorder with and without a history of suicide attempt. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;110:110324. doi: 10.1016/j.pnpbp.2021.110324. [DOI] [PubMed] [Google Scholar]
- 60.Martinotti G., Pettorruso M., De Berardis D., Varasano P.A., Lucidi Pressanti G., De Remigis V., Valchera A., Ricci V., Di Nicola M., Janiri L., Biggio G., Di Giannantonio M. Agomelatine increases BDNF serum levels in depressed patients in correlation with the im-provement of depressive symptoms. Int. J. Neuropsychopharmacol. 2016;19(5):pyw003. doi: 10.1093/ijnp/pyw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang B., Ren Q., Zhang J.C., Chen Q.X., Hashimoto K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: rethinking the brain-liver axis. Transl. Psychiatry. 2017;7(5):e1128. doi: 10.1038/tp.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen T., Zheng M., Li Y., Liu S., He L. The role of CCR5 in the protective effect of Esculin on lipopolysaccharide-induced depressive symptom in mice. J. Affect. Disord. 2020;277:755–764. doi: 10.1016/j.jad.2020.08.065. [DOI] [PubMed] [Google Scholar]
- 63.Chen H.L., Lan Y.W., Tu M.Y., Tung Y.T., Chan M.N., Wu H.S., Yen C.C., Chen C.M. Kefir peptides exhibit antidepressant-like activity in mice through the BDNF/TrkB pathway. J. Dairy Sci. 2021;104(6):6415–6430. doi: 10.3168/jds.2020-19222. [DOI] [PubMed] [Google Scholar]
- 64.Wu L., Zhang T., Chen K., Lu C., Liu X.F., Zhou J.L., Huang Y.K., Yan H., Chen Y., Zhang C.J., Li J.F., Shi S.Q., Ren P., Huang X. Rapid antidepressant-like effect of Fructus Aurantii depends on cAMP-response element binding protein/Brain-derived neurotrophic facto by mediating synaptic transmission. Phytother. Res. 2021;35(1):404–414. doi: 10.1002/ptr.6812. [DOI] [PubMed] [Google Scholar]
- 65.Hao Y., Ge H., Sun M., Gao Y. Selecting an appropriate animal model of depression. Int. J. Mol. Sci. 2019;20(19):4827. doi: 10.3390/ijms20194827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casarotto P.C., Girych M., Fred S.M., Kovaleva V., Moliner R., Enkavi G., Biojone C., Cannarozzo C., Sahu M.P., Kaurinkoski K., Brunello C.A., Steinzeig A., Winkel F., Patil S., Vestring S., Serchov T., Diniz C.R.A.F., Laukkanen L., Cardon I., Antila H., Rog T., Piepponen T.P., Bramham C.R., Normann C., Lauri S.E., Saarma M., Vattulainen I., Castrén E. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184(5):1299–1313.e19. doi: 10.1016/j.cell.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shirayama Y., Chen A.C., Nakagawa S., Russell D.S., Duman R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marshall J., Zhou X.Z., Chen G., Yang S.Q., Li Y., Wang Y., Zhang Z.Q., Jiang Q., Birnbaumer L., Cao C. Antidepression action of BDNF requires and is mimicked by Gαi1/3 expression in the hippocampus. Proc. Natl. Acad. Sci. USA. 2018;115(15):E3549–E3558. doi: 10.1073/pnas.1722493115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erkkinen M.G., Kim M.O., Geschwind M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018;10(4):a033118. doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Hu M.T.M. From dreams to parkinsonism: tracking the journey. Brain. 2019;142(7):1850–1852. doi: 10.1093/brain/awz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hane F.T., Lee B.Y., Leonenko Z. Recent progress in Alzheimer’s disease research, Part 1: Pathology. J. Alzheimers Dis. 2017;57(1):1–28. doi: 10.3233/JAD-160882. [DOI] [PubMed] [Google Scholar]
- 73.Mucke L. Neuroscience: Alzheimer’s disease. Nature. 2009;461(7266):895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 74.Barbereau C., Yehya A., Silhol M., Cubedo N., Verdier J.M., Maurice T., Rossel M. Neuroprotective brain-derived neurotrophic fac-tor signaling in the TAU-P301L tauopathy zebrafish model. Pharmacol. Res. 2020;158:104865. doi: 10.1016/j.phrs.2020.104865. [DOI] [PubMed] [Google Scholar]
- 75.Bloem B.R., Okun M.S., Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 76.Ng T.K.S., Ho C.S.H., Tam W.W.S., Kua E.H., Ho R.C. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): A systematic review and meta-analysis. Int. J. Mol. Sci. 2019;20(2):257. doi: 10.3390/ijms20020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei H., Zhu X., Li Y. Application value of serum biomarkers for choosing memantine therapy for moderate AD. J. Neurol. 2018;265(8):1844–1849. doi: 10.1007/s00415-018-8926-4. [DOI] [PubMed] [Google Scholar]
- 78.Menet R., Bourassa P., Calon F., ElAli A. Dickkopf-related protein-1 inhibition attenuates amyloid-beta pathology associated to Alz-heimer’s disease. Neurochem. Int. 2020;141:104881. doi: 10.1016/j.neuint.2020.104881. [DOI] [PubMed] [Google Scholar]
