Abstract
Background
Osteoporosis is a condition where bones become fragile due to low bone density and impaired bone quality. This results in fractures that lead to higher morbidity and reduced quality of life. Osteoporosis is considered a major public health concern worldwide. For this reason, preventive measurements need to be addressed throughout the life course. Exercise and a healthy diet are among the lifestyle factors that can help prevent the disease, the latter including intake of key micronutrients for bone, such as calcium and vitamin D. The evidence on whether supplementation with calcium and vitamin D improves bone mineral density (BMD) in premenopausal women is still inconclusive. In this age group, bone accrual is considered to be the goal of supplementation, so BMD is relevant for the future stages of life.
Objectives
To evaluate the benefits and harms of calcium and vitamin D supplementation, alone or in combination, to increase the BMD, reduce fractures, and report the potential adverse events in healthy premenopausal women compared to placebo.
Search methods
We used standard, extensive Cochrane search methods. The latest search was 12 April 2022.
Selection criteria
We included randomised controlled trials in healthy premenopausal women (with or without calcium or vitamin D deficiency) comparing supplementation of calcium or vitamin D (or both) at any dose and by any route of administration versus placebo for at least three months. Vitamin D could have been administered as cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2).
Data collection and analysis
We used standard Cochrane methods. Outcomes included total hip bone mineral density (BMD), lumbar spine BMD, quality of life, new symptomatic vertebral fractures, new symptomatic non‐vertebral fractures, withdrawals due to adverse events, serious adverse events, all reported adverse events and additional withdrawals for any reason.
Main results
We included seven RCTs with 941 participants, of whom 138 were randomised to calcium supplementation, 110 to vitamin D supplementation, 271 to vitamin D plus calcium supplementation, and 422 to placebo. Mean age ranged from 18.1 to 42.1 years. Studies reported results for total hip or lumbar spine BMD (or both) and withdrawals for various reasons, but none reported fractures or withdrawals for adverse events or serious adverse events. Results for the reported outcomes are presented for the three comparisons: calcium versus placebo, vitamin D versus placebo, and calcium plus vitamin D versus placebo. In all comparisons, there was no clinical difference in outcomes, and the certainty of the evidence was moderate to low. Most studies were at risk of selection, performance, detection, and reporting biases.
Calcium versus placebo
Four studies compared calcium versus placebo (138 participants in the calcium group and 123 in the placebo group) with mean ages from 18.0 to 47.3 years. Calcium supplementation may have little to no effect on total hip or lumbar spine BMD after 12 months in three studies and after six months in one study (total hip BMD: mean difference (MD) −0.04 g/cm2, 95% confidence interval (CI) −0.11 to 0.03; I2 = 71%; 3 studies, 174 participants; low‐certainty evidence; lumbar spine BMD: MD 0 g/cm2, 95% CI −0.06 to 0.06; I2 = 71%; 4 studies, 202 participants; low‐certainty evidence). Calcium alone supplementation does not reduce or increase the withdrawals in the trials (risk ratio (RR) 0.78, 95% CI 0.52 to 1.16; I2 = 0%; 4 studies, 261 participants: moderate‐certainty evidence).
Vitamin D versus placebo
Two studies compared vitamin D versus placebo (110 participants in the vitamin D group and 79 in the placebo group), with mean ages from 18.0 to 32.7 years. These studies reported lumbar spine BMD as a mixture of MDs and percent of change and we were unable to pool the results. In the original studies, there were no differences in lumbar BMD between groups. Vitamin D alone supplementation does not reduce or increase withdrawals for any reason between groups (RR 0.74, 95% CI 0.46 to 1.19; moderate‐certainty evidence).
Calcium plus vitamin D versus placebo
Two studies compared calcium plus vitamin D versus placebo (271 participants in the calcium plus vitamin D group and 270 in the placebo group; 220 participants from Woo 2007 and 50 participants from Islam 2010). The mean age range was 18.0 to 36 years. These studies measured different anatomic areas, one study reported total hip BMD and the other study reported lumbar spine BMD; therefore, data were not pooled for this outcome. The individual studies found no difference between groups in percent of change on total hip BMD (−0.03, 95% CI −0.06 to 0; moderate‐certainty evidence), and lumbar spine BMD (MD 0.01, 95% CI −0.01 to 0.03; moderate‐certainty evidence). Calcium plus vitamin D supplementation may not reduce or increase withdrawals for any reason (RR 0.82, 95% CI 0.29 to 2.35; I2 = 72%; 2 studies, 541 participants; low‐certainty evidence).
Authors' conclusions
Our results do not support the isolated or combined use of calcium and vitamin D supplementation in healthy premenopausal women as a public health intervention to improve BMD in the total hip or lumbar spine, and therefore it is unlikely to have a benefit for the prevention of fractures (vertebral and non‐vertebral).
The evidence found suggests that there is no need for future studies in the general population of premenopausal women; however, studies focused on populations with a predisposition to diseases related to bone metabolism, or with low bone mass or osteoporosis diagnosed BMD would be useful.
Plain language summary
Calcium and vitamin D for improving bone health in healthy premenopausal women
Key messages
The evidence suggests that calcium, vitamin D, or calcium plus vitamin D supplementation has no effect on bone mineral density at any site (hip or spine) in healthy premenopausal women.
What is osteoporosis?
Osteoporosis is characterised by low levels of calcium and other types of minerals in the bones (called bone mineral density). This causes holes to form inside the bones and the outer walls of the bone to become thin making the bones more fragile, which may lead to increased fractures and breaks.
Osteoporosis constitutes a major public health problem and contributes to more than 8.9 million broken bones annually, which means that on average, an osteoporotic fracture occurs every three seconds. Supplements of calcium and vitamin D are often recommended for women after menopause (although not everyone agrees), but adequate supplementation of calcium and vitamin D is always recommended in institutionalised people (e.g. people living in care homes) and people taking osteoporosis treatment. Little is known about the effect of calcium and vitamin D on the bone density of women who have not yet started menopause. There are few studies in this age group and the results are inconclusive. In this age group, increasing bone strength and health is considered the goal of supplementation, so BMD is relevant.
What did we want to find out?
We wanted to determine if calcium and vitamin D were able to increase the mineral content of bones and reduce the risk of fractures, and to report potential side effects of supplementation.
What did we do?
We searched medical databases for well‐designed clinical studies of calcium and vitamin D supplementation alone or in combination compared with placebo (dummy treatment) in healthy women aged 18 to 45 years (premenopausal). We analysed three combinations: calcium versus placebo, vitamin D versus placebo, and calcium plus vitamin D versus placebo, administered for at least three months. We looked at their effects on increasing minerals in the bones of the hip and spine, if the women had vertebral (backbone) or any other fractures during the study, effects on quality of life, and if these women had to stop the supplementation because of side effects.
What did we find?
We included seven studies with 941 healthy premenopausal women with an average age per study of 18 to 42.1 years. The women were randomly assigned to receive supplementation of calcium, vitamin D, or vitamin D plus calcium, or placebo.
Main results
There was no difference in bone mineral density in any of the groups being supplemented with calcium, vitamin D, or calcium plus vitamin D compared with placebo. The studies did not report fractures (from any anatomical site), quality of life, or stopping the supplementation for side effects.
What are the limitations of the evidence?
The common limitations in the methods of the studies included small numbers of participants, studies, and data; problems in adherence to treatment, participants may have known which treatment they received; and lack of information for withdrawals from treatment. The funding for the studies was provided by institutional, academic, government, and pharmaceutical industries.
How up to date is this evidence?
The evidence is up to date to April 2022.
Summary of findings
Summary of findings 1. Calcium compared to placebo for increasing bone mineral density in premenopausal women.
| Calcium compared to placebo for increasing bone mineral density in premenopausal women | |||||||
|
Patient or population: premenopausal women (age 18−47.3 years)
Setting: community
Intervention: calcium
Comparison: placebo Dosage: calcium carbonate 1500 mg (3 capsules of 500 mg with each meal); calcium elemental 1000 mg (1 tablet a day), citrate 1000 mg (1 tablet a day), and carbonate 1000 mg (2 chewable tablets a day) | |||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens | ||
| Without calcium | With calcium | Difference | |||||
| Total hip BMD assessed with: DXA (g/cm2) Follow‐up: mean 12 months | — | — | — | MD 0.04 g/cm2 lower (0.01 lower to 0.03 higher) | 174 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | Calcium alone supplementation may have little to no effect on total hip BMD in healthy premenopausal woman. |
| Lumbar spine BMD (mean SD and % change) assessed with: DXA (g/cm2) Follow‐up: mean 12 months | — | — | — | MD 0 g/cm2 (0.06 lower to 0.06 higher) | 202 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | Calcium alone supplementation may have little to no effect on BMD lumbar spine in healthy premenopausal woman. |
| Quality of life | — | — | — | — | — | — | Not reported |
| Vertebral fracture | — | — | — | — | — | — | Not reported |
| Non‐vertebral fracture | — | — | — | — | — | — | Not reported |
| Withdrawals from the study for any reason | RR 0.78 (0.52 to 1.16) | 27.6% | 21.6% (14.4% to 32.1%) | 6.1% lower (13.3% lower to 4.4% higher) | 261 (4 RCTs) | ⊕⊕⊕⊝ Moderatec | Calcium alone supplementation does not reduce or increase the withdrawals in the trials. |
| Serious adverse events | — | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; DXA: dual‐energy X‐ray absorptiometry; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level for studies that carried large weight for the overall effect estimate rated as high risk of bias due to attrition and other bias: problem with adherence to treatment in one of three studies. bDowngraded one level for inconsistency due to one study supplemented with calcium 1500 mg versus two studies supplemented with calcium 1000 mg, a difference that was supported by non‐overlapping CIs, I2 greater than 70%, and substantial heterogeneity of effect estimates. cDowngraded one level for the studies that carried large weight for the overall effect estimate rated as unclear risk of bias in almost all domains.
Summary of findings 2. Vitamin D compared to placebo for increasing bone mineral density in premenopausal women.
| Vitamin D compared to placebo for increasing bone mineral density in premenopausal women | |||||||
|
Patient or population: premenopausal women (age 18–32.7 years)
Setting: community
Intervention: vitamin D
Comparison: placebo Dosage: vitamin D3 10 μg (400 IU) or 20 μg (800 IU) (1 tablet a day) | |||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens | ||
| Without vitamin D | With vitamin D | Difference | |||||
|
Total hip BMD assessed with: DXA (g/cm2) |
— | — | — | — | — | — | Not reported. |
| Lumbar spine BMD assessed with: DXA g/cm2 Follow‐up: median 12 months | — | — | — | — | — | — | Vitamin D alone supplementation does not increase the BMD mean difference in the lumbar spine in healthy premenopausal woman.a |
| Quality of life | — | — | — | — | — | — | Not reported. |
| Vertebral fracture | — | — | — | — | — | — | Not reported. |
| Non‐vertebral fracture | — | — | — | — | — | — | Not reported. |
| Withdrawals from the study for any reason Follow‐up: median 12 months | RR 0.74 (0.46 to 1.19) | 21.5% | 15.9% (9.9% to 25.6%) | 5.6% lower (11.6% lower to 4.1% higher) | 189 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | Vitamin D alone supplementation does not reduce or increase the withdrawals in the trials. |
| Serious adverse events | — | — | — | — | — | — | Not reported. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; DXA: dual‐energy X‐ray absorptiometry; RCT: randomised controlled trial; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aThe studies included in these comparisons did not report the same outcomes and reported different units of measure. bDowngraded one level for imprecision, the CIs included both increased and decreased withdrawals.
