Abstract
Background
Extrachromosomal circular DNA (eccDNA) is a kind of DNA that widely exists in eukaryotic cells. Studies in recent years have shown that eccDNA is often enriched during tumors and aging, and participates in the development of cell physiological activities in a special way, so people have paid more and more attention to the eccDNA, and it has also become a critical new topic in modern biological research.
Description
We built a database to collect eccDNA, including animals, plants and fungi, and provide researchers with an eccDNA retrieval platform. The collected eccDNAs were processed in a uniform format and classified according to the species to which it belongs and the chromosome of the source. Each eccDNA record contained sequence length, start and end sites on the corresponding chromosome, order of the bases, genomic elements such as genes and transposons, and other information in the respective sequencing experiment. All the data were stored into the TeCD (The eccDNA Collection Database) and the BLAST (Basic Local Alignment Search Tool) sequence alignment function was also added into the database for analyzing the potential eccDNA sequences.
Conclusion
We built TeCD, a platform for users to search and obtain eccDNA data, and analyzed the possible potential functions of eccDNA. These findings may provide a basis and direction for researchers to further explore the biological significance of eccDNA in the future.
Keywords: Circular DNA, Extrachromosomal DNA, eccDNA
Background
In eukaryotes, a particular class of circular DNA molecules is separated from the normal genome, dissociated from the chromosome, and participates in physiological or pathological processes with a special way [1]. Because these molecules are independent DNA molecules outside the chromosome, and, they are often circular, so they are called extrachromosomal circular DNA, or eccDNA [2].
A lot of studies have shown that eccDNA can be involved in a wide range of biological processes [3–5]. For example, in cancer, eccDNA is the main factor leading to tumor heterogeneity and is regarded as a marker of genomic instability. However, eccDNA is also detected in healthy cells and can appear in a tissue-specific or developmentally regulated mode, which implies possible physiological effects [3]. Although researchers have found a large amount of eccDNA in normal and cancer cells [6, 7], people are still paying more and more attention to the connection between eccDNA and cancer [8–10]. Many studies have shown that eccDNA plays an important role in genetic variation, evolution, genomic instability, genomic plasticity, drug resistance, environmental adaptation, mutation and tumorigenesis [8]. An eccDNA usually contains tumor genes or drug resistance genes [9–13], so it can promote cancer cells to gain growth or survival advantages, and it can also lead to gene deletion, mutation, duplication, amplification or migration, intercellular genetic heterogeneity, and adaptive evolution [9]. Through the study of colon cancer HT29 cells, ovarian cancer, and breast cancer tissues, researchers found that eccDNA may promote the drug resistance of cancer or tumor cells by amplifying tumor genes [10]. One characteristic of the plasticity of the genome is the presence of eccDNA [12], which is the ability of the genome to produce different phenotypes based on different environmental cues and is related to a variety of human diseases and related phenotypes. High levels of eccDNA are related to genome instability, exposure to carcinogens, and cell aging [11]. The study of Fanconi’s anemia in DNA repair deficiency syndrome has shown genetic instability and increased levels of associated eccDNA molecules [13]. In summary, researchers are still exploring and studying the functions of eccDNA, and there is still a lot of unknown information that they have not grasped. The research of eccDNA may have a certain impact on traditional genetics and genomics in the future.
However, the integrative resources that already exist for eccDNA, such as eccDNAdb [14] and CircleBase [15], contain only human data or specifically human tumor data as far as we know. We would like to build a database platform containing eccDNA from a variety of eukaryotes. Thus, we established a database named TeCD (The eccDNA Collection Database) to integrate the eccDNA data scattered in public literature. The species distribution of the data in TeCD covers animals, plants and fungi. Researchers can obtain the genomic elements or locations of eccDNA and detect the related sequences by BLAST (Basic Local Alignment Search Tool) from TeCD.
Construction and content
Database implementation
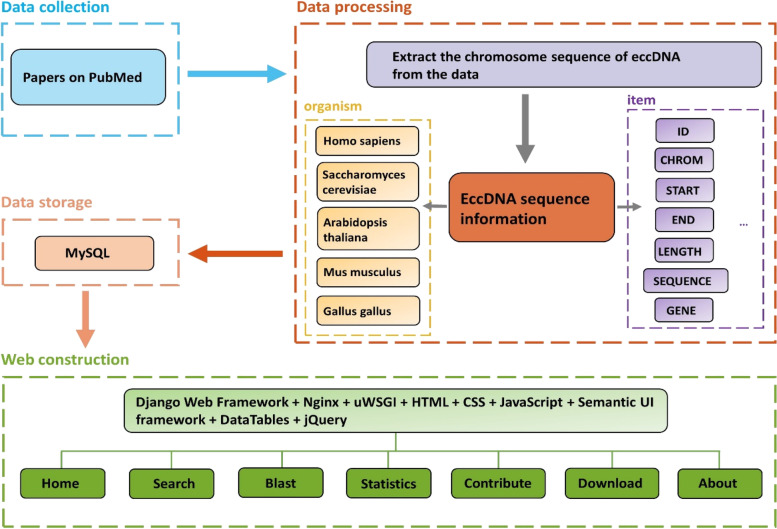
The web server was deployed based on Nginx 1.18.0 (http://nginx.org/) and uWSGI 2.0.19.1 (https://pypi.org/project/uWSGI/), and the Django 2.2.12 framework (https://www.djangoproject.com/) was used to implement Python language interface on a back-end server. And all data were stored in MySQL 8.0.26 database ( https://dev.mysql.com/) for Linux on x86_64 (Ubuntu Server 20.04.2 LTS). The web page templates were used on Semantic UI framework (https://semantic-ui.com/), DataTables (http://datatables.net), and jQuery (http://jquery.com) to establish a user-friendly front-end interface. HTML, CSS, and JavaScript were selected as the client-side languages for front-end design (Fig. 1).