- 79.Jiao S.S., Shen L.L., Zhu C., Bu X.L., Liu Y.H., Liu C.H., Yao X.Q., Zhang L.L., Zhou H.D., Walker D.G., Tan J., Götz J., Zhou X.F., Wang Y.J. Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’s disease. Transl. Psychiatry. 2016;6(10):e907. doi: 10.1038/tp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Liu H., Zhang B.S., Soares J.C., Zhang X.Y. Low BDNF is associated with cognitive impairments in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2016;29:66–71. doi: 10.1016/j.parkreldis.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 81.Huang Y., Huang C., Yun W. Peripheral BDNF/TrkB protein expression is decreased in Parkinson’s disease but not in essential tremor. J. Clin. Neurosci. 2019;63:176–181. doi: 10.1016/j.jocn.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 82.Hernández-Vara J., Sáez-Francàs N., Lorenzo-Bosquet C., Corominas-Roso M., Cuberas-Borròs G., Lucas-Del Pozo S., Carter S., Armengol-Bellapart M., Castell-Conesa J. BDNF levels and nigrostriatal degeneration in “drug naïve” Parkinson’s disease patients. An “in vivo” study using I-123-FP-CIT SPECT. Parkinsonism Relat. Disord. 2020;78:31–35. doi: 10.1016/j.parkreldis.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Liu H., Du X.D., Zhang Y., Yin G., Zhang B.S., Soares J.C., Zhang X.Y. Association of low serum BDNF with depression in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2017;41:73–78. doi: 10.1016/j.parkreldis.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 84.Zhu G., Li J., He L., Wang X., Hong X. MPTP-induced changes in hippocampal synaptic plasticity and memory are prevented by me-mantine through the BDNF-TrkB pathway. Br. J. Pharmacol. 2015;172(9):2354–2368. doi: 10.1111/bph.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu S.F., Zhang Y.H., Wang S., Pang Z.Q., Fan Y.G., Li J.Y., Wang Z.Y., Guo C. Lactoferrin ameliorates dopaminergic neurodegen-eration and motor deficits in MPTP-treated mice. Redox Biol. 2019;21:101090. doi: 10.1016/j.redox.2018.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vyas Y., Montgomery J.M., Cheyne J.E. Hippocampal deficits in amyloid-β-related rodent models of Alzheimer’s disease. Front. Neurosci. 2020;14:266. doi: 10.3389/fnins.2020.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Notaras M., van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry. 2020;25(10):2251–2274. doi: 10.1038/s41380-019-0639-2. [DOI] [PubMed] [Google Scholar]
- 88.Keyan D., Bryant R.A. The capacity for acute exercise to modulate emotional memories: A review of findings and mechanisms. Neurosci. Biobehav. Rev. 2019;107:438–449. doi: 10.1016/j.neubiorev.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 89.Miranda M., Morici J.F., Zanoni M.B., Bekinschtein P. Brain-Derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019;13:363. doi: 10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang S.J., Song Z.J., Wang X.C., Zhang Z.R., Wu S.B., Zhu G.Q. Curculigoside facilitates fear extinction and prevents depression-like behaviors in a mouse learned helplessness model through increasing hippocampal BDNF. Acta Pharmacol. Sin. 2019;40(10):1269–1278. doi: 10.1038/s41401-019-0238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amraie E., Pouraboli I., Rajaei Z. Neuroprotective effects of Levisticum officinale on LPS-induced spatial learning and memory impair-ments through neurotrophic, anti-inflammatory, and antioxidant properties. Food Funct. 2020;11(7):6608–6621. doi: 10.1039/D0FO01030H. [DOI] [PubMed] [Google Scholar]
- 92.López-Benito S., Sánchez-Sánchez J., Brito V., Calvo L., Lisa S., Torres-Valle M., Palko M.E., Vicente-García C., Fernández-Fernández S., Bolaños J.P., Ginés S., Tessarollo L., Arévalo J.C. Regulation of BDNF release by ARMS/Kidins220 through modulation of synaptotagmin-IV levels. J. Neurosci. 2018;38(23):5415–5428. doi: 10.1523/JNEUROSCI.1653-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El Hayek L., Khalifeh M., Zibara V., Abi A.R., Emmanuel N., Karnib N., El-Ghandour R., Nasrallah P., Bilen M., Ibrahim P., Younes J., Abou Haidar E., Barmo N., Jabre V., Stephan J.S., Sleiman S.F. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019;39(13):2369–2382. doi: 10.1523/JNEUROSCI.1661-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mokhtari-Zaer A., Saadat S., Marefati N., Hosseini M., Boskabady M.H. Treadmill exercise restores memory and hippocampal synap-tic plasticity impairments in ovalbumin-sensitized juvenile rats: Involvement of brain-derived neurotrophic factor (BDNF). Neurochem. Int. 2020;135:104691. doi: 10.1016/j.neuint.2020.104691. [DOI] [PubMed] [Google Scholar]
