Abstract
Animals can be rendered immune to Ascaris parasites by immunization with infectious-stage larvae. The specific parasite gene products that mediate protective responses in ascariasis are unknown. We have identified a cDNA encoding Ascaris suum 14-kDa antigen (As14) and evaluated the vaccinal effect of the Escherichia coli-expressed recombinant protein (rAs14). GenBank analysis showed that As14 has low similarity at the amino acid level to a Caenorhabditis elegans gene product and to antigens of the filarial nematodes but not to other known proteins. In addition, As14 homologues were found to be expressed in human and dog roundworms. In mice that received intranasal administration of rAs14 coupled with cholera toxin B subunit (rAs14-CTB), there was a 64% reduction of recovery of larvae compared with that in the nontreated group. The vaccinated mice showed a significant increase in the total serum immunoglobulin G (IgG) levels and the mucosal IgA responses. Elevation of the rAs14-specific IgE response was also seen. Measurement of the IgG subclasses showed a higher level of IgG1 and a lower level of IgG2a antibody response in the sera of the immunized mice, suggesting that protection was associated with a type II immune response. As14 is the first protective antigen against A. suum infection to be identified. Our immunization trial results in laboratory animals suggest the possibility of developing a mucosal vaccine for parasitic diseases caused by ascarid nematodes.
Ascaris roundworms are gastrointestinal nematodes that are widely distributed in both humans and animals worldwide. It is estimated that over 1.5 billion people are infected with Ascaris lumbricoides, mainly in tropical and subtropical areas (6). Ascarids are responsible for significant morbidity and economic loss in animals (11). One of these roundworms, Ascaris suum, was originally identified as a ubiquitous, pathogenic parasite of swine and is biochemically well characterized (46). Studies of A. suum provide important information about the biology of other ascarid nematodes, especially human-pathogenic ascarids. A. suum infection is established orally by infective third-stage larvae (L3) after their development from embryonated eggs (16). The L3 invade the small intestine of the host, migrate into the liver and the lung, and finally arrive at the small intestine, where they develop into adult worms. Recent studies have revealed that A. suum of swine origin can develop in humans, indicating its zoonotic importance (2, 39). Since A. suum embryonated eggs can hatch and their larvae can migrate into a wide range of hosts, experimental animal-A. suum infection models have been used for immunological and chemotherapeutic experiments (10, 24, 25, 45).
Prior studies have shown that pigs can be rendered immune to A. suum infection by immunization with radiation-attenuated infective larvae or by chemically abbreviated infection (22, 27, 57). Passive transfer of sera from immune pigs is effective for killing and stunting larvae in pigs (32). In addition, crude larval antigens can induce protective immunity (58). Similar findings were observed in an A. suum-laboratory animal infection model (28). These data suggest that larvae at various stages possess antigens that induce protective immunity against the infection and that the A. suum-mouse infection model can be used for identifying immune protective molecules.
Intranasal or oral routes for vaccination are among the convenient routes for immunization against pathogenic organisms. The initial phase of A. suum infection occurs in the mucosal surface of the small intestine of the host, and this phase is followed by the tissue migratory phase. It has been shown elsewhere that local antibodies present at the site where the L3 enter the host can induce partial protection against A. suum L3 infection in mice (25). Thus, intestinal immunity appears to be an important primary defense against the invasion of A. suum L3 into the host, while systemic immunity mediated by serum antibodies may protect the host against larval migration. Experimental animal studies have demonstrated that mucosal administration of several antigens fused to cholera toxin B subunit (CTB) can induce vigorous mucosal immunoglobulin A (IgA) and systemic immune responses (33, 59). CTB is a nontoxic binding moiety of cholera toxin; it is composed of a ring of five identical polypeptides that bind with high affinity to GM1 and other ganglioside cell surface receptors and promote the entry of the A subunit into the cell (47, 50). Oral or intranasal immunization has been shown elsewhere to successfully induce protective immunity against a variety of viral, bacterial, and protozoan infections (29, 31, 39, 42, 49, 61). More recently, the possibility of using CTB as a mucosal adjuvant in humans has been reported (8). Our aim in this study was to identify vaccine molecules whose mucosal administration could induce protection against A. suum infection.
In this study, we isolated a cDNA encoding a 14-kDa antigen from A. suum L3 (As14). We found As14-related antigens in a human roundworm, A. lumbricoides, and a dog roundworm, Toxocara canis. We performed L3-challenge infection using CTB as a mucosal adjuvant in a mouse-A. suum model. Mice immunized with Escherichia coli-expressed recombinant As14 (rAs14) coupled with CTB showed protection against challenge infection with A. suum L3; they had mucosal and systemic immune responses and reduced recovery of larvae from the lung. Based on these data, we suggest that rAs14 is the most promising vaccine candidate from ascarid nematodes.
MATERIALS AND METHODS
Parasites.
The A. suum used in the present study was originally derived from infected pigs at a slaughterhouse. Unembryonated and embryonated eggs were obtained essentially as described elsewhere (11). L3 and lung-stage larvae were obtained as previously described (16, 56). Excretory and secretory (ES) products from larval stages and adult worms were collected essentially as described elsewhere (14). RNA was isolated from embryonated eggs using an RNA isolation kit (Clontech, Palo Alto, Calif.). Poly(A)+ mRNA was prepared from total RNA using the Polytract mRNA isolation kit (Clontech), and first-strand cDNA synthesis was performed using a cDNA synthesis kit and an oligo(dT)15 primer from Amersham Pharmacia Biotech (Piscataway, N.J.). An L3 cDNA library was constructed in the UniZap XR vector (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions as previously described (56). Adult A. lumbricoides and adult T. canis were recovered from a naturally infected human in Bangladesh and an infected dog in Miyazaki, Japan, respectively. The protein concentrations of phosphate-buffered saline (PBS)-soluble parasite antigens and ES products were measured using the Micro BCA (bicinchoninic acid) protein assay reagent (Pierce, Rockford, Ill.).
Production of rabbit immune sera.
The rabbit immune serum was obtained by inoculating a rabbit with A. suum embryonated eggs as previously described (54). A Japanese White rabbit was inoculated with 2,000 eggs, followed by repeated inoculation every 2 weeks for a total of four inoculations. The rabbit was bled 2 weeks after the final inoculation, and the serum was stored at −20°C until use.
