Abstract
Epilepsy is commonly recognized as a disease driven by generalized hyperexcited and hypersynchronous neural activity. Sodium-activated potassium channels (KNa channels), which are encoded by the Slo 2.2 and Slo 2.1 genes, are widely expressed in the central nervous system and considered as “brakes” to adjust neuronal adaptation through regulating action potential threshold or after-hyperpolarization under physiological condition. However, the variants in KNa channels, especially gain-of-function variants, have been found in several childhood epileptic conditions. Most previous studies focused on mapping the epileptic network on the macroscopic scale while ignoring the value of microscopic changes. Notably, paradoxical role of KNa channels working on individual neuron/microcircuit and the macroscopic epileptic expression highlights the importance of understanding epileptogenic network through combining microscopic and macroscopic methods. Here, we first illustrated the molecular and physiological function of KNa channels on preclinical seizure models and patients with epilepsy. Next, we summarized current hypothesis on the potential role of KNa channels during seizures to provide essential insight into what emerged as a micro-macro disconnection at different levels. Additionally, we highlighted the potential utility of KNa channels as therapeutic targets for developing innovative anti-seizure medications.
Keywords: Epilepsy, KNa channels, microcircuit, rhythm, micro-macro disconnection, quinidine
1. INTRODUCTION
Characterized by repeated and unprovoked epileptic seizures, epilepsy is one of the most prevalent neurological disorders affecting 70 million people globally [1, 2]. It is estimated that more than 80 persons per 100,000 are diagnosed with epilepsy each year and anti-seizure medications (ASMs) are the first-line treatment for patients with epilepsy [3, 4]. However, up to one third of patients with epilepsy will eventually develop into intractable epilepsy due to lack of response to a range of ASMs and will revert to surgical intervention for the remission [4, 5]. The development of advanced ASMs and favorable surgical outcomes both depend on deeper insight into the underlying mechanism of epilepsy. Although the detailed mechanism is still largely unknown, it is commonly recognized that epilepsy is driven by generalized hyperexcitability and hypersynchronous neuronal activity [6].
How does the brain temporally and spatially keep the right synaptic rhythm across different brain regions and even among individual neurons to prevent hyperexcitability? It is established that epilepsy is caused by an imbalance between excitatory and inhibitory neurotransmission in the brain. However, recent studies with more advanced technologies tended to regard epilepsy as a sort of network disease not only at the macroscopic level but also at the microscopic level. A range from microscale (cellular signaling and communication) to macroscale (clinical symptoms, electroencephalography patterns, neuroimaging findings) associated with epilepsy has been reported in detail. These studies show that uncovering the exact epileptogenic mechanism at the microscale helps us explain macroscopic epilepsy expression [7]. However, the micro-macro disconnection develops when we focus on recognizing epilepsy at the macroscale and neglect its microscopic changes. As basic units of brain function, numerous neurons interact together to keep the normal rhythm via generating action potential (AP) and transferring electrical signals into chemical information [8]. Among the types of potassium channels expressed in the plasma membrane to regulate neurotransmitter release or control neuronal excitability, variants in sodium-activated potassium channels (KNa channels) have been reported on neurological disorders. In particular, the gain-of-function (GOF) variants in the Slack channel, which is a subtype of KNa channels, have been reported to be associated with several early onset epileptic encephalopathies: epilepsy of infancy with migrating focal seizures, EIMFS; autosomal dominant nocturnal frontal lobe epilepsy, ADNFLE; Ohtahara syndrome, OS; West syndrome; other early infantile epileptic encephalopathies, EOEE [9-15]. Paradoxically, it has been reported that KNa channels adjust neuronal adaptation as ‘brakes’ at the single cell level in response to repeated APs and regulate repolarization or afterhyperpolarization [16, 17]. Notably, this discrepancy of KNa channels timing the rhythm between individual neuron and neuronal populations may reflect the micro-macro disconnection in evaluating the epileptogenic network. Furthermore, microcircuits composed of neuronal clusters have different motifs which interact tightly and balance the dynamics of larger networks [18]. Do the distribution difference or other changes in KNa channels in types of neurons dispute the fine balance between these motifs and induce epileptic seizures from dysregulated microcircuits? Could seizures be terminated by normalizing the increased KNa currents (IKNa) pharmaceutically in Slack-associated epilepsy?
In this review, we aim to summarize studies with respect to the physiological role of KNa channels and their underlying pathophysiological effect in epileptogenic mechanisms at the macroscopic and microscopic levels to find the micro-macro disconnection. Particularly, we discussed evidence of functional and phenotypic alterations of KNa channels among different cell types within microcircuits at the microscopic level and deduced that dysfunction on the microscopic scale might be a critical source of differences in macroscopic epilepsy manifestation. Moreover, we reviewed current animal and human studies associated with variants in KNa channels and discussed the potential value of utilizing KNa channels as therapeutic targets for the discovery and development of novel ASMs.
2. PROPERTIES AND LOCALIZATIONS OF KNA CHANNELS
IKNa was first discovered in mammalian cardiac cells in 1984 [19] and then described to function in neurons in avian trigeminal ganglion in 1985 [20]. When the intracellular concentration of sodium ions ([Na+]i) increases as a result of repeated APs, potassium ions inflow via KNa channels to generate IKNa. Currently, KNa channels are known to be encoded by two genes belonging to the Slo family-Slo 2.2 and Slo 2.1, which are also termed as Slack (KCNT1) and Slick (KCNT2) channels, respectively [21-23]. With sequences like the other types of potassium channels, KNa channels are the fourfold symmetric tetramers composed of co-assembly of subunits with an S1-S4 domain and pore domain (S5-S6) within the membrane and two ‘regulator of K+ conductance’ (RCK) domains outside the membrane in the cytoplasmic side [23]. Both Slack and Slick channels contain a highly conserved PDZ-binding domain that grapples channels to specific locations. Although there is extensive homology in the transmembrane domains and the RCK domains of Slack and Slick channels, these two channels appear to have distinctly different molecular and electrical properties [22]. Here, we made a list to summarize those similarities and differences Table 1, [21-26].
Table 1.
Comparison of properties between Slack and Slick channels.
| Properties | Slack (KCNT1) | Slick (KCNT2) |
|---|---|---|
| Molecular weight | 138 kDa, with a larger distal C-terminal region. | 130 kDa, with a relatively smaller distal C-terminal region. |
| Unity conductance | ~180 pS | ~140 pS |
| Sub-conductance states | Multiple | Multiple |
| Voltage sensitivity | Activated slowly in depolarization without charged S4 membrane domain. | Activated rapidly in depolarization with charged S4 membrane domain. |
| EC50 values for [Na+]i | ~40 mM | ~89 mM |
| Na+ dependence | Highly dependent | Less dependent, Slick channels had a basal activity with no Na+. |
| [Cl-]i sensitivity | Less | More |
| Cytoplasmic ATP sensitivity | No ATP binding site. | ATP binding site in the second RCK region. |
| Isoforms | 5 | - |
Abbreviation: [Na+]i, intracellular concentration of sodium ions; [Cl-]i, intracellular concentration of chloride ions; RCK, regulator of conductance of K+.
Additionally, KNa channels were expressed in a vast array of neuronal cell types and in different locations, indicating a reasonably conservative evolutionary history across species [20, 24, 27-31]. The channels are highly expressed in neurons rather than glia cells [32]. Slick channels may act as an autonomous entity because of their heterogeneity of expression in several brain areas, including hippocampal CA1, CA2, and CA3 regions, the dentate gyrus, and cortical layer II, III, and V [27, 28]. It is known that pyramidal neurons are more or less homogeneous, and interneurons are diverse in molecular expression playing distinct roles in coordinating neuronal networks [33]. Recently, Shore et al. observed reduced excitability and AP firing rate only in inhibitory interneurons versus excitatory pyramidal neurons, especially in non-fast-spiking γ-aminobutyric acidergic (GABAergic) neurons, on an established GOF variant of Slack channels (Y796H) mouse model with strikingly similar epileptic manifestation to those patients with ADNFLE [17].
As for subcellular localization, Slick channels were mostly seen in processes, varicosities, and neuronal cell bodies, while Slack channels exhibited a diffuse immunostaining pattern with some labeling of cell stomata and processes [24]. Wu et al. assumed that Slack channels accounted for most of the IKNa in dorsal root ganglion (DRG) neurons, while some residual IKNa might be derived from Slick channels, which were located on more peripheral locations on the DRG neurons [16]. However, there is a dearth of research emphasizing the subcellular differences of Slack and Slick channels across different types of neurons? Given that the very high homology, the similar expression pattern between these two channels, and heteromultimeric channels, which consist of Slick channels and splicing of Slack channels, the function of KNa channels depends on their composition, distribution, cellular and subcellular localizations [34]. To determine the more specific antibodies targeting at different structural sites are needed. For example, to recognize different isoforms of Slack channels, a polyclonal antibody against the amino-terminus of Slack-B was applied to identify the Slack-B isoform, while polyclonal antibody against the carboxyl-terminus region of Slack channels was used to identify all Slack amino isoforms [26]. Moreover, more precise functional measurements are required to uncover the differences between KNa channels among multiple regions and even subcellular localizations. Performing patch clamp recordings on different subcellular sites such as the soma and the distal apical dendrites, Hu et al. determined that M-channel, which is another kind of K+ channel in the soma of CA1 pyramidal neurons, is mainly responsible for somatic excitability, whereas this channel seems to have little or no impact in the distal apical dendrites [35]. Nevertheless, whether or not these distribution changes caused functional differences between Slack and Slick channels in generating AP still remains a hot issue.