- 95.Rana T., Behl T., Sehgal A., Srivastava P., Bungau S. Unfolding the role of BDNF as a biomarker for treatment of depression. J. Mol. Neurosci. 2021;71(10):2008–2021. doi: 10.1007/s12031-020-01754-x. [DOI] [PubMed] [Google Scholar]
- 96.Mitre M., Mariga A., Chao M.V. Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin. Sci. (Lond.) 2017;131(1):13–23. doi: 10.1042/CS20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shirayama Y., Yang C., Zhang J.C., Ren Q., Yao W., Hashimoto K. Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antago-nist. Eur. Neuropsychopharmacol. 2015;25(12):2449–2458. doi: 10.1016/j.euroneuro.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 98.Zhang J.C., Wu J., Fujita Y., Yao W., Ren Q., Yang C., Li S.X., Shirayama Y., Hashimoto K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2014;18(4):pyu077. doi: 10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang M.W., Zhang S.F., Li Z.H., Han F. 7,8-Dihydroxyflavone reverses the depressive symptoms in mouse chronic mild stress. Neurosci. Lett. 2016;635:33–38. doi: 10.1016/j.neulet.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 100.Yao W., Zhang J.C., Ishima T., Dong C., Yang C., Ren Q., Ma M., Han M., Wu J., Suganuma H., Ushida Y., Yamamoto M., Hash-imoto K. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci. Rep. 2016;6:30659. doi: 10.1038/srep30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amin N., Xie S., Tan X., Chen Y., Ren Q., Botchway B.O.A., Hu S., Ma Y., Hu Z., Fang M. Optimized integration of fluoxetine and 7, 8-dihydroxyflavone as an efficient therapy for reversing depressive-like behavior in mice during the perimenopausal period. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;101:109939. doi: 10.1016/j.pnpbp.2020.109939. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J.C., Yao W., Dong C., Yang C., Ren Q., Ma M., Han M., Hashimoto K. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl.) 2015;232(23):4325–4335. doi: 10.1007/s00213-015-4062-3. [DOI] [PubMed] [Google Scholar]
- 103.Li S., Luo X., Hua D., Wang Y., Zhan G., Huang N., Jiang R., Yang L., Zhu B., Yuan X., Luo A., Yang C. Ketamine alleviates postoperative depression-like symptoms in susceptible mice: The role of BDNF-TrkB signaling. Front. Pharmacol. 2020;10:1702. doi: 10.3389/fphar.2019.01702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ren Q., Ma M., Yang C., Zhang J.C., Yao W., Hashimoto K. BDNF-TrkB signaling in the nucleus accumbens shell of mice has key role in methamphetamine withdrawal symptoms. Transl. Psychiatry. 2015;5(10):e666. doi: 10.1038/tp.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J.Q., Mao L. The ERK Pathway: Molecular mechanisms and treatment of depression. Mol. Neurobiol. 2019;56(9):6197–6205. doi: 10.1007/s12035-019-1524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barker R.A., Götz M., Parmar M. New approaches for brain repair-from rescue to reprogramming. Nature. 2018;557(7705):329–334. doi: 10.1038/s41586-018-0087-1. [DOI] [PubMed] [Google Scholar]
- 107.Chen C., Li X.H., Zhang S., Tu Y., Wang Y.M., Sun H.T. 7,8-dihydroxyflavone ameliorates scopolamine-induced Alzheimer-like pathologic dysfunction. Rejuvenation Res. 2014;17(3):249–254. doi: 10.1089/rej.2013.1519. [DOI] [PubMed] [Google Scholar]
- 108.Gao L., Tian M., Zhao H.Y., Xu Q.Q., Huang Y.M., Si Q.C., Tian Q., Wu Q.M., Hu X.M., Sun L.B., McClintock S.M., Zeng Y. TrkB activation by 7, 8-dihydroxyflavone increases synapse AMPA subunits and ameliorates spatial memory deficits in a mouse model of Alzheimer’s disease. J. Neurochem. 2016;136(3):620–636. doi: 10.1111/jnc.13432. [DOI] [PubMed] [Google Scholar]
- 109.Castello N.A., Nguyen M.H., Tran J.D., Cheng D., Green K.N., LaFerla F.M. 7,8-Dihydroxyflavone, a small molecule TrkB agonist, improves spatial memory and increases thin spine density in a mouse model of Alzheimer disease-like neuronal loss. PLoS One. 2014;9(3):e91453. doi: 10.1371/journal.pone.0091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Devi L., Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37(2):434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen C., Ahn E.H., Kang S.S., Liu X., Alam A., Ye K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci. Adv. 2020;6(31):eaba0466. doi: 10.1126/sciadv.aba0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He J., Xiang Z., Zhu X., Ai Z., Shen J., Huang T., Liu L., Ji W., Li T. Neuroprotective effects of 7,8-dihydroxyflavone on midbrain dopaminergic neurons in MPP+-treated monkeys. Sci. Rep. 2016;6:34339. doi: 10.1038/srep34339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Massaquoi M.S., Liguore W.A., Churchill M.J., Moore C., Melrose H.L., Meshul C.K. Gait deficits and loss of striatal tyrosine hy-droxlase/Trk-B are restored following 7,8-dihydroxyflavone treatment in a progressive MPTP mouse model of Parkinson’s disease. Neuroscience. 