Fig. 1.
The flow chart of TeCD. The raw data were collected from published literature. The chromosome sequence corresponding to eccDNA was obtained according to the locus. The gene, locus information, number of reads variants, and other information were extracted from the data and stored in MySQL 8.0.26 database. The information could be browsed and retrieved according to organism and item. Finally, the TeCD website was built with Django web framework for users
Data collection
Many studies have proven that eccDNA comes from the chromosome, not from any pre-existing extrachromosomal precursors [16, 17], so each detected eccDNA can be matched to a corresponding site on chromosome. At present, most the studies about eccDNA only focus on both the main characteristics of eccDNA (such as length, structure, and expressive characteristics) and the development of isolating methods that can be used to detect eccDNA [18, 19]. The research for the function of eccDNA is still few. To facilitate the further exploration of the function of eccDNA, we collected the existing information of eccDNA and built a database to organize them for acquirement.
The eccDNA data were collected from the published literature related to eccDNA for eukaryotes [7, 17, 20–35], and included the information such as eccDNA type, length, locus shedding from chromosomes, sequencing approach and so on. These data are relatively scattered in different eccDNA studies and need further process after the collection of the data (Fig. 1).
Data processing
There were about 2,343,000 eccDNAs to be collected from public literature. The eccDNAs were filtered according to whether they contained the start and end sites of eccDNA on the corresponding chromosome, and a total of 1,846,905 eccDNAs were obtained. We matched the eccDNAs through their start and end sites to the reference genome (Homo sapiens GRCh38.p13, Saccharomyces cerevisiae S288C, Arabidopsis thaliana TAIR10.1, Gallus gallus bGalGal1.mat.broiler. GRCg7b and Mus musculus GRCm39) from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/). Then, the filtered data were divided into groups according to species (Table 1). Then the repeated sequences were identified by BLAST tool [36] with a threshold of e-value in individual species, and were removed from the database. After the filter, we obtained a total of 948,469 eccDNAs. The amount of eccDNA for each species is shown in Table 1.
Table 1.
The amount of eccDNA for each organism
| Homo sapiens | Saccharomyces cerevisiae | Arabidopsis thaliana | Gallus gallus | Mus musculus | |
|---|---|---|---|---|---|
| Obtained data | 379,148 | 2111 | 702 | 1,087,503 | 377,441 |
| After BLAST | 193,294 | 1266 | 591 | 433,993 | 319,325 |
In this database, we assigned a unique identification ID which is composed of species information and alphanumeric ordering to each eccDNA. For example, ecc_Hs_100000001 is the ID of eccDNA from Homo sapiens (where ‘Hs’ represents Homo sapiens), and ecc_Sc_100000001 is the ID of eccDNA from Saccharomyces cerevisiae (where ‘Sc’ represents Saccharomyces cerevisiae). Besides, we also marked the source of each eccDNA based on the tissue and cell type of the sample, and statistically showed that there is a total of 42 categories for the source of tissue and cell type in the database. In addition, each eccDNA was marked the potential source of chromosomes and start/end sites in the chromosome. These classifications can help any scientific researcher to accurately retrieve information on the TeCD platform and carry out research work.
Next, each eccDNA was accurately matched to the corresponding genes or partially covered genes by searching the NCBI genome database. In order to facilitate scientific researchers for obtaining relevant gene information instantly when browsing and retrieving eccDNA, we have made a direct link to each matched gene, and NCBI’s gene bank will provide a comprehensive and authoritative explanation for the gene (Fig. 1). Specially, the Arabidopsis gene models are linked to TAIR (The Arabidopsis Information Resource https://www.arabidopsis.org/), the most authoritative Arabidopsis genome database and Arabidopsis genome annotation system in the world [37, 38]. Besides, the transposable elements information of the sequence is also included in TeCD.