Summary of findings 3. Calcium and vitamin D compared to placebo for increasing bone mineral density in premenopausal women.
| Calcium and vitamin D compared to placebo for increasing bone mineral density in premenopausal women | |||||||
|
Patient or population: premenopausal women (ages 18–36 years)
Setting: community
Intervention: calcium and vitamin D
Comparison: placebo Dosage: vitamin D 10 μg (400 IU) + calcium 600 mg (1 tablet a day); vitamin D3 5 μg (200 IU) + calcium 1000 mg (2 sachets per day) | |||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens | ||
| Without calcium + vitamin D | With calcium + vitamin D | Difference | |||||
| Total hip BMD assessed with: DXA (g/cm2) Follow‐up: median 12 months | — | — | — | MD 0.03 g/cm2 lower (0.06 lower to 0) | 408 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Calcium and vitamin D combined supplementation does not increase total hip BMD mean difference in healthy premenopausal woman.b |
| Lumbar spine BMD assessed with: DXA (g/cm2) Follow‐up: median 12 months | — | — | — | MD 0.01 g/cm2 higher (0.01 lower to 0.03 higher) | 76 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Calcium and vitamin D combined supplementation does not increase BMD mean difference in the lumbar spine in healthy premenopausal woman.b |
| Quality of life | — | — | — | — | — | — | Not reported. |
| Vertebral fracture | — | — | — | — | — | — | Not reported. |
| Non‐vertebral fracture | — | — | — | — | — | — | Not reported. |
| Withdrawals from the study for any reason Follow‐up: median 12 months | RR 0.82 (0.29 to 2.35) | 10.0% | 8.2% (2.9% to 23.5%) | 1.8% lower (7.1% lower to 13.5% higher) | 541 (2 RCTs) | ⊕⊕⊝⊝ Lowc,d | Calcium plus vitamin D supplementation may not reduce or increase withdrawals for any reason. |
| Serious adverse events | — | — | — | — | — | — | Not reported. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMD: bone mineral density; CI: confidence interval; DXA: dual‐energy X‐ray absorptiometry; RCT: randomised controlled trial; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level for the studies analysed that carried large weight for the overall effect estimate rated as unclear risk of bias in more than two domains. bOutcome included in only one study. cDowngraded one level, the CIs included both increased and decreased withdrawals. dDowngraded one level for inconsistency due to the studies reporting different outcomes measured (BMD mean difference of total hip and BMD in the lumbar spine), a difference that was supported by non‐overlapping CIs, I2 greater than 70%, and substantial heterogeneity of effect estimates.
Background
Description of the condition
Osteoporosis is characterised by low bone mineral density (BMD) and impaired quality of bone and is considered a major public health concern worldwide. The main consequence of low BMD are fragility fractures, mainly at the hip, spine, and wrist (NIH 1993). Fragility fractures may lead to excess mortality, morbidity, low quality of life (QoL), and chronic pain (Borgström 2013; Papaioannou 2010).
In 2007, it was estimated that osteoporosis affected about 200 million people worldwide, and that 75 million of them were from high‐income countries (Europe, Japan, and the USA) (Kanis 2007). Nine million new fragility fractures occurred in 2000, including 1.6 million of the hip, 1.7 million of the wrist, and 1.4 million of the spine (Johnell 2004), and impacted in the number of years lived with disability (YLD) by musculoskeletal disorders (IHME 2018). More‐recent data from the Global Burden of Disease Study 2019, reported an estimation of 172 million incident fractures (95% uncertainty intervals (UI) 162 to 196), 455 million (95% UI 428 to 484) prevalent cases of acute or long‐term symptoms of a fracture, and 25.8 million (95% UI 17.8 to 35.8) YLDs were documented. The increments in these indicators were huge from 33.4% to 70.1% and age‐specific rates of fractures were highest in the oldest age groups (GBD 2021).
Premenopausal bone mass is an important determinant of bone density in the postmenopausal period. Osteoporosis prevention is feasible and should be addressed throughout life, improving peak bone mass in adolescence and early adulthood, and decreasing age‐related bone loss over adult life, including in the premenopausal period.
Description of the intervention
Dietary intake and supplementation therapies are common strategies for maintaining bone mass and consequently preventing osteoporosis. These strategies include calcium and vitamin D supplementation. These micronutrients are primarily obtained from three sources: food, body synthesis process, and supplements. There are multiple nutritional sources of products that contribute calcium to the diet, dairy products are a good source of dietary calcium. Regarding vitamin D, the main source in humans is the synthesis of this prohormone in the skin through sun exposure, since vitamin D is only found in small quantities in certain foods.
Calcium supplements are most commonly available as calcium citrate or calcium carbonate, and vitamin D can be found in supplementation products such as ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). A combination of both nutrients is available in different doses and presentations. Calcium and vitamin D supplements are also prescribed with anti‐osteoporotic medications (e.g. oestrogens, calcitonin, anabolic steroids, etc.) as they are thought to have additive effects on the regeneration of bone (Sánchez 2003).
Clinical trials of calcium and vitamin D supplementation have been conducted to assess the efficacy of this strategy for improving BMD in children and postmenopausal women; the evidence is variable and reported in Cochrane Reviews and meta‐analyses (Rizzoli 2013; Winzenberg 2006; Winzenberg 2010).
In children, there is no known effect of calcium supplementation on the femoral neck or lumbar spine BMD, but review authors found a small effect on total body bone mineral content (BMC) and upper limb BMD in this population (Winzenberg 2006). Vitamin D supplementation in children showed no effect on BMC, hip BMD, and forearm BMD. There was a non‐significant tendency for BMD improvement in children with low mean levels of vitamin D. However, the review concluded that these results do not support vitamin D supplementation to improve BMD in healthy children with normal vitamin D levels (Winzenberg 2010).
The review of calcium supplementation for postmenopausal women showed that calcium had a small effect on BMD compared to placebo (Rizzoli 2013), and USPSTF 2018 argues that a combination of both nutrients has a beneficial role by increasing BMD and muscle strength and reduction in the number of falls in elderly people. However, the evidence remains inconclusive, as one meta‐analysis concluded that recommending vitamin D supplements to prevent fractures or falls in adults is not justified (Heneghan 2019), and one Cochrane Review concluded that vitamin D supplementation probably reduces the rate of falls but not the risk of falling (Cameron 2018).
How the intervention might work
Calcium is needed for bone formation and other important physiological processes and the bones are the main storage site of calcium in the body. Because calcium is lost each day through the urine, it is important to replace it to maintain adequate levels in the body. Low serum calcium leads to increased parathyroid hormone (PTH) and activates 1,25 dihydroxyvitamin D to increase calcium absorption starting the metabolic pathway of bone metabolism that includes the calcium and phosphate flux across bone, the gastrointestinal tract, and the kidneys (ASBMR 2019; IOM 2011).
In addition to the direct effects on bone, vitamin D has been associated with muscle strength and the prevention of the risk of falls. As vitamin D receptors are found in muscle tissue, their activation leads to muscle protein synthesis. In this way, vitamin D supplements may improve muscle strength, and decrease the risk of falls (Bischoff‐Ferrari 2009; Gupta 2010; Zhu 2010). However, as mentioned in the Description of the intervention section, the evidence on whether supplementation of vitamin D is associated with fall reduction is inconclusive.
Calcium and vitamin D are universally adopted as simple and inexpensive interventions to improve bone health, and the change in BMD is measured by dual‐energy x‐ray absorptiometry (DXA) appointed as the gold standard (Dunfield 2007; Lewiecki 2016).
Why it is important to do this review
The effect of calcium and vitamin D on BMD, BMC, or fractures has been studied in children and postmenopausal women. However, less is known about the effects of calcium and vitamin D in premenopausal women. In February 2013, the US Preventive Services Task Force (USPSTF) published a systematic review and meta‐analysis of the use of calcium and vitamin D supplements to prevent fractures in adults, and they concluded that the evidence was insufficient to assess the benefits and harms of the prevention of fractures (Moyer 2013). Cochrane Reviews have evaluated calcium and vitamin D in children (Winzenberg 2006; Winzenberg 2010). One recent systematic review focussed on vitamin D and vitamin D analogues for preventing fractures in postmenopausal women and older men (Abshirini 2020), and another study examined the effect of calcium supplementation on femoral and lumbar BMD in postmenopausal women (Avenell 2014). Also in this age group, bone accrual is considered to be the goal of supplementation, so BMD is relevant when fracture data are not available. No systematic review has specifically evaluated premenopausal women; therefore, the primary focus of this systematic review was whether calcium or vitamin D supplementation alone or in combination has a positive effect on BMD or BMC measured with DXA. In addition, we considered on vertebral and non‐vertebral fractures, QoL, and potential adverse events in healthy premenopausal women. This review was conducted according to the guidelines recommended by the Cochrane Musculoskeletal Group (Ghogomu 2014).
Objectives
To evaluate the benefits and harms of calcium and vitamin D supplementation, alone or in combination, to increase the BMD, reduce fractures, and report the potential adverse events in healthy premenopausal women compared to placebo.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) reported as full‐text and with no language restrictions.
Types of participants
We included trials conducted in healthy premenopausal women, with or without calcium or vitamin D deficiency, and studies where the menopausal status was not specified but the age was reported (commonly classified as 18 to 45 years). Healthy women were defined as those without an osteoporosis or osteopenia diagnosis, chronic conditions, cardiovascular disorders, or autoimmune or inflammatory diseases (e.g. rheumatoid arthritis, osteoarthritis, fibromyalgia, multiple sclerosis, systemic lupus erythematosus, diabetes mellitus, and asthma). We excluded studies in pregnant and lactating women; men; studies where participants had coexisting medical conditions; secondary causes of osteoporosis; and corticosteroid‐induced osteoporosis. We included studies with men when information on women was available separately.
Types of interventions
We included trials comparing calcium, vitamin D, or calcium plus vitamin D with placebo, focusing on three comparisons, regardless of type or dose of supplementation.
Calcium alone versus placebo
Vitamin D alone versus placebo
Calcium plus vitamin D versus placebo
We excluded trials with a treatment period of less than three months. We excluded studies with the following co‐interventions of specific anti‐osteoporosis therapy: bisphosphonates, hormone replacement therapy, PTH, selective oestrogen receptor modulators (SERMs), and strontium ranelate.
Types of outcome measures
Major outcomes
Total hip BMD measured with DXA in grams/centimetre squared
Lumbar spine BMD measured with DXA in grams/centimetre squared
Quality of life (QoL) for example measured with 36‐item Short Form (SF‐36), Menopause Rating Scale, or other types of instruments for measuring health‐related quality of life
New symptomatic vertebral fractures confirmed by imaging
New symptomatic non‐vertebral fractures confirmed by imaging
Withdrawals due to adverse events (all adverse events attributed directly to supplementation or placebo group)
Serious adverse events (i.e. hospitalisations, or those resulting in disability or death).
Minor outcomes
All reported adverse events
Withdrawals for any reason
Search methods for identification of studies
Electronic searches
We designed a search strategy for the following databases.
Cochrane Central Register of Controlled Trials (April 2022) including Ovid and Database of Reviews of Effects (DARE) (Appendix 1).
MEDLINE Ovid (1946 to 12 April 2022; Appendix 2).
Embase Ovid (1947 to 12 April 2022; Appendix 3).
Additionally, we conducted a search on the ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/clinical-trials-registry-platform).
The electronic search strategy for MEDLINE is outlined in Appendix 2. We adapted this search strategy for application to other databases. We used the 'sensitivity and precision maximising version' filter designed to identify clinical trials described by Lefebvre 2017.
We examined all databases from inception to April 2022 imposing no language restrictions.
Searching other resources
We searched the reference lists of other reviews related to our topic and examined the reference lists of the studies included in this review.
Data collection and analysis
Selection of studies
Two review authors (LM‐S and PC) independently screened titles and abstracts of all potentially relevant studies, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications, and two review authors (LM‐S and PC) screened the full‐text identifying studies for inclusion, recording reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion and, if required, we asked a third review author (PT). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process with sufficient detail to complete a PRISMA flow diagram (prisma-statement.org/PRISMAStatement/Default.aspx) and the Characteristics of excluded studies table.
Data extraction and management
We used a data collection form for recording study characteristics and outcome data, which was piloted on at least one study in the review. One review author (LM‐S) extracted study characteristics from included studies. A second review author (PC) spot‐checked study characteristics for accuracy against the trial report. We extracted the following study characteristics.
Methods: study design, the total duration of the study, details of any 'run‐in' period, number of study centres and location (country), study setting, withdrawals, and date of study.
Participants: sample size, mean age, age range, sex, ethnicity, vitamin D status (if available), calcium and vitamin D intake (if available), and inclusion and exclusion criteria.
Interventions: type of intervention (calcium alone, vitamin D alone, or calcium plus vitamin D); comparison (placebo or no intervention group). If data were available, we reported other alternative comparisons (e.g. calcium plus vitamin D versus vitamin D alone, or calcium alone). We described the dosage or type of vitamin D (ergocalciferol or cholecalciferol), dosage and type of calcium, supplementation period, and concomitant medications. We excluded comparisons with other interventions, if it was available the type of comparisons found were reported.
-
Outcomes: in all cases, we extracted the mean or percent of change from baseline to the endpoint that had been analysed on an intention‐to‐treat basis. We analysed data using Review Manager 2020 and Review Manager Web 2022.