2020;433:53–71. doi: 10.1016/j.neuroscience.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 114.Li X.H., Dai C.F., Chen L., Zhou W.T., Han H.L., Dong Z.F. 7,8-dihydroxyflavone ameliorates motor deficits via suppressing α-synuclein expression and oxidative stress in the MPTP-induced mouse model of Parkinson’s disease. CNS Neurosci. Ther. 2016;22(7):617–624. doi: 10.1111/cns.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sconce M.D., Churchill M.J., Moore C., Meshul C.K. Intervention with 7,8-dihydroxyflavone blocks further striatal terminal loss and restores motor deficits in a progressive mouse model of Parkinson’s disease. Neuroscience. 2015;290:454–471. doi: 10.1016/j.neuroscience.2014.12.080. [DOI] [PubMed] [Google Scholar]
- 116.Nie S., Ma K., Sun M., Lee M., Tan Y., Chen G., Zhang Z., Zhang Z., Cao X. 7,8-dihydroxyflavone protects nigrostriatal dopamin-ergic neurons from rotenone-induced neurotoxicity in rodents. Parkinsons Dis. 2019;2019:9193534. doi: 10.1155/2019/9193534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luo D., Shi Y., Wang J., Lin Q., Sun Y., Ye K., Yan Q., Zhang H. 7,8-dihydroxyflavone protects 6-OHDA and MPTP induced do-paminergic neurons degeneration through activation of TrkB in rodents. Neurosci. Lett. 2016;620:43–49. doi: 10.1016/j.neulet.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 118.Cho S.J., Kang K.A., Piao M.J., Ryu Y.S., Fernando P.D.S.M., Zhen A.X., Hyun Y.J., Ahn M.J., Kang H.K., Hyun J.W. 7,8-dihydroxyflavone protects high glucose-damaged neuronal cells against oxidative stress. Biomol. Ther. (Seoul) 2019;27(1):85–91. doi: 10.4062/biomolther.2018.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krishna G., Agrawal R., Zhuang Y., Ying Z., Paydar A., Harris N.G., Royes L.F.F., Gomez-Pinilla F. 7,8-Dihydroxyflavone facilitates the action exercise to restore plasticity and functionality: Implications for early brain trauma recovery. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863(6):1204–1213. doi: 10.1016/j.bbadis.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao S., Yu A., Wang X., Gao X., Chen J. Post-injury treatment of 7,8-dihydroxyflavone promotes neurogenesis in the hippocampus of the adult mouse. J. Neurotrauma. 2016;33(22):2055–2064. doi: 10.1089/neu.2015.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seppa K., Jagomäe T., Kukker K.G., Reimets R., Pastak M., Vasar E., Terasmaa A., Plaas M. Liraglutide, 7,8-DHF and their co-treatment prevents loss of vision and cognitive decline in a Wolfram syndrome rat model. Sci. Rep. 2021;11(1):2275. doi: 10.1038/s41598-021-81768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bollen E., Vanmierlo T., Akkerman S., Wouters C., Steinbusch H.M., Prickaerts J. 7,8-Dihydroxyflavone improves memory consoli-dation processes in rats and mice. Behav. Brain Res. 2013;257:8–12. doi: 10.1016/j.bbr.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 123.Pandey S.N., Kwatra M., Dwivedi D.K., Choubey P., Lahkar M., Jangra A. 7,8-Dihydroxyflavone alleviated the high-fat diet and alco-hol-induced memory impairment: behavioral, biochemical and molecular evidence. Psychopharmacology (Berl.) 2020;237(6):1827–1840. doi: 10.1007/s00213-020-05502-2. [DOI] [PubMed] [Google Scholar]
- 124.Tan Y., Nie S., Zhu W., Liu F., Guo H., Chu J., Cao X.B., Jiang X., Zhang Y., Li Y. 7,8-dihydroxyflavone ameliorates cognitive impairment by inhibiting expression of tau pathology in apoe-knockout mice. Front. Aging Neurosci. 2016;8:287. doi: 10.3389/fnagi.2016.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zeng Y., Liu Y., Wu M., Liu J., Hu Q. Activation of TrkB by 7,8-dihydroxyflavone prevents fear memory defects and facilitates amygdalar synaptic plasticity in aging. J. Alzheimers Dis. 2012;31(4):765–778. doi: 10.3233/JAD-2012-120886. [DOI] [PubMed] [Google Scholar]