Cloning of A. suum 14-kDa antigen.
An L3 cDNA library constructed in the UniZap XR vector (Stratagene) according to the manufacturer's instructions was used for immunoscreening. The library was screened with a 1:200 dilution of the rabbit immune serum as described by Sambrook et al. (41). Several clones were obtained by immunoscreening 5 × 105 plaques. The initial cDNA clone obtained for the A. suum 14-kDa antigen was a partial clone, approximately 800 bp in length and lacking its 5′ end. To obtain the missing 5′ region of the message, the first-strand A. suum L3 cDNA was amplified by PCR using the nematode splice leader sequence (4) as the sense SL1 primer (5′-GGT TTA ATT ACC CAA GTT TGA G-3′) and an antisense primer (5′-GTG TTC TGG CTT GTC CCA ATC TTC-3′) derived from the initial clone. The PCR fragments were ligated into pCRII vector (Invitrogen, Carlsbad, Calif.) as described in the manufacturer's protocol.
DNA sequence analysis.
The nucleotide sequences of the cDNAs were determined by the Sanger dideoxy chain termination method using a PRISM Ready Dye Terminator Cycle sequencing kit (Perkin-Elmer, Branchburg, N.J.). DNA samples were analyzed using an automated sequencer (373A DNA sequencer; Applied Biosystems, Foster City, Calif.). The GENETYX-WIN DNA sequence analysis software system (Software Inc., Tokyo, Japan) and the BLAST (1) network server of the National Center for Biotechnology Information (National Institutes of Health, Bethesda, Md.) were used to analyze the nucleotide sequence and deduce the amino acid sequences in determining similarities with previously reported sequences in GenBank. A primary sequence motif was identified using the PROSITE (3) network server at EMBL. Analysis of the signal sequence (37) was performed using SignalP V1.1 at the Center for Biological Sequence Analysis (http://www.cbs.dtu.dk/services/SignalP/index.html).
Expression and purification of recombinant A. suum 14-kDa fusion protein.
A partial coding region of As14 cDNA was amplified by PCR as previously described (55). A sense primer (5′-CCG AGC TCG AGA CAA GGA CCT CAA GGA CCA CCA C-3′) which contains an XhoI (Promega, Madison, Wis.) site upstream of the start codon and an antisense primer (5′-CAG CCA AGC TTC CTA GCC TTG CAT CTC TTT TTG-3′) which contains a HindIII (Promega) site just downstream of amino acid residue 156 were used. The PCR fragments were digested with XhoI and HindIII and ligated into plasmid expression vector pTrcHisB (Invitrogen), which had also been digested with the same enzymes as described in the manufacturer's protocol. The resultant plasmid was transferred into E. coli strain TOP10F′ (Invitrogen). Transformed cells were grown to an optical density at 600 nm (OD600) of 1.0 at 37°C in SOB medium supplemented with 50 μg of ampicillin per ml. Isopropyl-β-d-thiogalactoside (IPTG) was then added to a final concentration of 1 mM, and the culture was grown for an additional 4 h at 37°C. The E. coli cells were pelleted and resuspended in lysis buffer (50 mM NaH2PO4 [pH 8.0], 10 mM Tris-HCl [pH 8.0], 100 mM NaCl). Lysozyme was added to 100 μg/ml, and the cell suspension was incubated on ice for 20 min. The suspension was disrupted with an ultrasonic processor (VP-5; TAITEC, Tokyo, Japan) on ice. The lysate was centrifuged at 25,000 × g for 30 min at 4°C. rAs14 protein in the supernatant was purified using metal chelation chromatography (Invitrogen) under nondenaturing conditions as described in the manufacturer's protocol. Protein eluted with imidazole was concentrated using Centrisart I (molecular weight cutoff, 10,000; Sartorius, Göttingen, Germany) and then dialyzed against PBS in a Slide-A-Lyzer Dialysis Cassette (Pierce). The purification process was monitored by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (30) and immunoblotting (54) using a T7 Taq monoclonal antibody (Novergen). Protein concentrations were measured with the Micro BCA protein assay reagent (Pierce).
Production of an antiserum against rAs14.
Antiserum against rAs14 was prepared by subcutaneous injection of BALB/c mice with 50 μg of rAs14 purified as described above and mixed with TiterMax Gold (CytRx, Norcross, Ga.), followed by another injection 2 weeks later in the same adjuvant. The mice were bled 2 weeks after the second injection. The antisera from the mice were mixed and stored at −20°C until use.
Immunoblot analysis.
Immunoblot analysis was performed as previously described (55). Parasite antigens or rAs14 separated by SDS–14% PAGE were transferred onto nitrocellulose membranes, and the membranes were incubated for 30 min with 5% skim milk. For detection of parasite-derived As14, the membranes were incubated with the mouse anti-rAs14. Pig sera from animals with drug-abbreviated infection or mouse or rabbit sera from animals repeatedly inoculated with A. suum embryonated eggs were used for detection of the antigenicity of rAs14. After membranes were washed with Tris-buffered saline–Tween 20, they were incubated with alkaline phosphatase-conjugated goat anti-mouse, anti-pig, or anti-rabbit IgG (ICN Pharmaceuticals, Aurora, Ohio) as a secondary antibody. After the membranes were washed, the proteins bound to the secondary antibody were visualized with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP; GIBCO/BRL, Rockville, Md.).
Challenge infection and sampling.