For dichotomous outcomes, we extracted the number of events and the number of participants per treatment group. For withdrawals, we extracted data on both the number of events and the number of participants who were withdrawn from the studies with a report of withdrawals (for the analysis we used the final value).
For continuous outcomes, such as a change in BMD at the total hip and lumbar spine, we recorded mean and standard deviations (SD) of the percentage of change, median and 25th to 75th percentiles, and the number of participants per treatment group. For the analysis, we used the final change data per treatment group. We extracted only crude results.
When hip and vertebral data were available, we conducted analyses separately by intervention, population, dosage, type of vitamin D, and type of calcium supplement.
When adverse event data were not available for the withdrawals, we obtained their data for additional analyses (withdrawals for any reason).
Characteristics of the design of the trial as outlined in the Assessment of risk of bias in included studies section. We reported in the Characteristics of included studies table whether outcome data were not provided in a usable form and when data were transformed or estimated from a graph.
Notes: we recorded trial funding and statements of competing interests.
We resolved disagreements by consensus or by involving a third review author (PT). One review author (LM‐S) transferred the data into Review Manager 2020. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports.‐
Two review authors (LM‐S, PC) extracted data in duplicate from graphs or figures using Plot Digitizer 2015.
Assessment of risk of bias in included studies
Two review authors (LM‐S and PC) independently assessed the risk of bias for each study using the Cochrane RoB 1 tool using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved disagreements by discussion or by involving a third review author (PT). We assessed the risk of bias according to the following domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective outcome reporting (reporting bias)
We graded each potential source of bias as high, low, or unclear risk, and provided a quote from the study report along with an explanation for our decision in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. Likewise, we considered the impact of missing data on key outcomes under the limitations and scope of this systematic revision.
In case important study information was lacking, we contacted the study authors to obtain such data, using open‐ended questions. When the information on the risk of bias came from unpublished data or correspondence with trial lists, we reported this in the risk of bias table.
When considering treatment effects, we accounted for the risk of bias for the studies that contributed to that outcome.
We presented the figures generated by the risk of bias tool to provide summary assessments.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Méndez‐Sánchez 2017), and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RR) with 95% confidence intervals (CIs). We analysed continuous data as mean difference (MD) when the same scale was used to measure an outcome, along with 95% CIs. If studies used different scales, we planned to use the standardised mean difference. Data were presented as a scale with a consistent direction of effect across studies.
Unit of analysis issues
Where a trial reported multiple trial arms, we included only the relevant arms but listed the other treatment arms in the Characteristics of included studies table. When two comparisons (e.g. calcium versus placebo and vitamin D versus placebo) were combined using the same units in the same meta‐analysis, both were included. If the same comparisons (e.g. vitamin D versus different dosages of vitamin D) were combined in the same meta‐analysis, we followed the procedures recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022).
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only, when data were not available for all participants, or the data were in a graphical analysis or adjusted). If this was not possible, and the missing data were considered to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by conducting a sensitivity analysis. Any assumptions and imputations to handle missing data would have been clearly described and the effect of imputation explored using sensitivity analyses.
For dichotomous outcomes (e.g. number of withdrawals due to adverse events), we calculated the withdrawal rate using the number of participants randomised in the group as the denominator. For continuous outcomes (e.g. mean change in BMD), we calculated the MD based on the number of participants analysed at that time point. If the number of participants analysed was not presented for each time point, we used the number of randomised participants in each group at baseline.
Where feasible (in graphic data), we computed missing SDs from other statistics such as standard errors, CIs, or P values, according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). If SDs could not be calculated, we imputed them (e.g. from other studies in the meta‐analysis)
Assessment of heterogeneity
We assessed clinical and methodological diversity in terms of participants, interventions, outcomes, and study characteristics for the included studies to determine whether a meta‐analysis was appropriate by observing these data from the data extraction tables. We assessed statistical heterogeneity using the I² statistic and Chi² test.
According to the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022), the interpretation of an I² value of 0% to 40% might 'not be important'; 30% to 60% represents 'moderate' heterogeneity; 50% to 90% represents 'substantial' heterogeneity; and 75% to 100% represents 'considerable' heterogeneity. We keep in mind that the importance of the I2 statistic depended on the magnitude and direction of effects, and strength of evidence for heterogeneity. The Chi2 test was interpreted where a P ≤ 0.10 indicated evidence of statistical heterogeneity.
Assessment of reporting biases
We planned to create and examine with a funnel plot to detect possible small‐study biases, but there was an insufficient number of studies to allow this (Deeks 2019).
To assess outcome reporting bias, we checked trial protocols against published reports. For studies published after 1 July 2005, we screened ClinicalTrial.gov for the a priori trial protocol. We assessed whether selective reporting of outcomes was present.
Data synthesis
We conducted a meta‐analysis only where this was meaningful (i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We did not find sufficient data to perform the following planned subgroup analysis:
dosage (vitamin D: 600 IU or less and greater than 600 IU; calcium 1000 mg or less and greater than 1000 mg);
supplementation time (short term less than 12 months, long term 12 months or greater);
baseline vitamin D levels or dietary calcium intake (sufficiency greater than 30 ng/mL; insufficiency 11 ng/mL to 29 ng/mL; or deficiency less than 10 ng/mL).
Sensitivity analysis
We conducted sensitivity analyses to test the robustness of the treatment effects on major outcomes, by type of intervention.
Summary of findings and assessment of the certainty of the evidence
Two review authors (LM‐S and PC) independently assessed the certainty of the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence as it related to the studies that provided data to the meta‐analysis. This was reported as high, moderate, low, or very low. We used methods and recommendations described in Sections 8.5 and 8.7, and Chapters 11 and 12, of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019; McKenzie 2019). We used GRADEpro GDT software to prepare the summary of findings tables (GRADEpro GDT; Schünemann 2013). We explained all decisions to downgrade the certainty of the evidence using footnotes and made comments to expedite the reader's understanding of the review in the risk of bias table.
We created summary of findings tables using the major outcomes for each of the three comparisons.
Total hip BMD
Lumbar spine BMD
QoL
New symptomatic vertebral fractures
New symptomatic non‐vertebral fractures
Withdrawals due to adverse events
Serious adverse events
Results
Description of studies
We summarised the study characteristics under Included studies and Excluded studies. Full details of each study can be found in the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We performed an electronic search on 12 April 2022. Figure 1 presents the results from the screened process of this review.
1.
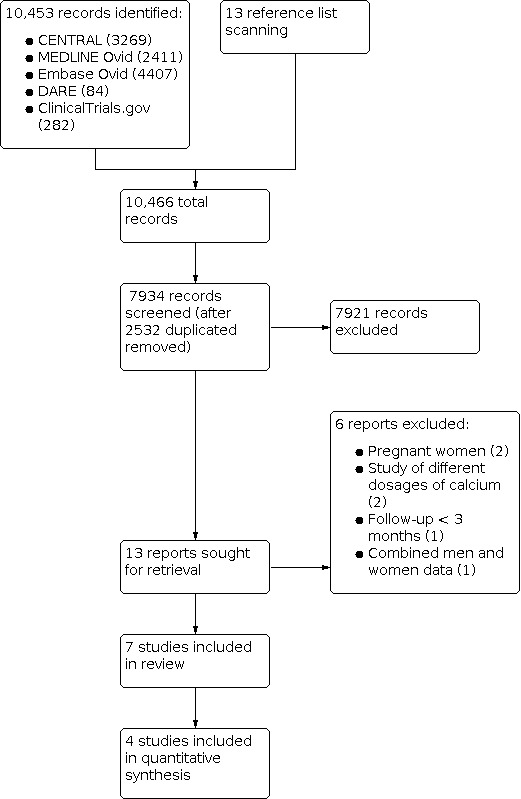
PRISMA flow diagram.
The search strategy identified 10,466 citations. After exclusion of 2532 duplicates and screening of the 7934 abstracts and titles for eligibility, we identified 13 studies for full‐text review. We excluded six studies for the following reasons: two focused on pregnant women, two compared different doses of calcium, one followed the intervention for less than three months, and one only included combined data for men and women.
We included seven RCTs in the review (Andersen 2008; Barger‐Lux 2005; Islam 2010; Rourke 1998; Shapses 2001; Winters‐Stone 2004; Woo 2007). Of these seven RCTs, four assessed the intervention in the general population, one assessed women on a weight loss programme, and two in female athletes. These studies reported data on 941 participants (138 randomised to calcium supplementation, 110 to vitamin D supplementation, 271 to vitamin D plus calcium supplementation, and 422 to placebo). All trials used DXA to assess BMD. Six studies reported BMD at the lumbar spine (Andersen 2008; Barger‐Lux 2005; Islam 2010; Rourke 1998; Shapses 2001; Winters‐Stone 2004), and four reported changes at the total hip (Barger‐Lux 2005; Rourke 1998; Winters‐Stone 2004; Woo 2007). We contacted two study authors to request missing information but received no answer.
Included studies
A full description of included trials and participants is provided in the Characteristics of included studies table. The principal outcomes are reported in Table 4, and characteristics of the type and doses of calcium and vitamin D supplementation in Table 5.
1. Main outcomes reported in the included studies.
| Study | BMD total hip | BMD lumbar spine | Quality of life | Vertebral fractures | Non‐vertebral Fractures | Withdrawals | Serious adverse events |
| Calcium compared to placebo | |||||||
| Barger‐Lux 2005 | BMDa | BMDa | NR | NR | NR | Yes | NR |
| Rourke 1998 | BMDb | BMDb | NR | NR | NR | NR | NR |
| Shapses 2001 | BMDa | BMDa | NR | NR | NR | NR | NR |
| Winters‐Stone 2004 | BMDb | BMDb | NR | NR | NR | Yes | NR |
| Vitamin D compared to placebo | |||||||
| Islam 2010 | NR | BMDa | NR | NR | NR | NR | NR |
| Andersen 2008 | NR | BMDc | NR | NR | NR | Yes | NR |
| Calcium plus vitamin D compared to placebo | |||||||
| Woo 2007 | BMDa | BMDa | NR | NR | NR | Yes | NR |
| Islam 2010 | NR | BMDa | NR | NR | NR | NR | NR |
BMD: bone mineral density; NR: not reported. BMD values reported as:a% change; bmean; c25th to 75th percentiles.
2. Type and dosage of supplementation of calcium and vitamin D.
| Author | Type of intervention/dosage | Type of administration | Follow‐up |
| Calcium compared to placebo | |||
| Barger‐Lux 2005 | Calcium carbonate 1500 mg | 3 capsules of 500 mg with each meal | 36 monthsa |
| Rourke 1998 | Calcium elemental 1000 mg | 1 tablet a day | 12 months |
| Shapses 2001 | Calcium citrate 1000 mg | 1 tablet a day | 6 months |
| Winters‐Stone 2004 | Calcium carbonate 1000 mg | 2 chewable tablets a day | 12 months |
| Vitamin D compared to placebo | |||
| Islam 2010 | Vitamin D3 10 μg (400 IU) | 1 tablet a day | 12 months |
| Andersen 2008 | Vitamin D3 10 μg (400 IU) or 20 μg (800 IU) | 1 tablet a day | 12 months |
| Calcium + vitamin D compared to placebo | |||
| Woo 2007 | Calcium 1000 mg + vitamin D3 5 μg (200 IU) | 2 sachets per day | 24 months |
| Islam 2010 | Vitamin D3 10 μg (400 IU) + calcium lactate 600 mg | 1 tablet a day | 12 months |
aOnly the first 12 months period of this study were included in the analysis.
Trial design
The length of these RCTs was three years in two studies (Barger‐Lux 2005; Woo 2007), 12 months in four studies (Andersen 2008; Islam 2010; Rourke 1998; Winters‐Stone 2004), and six months in one study (Shapses 2001).
Trial setting
Four studies were performed in North America (Barger‐Lux 2005; Rourke 1998; Shapses 2001; Winters‐Stone 2004), one in China (Woo 2007), one in Denmark (Andersen 2008 including Pakistani population), and one in India (Islam 2010).
Trial size
The review included 941 participants and the sample size of the individual studies ranged from 28 to 441.
Participants
The mean age of participants ranged from 19.2 to 42.1 years. Four studies included healthy women from open populations (Andersen 2008; Barger‐Lux 2005; Islam 2010; Woo 2007), one study enrolled women in a weight loss programme (Shapses 2001), and two studies included non‐professional female athletes from a running community (Rourke 1998: athletes from Division I and Division III collegiate teams; and Winters‐Stone 2004: ran a minimum of 10 miles per week and competed in regional or national running events). All women were premenopausal.