Classification and statistics of eccDNA
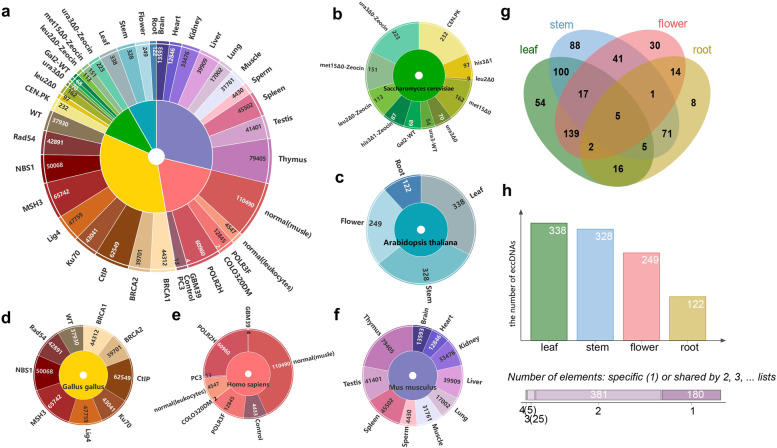
The tissue and cell type to which the sample belongs is an important indicator of eccDNA classification, according to which 948,469 eccDNA records in TeCD could be summarized into 42 types (Fig. 2a). And Fig. 2b-e show sample types for five organisms. The inner circle color of each figure corresponds to the inner circle color of Fig. 2a. Samples from Saccharomyces cerevisiae are from 11 categories which consisted of the CEN.PK strain and the S288C strain, and we named samples which from CEN.PK strains as ‘CEN.PK’ and further classified samples which from the S288C strain [21, 23]. In the S288C Saccharomyces cerevisiae (Fig. 2b), ‘MATa Gal2 isogenic WT population’ and ‘MATa ura3 isogenic WT population’ are two wild-type Saccharomyces cerevisiae respectively and are in contrast to ‘MATa his3Δ1 gene deletion yeast’, ‘MATa leu2Δ0 gene deletion yeast’, ‘MATa met15Δ0 gene deletion yeast’ and ‘MATa ura3Δ0 gene deletion yeast’. ‘MATa his3Δ1 gene deletion yeast’, ‘MATa leu2Δ0 gene deletion yeast’, ‘MATa met15Δ0 gene deletion yeast’ and ‘MATa ura3Δ0 gene deletion yeast’ are single-gene deletion mutants without his3Δ1, leu2Δ0, met15Δ0 and ura3Δ0. ‘MATa his3Δ1 gene deletion with Zeocin’, ‘MATa leu2Δ0 gene deletion with Zeocin’, ‘MATa met15Δ0 gene deletion with Zeocin’ and ‘MATa ura3Δ0 gene deletion with Zeocin’ are the samples in which DNA-damaging agent Zeocin is added to the corresponding single-gene deletion mutants [23]. MATa is a mating type of Saccharomyces cerevisiae [39]. The samples from Arabidopsis thaliana contain 4 categories [34], which are leaf, stem, flower, root (Fig. 2c). Notably, each eccDNA of Arabidopsis thaliana was detected from one or more samples. We intersected the number of eccDNA detected in different sample types and performed a Wayne diagram analysis of the results (Fig. 2g). The different colored circles represent the samples of different parts of Arabidopsis. As shown in Fig.2 h, 381 eccDNAs were detected in 2 samples, 25 eccDNAs were detected in 3 samples, and 5 eccDNA were detected in 4 samples. The samples from Gallus gallus contain 9 categories (Fig. 2d). These samples were derived from mutants of the DT-40 cell line [35]. For example, ‘BRCA1’ means DT40-Chicken cell line-mutated BRCA1. Samples from Homo sapiens come from 8 categories(Fig. 2e), of which ‘muscle’ represents human samples from healthy human muscle tissue, ‘leukocytes’ means leukocytes collected after centrifugation from the venous blood (40 ml) of healthy human arm veins [20]. The human embryonic kidney (HEK293) cells are easily transfected and ‘HEK293’ is the control of ‘HEK293 (+ POLR2H)’ and ‘HEK293 (+ POLR3F)’. ‘HEK293 (+ POLR2H)’ means that the POLR2H (RNA Polymerase II, I And III Subunit H) protein-coding gene was transfected into HEK293 cells and ‘HEK293 (+ POLR3F)’ means that the POLR3F (RNA Polymerase III Subunit F) protein-coding gene was transfected into HEK293 cells [22]. There is also human prostate cancer cell line ‘PC3’, colon cancer cell line ‘COLO320DM’, and human glioblastoma ‘GBM39’ tumor spheroids derived from patient tissues [24]. The samples from Mus musculus contain 10 categories (Fig. 2d). All samples are from organs or tissues of 6-months-old adult mice, including brain, heart, kidney, liver, lung, muscle, sperm, spleen, testis and thymus [35].
Fig. 2.
Sample types for different tissues and cells. a Sample types for all organisms and the corresponding number of eccDNA. The 5 colors of the inner ring correspond to 5 organisms. The outer ring represents the sample type for each organism. b-f Sample types of Saccharomyces cerevisiae, Arabidopsis thaliana, Gallus gallus, homo sapiens, and Mus musculus. And the color of its inner ring corresponds to the inner ring of the (a). g The number of eccDNA shared by samples from different parts of Arabidopsis. The different colored circles represent samples of different parts of Arabidopsis. h The figure above shows the number of eccDNA from four samples of Arabidopsis. The figure below shows the number of eccDNA that belongs to 1–4 different samples in common
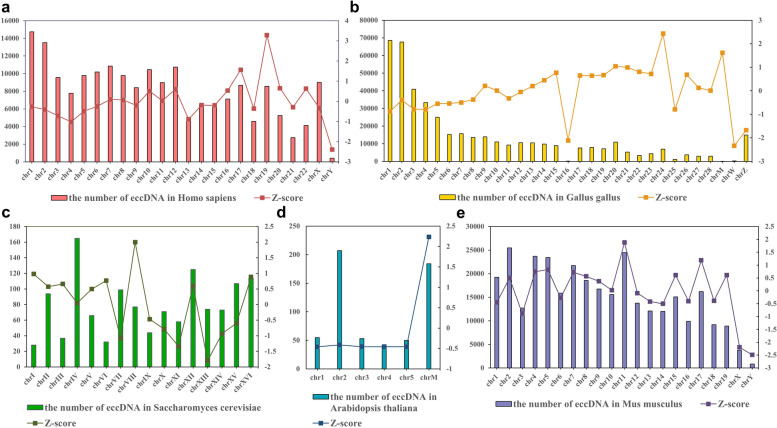
Additionally, we recorded the quantitative distribution of eccDNA on chromosomes, where the histogram represents the specific amount of eccDNA on different chromosomes and the line chart represents the standardized data (Fig. 3). As each chromosome has a different length, in order to eliminate the comparative baseline heterogeneity caused by chromosome heterogeneity, we calculated the ratio of the number of eccDNA in chromosomes to the length of chromosomes and standardized the ratio by Z-score to compare the amount of eccDNA formed by shedding from different chromosomes. The standardized results were plotted into a line chart. The source of eccDNA is dispersed to 22 autosomes, 2 sexual chromosomes, and mitochondria in humans, and the amount of eccDNA from chromosomes 1, 2, 7, 12 are obviously more than other chromosomes. The eccDNA from X chromosome significantly exceeds the eccDNA from Y chromosome (Fig. 3a). From the line chart of human data, it is not difficult to find that the amount of eccDNA on chromosomes 17 and 19 accounts for the largest proportion (Fig. 3a). The genome of Gallus gallus has 39 autosomes and two sex chromosomes, but in the collected data, eccDNA only comes from 28 autosomes and 2 sex chromosomes (Fig. 3b). Mostly, the number of eccDNA on each chromosome of Saccharomyces cerevisiae was 50–150. It was found on the eighth chromosome "ChrVIII" of Saccharomyces cerevisiae, the ratio of eccDNA to chromosome length was the highest, reaching 0.13‰ (Fig. 3c). Most of the eccDNA of Arabidopsis thaliana comes from chromosome 2 and mitochondria. Although the length of mitochondria is the shortest, the eccDNA produced by shedding from mitochondria accounts for about one-third of the total (Fig. 3d). The eccDNA of Mus musculus is mainly derived from autosomes. Through comparison, it is not difficult to find that the source distribution of eccDNA on chromosomes of different species is diverse.