- 126.Yang P., Leu D., Ye K., Srinivasan C., Fike J.R., Huang T.T. Cognitive impairments following cranial irradiation can be mitigated by treatment with a tropomyosin receptor kinase B agonist. Exp. Neurol. 2016;279:178–186. doi: 10.1016/j.expneurol.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi D.C., Maguschak K.A., Ye K., Jang S.W., Myers K.M., Ressler K.J. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc. Natl. Acad. Sci. USA. 2010;107(6):2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou J., Wang F., Zhang J., Li J., Ma L., Dong T., Zhuang Z. The interplay of BDNF-TrkB with NMDA receptor in propofol-induced cognition dysfunction: Mechanism for the effects of propofol on cognitive function. BMC Anesthesiol. 2018;18(1):35. doi: 10.1186/s12871-018-0491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tian M., Zeng Y., Hu Y., Yuan X., Liu S., Li J., Lu P., Sun Y., Gao L., Fu D., Li Y., Wang S., McClintock S.M. 7, 8-Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome. Neuropharmacology. 2015;89:43–53. doi: 10.1016/j.neuropharm.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 130.Seese R.R., Le A.A., Wang K., Cox C.D., Lynch G., Gall C.M. A TrkB agonist and ampakine rescue synaptic plasticity and multiple forms of memory in a mouse model of intellectual disability. Neurobiol. Dis. 2020;134:104604. doi: 10.1016/j.nbd.2019.104604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang X., Romine J.L., Gao X., Chen J. Aging impairs dendrite morphogenesis of newborn neurons and is rescued by 7, 8-dihydroxyflavone. Aging Cell. 2017;16(2):304–311. doi: 10.1111/acel.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu G., Yang S., Xie Z., Wan X. Synaptic modification by L-theanine, a natural constituent in green tea, rescues the impairment of hip-pocampal long-term potentiation and memory in AD mice. Neuropharmacology. 2018;138:331–340. doi: 10.1016/j.neuropharm.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 133.Liu X., Obianyo O., Chan C.B., Huang J., Xue S., Yang J.J., Zeng F., Goodman M., Ye K. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor. J. Biol. Chem. 2014;289(40):27571–27584. doi: 10.1074/jbc.M114.562561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marongiu D., Imbrosci B., Mittmann T. Modulatory effects of the novel TrkB receptor agonist 7,8-dihydroxyflavone on synaptic trans-mission and intrinsic neuronal excitability in mouse visual cortex in vitro. Eur. J. Pharmacol. 2013;709(1-3):64–71. doi: 10.1016/j.ejphar.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 135.Gudasheva T.A., Povarnina P., Tarasiuk A.V., Seredenin S.B. The low molecular weight brain-derived neurotrophic factor mimetics with antidepressant-like activity. Curr. Pharm. Des. 2019;25(6):729–737. doi: 10.2174/1381612825666190329122852. [DOI] [PubMed] [Google Scholar]
- 136.Liu X., Chan C.B., Jang S.W., Pradoldej S., Huang J., He K., Phun L.H., France S., Xiao G., Jia Y., Luo H.R., Ye K. A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J. Med. Chem. 2010;53(23):8274–8286. doi: 10.1021/jm101206p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Di Stefano A., Sozio P., Cerasa L.S., Iannitelli A. L-Dopa prodrugs: An overview of trends for improving Parkinson’s disease treat-ment. Curr. Pharm. Des. 2011;17(32):3482–3493. doi: 10.2174/138161211798194495. [DOI] [PubMed] [Google Scholar]
- 138.Liu X., Qi Q., Xiao G., Li J., Luo H.R., Ye K. O-methylated metabolite of 7,8-dihydroxyflavone activates TrkB receptor and displays antidepressant activity. Pharmacology. 2013;91(3-4):185–200. doi: 10.1159/000346920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sanches B.M.A., Ferreira E.I. Is prodrug design an approach to increase water solubility? Int. J. Pharm. 2019;568:118498. doi: 10.1016/j.ijpharm.2019.118498. [DOI] [PubMed] [Google Scholar]
- 140.Geng W.C., Sessler J.L., Guo D.S. Supramolecular prodrugs based on host-guest interactions. Chem. Soc. Rev. 2020;49(8):2303–2315. doi: 10.1039/C9CS00622B. [DOI] [PubMed] [Google Scholar]
- 141.Chen C., Wang Z., Zhang Z., Liu X., Kang S.S., Zhang Y., Ye K. The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2018;115(3):578–583. doi: 10.1073/pnas.1718683115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thomford N.E., Senthebane D.A., Rowe A., Munro D., Seele P., Maroyi A., Dzobo K. Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int. J. Mol. Sci. 2018;19(6):1578. doi: 10.3390/ijms19061578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen C., Ahn E.H., Liu X., Wang Z.H., Luo S., Liao J., Ye K. Optimized TrkB agonist ameliorates Alzheimer’s disease pathologies and improves cognitive functions via inhibiting delta-secretase. ACS Chem. Neurosci. 2021;12(13):2448–2461. doi: 10.1021/acschemneuro.1c00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pandey R.P., Parajuli P., Pokhrel A.R., Sohng J.K. Biosynthesis of novel 7,8-dihydroxyflavone glycoside derivatives and in silico study of their effects on BACE1 inhibition. Biotechnol. Appl. Biochem. 2018;65(2):128–137. doi: 10.1002/bab.1570. [DOI] [PubMed] [Google Scholar]
