Abstract
The eib genes of Escherichia coli encode surface-exposed proteins which bind immunoglobulins (Ig) such as the Fc fragment of human IgG (IgG Fc) in a nonimmune manner. The Eib proteins belong to a family which includes YadA of Yersinia, UspA2 of Moraxella, and DsrA of Haemophilus ducreyi. This family of surface-exposed proteins shares several features, such as the ability to impart resistance to human serum complement and a tendency to exist as stable multimers. Four genes, eibA, eibC, eibD and eibE, were previously identified and cloned from ECOR-9, a strain from the E. coli reference collection. EibC, -D, and -E bind human serum IgA in addition to IgG, but no IgA binding has been observed for EibA. Here, we report the cloning of a new eib gene, eibF, from a second strain of E. coli, ECOR-2. The product, EibF, has a relatively strong preference for IgA. Like the other eib genes, eibF attenuates serum sensitivity, occurs as a stable multimer, and is associated with a prophage. By subcloning portions of the eibA and eibF genes, we have identified distinct sequence segments sufficient to cause Ig binding, multimerization, and discrimination between IgA and IgG. The ability to multimerize is associated with a sequence close to the C terminus that is homologous to other family members such as YadA. Binding of IgG Fc is associated with a sequence that is highly conserved among all Eib proteins but otherwise unique. Binding of IgA is associated with a sequence of EibF that is not similar to any EibA sequence.
The Eib (for Escherichia coli immunoglobulin binding) proteins of E. coli are members of a family of surface-exposed proteins which includes YadA of Yersinia (15, 18, 19), UspA2 of Moraxella (1, 2), and DsrA of Haemophilus ducreyi (5). The Eib proteins have several phenotypic features in common with these proteins, such as the ability to impart resistance to human serum complement and a tendency to exist as highly stable multimers. In addition to the properties shared with other members of this protein family, the Eib proteins have the ability to bind immunoglobulins (Ig) such as the Fc fragment of human IgG (IgG Fc) in a nonimmune manner; i.e., a mechanism that does not require specific recognition by antibody (17). The Eib proteins were originally identified in 6 of 72 strains of the E. coli reference (ECOR) strain collection (13). At that time, one of six strains, ECOR-9, was selected for study, and it was found to produce several distinct Ig binding proteins, each encoded by a different member of a set of related prophages. Four genes, eibA, eibC, eibD, and eibE, were cloned from ECOR-9. In multicopy form, each conferred Ig binding activity on and attenuated the serum sensitivity of a test recipient. In each instance, the Eib proteins were observed as multiple bands in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), some of the bands exceeding 200 kDa in apparent molecular mass (16). Consequently, the sizes of the Eib proteins appeared much greater than predicted from the gene sequences, implying stable multimerization. Three of the four Eib proteins bound human serum IgA in addition to IgG. The IgA binding signal was strongest for EibD, and no signal was observed for EibA (16). Here, we report the cloning and characterization of a new eib gene, eibF, whose product has a particularly strong preference for IgA. This gene was cloned from E. coli strain ECOR-2, a strain originally isolated from the feces of a healthy human host (13) and belonging to phylogenetic group A (7). ECOR-2 differs from most group A ECOR strains in having genes for several extraintestinal virulence-associated traits, which are more common among group B2 strains (10). Like the eib genes of ECOR-9, eibF attenuates serum sensitivity. By subcloning portions of the eibA and eibF genes, we have identified sequence segments sufficient to cause Ig binding, multimerization, and discrimination between IgA and IgG. We also report that no binding to IgM or IgE can be detected in extracts of the ECOR strains previously shown to bind Ig (17) or in strains hosting the cloned eib genes.
MATERIALS AND METHODS
Strains and culture conditions.
The ECOR collection of E. coli strains was obtained from Robert Selander and Thomas Whittam (13). E. coli K-12 strain DH5α was used for cloning of all pOK12-based constructs and for expression of eibF fusion constructs. E. coli strain JM109 was used as the background strain for expression of eibA fusion constructs. E. coli strain AB1157 was used as the background strain for studies of serum resistance and accessibility to trypsin. For expression of Ig binding activity in cells hosting pOK12 derivatives, 24-h Luria-Bertani (LB) broth cultures grown at 37°C with agitation were used. For cells hosting pMal-c2X-based fusion plasmids, cells were similarly grown to an optical density at 595 nm of 0.5 and induced with 0.3 mM IPTG (isopropyl-α-d-galactopyranoside) for 2 h. Cells were harvested by centrifugation at 4°C. LB broth containing ampicillin, 50 μg per ml, was used for the maintenance of pMal-c2X fusion plasmids and pUC21 derivatives. LB broth containing kanamycin, 50 μg per ml (LBKm broth), was used to maintain pOK12 derivatives.