Fig. 3.
Data distribution plot. a-e The histogram shows the quantitative distribution of eccDNA of different organisms on chromosomes. These species are Homo sapiens, Gallus gallus, Saccharomyces cerevisiae, Arabidopsis thaliana and Mus musculus in turn. The line chart shows the ratio standardized with Z-score. And this ratio is the number of eccDNA in the chromosome divided by the length of the chromosome
Utility and discussion
Web interface
To ensure that users can use these datasets of eccDNA which we collected and processed, we have developed an online website for browsing and querying the information. The whole website is divided into home, search, blast, statistics, contribute, download, and about.
Search
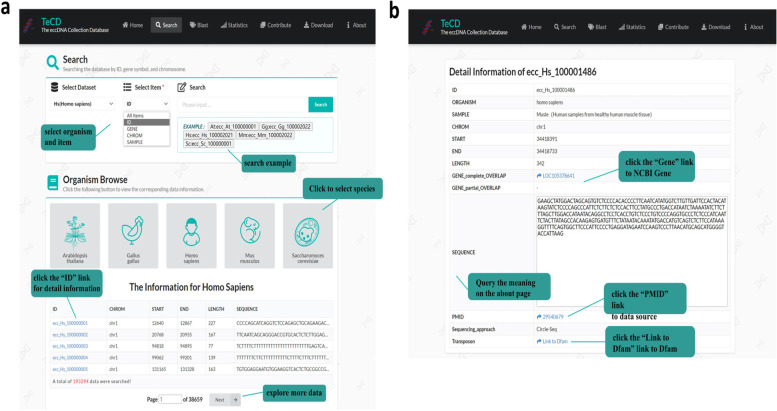
On the search page, a user can search and browse the basic information sets of eccDNA molecule (Fig. 4a and b). Users can select species or enter ID, gene, chromosome and sample type to retrieve eccDNA directionally. The retrieved eccDNA will be displayed at the bottom of the page. We have also placed examples for users’ reference by class under the input box of retrieval. Of course, users can directly view the information of eccDNAs related to a species by clicking the button in “Organism Browse”, and the information includes the ID of the eccDNA in the TeCD database, the chromosome to which it belongs, start and end sites in the chromosome, length of the sequence, nucleotide arrangement of the sequence (Fig. 4a). By clicking the ID of the eccDNA, it would jump to a detailed information page of the eccDNA. The detail page provides more information about the eccDNA (Fig. 4b). Users can find the notes of each row of information on the “About” page.
Fig. 4.
Screenshot of search page from the TeCD. a Search and browse pages. Users can search by organization and item, and we also provide some examples. Selected entry information will be displayed at the bottom of the page for users to browse. b Details of an eccDNA. Here, we provide a lot of information about eccDNA, including complete sequence, contained genes, transposons, data sources, etc. All information categories can be queried and explained on the about page. c JBrowse style visualization of individual eccDNAs and their location on the host genome
The information of genes covered by a retrieval eccDNA can be obtained by clicking the gene symbol and jumping to detailed page of gene in NCBI, and detailed function of the gene can be shown in the NCBI page. Each transposable element of eccDNA is linked to a visualization page of Dfam (https://www.dfam.org/) which is a eukaryotic transposable element database [40, 41]. It can visualize the distribution of transposable subcomponents on a sequence, provide a detailed interpretation of components and download the data. And the retrieval data of Dfam is highly matched with the data in TeCD such as eccDNA sites. The sequencing approach of each eccDNA is also documented on the detail page. We also provide the source literature of each eccDNA, and user can use the link of PMID to obtain the literature in PubMed database (https://pubmed.ncbi.nlm.nih.gov/) (Fig. 4b). Those links enhance the interoperability of data resources and improves the convenience of user retrieval.