3. PHYSIOLOGICAL ROLE OF KNa CHANNELS IN NEURONS
KNa channels have been reported to play a “conducting” role in three main phases during AP generation at the single cell level. Firstly, IKNa, which is blocked by extracellular Li+, produces an approximately 30% increase in burst length and amplitude of after-hyperpolarization (AHP), especially in slow AHP (sAHP), following a single action potential [36, 37]; Secondly, KNa channels modify intrinsic neuronal excitability during and following depolarization and produce an adaptation of firing rate following repetitive firing [30, 38-41]; additionally, activation of KNa channels at subthreshold voltages can shape intrinsic bursting [16, 17, 42, 43]. Early single-channel investigations demonstrated that activation of the KNa channels required a higher [Na+]i considerably beyond physiological value. Budelli et al. showed that IKNa was strongly activated by persistent Na+ currents (INaP), which played a key role in epileptic seizures during long-lasting depolarizations and repetitive AP firing. This effect persisted even when neurons had been already loaded with high [Na+]i [44, 45]. Based on the physiological properties of KNa channels concluded above, preferential activation of IKNa by persistent INaP during seizures may be an eradicator in seizure termination [46]. However, the proposition of whether ‘activity-dependent’ or ‘pathology-dependent’ KNa channels participate in epileptogenesis is worth exploring and provides possible directions for the development of novel ASMs.
KNa channels have large cytoplasmic C-terminal domains containing 913 amino acids as well as the largest known potassium channel subunits. The transmembrane spanning domains of the Slack channels comprise only 18% of the entire protein [22]. This structural feature raises the possibility that KNa channels with the extended cytoplasmic domains have “non-conducting” function by interacting with cytoplasmic signaling pathways. Thus, its opening can influence cellular signaling beyond controlling channel gating alone [47]. Fragile X mental retardation protein (FMRP) which is deleted in patients with Fragile X syndrome (FXS), is mainly used to regulate the translation of many other neuronal mRNAs via interacting directly with its distal cytoplasmic C-terminal domain. It has been found that the activity of Slack channels is reduced due to the absence of FMRP in patients with FXS [48]. This is also confirmed by increased IKNa currents and hyperpolarized resting membrane potential as a result of introducing the N-terminal 1-298 fragment of FMRP into bag cell neurons of Aplysia [49].
4. CELLULAR BINDING PARTNERS OF KNa CHANNELS
As mentioned earlier, KNa channels could alter other biochemical interactions of the channel with its cytoplasmic partners to contribute to neuronal dysfunction [48, 50]. Numerous proteins have been discovered as Slack-interacting binding partners, including phosphatase and actin regulator 1 (Phactr1), postsynaptic density-95 protein (PSD-95), FMRP, cytoplasmic FMRP interacting protein (CYFIP1), and transmembrane protein 16C (TMEM16C) [48, 50-52]. Phactr1, an ancillary protein belonging to a phosphatase and actin regulator family, is highly expressed in neurons of the cerebral cortex, where it plays a role in controlling the synaptic activity and the synapse morphology through regulating protein phosphatase 1 (PP1) and actin-binding [53]. Phactr1 has been identified as a binding partner of the Slack channels by yeast two-hybrid assay [51]. Ali et al. provided direct evidence that Phactr1 reduced the maximal current amplitude of Slack channels by linking PP1 to the channel instead of actin-binding. However, Phactr1 suppressed Slack channels with a variant phosphorylation site (S407) of conserved protein kinase C (PKC), which could mimic GOF variants of Slack channels and rapidly increase IKNa [9, 54]. Loss of function (LOF) variant of phactr1, which is considered to be connected to the pathophysiology of West Syndrome, may induce morphological and functional deficiency of cortical neurons during development [55] and other neurodevelopmental disorders [56]. Recently, it has been reported that the expression of Phactr1 mRNA is absent after traumatic brain injury (TBI) [57]. Whether or not Slack channels are altered in patients with TBI remains unknown. PSD-95 is a crucial postsynaptic scaffolding protein in excitatory neurons which regulates the trafficking and localization of glutamate receptors in synaptic development and plasticity [58, 59]. It clearly showed KNa channels could directly interact with PSD-95 and co-localize with PSD-95 in cultured neocortical neurons [52]. TMEM16C, which is a member of the TMEM16 family and abundantly expressed in the central and peripheral nervous systems, regulates the processing of pain messages by altering Slack channels' single-channel activity and enhances sodium sensitivity in DRG neurons [50]. Future studies are inspired to take further steps in detecting more sites associated with KNa channels.
5. PATHOLOGICAL ROLE OF KNa CHANNELS IN EPILEPSY NETWORK
Generally, it is considered that variants of K+ channels resulting in reduced K+ currents and rapid repolarization are associated with abnormally increased neuronal excitability [60]. It is reasonable to speculate that dysregulated firing rates contribute to epileptogenesis in patients with LOF variants of KNa channels [61]. However, a major and well-recognized clinical problem is that most epilepsy patients with Slack or Slick variants are reported to carry GOF variants [9, 10, 12, 14, 62]. Although the precise mechanism underlying the GOF variants increasing IKNa remains obscure, Kim et al. suggested that the GOF variants of Slack channels increased gate opening and mediating current by interacting with nearby channels even in a variant with a decreased channel conductance [31]. In another study, researchers found that over-activity of the GOF variants of the Slack channels was caused by two diverse mechanisms: amplified sodium sensitivity and increased maximum probability of opening [63].
How do GOF variants of KNa channels lead to epileptic seizures? Below we discuss some hypotheses which have been proposed previously: first, accelerating AP repolarization due to increased K+ current and increased fast AHP, as well as attenuating Na+ channel inactivation in excitatory neurons contribute to a higher AP firing frequency [40, 64]. A large delayed AHP produced by IKNa after a series of APs can also synchronize excitatory networks [30, 65]. Second, reduced membrane excitability and induced AP firing adaptation selectively in inhibitory interneurons result in disinhibition and overall network excitability [66, 67]. Those two hypotheses can be summarized as the imbalance of excitation and inhibition (E/I). Some studies have found differences in types of neurons with increased IKNa using Slack GOF variant mouse models. For instance, Quraishi et al. illustrated the peak of AP firing rate increased following a decrease in AP width and AHP with increased IKNa, in what were presumably immature glutamatergic neurons only at voltages above +40 mV on a P942L variant mouse model [68]. In another study, reduced excitability and increased subthreshold IKNa was selectively found in inhibitory interneurons, especially non-fast-spiking GABAergic neuron on a Y777H variant mouse model [17]. Consistent with previous studies, GOF variants of Slack channels had a stronger inhibitory effect on GABAergic interneurons than glutamatergic pyramidal neurons using biomathematical models. Increased E/I ratio in a cortical micro-network triggered a seizure and depolarizing GABA was required to migrate seizures on the macroscopic scale [69]. Thirdly, given that KNa channels are abundantly expressed in embryonic hippocampal and cortical neurons, changes in AP firing during neuronal development can produce alterations in synaptic connectivity, thus leading to the local epileptic foci and hyperexcitable network [9, 70]. Y777H variant mice have increased homotypic synaptic connectivity and hyperexcited network [17]. Finally, given that “non-conducting” function of KNa channels, variants in KNa channels could alter other biochemical interactions of the channel with its cytoplasmic partners. For instance, in addition to directly altering the excitability of neurons, variants in KNa channels could disrupt intracellular signals related to FMRP and activity-dependent protein translation [48, 50].