Six-week-old female BALB/c mice (SLC, Hamamatsu, Japan) from a pathogen-free colony were used for challenge infection studies. Mice were divided into four groups of five animals each. For preparation of conjugation, rAs14 was coupled with CTB (C-9903; Sigma, St. Louis, Mo.) in darkness at 4°C for 16 h. The immunized group of mice was inoculated intranasally with 50 μg of rAs14 coupled with 20 μg of CTB under light ether anesthesia. On day 21, a booster inoculation of 30 μg of rAs14 coupled with 10 μg of CTB was given. A final boost of 30 μg of rAs14 coupled with 10 μg of CTB was given on day 35. The second and third groups were inoculated with the same doses of CTB or rAs14 alone, respectively, on the same days as the immunized group. The fourth group was given PBS alone. Two weeks after the final immunization, all animals, including those in the fourth group, were inoculated orally with 5,000 A. suum infective embryonated eggs. The mice were euthanatized on day 7, and their sera were collected and stored at −20°C. Their lungs were removed and minced with a surgical knife, and larvae were recovered by the method of Baermann (43, 44) and counted under a microscope. The small intestine was removed and put on an ice pack, and mucous tissues were removed with a surgical knife. The mucosal tissues were placed in an equal volume of PBS containing a protein inhibitor cocktail (Complete; Boehringer, Mannheim, Germany) and vortexed until the tissues were disrupted. The mixture was centrifuged at 24,500 × g for 60 min at 4°C, and the supernatant was stored at −80°C. Animal studies were approved by the National Institute of Animal Health Animal Care and Use Committee.
Antibody assays.
Measurement of mucosal IgA, serum IgG, serum IgE, and IgG subclass specific antibodies to rAs14 was performed by enzyme-linked immunosorbent assay. The wells of polystyrene microplates (AE1640; Sumitomo, Tokyo, Japan) were coated with 100 μl of 2-μg/ml rAs14 in 0.1 M carbonate buffer, pH 9.6. The plates were incubated at 4°C for 16 h and washed three times with PBS containing 0.05% Tween 20 (PBS-T). The wells were blocked with 100 μl of PBS–1% bovine serum albumin (Sigma) for 1 h at 37°C. After the wells were washed five times with PBS-T, serial dilutions of the mucosal extract from the small intestine or the serum were added and incubated for 1 h at 37°C. After the incubation, the wells were washed five times with PBS-T, and 100 μl of horseradish peroxidase (HRP)-conjugated anti-mouse IgA or IgG (Bethyl Laboratories, Montgomery, Tex.) diluted 1:10,000 was added to the wells. The plates were incubated for 1 h at 37°C and washed five times with PBS-T. Detection was performed at 37°C with 100 μl of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) substrate solution (ABTS; Kirkegaard & Perry Laboratories, Gaithersburg, Md.), and the coloring reaction was terminated with 100 μl of 1% SDS. Plates were read at 405 nm in a microplate reader (Spectrafluor; Wako, Tokyo, Japan).
The IgE response was examined using plates coated with rAs14 as described above. After antigens were bound to the wells, a diluted serum sample and rat monoclonal anti-mouse IgE antibody (American Research Products, Belmont, Mass.) diluted 1:10,000 were added and incubated for 1 h at 37°C. After the plates were washed five times with PBS-T, they were incubated with HRP-conjugated goat anti-rat IgG (Bethyl Laboratories) at 37°C for 1 h. The plates were washed five times with PBS-T, and then color development was performed as described above. IgG subclass responses were detected using plates coated with rAs14 as described above. After the antigens were bound to the wells, a diluted serum sample and rabbit anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (Bethyl Laboratories) were added and incubated for 1 h at 37°C. After the plates were washed five times with PBS-T, they were incubated with HRP-conjugated goat anti-rabbit IgG at 37°C for 1 h. The plates were washed five times with PBS-T, and then color development was performed as described above. The endpoint titer was determined as the reciprocal log2 of the last dilution that gave an OD405 above the OD405 of negative control samples from the fourth experimental group.
Statistical analysis.
The data are expressed as means ± standard deviations for each experimental group. Comparisons between experimental groups were performed by two-tailed Student's t test.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper have the DDBJ/EMBL/GenBank accession no. AB057441.
RESULTS
Molecular characterization of the cDNA encoding As14.
Several cDNA clones were obtained by immunoscreening 5 × 105 plaques, and their DNA sequences were determined. BLASTX searches were performed to obtain cDNA clones with low similarity to mammalian proteins whose sequences are stored in the current database. A clone designated L2R59 was selected from among these clones for further analysis in the present study. Sequence analysis showed that L2R59 was 450 bp long and contained an open reading frame coding for 97 amino acids with a complete 3′ end but appeared to be lacking the 5′ end of the sequence. Therefore, the missing 5′ end was obtained by PCR using the 22-nucleotide nematode spliced leader (SL1) and an L2R59-specific oligonucleotide as the primers and A. suum L3 cDNA as the template. A 486-bp product amplified by the PCR contained the SL1 sequence at its 5′ end. We confirmed the complete overlapping of 294 bp of the 486-bp PCR product with the 5′ end of the original L2R59. The composite cDNA, representing an apparently full-length cDNA for A. suum 14-kDa antigen, was assembled by overlapping the initial clone (L2R59) and the 486-bp SL1-PCR product, resulting in a 623-bp-long As14 cDNA that contains a single open reading frame of 436 bases. The ATG initiation codon is predicted to be at nucleotides 72 to 74 and is followed by a region encoding a hydrophobic sequence of 16 amino acids, which may function as a signal peptide. There is one potential site (residue 108) for N-glycosylation in the putative polypeptide encoded by As14 cDNA. As14 cDNA encodes a putative polypeptide of 146 amino acids with a molecular mass of 15,737 Da and a pI of 10.12 (Fig. 1). Removal of the signal peptide would result in a putative mature protein with a molecular mass of 14,009 Da and a pI of 10.0.
FIG. 1.
Comparison of the deduced As14 amino acid sequence with the amino acid sequences of selected homologous molecules. Sequences are from A. vitae (AF000609), C. elegans (T15428), Onchocerca volvulus (P36991), and Wuchereria bancrofti (AF098861). Identical residues are marked with asterisks, and conserved residues are marked with a dot. Gaps, marked by hyphens, have been introduced for better alignment.
A search of the protein database conducted using the information obtained from the National Center for Biotechnology Information revealed that As14 has amino acid sequence similarity with a Caenorhabditis elegans gene product and antigens from filarial parasites. As14 shared the highest amino acid sequence similarity with a RAL-2 homologue from the rodent filarial parasite Acanthocheilonema vitae (41%) (GenBank accession no. AF000609). As14 shared 38% similarity with the amino acid sequence of a C. elegans gene product (T15428). As14 had no significant amino acid sequence similarity with any known sequence except with those of the C. elegans gene product and filarial antigens, suggesting that the As14 antigen predicted by the As14 cDNA sequence is a nematode-specific gene product.
