Abstract
Two almost-identical strains of Eubacterium aerofaciens isolated from the normal human gut flora were used. The cell wall (CW) of one strain with a peptidoglycan (PG) type A4α induces chronic arthritis in the rat after a single intraperitoneal injection, whereas CW of the other with PG type A4β induces only a transient acute arthritis. The CW of the arthritogenic E. aerofaciens was a twofold-more-potent stimulator of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and monocyte chemoattractant protein 1 (MCP-1) than the nonarthritogenic CW. After degradation with mutanolysin, the capacity of the arthritogenic PG to stimulate production of TNF-α and MCP-1 was significantly increased, whereas that of the nonarthritogenic PG was significantly decreased. In other words, after enzyme degradation the arthritogenic PG had a four- to fivefold-stronger stimulatory capacity than that of the enzyme-treated nonarthritogenic PG. These findings indicate that the arthritogenicity of CW or a PG is not dependent on the enzyme resistance alone but also on how the PG fragments released by enzyme degradation stimulate the production of proinflammatory cytokines.
Bacterial cell wall (CW) arthritis induced by a single intraperitoneal (i.p.) injection of gram-positive CWs in the rats is an experimental model closely resembling human rheumatoid arthritis. Using this model, we have described two bacterial strains of the same species, Eubacterium aerofaciens, one with a CW causing chronic arthritis and another with a CW causing only a transient acute arthritis. Both strains were isolated as a part of the normal human intestinal flora. They are 100% identical by the 16S ribosomal DNA (rDNA) analysis and have slightly different cellular fatty acid and biochemical profiles, indicating that they are clones of the same species (53). Chronic arthritis can be induced by using peptidoglycan (PG)-polysaccharide polymers isolated from the CW. The significance of polysaccharides seems to be to protect PG from enzyme degradation (4, 10, 35, 52), and for the induction of chronic arthritis the chemical structure of the PG moiety is decisive (52). The E. aerofaciens CW causing chronic arthritis has a PG of type A4α, and the CW of the strain causing only a mild, transient arthritis has a PG of type A4β. These two strains of E. aerofaciens have been designated arthritogenic and nonarthritogenic strains, respectively (53).
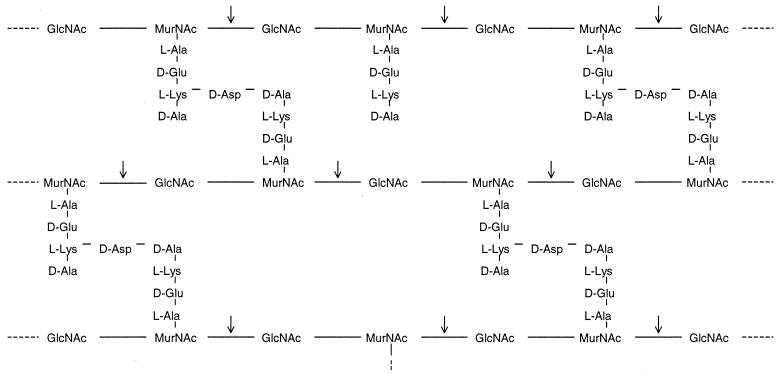
PG in gram-positive CWs consists of several layers (up to 70) of sugar chains containing N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) alternatively linked to each other by β-1,4-glycosidic bonds (Fig. 1). Stem peptides, also called muramyl peptides, are short peptides of four to five amino acids bound to MurNAc. PG and muramyl peptides possess multiple immune activities (3, 17, 24, 25, 42). For instance, PG can bind to CD14 (6) and to Toll-like receptor 2 (36, 51) to stimulate T cells and monocytes to produce inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin (IL-8), and monocyte chemoattractant protein 1 (MCP-1) (48, 49), and to cause gram-positive shock (5, 16). Stem peptides are linked to each other by interpeptide bridges (cross-linkage) or exist as free peptides (non-cross-linking). The degree of cross-linkage varies between different bacterial strains. The glycosidic bonds between MurNAc and GlcNAc are cut by the PG hydrolyzing enzymes mutanolysin and lysozyme. However, due to steric hindrance by associated polysaccharides and proteins, all bonds may not be reached by enzymes, resulting in fragments of different sizes.
FIG. 1.
Schematic picture of PG from the arthritogenic E. aerofaciens. PG in gram-positive CWs consists of several layers (up to 70) of sugar chains containing of GlcNAc and MurNAc alternately linked to each other by β-1,4-glycosidic bonds. Stem peptides, also called muramyl peptides, are the short peptides of four to five amino acids bound to MurNAc. Stem peptides may be linked to each other by interpeptide bridges (cross-linkage) or exist as free peptides (non-cross-linking). The degree of cross-linkage varies between different bacterial strains. Mutanolysin and lysozyme cut glycosidic bonds between MurNAc and GlcNAc (arrows). Due to steric hindrance by associated polysaccharides and proteins, all bonds may not be reached by enzymes, resulting in fragments of different sizes.
Since there is increasing attention on intestinal bacteria as potential triggers of rheumatoid arthritis (7, 14, 28, 31, 37, 46), we have studied here why two nearly identical representatives of the human normal gut flora are so different in their arthritogenic capacity. For this purpose, CWs of the two E. aerofaciens strains were examined for degradation by mutanolysin and lysozyme and for their capacity to stimulate production of proinflammatory cytokines. TNF-α and MCP-1 were chosen as the cytokines, since TNF-α plays a key role in the pathogenesis of rheumatoid arthritis (2) and since the development of experimental arthritides, including bacterial CW arthritis, depends on the upregulation of MCP-1 (13, 27, 32, 33). In addition, the distribution of the CWs in the liver, spleen, synovial tissue, and synovial fluid was studied by using muramic acid as a marker.
MATERIALS AND METHODS
Bacteria.
E. aerofaciens ATCC 25986 (now recognized as Collinsella aerofaciens [15]) was obtained from the Culture Collection, University of Gothenburg, Gothenburg, Sweden, with the designation CCUG 28087. E. aerofaciens ATCC 35085 was purchased from the American Type Culture Collection, Rockville, Md. Both strains were grown overnight under strictly anaerobic conditions at 37°C in BBL Schaedler Broth (Becton Dickinson Microbiology Systems, Sparks, Md.) to the late logarithmic phase. Both strains originate in the human intestinal flora and are nonpathogenic. They were characterized by 16S rDNA sequence analysis, by biochemical reactions, and by gas chromatographic analysis of cellular fatty acid (53).
