Abstract
Neurosteroids are endogenous modulators of GABAA receptors that mediate anxiety, pain, mood and arousal. The 3-hydroxyl epimers, allopregnanolone (3α-OH) and epi-allopregnanolone (3β-OH) are both prevalent in the mammalian brain and produce opposite effects on GABAA receptor function, acting as positive and negative allosteric modulators, respectively. This Perspective provides a model to explain the actions of 3α-OH and 3β-OH neurosteroids. The model is based on evidence that the neurosteroid epimers bind to an overlapping subset of specific sites on GABAA receptors, with their net functional effect on channel gating being the sum of their independent effects at each site.
Keywords: Neurosteroids, GABAA receptors, ion channels, affinity labeling, desensitization, structural biology
1. INTRODUCTION
Neurosteroids are cholesterol metabolites produced in neurons and glia that act as modulators of a number of cell surface receptors and ion channels [1]. The chloride-permeable GABAA receptor is a major target of neurosteroids; 3α-OH steroids enhance, whereas 3β-OH and sulfated steroids inhibit GABA-elicited currents [2-4]. Modulation of GABAA receptor activity by potentiating neurosteroids and their synthetic analogues controls neuronal excitability and contributes to anxiolysis, sedation, and anesthesia [5, 6]. The focus of this Perspective is the molecular basis for the actions of 3α-OH and 3β-OH neurosteroids, and how steroid interactions with distinct binding sites underlie the net modulatory effect observed in functional assays.
2. FUNCTIONAL EFFECTS OF 3α-OH PAM NEUROSTEROIDS
3α-OH neurosteroids such as allopregnanolone (3α5α-P) and tetrahydrodeoxycorticosterone (THDOC) are positive allosteric modulators (PAM) of GABAA receptors, augmenting the response of the receptor to a sub-saturating concentration of GABA (Fig. 1). The effect manifests as increased peak and steady-state responses in macroscopic recordings, and increased opening frequency and prolonged mean open duration of the channel in single-channel recordings [7-9]. Single-channel kinetic analysis has identified up to three specific changes in open and closed time properties in the presence of PAM steroids that collectively underlie the increase in receptor open probability. There is an increase in the mean duration and prevalence of the longest-lived open time component and a decrease in the prevalence of the closed time component associated with agonist binding and channel opening [9]. Whether the individual kinetic effects are mediated by unique sites or a single, common interaction site remains unclear [10]. In radioligand binding assays, exposure to a PAM steroid enhances the binding of [3H]muscimol to the orthosteric binding site and [3H]flunitrazepam to the benzodiazepine site [11-14]. Conversely, the binding of [35S]t-butylbicyclophosphorothionate, a cage convulsant and noncompetitive GABAA receptor antagonist, is reduced in the presence of PAM neurosteroids [12, 15, 16].
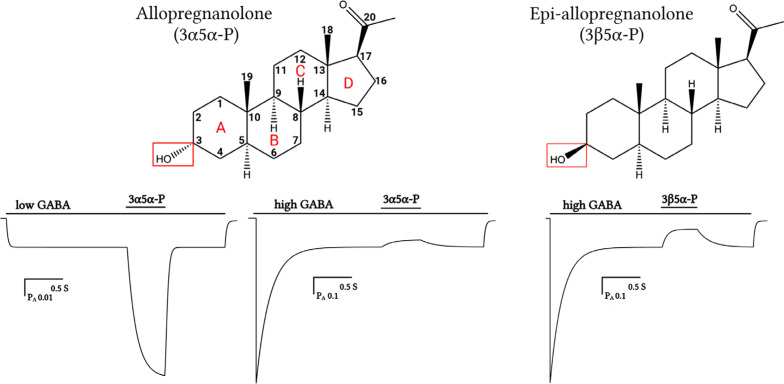
Fig. (1).