BLAST
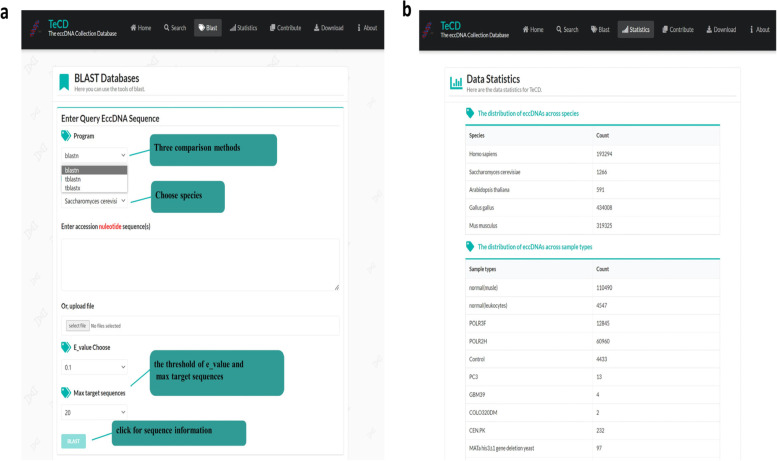
BLAST (basic local alignment search tool) is a set of analysis tools for similarity comparison in protein or DNA databases [42]. There are three BLAST tools to be provided in alignment in TeCD: BLASTN, TBLASTN, and TBLASTX. The BLASTN can compare and aligns an input nucleic acid sequence, and return the most similar nucleic acid sequence in the TeCD [43, 44]. TBLASTN translates all nucleic acid sequences in TeCD into protein sequences and then align an input protein sequence with the translated sequences [45, 46]. TBLASTX can align a translated input nucleic acid sequence with all translated nucleic acid sequences in TeCD and return the most similar sequences [46]. Users can choose the tool according to sequence information and needs (Fig. 5a).
Fig. 5.
Screenshot of BLAST and statistics page. a Screenshot of blast page from the TeCD. Users can use BLAST to search the database by sequence alignment. b Screenshot of statistics page from the TeCD
Statistics
The statistics page presents the aggregated data in TeCD by tables. It includes the number of genes covered on the eccDNA of each species, percentage of eccDNA with complete gene overlap and partial gene overlap among species, the distribution of eccDNAs across species and sample types. Moreover, the distribution of eccDNAs on chromosomes of each species and the sequencing approach of eccDNAs are also summarized.
Contribute, download and about
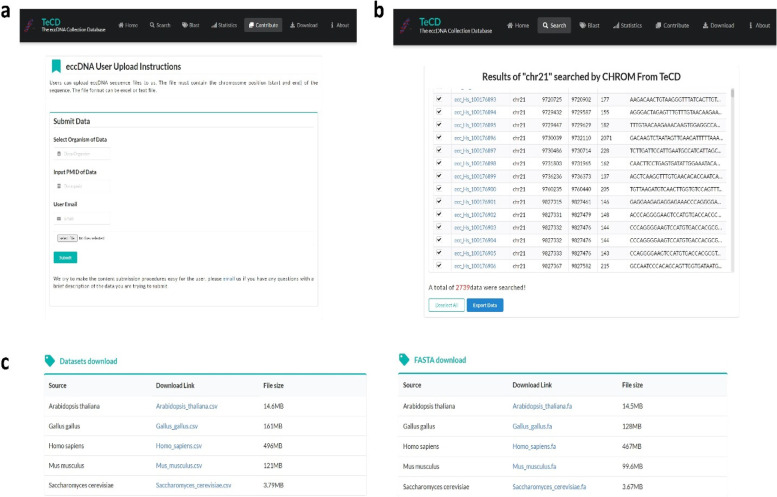
To continuously expand and improve the database, we have built a submission and upload interface, and allow users to upload new eccDNA sequences into the TeCD database (Fig. 6a). We will process the upload eccDNA sequences and integrate them into TeCD in the background.
Fig. 6.
Screenshot of contribute and download page. a Screenshot of contribute page from the TeCD. b Screenshot of search results page from the TeCD. Users can export some data optionally. c Screenshot of download page from the TeCD. TeCD provides download links of all data by species, and the data formats are. csv and. fa
All data in TeCD are shared under the knowledge sharing license agreement, and we provide a download interface for users to download the data from TeCD (Fig. 6c). Of course, if users want to directly download part of the data they retrieved, they can also check download on the search page. As shown in Fig. 6b, by searching eccDNA data on the human chromosome 21, users can manually select some or all of the data to export. To facilitate users to understand and use our database, we have developed an “About” page to query the meaning of specific items in the detailed sequence information in time.
Analyze database sequences using the BLAST
In the Saccharomyces cerevisiae data from the TeCD, there were experimental groups with the DNA damaging agent zeocin and control groups without the DNA damaging agent, to explore the effects of DNA damaging agents on eccDNA. As shown in Fig. 7, the control group had an average of 147 eccDNAs per group (181, 42, 248, and 118 eccDNAs); whereas the group with the DNA damaging agent zeocin had an average of 219 eccDNAs (159, 210, 218, and 288 eccDNAs). Thus, the samples treated with the DNA damaging agent zeocin (219 eccDNAs) showed more eccDNA than untreated samples (147 eccDNAs). We further processed the data and performed bidirectional comparisons of eccDNA sequences obtained from each experimental group and its control group using the platform-built BLAST tool. The result showed that the eccDNA matched by bidirectional comparisons in each group to account for 11%-13% of the average eccDNA in the control and experimental groups. Thus, this result can not only verify that the DNA damaging agent Zeocin can damage the single-strand and double-strand DNA of cells to form eccDNA [23], but also can be used to evaluate the level of DNA strand breakage induced by the DNA damaging agent Zeocin and its effect on eccDNA formation.
Fig. 7.