6. POSSIBLE SOURCES OF DYSREGULATION WITHIN MICROCIRCUITS RELATED TO CHANGES IN KNa CHANNELS
Recently, the time-honored opinion no longer sufficiently holds up that epilepsy simply results from an E/I imbalance, given that epilepsy might be a network disease that can result from imbalances among different microcircuit motifs composed of different types of neurons that are embedded in the larger network [18]. It is well-known that there are prominent differences between interneurons and pyramidal neurons. With local morphological features, interneurons coordinate local circuits within functionally and anatomically confined subregions. By contrast, pyramidal neurons are responsible for transferring signals on greater spatial scales [33]. According to the different arrangements of these neurons, there are four microcircuit motifs: 1) Feed-forward inhibition which is composed of excitatory inputs, recruits local inhibitory networks and tunes the efferent signal; 2) Locally stimulated inhibitory neurons to influence recurrent excitatory activity via feed-back inhibition; 3) Counter-inhibition is shown as local connections between inhibitory neurons; 4) Local recurrent excitatory circuits are a prevalent pattern in cortical networks, where around 80% of neurons and synapses are excitatory [18]. Spike frequency adaptation with a slow time course could be induced within fast-spiking (FS) neurons following approximately 20s current injection, which is coincided with the formation of the sAHP following prolonged stimulation and hypothesized to be caused by KNa channel activation [67]. Consistent with FS neurons, an sAHP of 12-75s following long (20-60s) discharges in non-fast-spiking GABAergic neurons also induces a long-lasting decrease of excitability, which is mainly due to the activation of KNa channels [71]. To conclude, the sustained activation of KNa channels depresses interneuron activity, leading to disinhibition and reduced feed-forward inhibition of cortical pyramidal neurons. The physiologic role of KNa channels suggests that the involvement of those channels in distinct disorders may cause a spectrum of focal epilepsies through triggering critical circuit junctures or choke points and contributing to development of epilepsy [61, 72]. As mentioned above, increased homotypic synaptic connectivity in patients with Slack-associated epilepsy, subsequently participate in enhanced counter-inhibition inducing disinhibition or altering oscillatory coupling, or intensive connections of local recurrent excitatory circuits. It not only causes hyperexcitation in local microcircuits, but also activates neuronal activity in the distal regions [17].The hypothesis that epileptic seizures begin with the malfunction of certain microcircuits and develop to the activation of the whole seizure network may help explain why those patients with Slack-associated epilepsy frequently have focal or multifocal macroscopic epileptiform origins. However, the discrepancies between excitatory and inhibitory neurons in the KNa channels should be noted.
Selective expression might not be the major cause of the differential effects on neuronal populations due to the widespread expression of KNa channels in the central nervous system (CNS) [24, 28]. Previous studies have underlined the diversity in the properties of neuronal KNa channels. Based on observing at least five different alternative splicing of Slack channels [26], Chen et al. found that Slick and Slack-B subunits could assemble to form heteromeric channels that differed in biophysical properties of the homomers on many central neurons [34]. Therefore, candidate mechanisms involving glutamatergic and GABAergic neurons specifically express unique splice forms of Slack channels that interact with channels/proteins, which attributes to the differences of KNa channels in neurons.
7. ESTABLISHING MODELS TO BRIDGE THE CLINICAL PHENOTYPES WITH BASIC RESEARCHES
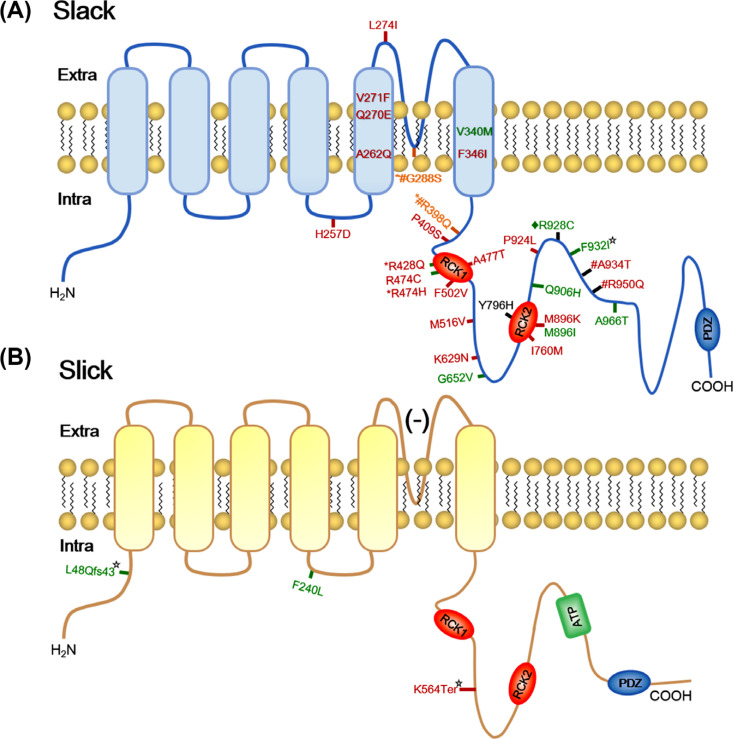
Genetic models, including cellular and animal models, permit researchers to span the gap between micro- and macro- networks, although they lack cell-type-targeted control. Twelve studies have identified 22 Slack pathogenic variants, and 2 studies have identified 3 Slick pathogenic variants in patients with EIMFS, ADNFLE, OS, leukoencephalopathy, and other severe epilepsy (Fig. 1 and Table 2) [9-11, 14, 31, 61-63, 68, 73-77].
Fig. (1).
Human variants in KNa channels subunits topography. (A) Schematic diagram of Slack channels subunits topography. (B) Schematic diagram of Slick channels subunits topography. Red sites represent variants which are found in patients with EIMFS. Black sites represent variants which are found in patients with ANDFLE or NFLE. Green sites represent mutation sites that are found in patients with other severe epilepsy, such as OS, EOEE, West syndrome, leuko-encephalopathy et al. Orange sites represent mutation sites that are found in three phenotypes. *sites represent mutation are showed in EIMFS and other severe epilepsy patients. # sites represent variants are showed in EIMFS and ADNFLE patients. ¨represent variants are showed in ADNFLE and other severe epilepsy patients. ☆ sites represent variants with loss of function.
Table 2.
Variants in KNa channels associated with human epileptic phenotypes.
| Epileptic phenotypes | Homo Variants |
Rat
Variants |
Models | Results |
|---|---|---|---|---|
| Slack/GOF | ||||
| EIMFS [9] |
☆p.Arg428Gln p.Arg474His p.Ile760Me ☆p.Ala934Thr |
★p.Arg409Gln ★p.Ala913Thr |
Transfected Xenopus oocytes | They produced two to threefold greater than IKNa in wild-type, similar with phosphorylated at Ser407. |
| EIMFS [11] |
★p.Gly288Ser ★p.Met516Val |
- | Transfected CHO cells | Variants carried larger currents and showed higher sensitivity to blockade compared to wild-type. |
| EIMFS [31] |
☆p.Gly288Ser ☆p.Arg398Gln ☆p.Arg428Gln ☆p.Arg474His ☆p.Ala934Thr |
★p.Gly269Ser ★p.Arg379Gln ★p.Arg409Gln ★p.Arg455His ★p. Ala913Thr |
Transfected Xenopus oocytes | All produced increases in current amplitude between 3-22 folds relative to the wild-type channel. |
| EIMFS [62] |
★p.Arg428Gln ★p.Ala934Thr ★p.Pro924Leu |
- | Transfected Xenopus oocytes | All Slack variants caused a marked increase in function. |
| EIMFS [63] |
☆p.Val271Phe ☆p.Gly288Ser ☆p.Arg428Gln ☆p.Arg474His ☆p.Ile760Met ☆p.Ala934Thr |
★p.Val252Phe ★p.Gly269Ser ★p.Arg409Gln ★p.Arg455His ★Ile739Met ★p.Ala913Thr |
Transfected Xenopus oocytes | All variants caused increases in function due to enhanced sodium sensitivity. |
| EIMFS [68] | ★p.Pro924Leu | - | iPSC-derived neurons | The variant caused an increased KNa current by shortening the duration of AP and increasing the amplitude of AHP. |
| EIMFS [73] |
★p.Tyr796His ★p.Arg428Gln ★p.Lys629Asn |
- | Transfected Xenopus oocytes | The variants caused the increased KNa current, which could be reduced by quinidine. |
| EIMFS [75] | ☆p.Arg474His | ★p.Arg455His | Transgeneic KCNT1+/R455H, KCNT1R455H/R455H mice | KCNT1+/R455H mice showed persistent IEDs, spontaneous seizures, and a substantially decreased threshold for PTZ-induced seizures. |
| EIMFS [76] |
★p.Leu274Ile ★p.Val271Phe ★p.Phe346Leu ★p.Phe502Val ★p.Met896Lys p.Ala934Thr |
- | Transfected Xenopus oocytes; Homology model (★p.Phe346Leu; ★p.Phe502Val) |
All variants resulted in significantly increased channel amplitude and variable blockade by quinidine. |
| EIMFS [77] | ☆p.Gly288Ser | - | - | Computational analysis suggested possible changes in the molecular structure and ion channel property due to trapping potassium ions. |
| ADNFLE [10] |
☆p.Arg398Gln ☆p.Tyr796His ☆p.Met896Ile ☆p.Arg928Cys |
- | - | Whole-exome sequencing firstly identified four variants of Slack channels in ADNFLE. |
| ADNFLE [31] |
☆p.Gly288Ser ☆p.Arg398Gln ☆p.Tyr796His ☆p.Met896Ile ☆p.Arg928Cys |
★p.Gly269Ser ★p.Arg379Gln ★p.Tyr775His ★p.Met875Ile ★p.Arg907Cys |
Transfected Xenopus oocytes | All produced ranged from 1.5-11 folds increases in current amplitude compared to the wild-type channel. |
| ADNFLE [62] |
★p.Arg398Gln ★p.Tyr796His ★p.Met896Ile ★p.Arg928Cys |
- | Transfected Xenopus oocytes | All variants caused increases in function, which are much smaller compared with that in EIMFS. |
| ADNFLE [63] |
☆p.Arg398Gln ☆p.Tyr796His ☆p.Met896Ile ☆p.Arg928Cys |
★p.Arg379Gln ★p.Tyr775His ★p.Met875Ile ★p.Arg907Cys |
Transfected Xenopus oocytes | All variants caused an increase in function due to enhanced sodium sensitivity or increased Maximal Po. |
| Childhood idiopathic epilepsy [63] | ☆p.Ala945Thr | ★p.Ala924Thr | ||
| Leukoencephalopathy [63] | ☆p.Phe932Ile | ★p.Phe911Ile | ||
| ADNFLE [17] | ☆p.Tyr796His | ★p.Tyr777His | Transgeneic KCNT1Y777H/Y777H mice | KCNT1Y777H/Y777H mice caused epileptic and behavioral phenotypes similar to those of patients. |