Four trials reported the mean 25(OH)D levels of both groups at baseline: Shapses 2001 reported levels greater than 60 nmol/L; Woo 2007 and Islam 2010 reported levels of less than 27 nmol/L, and Andersen 2008 reported levels between 9.9 nmol/L and 14 nmol/L. The remaining trials did not report these data.
Five trials provided baseline data on dietary calcium intake for all groups. Andersen 2008 reported a median intake of 495 mg/day to 533 mg/day. Four studies reported the mean baseline: Barger‐Lux 2005 reported an overall mean (both groups) of 605 (SD 181) mg/day; Winters‐Stone 2004 reported a mean calcium intake of 1006 (SD 454) mg/dL in the intervention group and 1294 (SD 263) mg/dL in the placebo group; Woo 2007 reported a mean in one city of 446 (SD 249) mg/day and another city of 446 (SD 260) mg/day, and Shapses 2001 reported a mean of 810 (SD 335) mg/day in the intervention group and 1005 (SD 390) mg/day in the placebo group. The remaining trials did not report these data.
Interventions
Calcium
Four trials administered calcium: Winters‐Stone 2004 used calcium carbonate 1000 mg/day in the intervention group (chewable pills 500 mg twice a day); Barger‐Lux 2005 calcium carbonate 1500 mg/day (one 500 mg tablet with each of three meals); Shapses 2001 calcium citrate 1000 mg/day (one tablet a day), and Rourke 1998 elemental calcium 1000 mg once daily (Table 5).
Vitamin D
Two trials administered vitamin D as cholecalciferol (vitamin D₃; Andersen 2008; Islam 2010). One of the trials tested two different doses of vitamin D: 10 μg (400 IU) once a day and 20 μg (800 IU) once a day (Andersen 2008). Islam 2010 was a four‐arm study with one arm including vitamin D 10 μg (400 IU) in tablets (Table 5).
Calcium plus vitamin D
Two trials administered calcium plus vitamin D; Woo 2007 administered calcium 1000 mg plus vitamin D 5 μg (200 IU) in two sachets of milk powder to the intervention group once a day and Islam 2010 administered calcium lactate 600 mg plus vitamin D 10 μg (400 IU) in tablets once a day (Table 5).
Comparators
Six trials administered a placebo (Andersen 2008; Barger‐Lux 2005; Islam 2010; Rourke 1998; Shapses 2001; Winters‐Stone 2004), and in one trial, participants received no intervention (Woo 2007).
Outcomes
All trials reported BMD; three reported two regions (total hip and lumbar spine) (Barger‐Lux 2005; Rourke 1998; Winters‐Stone 2004), one reported total hip only (Woo 2007), and three reported lumbar spine only (Andersen 2008; Islam 2010; Shapses 2001). All trials assessed BMD using DXA measured in grams per centimetre squared. Four studies reported percentage change (Barger‐Lux 2005; Islam 2010; Shapses 2001; Woo 2007), two reported mean and SD (Rourke 1998; Winters‐Stone 2004), and one reported median and 25th to 75th percentiles (Andersen 2008).
None of the trials reported QoL, vertebral fractures, non‐vertebral fractures, withdrawals due to adverse events, or serious adverse events (Table 4).
Funding
The studies' sources of funding were institutional, academic, government, and pharmaceutical industries (SmithKline Beecham, Fonterra Brands Ltd, and Johnson and Johnson).
Excluded studies
We excluded six studies following a full‐text review (Characteristics of excluded studies table). Two studies included pregnant women (Jarjou 2010; Liu 2011), two compared two different doses of calcium (from 200 mg/day to 1300 mg/day) without a placebo or no intervention group (Riedt 2007; Teegarden 2005), one with less than three months of follow‐up time (Mesinovic 2019), and one included the total data of men and women without separate analysis (Gaffney‐Stomberg 2022).
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the risk of bias assessments across all included trials and individual ratings for each trial. Full descriptions and review authors' justifications for the assigned rating are included in the risk of bias tables within the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
For sequence generation, three studies were at low risk (Andersen 2008; Islam 2010; Woo 2007), and four studies were at unclear risk of bias for not specifying the methods used for the sequence generation (Barger‐Lux 2005; Rourke 1998; Shapses 2001; Winters‐Stone 2004). The method for randomisation was equal size block randomisation (Andersen 2008; Islam 2010), and permuted block randomisation (Woo 2007).
Allocation concealment
Three studies described allocation concealment adequately and were at low risk of bias (Andersen 2008; Barger‐Lux 2005; Islam 2010). Two studies reported allocation by uninvolved third‐parties (Andersen 2008; Islam 2010), and one study reported central allocation (pharmacy‐controlled) (Barger‐Lux 2005). Four studies did not describe allocation concealment or it was described insufficiently; therefore, the risk of bias was unclear (Rourke 1998; Shapses 2001; Winters‐Stone 2004; Woo 2007).
Blinding
Performance bias
Three studies were at low risk of performance bias as they reported that the drug manufacturer provided the intervention and placebo and they had the same appearance (Andersen 2008; Barger‐Lux 2005; Islam 2010). Four studies were at unclear risk as they did not provide sufficient information about participants and personnel blinding (Rourke 1998; Shapses 2001; Winters‐Stone 2004; Woo 2007); specifically, Rourke 1998 reported having "probably problems" with contamination of the groups.
Detection bias
All seven studies were at unclear risk of detection bias due to insufficient information about outcome assessment.
Incomplete outcome data
Four studies were at low risk of attrition bias (Barger‐Lux 2005; Islam 2010; Winters‐Stone 2004; Woo 2007). Two studies were at high risk of attrition bias; Rourke 1998 lost 33% of participants and provided an explanation, and they only performed a per‐protocol analysis; in Shapses 2001, the dropouts in the calcium group due to poor adherence were reassigned to the placebo group in the analysis. Andersen 2008 was at unclear risk of attrition bias as it lacked information about withdrawals.
Selective reporting
We assessed the risk of bias due to selective reporting to be unclear in six studies (Andersen 2008; Barger‐Lux 2005; Islam 2010; Rourke 1998; Winters‐Stone 2004; Woo 2007). Shapses 2001 was at high risk of selective reporting bias as the placebo group showed substantial deviations from the randomised intervention since 37% from the intervention group who did not comply were then reassigned to the placebo group.
Other potential sources of bias
Five trials were at low risk of other bias (Andersen 2008; Barger‐Lux 2005; Islam 2010; Winters‐Stone 2004; Woo 2007). Shapses 2001 was at unclear risk of other bias since the final allocation did not match with the baseline allocation of participants. Rourke 1998 was at high risk of other bias due to the report of poor adherence and compliance with treatment; participants in the placebo group had a higher dietary calcium intake than the calcium supplementation group and there was suspicion of contamination between groups.
Effects of interventions
See: Table 1; Table 2; Table 3
Calcium versus placebo
Four studies compared calcium versus placebo, which included 261 healthy premenopausal women (138 received calcium and 123 received placebo) (Table 1; Barger‐Lux 2005; Rourke 1998; Shapses 2001; Winters‐Stone 2004).
The studies used elemental calcium 1000 mg, carbonate calcium 1500 mg, carbonate calcium 1000 mg, and calcium citrate 1000 mg compared with placebo (Barger‐Lux 2005; Rourke 1998; Shapses 2001; Winters‐Stone 2004; see Table 5 for further details on the intervention).
Total hip bone mineral density
Three studies including 174 participants reported the effect of calcium on total hip BMD (Barger‐Lux 2005; Rourke 1998; Winters‐Stone 2004). Calcium supplementation may have little to no effect on total hip BMD at 12 months (MD −0.04 g/cm2, 95% CI −0.11 to 0.03; I2 = 71%; low‐certainty evidence; Analysis 1.1; Figure 4). Barger‐Lux 2005 reported percent change and we calculated the equivalent mean and SD using the Review Manager calculator (Review Manager Web 2022).
1.1. Analysis.

Comparison 1: Calcium versus placebo, Outcome 1: Total hip bone mineral density (BMD)
4.

Forest plot of comparison: 1 Calcium versus placebo, outcome: 1.1 Total hip BMD.
Lumbar spine bone mineral density
Four studies including 202 participants reported the effect of calcium on lumbar spine BMD (Barger‐Lux 2005; Rourke 1998; Shapses 2001; Winters‐Stone 2004). Calcium supplementation may have little to no effect on lumber spine BMD at 12 months (MD 0 g/cm2, 95% CI −0.06 to 0.06; I2 = 71%; low‐certainty evidence; Analysis 1.2; Figure 5). Barger‐Lux 2005 and Shapses 2001 reported percent change and we calculated the equivalent mean and SD using the Review Manager calculator (Review Manager Web 2022).
1.2. Analysis.

Comparison 1: Calcium versus placebo, Outcome 2: Lumbar spine BMD
5.

Forest plot of comparison: 1 Calcium versus placebo, outcome: 1.2 Lumbar spine BMD.
Quality of life
No studies reported the effects of calcium versus placebo on QoL.
New symptomatic vertebral fractures
No studies reported the effects of calcium versus placebo on new symptomatic vertebral fractures.
New symptomatic non‐vertebral fractures
No studies reported the effects of calcium versus placebo on new symptomatic non‐vertebral fractures.
Withdrawals due to adverse events
No studies reported the effects of calcium versus placebo on withdrawals due to adverse events.
Serious adverse events
No studies reported the effects of calcium versus placebo on serious adverse events.
All reported adverse events
No studies reported the effects of calcium versus placebo on all reported adverse events.
Withdrawals for any reason
Four studies including 261 participants reported the effect of calcium on withdrawals for any reason (Barger‐Lux 2005; Rourke 1998; Shapses 2001; Winters‐Stone 2004). There were 63 withdrawals for any reason (29 in the intervention group and 34 in the placebo group) but the original articles did not report the specific reasons for withdrawals. Calcium supplementation does not reduce or increase withdrawals (RR 0.78, 95% CI 0.52 to 1.16; I2 = 0%; moderate‐certainty evidence; Analysis 1.3; Figure 6).
1.3. Analysis.

Comparison 1: Calcium versus placebo, Outcome 3: Withdrawals for any reason
6.

Forest plot of comparison: 1 Calcium versus placebo, outcome: 1.3 Withdrawals for any reason.
Sensitivity analyses
In addition, this comparison included a population of athletes (Rourke 1998; Winters‐Stone 2004), and it should be noted that Rourke 1998 reported potential contamination of groups, which could cause problems in the interpretation of the results. Therefore, we performed a sensitivity analysis including only these two studies in the forest plot, where there remained no evidence of a difference between groups in either mean and total hip BMD (MD 0, 95% CI −0.05 to 0.05) and lumbar spine BMD (MD 0, 95% CI −0.05 to 0.06).
Vitamin D alone versus placebo
Two studies compared vitamin D versus placebo, which included 189 healthy premenopausal women (110 received vitamin D and 79 received placebo) (Table 2; Andersen 2008; Islam 2010). The intervention groups included supplementation with cholecalciferol (vitamin D3) 10 μg to 20 μg (400 IU to 800 IU) in Andersen 2008 (two intervention groups) and 10 μg (400 IU) in Islam 2010 (see Table 5 for further details).
Total hip bone mineral density
No studies reported total hip BMD.
Lumbar spine bone mineral density
Both studies reported lumbar spine BMD, although it was not possible to combine the data in a meta‐analysis since the units were different. Islam 2010 reported results as a percentage of change and Andersen 2008 reported medians with 25th to 75th percentiles.
Neither study reported any difference in lumbar spine BMD. Islam 2010 reported the percentage of change (mean: 0.013 (SD 0.036) with vitamin D versus −0.003 (SD 0.049) with placebo; P = 0.205; MD 0.01, 95% CI −0.01 to 0.03; Analysis 2.1). Andersen 2008 reported a no difference in lumbar spine BMD using median and 25th to 75th percentiles (vitamin D 10 μg (400 IU): median 1.06, 25th to 75th percentiles 1.01 to 1.11; vitamin D 20 μg (800 IU): median 0.99, 25th to 75th percentiles 0.93 to 1.08; placebo: median 1.00, 25th to 75th percentiles 0.89 to 1.16).
