To the Editor:
Assessment of predonation glomerular filtration rate (GFR) is a key aspect of the evaluation of potential living kidney donors. In the United States, measurement of donor GFR is a regulatory requirement and most commonly assessed using 24-hour timed creatinine clearance (CrCl24), despite the potential for error due to incorrectly timed urine sample collection and tubular creatinine secretion.1,2 We aimed to determine the real-world performance of CrCl24 in living donor candidates.
We performed a retrospective cross-sectional study of living kidney donor candidates evaluated at our center. This study was approved by the Columbia University Medical Center institutional review board (#AAAI1288). We identified 279 consecutive candidates who underwent cold iothalamate clearance testing from 2018-2021 for GFR assessment as part of living kidney donation evaluation. At our center, a GFR ≥80 mL/min/1.73 m2 is used to determine suitability for donation for most candidates. Donor candidates were referred for iothalamate clearance testing if either Chronic Kidney Disease Epidemiology Collaboration 2009 creatinine-based estimated GFR (eGFR) or CrCl24 was <90 mL/min/1.73 m2, if the candidate was unable to perform a timed urinary collection, or if the testing was requested by the evaluating nephrologist. After excluding donors with incomplete data (see detailed methods in Item S1), we analyzed a final cohort of 212 donor candidates.
Demographic information was obtained from the medical record. Body surface area was calculated using the Gehan & George formula.3 Donor candidates performed ambulatory 24-hour urine collections, CrCl24 was calculated as the product of 24-hour urinary creatinine concentration and urine volume divided by serum creatinine concentration, then adjusted for body surface area. Serum creatinine and cystatin C values were used to calculate eGFR using the Chronic Kidney Disease Epidemiology Collaboration 2021 combined creatinine and cystatin C equation (eGFRcrcys).4 “Measured” GFR (mGFR) was determined based on cold iothalamate clearance using the Bröchner-Mortensen correction and adjusted for body surface area (Item S1).5 Bias for each GFR estimate equation was calculated as [mGFR - estimate]. All GFR and bias values below are presented in units mL/min/1.73 m2.
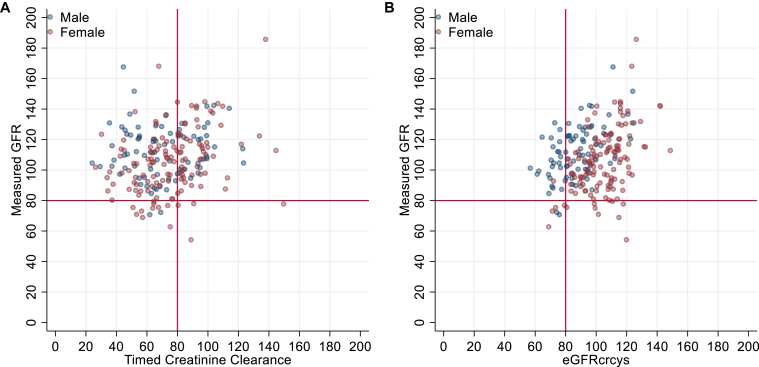
Among 212 donor candidates analyzed, median age was 54 years, and 62% were female. Body size parameters are presented in Table 1. Median mGFR was 107 (IQR, 95-120). Median weight-indexed 24-hour creatinine excretion was 21.9 mg/kg (IQR, 16.5-26.0) for males and 15.9 (IQR, 12.8-18.7) for females, and median CrCl24 was 73 (IQR, 58-89). Median serum creatinine was 0.89 mg/dL and median cystatin C was 0.8 mg/L, corresponding to median eGFRcrcys 97 (IQR, 85-111). Scatterplots of mGFR versus CrCl24 and eGFRcrcys are shown in Fig 1. Overall, median bias for CrCl24 was 33.9 (IQR, 16.3-50.7), including 40.0 (IQR, 20.5-63.3) for males and 32.1 (IQR, 14.2-46) for females. Median bias for eGFRcrcys was 10.5 (IQR, -1.7 to 25.4), including 25.6 (IQR, 13.4-36.0) for males and 2.7 (IQR, -11.0 to 13.6) for females.
Table 1.
Characteristics of Donor Candidates Analyzed
|
n (col %) or Median (IQR) |
All |
Male |
Female |
|---|---|---|---|
| n = 212 (100%) | n = 80 (38%) | n = 132 (62%) | |
| Age, y | 54 (43-61) | 49 (37-58) | 57 (47-62) |
| Race | |||
| White | 138 (65%) | 46 (58%) | 92 (70%) |
| Black/African American | 19 (9%) | 11 (14%) | 8 (6%) |
| All others | 55 (26%) | 23 (29%) | 32 (24%) |
| Height, cm | 168 (163-175) | 175 (170-180) | 163 (159-170) |
| Weight, kg | 79 (66-88) | 76 (63-85) | 82 (74-93) |
| Body mass index, kg/m2 | 27 (24-31) | 27 (24-30) | 28 (24-32) |
| Body surface area, m2 | 2.06 (1.90-2.19) | 2.16 (2.05-2.30) | 1.98 (1.87-2.13) |
| 24-h creatinine excretion, g | 1.29 (1.06-1.67) | 1.75 (1.39-2.22) | 1.16 (0.96-1.36) |
| Weight-indexed 24-h creatinine excretion, mg/kg | 17.4 (13.5-21.8) | 21.9 (16.5-26.0) | 15.9 (12.8-18.7) |
| Serum creatinine, mg/dL | 0.89 (0.76-1.00) | 1.07 (0.93-1.15) | 0.81 (0.73-0.90) |
| Cystatin C, mg/L | 0.8 (0.8-0.9) | 0.8 (0.8-1.0) | 0.8 (0.7-0.9) |
| GFR assessments, mL/min/1.73 m2 | |||
| Measured GFR (iothalamate) | 107 (95-120) | 111 (100-123) | 106 (91-117) |
| CKD-EPI 2021 (creatinine) | 90 (77-104) | 88 (79-103) | 91 (76-104) |
| CKD-EPI 2012 (cystatin C) | 99 (83-110) | 105 (86-116) | 98 (82-105) |
| CKD-EPI 2021 (combined) | 97 (85-111) | 85 (76-96) | 106 (94-115) |
| Timed creatinine clearance | 73 (58-89) | 67 (54-86) | 75 (63-89) |
Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; GFR, glomerular filtration rate.