Characterization of rAs14.
The open reading frame of As14 except for the signal sequence was subcloned into the pTrcHisB protein expression vector (Invitrogen). rAs14 was expressed in E. coli and found to migrate as an 18-kDa fusion protein with a hexahistidine tag by SDS-PAGE. Immunoblot analysis was performed using T7 Tag monoclonal antibody directed against the amino-terminal fusion peptide of rAs14. The epitope tag fusion peptide in rAs14 was found to be approximately 4 kDa in size. Thus, rAs14 has an approximate molecular mass of 14 kDa, similar to the mass predicted from the amino acid sequence of As14. rAs14 was purified by metal chelation chromatography under native conditions. One milligram of purified rAs14 was obtained from a liter of bacterial culture. The rAs14 was 99% pure as judged by SDS-PAGE analysis. The purified rAs14 was used for the production of polyclonal antibodies in mice and for a vaccine trial using the mouse-A. suum infection model.
Identification of parasite-derived antigen corresponding to rAs14 in A. suum and an rAs14 homologue in ascarids.
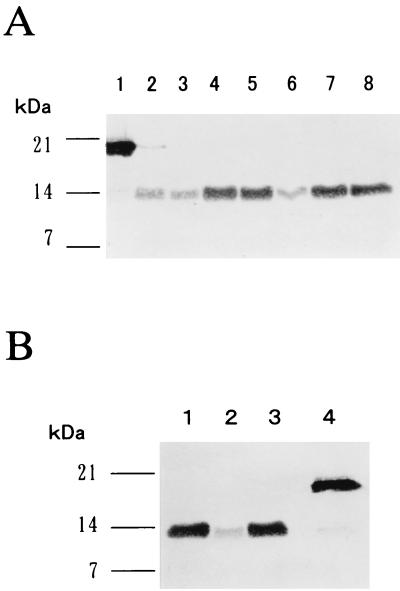
The parasite-derived antigen corresponding to rAs14 was identified in various developmental stages of A. suum. Expression of the parasite-derived antigen corresponding to rAs14 was evaluated by immunoblot analysis using parasite extracts prepared from embryonated eggs, L3, lung-stage larvae, and female and male adult worms. Mouse anti-rAs14 serum bound strongly to a 14-kDa parasite-derived antigen in parasite extracts from all stages and in larval and adult ES products (Fig. 2A). Serum from a preimmune mouse did not react with any antigens in the parasite extract (data not shown). These findings suggest that As14 is ubiquitously expressed in A. suum at all developmental stages and is also released by larvae and adults. In addition, we performed immunoblot analysis of a human roundworm, A. lumbricoides, and a dog roundworm, T. canis, with mouse anti-rAs14 serum. The mouse serum immunoreacted with a 14-kDa PBS-soluble antigen from A. lumbricoides that was the same size as parasite-derived As14. The identical intensities of the immunoblot bands suggest the presence of an As14 homologue in the human roundworm (Fig. 2B). A 14-kDa immunoreactive band was also detected in the PBS-soluble protein from T. canis. Serum from a preimmune mouse did not react with any of the antigens present in the parasite extracts (data not shown).
FIG. 2.
(A) Identification of parasite-derived As14 in A. suum at various developmental stages. The parasite extract was prepared essentially as described in Materials and Methods. Twenty micrograms of parasite extract or ES products was separated by SDS-PAGE, and the proteins were then transferred to a nitrocellulose membrane. Lane 1, rAs14 (50 ng); lane 2, L3; lane 3, lung-stage larvae; lane 4, female worm; lane 5, male worm; lane 6, larval ES; lane 7, female worm ES; lane 8, male worm ES. The antigen bound to the mouse anti-rAs14 serum was detected using NBT-BCIP. (B) Expression of As14 homologues in ascarid nematodes. Lane 1, A. lumbricoides; lane 2, T. canis; lane 3, A. suum; lane 4, rAs14 (50 ng). Forty micrograms of protein equivalents of each parasite extract was used for immunoblot analysis with mouse anti-rAs14 serum.
Reactivity of rAs14 with pig immune sera.
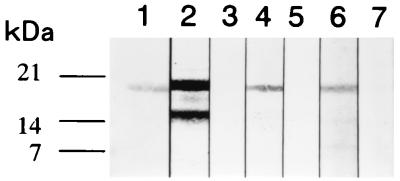
The reactivity of rAs14 with serum from pigs with flubendazole-abbreviated infection was examined using immunoblot analysis. The serum reacted with rAs14, suggesting that rAs14 was antigenic in the natural host (Fig. 3). Pig preimmune sera did not react with rAs14. Sera from repeatedly inoculated rabbits and mice with A. suum embryonated eggs also reacted with rAs14. Rabbit and mouse preimmune sera did not react with rAs14.
FIG. 3.
Reactivity of recombinant As14 with sera from rabbits, mice, and pigs repeatedly infected with A. suum infective embryonated eggs. Fifty nanograms of protein was electrophoresed in each lane of an SDS–12% polyacrylamide gel and blotted onto a nitrocellulose membrane. The recombinant As14 bound to serum samples was detected using NBT-BCIP. Lane 1, purified As14 stained with amido black; lane 2, immune rabbit; lane 3, preimmune rabbit; lane 4, immune mouse; lane 5, preimmune mouse; lane 6, immune pig; lane 7, preimmune pig.
Intranasal vaccination against A. suum L3.
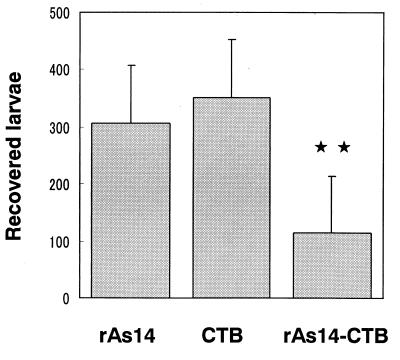
In our preliminary experiments, we observed an apparent reduction in recovery of larvae from the lung in mice subcutaneously injected with rAs14-precipitated Freund's complete adjuvant (FCA). Mice which were immunized with rAs14-FCA and which received two booster doses at 2-week intervals showed a 50% reduction of recovered larvae from the lungs following the challenge infection, compared to either a nonimmunized group of mice or mice that had received FCA alone. In addition, a 99% reduction of recovered larvae was found for a group of mice orally immunized with A. suum L3, compared with the number in groups of nonimmunized mice or mice receiving FCA alone. We therefore tested whether nasal administration of rAs14 coupled with CTB would be more effective. As shown in Fig. 4, a group of mice inoculated intranasally with rAs14 coupled with CTB showed a significant reduction in recovery of larvae from the lung compared with the CTB-control group after A. suum L3 challenge (P < 0.01 by Student's t test). The same level of larval reduction was observed in three other repeated-challenge experiments.