- 145.Chen G., Seukep A.J., Guo M. Recent advances in molecular docking for the research and discovery of potential marine drugs. Mar. Drugs. 2020;18(11):E545. doi: 10.3390/md18110545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saikia S., Bordoloi M. Molecular docking: challenges, advances and its use in drug discovery perspective. Curr. Drug Targets. 2019;20(5):501–521. doi: 10.2174/1389450119666181022153016. [DOI] [PubMed] [Google Scholar]
- 147.Mohankumar T., Chandramohan V., Lalithamba H.S., Jayaraj R.L., Kumaradhas P., Sivanandam M., Hunday G., Vijayakumar R., Balakrishnan R., Manimaran D., Elangovan N. Design and Molecular dynamic investigations of 7,8-dihydroxyflavone derivatives as po-tential neuroprotective agents against alpha-synuclein. Sci. Rep. 2020;10(1):599. doi: 10.1038/s41598-020-57417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen Y., Xia G., Zhao Z., Xue F., Chen C., Zhang Y. Formation, structural characterization, stability and in vitro bioaccessibility of 7,8-dihydroxyflavone loaded zein-/sophorolipid composite nanoparticles: effect of sophorolipid under two blending sequences. Food Funct. 2020;11(2):1810–1825. doi: 10.1039/C9FO02704A. [DOI] [PubMed] [Google Scholar]
- 149.Chen Y., Zhao Z., Xia G., Xue F., Chen C., Zhang Y. Fabrication and characterization of zein/lactoferrin composite nanoparticles for encapsulating 7,8-dihydroxyflavone: Enhancement of stability, water solubility and bioaccessibility. Int. J. Biol. Macromol. 2020;146:179–192. doi: 10.1016/j.ijbiomac.2019.12.251. [DOI] [PubMed] [Google Scholar]
- 150.Tian Z., Mai Y., Meng T., Ma S., Gou G., Yang J. Nanocrystals for improving oral bioavailability of drugs: intestinal transport mecha-nisms and influencing factors. AAPS PharmSciTech. 2021;22(5):179. doi: 10.1208/s12249-021-02041-7. [DOI] [PubMed] [Google Scholar]
- 151.Williams H.D., Ford L., Igonin A., Shan Z., Botti P., Morgen M.M., Hu G., Pouton C.W., Scammells P.J., Porter C.J.H., Benameur H. Unlocking the full potential of lipid-based formulations using lipophilic salt/ionic liquid forms. Adv. Drug Deliv. Rev. 2019;142:75–90. doi: 10.1016/j.addr.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 152.Schittny A., Huwyler J., Puchkov M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020;27(1):110–127. doi: 10.1080/10717544.2019.1704940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Paul R., Nath J., Paul S., Mazumder M.K., Phukan B.C., Roy R., Bhattacharya P., Borah A. Suggesting 7,8-dihydroxyflavone as a promising nutraceutical against CNS disorders. Neurochem. Int. 2021;148:105068. doi: 10.1016/j.neuint.2021.105068. [DOI] [PubMed] [Google Scholar]
- 154.Han M., Zhang J.C., Yao W., Yang C., Ishima T., Ren Q., Ma M., Dong C., Huang X.F., Hashimoto K. Intake of 7,8-Dihydroxyflavone during juvenile and adolescent stages prevents onset of psychosis in adult offspring after maternal immune activation. Sci. Rep. 2016;6:36087. doi: 10.1038/srep36087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Han M., Zhang J.C., Huang X.F., Hashimoto K. Intake of 7,8-dihydroxyflavone from pregnancy to weaning prevents cognitive deficits in adult offspring after maternal immune activation. Eur. Arch. Psychiatry Clin. Neurosci. 2017;267(5):479–483. doi: 10.1007/s00406-017-0802-1. [DOI] [PubMed] [Google Scholar]
- 156.Han M., Zhang J.C., Hashimoto K. Increased levels of C1q in the prefrontal cortex of adult offspring after maternal immune activation: prevention by 7,8-dihydroxyflavone. Clin. Psychopharmacol. Neurosci. 2017;15(1):64–67. doi: 10.9758/cpn.2017.15.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang Y.J., Li Y.K., Wang W., Wan J.G., Yu B., Wang M.Z., Hu B. Small-molecule TrkB agonist 7,8-dihydroxyflavone reverses cogni-tive and synaptic plasticity deficits in a rat model of schizophrenia. Pharmacol. Biochem. Behav. 2014;122:30–36. doi: 10.1016/j.pbb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 158.Waltman S.H., Shearer D., Moore B.A. Management of post-traumatic nightmares: A review of pharmacologic and nonpharmacologic treatments since 2013. Curr. Psychiatry Rep. 2018;20(12):108. doi: 10.1007/s11920-018-0971-2. [DOI] [PubMed] [Google Scholar]