Figure 1.
Measured glomerular filtration rate (GFR) versus 24-hour timed creatinine clearance (A) and estimated GFR based on the Chronic Kidney Disease Epidemiology Collaboration 2021 creatinine-cystatin C equation (eGFRcrcys) (B). Red lines indicate 80 mL/min/1.73 m2, a typical threshold used for suitability for living kidney donation.
Using a GFR-based donation eligibility threshold of 80, 119 (56%) donors had discordant classification using CrCl24 versus mGFR (Table S1). Of these, 115 (54% of all candidates and 97% of those with discordant classification) had mGFR ≥ 80 but CrCl24 < 80, likely a reflection of the underlying selection bias of the cohort.
We next sought to determine whether urine collection adequacy (as reflected by weight-indexed 24-hour creatinine excretion) or similarity in CrCl24 and eGFRcrcys results could be used as indicators of low CrCl24 bias. Among males with creatinine excretion 20-25 mg/kg (n=23) and females with creatinine excretion 15-20 mg/kg (n=49), median bias was 32.2 (IQR, 14.5-46.7) (Fig S1).
Only 70 (33%) candidates had eGFRcrcys within 20% of CrCl24. Although there was a positive relationship between the absolute bias of CrCl24 and the absolute difference between CrCl24 and eGFRcrcys (r2 = 0.34, P < 0.001, Fig 1, Fig S2), CrCl24 bias remained high even when the difference between both estimates was small. Even among the 89 donor candidates with eGFRcrcys within 20 mL/min/1.73 m2 of CrCl24, median bias was 22.1 (IQR, 11.5-37.2), suggesting that similarity between CrCl24 and eGFRcrcys does not imply that CrCl24 approximates mGFR well.
Given the large median bias we observed, CrCl24 appears to be a suboptimal method of “measuring” GFR in a subset of potential living kidney donors despite current regulatory policies requiring GFR assessment using “isotopic methods or a creatinine clearance calculated from a 24-hour urine collection.”6 This inaccuracy likely stems from the challenges of accurately collecting timed urine samples in an ambulatory setting. Our study may be limited by selection bias, given that participants were healthy and only selected donor candidates were referred for iothalamate clearance testing, thereby enriching our cohorts for individuals with eGFR or CrCl24 that underestimated mGFR. Additionally, potential deviation of iothalamate-based mGFR from true GFR may influence our results. However, given that CrCl24 does not appear to accurately reflect GFR in a subset of candidates—and that CrCl24 bias remained large even among those with creatinine excretion suggesting “adequate” urinary collection and those with agreement between CrCl24 and eGFRcrcys results—additional study is needed to determine how to best evaluate kidney function during living kidney donor evaluations and identify which donor candidates may warrant more accurate GFR assessments.
Article Information
Authors’ Contributions
Research idea and study design: SAH, KLK, AKL, SC, SM; Data acquisition: SAH, JSS, SYRJ, MC; Statistical analysis: SAH; Data analysis/interpretation: SAH, JSS, KLK, SYRJ, MC, AKL, SC, SM; Supervision or mentorship: AKL, SC, SM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Dr Husain was supported by Nelson Family Foundation and a Nelson Family Faculty Development Award and NIH grant K23DK133729. Dr Mohan was supported by NIH grants DK114893, DK116066, DK126739, DK130058, and MD014161 and a Nelson Family Faculty Development Award. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received August 5, 2022 as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form September 18, 2022.
Footnotes
Figure S1: Bias of 24-hour timed creatinine clearance versus weight-indexed 24-hour creatinine excretion.
Figure S2: Absolute value of the bias of 24-hour timed creatinine clearance (CrCl24) versus the absolute difference between the 24-hour timed creatinine clearance and the estimated glomerular filtration rate based on the 2021 CKD-EPI creatinine-cystatin C equation (eGFRcrcys).
Item S1: Supplementary Methods.
Table S1: Reclassification of Glomerular Filtrate Rate (GFR) Based Donor Eligibility Using Measured GFR Versus Timed Creatinine Clearance.
Supplementary Material
Fig S1-S2; Item S1; Table S1.
References
- 1.Garg N., Lentine K.L., Inker L.A., et al. The kidney evaluation of living kidney donor candidates: US practices in 2017. Am J Transplant. 2020;20(12):3379–3389. doi: 10.1111/ajt.15951. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X., McCulloch C.E., Lin F., et al. Measurement error as alternative explanation for the observation that CrCl/GFR ratio is higher at lower GFR. Clin J Am Soc Nephrol. 2016;11(9):1574–1581. doi: 10.2215/CJN.12821215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gehan E.A., George S.L. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54(4):225–235. [PubMed] [Google Scholar]
- 4.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bröchner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest. 1972;30(3):271–274. doi: 10.3109/00365517209084290. [DOI] [PubMed] [Google Scholar]
- 6.Organ Procurement and Transplantation Network (OPTN). Policies. Accessed May 6, 2022. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1-S2; Item S1; Table S1.