| West syndrome [15] | ☆p.Gly652Val | Quinidine decreased epileptic spasms and decreased epileptiform paroxysmal activity in patients. | ||
| OS [31] | ☆p.Ala966Thr | ★p.Ala945Thr | Transfected Xenopus oocytes | It produced 13 folds increase in current amplitude. |
| Slack/LOF | ||||
| EOEE [74] | ☆p.Phe932Ile | ★p.Phe911Ile | Transfected CHO cells | The variants produced a LOF phenotype that was insensitive to the established opener. |
| Slick/GOF | ||||
| EOEE [14] | ★pPhe240Leu | - | Transfected CHO cells, oocytes, and rat DRG neurons | Reversed [Cl-]i sensitivity and loss of exclusive selectivity to K+ and a larger inward conductance caused increased ISlick at rest state. |
| Slick/LOF | ||||
| EIMFS [61] | ★pLys564Ter | - | Transfected CHO cells |
It decreased the global current density of heteromeric channels by ∼25%. |
| EOEE [61] | ★p.Leu48Qfs43 | - | Transfected CHO cells |
It decreased the global current density of heteromeric channels by ∼55%. |
Abbreviation: ADNFLE: Autosomal dominant nocturnal frontal lobe epilepsy; AHP: afterhyperpolarization; AP: action potential; EIMFS: Epilepsy of infancy with migrating focal seizures; EOEE: early onset epileptic encephalopathy; GOF: gain of function; IED: interictal discharge; LOF: loss of function; OS: Ohtahara syndrome; Po: open probability; PTZ: pilocarpine. ★ indicated mutant sites which have done functional tests; ☆indicated corresponding heterologous homo mutant sites but without functional tests.
Among those variants, several sites have been transferred on cellular or animal models to explore their role in regulating IKNa, including the details regarding how the variants cause epilepsy at the molecular, (sub)cellular, and network scales. Those variant sites are mainly located on the pore region between S5 and S6, and the C-terminus contains two RCK domains or binding sites of the nicotinamide adenine (NAD+). It is obvious that Slack and Slick channels associated variants were highly pleiotropic among multiple epileptic diseases. Interestingly, genotype-phenotype relationships of Slack variants are not represented as one-to-one correspondence. For example, although ADNFLE and EIMFS are strikingly different epilepsy syndromes, it has been reported that p.Arg398Gln and p.Gly288Ser are associated with these two distinct phenotypes, even within the same family [31, 72, 77]. Furthermore, whether or not there are some relations between variants and clinical characters of patients with different epileptic phenotypes are unknown. On the one hand, facing EIMFS beginning in earlier infancy is more detrimental when compared with ADNFLE beginning in mid-childhood. Milligan et al. deduced that this difference might be caused by a 5-fold increase in IKNa produced by EIMFS variants, whereas a 3-fold change was observed with ADNFLE variants. The larger increase in EIMFS might enhance network excitability in the immature infant brain, thus resulting in ongoing multifocal seizures. In contrast, the smaller increase in ADNFLE may not be adequate to induce seizures until a later stage of development, even potentially limiting pathology of the frontal lobe [62]. However, this viewpoint was challenged by Kim et al. in their study, contrary to the clinical severity, there was a 3-fold increase in Slack channels’ activity produced by p. Arg409Gln in EIMFS while an 11-fold increase produced by p.Tyr775His in ADNFLE. It showed that the magnitude of the increase in Slack variants in an expression system could not easily be associated with the clinical manifestations [31].
On the other hand, it is possible to connect the location of the variant sites with clinical symptoms. ADNFLE is characterized by clusters of focal motor seizures arising from sleep without intellectual disability frequently [78]. However, it has been reported that ADNFLE associated with GOF variants of Slack channels often display a severe delay in cognitive development [10]. There are some interesting connections between clinical features and basic research. Firstly, consistent with recurrent frontal lobe seizures, higher expression of Slack channels is located in the frontal cortex [27]. Meanwhile, it has been reported that NAD+ and nicotinamide adenine dinucleotide phosphate (NADP) can activate Slack channels expressed in HEK293 cells [79]. The level of NAD+ regulated by the core clock machinery becoming higher in darkness most likely accounts for ADNFLE patients with GOF variants of Slack channels, including M896I, Y796H, and R928C, which are adjacent to or within a putative NAD+-binding site, ongoing nocturnal seizures [62, 80]. Secondly, FXS is an inherited neurological disease featuring salient intellectual disability and autism spectrum disorder (ASD) due to the absence of FMRP, which is known to interact with target mRNAs and other binding partners in nuclear and cytosolic processing [81]. Brown et al. found that FMRP activated the Slack channels by binding to and interacting with its C-terminal domain [48]. In subsequent studies, Slack channels might serve as a developmental modulator of cell plasticity underlying normal cognitive development without affecting the autistic phenotype [82, 83]. Considering that the co-diagnosis rate between FXS and epilepsy patients is up to 18%-25%, it is worth exploring whether mutated Slack channels still interact with FMRP to contribute to normal cognitive development [84, 85]. Furthermore, it is reasonable to deduce that multiple epileptic phenotypes with variant sites located on the C-terminal domain of Slack channels tend to present with intellectual disability. This may inform clinicians to make notes of Slack-associated variants, especially those sites within the C-terminal domain of Slack channels when treating patients with epilepsy associated with intellectual disability.
8. NOVEL APPROACHES FOR DISRUPTING EPILEPTOGENIC NETWORK
Although existing genetic models provide more details about changes at the single cell level to explain macroscopic epileptic expressions, they are limited by the lack of cell-type-targeted control, synchronized observation on several scales with varying spatio-temporal resolution, and optimal models simulating human disease to evaluate how Slack and Slick channels associated changes cause subsequent seizures.
Optogenetics may help utilize cell-type-targeted control by using light to mediate the genetically defined cell populations with spatio-temporal precision [86-88] and become a prospective tool to control seizures [89, 90]. Additionally, with more or less differences from human patients, established genetic animal models within Slack- associated variants suggest analogous underlying processes [17, 75]. Using a multiplatform approach, it is possible to bridge phenotyping of the Slack-associated variant mouse model and deeper cellular mechanism on a discrete time and place. As mentioned above, one of the hypotheses regarding how increasing IKNa causes the excitability of neuronal networks is focused on the imbalanced expression of KNa channels between excitatory and inhibitory neurons. In the future, combining optogenetics and genetic animal models could determine which type of neurons fails in tuning the regular rhythm on Slack-associated variant mouse model.
In addition, it can also help to detect the specific cell type by identifying the cellular sources of neuroimaging signals, which requires high-density microelectrode arrays that offer a way to track the activity of neuron populations and identify individual neurons according to their unique character of discharge [17, 91]. Unfortunately, it is difficult to separate overlapping spike waveforms during seizures for microelectrode recordings [92]. Combining genetic tools with optical methods make it more feasible to observe specific neuron population activity at larger scales with varying spatio-temporal resolution than separate applications [93]. However, the technologies mentioned above are still limited in transferring microscopic observation to corresponding macroscopic EEG patterns. New techniques and a more comprehensive understanding of seizure dynamics are in demand for basic epilepsy research. In silico computational model enables researchers to simulate the immature brain, reproduce the interictal EEG pattern of patients, and connect the microscopic scale with macroscopic scale to observe how changes at the microcircuit level influence and contribute to macroscopic seizure properties [94]. According to frequency-intensity curves in each neuronal type, Kuchenbuch et al. constructed a computational model by modifying the wave-to-pulse functions of the various sub-populations in patients using GOF variants of Slack channels [69]. To establish a more precise computational model, more electrophysiological properties of KNa channels based on various neuron types are in urgent need to simulate the complexities of the human brain.