Preparation of crude CW.
Bacterial CWs were isolated as described previously (53). Briefly, the cells were broken with glass beads in an MSK Cell Homogenizer (B. Braun Melsungen AG, Melsungen, Germany). The CWs were collected; treated with DNase I, RNase A, and trypsin; and sonicated in an ice bath for 75 min. The sonicated CW suspension was centrifuged at 10,000 × g at 4°C for 20 min. The supernatant was centrifuged by ultracentrifugation at 100,000 × g at 4°C for 60 min. The pellet containing the CW was resuspended in phosphate-buffered saline (PBS). For i.p. injection into rats, the CW suspension was sterilized by heating at 90°C for 30 min (30). The sterility was confirmed by cultures on agar plates at 37°C and at room temperature under aerobic and anaerobic conditions; no bacterial growth was detected after 48 h of culture. The endotoxin tests (E-TOXATE; Sigma Chemical Co., St. Louis, Mo.) were also negative.
Preparation of soluble PG polymers.
PG was isolated from the CW as described previously (30, 52). The lyophilized CWs were extracted with 10% trichloroacetic acid at 60°C for 4 h. The suspension was centrifuged at 38,800 × g at 4°C for 30 min, and the supernatant was discarded. The pellet containing PG was further treated with proteolytic enzymes, protease, proteinase K, pepsin, and papain (Sigma) as previously described (52) and then lyophilized. Thereafter, the PG was purified with chloroform-methanol, 8 M lithium chloride, 0.1 M EDTA, and acetone to remove possible contaminating proteins, lipoteichoic acid, and endotoxin. The purified PG was sonicated (Branson Sonifier; Smith Kline Co., Danbury, Conn.) for 180 min (18 10-min cycles with breaks for cooling between cycles) in an ice bath. The remaining insoluble PG was removed by centrifugation at 10,000 × g, 4°C, for 30 min. The supernatant was collected and dialyzed against distilled water for 72 h, resulting in soluble PG polymers that were mostly in the Mr range of 5 × 106 to 10 × 106 (30).
Digestion of soluble PG polymers.
Mutanolysin from Streptomyces globisporus (Sigma) was used to digest PG (25, 38). Mutanolysin was added to a final concentration of 500 U/ml/mg of PG in 0.02 M Tris-HCl buffer (pH 6.8). The reaction mixture was incubated at 37°C for 24 h with gentle shaking. The mixture was spun down for 10 min at 15,000 × g, and the supernatant was collected and boiled for 10 min and further centrifuged for 10 min at 15,000 × g to remove the enzyme. The supernatant containing a mixture of PG fractions was dried by rotary evaporation and dissolved in sterile water.
Sensitivity of bacterial CW to lysozyme and mutanolysin.
The sensitivity of bacterial CWs to lysozyme derived from chicken egg white (Sigma) was tested as described previously (44). The CWs were suspended in 0.1 M sodium acetate buffer (pH 5.0) at a concentration of 4 mg/ml. Lysozyme was added to a final concentration of 400 μg/ml, and the suspension was incubated at 37°C with gentle shaking. The optical density at 560 nm (OD560) was measured after 5 and 24 h of incubation. The sensitivity to mutanolysin derived from S. globisporus was also tested as previously described (38). The CWs were suspended in 0.02 M Tris-HCl buffer (pH 6.8) at a concentration of 4 mg/ml. The suspension was incubated with mutanolysin at a final concentration of 500 U/ml at 37°C with gentle shaking. The OD600 of the suspension was recorded after 5 and 24 h of incubation.
Isolation and stimulation of rat peritoneal macrophages.
Naive rats were injected i.p. with 5 ml of 3% sterile Brewer thioglycolate (Becton Dickinson) medium. Peritoneal exudate cells were harvested 6 days later by peritoneal lavage by using 30 ml of Dulbecco modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum (FCS). The cells were washed, resuspended in DMEM supplemented with 10% FCS, and seeded at 1.5 × 106 cells in 1.5 ml per well on 24-well Costar culture plates (Costar, Cambridge, Mass.). The cells were allowed to adhere for 1 h in a humidified incubator at 37°C with 5% CO2, whereafter the nonadherent cells were removed by aspiration. The remaining monolayers of macrophages (judged to be >90% macrophages by microscopy and >90% viable by trypan blue staining) were then stimulated with preparations of the crude CW, soluble PG polymer, or a mixture of digested PG fractions with a final concentration of 100 μg (dry weight)/ml. Lipopolysaccharide (LPS) from E. coli O127:B8 (Sigma) at 100 ng/ml was used for comparison. Supernatants were collected after 8 h of incubation in 5% CO2 at 37°C and stored at −70°C for the assays of TNF-α and MCP-1 production.
Detection of TNF-α and MCP-1.
The concentration of rat TNF-α in the cell culture supernatants was measured by using the Quantikine M rat TNF-α immunoassay kit from R&D Systems Europe, Ltd. (Abington, United Kingdom). The OD450 was determined and then corrected at 540 nm by using a microplate reader (Labsystems Multiskan RC, Helsinki, Finland). The detection limit was 5 pg/ml, as indicated in the manufacturer's instructions. The concentration of rat MCP-1 in the cell culture supernatants was measured by rat MCP-1 enzyme-linked immunosorbent assay kit from BioSource International, Inc. (Camarillo, Calif.). The absorbance was determined at a wavelength of 450 nm by using a Titertek Multiscan plus spectrophotometer (ThermoLabsystems Oy, Helsinki, Finland). The detection limit was 8 pg/ml, as described in the manufacturer's instructions. All tests were performed in duplicate.
Animals and induction of arthritis.