FIG. 4.
Number of larvae recovered from mice immunized with rAs14. Mice were immunized as described in Materials and Methods. Data were expressed as the mean value ± the standard deviation in each group of five mice. Stars indicate that the mean value was significantly lower than that of the group immunized with rAs14 or CTB alone (P < 0.01).
Immune response to intranasal vaccination.
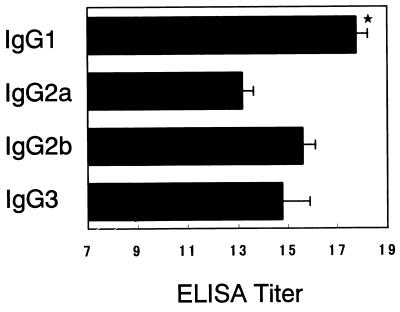
In preliminary experiments, a significant rAs14-specific IgG titer in serum was found in the group of mice immunized with rAs14-FCA (18.2 ± 1.2) and the group immunized with A. suum L3 (13.2 ± 2.2), suggesting that protection against A. suum L3 infection may be immunologically induced. In the present study, we measured the levels of rAs14-specific antibody responses in the mucous fluid from the small intestine and in the sera from mice intranasally immunized with rAs14-CTB. As shown in Table 1, predominantly rAs14-specific IgA responses were seen in mucosal extracts, while predominantly IgG responses and IgE responses were seen in serum. No detectable anti-As14 IgA antibody responses in mucosal extracts, or anti-As14 IgG antibody responses or anti-As14 IgE antibody responses, were seen in sera from mice immunized with rAs14 alone or CTB alone. Furthermore, we examined rAs14-specific serum IgG subclass responses. Mice immunized with rAs14-CTB showed a significant anti-As14 IgG1 response and weak anti-As14 IgG2a, IgG2b, and IgG3 antibody responses (Fig. 5). No detectable IgG subclass anti-As14 antibody response was seen for mice immunized with rAs14 alone or CTB alone.
TABLE 1.
Antigen-specific serum IgG and IgE and mucosal IgA antibody titers to rAs14 in mice intranasally immunized with rAs14a
| Antigen | Titer
|
||
|---|---|---|---|
| Serum IgG | Serum IgE | Mucosal IgA | |
| rAs14 | <8 | <5 | <4 |
| CTB | <8 | <5 | 8.8 ± 1.5 |
| rAs14 + CTB | 18.6 ± 0.55∗ | 10.3 ± 1.3∗ | 9.2 ± 1.3 |
Mice were immunized as described in Materials and Methods. The titer shows the reciprocal log2 of the highest dilution of serum that gave an OD405 greater than that seen with control (no serum added to the well). Values are expressed as the reciprocal log2 titer ± the standard deviation of the mean for each group of five mice. Asterisks indicate that the mean value was significantly higher than that of the rAs14-alone or CTB-alone group (P < 0.001).
FIG. 5.
Serum IgG subclass responses to rAs14. The titer of each subclass is shown as the reciprocal log2 as described in Materials and Methods. Data were expressed as the mean value ± the standard deviation of the mean for each group of five mice. The star indicates that the mean value was significantly higher than that of the other groups (P < 0.001). ELISA, enzyme-linked immunosorbent assay.
DISCUSSION
Numerous reports have shown that protective immune responses to A. suum infection can be achieved in pigs by immunization with irradiated A. suum L3 or by chemically abbreviated larval infection (22, 27, 48, 57, 59). These findings suggest an important role for larval antigens in protective immunity against swine ascariasis. In order to isolate the immunoreactive antigens from various larval stages, we used a nonpermissive host rabbit to raise antibodies against larvae by inoculating the rabbit repeatedly with A. suum L3. Serum from the rabbit reacted with several recombinant clones in an A. suum L3 cDNA library. Among the several cDNA clones that reacted with the rabbit immune sera, clone L2R59 was selected for further analysis because of its low similarity to mammalian proteins. An antibody raised in mice against the recombinant protein produced using a composite cDNA derived from L2R59 was tested for its ability to bind the parasite-derived antigen in immunoblot analysis of A. suum L3 extracts. The results showed that serum from immunized mice reacted with the 14-kDa antigen, which is now designated As14. Mice immunized with rAs14 showed protection against A. suum L3 infection.
Though As14 has amino acid sequence similarity with human and rodent filarial parasite antigens (5, 7) and with a gene product of the free-living nematode C. elegans, extensive database searches failed to detect similarity to any protein of known function. Analysis of developmental-stage-specific expression of As14 showed that high levels of As14 were released in the ES products in larval and adult stages. A number of reports concerning ES products from nematode parasitic stages showed that they change the host physiology and suppresse host immune responses (12, 20, 38). Moreover, some investigators believe that they may be associated with parasite survival (21, 60).
Recently, abundant larval transcript (ALT) antigen, a highly immunoprotective antigen, was identified from the human filarial parasite Brugia malayi (17). A vaccination study demonstrated that ALT gave the highest protection among recombinant antigens that have been cloned from parasitic filarial nematodes (18). ALT has no similarity to mammalian proteins, suggesting that it is a parasite-specific molecule. Parasite-specific antigens with no similarity to host proteins are desirable as parasite vaccine antigens because antibodies against them should not cross-react with host proteins. In the present study, immunoblot analysis using sera from a variety of hosts immunized against A. suum L3 showed that rAs14 was antigenic. In addition, As14 homologues were detected in A. lumbricoides and T. canis, suggesting that ascarid nematodes possess As14-related molecules. Therefore, we examined whether vaccination with rAs14 induces protection in a mouse-A. suum model in order to evaluate rAs14 as a new vaccine candidate for parasitic diseases caused by ascarid nematodes.