9. IMPLYING VALUE OF KNa CHANNELS FOR ASMS DISCOVERY
Slack and Slick-associated epilepsies often manifest as multidrug-resistant genetic neurological diseases with psychiatric, behavioral, and cognitive disabilities, such as EIMFS [95, 96]. Recent studies found that there were more patients with GOF variants of Slack channels in clinical practice, especially in patients with EIMFS, suggesting that Slack channels might be promising drug targets for Slack-positive patients [9, 10]. Quinidine, a known antiarrhythmic drug, has been reported to be able to normalize the increased KNa currents by nonspecifically inhibiting K+ current on the rodent or human Slack variant heterologous systems in previous studies [62, 97]. Below are several clinical studies that investigated the efficacy of quinidine on Slack-associated epilepsies (see in [98]). Based on the published inconsistent results, the causes of differences in the efficacy of quinidine among Slack-associated epilepsies remain unknown. According to the review by Wang et al., compared to patients with ADNFLE and other epileptic syndromes, quinidine therapy tended to be more effective in patients with EIMFS and West syndrome carrying the variants of Slack channels. It may suggest that the efficacy of quinidine was dependent on the epilepsy phenotype [98]. In addition, based on the patients recruited in their center, Mikati et al. observed that 4/4 patients <4 years of age showed quinidine responsiveness, while 0/4 patients >4 years showed no response to quinidine. This suggested that age may be a factor since quinidine may have a greater permeability across the blood brain barrier (BBB) in younger patients. This potentially affected the response to quinidine therapy [73, 99]. Other considerations including cerebral drug levels, dose-dependent prolonged QT interval (QTc), injury age of onset, and past neuronal injury may affect the response to quinidine. The efficacy of quinidine might also be caused by the frequency of seizures from the early neonatal phase, which may have already resulted in persistent brain injury prior to the administration of quinidine [100]. A recent study showed that GOF variants of Slack channels in animal models might cause changes in neuron-subtype-specific membrane currents and synaptic connections [17]. Early therapy with quinidine will be recommended in order to facilitate the repairment of ion channel currents since these deficits are more difficult to reverse later [101]. Due to limited cases performed previously, randomized controlled trials (RCT) are needed to identify the relationship between the multi-factors and the efficacy of quinidine therapy.
However, quinidine is limited by its poor specificity, poor BBB penetration, and interaction with other ASMs. Other antiarrhythmic drugs such as bepridil work as a more potent blocker of Slack channels with an IC50 of 1.0 μM, whereas quinidine inhibits currents with an IC50 of 89.6 μM in HEK cells [97]. Clofilium, which is also an antiarrhythmic drug, crosses the BBB with its large hydrophobic alkyl side chains, as well as inhibits KNa channels expressed in Xenopus oocytes [102]. Nevertheless, these three drugs mentioned above are presented with low-specific selectivity in KNa channels inhibition. Cole et al. summarized new inhibitors of Slack channels according to high-resolution cryogenic electron microscopy (cryo-EM) structure-based virtual screening [103]. Two out of 100 000 compounds-BC13 and BC14, were identified to show limited cytotoxicity and specificity in preliminary toxicity screens [104]. In a recent study, using a high-throughput thallium flux assay, Spitznagel et al. found low micromolar VU0606170 reduced excitability and spontaneously firing rate of cortical neurons by raising inhibitory tone in the brain [105]. Similarly, Griffin et al. found that Compound 31 reduced both seizure frequency and interictal spikes in a P924L variant mouse model [106]. However, it requires future clinical studies to explore the effects of these drugs.
CONCLUSION
In this paper, we reviewed the molecular and cellular properties of two KNa channels, Slack and Slick, which are widely expressed in CNS and uniquely expressed in neurons. At the single cell level, they modulate neuronal firing rates and tune neuronal rhythm under physiological state. However, more studies reported that patients with ADNFLE and EIMFS tend to carry GOF variants of Slack channels needed. This emphasized its double-edged role and inconsistency in tuning the individual neuron and populations at microscopic and macroscopic scales. Furthermore, it suggested that variants in Slack channels may produce profound effects on neuronal function through cytoplasmic biochemical pathways, circuits, synaptic connections, and development patterns instead of simply setting the level of neuronal excitability. Here we summarized the proposed hypotheses regarding how GOF variants of KNa channels lead to epileptic seizures from varying scales, including a single cell as well as a microcircuit level, even a larger macroscopic scale. To resolve this micro-macro disconnection, advanced techniques, such as combining genetics and other neuroimaging tools for basic epilepsy research, are in need. Finally, due to the severity of Slack and Slick-associated epilepsies, we also explored whether quinidine could be a promising drug for Slack-positive epilepsies. Future studies should take greater insights into the underlying mechanisms of these variants and provide us with validated targets for developing novel ASMs.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by grants from the Natural Science Foundation of Beijing (7214224, MJ) and the National Natural Science Foundation of China (81870935, JW).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Thijs R.D., Surges R., O’Brien T.J., Sander J.W. Epilepsy in adults. Lancet. 2019;393(10172):689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 2.Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., Engel J., Jr, Forsgren L., French J.A., Glynn M., Hesdorffer D.C., Lee B.I., Mathern G.W., Moshé S.L., Perucca E., Scheffer I.E., Tomson T., Watanabe M., Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 3.Wallace H., Shorvon S., Tallis R. Age-specific incidence and prevalence rates of treated epilepsy in an unselected population of 2,052,922 and age-specific fertility rates of women with epilepsy. Lancet. 1998;352(9145):1970–1973. doi: 10.1016/S0140-6736(98)04512-7. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P., Arzimanoglou A., Berg A.T., Brodie M.J., Allen Hauser W., Mathern G., Moshe S.L., Perucca E., Wiebe S., French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 5.Jette N., Reid A.Y., Wiebe S. Surgical management of epilepsy. CMAJ. 2014;186(13):997–1004. doi: 10.1503/cmaj.121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boison D., Steinhäuser C. Epilepsy and astrocyte energy metabolism. Glia. 2018;66(6):1235–1243. doi: 10.1002/glia.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell J.S., Nguyen Q.A., Soltesz I. Resolving the micro-macro disconnect to address core features of seizure networks. Neuron. 2019;101(6):1016–1028. doi: 10.1016/j.neuron.2019.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart G., Spruston N., Sakmann B., Häusser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20(3):125–131. doi: 10.1016/S0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- 9.Barcia G., Fleming M.R., Deligniere A., Gazula V.R., Brown M.R., Langouet M., Chen H., Kronengold J., Abhyankar A., Cilio R., Nitschke P., Kaminska A., Boddaert N., Casanova J.L., Desguerre I., Munnich A., Dulac O., Kaczmarek L.K., Colleaux L., Nabbout R. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat. Genet. 2012;44(11):1255–1259. doi: 10.1038/ng.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heron S.E., Smith K.R., Bahlo M., Nobili L., Kahana E., Licchetta L., Oliver K.L., Mazarib A., Afawi Z., Korczyn A., Plazzi G., Petrou S., Berkovic S.F., Scheffer I.E., Dibbens L.M. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 2012;44(11):1188–1190. doi: 10.1038/ng.2440. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo F., Ambrosino P., Guacci A., Chetta M., Marchese G., Rocco T., Soldovieri M.V., Manocchio L., Mosca I., Casara G., Vecchi M., Taglialatela M., Coppola G., Weisz A. Characterization of two de novoKCNT1 mutations in children with malignant migrating partial seizures in infancy. Mol. Cell. Neurosci. 2016;72:54–63. doi: 10.1016/j.mcn.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosino P., Soldovieri M.V., Bast T., Turnpenny P.D., Uhrig S., Biskup S., Döcker M., Fleck T., Mosca I., Manocchio L., Iraci N., Taglialatela M., Lemke J.R. De novo gain-of-function variants in KCNT2 as a novel cause of developmental and epileptic encephalopathy. Ann. Neurol. 2018;83(6):1198–1204. doi: 10.1002/ana.25248. [DOI] [PubMed] [Google Scholar]