Inbred pathogen-free female Lewis rats weighing ca. 150 g were purchased from Harlan Sprague-Dawley, Inc., Indianapolis, Ind. The animals were kept in Macrolon III cages with disposable filter tops (Scanbur, Denmark) and given autoclaved standard diet and water. All handling was performed in a laminar flow hood. Arthritis was induced by a single i.p. injection of the CW preparations (200 μg [dry weight] of CW/g of body weight) suspended in sterile PBS. Regarding the arthritogenic CW (E. aerofaciens ATCC 25986), this dose contains 8.6 μg of muramic acid per g of body weight. For the nonarthritogenic CW (E. aerofaciens ATCC 35085), the muramic acid dose was 9.1 μg per g of body weight. Control rats were injected with an equal volume of sterile PBS. To evaluate the severity of arthritis, each limb was assessed by a visual scoring from 0 to 4 by two independent observers as described previously (54). A score of 1 indicates a minimal inflammation, and a score of 4 represents extensive swelling and erythema of the ankle and metatarsal joints (wrist and metacarpal joints). The animals were observed for 4 weeks.
GC-MS.
The muramic acid concentration in the CW preparations, as well as in the rat tissues after injection of the bacterial CW, was determined by gas chromatography-mass spectrometry (GC-MS) by using negative chemical ionization as described previously (9), with some modifications. Spleens, livers, synovial tissues, and fluids of ankle joints were collected at five time points after the injection (day 1, day 3, week 1, week 2, and week 4). Spleens and livers were homogenized with an Ultra Turrax T25 tissue homogenizer (Janke & Kunkel, IKA-Labortechnik, Staufen, Germany) in 2 and 5 ml of sterile water, respectively. Synovial tissues and fluids were taken from the ankle joints immediately after the rats were killed. After the skinned ankle joints were opened under sterile conditions, synovial fluids were first drawn by pipette and then combined with sterile water used to rinse the joints. Synovial tissues were separated from skinned ankle joints under sterile conditions. Aliquots of diluted suspension of spleen, liver, and both synovial fluids and tissues were evaporated to dryness under a nitrogen stream at 40°C. After being dried, all of the samples were methanolyzed under a nitrogen atmosphere at 85°C for 24 h in 2 ml of 4 M methanolic hydrochloric acid. The methanolysate was extracted with 3 ml of hexane (SupraSolv purity; Merck & Co., Inc., Rahway, N.J.). After evaporation to dryness at 40°C under a stream of nitrogen, 50 μl of acetonitrile and 50 μl of trifluoroacetic anhydride were added to all samples, standards, and internal standards. The derivatization was performed at 80°C for 5 min. Subsequently, the derivatized samples were allowed to stand for 5 min at room temperature, whereafter 400 μl of toluene was added. In negative chemical ionization analysis, N-methyl-d-glucamine (Sigma) was used as the internal standard and prepared separately, and its concentration was either 30 or 100 pg (final injected amount) depending on the standard curve used in the analysis. Since free muramic acid cannot be used as a standard (9), Eubacterium limosum CW was used as a standard for GC-MS analysis by negative chemical ionization. The muramic acid concentration of this CW was quantified by using electron impact ionization with authentic muramic acid (Sigma) as the standard (53) and processed as described above.
Next, 50-μl portions of derivatized samples and standards were added into vials with 50 μl of derivatized internal standard, and 1 μl was injected into a GC-MS apparatus by pulsed splitless mode with a slow plunger speed. To avoid possible contamination from a previous sample, the cleaning run with pure solvent (toluene) was performed after each sample run. The analysis was performed by negative chemical ionization with selected ion monitoring. The gas chromatograph (Model 6890; Hewlett-Packard, Little Falls, Del.) was coupled to mass selective detector (Model 5973; Hewlett-Packard, Palo Alto, Calif.). The GC-MS apparatus was equipped with an HP-5MS capillary column (30 m by 0.25 mm by 0.25-μm film thickness; Agilent Technologies). The initial oven temperature 80°C was programmed to reach 210°C at the rate of 8°C/min and then held for 1 min; after the run, the oven was heated to 300°C and held for 7 min. The inlet temperature was 250°C. The temperature of MS quadrupole was 106°C, and that of the ion source was 150°C. Helium was used as the carrier gas, and isobutane (ionized at an energy of 150 eV) was used as the reagent gas. The mass-to-charge ratio 567 was used as the target ion for muramic acid quantification, whereas ions with mass-to-charge ratios of 480 and 453 were were used as qualifier ions. The quantification for muramic acid was carried out by a computerized quantitative internal method as described in the Hewlett-Packard G1701AA MS Chemstation workbook (DOS series). The two standard curves used were linear with muramic acid concentrations at 3.7 to 47 pg and at 56 to 150 pg (final injected amount). The concentrations of internal standard were 30 and 100 pg, respectively. The detection limit for muramic acid was defined as 1 pg, giving a signal-to-noise ratio of from 20 to 30.
All glassware used was treated with DECON 90 (Decon Laboratories, Ltd., Sussex, United Kingdom) and 10% DECONEX 11 UNIVERSAL (Borer Chemie Ag, Zuchwil, Switzerland), washed with water, heated at 170°C for 2 h, and autoclaved for 20 min.
Statistics.
Differences between study groups were analyzed by using the Student's t test. P values of <0.05 were considered significant.
RESULTS
Enzyme degradation.
It has been speculated that a CW should be relatively resistant to degradation by lysozyme in order to induce chronic bacterial CW arthritis (19, 35). A recent review of literature (E. Šimelyte et al., unpublished data), however, casts doubt on this view. In the present study, we have tested susceptibility of the arthritogenic and nonarthritogenic CWs to two enzymes hydrolyzing PG: lysozyme and mutanolysin. The results obtained indicate that both of the CWs are affected by lysozyme and mutanolysin (Table 1). After 24 h of digestion, 20.7% of the arthritogenic CW was degraded by lysozyme and 34.7% was degraded by mutanolysin, as judged from changes in OD. Regarding the nonarthritogenic CW, the degradation was considerably greater, i.e., 77.7% by lysozyme and 76.6% by mutanolysin.
TABLE 1.
Enzyme degradation of the arthritogenic and nonarthritogenic CWs
| Origin of CW (strain) | % Decreasea
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Lysozyme (OD560) at:
|
Mutanolysin (OD600) at:
|
|||||||
| 5 h
|
24 h
|
5 h
|
24 h
|
|||||
| − | + | − | + | − | + | − | + | |
| E. aerofaciens 25986 | 2.0 | 15.6 | 1.9 | 20.7 | 0 | 32.2 | 0 | 34.7 |
| E. aerofaciens 35085 | 6.0 | 44.5 | 12.3 | 77.7 | 2.1 | 72.2 | 7.8 | 76.6 |
Results are given the percent decrease of OD after 5 and 24 h of incubation without or with the enzymes compared to the OD at time zero. Mean values from triplicate experiments are given.