Protein extraction and Ig binding.
Preparation of cell extracts, determination of protein concentration, SDS-PAGE, and immunoblotting were as described previously (17). It is important to note that the immunoblotting procedure used to detect nonimmune Ig binding differs from traditional immunoblotting procedures used to detect the binding of specific antibody to an antigen (17). Our standard immunoblotting procedure entails a one-step incubation with nonimmune antibody (such as normal serum IgA or the IgG Fc) conjugated with horseradish peroxidase (HRP). There is no incubation with primary antibody specifically directed against an antigen.
Purified IgG Fc conjugated with HRP (IgG Fc-HRP) (Rockland) was used at a concentration of 20 ng of antibody per ml; purified whole human serum IgA conjugated with HRP (IgA-HRP) (Jackson ImmunoResearch Laboratories) was used at 50 ng per ml. Human myeloma IgM Fc conjugated with horseradish peroxidase (IgM Fc-HRP) (Jackson ImmunoResearch Laboratories) was used at 4 μg per ml. Human monoclonal IgE (Biodesign International) was conjugated with HRP using the EZ Link Activated Peroxidase Antibody Labeling kit (Pierce) according to the manufacturer's instructions and was used at 250 ng or 2.5 μg per ml. Antiserum directed against maltose binding protein (MBP) (anti-MBP) developed in rabbit (New England Biolabs) was used at a dilution of 1/10,000 and was detected by HRP-conjugated anti-rabbit Ig developed in donkey (Amersham-Pharmacia Biotech) used at 100 ng per ml. Human polyclonal IgE (Biodesign International) was used at 33 μg per ml and detected by use of HRP-conjugated mouse monoclonal anti-IgE (United States Biological) at 50 ng per ml.
DNA cloning and analysis.
Techniques used for DNA isolation, cloning, and sequence analysis involve minor modifications of those indicated elsewhere (8, 16, 21). The plasmid vectors for cloning were pOK12 (20) and pMal-c2X (New England Biolabs). Cloning of the eibF gene utilized a partial Sau3A digest of ECOR-2 genomic DNA. Fragments in the desired size range were purified by agarose gel electrophoresis and with the Qiaex II Gel Extraction kit (Qiagen), ligated into the BamHI site of pOK12, and electroporated into E. coli strain DH5α. Colony blots of transformants were screened for IgA binding by procedures based on published protocols (6, 16). Briefly, colonies were blotted to nitrocellulose and lysed in situ with 1% SDS at 65°C for 30 min. The membranes were blocked with 10% (wt/vol) nonfat dry milk in phosphate-buffered saline (PBS) for 1 h, washed, and incubated for 1 h with affinity-purified human serum IgA conjugated with HRP. The blots were washed, and film was exposed. Deletions within the original cloned fragment were made using convenient restriction sites; key plasmids are listed in Table 1. Oligonucleotide synthesis and automated DNA sequencing were done by the Macromolecular Core Facility of the Penn State College of Medicine. For all new sequences, both strands were sequenced; for repetitive sequencing of essentially identical segments, sequencing was sometimes limited to a single strand. Nucleic acid and amino acid sequence similarity searches were done with the BLAST programs (3) without filters.
TABLE 1.
Plasmids
| Plasmid | Associated eib gene | Amino acids | Vector | Insert source | Insert identification |
|---|---|---|---|---|---|
| pCS6379a | eibA | 1–392 | pOK12 | pCS6102 | 1,390-bp XmnI-MscI |
| pDC2240a | eibA | 254–392 | pMal-c2X | pDC6165 | 526-bp HpaI-MscI |
| pDC2252 | eibA | 301–392 | pMal-c2X | pDC2240 | 386-bp PstI-MscI |
| pDC2273b | eibA | 254–392 | pMal-c2X | pDC2240 | 526-bp HpaI-MscI |
| pDC2283b | eibA | 254–344 | pMal-c2X | pDC2273 | 273-bp HpaI-MluI |
| pCS7299 | eibA | 254–298 | pMal-c2X | pDC2240 | 136-bp HpaI-PstI |
| pCS6431a | eibC | 1–504 | pOK12 | pCS6351 | 2,463-bp DraI-XmnI |
| pCS6365a | eibD | 1–511 | pOK12 | pCS6350 | 1,894-bp DraI-BglI |
| pCS6432a | eibE | 1–487 | pOK12 | pCS6398 | 1,891-bp DraI-KasI |
| pCS7164 | eibF | 1–459 | pOK12 | ECOR-2 | 3,167-bp partial Sau3A |
| pCS7180 | eibF | 1–459 | pOK12 | pCS7164 | 2,887-bp MluI-Sau3A |
| pCS7216 | eibF | 1–459 | pOK12 | pCS7164 | 2,049-bp XmnI-Sau3A |
| pCS7217 | eibF | 1–459 | pUC21 | pCS7164 | 2,115-bp NruI-Sau3A |
| pCS7227 | eibF | 97–459c | pMal-c2X | pCS7217 | 1,657-bp EcoRI-Sau3A |
| pCS7269 | eibF | 97–459 | pMal-c2X | pCS7227 | 1,657-bp EcoRI-Sau3Ad |
| pCS7280 | eibF | 318–459 | pMal-c2X | pCS7217 | 1,331-bp BsgI-Sau3A |
| pCS7284 | eibF | 400–459 | pMal-c2X | pCS7269 | 748-bp StuI-Sau3A |
| pCS7285 | eibF | 97–399 | pMal-c2X | pCS7269 | 909-bp EcoRI-StuI |
| pCS7286 | eibF | 181–280 | pMal-c2X | pCS7164 | 300-bp amplicond |
| pCS7296 | eibF | 97–353 | pMal-c2X | pCS7269 | 773-bp amplicond |
Protein fusions.