When A. suum L3 are orally administered to mice, the larvae penetrate the gastrointestinal tract after approximately 24 h, and the administered larvae reach the lungs, where they cause pulmonary hemorrhage after 72 h (45). Mice vaccinated orally with A. suum L3 were found to be protected against verminous pneumonitis after challenge infection (19). These results show that A. suum L3 vaccination results in a protective immune response associated with a reduction in the number of larvae reaching the lung. In the present study, we performed challenge infection in BALB/c mice after oral administration of A. suum L3. The mice immunized with A. suum L3 showed a 99% reduction in the number of larvae recovered from the lung. Therefore, we examined the protective efficacy of rAs14 administration in BALB/c mice against challenge infection using A. suum L3 in the present study. The number of larvae recovered from the lung was reduced by approximately 63% compared to that recovered from the control group, suggesting that nasal immunization with rAs14 prevents the migration of larvae to the lung.
The generation of protective immune responses at the mucosal surface by nasal or oral administration is a critical goal in the development of a vaccine against intestinal pathogens. Since the mucosal surface of the small intestine is the initial site of the A. suum infection, it is important to establish protective immunity there (27). It has been reported elsewhere that administration of A. suum L3 to animals results in induction of an A. suum L3-specific IgA response in the small intestine (25). However, a major problem with the delivery of antigens to the intestinal mucosa is that oral administration of soluble proteins gives rise either to no immune response or to the development of tolerance (59). In contrast, numerous reports have demonstrated that CTB induces both mucosal and systemic immunity after oral or nasal immunization (40, 53). In the present study, we performed nasal immunization with CTB as a mucosal adjuvant in a BALB/c mouse-A. suum model. The number of larvae recovered from the lungs of the vaccinated mice was significantly lower than that for the parenterally immunized group using FCA alone and lower than that for a nontreated group. In addition, we found that mice vaccinated with rAs14-CTB had high titers of rAs14-specific mucosal IgA and IgG in serum, suggesting that As14-CTB induced both local and systemic protective immune responses against A. suum. In fact, the degree of protection in mice immunized with rAs14-CTB was higher than that in mice parenterally immunized with rAs14 plus FCA (data not shown). It is also worth noting that elevation of the rAs14-specific IgE titer was seen in mice vaccinated with rAs14-CTB. It has been shown elsewhere that Ascaris-specific IgE is associated with protection against Ascaris infection (34). The mechanism by which rAs14 antigen induces protective immunity against A. suum infection was not determined in the present study.
Mice immunized with rAs14 coupled with CTB had a high level of anti-rAs14 IgG1 antibody and a low level of anti-rAs14 IgG2a antibody. CTB used as a mucosal adjuvant induces antigen-specific IgG1 and IgG2 responses, suggesting that CTB activates a type II immune response in mice (36, 52, 53). Protective immunity to A. suum infection in mice may be associated with type II immune responses (35). Recently, it was reported that type II cytokine responses against adult A. lumbricoides were predominantly noted in human ascariasis (9). Further analysis of cytokine profiles may reveal whether type I or type II immune responses predominate. On the other hand, the life cycle of ascarid nematodes involves two different phases that proceed in internal and external environments. Particular events in these two phases may provoke different host immune responses against the larval stages in the tissues and the adult worms in the small intestine of the natural host. The development of A. suum in mice includes passage through larval stages before the development of adult worms. Recently, it was demonstrated that immunization against the parasite in the migratory phase that occurs between L3 and the larval stage resulted in protective immunity against A. suum infection, but not against adult worms, in pigs (26). Further analysis of mice immunized with As14-CTB may provide insight into the immunological mechanisms that function in host resistance against infection with ascarid larval-stage parasites. In fact, immune responses against tissue helminths are different from those against gastrointestinal parasites (15, 23).
Recombinant parasite antigens have been identified as vaccine candidates for a variety of helminths. Recent studies demonstrated that CTB fused with Schistosoma mansoni 28-kDa glutathione S-transferase, which is a candidate vaccine antigen for schistosomiasis, suppressed pathological lesions caused by parasites and reduced animal mortality, not merely by inducing protection against the parasite infection but also through therapeutic effects (51). In addition, the number of infective-stage larvae administered to the host may be an important factor when candidate molecules are evaluated for their vaccine effects against parasitic challenge infections. In fact, animals vaccinated with Trichinella spiralis antigen showed reductions of worm fecundity and worm size (13).
In conclusion, we have cloned a novel 14-kDa immune protective antigen from A. suum that is the first recombinant protective antigen to be identified from ascarid nematodes. In addition, protection against A. suum infection was achieved by mucosal administration of this antigen. One of the current goals in the field of human vaccines is the development of a noninvasive and practical route of administration via mucosal surfaces. Further analysis of mucosally administered As14 should expand our understanding of the induction of protective immunity against parasitic infections caused by ascarid nematodes.
ACKNOWLEDGMENTS
We thank W. Abebe, T. Fujisawa, and Y. Kinoshita for excellent technical assistance.
This study was supported in part by a grant (Parasite Protein) from the Ministry of Agriculture, Forestry and Fishery and by a grant (Edible Vaccine) from the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN).