Production of TNF-α and MCP-1 by macrophages stimulated with CWs.
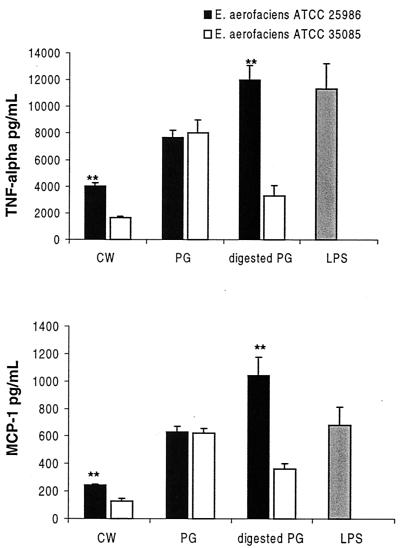
On the basis of the difference observed in the enzyme susceptibility, we decided to test the capacity of the arthritogenic and nonarthritogenic CWs to stimulate the production of the proinflammatory cytokines TNF-α and MCP-1. For this purpose, crude CWs containing PG-polysaccharide complex, isolated PGs, and PGs digested with mutanolysin for 24 h were used. For a comparison, LPS from E. coli was also applied. The following findings are evident (Fig. 2). (i) The crude CW of the arthritogenic E. aerofaciens is a significantly more potent stimulator of TNF-α and MCP-1 than is the crude nonarthritogenic CW (P < 0.01). Such a difference was not observed using isolated PGs. (ii) Mutanolysin digestion affects arthritogenic and nonarthritogenic PGs differently. When the arthritogenic PG was digested, a considerable increase (P < 0.02) occurs in its capacity to stimulate the production of the proinflammatory cytokines. In contrast, the same treatment of the nonarthritogenic PG results in a decreased stimulatory capacity (P < 0.02). The stimulatory power of LPS was in the same range as that of the PGs.
FIG. 2.
Production of TNF-α and MCP-1 by rat peritoneal macrophages after stimulation with crude CW, isolated PG, PG digested with mutanolysin, and LPS. CWs derived from an arthritogenic strain (E. aerofaciens ATCC 25986) and a nonarthritogenic strain (E. aerofaciens ATCC 35085) were used. Asterisks indicate significant difference between the arthritogenic and nonarthritogenic preparations (∗∗, P < 0.01). The results are given as mean values plus the standard error of the mean (SEM) of three separate experiments. For each experiment, macrophages were pooled from two to three rats, and the cytokine production was determined in duplicate.
Tissue distribution of arthritogenic and nonarthritogenic CWs.
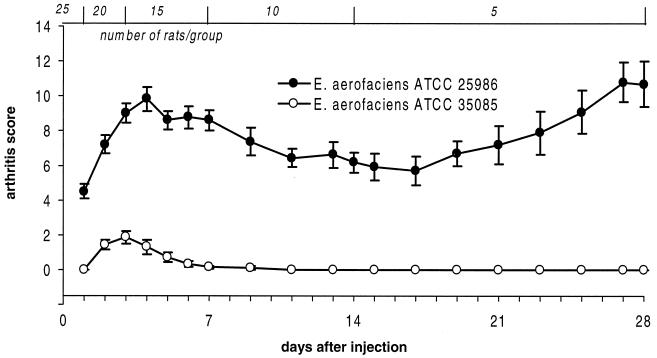
Previous findings have suggested that bacterial CWs that are able to induce chronic arthritis accumulate in the tissues in large quantities and persist there for a long time (8, 40, 41, 43). Regarding their presence in the joint tissues, different results have been presented (8, 12, 41, 43). Therefore, we undertook to clarify the tissue distribution of the arthritogenic and nonarthritogenic Eubacterium CWs after an i.p. injection. As expected, a single i.p. injection of the CW of E. aerofaciens ATCC 25986 induced a severe acute arthritis, followed by a mild remission phase, and developed further toward chronicity. In contrast, the CW of the nonarthritogenic E. aerofaciens ATCC 35085 only induced weak signs of acute arthritis that completely subsided in 7 days without any evidence of chronicity (Fig. 3). None of the rats injected with PBS alone developed arthritis.
FIG. 3.
Development of arthritis after a single i.p. injection of CW isolated from an arthritogenic or nonarthritogenic E. aerofaciens. Each circle represents the mean ± the SEM for 5 to 25 rats, as indicated across the top.