The pMAL protein fusion and purification system (New England Biolabs) was used. The fusion constructs are shown in Table 1 (see also Fig. 3). For the eibA fusion, an HpaI site near the center of block 2 of EibA was used to make an in-frame fusion between the N terminus of malE (410 codons) at the vector XmnI site and the C terminus of eibA (141 codons), producing pDC2240 (Table 1; see Fig. 3A through C). A set of plasmids with more limited inserts was derived (Table 1; see Fig. 3C). For eibF, the initial fusion construct pCS7269 was prepared in two steps. The first involved fusion of eibF at an EcoR I site at codon 97 to an EcoRI site in pMal-c2X. The second step involved deletion between XmnI of the vector and the EcoRI site, which brought 363 codons of the eibF sequence into frame with the malE open reading frame (ORF) (Table 1; see Fig. 3A, D, and E). A set of plasmids with more limited inserts was derived from pCS7269 (Table 1; see Fig. 3E). PCR was used to construct pCS7286 and pCS7296 using primers which introduced appropriate restriction sites for cloning (Table 2).
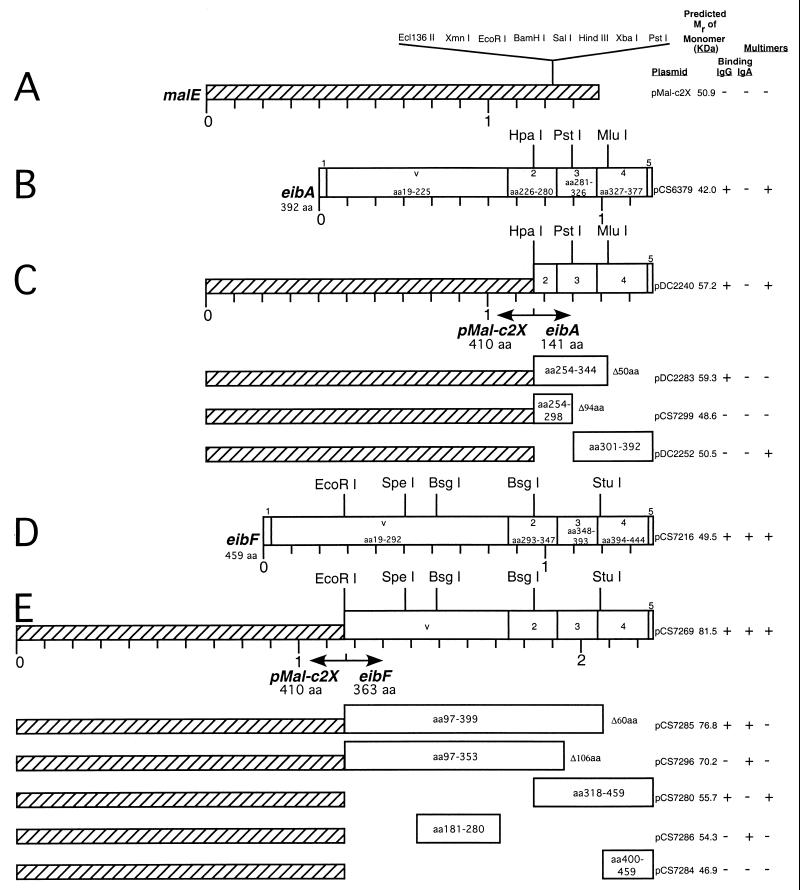
FIG. 3.