Structure and actions of neurosteroids. Allopregnanolone (3α5α-P; top left) is an endogenous PAM neurosteroid characterized by a 3α-hydroxyl group. Its epimer, epi-allopregnanolone (3β5α-P; top right), is a NAM neurosteroid characterized by a 3β-hydroxyl group. The lower panels show simulated electrophysiological tracings of GABAA-receptor currents constructed using a three-state (resting-active-desensitized) Monod-Wyman-Changeux model (simulation performed in ChanneLab™ ver. 2 software provided by Stephen F. Traynelis, Emory University School of Medicine). At low open probability (PA), (left lower panel) 3α5α-P significantly potentiates the channel activation elicited by a low concentration of the orthosteric agonist GABA, whereas 3β5α-P (not shown) has no effect. In contrast, at high PA (such as that produced by a saturating concentration of GABA) 3α5α-P weakly (lower middle panel) and 3β5α-P substantially desensitize the receptor, thus inhibiting the steady-state current.
3. THE BINDING SITES FOR PAM NEUROSTEROIDS
By comparing the modulatory effect of THDOC on the mouse α1β2γ2 GABAA receptor and the neurosteroid-insensitive Drosophila GABA receptor, Hosie et al. [17] identified residues in the transmembrane region, notably α1(Q242) that are essential for the PAM effects of neurosteroids. Subsequent studies using neurosteroid analogue photolabeling [18-20], X-ray crystallography [21-23] and scanning cysteine mutagenesis [24] have localized a major neurosteroid binding site at the interface between the β(+) and α(-) subunits. The site is defined by the α1(Q242), α1(W246) and β3(F301) residues, and the actions of neurosteroids are sensitive to amino acid substitutions at these positions [25]. The hydrogen bond between the 3α-OH of the steroid and the α1(Q242) residue is a key interaction and determinant of functional modulation.
Photolabeling studies have identified additional binding sites for PAM neurosteroids within the transmembrane domains in α1 and β3 subunits [19], encompassing the remaining neurosteroid-sensitive residues originally described by Hosie [17] (Fig. 2). In the α1 subunit, a 3α5α-P photolabeling analogue binds between transmembrane α-helices (TM) TM1 and TM4. The A-ring of the steroid is oriented towards the extracellular domain (ECD) with the sides of the predicted binding site lined by the α1(N408) and α1(Y415) residues of TM4 and α1(V227) of TM1. In the β3 subunit, the preferred pose of the steroid is between TM3 and TM4, with the A-ring positioned towards the ECD near β3(Y442) and the D-ring near β3(V290) of TM3. Amino acid substitutions introduced within the intra-α subunit site (α1(V227W) or α1(N408A+Y411F)) impair receptor potentiation by the neurosteroid 3α5α-P. Amino acid substitutions within the intra-β subunit site have little effect on receptor potentiation by 3α5α-P. Instead, the intra-β subunit site may be involved in receptor desensitization. Application of 3α5α-P to receptors containing mutations to the β-α intersubunit (α1(Q242L)) and intra-α subunit sites (α1(N408A+Y411F)) reduces steady-state current elicited by agonists that generate a response with a high open probability [26]. This effect of 3α5α-P is obscured by potentiation in the wild-type receptor with intact β-α and intra-α subunits sites, or at low levels of activity when most receptors are in the resting state.
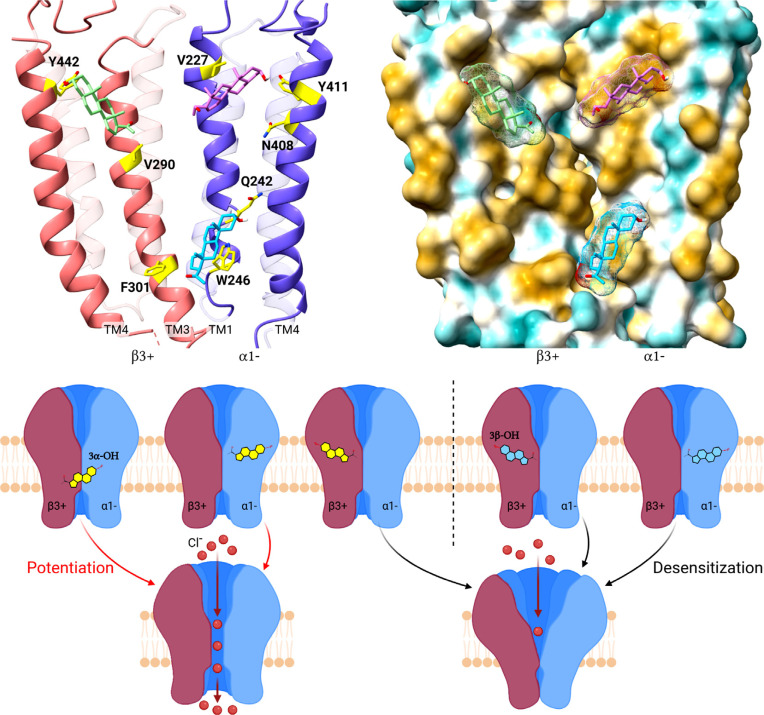
Fig. (2).