- 13.Ohba C., Kato M., Takahashi N., Osaka H., Shiihara T., Tohyama J., Nabatame S., Azuma J., Fujii Y., Hara M., Tsurusawa R., Inoue T., Ogata R., Watanabe Y., Togashi N., Kodera H., Nakashima M., Tsurusaki Y., Miyake N., Tanaka F., Saitsu H., Matsumoto N. De novo KCNT1 mutations in early-onset epileptic encephalopathy. Epilepsia. 2015;56(9):e121–e128. doi: 10.1111/epi.13072. [DOI] [PubMed] [Google Scholar]
- 14.Gururaj S., Palmer E.E., Sheehan G.D., Kandula T., Macintosh R., Ying K., Morris P., Tao J., Dias K.R., Zhu Y., Dinger M.E., Cowley M.J., Kirk E.P., Roscioli T., Sachdev R., Duffey M.E., Bye A., Bhattacharjee A. A de novo mutation in the sodium-activated potassium channel KCNT2 alters ion selectivity and causes epileptic encephalopathy. Cell Rep. 2017;21(4):926–933. doi: 10.1016/j.celrep.2017.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuoka M., Kuki I., Kawawaki H., Okazaki S., Kim K., Hattori Y., Tsuji H., Nukui M., Inoue T., Yoshida Y., Uda T., Kimura S., Mogami Y., Suzuki Y., Okamoto N., Saitsu H., Matsumoto N. Quinidine therapy for West syndrome with KCNTI mutation: A case report. Brain Dev. 2017;39(1):80–83. doi: 10.1016/j.braindev.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Espinosa P.L., Wu J., Yang C., Gonzalez-Perez V., Zhou H., Liang H., Xia X.M., Lingle C.J. Knockout of Slo2.2 enhances itch, abolishes KNa current, and increases action potential firing frequency in DRG neurons. eLife. 2015;4:e10013. doi: 10.7554/eLife.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shore A.N., Colombo S., Tobin W.F., Petri S., Cullen E.R., Dominguez S., Bostick C.D., Beaumont M.A., Williams D., Khodagholy D., Yang M., Lutz C.M., Peng Y., Gelinas J.N., Goldstein D.B., Boland M.J., Frankel W.N., Weston M.C. Reduced GABAergic neuron excitability, altered synaptic connectivity, and seizures in a KCNT1 gain-of-function mouse model of childhood epilepsy. Cell Rep. 2020;33(4):108303. doi: 10.1016/j.celrep.2020.108303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paz J.T., Huguenard J.R. Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat. Neurosci. 2015;18(3):351–359. doi: 10.1038/nn.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- 20.Bader C.R., Bernheim L., Bertrand D. Sodium-activated potassium current in cultured avian neurones. Nature. 1985;317(6037):540–542. doi: 10.1038/317540a0. [DOI] [PubMed] [Google Scholar]
- 21.Yuan A., Santi C.M., Wei A., Wang Z.W., Pollak K., Nonet M., Kaczmarek L., Crowder C.M., Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron. 2003;37(5):765–773. doi: 10.1016/S0896-6273(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharjee A., Joiner W.J., Wu M., Yang Y., Sigworth F.J., Kaczmarek L.K. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J. Neurosci. 2003;23(37):11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y., Pico A., Cadene M., Chait B.T., MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29(3):593–601. doi: 10.1016/S0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 24.Rizzi S., Knaus H.G., Schwarzer C. Differential distribution of the sodium-activated potassium channels slick and slack in mouse brain. J. Comp. Neurol. 2016;524(10):2093–2116. doi: 10.1002/cne.23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hite R.K., MacKinnon R. Structural titration of Slo2.2, a Na+-dependent K+ channel. Cell. 2017;168(3):390–399.e11. doi: 10.1016/j.cell.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown M.R., Kronengold J., Gazula V.R., Spilianakis C.G., Flavell R.A., von Hehn C.A., Bhattacharjee A., Kaczmarek L.K. Amino-termini isoforms of the Slack K+ channel, regulated by alternative promoters, differentially modulate rhythmic firing and adaptation. J. Physiol. 2008;586(21):5161–5179. doi: 10.1113/jphysiol.2008.160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharjee A., Gan L., Kaczmarek L.K. Localization of the Slack potassium channel in the rat central nervous system. J. Comp. Neurol. 2002;454(3):241–254. doi: 10.1002/cne.10439. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharjee A., von Hehn C.A., Mei X., Kaczmarek L.K. Localization of the Na+-activated K+ channel Slick in the rat central nervous system. J. Comp. Neurol. 2005;484(1):80–92. doi: 10.1002/cne.20462. [DOI] [PubMed] [Google Scholar]
- 29.Evely K.M., Pryce K.D., Bausch A.E., Lukowski R., Ruth P., Haj-Dahmane S., Bhattacharjee A. Slack KNa channels influence dorsal horn synapses and nociceptive behavior. Mol. Pain. 2017;13:1744806917714342. doi: 10.1177/1744806917714342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwindt P.C., Spain W.J., Crill W.E. Long-lasting reduction of excitability by a sodium-dependent potassium current in cat neocortical neurons. J. Neurophysiol. 1989;61(2):233–244. doi: 10.1152/jn.1989.61.2.233. [DOI] [PubMed] [Google Scholar]
- 31.Kim G.E., Kronengold J., Barcia G., Quraishi I.H., Martin H.C., Blair E., Taylor J.C., Dulac O., Colleaux L., Nabbout R., Kaczmarek L.K. Human slack potassium channel mutations increase positive cooperativity between individual channels. Cell Rep. 2014;9(5):1661–1672. doi: 10.1016/j.celrep.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joiner W.J., Tang M.D., Wang L.Y., Dworetzky S.I., Boissard C.G., Gan L., Joiner W.J., Tang M.D., Wang L.Y., Dworetzky S.I., Boissard C.G., Gan L., Gribkoff V.K., Kaczmarek L.K. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nat. Neurosci. 1998;1(6):462–469. doi: 10.1038/2176. [DOI] [PubMed] [Google Scholar]
- 33.Xu N.L. Deciphering pyramidal neuron diversity: delineating perceptual functions of projection-defined neuronal types. Neuron. 2020;105(2):209–211. doi: 10.1016/j.neuron.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Chen H., Kronengold J., Yan Y., Gazula V.R., Brown M.R., Ma L., Ferreira G., Yang Y., Bhattacharjee A., Sigworth F.J., Salkoff L., Kaczmarek L.K. The N-terminal domain of Slack determines the formation and trafficking of Slick/Slack heteromeric sodium-activated potassium channels. J. Neurosci. 2009;29(17):5654–5665. doi: 10.1523/JNEUROSCI.5978-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu H., Vervaeke K., Storm J.F. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J. Neurosci. 2007;27(8):1853–1867. doi: 10.1523/JNEUROSCI.4463-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franceschetti S., Lavazza T., Curia G., Aracri P., Panzica F., Sancini G., Avanzini G., Magistretti J. Na+-activated K+ current contributes to postexcitatory hyperpolarization in neocortical intrinsically bursting neurons. J. Neurophysiol. 2003;89(4):2101–2111. doi: 10.1152/jn.00695.2002. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Stan Leung L. Sodium-activated potassium conductance participates in the depolarizing afterpotential following a single action potential in rat hippocampal CA1 pyramidal cells. Brain Res. 2004;1023(2):185–192. doi: 10.1016/j.brainres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Wallén P., Robertson B., Cangiano L., Löw P., Bhattacharjee A., Kaczmarek L.K., Grillner S. Sodium-dependent potassium channels of a Slack-like subtype contribute to the slow afterhyperpolarization in lamprey spinal neurons. J. Physiol. 2007;585(Pt 1):75–90. doi: 10.1113/jphysiol.2007.138156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim U., McCormick D.A. Functional and ionic properties of a slow afterhyperpolarization in ferret perigeniculate neurons in vitro. J. Neurophysiol. 1998;80(3):1222–1235. doi: 10.1152/jn.1998.80.3.1222. [DOI] [PubMed] [Google Scholar]
- 40.Yang B., Desai R., Kaczmarek L.K. Slack and Slick K(Na) channels regulate the accuracy of timing of auditory neurons. J. Neurosci. 2007;27(10):2617–2627. doi: 10.1523/JNEUROSCI.5308-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaczmarek L.K. Slack, slick and sodium-activated potassium channels. ISRN Neurosci., 2013, 2013(2013), 2013. [DOI] [PMC free article] [PubMed]
- 42.Reijntjes D.O.J., Lee J.H., Park S., Schubert N.M.A., van Tuinen M., Vijayakumar S., Jones T.A., Jones S.M., Gratton M.A., Xia X.M., Yamoah E.N., Pyott S.J. Sodium-activated potassium channels shape peripheral auditory function and activity of the primary auditory neurons in mice. Sci. Rep. 2019;9(1):2573. doi: 10.1038/s41598-019-39119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hage T.A., Salkoff L. Sodium-activated potassium channels are functionally coupled to persistent sodium currents. J. Neurosci. 2012;32(8):2714–2721. doi: 10.1523/JNEUROSCI.5088-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budelli G., Hage T.A., Wei A., Rojas P., Jong Y.J., O’Malley K., Salkoff L. Na+-activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat. Neurosci. 2009;12(6):745–750. doi: 10.1038/nn.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stafstrom C.E. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007;7(1):15–22. doi: 10.1111/j.1535-7511.2007.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Igelström K.M. Is slack an intrinsic seizure terminator? Neuroscientist. 2013;19(3):248–254. doi: 10.1177/1073858412446311. [DOI] [PubMed] [Google Scholar]