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Anderson T J, Romero-Abal M E, Jaenike J. Genetic structure and epidemiology of Ascaris populations: patterns of host affiliation in Guatemala. Parasitology. 1993;107:319–334. doi: 10.1017/s0031182000079294. [DOI] [PubMed] [Google Scholar]
- 3.Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992;20:2013–2018. doi: 10.1093/nar/20.suppl.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaxter M, Liu L. Nematode spliced leaders—ubiquity, evolution and utility. Int J Parasitol. 1996;26:1025–1033. [PubMed] [Google Scholar]
- 5.Bradley J E, Tuan R S, Shepley K J, Tree T I, Maizels R M, Helm R, Gregory W F, Unnasch T R. Onchocerca volvulus: characterization of an immunodominant hypodermal antigen present in adult and larval parasites. Exp Parasitol. 1993;77:414–424. doi: 10.1006/expr.1993.1101. [DOI] [PubMed] [Google Scholar]
- 6.Chan M S. The global burden of intestinal nematode infections: fifty years on. Parasitol Today. 1997;13:438–443. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- 7.Chandrashekar R, Curtis K C, Ramzy R M, Liftis F, Li B W, Weil G J. Molecular cloning of Brugia malayi antigens for diagnosis of lymphatic filariasis. Mol Biochem Parasitol. 1994;64:261–271. doi: 10.1016/0166-6851(94)00035-2. [DOI] [PubMed] [Google Scholar]
- 8.Cohen D, Orr N, Haim M, Ashkenazi S, Robin G, Green M S, Ephros M, Sela T, Slepon R, Ashkenazi I, Taylor D N, Svennerholm A M, Eldad A, Shemer J. Safety and immunogenicity of two different lots of the oral, killed enterotoxigenic Escherichia coli-cholera toxin B subunit vaccine in Israeli young adults. Infect Immun. 2000;68:4492–4497. doi: 10.1128/iai.68.8.4492-4497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper P J, Chico M E, Sandoval C, Espinel I, Guevara A, Kennedy M W, Urban J F, Jr, Griffin G E, Nutman T B. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J Infect Dis. 2000;182:1207–1213. doi: 10.1086/315830. [DOI] [PubMed] [Google Scholar]
- 10.Crandall C A, Crandall R B. Ascaris suum: immunoglobulin responses in mice. Exp Parasitol. 1971;30:426–437. doi: 10.1016/0014-4894(71)90107-x. [DOI] [PubMed] [Google Scholar]
- 11.Crompton D W. Ascaris and ascariasis. Adv Parasitol. 2001;48:285–375. doi: 10.1016/s0065-308x(01)48008-0. [DOI] [PubMed] [Google Scholar]
- 12.Deehan M R, Harnett M M, Harnett W. A filarial nematode secreted product differentially modulates expression and activation of protein kinase C isoforms in B lymphocytes. J Immunol. 1997;159:6105–6111. [PubMed] [Google Scholar]
- 13.DeVos T, Dick T A. Trichinella spiralis: the effect of oral immunization and the adjuvancy of cholera toxin on the mucosal and systemic immune response of mice. Exp Parasitol. 1994;76:182–191. doi: 10.1006/expr.1993.1021. [DOI] [PubMed] [Google Scholar]
- 14.Douvres F W, Urban J F., Jr . Nematoda except parasites of insects. In: Taylor A E R, Baker J R, editors. In vitro methods for parasite cultivation. London, United Kingdom: Academic Press; 1987. pp. 318–378. [Google Scholar]
- 15.Garside P, Kennedy M W, Wakelin D, Lawrence C F. Immunopathology of intestinal helminth infection. Parasite Immunol. 2000;22:605–612. doi: 10.1046/j.1365-3024.2000.00344.x. [DOI] [PubMed] [Google Scholar]
- 16.Geenen P L, Bresciani J, Boes J, Pedersen A, Eriksen L, Fagerholm H P, Nansen P. The morphogenesis of Ascaris suum to the infective third-stage larvae within the egg. J Parasitol. 1999;85:616–622. [PubMed] [Google Scholar]
- 17.Gregory W F, Blaxter M L, Maizels R M. Differentially expressed, abundant trans-spliced cDNAs from larval Brugia malayi. Mol Biochem Parasitol. 1997;87:85–95. doi: 10.1016/s0166-6851(97)00050-9. [DOI] [PubMed] [Google Scholar]
- 18.Gregory W F, Atmadja A K, Allen J E, Maizels R M. The abundant larval transcript-1 and -2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infect Immun. 2000;68:4174–4179. doi: 10.1128/iai.68.7.4174-4179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerrero J, Silverman P H. Active immunization against Ascaris suum minimum lethal dose in mice. Z Parasitenkd. 1972;39:339–344. doi: 10.1007/BF00329095. [DOI] [PubMed] [Google Scholar]
- 20.Harnett W, Harnett M M. Phosphorylcholine: friend or foe of the immune system? Immunol Today. 1999;20:125–129. doi: 10.1016/s0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- 21.Hawdon J M, Jones B F, Hoffman D R, Hotez P J. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J Biol Chem. 1996;271:6672–6678. doi: 10.1074/jbc.271.12.6672. [DOI] [PubMed] [Google Scholar]
- 22.Hill D E, Fetterer R H, Romanowski R D, Urban J F., Jr The effect of immunization of pigs with Ascaris suum cuticle components on the development of resistance to parenteral migration during a challenge infection. Vet Immunol Immunopathol. 1994;42:161–169. doi: 10.1016/0165-2427(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann K F, Cheever A W, Wynn T A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 24.Jeska E L, Williams J F, Cox D F. Ascaris suum: larval returns in rabbits, guinea pigs and mice after low-dose exposure to eggs. Exp Parasitol. 1969;26:187–192. doi: 10.1016/0014-4894(69)90111-8. [DOI] [PubMed] [Google Scholar]
- 25.Jeska E L, Stankiewicz M. Responses of NFR/N inbred mice to very low-dose infections with Ascaris suum. Int J Parasitol. 1989;19:85–89. doi: 10.1016/0020-7519(89)90025-8. [DOI] [PubMed] [Google Scholar]
- 26.Jungersen G, Eriksen L, Roepstorff A, Lind P, Meeusen E N, Rasmussen T, Nansen P. Experimental Ascaris suum infection in the pig: protective memory response after three immunizations and effect of intestinal adult worm population. Parasite Immunol. 1999;21:619–630. doi: 10.1046/j.1365-3024.1999.00261.x. [DOI] [PubMed] [Google Scholar]
- 27.Kelly G, Nayak D P. Acquired immunity to migrating larvae of Ascaris suum induced in pigs by repeated oral inoculation of infective eggs. J Parasitol. 1964;50:499–503. [PubMed] [Google Scholar]
- 28.Khoury P B, Stromberg B E, Soulsby E J. Immune mechanisms to Ascaris suum in inbred guinea-pigs. I. Passive transfer of immunity by cells or serum. Immunology. 1977;32:405–411. [PMC free article] [PubMed] [Google Scholar]