Fusion of eibA and eibF to malE. In-frame fusions between eibA or eibF and malE of vector pMal-c2X were made, and deletion constructs were derived from them as described in Materials and Methods. (A) malE, showing restriction sites and position of cloned inserts; (B) eibA, showing restriction sites, blocks, and amino acids encoded by each block (see text); (C) malE-eibA fusion and derived deletion constructs; (D) eibF, showing restriction sites, blocks, and amino acids encoded by each block (see text); (E) malE-eibF fusion and derived deletion constructs. Map units are in kilobases. These constructs were used in the experiments shown in Fig. 5 and 6, whose results are summarized in the column on the right as binding (+) or nonbinding (−) of IgG Fc and IgA, and presence (+) or absence (−) of stable multimerization.
TABLE 2.
PCR primers used for cloning
| Plasmid | PCR primera
|
Coordinates of amplicon in eibF ORF
|
Restriction sites used for cloning
|
Template | |||
|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||
| pCS7286 | 248 | 249 | 541 | 840 | BamHI | HindIII | pCS7164 |
| pCS7296 | 244 | 250 | 4 | 1059 | SpeI | XbaI | pCS7164 |
Primers, sequences, and restriction sites (in parentheses) are as follows: 248, CACCACGGATCCGAATATAACCGCATTGTAACC (BamHI); 249, CACCACAAGCTTATCAGTTCGACCAGAAAGATTTGC (HindIII); 244, GAGCAAGAAATTTACAAAGAC; and 250, CACCACTCTAGACTACTCAATGGATTGCGTGTTAC (XbaI). Restriction sites in primer sequences are underlined.
Trypsin treatment of intact cells.
Trypsinization of intact cells was as described previously (17). Briefly, cells were diluted to 10% (wt/vol) in 50 mM potassium phosphate, pH 6.1, containing trypsin at concentrations of 5, 50, or 500 μg per ml and incubated for 1 h at 37°C. The digestion was stopped with 2 mM phenylmethylsulfonyl fluoride, and the cells were harvested by centrifugation. Control cells lacking trypsin were similarly treated.
Serum sensitivity assay.
Serum sensitivity was determined as described previously (16). In brief, 24-h cultures grown in LBKm broth were washed in PBS and thoroughly resuspended in PBS by vortexing. Control cells harboring vector pOK12 alone or test cells harboring a cloned eib gene were seeded in 150 μl of 25% unheated human serum complement (Sigma) or PBS at a concentration of 2 × 108 CFU per ml. Five replicate samples of cells were incubated with serum, and three samples were incubated with PBS. The cells were shaken at 37°C for 1 h in PBS. Serial dilutions were prepared from each replicate and plated on LBKm agar.
Nucleotide sequence accession number.
A 3,167-bp segment of DNA containing eibF and adjacent DNA was sequenced. The GenBank accession number is AF399847.
RESULTS
Cloning of a gene for an IgA binding protein from ECOR-2.
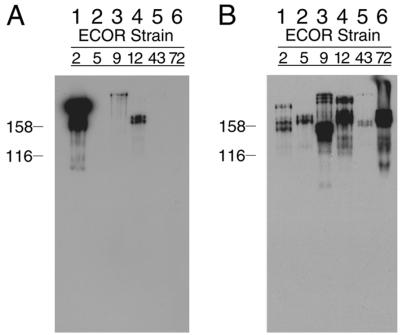
In a screen of ECOR strains for the binding of human serum IgA, we observed that ECOR-2 extracts bound IgA with an unusually strong signal (Fig. 1A, compare lane 1 to lanes 2 through 6). On the other hand, the signal for binding the IgG Fc was relatively weak for ECOR-2 (Fig. 1B, compare lane 1 to lanes 3, 4, and 6). Because of its strong IgA binding signal, ECOR-2 was chosen as the source strain to clone a gene for an Ig binding protein with a strong preference for IgA. A 3,167-bp segment of DNA was cloned into pOK12 and detected in a direct screen of colonies for IgA binding (see Materials and Methods). This segment contained three ORFs. The amino acid sequence predicted by one of these ORFs was related to that of the eib genes previously cloned from ECOR-9, and it was designated eibF. Similarity was particularly strong for the C-terminal 167 codons, where sequence conservation among the eib genes of ECOR-9 is greatest. Sequencing of the regions adjoining eibF revealed exactly the same organization of genes as previously seen with the P-Eib prophages of ECOR-9 (16). This suggested that eibF is carried by a prophage similar to those described for ECOR-9 (see below).
FIG. 1.
Ig binding of ECOR strains. Whole-cell extracts from 24-h broth cultures were fractionated by SDS-PAGE (8% acrylamide) and blotted to polyvinylidene difluoride (PVDF). The blot was incubated sequentially with human IgG Fc-HRP (B) and IgA-HRP (A). Lanes: 1, ECOR-2; 2, ECOR-5; 3, ECOR-9; 4, ECOR-12; 5, ECOR-43; 6, ECOR-72. Each lane contained approximately 30 μg of protein.