- 47.Kaczmarek L.K. Non-conducting functions of voltage-gated ion channels. Nat. Rev. Neurosci. 2006;7(10):761–771. doi: 10.1038/nrn1988. [DOI] [PubMed] [Google Scholar]
- 48.Brown M.R., Kronengold J., Gazula V.R., Chen Y., Strumbos J.G., Sigworth F.J., Navaratnam D., Kaczmarek L.K. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat. Neurosci. 2010;13(7):819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Brown M.R., Hyland C., Chen Y., Kronengold J., Fleming M.R., Kohn A.B., Moroz L.L., Kaczmarek L.K. Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels. J. Neurosci. 2012;32(44):15318–15327. doi: 10.1523/JNEUROSCI.2162-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang F., Wang X., Ostertag E.M., Nuwal T., Huang B., Jan Y.N., Basbaum A.I., Jan L.Y. TMEM16C facilitates Na(+)-activated K+ currents in rat sensory neurons and regulates pain processing. Nat. Neurosci. 2013;16(9):1284–1290. doi: 10.1038/nn.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fleming M.R., Brown M.R., Kronengold J., Zhang Y., Jenkins D.P., Barcia G., Nabbout R., Bausch A.E., Ruth P., Lukowski R., Navaratnam D.S., Kaczmarek L.K. Stimulation of slack K(+) channels alters mass at the plasma membrane by triggering dissociation of a phosphatase-regulatory complex. Cell Rep. 2016;16(9):2281–2288. doi: 10.1016/j.celrep.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchino S., Wada H., Honda S., Hirasawa T., Yanai S., Nakamura Y., Ondo Y., Kohsaka S. Slo2 sodium-activated K+ channels bind to the PDZ domain of PSD-95. Biochem. Biophys. Res. Commun. 2003;310(4):1140–1147. doi: 10.1016/j.bbrc.2003.09.133. [DOI] [PubMed] [Google Scholar]
- 53.Allen P.B., Greenfield A.T., Svenningsson P., Haspeslagh D.C., Greengard P. Phactrs 1-4: A family of protein phosphatase 1 and actin regulatory proteins. Proc. Natl. Acad. Sci. USA. 2004;101(18):7187–7192. doi: 10.1073/pnas.0401673101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali S.R., Malone T.J., Zhang Y., Prechova M., Kaczmarek L.K. Phactr1 regulates Slack (KCNT1) channels via protein phosphatase 1 (PP1). FASEB J. 2020;34(1):1591–1601. doi: 10.1096/fj.201902366R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamada N., Ogaya S., Nakashima M., Nishijo T., Sugawara Y., Iwamoto I., Ito H., Maki Y., Shirai K., Baba S., Maruyama K., Saitsu H., Kato M., Matsumoto N., Momiyama T., Nagata K.I. De novo PHACTR1 mutations in West syndrome and their pathophysiological effects. Brain. 2018;141(11):3098–3114. doi: 10.1093/brain/awy246. [DOI] [PubMed] [Google Scholar]
- 56.de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., de Vries P., Gilissen C., del Rosario M., Hoischen A., Scheffer H., de Vries B.B., Brunner H.G., Veltman J.A., Vissers L.E. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367(20):1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 57.Kim J.Y., Choi S.Y., Moon Y., Kim H.J., Chin J.H., Kim H., Sun W. Different expression patterns of Phactr family members in normal and injured mouse brain. Neuroscience. 2012;221:37–46. doi: 10.1016/j.neuroscience.2012.06.059. [DOI] [PubMed] [Google Scholar]
- 58.Zheng C.Y., Seabold G.K., Horak M., Petralia R.S. MAGUKs, synaptic development, and synaptic plasticity. Neuroscientist. 2011;17(5):493–512. doi: 10.1177/1073858410386384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang P., Lisman J.E. Activity-dependent regulation of synaptic strength by PSD-95 in CA1 neurons. J. Neurophysiol. 2012;107(4):1058–1066. doi: 10.1152/jn.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan D.P., Ptácek L.J. Episodic neurological channelopathies. Neuron. 2010;68(2):282–292. doi: 10.1016/j.neuron.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Mao X., Bruneau N., Gao Q., Becq H., Jia Z., Xi H., Shu L., Wang H., Szepetowski P., Aniksztejn L. The epilepsy of infancy with migrating focal seizures: identification of de novo mutations of the KCNT2 gene that exert inhibitory effects on the corresponding heteromeric KNa1.1/KNa1.2 potassium channel. Front. Cell. Neurosci. 2020;14:1. doi: 10.3389/fncel.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milligan C.J., Li M., Gazina E.V., Heron S.E., Nair U., Trager C., Reid C.A., Venkat A., Younkin D.P., Dlugos D.J., Petrovski S., Goldstein D.B., Dibbens L.M., Scheffer I.E., Berkovic S.F., Petrou S. KCNT1 gain of function in 2 epilepsy phenotypes is reversed by quinidine. Ann. Neurol. 2014;75(4):581–590. doi: 10.1002/ana.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang Q.Y., Zhang F.F., Xu J., Wang R., Chen J., Logothetis D.E., Zhang Z. Epilepsy-related slack channel mutants lead to channel over-activity by two different mechanisms. Cell Rep. 2016;14(1):129–139. doi: 10.1016/j.celrep.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown M.R., Kaczmarek L.K. Potassium channel modulation and auditory processing. Hear. Res. 2011;279(1-2):32–42. doi: 10.1016/j.heares.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández de Sevilla D., Garduño J., Galván E., Buño W. Calcium-activated afterhyperpolarizations regulate synchronization and timing of epileptiform bursts in hippocampal CA3 pyramidal neurons. J. Neurophysiol. 2006;96(6):3028–3041. doi: 10.1152/jn.00434.2006. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z., Rosenhouse-Dantsker A., Tang Q.Y., Noskov S., Logothetis D.E. The RCK2 domain uses a coordination site present in Kir channels to confer sodium sensitivity to Slo2.2 channels. J. Neurosci. 2010;30(22):7554–7562. doi: 10.1523/JNEUROSCI.0525-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Descalzo V.F., Nowak L.G., Brumberg J.C., McCormick D.A., Sanchez-Vives M.V. Slow adaptation in fast-spiking neurons of visual cortex. J. Neurophysiol. 2005;93(2):1111–1118. doi: 10.1152/jn.00658.2004. [DOI] [PubMed] [Google Scholar]
- 68.Quraishi I.H., Stern S., Mangan K.P., Zhang Y., Ali S.R., Mercier M.R., Marchetto M.C., McLachlan M.J., Jones E.M., Gage F.H., Kaczmarek L.K. An epilepsy-associated KCNT1 mutation enhances excitability of human iPSC-derived neurons by increasing slack KNa currents. J. Neurosci. 2019;39(37):7438–7449. doi: 10.1523/JNEUROSCI.1628-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuchenbuch M., Nabbout R., Yochum M., Sauleau P., Modolo J., Wendling F., Benquet P. In silico model reveals the key role of GABA in KCNT1-epilepsy in infancy with migrating focal seizures. Epilepsia. 2021;62(3):683–697. doi: 10.1111/epi.16834. [DOI] [PubMed] [Google Scholar]
- 70.Katz L.C., Shatz C.J. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez-Vives M.V., Nowak L.G., McCormick D.A. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J. Neurosci. 2000;20(11):4286–4299. doi: 10.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Møller R.S., Heron S.E., Larsen L.H., Lim C.X., Ricos M.G., Bayly M.A., van Kempen M.J., Klinkenberg S., Andrews I., Kelley K., Ronen G.M., Callen D., McMahon J.M., Yendle S.C., Carvill G.L., Mefford H.C., Nabbout R., Poduri A., Striano P., Baglietto M.G., Zara F., Smith N.J., Pridmore C., Gardella E., Nikanorova M., Dahl H.A., Gellert P., Scheffer I.E., Gunning B., Kragh-Olsen B., Dibbens L.M. Mutations in KCNT1 cause a spectrum of focal epilepsies. Epilepsia. 2015;56(9):e114–e120. doi: 10.1111/epi.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mikati M.A., Jiang Y.H., Carboni M., Shashi V., Petrovski S., Spillmann R., Milligan C.J., Li M., Grefe A., McConkie A., Berkovic S., Scheffer I., Mullen S., Bonner M., Petrou S., Goldstein D. Quinidine in the treatment of KCNT1-positive epilepsies. Ann. Neurol. 2015;78(6):995–999. doi: 10.1002/ana.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evely K.M., Pryce K.D., Bhattacharjee A. The Phe932Ile mutation in KCNT1 channels associated with severe epilepsy, delayed myelination and leukoencephalopathy produces a loss-of-function channel phenotype. Neuroscience. 2017;351:65–70. doi: 10.1016/j.neuroscience.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quraishi I.H., Mercier M.R., McClure H., Couture R.L., Schwartz M.L., Lukowski R., Ruth P., Kaczmarek L.K. Impaired motor skill learning and altered seizure susceptibility in mice with loss or gain of function of the Kcnt1 gene encoding Slack (KNa1.1) Na+-activated K+ channels. Sci. Rep. 2020;10(1):3213. doi: 10.1038/s41598-020-60028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McTague A., Nair U., Malhotra S., Meyer E., Trump N., Gazina E.V., Papandreou A., Ngoh A., Ackermann S., Ambegaonkar G., Appleton R., Desurkar A., Eltze C., Kneen R., Kumar A.V., Lascelles K., Montgomery T., Ramesh V., Samanta R., Scott R.H., Tan J., Whitehouse W., Poduri A., Scheffer I.E., Chong W.K.K., Cross J.H., Topf M., Petrou S., Kurian M.A. Clinical and molecular characterization of KCNT1-related severe early-onset epilepsy. Neurology. 2018;90(1):e55–e66. doi: 10.1212/WNL.0000000000004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishii A., Shioda M., Okumura A., Kidokoro H., Sakauchi M., Shimada S., Shimizu T., Osawa M., Hirose S., Yamamoto T. A recurrent KCNT1 mutation in two sporadic cases with malignant migrating partial seizures in infancy. Gene. 2013;531(2):467–471. doi: 10.1016/j.gene.2013.08.096. [DOI] [PubMed] [Google Scholar]