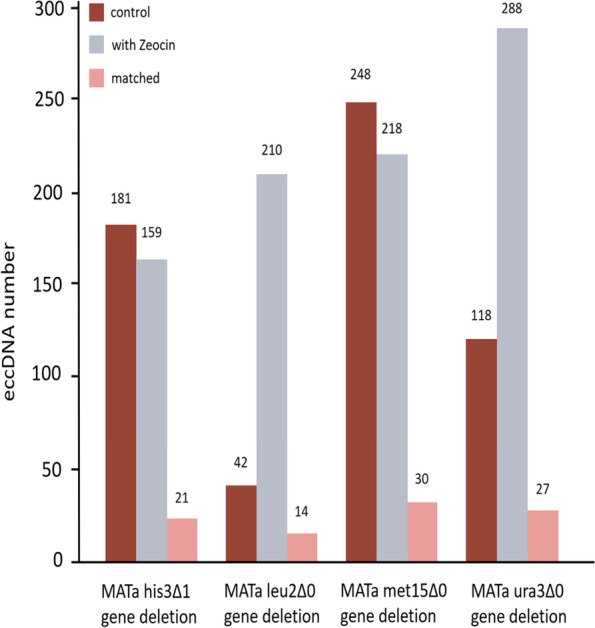
The number of eccDNA with and without DNA damaging. Taking Saccharomyces cerevisiae as an example, a bidirectional comparison of nucleic acid sequences was performed by BLAST
Conclusion
To provide the chromosome source, complete base sequence, and related gene information of eccDNA in different species, we have carried out a large amount of data collection and sorting out, and developed TeCD. We obtained the site information of eccDNA from published literature and matched it with reference genome and gene library data. Thus, we obtained the partially overlapped genes, complete overlapped genes, and transposons. In addition, the sequencing approaches for obtaining eccDNA were also collated and recorded. TeCD web interface allows users to query on-demand and browse the details of each eccDNA. To facilitate users to compare and identify sequences by themselves, we have provided the BLAST function on the web page and all the processed data in TeCD can be downloaded directly by users. TeCD will be updated regularly according to the latest sequencing data and data submitted by users as an interactive website. Based on our knowledge, TeCD is the first database containing the sequence and genomic information of extrachromosomal circular DNA of eukaryotes and BLAST sequence alignment function. Anyway, TeCD will become a powerful tool for researchers to browse and retrieve eccDNA data and further explore the biological functions and contributions of eccDNA.
Acknowledgements
Not applicable.
Abbreviations
- EccDNA
Extrachromosomal circular DNA
- TeCD
The eccDNA Collection Database
- BLAST
Basic Local Alignment Search Tool
- NCBI
National Center for Biotechnology Information
- ID
Identifier
- TAIR
The Arabidopsis Information Resource
- HEK
Human Embryonic Kidney
- POLR2H
RNA Polymerase II, I And III Subunit H
- POLR3F
RNA Polymerase III Subunit F
- PC
Prostate Cancer
- COLO
Colon Cancer
- GBM
Glioblastoma
- PMID
PubMed Unique Identifier
Authors’ contributions
JG: collection of data, analysis and interpretation of data, writing of the manuscript. ZZ: construction and maintenance of the database. QL: collection of data. XC and XL: conception and design of the database, manuscript revision. All authors read and approved the final manuscript.
Funding
This work was supported by grants from Zhejiang Provincial Natural Science Foundation of China (No. LZ22C060001), the research funds of Hangzhou Institute for advanced study, UCAS (No. 2022ZZ01013), the Key Project of Natural Science of Anhui Provincial Education Department (No. KJ2020A0018), the Key project of Anhui Finance and Economics University (No. ackyb20015), and Project of teaching and research of Department of Education of Anhui Province (No.2020xsxxkc014).
Availability of data and materials
All the data are free to use for academic purpose at http://www.eccdna.org:2022 or http://122.224.251.240:2022.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Guo and Ze Zhang contributed equally to this study as the first authors.
Contributor Information
Xiao Chang, Email: chxlaugh@163.com.
Xiaoping Liu, Email: xpliu@ucas.ac.cn.
References
- 1.Koche RP, Rodriguez-Fos E, Helmsauer K, Burkert M, MacArthur IC, Maag J, Chamorro R, Munoz-Perez N, Puiggros M, Dorado Garcia H, et al. Extrachromosomal circular DNA drives oncogenic genome remodeling in neuroblastoma. Nat Genet. 2020;52(1):29–34. doi: 10.1038/s41588-019-0547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan Y, Guo G, Huang J, Gao M, Zhu Q, Zeng S, Gong Z, Xu Z. Current understanding of extrachromosomal circular DNA in cancer pathogenesis and therapeutic resistance. J Hematol Oncol. 2020;13(1):124. doi: 10.1186/s13045-020-00960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner KM, Deshpande V, Beyter D, Koga T, Rusert J, Lee C, Li B, Arden K, Ren B, Nathanson DA, et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature. 2017;543(7643):122–125. doi: 10.1038/nature21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansisidor A, Molinar T, Srivastava P, Dartis DD, Delgado AP, Blitzblau HG, Klein H, Hochwagen A. Genomic Copy-Number Loss Is Rescued by Self-Limiting Production of DNA Circles. Mol Cell. 2018;72(3):583. doi: 10.1016/j.molcel.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hull RM, Houseley J. The adaptive potential of circular DNA accumulation in ageing cells. Curr Genet. 2020;66(5):889–894. doi: 10.1007/s00294-020-01069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulsen T, Kumar P, Koseoglu MM, Dutta A. Discoveries of Extrachromosomal Circles of DNA in Normal and Tumor Cells. Trends Genet. 2018;34(4):270–278. doi: 10.1016/j.tig.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar P, Dillon LW, Shibata Y, Jazaeri AA, Jones DR, Dutta A. Normal and Cancerous Tissues Release Extrachromosomal Circular DNA (eccDNA) into the Circulation. Mol Cancer Res. 2017;15(9):1197–1205. doi: 10.1158/1541-7786.Mcr-17-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling X, Han Y, Meng J, Zhong B, Chen J, Zhang H, Qin J, Pang J, Liu L. Small extrachromosomal circular DNA (eccDNA): major functions in evolution and cancer. Mol Cancer. 