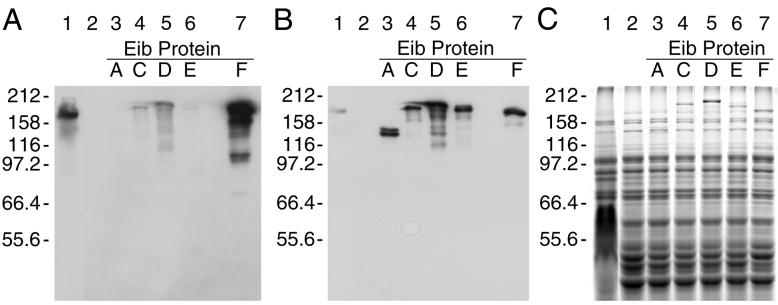
A subclone comprising 2,049 bp was sufficient to confer Ig binding. This segment contained eibF and extended 106 bp upstream from the translational start codon. The eibF sequence predicted a 49.5-kDa protein, considerably smaller than the Ig binding activities observed for its source, ECOR-2. However, the Ig binding activity conferred by cloned eibF occurred as two IgA binding bands whose sizes (158 kDa and larger) were similar to those found in ECOR-2 (Fig. 2A, compare lane 7 to lane 1). Of particular interest, the signal for IgA binding conferred by eibF was stronger than for any of the eib genes cloned from ECOR-9 (Fig. 2A, compare lane 7 to lanes 3 through 6), while the signal for IgG Fc binding by EibF was comparable to that of EibA, EibC, EibD, and EibE (Fig. 2B, compare lane 7 to lanes 3 through 6).
FIG. 2.
Ig binding conferred by cloned eib genes. Extracts were prepared and fractionated on duplicate gels by SDS-PAGE (8% acrylamide) as shown in Fig. 1. One gel was blotted to PVDF and the other was stained with Coomassie brilliant blue (C). The blot was incubated sequentially with IgG Fc-HRP (B) and IgA (A). Lanes: 1, positive control ECOR-2; 2, negative control pOK12; 3, eibA(pCS6379); 4, eibC(pCS6431); 5, eibD(pCS6364); 6, eibE(pCS6432); 7, eibF(pCS7216). Each lane contained approximately 10 μg of protein. E. coli DH5α was used as the background strain for all constructs (lanes 2 to 7).
Lack of binding human IgM and IgE.
In view of the differential binding of IgG Fc and IgA by proteins from the ECOR strains and cells harboring the cloned eib genes, we extended our efforts to test binding of human IgM and IgE. The binding of IgE was analyzed by two methods. The first involved a one-step incubation of immunoblots with monoclonal IgE-HRP; the second involved a two-step incubation, first with polyclonal IgE and second with an HRP-conjugated mouse monoclonal antibody directed against human IgE. The binding of IgM Fc was analyzed by direct binding of monoclonal IgM Fc-HRP to immunoblots. None of the Ig binding proteins present in the ECOR strains or clones bound human myeloma IgE, polyclonal IgE, or the monoclonal IgM Fc fragment with a signal above background (data not shown).
Comparison of EibF from ECOR-2 to Eib proteins from ECOR-9.
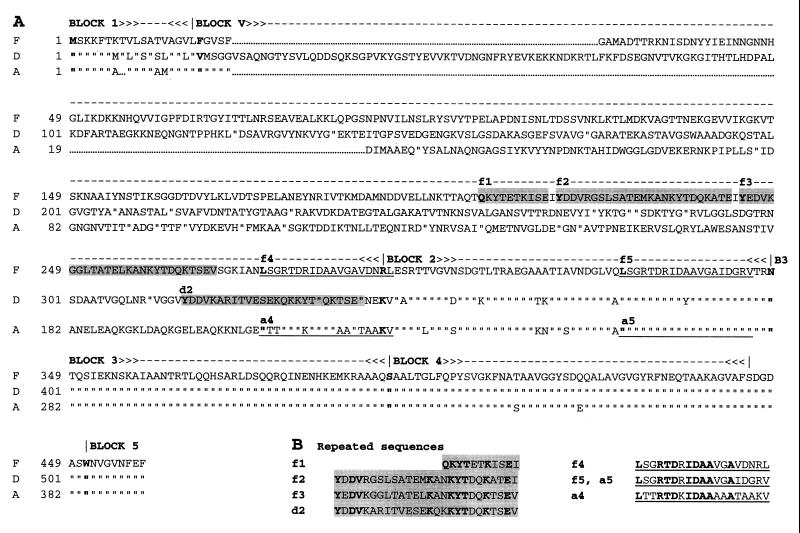
For purposes of discussion in our earlier report, five regions of amino acid homology shared by the various Eib proteins derived from ECOR-9 were designated blocks 1 through 5 (16). Figure 3B shows an eib gene with designated regions corresponding to blocks 1 through 5. For purposes of the present discussion, we have further designated the segment between blocks 1 and 2 block V (variable). Block 1 is part of a putative signal sequence for export of the proteins across the cytoplasmic membrane. Block 5 is an outer membrane-targeting motif consisting of nine alternating hydrophobic residues ending in phenylalanine. Block 4 is a region of homology shared by a family of cell surface proteins whose members include YadA of Yersinia (15, 18, 19), UspA2 of Moraxella (1, 2), and DsrA of H. ducreyi (5). Blocks 2 and 3 are regions of homology shared by the Eib proteins but not other proteins in the databases; block 3 is predicted to assume a coiled-coil structure (16). Analysis of the EibF sequence revealed the same overall organization and blocks of homology. The block 4 sequence of EibF is more similar to the corresponding region found in the other Eib proteins (87 to 100% identity) than to that found in the other members of this family of proteins (e.g., identities of 68% for UspA2 and 70% for YadA). Comparing EibF to the other Eib proteins, the 167-amino-acid sequence covering blocks 2 through 5 was most similar to EibA (94% identity) and least similar to EibE (78% identity). The portion of EibF designated block V was much more divergent than the last 167 amino acids, although block V contained a sequence with limited similarity to EibD (30% identity over 185 amino acids), EibC (33% identity over 130 amino acids), and EibE (25% identity over 142 amino acids); no homology to EibA was found in block V.