- 78.Scheffer I.E., Jones L., Pozzebon M., Howell R.A., Saling M.M., Berkovic S.F. Autosomal dominant rolandic epilepsy and speech dyspraxia: a new syndrome with anticipation. Ann. Neurol. 1995;38(4):633–642. doi: 10.1002/ana.410380412. [DOI] [PubMed] [Google Scholar]
- 79.Tamsett T.J., Picchione K.E., Bhattacharjee A. NAD+ activates KNa channels in dorsal root ganglion neurons. J. Neurosci. 2009;29(16):5127–5134. doi: 10.1523/JNEUROSCI.0859-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramsey K.M., Yoshino J., Brace C.S., Abrassart D., Kobayashi Y., Marcheva B., Hong H.K., Chong J.L., Buhr E.D., Lee C., Takahashi J.S., Imai S., Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324(5927):651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfeiffer B.E., Huber K.M. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15(5):549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bausch A.E., Dieter R., Nann Y., Hausmann M., Bausch A.E., Dieter R., Nann Y., Hausmann M., Meyerdierks N., Kaczmarek L.K., Ruth P., Lukowski R. The sodium-activated potassium channel Slack is required for optimal cognitive flexibility in mice. Learn. Mem. 2015;22(7):323–335. doi: 10.1101/lm.037820.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bausch A.E., Ehinger R., Straubinger J., Zerfass P., Nann Y., Lukowski R. Loss of sodium-activated potassium channel slack and FMRP differentially affect social behavior in mice. Neuroscience. 2018;384:361–374. doi: 10.1016/j.neuroscience.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 84.Musumeci S.A., Hagerman R.J., Ferri R., Bosco P., Dalla Bernardina B., Tassinari C.A., De Sarro G.B., Elia M. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40(8):1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 85.Sabaratnam M., Vroegop P.G., Gangadharan S.K. Epilepsy and EEG findings in 18 males with fragile X syndrome. Seizure. 2001;10(1):60–63. doi: 10.1053/seiz.2000.0492. [DOI] [PubMed] [Google Scholar]
- 86.Zemelman B.V., Lee G.A., Ng M., Miesenböck G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33(1):15–22. doi: 10.1016/S0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 87.Li X., Gutierrez D.V., Hanson M.G., Han J., Mark M.D., Chiel H., Hegemann P., Landmesser L.T., Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA. 2005;102(49):17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boyden E.S., Zhang F., Bamberg E., Nagel G., Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 89.Lu Y., Zhong C., Wang L., Wei P., He W., Huang K., Zhang Y., Zhan Y., Feng G., Wang L. Optogenetic dissection of ictal propagation in the hippocampal-entorhinal cortex structures. Nat. Commun. 2016;7:10962. doi: 10.1038/ncomms10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paz J.T., Davidson T.J., Frechette E.S., Delord B., Parada I., Peng K., Deisseroth K., Huguenard J.R. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 2013;16(1):64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buzsáki G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004;7(5):446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 92.Merricks E.M., Smith E.H., McKhann G.M., Goodman R.R., Bateman L.M., Emerson R.G., Schevon C.A., Trevelyan A.J. Single unit action potentials in humans and the effect of seizure activity. Brain. 2015;138(Pt 10):2891–2906. doi: 10.1093/brain/awv208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Broussard G.J., Liang R., Tian L. Monitoring activity in neural circuits with genetically encoded indicators. Front. Mol. Neurosci. 2014;7:97. doi: 10.3389/fnmol.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suresh N.T. ; e R, V.; U, K. Multi-scale top-down approach for modelling epileptic protein-protein interaction network analysis to identify driver nodes and pathways. Comput. Biol. Chem. 2020;88:107323. doi: 10.1016/j.compbiolchem.2020.107323. [DOI] [PubMed] [Google Scholar]
- 95.Marsh E., Melamed S.E., Barron T., Clancy R.R. Migrating partial seizures in infancy: expanding the phenotype of a rare seizure syndrome. Epilepsia. 2005;46(4):568–572. doi: 10.1111/j.0013-9580.2005.34104.x. [DOI] [PubMed] [Google Scholar]
- 96.McTague A., Appleton R., Avula S., Cross J.H., King M.D., Jacques T.S., Bhate S., Cronin A., Curran A., Desurkar A., Farrell M.A., Hughes E., Jefferson R., Lascelles K., Livingston J., Meyer E., McLellan A., Poduri A., Scheffer I.E., Spinty S., Kurian M.A., Kneen R. Migrating partial seizures of infancy: expansion of the electroclinical, radiological and pathological disease spectrum. Brain. 2013;136(Pt 5):1578–1591. doi: 10.1093/brain/awt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang B., Gribkoff V.K., Pan J., Damagnez V., Dworetzky S.I., Boissard C.G., Bhattacharjee A., Yan Y., Sigworth F.J., Kaczmarek L.K. Pharmacological activation and inhibition of Slack (Slo2.2) channels. Neuropharmacology. 2006;51(4):896–906. doi: 10.1016/j.neuropharm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 98.Jia Y., Lin Y., Li J., Li M., Zhang Y., Hou Y., Liu A., Zhang L., Li L., Xiang P., Ye J., Huang Z., Wang Y. Quinidine therapy for lennox-gastaut syndrome With KCNT1 mutation. A case report and literature review. Front. Neurol. 2019;10:64. doi: 10.3389/fneur.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abdelnour E., Gallentine W., McDonald M., Sachdev M., Jiang Y.H., Mikati M.A. Does age affect response to quinidine in patients with KCNT1 mutations? Report of three new cases and review of the literature. Seizure. 2018;55:1–3. doi: 10.1016/j.seizure.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 100.Bearden D., Strong A., Ehnot J., DiGiovine M., Dlugos D., Goldberg E.M. Targeted treatment of migrating partial seizures of infancy with quinidine. Ann. Neurol. 2014;76(3):457–461. doi: 10.1002/ana.24229. [DOI] [PubMed] [Google Scholar]
- 101.Dilena R., DiFrancesco J.C., Soldovieri M.V., Giacobbe A., Ambrosino P., Mosca I., Galli M.A., Guez S., Fumagalli M., Miceli F., Cattaneo D., Darra F., Gennaro E., Zara F., Striano P., Castellotti B., Gellera C., Varesio C., Veggiotti P., Taglialatela M. Early treatment with quinidine in 2 patients with epilepsy of infancy with migrating focal seizures (EIMFS) due to gain-of-function KCNT1 mutations: functional studies, clinical responses, and critical issues for personalized therapy. Neurotherapeutics. 2018;15(4):1112–1126. doi: 10.1007/s13311-018-0657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Los Angeles Tejada M., Stolpe K., Meinild A.K., Klaerke D.A. Clofilium inhibits slick and slack potassium channels. Biologics. 2012;6:465–470. doi: 10.2147/BTT.S33827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cole B.A., Clapcote S.J., Muench S.P., Lippiat J.D. Targeting KNa1.1 channels in KCNT1-associated epilepsy. Trends Pharmacol. Sci. 2021;42(8):700–713. doi: 10.1016/j.tips.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 104.Cole B.A., Johnson R.M., Dejakaisaya H., Pilati N., Fishwick C.W.G., Muench S.P., Lippiat J.D. Structure-based identification and characterization of inhibitors of the epilepsy-associated KNa1.1 (KCNT1) potassium channel. iScience. 2020. 23(5), 101100. [DOI] [PMC free article] [PubMed]
- 105.Spitznagel B.D., Mishra N.M., Qunies A.M., Prael F.J., III, Du Y., Kozek K.A., Lazarenko R.M., Denton J.S., Emmitte K.A., Weaver C.D. VU0606170, a selective slack channels inhibitor, decreases calcium oscillations in cultured cortical neurons. ACS Chem. Neurosci. 2020;11(21):3658–3671. doi: 10.1021/acschemneuro.0c00583. [DOI] [PubMed] [Google Scholar]
- 106.Griffin A.M., Kahlig K.M., Hatch R.J., Hughes Z.A., Chapman M.L., Antonio B., Marron B.E., Wittmann M., Martinez-Botella G. Discovery of the first orally available, selective KNa1.1 inhibitor: in vitro and in vivo activity of an oxadiazole series. ACS Med. Chem. Lett. 2021;12(4):593–602. doi: 10.1021/acsmedchemlett.0c00675. [DOI] [PMC free article] [PubMed] [Google Scholar]