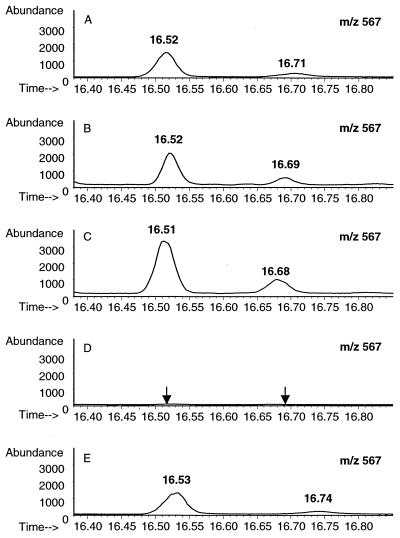
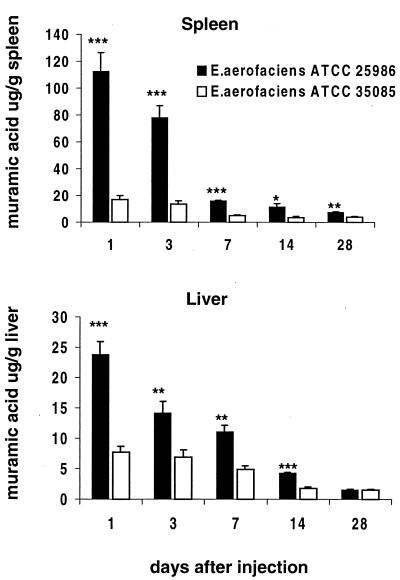
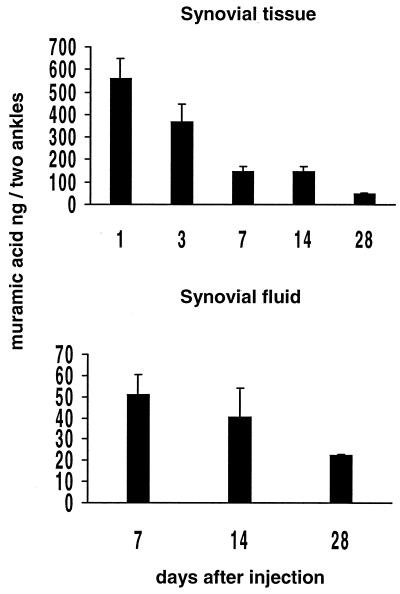
Muramic acid was used as a marker to monitor the tissue distribution of CWs after the i.p. injection. Muramic acid was identified by GC-MS based on the target ions and retention time and was further confirmed by mixing a known amount of muramic acid with the sample analyzed (Fig. 4). At different time points after the i.p. injection, the amount of muramic acid was significantly higher in the spleens and livers of the rats injected with the arthritogenic CW than in those from the rats injected with the nonarthritogenic CW (Fig. 5). The concentrations of muramic acid observed in the spleen and liver reflect each other, as has also been described previously (1, 8, 20, 40, 41, 43). Muramic acid was detected in the synovial tissues from day 1 to day 28 and in the synovial fluids analyzed on days 7, 14, and 28 (Fig. 6). The concentrations in the synovial tissue were always two- to fourfold higher than those in the synovial fluid. It is significant that muramic acid was not detected in the synovial tissues or fluids of the rats injected with the nonarthritogenic CW, not even on day 3 when the acute arthritis was at its peak. The results from these analyses as well as from those of the synovial tissues and fluids from the rats injected with PBS alone were below the detection limit of our method (1 pg of muramic acid giving a signal-to-noise ratio of 20 to 30).
FIG. 4.
Detection, by GC-MS, of muramic acid in the synovial fluid on day 28 after i.p. injection of the arthritogenic CW. The x axis represents the running time (min), and the y axis represents the absolute abundance of molecular ion of muramic acid (mass-to-charge ratio of 567). The samples were derivatized by use of trifluoroacetic anhydride and analyzed by using negative chemical ionization as described in Materials and Methods. Muramic acid was observed as a major and as a minor peak with different retention times as indicated. (A) Standard muramic acid (5.60 pg) from E. limosum CW. (B) Detection of muramic acid (11.09 pg) in the synovial fluid of a rat injected with the arthritogenic CW. (C) The detection of muramic acid in the synovial fluid was further confirmed by adding a known amount (5.60 pg) of muramic acid into the synovial fluid analyzed in panel B. The amount observed (16.35 pg) is approximately equal to the expected amount (5.60 + 11.09 pg). (D) Absence of muramic acid in the synovial fluid of a rat injected with the nonarthritogenic CW. (E) The absence of muramic acid in synovial fluid was confirmed by adding a known amount (5.60 pg) of muramic acid into the synovial fluid analyzed in panel D. The amount observed (5.42 pg) is approximately equal to the expected amount (5.60 + 0.00 pg).
FIG. 5.
Muramic acid in the spleen and liver at different time points after i.p. injection of arthritogenic or nonarthritogenic CWs. For each time point, the organs were taken from five rats. Mean values plus the SEM are indicated. Asterisks indicate significant differences between the arthritogenic and nonarthritogenic CWs on each day (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
FIG. 6.
Muramic acid in the synovial tissue and synovial fluid at different time points after i.p. injection of the arthritogenic CW. Muramic acid was not found in the synovial tissues or fluids of the rats injected with the nonarthritogenic CW. The detection limit was 1 pg of muramic acid, giving a signal-to-noise ratio of 20 to 30. For each time point, the synovial tissues and synovial fluids were collected from five rats. Mean values plus the SEM are shown.
DISCUSSION
Our results indicate that a crude CW preparation of the arthritogenic E. aerofaciens is a twofold-more-potent stimulator of the proinflammatory cytokines TNF-α and MCP-1 than is a similar preparation made from the nonarthritogenic strain of E. aerofaciens (Fig. 2). However, in trying to explain why only one of these nearly identical bacterial strains is arthritogenic, the effects of enzyme hydrolysis on the stimulatory capacity are even more remarkable. Instead of the CW, we used the enzyme-digested PG to test production of proinflammatory cytokines in vitro, due to the observation that the PG moiety determines arthritogenicity of the bacterial CW (52). Both arthritogenic and nonarthritogenic PGs were degraded by mutanolysin to a roughly similar extent, in 52 to 65%, respectively. The resulting fragments of both were collected by the same procedure and are assumed to be of similar sizes. However, the fragments of the arthritogenic PG have a significantly higher stimulatory capacity than the undigested PG. With the nonarthritogenic PG the effect of the degradation on the stimulatory capacity is completely opposite, which is a considerable decrease. This effect can be summarized by concluding that after enzyme degradation the arthritogenic PG has a four- to fivefold-stronger stimulatory capacity than that of the degraded nonarthritogenic PG. This applies to the production of both TNF-α and MCP-1.