2.1. Analysis.

Comparison 2: Vitamin D versus placebo, Outcome 1: Lumbar spine bone mineral density (BMD) mean standard deviation
Quality of life
No studies reported the effects of vitamin D versus placebo on QoL.
New symptomatic vertebral fractures
No studies reported the effects of vitamin D versus placebo on new symptomatic vertebral fractures.
New symptomatic non‐vertebral fractures
No studies reported the effects of vitamin D versus placebo on new symptomatic non‐vertebral fractures.
Withdrawals due to adverse events
No studies reported the effects of calcium versus vitamin D on withdrawals due to adverse events.
Serious adverse events
No studies reported the effects of vitamin D versus placebo on serious adverse events.
All reported adverse events
No studies reported the effects of vitamin D versus placebo on all reported adverse events.
Withdrawals for any reason
Two studies including 189 participants reported the effect of vitamin D on withdrawals for any reason (Andersen 2008; Islam 2010). There were 62 withdrawals for any reason (27 in the intervention group and 35 in the placebo group). Vitamin D supplementation does not reduce or increase withdrawals (0.74, 95% CI 0.46 to 1.19, I2 = 0%; moderate‐certainty evidence; Analysis 2.2; Figure 7). Reported reasons for withdrawals included "lost job," "disliked capsule pills," moved to another location, or unclear.
2.2. Analysis.

Comparison 2: Vitamin D versus placebo, Outcome 2: Withdrawals for any reason
7.

Forest plot of comparison: 2 Vitamin D versus placebo, outcome: 2.2 Withdrawals for any reason.
Calcium plus vitamin D versus placebo
We identified two studies comparing calcium plus vitamin D versus placebo including 541 healthy premenopausal women (271 received vitamin D plus calcium group and 270 received placebo) (Table 3; Islam 2010; Woo 2007). These studies measured different anatomic areas, one study reported total hip BMD and the other study reported lumbar spine BMD; therefore, data were not pooled for this outcome. Doses administered were calcium lactate 600 mg plus vitamin D3 10 μg (400 IU) (Islam 2010), and calcium 1000 mg and vitamin D3 5 μg (200 IU) (Woo 2007).
Total hip bone mineral density
One study including 408 participants reported the effect of calcium plus vitamin D on total hip BMD (Woo 2007). The study reported no difference between groups in percent of change on total hip BMD at six months (0.16 (SD 0.12) in the intervention group and 0.19 (SD 0.19) in the placebo group; P value not reported; MD −0.03%, 95% CI −0.06% to 0%; Analysis 3.1).
3.1. Analysis.

Comparison 3: Calcium plus vitamin D versus placebo, Outcome 1: Total hip bone mineral density (BMD) % change
Lumbar spine bone mineral density
One study including 76 participants reported the effect of calcium plus vitamin D on lumbar spine BMD (Islam 2010). The study reported no difference between groups in percent of change on lumbar spine BMD (0.01 (SD 0.04) in the intervention group and −0.003 (SD 0.049) in the placebo group; MD 0.01, 95% CI −0.01 to 0.03; moderate‐certainty evidence Analysis 3.2).
3.2. Analysis.

Comparison 3: Calcium plus vitamin D versus placebo, Outcome 2: BMD lumbar spine
Quality of life
No studies reported the effects of calcium plus vitamin D versus placebo on QoL.
New symptomatic vertebral fractures
No studies reported the effects of calcium plus vitamin D versus placebo on new symptomatic vertebral fractures.
New symptomatic non‐vertebral fractures
No studies reported the effects of calcium plus vitamin D versus placebo on new symptomatic non‐vertebral fractures.
Withdrawals due to adverse events
No studies reported the effects of calcium plus vitamin D versus placebo on withdrawals due to adverse events.
Serious adverse events
No studies reported the effects of calcium plus vitamin D versus placebo on serious adverse events.
All reported adverse events
No studies reported the effects of calcium plus vitamin D versus placebo on all reported adverse events.
Withdrawals for any reason
Two studies including 541 participants reported the effect of calcium plus vitamin D on withdrawals for any reason (Islam 2010; Woo 2007). There were 52 withdrawals for any reason (25 in the intervention group and 27 in the placebo group). Calcium plus vitamin D supplementation may not reduce or increase withdrawals for any reason (RR 0.82, 95% CI 0.29 to 2.35; I2 = 72%; low‐certainty evidence; Analysis 3.3; Figure 8).
3.3. Analysis.

Comparison 3: Calcium plus vitamin D versus placebo, Outcome 3: Withdrawals for any reason
8.

Forest plot of comparison: 3 Calcium and vitamin D versus placebo, outcome: 3.3 Withdrawals for any reason.
Islam 2010 did not report the reasons for withdrawals. Woo 2007 reported withdrawals such as pregnancy, change of residence, or illness. See Characteristics of included studies table.
Discussion
Summary of main results
Our review included seven RCTs with 941 participants. The comparisons were calcium versus placebo (four studies), vitamin D versus placebo (two studies), and calcium plus vitamin D versus placebo (one study). From our outcomes of interest, the studies only reported BMD at the lumbar spine, total hip, or both. Calcium doses varied from 600 mg to 1500 mg and included various types of calcium substrates (elemental, citrate, lactate, and carbonate). Regarding vitamin D, all the studies used cholecalciferol (vitamin D3); doses varied from 10 µg to 20 µg (400 IU to 800 IU). The length of the studies varied from six to 12 months. We were able to combine the data for meta‐analysis on four studies (Barger‐Lux 2005; Rourke 1998; Shapses 2001; Winters‐Stone 2004).
Calcium versus placebo
Calcium supplementation may have little to no effect on total hip or lumbar spine BMD (total hip BMD: MD −0.04 g/cm2, 95% CI −0.11 to 0.03; 3 studies, 174 participants; lumbar spine BMD: MD 0 g/cm2, 95% CI −0.06 to 0.06; 4 studies, 202 participants). None of the seven RCTs reported our other major outcomes including QoL, new symptomatic vertebral fracture, new symptomatic non‐vertebral fracture, withdrawals due to adverse events, or serious adverse events.
Amongst the studies included for the comparison of calcium versus placebo, we found two studies that recruited female athletes (Rourke 1998; Winters‐Stone 2004), which although in both cases report being athletes of low performance, could provide results that could not be generalised to the rest of the population, for multiple reasons, amongst them the biological fact that exercise can lead to a greater accumulation of bone. In addition, many female athletes experience amenorrhoea, which will affect bone density. However, both studies reported equal menstrual irregularities (defined as menstrual cycles occurring at intervals greater than 40 days or at inconsistent intervals) ranging from three to four occurrences in both groups (Rourke 1998); or oligomenorrhoeic problems (four to 10 menstrual cycles per year) reported in ranges of one to three occurrences in both groups (Winters‐Stone 2004).
Vitamin D versus placebo
In the vitamin D versus placebo comparison, studies reported lumbar spine BMD using different units: Islam 2010 used MDs of the percentage change and Andersen 2008 used 25th to 75th percentiles; therefore, we were unable to pool data. In the original studies, there were no differences in lumbar BMD between groups of healthy premenopausal women; therefore, supplementation with vitamin D had no effect on lumbar BMD. Regarding withdrawals between groups, vitamin D supplementation did not reduce or increase the withdrawals in the trials reported (RR 0.74, 95% CI 0.46 to 1.19; moderate‐certainty evidence).
Calcium plus vitamin D versus placebo
Among the calcium plus vitamin D versus placebo comparison, one study reported total hip BMD, and another study reported lumbar spine BMD. These individual studies reported no difference between groups in percent of change on total hip BMD or lumbar spine BMD (total hip BMD: MD −0.03, 95% CI −0.06 to 0; moderate‐certainty evidence; Woo 2007; lumbar spine BMD: 0.01, 95% CI −0.01 to 0.03; moderate‐certainty evidence; Islam 2010). We concluded that calcium plus vitamin D did not increase or reduce BMD for the total hip or lumbar spine compared with placebo. Results for withdrawals for any reason found no difference (RR 0.82, 95% CI 0.29 to 2.35; low‐certainty evidence).
Overall completeness and applicability of evidence
We performed an exhaustive search for all studies available on this important topic. The clinical trials that we identified had serious problems in internal validity (bias, inconsistency, and imprecision) that in turn impacted external validity and applicability of the results in similar populations.
Our results would not apply to populations with known deficits in calcium or vitamin D intake or vulnerable populations (Holick 2011), such as people with eating disorders (Modan‐Moses 2014), bariatric surgery (Bacci 2010), coeliac disease (Haines 2008), or in populations with vegan diets (Fulgoni 2011; Morehouse‐Grand 2014).
Quality of the evidence
The body of evidence identified through these seven studies in premenopausal women did not allow a robust conclusion regarding the objectives selected for this review. None of the included studies reported new symptomatic vertebral and non‐vertebral fractures, QoL, withdrawals due to adverse events, and serious adverse events.
We have concerns about the methodological quality and the risk of bias in all studies (Figure 3): the percentages vary for each type of risk from 10% unclear risk to 100% as follows: random sequence generation (40% low risk and 60% unclear risk), allocation concealment (40% low risk and 60% unclear risk), blinding of participants and personnel (40% low risk and 60% unclear risk), blinding of outcome assessment (100% unclear risk), incomplete outcome data (60% low risk, 10% unclear risk, and 30% high risk), selective reporting (90% unclear risk and 10% high risk), and other bias (70% low risk, 15% unclear risk, and 15% high risk).
We presented the findings of trials that reported the major outcomes of interest in the summary of findings tables for each comparison and used the GRADE approach to assess the certainty of evidence for each major outcome (Schünemann 2011b). Most of the evidence was downgraded to low or moderate certainty based on three factors: risk of bias, inconsistency generated by heterogeneity, and imprecision with small trials and the CIs included 0 (no appreciable effect on total hip BMD or lumbar spine BMD).
Potential biases in the review process
This review was conducted according to the previously published protocol (Méndez‐Sánchez 2017). While our search was comprehensive, we might have failed to include some RCTs when the data were not available by age or specific subgroup. However, we are quite certain that the substantial RCTs that could provide the definitive evidence needed in this area were identified by our search strategy. We undertook systematic processes throughout the review and a cautious approach when interpreting the evidence.
Agreements and disagreements with other studies or reviews
The prevention of osteoporosis in the early stages is a vital strategy for the prevention of fragility fractures in postmenopausal women; therefore, decision‐makers and medical services need low‐cost and straightforward interventions that could impact bone health (Burger 2007).
However, defining the efficacy and safety of calcium or vitamin D (or both) supplementation is a must before any implementation regarding supplementation of these nutrients to the general population or a segment of it is mandatory.
Supplementation strategies with calcium and vitamin D alone or in combination have been proposed to increase bone quality, prevent falls, and consequently prevent fractures (IOM 2011). However, the type of supplementation required must be followed by the target population that requires it and must be based on evidence of the efficacy of these interventions.
As far as we are concerned there are no other reviews that intentionally examined whether supplementation with calcium, vitamin D, or calcium plus vitamin D is effective for low BMD or in preventing fractures in premenopausal women. USPSTF 2018 found inadequate evidence to estimate the benefits of calcium, vitamin D, or calcium plus vitamin D to prevent fractures in community‐dwelling men and premenopausal women; however, we found no analysis of a specific subgroup of premenopausal women in the publication, and none of the trials included in this present Cochrane Review were cited in USPSTF 2018. It is important to mention that the focus of the USPSTF 2018 was on fractures, and none of the included studies in the present review reported vertebral and non‐vertebral fractures.
Authors' conclusions
Implications for practice.
The present systematic review aimed to evaluate the effects of calcium or vitamin D (or both) on total hip bone mineral density (BMD), lumber spine BMD, quality of life, new symptomatic vertebral fractures, new symptomatic non‐vertebral fractures, withdrawals due to adverse events, and serious adverse events. We reported the BMD outcomes in this population where fracture data were not available. In addition, in this age group, bone accrual is considered to be the goal of supplementation, so BMD is relevant.
The evidence suggests that compared to placebo, calcium or vitamin D alone or in combination used as a supplementation strategy results in little to no difference in all studies that reported BMD either at the lumbar spine or total hip in healthy premenopausal women. The certainty of the evidence is low to moderate. The clinical trials did not report our other clinically important outcomes (quality of life, new symptomatic vertebral fractures, new symptomatic non‐vertebral fractures, withdrawals due to adverse events, and serious adverse events).
Implications for research.