Two types of imperfect repeats were found in EibF. The first type consisted of a set of three tandem repeats located in block V. Figure 4 shows the position of these repeats, designated f1, f2, and f3 (Fig. 4A) and their alignments (Fig. 4B). Repeats f2 and f3 each contained 26 amino acids; f1 was a partial repeat containing 11 amino acids. Versions of this repeat were also found in EibC and EibD (designated c2 and d2) but not in EibA or EibE. Amino acid identities ranged from 62 to 69% in pairwise comparisons among the f1, f2, f3, and d2 repeats. A second type of imperfect repeat consisting of 19 amino acids in EibF and EibA was identified. In each case, the first copy (designated f4 in EibF and a4 in EibA) occurred within block V at a position immediately preceding block 2. The second copy (designated f5 in EibF and a5 in EibA) occurred within block 2; the sequence of f5 was identical to a5. Repeats f4 and f5 of EibF had 84% amino acid identity, whereas repeats a4 and a5 had 53% identity.
FIG. 4.
Sequence comparison for EibF, EibD, and EibA. (A) Amino acid sequence alignment showing positions of repeated sequences and blocks 1, V, and 2 to 5. Block designations are shown above the sequence. Repeats f1, f2, f3, and d2 are shaded; repeats a4, a5, f4, and f5 are underlined. The first amino acid in each block and repeat is shown in boldface. (B) Repeated sequence alignments. Invariant amino acids are shown in boldface.
Functional mapping.
Two striking properties of the Eib proteins are Ig binding (IgG Fc and/or IgA) and stable multimerization (defined as resistance to dissociation after being boiled in SDS-PAGE sample buffer containing 6% SDS and 2-mercaptoethanol). In every case previously described, Ig binding signals were associated with Eib protein bands representing much larger molecular masses than predicted from the eib gene sequences. This raised the possibility that multimerization might be important or even essential for Ig binding. If, on the other hand, Ig binding and multimerization are independent functions, then it should be possible to separate these functions experimentally. More specifically, it might be possible to identify sequence segments that can confer Ig binding without multimerization or vice versa. By further extension of this analysis to segments of EibA and EibF, it might be possible to identify portions of the sequences which allow discrimination between IgG and IgA. Accordingly, we constructed protein fusions to screen specific sequence segments. Our choice was constrained by previous observations. Namely, deletion of as few as five codons from the C terminus of the Eib proteins (i.e., the region encoding the outer membrane-targeting motif) abolished both the Ig binding activity and the high-molecular-mass Eib proteins observed on Coomassie brilliant blue-stained gels (16). This result suggested that transport of protein through the cytoplasmic membrane in the absence of a signal for insertion into the outer membrane caused complete loss. To avoid problems associated with Eib proteins lacking their normal C termini, we adopted a fusion strategy which would keep the proteins in the cytoplasm. Our choice was the pMAL-c2X protein fusion and purification system (New England Biolabs). The strategy involved fusing an eib gene to the N terminus of the malE gene of the plasmid vector pMal-c2X. Since the MBP encoded by malE of pMal-c2X lacks a signal sequence, the fusion proteins derived from it are retained in the cytoplasm. In this system, expression of the MBP-Eib fusion proteins is controlled by IPTG. A significant advantage of the system is that antibody directed against MBP provides a means of detecting derivatives of the Eib proteins even when nonimmune Ig binding is abrogated. The fusion plasmids and deletion derivatives are described in Table 1. Figure 3 shows the amino acid coordinates within the amino acid sequences and the relationship to blocks 2 to 5 of EibA (Fig. 3C) and EibF (Fig. 3E). The amino acid coordinates for these blocks are given in Fig. 3B and D.