Models of neurosteroid binding sites on α1β3 GABAA receptors and the effects of their occupancy on channel gating. (Top left) Ribbon diagram of the interface between the β3(+) (salmon) and α1(-) (blue) subunits of a GABAA receptor (based on pdb 6HUO) with the neurosteroid 3α5α-P in its preferred docking poses in: an intra-β-subunit site between β3(V290) on TM3 and β3(Y442) on TM4; a β3(+)/α1(-) intersubunit site between α1(Q242), α1(W246) on TM1 and β3(F301) on TM3 and; an intra-α-subunit site between α1(V227) on TM1 and α1(Y411 and N408) on TM4. (Top right) Hydrophobic surface representation of 3α5α-P docked to the α1β3 GABAA receptor (brown most and turquoise least hydrophobic) illustrating that neurosteroids bind between the hydrophobic transmembrane α-helices on the receptor surface, interacting with both protein and annular lipid. (Lower panel) Cartoon illustrating that 3α5α-P (yellow steroid) occupancy of the β3(+)/α1(-) intersubunit site and/or the intra-α1-subunit site promotes channel activation. In contrast, occupancy of the intra-β3-subunit site by either 3α5α-P or 3β5α-P (blue steroid) and occupancy of the intra-α1-subunit site by 3β5α-P inhibits the receptor by promoting desensitization.
4. INDEPENDENT ACTIONS OF 3α5α-P AT THE BINDING SITES
Amino acid substitutions introduced to the neurosteroid sites at either the β-α interface (α1(Q242L)) or within the α subunit (α1(V227W)) impair receptor potentiation by the neurosteroid 3α5α-P. Comparison of the magnitude of the effects suggests that the free energy change contributed by 3α5α-P via the β-α intersubunit site (-1.3 kcal/mol) is approximately double that of the intra-α subunit site (-0.6 kcal/mol). The effects are energetically additive, indicating that the sites are independent (i.e. not allosterically coupled) [27]. Biochemical evidence from photolabeling studies supports the absence of allosteric coupling [28]. The β-α intersubunit site mutations, α1(Q242L) and α1(W246L), drastically reduce receptor potentiation by PAM neurosteroids but change neither the labeling efficiency nor the orientation of a PAM-neurosteroid analogue photolabeling reagent (KK200) in the intra-α subunit site. Conversely, the α1(V227W) substitution in the intra-α subunit site reduces receptor potentiation by 3α5α-P and has no effect on steroid occupancy or orientation in the β-α intersubunit site. Interestingly, the amino acid substitutions in both the β-α intersubunit and intra-α subunit sites change the residues labeled by KK200 but not the efficiency of labeling within the mutated site, indicating that steroid orientation rather than ligand occupancy drives the functional effects of these mutations.