It should be emphasized that the bacterially derived mutanolysin breaks the same β-1,4-glycosidic bonds as does the mammalian lysozyme (38). Treatment of PG with either one of these two enzymes results in fragments of various sizes (3, 25, 45) (Fig. 1). The present results do not allow conclusions about the identity of the fragments with a low or a high stimulatory capacity. However, it is apparent that the high proinflammatory capacity of the fragments released from the arthritogenic PG is due to their composition and structure. The fragment sizes seem to be less important, since the level of mutanolysin degradation of both PGs was roughly equal. Studies with mutanolysin treated Streptococcus pneumoniae CW have indicated that fragments containing stem peptides carry a high TNF-α stimulating activity (25). Such fragments of the arthritogenic E. aerofaciens PG but not of the nonarthritogenic one have lysine in position 3 of the stem peptide (53), which is known to be associated with high proinflammatory activity (3, 25). On the other hand, stem peptides alone are 10-fold less active than is the undigested PG (25). Currently, isolation of the proinflammatory PG fragments by using high-performance liquid chromatography with the purpose of identification is in progress. It has been shown that PG can bind CD14 on the macrophages but that PG fragments cannot (6). Therefore, the proinflammatory PG fragments may use a different strategy to stimulate macrophages for the cytokine production. When enzyme hydrolysis affects different CWs differently regarding their proinflammatory capacity, it becomes understandable that arthritogenicity of a CW cannot be dependent on its enzyme resistance or susceptibility alone. It is also decisive whether the PG fragments released in vivo can stimulate production of proinflammatory cytokines. In a recent study, no difference between arthritogenic and nonarthritogenic Lactobacillus CWs was observed in their capacity to stimulate production of TNF-α, IL-1β, or IL-10 (39). That study was performed with crude CWs with a relatively low stimulatory power. Therefore, those results do not contradict our present conclusions.
On the other hand, it is not known exactly how CWs are digested in vivo after they are injected into rats. It should be noted that when crude CWs were tested for cytokine stimulation, a difference between the arthritogenic and nonarthritogenic CWs was observed, whereas with isolated PGs the difference became manifest only after enzyme digestion (Fig. 2). Since the nonarthritogenic CW is effectively degraded by muralytic enzymes, it is apparent that the resulting CW fragments are smaller than those released from the arthritogenic CW. Such a difference indicates that only part of the susceptible bonds are broken. The small CW fragments are eliminated from the body more rapidly than the larger fragments (10, 43). This is reflected in the concentration and persistence of the muramic acid in the spleen, liver, synovial tissue, and synovial fluid. The concentrations of muramic acid derived from the arthritogenic CW are throughout our study, almost without exception, significantly higher than those of the muramic acid derived from the nonarthritogenic strain (Fig. 5 and 6). In the synovial tissue or synovial fluid, muramic acid derived from the nonarthritogenic strain was observed not at all, not even on day 3 when the acute mild arthritis was at its peak. It still remains possible that degradation products of nonarthritogenic CWs reach synovial tissues in minute quantities, since, by immunofluorescence, antigens of E. limosum have been detected in the synovial tissue at day 14 after a single i.p. injection (41).
Compared to earlier studies in which the presence of CW degradation products in the joint tissues was measured quantitatively (1, 8, 11, 12, 20, 43), our results allow a direct comparison between the injected amount and that present in the joints. The sensitivity of GC-MS in the detection of muramic acid was improved by using negative chemical ionization; the detection limit was ca. 1 pg of the derivatized muramic acid, giving a signal-to-noise ratio of 20 to 30. The identification of muramic acid was made not only by retention time but also by spiking the tissues with standard muramic acid (Fig. 4). Our results are consistent with those of Kozar et al. (18), who found muramic acid to be absent in the normal rat spleens and brains. It appears that on day 1 after an i.p. injection ca. 0.04% of the injected muramic acid was observed in the tissues of the two ankles (Fig. 6). An open, interesting question remains as to why such an amount of muramic acid representing phlogistic PG fragments does not lead to chronic arthritis also in arthritis resistance strains of rats (1, 20, 39).
Our results are in line with the view that the development of chronic bacterial CW arthritis is dependent upon deposition and the persistence of CWs in the synovial tissues (8, 43, 50), with the liver and spleen acting as reservoirs releasing CW fragments to circulation and the joint tissues (43). Regarding the pathogenesis of human rheumatoid arthritis, it should be emphasized that (i) the E. aerofaciens strains used here originate in the normal gut flora of humans, (ii) degradation products derived from the normal gut flora are present in the circulation of healthy individuals (21, 22), and (iii) bacterial CW debris has been demonstrated in the synovial tissues of patients with inflammatory arthritis (23, 26, 29, 34, 47). Therefore, the bacterial composition of the individual intestinal flora might represent a crucial factor contributing to the potential role of intestinal bacteria in the pathogenesis of rheumatoid arthritis.
ACKNOWLEDGMENTS
We gratefully thank L. Kivistö, M.-R. Teräsjärvi, M. Suominen, and S. Niittoaho for excellent technical assistance; S. Lindqvist for taking care of the animals; Jari Jalava for 16S rDNA sequence analysis; and J. Uksila for kind advice and helpful discussion.
This work was supported by EVO of Turku University Central Hospital.
REFERENCES
- 1.Anderle S K, Allen J B, Wilder R L, Eisenberg R A, Cromartie W J, Schwab J H. Measurement of streptococcal cell wall in tissue of rats resistant or susceptible to cell wall-induced chronic erosive arthritis. Infect Immun. 1985;49:836–837. doi: 10.1128/iai.49.3.836-837.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan F M, Maini R N, Feldmann M. Role of pro-inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:133–147. doi: 10.1007/BF00832003. [DOI] [PubMed] [Google Scholar]