Even though the studies included in this review had some methodological weaknesses, it is noteworthy that they all had the same direction in the outcomes reported, and there was no positive effect in the supplemented group compared with the placebo group in a diverse population of premenopausal women including women from the general population, various ethnic groups (from India, China, Pakistan, and North America), and other special groups such as non‐professional athletes and obese women.
Despite the low‐certainty evidence, we do not think it is justified to conduct more studies on healthy premenopausal women. It would be desirable that the next generation of randomised controlled trials evaluate these interventions in populations of premenopausal women with vulnerable conditions, such as people with eating disorders, bariatric surgery, coeliac disease, or in populations with special diets such as vegans where it might be more likely that supplementation might be beneficial. New studies should measure baseline levels of vitamin D and dietary intake of calcium to be able to identify population subgroups with a higher chance of benefitting from the intervention.
History
Protocol first published: Issue 5, 2017
Acknowledgements
Dr Lucia Méndez‐Sánchez (LM‐S) is grateful for her training in systematic reviews and meta‐analysis at the universities of Ottawa and Cornell and to the PhD Program in Health Sciences from the Universidad Nacional Autónoma de Mexico (UNAM) with the support of the Consejo Nacional de Ciencia y Tecnología (CONACyT) and Hospital Infantil de México Federico Gómez‐UNAM, Unidad de Epidemiología Clínica.
Special thanks to Dr Joanne Homik, Dr Lara Maxwell, and Jordi Pardo, for their comments on this review. We acknowledge the Copy Editor: Anne Lawson, Central Production Service, Cochrane.
Appendices
Appendix 1. CENTRAL search strategy
C1 ‐ Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <April 2022>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp vitamin d/ (4359)
2 vitamin d.tw. (7009)
3 vitamin d2.tw. (167)
4 vitamin d3.tw. (1739)
5 exp Ergocalciferols/ (1116)
6 ergocalciferol$.tw. (157)
7 exp Cholecalciferol/ (2554)
8 cholecalciferol.tw. (878)
9 hydroxycholecalciferol.tw. (104)
10 calcitriol.tw. (778)
11 dihydroxyvitamin D3.tw. (161)
12 alphacalcidol.tw. (39)
13 Calcium, Dietary/ or Calcium/ (4269)
14 calcium.tw. (16630)
15 Calcium carbonate/ (573)
16 Calcium citrate/ (43)
17 or/1‐16 (22739)
18 exp Osteoporosis/ (3661)
19 (bone adj3 loss).tw. (3374)
20 (bone adj3 mineral).tw. (6464)
21 bone mineral densit$.tw. (5500)
22 bmd.tw. (4083)
23 bmc.tw. (668)
24 osteop$.tw. (7612)
25 Fractures, Bone/ (1749)
26 exp Osteoporotic Fractures/ (241)
27 fractur$.tw. (13677)
28 or/18‐27 (23723)
29 17 and 28 (4267)
30 exp Men/ not women.sh. (43)
31 postmenopause/ not premenopause/ (4232)
32 "Aged, 80 and over"/ or Aged/ (193254)
33 or/30‐32 (195248)
34 29 not 33 (2796)
Appendix 2. MEDLINE search strategy
C1 ‐ Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to April 12, 2022>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp vitamin d/ (54939)
2 vitamin d.tw. (55639)
3 vitamin d2.tw. (1311)
4 vitamin d3.tw. (9283)
5 exp Ergocalciferols/ (4240)
6 ergocalciferol$.tw. (538)
7 exp Cholecalciferol/ (25483)
8 cholecalciferol.tw. (2243)
9 hydroxycholecalciferol.tw. (1325)
10 calcitriol.tw. (4600)
11 dihydroxyvitamin D3.tw. (7081)
12 alphacalcidol.tw. (95)
13 Calcium, Dietary/ or Calcium/ (267509)
14 calcium.tw. (349385)
15 Calcium carbonate/ (6831)
16 Calcium citrate/ (164)
17 or/1‐16 (528515)
18 exp Osteoporosis/ (52807)
19 (bone adj3 loss).tw. (31129)
20 (bone adj3 mineral).tw. (48238)
21 bone mineral densit$.tw. (37275)
22 bmd.tw. (26669)
23 bmc.tw. (6135)
24 osteop$.tw. (102714)
25 Fractures, Bone/ (61461)
26 exp Osteoporotic Fractures/ (4501)
27 fractur$.tw. (235180)
28 or/18‐27 (373337)
29 randomized controlled trial.pt. (474740)
30 controlled clinical trial.pt. (92739)
31 randomized.ab. (432962)
32 placebo.ab. (194801)
33 clinical trials as topic.sh. (185287)
34 randomly.ab. (304454)
35 trial.ti. (193546)
36 or/29‐35 (1194110)
37 exp animals/ not humans.sh. (4534465)
38 36 not 37 (1098239)
39 17 and 28 and 38 (4176)
40 exp Men/ not women.sh. (3215)
41 *postmenopause/ (11189)
42 "Aged, 80 and over"/ or Aged/ (2898252)
43 or/40‐42 (2907023)
44 39 not 43 (2309)
Appendix 3. Embase search strategy
C1 ‐ Database: Embase Classic+Embase <1947 to April 12, 2022>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 vitamin D/ (72514)
2 vitamin d.tw. (85247)
3 vitamin d2.tw. (2348)
4 vitamin d3.tw. (14743)
5 ergocalciferol/ (9929)
6 rgocalciferol$.tw. (0)
7 colecalciferol/ (20044)
8 cholecalciferol.tw. (3586)
9 hydroxycholecalciferol.tw. (1702)
10 calcitriol.tw. (6606)
11 dihydroxyvitamin D3.tw. (9392)
12 alphacalcidol.tw. (162)
13 calcium intake/ (16449)
14 calcium carbonate/ or calcium/ (312839)
15 calcium.ti,ab. (452819)
16 citrate calcium/ (1325)
17 or/1‐16 (661825)
18 osteoporosis/ (112550)
19 (bone adj3 loss).tw. (40468)
20 (bone adj3 mineral).tw. (70019)
21 bone mineral densit$.tw. (53277)
22 bmd.tw. (44378)
23 bmc.tw. (9740)
24 osteop$.tw. (157218)
25 fracture/ (104754)
26 fragility fracture/ (16096)
27 fractur$.tw. (307584)
28 or/18‐27 (517698)
29 random$.tw. (1392476)
30 factorial$.tw. (35030)
31 crossover$.tw. (69987)
32 cross over.tw. (30699)
33 cross‐over.tw. (30699)
34 placebo$.tw. (290468)
35 (doubl$ adj blind$).tw. (200749)
36 (singl$ adj blind$).tw. (22523)
37 assign$.tw. (360531)
38 allocat$.tw. (137123)
39 volunteer$.tw. (247074)
40 crossover procedure/ (58579)
41 double blind procedure/ (160661)
42 randomized controlled trial/ (538520)
43 single blind procedure/ (33968)
44 or/29‐43 (2145428)
45 17 and 28 and 44 (8753)
46 exp Male/ not exp Female/ (2769384)
47 aged/ (2905059)
48 *postmenopause/ or *postmenopause osteoporosis/ (29373)
49 or/46‐48 (5383871)
50 45 not 49 (4855)
Data and analyses
Comparison 1. Calcium versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total hip bone mineral density (BMD) | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 1.1.1 Total hip BMD | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 1.2 Lumbar spine BMD | 4 | 202 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 1.2.1 Lumbar spine BMD | 4 | 202 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 1.3 Withdrawals for any reason | 4 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.16] |
Comparison 2. Vitamin D versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Lumbar spine bone mineral density (BMD) mean standard deviation | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 2.2 Withdrawals for any reason | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.19] |
Comparison 3. Calcium plus vitamin D versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Total hip bone mineral density (BMD) % change | 1 | 408 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.06, 0.00] |
| 3.2 BMD lumbar spine | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 3.3 Withdrawals for any reason | 2 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.29, 2.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersen 2008.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial (3 groups) Duration of study: not described Duration of follow‐up: 1 year |
|
| Participants |
Setting characteristic: open population Inclusion criteria: healthy girls, women, and men (divided for age and groups), of Pakistani origin (immigrants or descendants of Pakistani parents) primarily living in the Copenhagen area of Denmark Exclusion criteria: medications known to affect bone metabolism or S‐25OHD concentrations (antiepileptic drugs, intervention vitamin D metabolites, corticosteroids, thyroid hormones, bisphosphonate, oestrogens), serious illness (cancer, liver, or kidney insufficiency), pregnancy, planning pregnancy within 1 year, breastfeeding, and serum ionised calcium concentrations 1.5 mmol/L. Age: 36.2 (range 18.1–52.7) years Sample: Total included 199
Country: Denmark |
|
| Interventions |
Comparison: vitamin D3 10 µg (400 IU) or 20 µg (800 IU) vs placebo Intervention groups:
Control group:
Administration: daily Duration of treatment: 12 months |
|
| Outcomes | BMD: whole body and lumbar spine BMC: whole body and lumbar spine Biomarkers: serum 25‐hydroxyvitamin D, serum intact parathyroid hormone, osteocalcin, urinary pyridinoline Anthropometric measured: weight, BMI Intake: vitamin D and calcium |
|
| Notes | Study authors reported separate data for girls, women, and men. This review and the meta‐analysis included only women‐specific data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects … were randomized … in blocks of six using random numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "The subjects (girls, women, and men separately) were randomized (by an impartial scientist) …" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Scanpharm A/S produced the placebo and vitamin D3 tablets especially for the present study" Comment: similar in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors reported withdrawals in all groups and reasons were not reported. At 12 months, 8 (26%) participants in vitamin D 10 µg (400 IU) group lost, 9 (30%) in vitamin D 20 µg (800 IU) group and 10 (34%) in placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | Low risk | Population balanced on baseline characteristics. |
Barger‐Lux 2005.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial (2 groups) Duration of study: May 1995 to April 2000 Duration of follow‐up: 36 months |
|
| Participants |
Setting characteristic: open population Inclusion criteria: healthy women, non‐smoking, non‐pregnant Exclusion criteria: height and weight that yielded a BMI > 30 kg/m2, binge‐drinking, significant risk of pregnancy, or more than occasional use of calcium supplements or calcium‐containing antiacids Age: 23.1 (SD 2.7) years Sample: Total included 152 (and total report of 87 followed at 36 months) (total of both groups not specified by group)
Country: USA |
|
| Interventions |
Comparison: calcium versus placebo Intervention group:
Control group:
Administration: daily consumption of food Duration of treatment: 36 months |
|
| Outcomes | BMD: total hip and lumbar spine BMC: total body, spine L1–L4, and total hip Biomarkers: serum calcium, urine calcium |
|
| Notes | Other intervention: (quote) "All participants were also supplied with fully‐labeled Geritol® multivitamin tablets, to be taken once daily to ensure at least minimal status for vitamin D and other trace nutrients. Ca, placebo, and multivitamin tablets were supplied without charge by the manufacturer (SmithKline Beecham)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. Quote: "152 were randomly assigned to groups and entered the study." |
| Allocation concealment (selection bias) | Low risk | Central allocation (pharmacy‐controlled) and sequentially numbered drug containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Tablets were packaged in bottles of 90 by the manufacturer; a research pharmacist selected and labeled the bottles." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported withdrawals in both groups and the reasons were not related to the intervention. At 12 months, data for 10 (12%) participants in calcium group and 21 (21%) participants in placebo group were missing. The reported reasons were pregnancy, unwillingness to follow protocol, loss of follow‐up, and others. |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | Low risk |
Baseline imbalance. Characteristics were imbalanced between groups. Quote: "The 2 groups were well matched except for urine calcium, which was significantly higher at baseline in those assigned to calcium (0.092 ± 0.007 g/g) than in those assigned to placebo (0.073 ± 0.005 g/g)." Comment: we judged this difference was not important in the study population. |
Islam 2010.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, parallel‐group trial (4 groups) Duration of study: 1 year Duration of follow‐up: 1 year |
|
| Participants |
Setting characteristic: open population Inclusion criteria: healthy women, garment factory located in an urban area belonging to Standard Group Bangladesh Exclusion criteria: no history of serious medical conditions, no history of medication known to affect bone metabolism, no current pregnancies, no lactation within previous 3 years, and residing in the city for ≥ 2 years Age: 18–36 years
Sample: Total included 200
Country: Bangladesh |
|
| Interventions | 4 groups Intervention group:
Control group:
Administration: daily Duration of treatment: 12 months |
|
| Outcomes | BMD: lumbar spine L2–L4, femoral neck, femoral neck T‐score, trochanter, wards triangle BMC: femoral neck, trochanter Biomarkers: 25‐hydroxyvitamin D, intact parathyroid hormone, calcium, phosphorus, alkaline phosphatase, creatinine. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was performed for equal‐size blocks and carried out …" |
| Allocation concealment (selection bias) | Low risk | Quote: "… carried out by a person who was not involved in the project." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both Ca [calcium] and VD [vitamin D] placebos were donated by the same companies and were identical to the active tablets". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported withdrawals in all groups and the reasons are not related to the intervention. At 12 months, 9 (21%) participants in the calcium plus vitamin D group were lost, 10 (25%) in the vitamin D group, and 15 (42%) in the placebo group. The reported reasons were lost job, disliked tablets, moved, no special reason, and became pregnant. |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | Low risk | No major baseline imbalance. |
Rourke 1998.