5. ACTIONS OF 3β-OH NAM NEUROSTEROIDS
The 3β-OH neurosteroid epi-allopregnanolone (3β5α-P) is a negative allosteric modulator (NAM) of GABAA receptors, producing a non-conducting liganded state characterized by enhanced orthosteric ligand ([3H] muscimol) binding and reduction of the steady-state current elicited by a saturating concentration of GABA [26] (Fig. 1). The synaptic [3] and single-channel [26] effects of 3β-OH neurosteroids suggest that these effects represent stabilization of a desensitized state of the receptor. 3β5α-P non-competitively inhibits potentiation by PAM neurosteroids, suggesting that the PAM and NAM effects of neurosteroids are mediated by distinct binding sites [3]. Photoaffinity labeling studies demonstrated that 3β5α-P binds to the intra-α and intra-β subunit neurosteroid binding sites, but not to the β-α intersubunit site, explaining the absence of a PAM effect [26]. Consistent with this explanation, amino acid substitutions in either the intra-α or intra-β subunit sites reduce 3β5α-P-mediated desensitization, whereas mutations in the β-α intersubunit site have no effect [26] (Fig. 2).
6. THE ROLE OF MEMBRANE LIPIDS IN NEUROSTEROID ACTION
All of the identified neurosteroid sites are on the protein surface between transmembrane α-helices of the same or adjoining subunits. This is consistent with observations indicating that neurosteroids must partition into the membrane and laterally diffuse to their protein binding sites [29]. This suggests that membrane lipid composition may influence both the kinetics and actions of neurosteroids [30]. Indeed, PAM neurosteroids are more effective at potentiating GABA-activated receptors in cholesterol-depleted membranes, and less effective in cholesterol-enriched membranes, leading to a hypothesis that cholesterol competitively or allosterically inhibits the binding of a PAM steroid [31]. Molecular docking studies predict that cholesterol can bind to multiple sites between the transmembrane α-helices on the surface of GABAA receptors, including all of the identified neurosteroid sites, and that neurosteroids and cholesterol compete for binding [32]. Indeed, in the pentameric ligand-gated ion channel from Gloeobacter violaceus (GLIC), cholesterol and neurosteroids compete for binding to an intrasubunit site analogous to the neurosteroid binding site in GABAA subunits [33].
7. SYNOPSIS AND REMAINING QUESTIONS
Three unique binding sites on the GABAA receptor (seven per α1β3 pentamer) account for the known actions of both the 3α-OH PAM and the 3β-OH NAM neurosteroids: 1) A β(+)-α(-) intersubunit site selectively binds 3α-OH neurosteroids and mediates their PAM effect. 2) An intra-β subunit site binds either 3α- or 3β-OH neurosteroids producing a NAM effect (desensitization). 3) An intra-α subunit site binds either 3α-OH or 3β-OH neurosteroids, but the epimers produce effects of opposite valence (i.e. PAM vs NAM), indicating state-dependent binding (Fig. 2). Finally, neurosteroid binding and effect at the various sites occur independently (i.e. the sites are not allosterically linked) with the net effect on GABAA receptor activity being the sum of the energetic contributions of binding at each site.
CONCLUSION
This model predicts a rich pharmacology in which neurosteroid analogues can differentially bind to the three sites, producing distinct effects (e.g. PAM, NAM or competitive antagonist) at each site that summate to produce a highly tunable GABAergic output. It remains to be determined whether there are additional unique sites on γ or δ subunits or isoform specificity in neurosteroid binding within the α1-6 or β1-3 subunits. Either of these possibilities could provide specific pharmacologic targets for GABAA receptor subtypes. Notably, the NAM effects of sulfated neurosteroids are unaltered by mutations in the identified binding sites [21, 25], suggesting that additional neurosteroid binding sites on GABAA receptors remain to be elucidated.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The study was supported by the National Institutes of General Medical Sciences grants RO1GM108799 (ASE), RO1GM108580 (GA) and R35GM140947 (GA) and funds from the Taylor Family Institute for Innovative Psychiatric Research.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Tuem K.B., Atey T.M. Neuroactive steroids: Receptor interactions and responses. Front. Neurol. 