- 159.Koek R.J., Luong T.N. Theranostic pharmacology in PTSD: Neurobiology and timing. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;90:245–263. doi: 10.1016/j.pnpbp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 160.Compean E., Hamner M. Posttraumatic stress disorder with secondary psychotic features (PTSD-SP): Diagnostic and treatment challeng-es. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;88:265–275. doi: 10.1016/j.pnpbp.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baker-Andresen D., Flavell C.R., Li X., Bredy T.W. Activation of BDNF signaling prevents the return of fear in female mice. Learn. Mem. 2013;20(5):237–240. doi: 10.1101/lm.029520.112. [DOI] [PubMed] [Google Scholar]
- 162.Andero R., Daviu N., Escorihuela R.M., Nadal R., Armario A. 7,8-dihydroxyflavone, a TrkB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus. 2012;22(3):399–408. doi: 10.1002/hipo.20906. [DOI] [PubMed] [Google Scholar]
- 163.Kutlu M.G., Cole R.D., Connor D.A., Natwora B., Gould T.J. Tyrosine receptor kinase B receptor activation reverses the impairing effects of acute nicotine on contextual fear extinction. J. Psychopharmacol. 2018;32(3):367–372. doi: 10.1177/0269881118758305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sanz-García A., Knafo S., Pereda-Pérez I., Esteban J.A., Venero C., Armario A. Administration of the TrkB receptor agonist 7,8-dihydroxyflavone prevents traumatic stress-induced spatial memory deficits and changes in synaptic plasticity. Hippocampus. 2016;26(9):1179–1188. doi: 10.1002/hipo.22599. [DOI] [PubMed] [Google Scholar]
- 165.Tohyama S., Matsuda S., Mizutani A. Sex-dependent opposite effects of a tropomyosin-related kinase B receptor (TrkB) agonist 7,8-dihydroxyflavone on cued fear extinction in mice. Neurosci. Lett. 2020;715:134670. doi: 10.1016/j.neulet.2019.134670. [DOI] [PubMed] [Google Scholar]
- 166.Choi J.W., Lee J., Park Y.I. 7,8-Dihydroxyflavone attenuates TNF-α-induced skin aging in Hs68 human dermal fibroblast cells via down-regulation of the MAPKs/Akt signaling pathways. Biomed. Pharmacother. 2017;95:1580–1587. doi: 10.1016/j.biopha.2017.09.098. [DOI] [PubMed] [Google Scholar]
- 167.Jin Z., Yang Y.Z., Chen J.X., Tang Y.Z. Inhibition of pro-inflammatory mediators in RAW264.7 cells by 7-hydroxyflavone and 7,8-dihydroxyflavone. J. Pharm. Pharmacol. 2017;69(7):865–874. doi: 10.1111/jphp.12714. [DOI] [PubMed] [Google Scholar]
- 168.Butterfield D.A. Perspectives on oxidative stress in Alzheimer’s disease and predictions of future research emphases. J. Alzheimers Dis. 2018;64(s1):S469–S479. doi: 10.3233/JAD-179912. [DOI] [PubMed] [Google Scholar]
- 169.Jiang T., Sun Q., Chen S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016;147:1–19. doi: 10.1016/j.pneurobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 170.Santos J.R., Gois A.M., Mendonça D.M., Freire M.A. Nutritional status, oxidative stress and dementia: the role of selenium in Alz-heimer’s disease. Front. Aging Neurosci. 2014;6:206. doi: 10.3389/fnagi.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Akhtar A., Dhaliwal J., Sah S.P. 7,8-Dihydroxyflavone improves cognitive functions in ICV-STZ rat model of sporadic Alzheimer’s disease by reversing oxidative stress, mitochondrial dysfunction, and insulin resistance. Psychopharmacology (Berl.) 2021;238(7):1991–2009. doi: 10.1007/s00213-021-05826-7. [DOI] [PubMed] [Google Scholar]
- 172.Ahmed S., Kwatra M., Gawali B., Panda S.R., Naidu V.G.M. Potential role of TrkB agonist in neuronal survival by promoting CREB/BDNF and PI3K/Akt signaling in vitro and in vivo model of 3-nitropropionic acid (3-NP)-induced neuronal death. Apoptosis. 2021;26(1-2):52–70. doi: 10.1007/s10495-020-01645-x. [DOI] [PubMed] [Google Scholar]
- 173.Li X., Chen C., Zhan X., Li B., Zhang Z., Li S., Xie Y., Song X., Shen Y., Liu J., Liu P., Liu G.P., Yang X. R13 preserves motor performance in SOD1G93A mice by improving mitochondrial function. Theranostics. 2021;11(15):7294–7307. doi: 10.7150/thno.56070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fang Y.Y., Luo M., Yue S., Han Y., Zhang H.J., Zhou Y.H., Liu K., Liu H.G. 7,8-dihydroxyflavone protects retinal ganglion cells against chronic intermittent hypoxia-induced oxidative stress damage via activation of the BDNF/TrkB signaling pathway. Sleep Breath. 2021;26(1):287–295. doi: 10.1007/s11325-021-02400-5. [DOI] [PubMed] [Google Scholar]
- 175.Mohankumar T., Lalithamba H.S., Manigandan K., Muthaiyan A., Elangovan N. DHF-BAHPC molecule exerts ameliorative antioxidant status and reduced cadmium-induced toxicity in zebrafish (Danio rerio) embryos. Environ. Toxicol. Pharmacol. 2020;79:103425. doi: 10.1016/j.etap.2020.103425. [DOI] [PubMed] [Google Scholar]