2021;20(1):113. doi: 10.1186/s12943-021-01413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Chen X, Yu F, Ding H, Zhang Y, Wang K. Extrachromosomal Circular DNAs: Origin, formation and emerging function in Cancer. Int J Biol Sci. 2021;17(4):1010–1025. doi: 10.7150/ijbs.54614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, Zhang H, Zhou Y, Shi J. Extrachromosomal circular DNA: a new potential role in cancer progression. J Transl Med. 2021;19(1):257. doi: 10.1186/s12967-021-02927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen S, Mechali M. A novel cell-free system reveals a mechanism of circular DNA formation from tandem repeats. Nucleic Acids Res. 2001;29(12):2542–2548. doi: 10.1093/nar/29.12.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaubatz JW. Extrachromosomal circular DNAs and genomic sequence plasticity in eukaryotic cells. Mutat Res. 1990;237(5–6):271–292. doi: 10.1016/0921-8734(90)90009-g. [DOI] [PubMed] [Google Scholar]
- 13.Motejlek K, Schindler D, Assum G, Krone W. Increased amount and contour length distribution of small polydisperse circular DNA (spcDNA) in Fanconi anemia. Mutat Res. 1993;293(3):205–214. doi: 10.1016/0921-8777(93)90071-n. [DOI] [PubMed] [Google Scholar]
- 14.Peng L, Zhou N, Zhang CY, Li GC, Yuan XQ. eccDNAdb: a database of extrachromosomal circular DNA profiles in human cancers. Oncogene. 2022;41(19):2696–2705. doi: 10.1038/s41388-022-02286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao XL, Shi LS, Ruan SS, Bi WJ, Chen YF, Chen L, Liu YF, Li MK, Qiao J, Mao FB. CircleBase: an integrated resource and analysis platform for human eccDNAs. Nucleic Acids Res. 2022;50(D1):D72–D82. doi: 10.1093/nar/gkab1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S, Segal D. Extrachromosomal circular DNA in eukaryotes: possible involvement in the plasticity of tandem repeats. Cytogenet Genome Res. 2009;124(3–4):327–338. doi: 10.1159/000218136. [DOI] [PubMed] [Google Scholar]
- 17.Hull RM, King M, Pizza G, Krueger F, Vergara X, Houseley J. Transcription-induced formation of extrachromosomal DNA during yeast ageing. PLoS Biol. 2019;17(12):e3000471. doi: 10.1371/journal.pbio.3000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moller HD. Circle-Seq: Isolation and Sequencing of Chromosome-Derived Circular DNA Elements in Cells. Methods Mol Biol. 2020;2119:165–181. doi: 10.1007/978-1-0716-0323-9_15. [DOI] [PubMed] [Google Scholar]
- 19.Kumar P, Kiran S, Saha S, Su Z, Paulsen T, Chatrath A, Shibata Y, Shibata E, Dutta A. ATAC-seq identifies thousands of extrachromosomal circular DNA in cancer and cell lines. Sci Adv. 2020;6(20):eaba2489. doi: 10.1126/sciadv.aba2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moller HD, Mohiyuddin M, Prada-Luengo I, Sailani MR, Halling JF, Plomgaard P, Maretty L, Hansen AJ, Snyder MP, Pilegaard H, et al. Circular DNA elements of chromosomal origin are common in healthy human somatic tissue. Nat Commun. 2018;9(1):1069. doi: 10.1038/s41467-018-03369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moller HD, Bojsen RK, Tachibana C, Parsons L, Botstein D, Regenberg B. Genome-wide Purification of Extrachromosomal Circular DNA from Eukaryotic Cells. J Vis Exp. 2016;110:e54239. doi: 10.3791/54239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulsen T, Shibata Y, Kumar P, Dillon L, Dutta A. Small extrachromosomal circular DNAs, microDNA, produce short regulatory RNAs that suppress gene expression independent of canonical promoters. Nucleic Acids Res. 2019;47(9):4586–4596. doi: 10.1093/nar/gkz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moller HD, Parsons L, Jorgensen TS, Botstein D, Regenberg B. Extrachromosomal circular DNA is common in yeast. P Natl Acad Sci USA. 2015;112(24):E3114–E3122. doi: 10.1073/pnas.1508825112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S, Turner KM, Nguyen N, Raviram R, Erb M, Santini J, Luebeck J, Rajkumar U, Diao Y, Li B, et al. Circular ecDNA promotes accessible chromatin and high oncogene expression. Nature. 2019;575(7784):699–703. doi: 10.1038/s41586-019-1763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta D, Cornet L, Hirsch-Hoffmann M, Zaidi SS, Vanderschuren H. Full-length sequencing of circular DNA viruses and extrachromosomal circular DNA using CIDER-Seq. Nat Protoc. 2020;15(5):1673–1689. doi: 10.1038/s41596-020-0301-0. [DOI] [PubMed] [Google Scholar]