2017;8:442. doi: 10.3389/fneur.2017.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majewska M.D., Harrison N.L., Schwartz R.D., Barker J.L., Paul S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 3.Wang M., He Y., Eisenman L.N., Fields C., Zeng C.M., Mathews J., Benz A., Fu T., Zorumski E., Steinbach J.H., Covey D.F., Zorumski C.F., Mennerick S. 3beta-hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists. J. Neurosci. 2002;22(9):3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majewska M.D., Mienville J.M., Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci. Lett. 1988;90(3):279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- 5.Belelli D., Lambert J.J. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005;6(7):565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 6.Olsen R.W. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology, 2018;136(Pt A):10–22. doi: 10.1016/j.neuropharm.2018.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison N.L., Simmonds M.A. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323(2):287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- 8.Twyman R.E., Macdonald R.L. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J. Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akk G., Bracamontes J.R., Covey D.F., Evers A., Dao T., Steinbach J.H. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J. Physiol. 2004;558(Pt 1):59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akk G., Covey D.F., Evers A.S., Mennerick S., Zorumski C.F., Steinbach J.H. Kinetic and structural determinants for GABA-A receptor potentiation by neuroactive steroids. Curr. Neuropharmacol. 2010;8(1):18–25. doi: 10.2174/157015910790909458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan N.C., Gee K.W., Bolger M.B., Chen J.S. Differential responses of expressed recombinant human gamma-aminobutyric acidA receptors to neurosteroids. J. Neurochem. 1991;57(5):1818–1821. doi: 10.1111/j.1471-4159.1991.tb06388.x. [DOI] [PubMed] [Google Scholar]
- 12.Turner D.M., Ransom R.W., Yang J.S., Olsen R.W. Steroid anesthetics and naturally occurring analogs modulate the gamma-aminobutyric acid receptor complex at a site distinct from barbiturates. J. Pharmacol. Exp. Ther. 1989;248(3):960–966. [PubMed] [Google Scholar]
- 13.Peters J.A., Kirkness E.F., Callachan H., Lambert J.J., Turner A.J. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br. J. Pharmacol. 1988;94(4):1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akk G., Germann A.L., Sugasawa Y., Pierce S.R., Evers A.S., Steinbach J.H. Enhancement of muscimol binding and gating by allosteric modulators of the GABAA receptor: Relating occupancy to state functions. Mol. Pharmacol. 2020;98(4):303–313. doi: 10.1124/molpharm.120.000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evers A.S., Chen Z-W., Manion B.D., Han M., Jiang X., Darbandi-Tonkabon R., Kable T., Bracamontes J., Zorumski C.F., Mennerick S., Steinbach J.H., Covey D.F. A synthetic 18-norsteroid distinguishes between two neuroactive steroid binding sites on GABAA receptors. J. Pharmacol. Exp. Ther. 2010;333(2):404–413. doi: 10.1124/jpet.109.164079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sapp D.W., Witte U., Turner D.M., Longoni B., Kokka N., Olsen R.W. Regional variation in steroid anesthetic modulation of [35S]TBPS binding to gamma-aminobutyric acidA receptors in rat brain. J. Pharmacol. Exp. Ther. 1992;262(2):801–808. [PubMed] [Google Scholar]
- 17.Hosie A.M., Wilkins M.E., da Silva H.M., Smart T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444(7118):486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z.W., Manion B., Townsend R.R., Reichert D.E., Covey D.F., Steinbach J.H., Sieghart W., Fuchs K., Evers A.S. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the β3 subunit of the GABA(A) receptor. Mol. Pharmacol. 2012;82(3):408–419. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z.W., Bracamontes J.R., Budelier M.M., Germann A.L., Shin D.J., Kathiresan K., Qian M.X., Manion B., Cheng W.W.L., Reichert D.E., Akk G., Covey D.F., Evers A.S. Multiple functional neurosteroid binding sites on GABAA receptors. PLoS Biol. 2019;17(3):e3000157. doi: 10.1371/journal.pbio.3000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayakar S.S., Chiara D.C., Zhou X., Wu B., Bruzik K.S., Miller K.W., Cohen J.B. Photoaffinity labeling identifies an intersubunit steroid-binding site in heteromeric GABA type A (GABAA) receptors. J. Biol. Chem. 2020;295(33):11495–11512. doi: 10.1074/jbc.RA120.013452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laverty D., Thomas P., Field M., Andersen O.J., Gold M.G., Biggin P.C., Gielen M., Smart T.G. Crystal structures of a GABAA-receptor chimera reveal new endogenous neurosteroid-binding sites. Nat. Struct. Mol. Biol. 2017;24(11):977–985. doi: 10.1038/nsmb.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller P.S., Scott S., Masiulis S., De Colibus L., Pardon E., Steyaert J., Aricescu A.R. Structural basis for GABAA receptor potentiation by neurosteroids. Nat. Struct. Mol. Biol. 2017;24(11):986–992. doi: 10.1038/nsmb.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Q., Wells M.M., Arjunan P., Tillman T.S., Cohen A.E., Xu Y., Tang P. Structural basis of neurosteroid anesthetic action on GABAA receptors. Nat. Commun. 2018;9(1):3972. doi: 10.1038/s41467-018-06361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziemba A.M., Szabo A., Pierce D.W., Haburcak M., Stern A.T., Nourmahnad A., Halpin E.S., Forman S.A. Alphaxalone binds in inner transmembrane beta+-alpha- interfaces of alpha1beta3gamma2 gamma-aminobutyric acid type A receptors. Anesthesiology. 2018;128(2):338–351. doi: 10.1097/ALN.0000000000001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akk G., Li P., Bracamontes J., Reichert D.E., Covey D.F., Steinbach J.H. Mutations of the GABA-A receptor α1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol. Pharmacol. 2008;74(3):614–627. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugasawa Y., Cheng W.W., Bracamontes J.R., Chen Z.W., Wang L., Germann A.L., Pierce S.R., Senneff T.C., Krishnan K., Reichert D.E., Covey D.F., Akk G., Evers A.S. Site-specific effects of neurosteroids on GABAA receptor activation and desensitization. eLife. 2020;9:e55331. doi: 10.7554/eLife.55331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Germann A.L., Pierce S.R., Tateiwa H., Sugasawa Y., Reichert D.E., Evers A.S., Steinbach J.H., Akk G. Intrasubunit and intersubunit steroid binding sites independently and additively mediate α1β2γ2L GABAA receptor potentiation by the endogenous neurosteroid allopregnanolone. Mol. Pharmacol. 2021;100(1):19–31. doi: 10.1124/molpharm.121.000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugasawa Y., Bracamontes J.R., Krishnan K., Covey D.F., Reichert D.E., Akk G., Chen Q., Tang P., Evers A.S., Cheng W.W.L. The molecular determinants of neurosteroid binding in the GABA(A) receptor. J. Steroid Biochem. Mol. Biol. 2019;192:105383. doi: 10.1016/j.jsbmb.2019.105383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chisari M., Eisenman L.N., Krishnan K., Bandyopadhyaya A.K., Wang C., Taylor A., Benz A., Covey D.F., Zorumski C.F., Mennerick S. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: Evidence for a low-affinity interaction. J. Neurophysiol. 2009;102(2):1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P., Shu H.J., Wang C., Mennerick S., Zorumski C.F., Covey D.F., Steinbach J.H., Akk G. Neurosteroid migration to intracellular compartments reduces steroid concentration in the membrane and diminishes GABA-A receptor potentiation. J. Physiol. 2007;584(Pt 3):789–800. doi: 10.1113/jphysiol.2007.142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sooksawate T., Simmonds M.A. Influence of membrane cholesterol on modulation of the GABA(A) receptor by neuroactive steroids and other potentiators. Br. J. Pharmacol. 2001;134(6):1303–1311. doi: 10.1038/sj.bjp.0704360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee A.G. Interfacial binding sites for cholesterol on GABAA receptors and competition with neurosteroids. Biophys. J. 2021;120(13):2710–2722. doi: 10.1016/j.bpj.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budelier M.M., Cheng W.W.L., Chen Z.W., Bracamontes J.R., Sugasawa Y., Krishnan K., Mydock-Magrane L., Covey D.F., Evers A.S. Common binding sites for cholesterol and neurosteroids on a pentameric ligand-gated ion channel. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864:128–136. doi: 10.1016/j.bbalip.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]