(i) Eib A protein fusions.
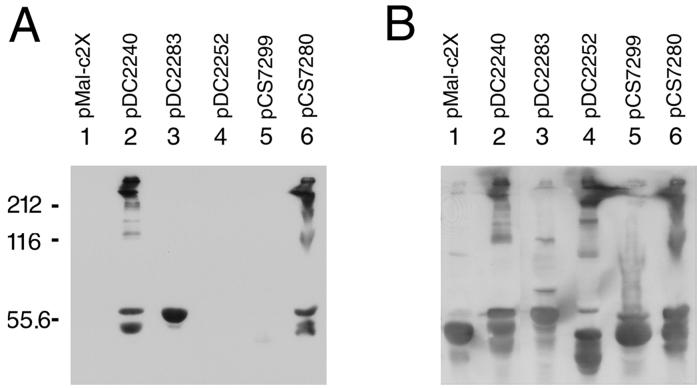
A fusion was made between the N-terminal 410 codons of malE (Fig. 3A) and the C-terminal 141 codons of eibA (Fig. 3B), producing pDC2240 (Fig. 3C). The eibA portion consequently retained 27 amino acids of block 2 and all of blocks 3 to 5. The construct was assessed by immunoblotting for its ability to confer binding of IgG Fc and multimerization of the encoded protein. Figure 5 shows an immunoblot which was incubated sequentially with IgG Fc-HRP (Fig. 5A) and rabbit anti-MBP (Fig. 5B). The fusion protein encoded by pDC2240 was expressed in the form of several discrete IgG Fc binding bands ranging in size up to and exceeding 200 kDa (Fig. 5A, lane 2). This indicated that domains sufficient for binding IgG Fc and for multimerization both lay within the last 141 amino acids of EibA. It should be mentioned parenthetically that the fusion protein has no cysteine residues, so it cannot participate in a disulfide bond. This initial construct was modified by deletion of the final 50 amino acids (pDC2283). This deletion eliminated the high-molecular-mass forms but not IgG Fc binding activity; a single IgG Fc binding band occupied the position close to that expected of a monomer (Fig. 5A, lane 3). A fusion protein which retained only the last 92 amino acids of EibA (pDC2252) failed to bind IgG Fc (Fig. 5A, lane 4) but was still able to form bands larger than 200 kDa (recognized by anti-MBP) (Fig. 5B, lane 4). A fusion protein lacking the last 94 amino acids of EibA (pCS7299) failed to bind IgG Fc (Fig. 5A, lane 5) and failed to form large bands, appearing instead as a monomer-sized band recognized by anti-MBP (Fig. 5B, lane 5). Combining these results, we conclude that a fragment retaining only the C-terminal 141 amino acids of the 392-amino-acid EibA protein can mediate formation of large multimeric structures which bind IgG Fc. Consequently, neither block V nor the first 28 amino acids of the sequence identified as block 2 are essential for these properties. Deletion of the last 50 amino acids abrogates multimerization but not IgG Fc binding, while deletion of the first 49 of those 141 amino acids abrogates IgG Fc binding but not multimerization, indicating that different parts of the protein are essential for binding IgG Fc and multimerization. Of the constructs tested, the smallest fragment retaining IgG Fc binding activity included 27 amino acids of block 2, all 46 amino acids of block 3, and the first 14 amino acids of block 4.
FIG. 5.
MBP-EibA fusions. Broth cultures were grown and induced with IPTG, and whole-cell were extracts prepared as described in Materials and Methods. Equivalent amounts of cell extracts were fractionated by SDS-PAGE (8% acrylamide) and blotted to PVDF. The blot was incubated first with human IgG Fc-HRP (A) and second with rabbit anti-MBP developed with donkey anti-rabbit Ig (B) (see Materials and Methods). Lanes: 1, pMal-c2X; 2, EibA (amino acids [aa] 254 to 392), pDC2240; 3, EibA (aa 254 to 344), pDC2283; 4, EibA (aa 300 to 392), pDC2252; 5, EibA (aa 254 to 298), pCS7299; 6, EibF (aa 318 to 459), pCS7280.
(ii) EibF protein fusions.