- 26.Morton AR, Dogan-Artun N, Faber ZJ, MacLeod G, Bartels CF, Piazza MS, Allan KC, Mack SC, Wang X, Gimple RC, et al. Functional Enhancers Shape Extrachromosomal Oncogene Amplifications. Cell. 2019;179(6):1330–1341 e1313. doi: 10.1016/j.cell.2019.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sin STK, Jiang P, Deng J, Ji L, Cheng SH, Dutta A, Leung TY, Chan KCA, Chiu RWK, Lo YMD. Identification and characterization of extrachromosomal circular DNA in maternal plasma. Proc Natl Acad Sci U S A. 2020;117(3):1658–1665. doi: 10.1073/pnas.1914949117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoura MJ, Gabdank I, Hansen L, Merker J, Gotlib J, Levene SD, Fire AZ. Intricate and Cell Type-Specific Populations of Endogenous Circular DNA (eccDNA) in Caenorhabditis elegans and Homo sapiens. G3 (Bethesda) 2017;7(10):3295–3303. doi: 10.1534/g3.117.300141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moller HD, Ramos-Madrigal J, Prada-Luengo I, Gilbert MTP, Regenberg B. Near-Random Distribution of Chromosome-Derived Circular DNA in the Condensed Genome of Pigeons and the Larger, More Repeat-Rich Human Genome. Genome Biol Evol. 2020;12(1):3762–3777. doi: 10.1093/gbe/evz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Werken HJ, Landan G, Holwerda SJ, Hoichman M, Klous P, Chachik R, Splinter E, Valdes-Quezada C, Oz Y, Bouwman BA, et al. Robust 4C-seq data analysis to screen for regulatory DNA interactions. Nat Methods. 2012;9(10):969–972. doi: 10.1038/nmeth.2173. [DOI] [PubMed] [Google Scholar]
- 31.Epstein L, Hunter JC, Arwady MA, Tsai V, Stein L, Gribogiannis M, Frias M, Guh AY, Laufer AS, Black S, et al. New Delhi metallo-beta-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014;312(14):1447–1455. doi: 10.1001/jama.2014.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grzywacz B, Chobanov DP, Maryanska-Nadachowska A, Karamysheva TV, Heller KG, Warchalowska-Sliwa E. A comparative study of genome organization and inferences for the systematics of two large bushcricket genera of the tribe Barbitistini (Orthoptera: Tettigoniidae: Phaneropterinae) Bmc Evol Biol. 2014;14:48. doi: 10.1186/1471-2148-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molin WT, Yaguchi A, Blenner M, Saski CA. The EccDNA Replicon: A Heritable, Extranuclear Vehicle That Enables Gene Amplification and Glyphosate Resistance in Amaranthus palmeri. Plant Cell. 2020;32(7):2132–2140. doi: 10.1105/tpc.20.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang KY, Tian H, Wang LQ, Wang L, Tan YC, Zhang ZT, Sun K, Yin M, Wei QG, Guo BH, et al. Deciphering extrachromosomal circular DNA in Arabidopsis. Comput Struct Biotec. 2021;19:1176–1183. doi: 10.1016/j.csbj.2021.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dillon LW, Kumar P, Shibata Y, Wang YH, Willcox S, Griffith JD, Pommier Y, Takeda S, Dutta A. Production of Extrachromosomal MicroDNAs Is Linked to Mismatch Repair Pathways and Transcriptional Activity. Cell Rep. 2015;11(11):1749–1759. doi: 10.1016/j.celrep.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boratyn GM, Camacho C, Cooper PS, Coulouris G, Fong A, Ma N, Madden TL, Matten WT, McGinnis SD, Merezhuk Y, et al. BLAST: a more efficient report with usability improvements. Nucleic Acids Res. 2013;41(Web Server issue):W29–33. doi: 10.1093/nar/gkt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Hernandez M, Berardini TZ, Chen G, Crist D, Doyle A, Huala E, Knee E, Lambrecht M, Miller N, Mueller LA, et al. TAIR: a resource for integrated Arabidopsis data. Funct Integr Genomics. 2002;2(6):239–253. doi: 10.1007/s10142-002-0077-z. [DOI] [PubMed] [Google Scholar]
- 38.Rhee SY, Beavis W, Berardini TZ, Chen GH, Dixon D, Doyle A, Garcia-Hernandez M, Huala E, Lander G, Montoya M, et al. The Arabidopsis Information Resource (TAIR): a model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucleic Acids Res. 2003;31(1):224–228. doi: 10.1093/nar/gkg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haber JE. Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics. 2012;191(1):33–64. doi: 10.1534/genetics.111.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheeler TJ, Clements J, Eddy SR, Hubley R, Jones TA, Jurka J, Smit AFA, Finn RD. Dfam: a database of repetitive DNA based on profile hidden Markov models. Nucleic Acids Res. 2013;41(D1):D70–D82. doi: 10.1093/nar/gks1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storer J, Hubley R, Rosen J, Wheeler TJ, Smit AF. The Dfam community resource of transposable element families, sequence models, and genome annotations. Mobile DNA-Uk. 2021;12(1):2. doi: 10.1186/s13100-020-00230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004;32:W20–W25. doi: 10.1093/nar/gkh435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36(Web Server issue):W5–9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ladunga I. Finding Similar Nucleotide Sequences Using Network BLAST Searches. Curr Protoc Bioinformatics. 2017;58:3 3 1–3 3 25. doi: 10.1002/cpbi.29. [DOI] [PubMed] [Google Scholar]
- 45.Zhang ZY, Zhang HM, Li DS, Xiong TY, Fang SG. Characterization of the beta-defensin genes in giant panda. Sci Rep. 2018;8(1):12308. doi: 10.1038/s41598-018-29898-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Mao Y, Huang Z, Qu M, Chen J, Ding S, Hong J, Sun T. Transcriptome analysis of the Octopus vulgaris central nervous system. PLoS One. 2012;7(6):e40320. doi: 10.1371/journal.pone.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are free to use for academic purpose at http://www.eccdna.org:2022 or http://122.224.251.240:2022.