| Study characteristics | ||
| Methods | Randomised, controlled, parallel‐group trial (2 groups) Duration of study: not specified Duration of follow‐up: 1 year |
|
| Participants |
Setting characteristic: athletes Included: healthy women, athletes from collegiate teams Excluded: screened athletes denied the use of steroids, anticonvulsants, or tobacco Age: 18–22 years
Sample: Total included 30
Country: USA |
|
| Interventions | Calcium versus placebo Intervention group:
Control group:
Administration: daily Duration of treatment: 1 year |
|
| Outcomes | BMD: lumbar spine (L1–L4), total hip, femoral neck, trochanter, wards triangle, and radius. Anthropometric measures: height, weight, BMI, % body fat, VO2max. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The reasons for the loss of 33% of the participants were not explained. The study began with 45 participants and finished with 30 participants. The study authors presented only the analysis per protocol (calcium group 17 participants and placebo group 13 participants). |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | High risk | Problems with adherence to treatment. "Compliance data suggest that the CS [calcium] group failed to consume the prescribed 1000 mg daily intake of calcium. Furthermore, subjects in the placebo group reported a higher intake of calcium when compared with the CS group." |
Shapses 2001.
| Study characteristics | ||
| Methods | Randomised, controlled, parallel‐group trial (2 groups) Duration of study: not specified Duration of follow‐up: 6 months |
|
| Participants |
Setting characteristic: participants were in a moderate weight‐loss programme Inclusion criteria: obese healthy women; stable weight for ≥ 3 months before the start of the study Exclusion: pregnant or lactating within the previous year; history of an irregular menstrual cycle; ill or taking medication known to interfere with bone metabolism, including oral contraceptives Age: 42.1 (SD 6.2) years Sample: Total included 60
Country: USA |
|
| Interventions |
Intervention group:
Control group:
Other intervention: reduced calorie diet was then individually created using the American Diabetic Association Exchange List. Administration: daily Duration of treatment: 6 months |
|
| Outcomes | BMD: lumbar and total body BMC: lumbar and total body Biomarkers: pyridinoline, deoxypyridinoline, N‐telopeptide, osteocalcin, parathyroid hormone, and 25‐hydroxyvitamin D Intake: energy, protein, fat, carbohydrates, calcium, phosphorus, vitamin D, and magnesium. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The analysis of the placebo group showed substantial deviations from the randomised intervention. |
| Selective reporting (reporting bias) | High risk | The groups assigned initially from the methodology, during the study were arbitrarily reassigned. |
| Other bias | Unclear risk | The final allocation did not coincide with the baseline allocation of participants. |
Winters‐Stone 2004.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial (2 groups) Duration of study: not specified Duration of follow‐up: 1 year |
|
| Participants |
Setting characteristic: athletes Inclusion criteria: healthy women, running a minimum of 10 miles per week (16 km per week), and competing in regional or national running events. The open population of running communities. Exclusion criteria: diseases known to affect bone metabolism, and were not taking medications known to alter bone or calcium metabolism (i.e. glucocorticoids, thyroid hormone, parathyroid hormone); menstrual status was not used as an inclusion criterion but rather was tracked and considered in statistical analyses; oral contraceptive use did not preclude participation in this study since its effect on BMD in premenopausal women is inconclusive. Age: 18−35 years
Sample: Total included 51
Country: USA |
|
| Interventions |
Intervention group:
Control group:
Administration: daily 1 in the morning and 1 in the evening Duration of treatment: 1 year |
|
| Outcomes | BMD: lumbar spine, total hip, femoral neck, greater trochanter, and femoral mid‐shaft Anthropometric measures: height, weight, body fat Intake: total energy, carbohydrate, protein, fat, calcium, and phosphorus |
|
| Notes | After 7 months, report 37 withdrawals were reported, not specified by group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported withdrawals in both groups and reasons were not related to the intervention. At 12 months, 13 (50%) participants in the calcium group and 15 (60%) in the placebo group were lost. Reported reasons were lack of interest, injury, relocation, and inability to travel. |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | Low risk | No major baseline imbalance. Quote: "Women in the two study groups were similar for age, height, weight, menstrual cycle status, and training mileage." |
Woo 2007.
| Study characteristics | ||
| Methods | Randomised, parallel‐group trial (2 groups) Duration of study: not specified Duration of follow‐up: 2 years |
|
| Participants |
Setting characteristic: open population Inclusion criteria: healthy women, open population of 2 community‐living areas in China (Beijing 220 and Hong Kong 221) invited to participate using posters in public places, and Internet mass mailing Exclusion criteria: medical history of metabolic bone, liver, endocrine, connective tissue, and respiratory diseases; cancer; previous operations; those taking medications (diuretics, oral hypoglycaemic agents, insulin injection, steroids, thyroxine, anticonvulsants, calcium or vitamin D supplements, heparin, oestrogens (except oral contraceptives and Depo‐Provera)); those with amenorrhoea or early menopause; current pregnancy, aiming to become pregnant during duration of trial, or breastfeeding Age: 20−35 years (from Hong Kong and Beijing combined)
Sample: Total included 441
Country: China |
|
| Interventions |
Intervention group:
Control group:
Administration: daily consumed with food Duration of treatment: 36 months |
|
| Outcomes | BMD: lumbar spine, total hip, and whole body Biochemical indices: serum calcium, phosphate, parathyroid hormone, 25‐hydroxyvitamin D, vitamin K1, calcium/creatinine, alkaline phosphatase, C‐terminal cross‐linking telopeptide of collagen, total osteocalcin, undercarboxylated osteocalcin, undercarboxylated osteocalcin/total osteocalcin, procollagen type I N propeptide, and N‐terminal cross‐linking telopeptide of collagen/creatinine. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation were permuted blocks (4 per block). |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported withdrawals in all groups and the reasons were not related to the intervention. At 12 months, 19 (8.6%) participants in the calcium group and 14 (6.3%) in the placebo group were lost. The reported reasons were busy, moved, pregnancy, problems related to milk, and illness. |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | Low risk | No major baseline imbalance. Quote: "P=0.057 for the difference between Beijing and Hong Kong in control groups." |
BMC: bone mineral content; BMD: bone mineral density; BMI: body mass index; IU: international units; VO2max: maximal oxygen consumption.
Characteristics of excluded studies [ordered by year]
| Study | Reason for exclusion |
|---|---|
| Teegarden 2005 | Population was women taking oral contraceptives. Study authors compared different doses of calcium: low < 800 mg/day; medium 1000–1100 mg/day, or high 1200–1300 mg/day. |
| Riedt 2007 | Included women aged 24–49 years, and compared calcium at different concentrations: 1000 mg vs 200 mg (with no placebo comparison). |
| Jarjou 2010 | Population was pregnant women at 20 weeks of gestation. |
| Liu 2011 | Population was pregnant women attending regular health examinations before delivery (and followed up > 6 weeks after delivery). |
| Gaffney‐Stomberg 2022 | Population was men and women, the total data were reported in unique analyses. |
| Mesinovic 2019 | Reported < 3 months of follow‐up. |
Differences between protocol and review
We made the following changes from the protocol (Méndez‐Sánchez 2017).
Karen López Casimiro left the review team and Paulina Correa‐Burrows and Rebecca Costello joined the review team.
We did not search "unpublished results" or perform "handsearching" of conference abstracts because the main focus of this review was on published studies.
Database of Reviews of Effects (DARE) was searched via Cochrane CENTRAL (Ovid).
Since withdrawals for adverse events or serious adverse events were not available, we used withdrawals for any reason.
We did not find enough data to perform the subgroup analysis or summary estimated proposed for the other characteristics included in the review protocol: dosage (vitamin D: 600 IU or less and greater than 600 IU; calcium 1000 mg or less and greater than 1000 mg); supplementation time (short term less than 12 months, long term 12 months or greater); and baseline vitamin D levels or dietary calcium intake (sufficiency greater than 30 ng/mL; insufficiency 11 ng/mL to 29 ng/mL; or deficiency less than 10 ng/mL).
We did not find data to calculate measures of the intervention in conceptual outcomes as quality of life and the number needed to treat for an additional beneficial outcome (NNTB) or harmful outcome (NNTH).
Contributions of authors
Conceiving, designing, and co‐ordinating the review: LM‐S, PC, TW, and PT.
Designing protocol, search strategies, and undertaking searches: LM‐S, PC, TW, PT.
Editor of English language in the protocol: TW, PT.
Screening search results and retrieved papers against inclusion criteria: LM‐S, PC.
Appraising the quality of papers: LM‐S, PC, PT.
Extracting data from papers: LM‐S, PC.
Writing to study authors for additional information: LM‐S, PC, PT.
Data management for the review and entering data into Review Manager Web: LM‐S, PC, PT.
Analysis and interpretation of data: LM‐S, PC, TW, PT.
Providing a research perspective: PC, TW, PT, RC.
Writing the review: LM‐S, PC, PT, TW, RC, P C‐B.
Providing general advice on the review: LM‐S, PC, TW, PT, RC, P C‐B.
Performing previous work that was the foundation of the current review: LM‐S, PC, TW, PT.
Sources of support
Internal sources
-
National Council of Science and Technology (CONACYT), Mexico
CONACYT funded the training of Lucía Méndez‐Sánchez at University of Ottawa
External sources
-
No external sources of support was provided, Mexico
No external sources of support was provided
Declarations of interest
LM‐S: none.
PC: none.
TW: Australian and New Zealand Bone Mineral Society membership; editor for Cochrane Musculoskeletal; Amgen Independent Contractor – Consultant. TW was not involved in the editorial decisions concerning this review.
PT: travel and accommodation for OMERACT meetings – a registered non‐profit independent medical research organisation whose goal is to improve and advance the health outcomes for people with musculoskeletal conditions. OMERACT receives unrestricted educational grants from the American College of Rheumatology, the European League of Rheumatology, and several pharmaceutical companies (listed below), which is used to support fellows, international patient groups, and major international bi‐annual conferences, which results in many peer‐reviewed publications; Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech/Roche, Genzyme/Sanofi, Horizon Pharma Inc, Merck, Novartis, Pfizer, PPD, Quintiles, Regeneron, Savient, Takeda Pharmaceutical, UCB Group, Vertex, Forest, Bioiberica. PT is an independent committee member for clinical trial Data Safety Monitoring Boards for US Food and Drug Administration‐approved trials, being conducted by UCB Biopharma GmbH and SPRL, Parexel International, and Prehealth Sciences. He is an independent medical consultation professional services for CHEOR Solutions (Canada) Ltd, Innovative Science Solutions LLC; and an advisory committee member of the Canadian Reformulary Group Inc, a company that reviews the evidence for health insurance companies' employer drug plans. PT was not involved in the editorial decisions concerning this review.
PC‐B: none.
RC: National Institutes of Health Independent Contractor – Consultant.