- 176.Hynd M.R., Scott H.L., Dodd P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004;45(5):583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 177.Lau A., Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460(2):525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 178.Beal M.F. Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann. Neurol. 1998;44(3) Suppl. 1:S110–S114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- 179.Chen J., Chua K.W., Chua C.C., Yu H., Pei A., Chua B.H., Hamdy R.C., Xu X., Liu C.F. Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci. Lett. 2011;499(3):181–185. doi: 10.1016/j.neulet.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 180.Zhao Z., Xue F., Gu Y., Han J., Jia Y., Ye K., Zhang Y. Crosstalk between the muscular estrogen receptor α and BDNF/TrkB signaling alleviates metabolic syndrome via 7,8-dihydroxyflavone in female mice. Mol. Metab. 2021;45:101149. doi: 10.1016/j.molmet.2020.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sharma P., Wu G., Kumaraswamy D., Burchat N., Ye H., Gong Y., Zhao L., Lam Y.Y., Sampath H. Sex-dependent effects of 7,8-dihydroxyflavone on metabolic health are associated with alterations in the host gut microbiome. Nutrients. 2021;13(2):637. doi: 10.3390/nu13020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sun T., Chen S., Huang H., Li T., Yang W., Liu L. Metabolic profile study of 7, 8-dihydroxyflavone in monkey plasma using high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1061-1062:97–102. doi: 10.1016/j.jchromb.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 183.Sanchez-Rangel E., Inzucchi S.E. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586–1593. doi: 10.1007/s00125-017-4336-x. [DOI] [PubMed] [Google Scholar]
- 184.Kamarudin M.N.A., Sarker M.M.R., Zhou J.R., Parhar I. Metformin in colorectal cancer: molecular mechanism, preclinical and clinical aspects. J. Exp. Clin. Cancer Res. 2019;38(1):491. doi: 10.1186/s13046-019-1495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kessing L.V., Rytgaard H.C., Ekstrøm C.T., Knop F.K., Berk M., Gerds T.A. Antidiabetes agents and incident depression: a nation-wide population-based study. Diabetes Care. 2020;43(12):3050–3060. doi: 10.2337/dc20-1561. [DOI] [PubMed] [Google Scholar]
- 186.Fang W., Zhang J., Hong L., Huang W., Dai X., Ye Q., Chen X. Metformin ameliorates stress-induced depression-like behaviors via enhancing the expression of BDNF by activating AMPK/CREB-mediated histone acetylation. J. Affect. Disord. 2020;260:302–313. doi: 10.1016/j.jad.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 187.Zilov A.V., Abdelaziz S.I., AlShammary A., Al Zahrani A., Amir A., Assaad K.S.H., Brand K., Elkafrawy N., Hassoun A.A.K., Jahed A., Jarrah N., Mrabeti S., Paruk I. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab. Res. Rev. 2019;35(7):e3173. doi: 10.1002/dmrr.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Itoh H., Inoue M. Comprehensive structure-activity relationship studies of macrocyclic natural products enabled by their total syntheses. Chem. Rev. 2019;119(17):10002–10031. doi: 10.1021/acs.chemrev.9b00063. [DOI] [PubMed] [Google Scholar]
- 189.Chen Y., Xue F., Xia G., Zhao Z., Chen C., Li Y., Zhang Y. Transepithelial transport mechanisms of 7,8-dihydroxyflavone, a small molecular TrkB receptor agonist, in human intestinal Caco-2 cells. Food Funct. 2019;10(8):5215–5227. doi: 10.1039/C9FO01007F. [DOI] [PubMed] [Google Scholar]
- 190.Ascherio A., Schwarzschild M.A. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 191.Crous-Bou M., Minguillón C., Gramunt N., Molinuevo J.L. Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res. Ther. 2017;9(1):71. doi: 10.1186/s13195-017-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kwon M.J., Kim J.H., Kim T., Lee S.B. Pharmacological intervention of early neuropathy in neurodegenerative diseases. Pharmacol. Res. 2017;119:169–177. doi: 10.1016/j.phrs.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 193.Aytan N., Choi J.K., Carreras I., Crabtree L., Nguyen B., Lehar M., Blusztajn J.K., Jenkins B.G., Dedeoglu A. Protective effects of 7,8-dihydroxyflavone on neuropathological and neurochemical changes in a mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 2018;828:9–17. doi: 10.1016/j.ejphar.2018.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Giacomini A., Stagni F., Emili M., Uguagliati B., Rimondini R., Bartesaghi R., Guidi S. Timing of treatment with the flavonoid 7,8-DHF critically impacts on its effects on learning and memory in the Ts65Dn mouse. Antioxidants. 2019;8(6):163. doi: 10.3390/antiox8060163. [DOI] [PMC free article] [PubMed] [Google Scholar]