- 3.Burroughs M, Rozdzinski E, Geelen S, Tuomanen E. A structure-activity relationship for induction of meningeal inflammation by muramyl peptides. J Clin Investig. 1993;92:297–302. doi: 10.1172/JCI116565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chetty C, Brown R R, Schwab J H. Edema-producing activity of group A streptococcal polysaccharide and its possible role in the pathogenesis of cell wall-induced polyarthritis. J Exp Med. 1983;157:1089–1100. doi: 10.1084/jem.157.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Kimpe S, Kengatharan M, Thiemermann C, Vane J. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dziarski R, Tapping R I, Tobias P S. Binding of bacterial peptidoglycan to CD14. J Biol Chem. 1998;273:8680–8690. doi: 10.1074/jbc.273.15.8680. [DOI] [PubMed] [Google Scholar]
- 7.Eerola E, Möttönen T, Hannonen P, Luukkainen R, Kantola I, Vuori K, Tuominen J, Toivanen P. Intestinal flora in early rheumatoid arthritis. Br J Rheumatol. 1994;33:1030–1038. doi: 10.1093/rheumatology/33.11.1030. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg R, Fox A, Greenblatt J J, Anderle S K, Cromartie W J, Schwab J H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982;38:127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmroth I, Fox A, Larsson L. Determination of bacterial muramic acid by gas chromatography-mass spetrometry with negative-ion detection. J Chromatogr. 1993;628:93–102. [Google Scholar]
- 10.Fox A, Brown R R, Anderle S K, Chetty C, Cromartie W J, Gooder H, Schwab J H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982;35:1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox A, Schwab J H, Cochran T. Muramic acid detection in mammalian tissues by gas-liquid chromatography-mass spectrometry. Infect Immun. 1980;29:526–531. doi: 10.1128/iai.29.2.526-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbart J, Fox A. Elimination of group A streptococcal cell walls from mammalian tissues. Infect Immun. 1987;55:1526–1528. doi: 10.1128/iai.55.6.1526-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong J-H, Ratkay L G, Waterfield J D, Clark-Lewis I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med. 1997;186:131–137. doi: 10.1084/jem.186.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazenberg M P, Klasen I S, Kool J, Ruseler-van Embden J G H, Severijnen A J. Are intestinal bacteria involved in the etiology of rheumatoid arthritis? APMIS. 1992;100:1–9. doi: 10.1111/j.1699-0463.1992.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 15.Kageyama A, Benno Y, Nakase T. Phylogenetic and phenotypic evidence for the transfer of Eubacterium aerofaciens to the genus Collinsella as Collinsella aerofaciens gen. nov., comb. nov. Int J Syst Bacteriol. 1999;49:557–565. doi: 10.1099/00207713-49-2-557. [DOI] [PubMed] [Google Scholar]
- 16.Kengatharan K M, De Kimpe S, Robson C, Foster S J, Thiemermann C. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J Exp Med. 1998;188:305–315. doi: 10.1084/jem.188.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotani S, Tsujimoto M, Koga T, Nagao S, Tanaka A, Kawata S. Chemical structure and biological activity relationship of bacterial cell walls and muramyl peptides. Fed Proc. 1986;45:2534–2540. [PubMed] [Google Scholar]
- 18.Kozar M P, Krahmer M T, Fox A, Gray B M. Failure to detect muramic acid in normal rat tissues but detection in cerebrospinal fluids from patients with pneumococcal meningitis. Infect Immun. 2000;68:4688–4698. doi: 10.1128/iai.68.8.4688-4698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehman T J, Allen J B, Plotz P H, Wilder R L. Bacterial cell wall composition, lysozyme resistance, and the induction of chronic arthritis in rats. Rheumatol Int. 1985;5:163–167. doi: 10.1007/BF00541517. [DOI] [PubMed] [Google Scholar]
- 20.Lehman T J, Allen J B, Plotz P H, Wilder R L. Lactobacillus casei cell wall-induced arthritis in rats: cell wall fragment distribution and persistence in chronic arthritis-susceptible LEW/N and -resistant F344/N rats. Arthritis Rheum. 1984;27:939–942. doi: 10.1002/art.1780270815. [DOI] [PubMed] [Google Scholar]
- 21.Lehtonen L, Eerola E, Oksman P, Toivanen P. Muramic acid in peripheral blood leukocytes of healthy human subjects. J Infect Dis. 1995;171:1060–1064. doi: 10.1093/infdis/171.4.1060. [DOI] [PubMed] [Google Scholar]
- 22.Lehtonen L, Eerola E, Toivanen P. Muramic acid in human peripheral blood leucocytes in different age groups. Eur J Clin Investig. 1997;27:791–792. doi: 10.1046/j.1365-2362.1997.1950732.x. [DOI] [PubMed] [Google Scholar]
- 23.Lehtonen L, Kortekangas P, Oksman P, Eerola E, Aro H, Toivanen A. Synovial fluid muramic acid in acute inflammatory arthritis. Br J Rheumatol. 1994;33:1127–1130. doi: 10.1093/rheumatology/33.12.1127. [DOI] [PubMed] [Google Scholar]
- 24.Luker K E, Collier J L, Kolodziej E W, Marshall G R, Doldman W E. Bordetella pertussis tracheal cytotoxin and other muramyl peptides: distinct structure-activity relationships for respiratory epithelial cytopathology. Proc Natl Acad Sci USA. 1993;90:2365–2369. doi: 10.1073/pnas.90.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majcherczyk P A, Langen H, Heumann D, Fountoulakis M, Glauser M P, Moreillon P. Digestion of Streptococcus pneumoniae cell walls with its major peptidoglycan hydrolase releases branched stem peptides carrying proinflammatory activity. J Biol Chem. 1999;274:12537–12543. doi: 10.1074/jbc.274.18.12537. [DOI] [PubMed] [Google Scholar]
- 26.Melief M-J, Hoijer M A, Van Paassen H C, Hazenberg M P. Presence of bacterial flora-derived antigen in synovial tissue macrophages and dendritic cells. Br J Rheumatol. 1995;34:1112–1116. doi: 10.1093/rheumatology/34.12.1112. [DOI] [PubMed] [Google Scholar]
- 27.Ogata H, Takeya M, Yoshimura T, Takagi K, Takahashi K. The role of monocyte chemoattractant protein-1 (MCP-1) in the pathogenesis of collagen-induced arthritis in rats. J Pathol. 1997;182:106–114. doi: 10.1002/(SICI)1096-9896(199705)182:1<106::AID-PATH816>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 28.Peltonen R, Kjeldsen Kragh J, Haugen M, Tuominen J, Toivanen P, Forre O, Eerola E. Changes of faecal flora in rheumatoid arthritis during fasting and one-year vegetarian diet. Br J Rheumatol. 1994;33:638–643. doi: 10.1093/rheumatology/33.7.638. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard D G, Settine R L, Bennett J C. Sensitive mass spectrometric procedure for the detection of bacterial cell wall components in rheumatoid joints. Arthritis Rheum. 1980;23:608–610. doi: 10.1002/art.1780230514. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal R S, Dziarski R. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol. 1994;235:253–285. doi: 10.1016/0076-6879(94)35146-5. [DOI] [PubMed] [Google Scholar]