- 29.Kodama S, Suenaga S, Hirano T, Suzuki M, Mogi G. Induction of specific immunoglobulin A and Th2 immune responses to P6 outer membrane protein of nontypeable Haemophilus influenzae in middle ear mucosa by intranasal immunization. Infect Immun. 2000;68:2294–2300. doi: 10.1128/iai.68.4.2294-2300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit–whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marbella C O, Gaafar S M. Production and distribution of immunoglobulin-bearing cells in the intestine of young pigs infected with Ascaris suum. Vet Parasitol. 1989;34:63–70. doi: 10.1016/0304-4017(89)90165-9. [DOI] [PubMed] [Google Scholar]
- 33.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 34.McSharry C, Xia Y, Holland C V, Kennedy M W. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect Immun. 1999;67:484–489. doi: 10.1128/iai.67.2.484-489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa I, Takahashi I, Kiyono H, McGhee J R, Hamada S. Oral immunization with the B subunit of the heat-labile enterotoxin of Escherichia coli induces early Th1 and late Th2 cytokine expression in Peyer's patches. J Infect Dis. 1996;173:1428–1436. doi: 10.1093/infdis/173.6.1428. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Pastrana D V, Raghavan N, FitzGerald P, Eisinger S W, Metz C, Bucala R, Schleimer R P, Bickel C, Scott A L. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun. 1998;66:5955–5963. doi: 10.1128/iai.66.12.5955-5963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng W, Anderson T J, Zhou X, Kennedy M W. Genetic variation in sympatric Ascaris populations from humans and pigs in China. Parasitology. 1998;117:355–361. doi: 10.1017/s0031182098003102. [DOI] [PubMed] [Google Scholar]
- 40.Ruedl C, Rieser C, Kofler N, Wick G, Wolf H. Humoral and cellular immune responses in the murine respiratory tract following oral immunization with cholera toxin or Escherichia coli heat-labile enterotoxin. Vaccine. 1993;14:792–798. doi: 10.1016/0264-410x(95)00231-o. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Shen X, Lagergard T, Yang Y, Lindblad M, Fredriksson M, Holmgren J. Systemic and mucosal immune responses in mice after mucosal immunization with group B streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate vaccine. Infect Immun. 2000;68:5749–5755. doi: 10.1128/iai.68.10.5749-5755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slotved H C, Roepstorff A, Barnes E H, Eriksen L, Nansen P. Comparison of two methods for recovering migrating Ascaris suum larvae from the liver and lungs of pigs. J Parasitol. 1996;82:612–615. [PubMed] [Google Scholar]
- 44.Slotved H C, Eriksen L, Murrell K D, Nansen P. Comparison of methods for recovery of Ascaris suum larvae from tissues of mice. Int J Parasitol. 1997;27:1305–1310. doi: 10.1016/s0020-7519(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 45.Slotved H C, Eriksen L, Murrell K D, Nansen P. Early Ascaris suum migration in mice as a model for pigs. J Parasitol. 1998;84:16–18. [PubMed] [Google Scholar]
- 46.Soulsby E J L. Helminths, arthropods and protozoa of domesticated animals. 7th ed. London, United Kingdom: Bailliere Tindall; 1986. [Google Scholar]
- 47.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stankiewicz M, Jeska E L. Evaluation of pyrantel-tartrate abbreviated Ascaris suum infections for the development of resistance in young pigs against migrating larvae. Int J Parasitol. 1990;20:77–81. doi: 10.1016/0020-7519(90)90176-n. [DOI] [PubMed] [Google Scholar]
- 49.Sultan F, Jin L L, Jobling M G, Holmes R K, Stanley S L., Jr Mucosal immunogenicity of a holotoxin-like molecule containing the serine-rich Entamoeba histolytica protein (SREHP) fused to the A2 domain of cholera toxin. Infect Immun. 1998;66:462–468. doi: 10.1128/iai.66.2.462-468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun J B, Holmgren J, Czerkinsky C. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–10799. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun J B, Mielcarek N, Lakew M, Grzych J M, Capron A, Holmgren J, Czerkinsky C. Intranasal administration of a Schistosoma mansoni glutathione S-transferase-cholera toxoid conjugate vaccine evokes antiparasitic and antipathological immunity in mice. J Immunol. 1999;163:1045–1052. [PubMed] [Google Scholar]
- 52.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 53.Tamura S, Samegai Y, Kurata H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6:409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 54.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuji N, Morales T H, Ozols V V, Carmody A B, Chandrashekar R. Molecular characterization of a calcium-binding protein from the filarial parasite Dirofilaria immitis. Mol Biochem Parasitol. 1998;97:69–79. doi: 10.1016/s0166-6851(98)00131-5. [DOI] [PubMed] [Google Scholar]
- 56.Tsuji N, Kasuga-Aoki H, Isobe T, Yoshihara S. Cloning and characterisation of a peroxiredoxin from the swine roundworm Ascaris suum. Int J Parasitol. 2000;30:125–128. doi: 10.1016/s0020-7519(99)00180-0. [DOI] [PubMed] [Google Scholar]
- 57.Urban J F, Jr, Tromba F G. Development of immune responsiveness to Ascaris suum antigens in pigs vaccinated with ultraviolet-attenuated eggs. Vet Immunol Immunopathol. 1982;3:399–409. doi: 10.1016/0165-2427(82)90022-8. [DOI] [PubMed] [Google Scholar]
- 58.Urban J F, Jr, Romanowski R D. Ascaris suum: protective immunity in pigs immunized with products from eggs and larvae. Exp Parasitol. 1985;60:245–254. doi: 10.1016/0014-4894(85)90028-1. [DOI] [PubMed] [Google Scholar]
- 59.Weiner H L. Oral tolerance. Proc Natl Acad Sci USA. 1994;91:10762–10765. doi: 10.1073/pnas.91.23.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whelan M, Harnett M M, Houston K M, Patel V, Harnett W, Rigley K P. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–6460. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 61.Zhang T, Li E, Stanley S L., Jr Oral immunization with the dodecapeptide repeat of the serine-rich Entamoeba histolytica protein (SREHP) fused to the cholera toxin B subunit induces a mucosal and systemic anti-SREHP antibody response. Infect Immun. 1995;63:1349–1355. doi: 10.1128/iai.63.4.1349-1355.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]