New
References
References to studies included in this review
Andersen 2008 {published data only}
- Andersen R, Molgaard C, Skovgaard LT, Brot C, Cashman KD, Jakobsen J, et al. Effect of vitamin D supplementation on bone and vitamin D status among Pakistani immigrants in Denmark: a randomised double-blinded placebo-controlled intervention study. British Journal of Nutrition 2008;100:197-207. [DOI] [PubMed] [Google Scholar]
Barger‐Lux 2005 {published data only}
- Barger-Lux MJ, Davies KM, Heaney RP. Calcium supplementation does not augment bone gain in young women consuming diets moderately low in calcium. Journal of Nutrition 2005;135(10):2362-6. [DOI] [PubMed] [Google Scholar]
Islam 2010 {published data only}
- Islam MZ, Shamim AA, Viljakainen HT, Akhtaruzzaman M, Jehan AH, Khan HU, et al. Effect of vitamin D, calcium and multiple micronutrient supplementation on vitamin D and bone status in Bangladeshi premenopausal garment factory workers with hypovitaminosis D: a double-blinded, randomised, placebo-controlled 1-year intervention. British Journal of Nutrition 2010;104(2):241-7. [DOI] [PubMed] [Google Scholar]
Rourke 1998 {published data only}
- Rourke K, Bowering J, Turkki P, Buckenmeyer P, Keller B, Sforzo G. Effect of calcium supplementation on bone mineral density in female athletes. Nutrition Research 1998;18(5):775-83. [Google Scholar]
Shapses 2001 {published data only}
- Shapses SA, Thun LV, Heymsfield SB, Ricci TA, Ospina M, Pierson RN, et al. Bone turnover and density in obese premenopausal women during moderate weight loss and calcium supplementation. Journal of Bone and Mineral Research 2001;16(7):1329-36. [DOI] [PubMed] [Google Scholar]
Winters‐Stone 2004 {published data only}
- Winters-Stone KM, Snow CM. One year of oral calcium supplementation maintains cortical bone density in young adult female distance runners. International Journal of Sport Nutrition & Exercise Metabolism 2004;14(1):7-17. [DOI] [PubMed] [Google Scholar]
Woo 2007 {published data only}
- Woo J, Lau W, Xu L, Lam CW, Zhao X, Yu W, et al. Milk supplementation and bone health in young adult Chinese women. Journal of Women's Health 2007;16(5):692-702. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gaffney‐Stomberg 2022 {published data only}
- Gaffney-Stomberg E, Hughes JM, Guerriere KI, Staab JS, Cable SJ, Bouxsein ML, et al. Once daily calcium (1000 mg) and vitamin D (1000 IU) supplementation during military training prevents increases in biochemical markers of bone resorption but does not affect tibial microarchitecture in Army recruits. Bone 2022;155(Suppl 1):116-269. [DOI: 10.1016/j.bone.2021.116269] [DOI] [PubMed] [Google Scholar]
Jarjou 2010 {published data only (unpublished sought but not used)}
- Jarjou LM, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with low calcium intake. American Journal of Clinical Nutrition 2010;92(2):450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2011 {published data only (unpublished sought but not used)}
- Liu Z, Qiu L, Chen YM, Su YX. Effect of milk and calcium supplementation on bone density and bone turnover in pregnant Chinese women: a randomized controlled trial. Archives of Gynecology & Obstetrics 2011;283(2):205-11. [DOI] [PubMed] [Google Scholar]
Mesinovic 2019 {published data only}
- Mesinovic J, Mousa A, Wilson K, Scragg R, Plebanski M, Courten M, et al. Effect of 16-weeks vitamin D replacement on calcium-phosphate homeostasis in overweight and obese adults. Journal of Steroid Biochemistry & Molecular Biology 2019;186:169-75. [DOI: 10.1016/j.jsbmb.2018.10.011] [DOI] [PubMed] [Google Scholar]
Riedt 2007 {published data only (unpublished sought but not used)}
- Riedt CS, Schlussel Y, Thun N, Ambia-Sobhan H, Stahl T, Field MP, et al. Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. American Journal of Clinical Nutrition 2007;85(4):972-80. Erratum in: American Journal of Clinical Nutrition 2007;86(3):808. [DOI: 10.1093/ajcn/85.4.972] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teegarden 2005 {published data only (unpublished sought but not used)}
- Teegarden D, Legowski P, Gunther CW, McCabe GP, Peacock M, Lyle RM. Dietary calcium intake protects women consuming oral contraceptives from spine and hip bone loss. Journal of Clinical Endocrinology & Metabolism 2005;90(9):5127-33. [DOI] [PubMed] [Google Scholar]
Additional references
Abshirini 2020
- Abshirini M, Mozaffari H, Kord-Varkaneh H, Omidian M, Kruger MC. The effects of vitamin D supplementation on muscle strength and mobility in postmenopausal women: a systematic review and meta-analysis of randomised controlled trials. Journal of Human Nutrition and Dietetics 2019;1:1-15. [DOI: 10.1111/jhn.12717] [DOI] [PubMed] [Google Scholar]
ASBMR 2019
- Bikle DD, Adams JS, Christakos S. Chapter 30. Vitamin D; Production, Metabolism, Action, and Clinical Requirements; Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 9th edition. Washington (DC): American Society for Bone and Mineral Research, 2018. [DOI: 10.1002/9781119266594] [DOI] [Google Scholar]
Avenell 2014
- Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD000227. [DOI: 10.1002/14651858.CD000227.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bacci 2010
- Bacci V, Silecchia G. Vitamin D status and supplementation in morbid obesity before and after bariatric surgery. Expert Review of Gastroenterology & Hepatology 2010;4(6):781-94. [DOI: 10.1586/egh.10.69] [DOI] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2009
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 2009;339:1-11. [DOI: 10.1136/bmj.b3692] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borgström 2013
- Borgström F, Lekander I, Ivergård M, Ström O, Svedbom A, Alekna V. The International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS) – quality of life during the first 4 months after fracture. Osteoporosis International 2013;24(3):811-23. [DOI] [PubMed] [Google Scholar]
Burger 2007
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. Journal of Bone Mineral Research 2007;22:465-75. [DOI: 10.1359/jbmr.061113] [DOI] [PubMed] [Google Scholar]
Cameron 2018
- Cameron ID, Dyer SM, Panagoda CE, Murray GR, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD005465. [DOI: 10.1002/14651858.CD005465.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 10. Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Vol. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:241-84. [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Dunfield 2007
- Dunfield L, Mierzwinski-Urban M, Hodgson A, Banks R. Diagnostic Performance and Cost Effectiveness of Technologies to Measure Bone Mineral Density in Postmenopausal Women. Technology Report Number 94. Ottawa (Canada): Canadian Agency for Drugs and Technologies in Health, 2007. [Google Scholar]
Fulgoni 2011
- Fulgoni VL, Keast DR, Auestad N, Quann EE. Nutrients from dairy foods are difficult to replace in diets of Americans: food pattern modeling and an analyses of the National Health and Nutrition Examination Survey 2003–2006. Nutrition Research 2011;31(0):759-65. [DOI: 10.1016/j.nutres.2011.09.017] [DOI] [PubMed] [Google Scholar]
GBD 2021
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longevity 2021;2(9):e580-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghogomu 2014
- Ghogomu EA, Maxwell LJ, Buchbinder R, Rader T, Pardo Pardo J, Johnston RV, et al. Updated method guidelines for Cochrane musculoskeletal group systematic reviews and meta-analyses. Journal of Rheumatology 2014;41(2):194-205. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed September 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gupta 2010
- Gupta R, Sharma U, Gupta N, Kalaivani M, Singh U, Guleria R. Effect of cholecalciferol and calcium supplementation on muscle strength and energy metabolism in vitamin D deficient Asian Indians: a randomised, controlled trial. Clinical Endocrinology 2010;73(4):445-51. [DOI] [PubMed] [Google Scholar]
Haines 2008
- Haines ML, Anderson RP, Gibson PR. Systematic review: the evidence base for long-term management of celiac disease. Alimentary Pharmacology & Therapeutics 2008;28(9):1042-66. [DOI: 10.1111/j.1365-2036.2008.03820.x] [DOI] [PubMed] [Google Scholar]
Heneghan 2019
- Heneghan C, Mahtani KR. Vitamin D does not prevent fractures and falls. BMJ Evidence-Based Medicine 2019;24:147-8. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2/.
Higgins 2019
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8. Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Vol. 2nd. Chichester (UK): John Wiley & Sons, 2019:205-28. [Google Scholar]
Higgins 2022
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Holick 2011
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al, Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical and Endocrinological Metabolism 2011;96(7):1911-30. [DOI: 10.1210/jc.2011-0385] [DOI] [PubMed] [Google Scholar]
IHME 2018
- Institute for Health Metrics and Evaluation. Findings from the Global Burden of Disease Study 2017. Seattle (WA): IHME, 2018. [Google Scholar]
IOM 2011
- Institute of Medicine. Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academies Press, 2011. [PubMed] [Google Scholar]
Johnell 2004
- Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporosis International 2004;15(11):897-902. [DOI] [PubMed] [Google Scholar]
Kanis 2007
- Kanis JA, on behalf of the World Health Organization Scientific Group. Assessment of osteoporosis at primary health-care level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK 2007:337.
Lefebvre 2017
- Lefebvre C, Glanville J, Beale S, Boachie C, Duffy S, Fraser C, et al. Assessing the performance of methodological search filters to improve the efficiency of evidence information retrieval: five literature reviews and a qualitative study. Health Technology Assessment 2017;21(69):1-148. [DOI: 10.3310/hta21690] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewiecki 2016
- Lewiecki EM, Binkley N, Morgan SL, Shuhart CR, Camargos BM, Carey JJ, et al. Best practices for dual-energy X-ray absorptiometry measurement and reporting: International Society for Clinical Densitometry Guidance. Journal of Clinical Densitometry 2016;19(2):127-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
McKenzie 2019
- McKenzie JE, Brennan SE. Chapter 12. Synthesizing and presenting findings using other methods. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Vol. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:321-47. [Google Scholar]
Modan‐Moses 2014
- Modan-Moses D, Levy-Shraga Y, Pinhas-Hamiel O, Kochavi B, Enoch-Levy A, Vered I, et al. High prevalence of vitamin D deficiency and insufficiency in adolescent inpatients diagnosed with eating disorders. International Journal of Eating Disorders 2015;48(6):607-14. [DOI: 10.1002/eat.22347] [DOI] [PubMed] [Google Scholar]
Morehouse‐Grand 2014
- Morehouse-Grand K, Grand S. Can vegans have healthy bones? A literature review. Topics in Integrative Health Care 2014;5(4):5.4003. [ISSN 2158-4222] [Google Scholar]
Moyer 2013
- Moyer VA, on behalf of the US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2013;158(9):691-6. [DOI] [PubMed] [Google Scholar]
NIH 1993
- National Institutes of Health. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. American Journal of Medicine 1993;94:646-50. [DOI] [PubMed] [Google Scholar]
Papaioannou 2010
- Papaioannou A, Morin S, Cheung A, Atkinson S, Brown JP, Feldman S, et al. 2010 Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. Canadian Medical Association Journal 2010;182(17):1829-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plot Digitizer 2015 [Computer program]
- Plot Digitizer. Free Software Foundation, Version accessed 14 November 2016. Free Software Foundation, Created: 3 June 2001; Modified: 24 October 2015. Available at plotdigitizer.com. [plotdigitizer.sourceforge.net]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Review Manager Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rizzoli 2013
- Rizzoli R, Boonen S, Brandi ML, Bruyère O, Cooper C, Kanis JA, et al. Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Current Medical Research and Opinions 2013;29(4):305-13. [DOI: 10.1185/03007995.2013.766162] [DOI] [PubMed] [Google Scholar]
Sánchez 2003
- Sánchez A, Puche R, Zeni S, Oliveri B, Galich AM, Maffei L, et al. Role of calcium and vitamin D in bone health (part II) [Papel del calcio y de la vitamina D en la salud ósea (parte II)]. Revista Española de Enfermedades Metabolicas Oseas 2003;12(1):16. [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
USPSTF 2018
- US Preventive Services Task Force (USPSTF) members. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults. JAMA 2018;319(15):1592-9. [DOI] [PubMed] [Google Scholar]
Winzenberg 2006
- Winzenberg TM, Shaw KA, Fryer J, Jones G. Calcium supplementation for improving bone mineral density in children. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD005119. [DOI: 10.1002/14651858.CD005119.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winzenberg 2010
- Winzenberg TM, Powell S, Shaw KA, Jones G. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD006944. [DOI: 10.1002/14651858.CD006944.pub2] [DOI] [PubMed] [Google Scholar]
Zhu 2010
- Zhu K, Austin N, Devine A, Bruce D, Prince RL. A randomised controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. Journal of the American Geriatrics Society 2010;58(11):2063-8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Méndez‐Sánchez 2017
- Méndez-Sánchez L, López Casimiro K, Winzenberg TM, Tugwell P, Clark P. Calcium and vitamin D for increasing bone mineral density in premenopausal women. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012664. [DOI: 10.1002/14651858.CD012664] [DOI] [PMC free article] [PubMed] [Google Scholar]