- 31.Sartor R B. Role of the enteric microflora in the pathogenesis of intestinal inflammation and arthritis. Aliment Pharmacol Ther. 1997;11:17–23. doi: 10.1111/j.1365-2036.1997.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 32.Schimmer R C, Schrier D J, Flory C M, Laemont K D, Tung D, Metz A L, Friedl H P, Conroy M C, Warren J S, Beck B, Ward P A. Streptococcal cell wall-induced arthritis: requirements for IL-4, IL-10, IFN-gamma, and monocyte chemoattractant protein-1. J Immunol. 1998;160:1466–1471. [PubMed] [Google Scholar]
- 33.Schrier D J, Schimmer R C, Flory C M, Tung D K, Ward P A. Role of chemokines and cytokines in a reactivation model of arthritis in rats induced by injection with streptococcal cell walls. J Leukoc Biol. 1998;63:359–363. doi: 10.1002/jlb.63.3.359. [DOI] [PubMed] [Google Scholar]
- 34.Schrijver I A, Melief M-J, Tak P P, Hazenberg M P, Laman J D. Antigen-presenting cells containing bacterial peptidoglycan in synovial tissues of rheumatoid arthritis patients coexpress costimulatory molecules and cytokines. Arthritis Rheum. 2000;43:2160–2168. doi: 10.1002/1529-0131(200010)43:10<2160::AID-ANR3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 35.Schwab J H. Phlogistic properties of peptidoglycan-polysaccharide polymers from cell walls of pathogenic and normal-flora bacteria which colonize humans. Infect Immun. 1993;61:4535–4539. doi: 10.1128/iai.61.11.4535-4539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 37.Severijnen A J, Kool J, Swaak A J, Hazenberg M P. Intestinal flora of patients with rheumatoid arthritis: induction of chronic arthritis in rats by cell wall fragments from isolated Eubacterium aerofaciens strains. Br J Rheumatol. 1990;29:433–439. doi: 10.1093/rheumatology/29.6.433. [DOI] [PubMed] [Google Scholar]
- 38.Siegel J L, Hurst S F, Liberman E S, Coleman S E, Bleiweis A S. Mutanolysin-induced spheroplasts of Streptococcus mutans are true protoplasts. Infect Immun. 1981;31:808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Šimelyte E, Isomäki P, Rimpiläinen M, Zhang X, Toivanen P. Cytokine production in arthritis susceptible and resistant rats: a study with arthritogenic and non-arthritogenic Lactobacillus cell walls. Scand J Immunol. 2001;53:132–138. doi: 10.1046/j.1365-3083.2001.00846.x. [DOI] [PubMed] [Google Scholar]
- 40.Šimelyte E, Rimpiläinen M, Lehtonen L, Zhang X, Toivanen P. Bacterial cell wall-induced arthritis: chemical composition and tissue distribution of four Lactobacillus strains. Infect Immun. 2000;68:3535–3540. doi: 10.1128/iai.68.6.3535-3540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Šimelyte E, Rimpiläinen M, Rantakokko K, Lehtonen L, Zhang X, Aho H, Isomäki P, Toivanen P. Tissue distribution and persistence of arthritogenic and nonarthritogenic Eubacterium cell walls. Clin Exp Rheumatol. 1999;17:281–288. [PubMed] [Google Scholar]
- 42.Stewart-Tull D E S. The immunological activities of bacterial peptidoglycans. Annu Rev Microbiol. 1980;34:311–340. doi: 10.1146/annurev.mi.34.100180.001523. [DOI] [PubMed] [Google Scholar]
- 43.Stimpson S A, Esser R E, Cromartie W J, Schwab J H. Comparison of in vivo degradation of 125I-labeled peptidoglycan-polysaccharide fragments from group A and group D streptococci. Infect Immun. 1986;52:390–396. doi: 10.1128/iai.52.2.390-396.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stimpson S A, Lerch R A, Cleland D R, Yarnall D P, Clark R L, Cromartie W J, Schwab J H. Effect of acetylation on arthropathic activity of group A streptococcal peptidoglycan-polysaccharide fragments. Infect Immun. 1987;55:16–23. doi: 10.1128/iai.55.1.16-23.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Striker R, Kline M E, Haak R A, Rest R F, Rosenthal R S. Degradation of gonococcal peptidoglycan by granule extract from human neutrophils: demonstration of N-acetylglucosaminidase activity that utilizes peptidoglycan substrates. Infect Immun. 1987;55:2579–2584. doi: 10.1128/iai.55.11.2579-2584.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toivanen P. From reactive arthritis to rheumatoid arthritis. J Autoimmun. 2001;16:369–371. doi: 10.1006/jaut.2000.0496. [DOI] [PubMed] [Google Scholar]
- 47.van der Heijden I M, Wilbrink B, Tchetverikov I, Schrijver I A, Schouls L M, Hazenberg M P, Breedveld F C, Tak P P. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000;43:593–598. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Wang J E, Jorgensen P F, Almlof M, Thiemermann C, Foster S J, Aasen A O, Solberg R. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect Immun. 2000;68:3965–3970. doi: 10.1128/iai.68.7.3965-3970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z-M, Liu C, Dziarski R. Chemokines are the main proinflammatory mediators in human monocytes activated by Staphylococcus aureus, peptidoglycan, and endotoxin. J Biol Chem. 2000;275:20260–20267. doi: 10.1074/jbc.M909168199. [DOI] [PubMed] [Google Scholar]
- 50.Wilder R L, Allen J B, Wahl L M, Calandra G B, Wahl S M. The pathogenesis of group A streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1983;26:1442–1451. doi: 10.1002/art.1780261205. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura A, Lien E, Ingalls R R, Tuomanen E, Dziarski R, Golenbock D. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 52.Zhang X, Rimpiläinen M, Šimelyte E, Toivanen P. Characterization of Eubacterium cell wall: peptidoglycan structure determines arthritogenicity. Ann Rheum Dis. 2001;60:269–274. doi: 10.1136/ard.60.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Rimpiläinen M, Šimelyte E, Toivanen P. What determines arthritogenicity of bacterial cell wall? A study on Eubacterium cell wall-induced arthritis. Rheumatology. 2000;39:274–282. doi: 10.1093/rheumatology/39.3.274. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Gripenberg Lerche C, Söderström K O, Toivanen A, Toivanen P. Antibiotic prophylaxis and treatment of reactive arthritis: lessons from an animal model. Arthritis Rheum. 1996;39:1238–1243. doi: 10.1002/art.1780390725. [DOI] [PubMed] [Google Scholar]