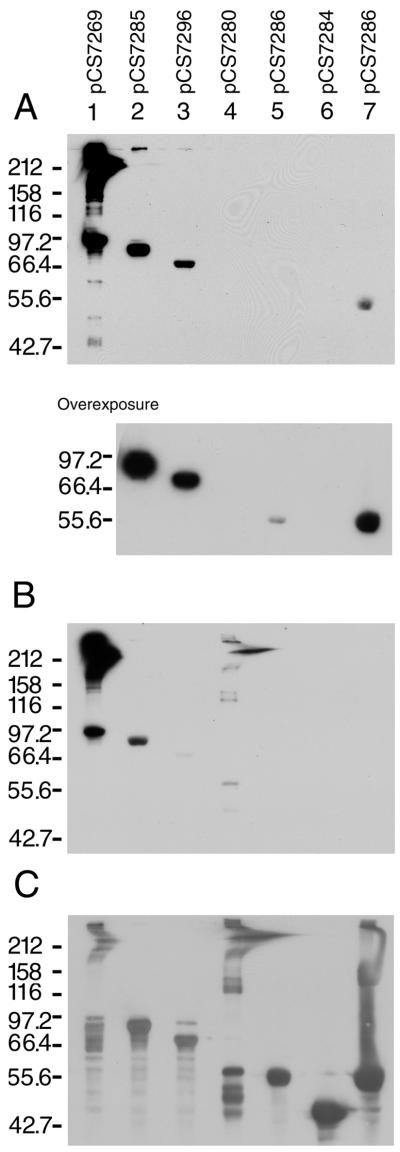
A malE-eibF fusion was constructed which contained the final 363 codons of the eibF sequence (amino acids 97 through 459); this construct (pCS7269) included 196 codons preceding block 2 (Fig. 3E). The fusion construct conferred both IgA binding (Fig. 6A, lane 1) and IgG Fc binding (Fig. 6B, lane 1), and both activities were associated with multimerized structures.
FIG. 6.
MBP-EibF fusions. Blots were prepared as described in the legend to Fig. 4 except that the amount of extract loaded in lane 7 was four times the volume loaded in other lanes. The blots were incubated sequentially, first with IgA-HRP (A), second with IgG Fc-HRP (B), and finally with rabbit anti-MBP developed with donkey anti-rabbit Ig-HRP (C) (see Materials and Methods). Lanes: 1, EibF (aa 97 to 459), pCS7269; 2, EibF (aa 97 to 399), pCS7285; 3, EibF (aa 97 to 353), pCS7296; 4, EibF (aa 318 to 459), pCS7280; 5, EibF (aa 181 to 280), pCS7286; 6, EibF (aa 400 to 459), pCS7284; 7, EibF (aa 181 to 280), pCS7286.
The preceding section has shown that only the C-terminal 141 amino acids of EibA were required for IgG Fc binding to the fusion protein. Since EibA and EibF differ by only 3 of these 141 amino acids, it was of obvious interest to test whether a comparable construct derived from EibF would bind IgG Fc and, more particularly, whether it would also retain the EibF feature of strong IgA binding. The construct used (pCS7280) actually contained the C-terminal 142 amino acids of EibF (Fig. 3C). When tested it conferred IgG Fc binding with multimerization (Fig. 5A, lane 6, and Fig. 6B, lane 4) but not IgA binding (Fig. 6A, lane 4). This suggested that segments nearer the N terminus of EibF were necessary for IgA binding. This was investigated by testing a series of additional fusions derived from the initial construct, pCS7269. Deletion of 60 amino acids from the C terminus (pCS7285) abrogated multimerization, but IgA and IgG Fc binding both remained (Fig. 6A and B, lanes 2). Deletion of 106 amino acids from the C terminus (i.e., an additional 46 amino acids) (pCS7296) eliminated detectable IgG Fc binding (Fig. 6B, lane 3), but the fusion protein retained IgA binding (Fig. 6A, lane 3). This indicated that the sequence located between amino acids 97 and 353 was sufficient for binding IgA. This region contained the repeats f1, f2, and f3 (Fig. 4) located between amino acids 207 and 269. We postulated that these repeats might be important for IgA binding and focused on cloning this region. A 100-codon region of eibF containing the repeats was amplified by PCR and cloned into pMal-c2X to produce pCS7286 (see Materials and Methods). Cells harboring this plasmid expressed a protein which bound IgA but not IgG Fc (Fig. 6A and B, lanes 5 and 7). The apparent molecular mass was consistent with that expected of a monomer (54.3 kDa). It is important to note that the binding signal was greatly diminished compared to the protein encoded by parent plasmid pCS7269 and was detectable only by overexposing the blot (Fig. 6A, lower panel, lane 5) or overloading the sample (Fig. 6A, lane 7). Ample protein was present (Fig. 6C, lanes 5 and 7), suggesting that the loss of binding signal was not due to a reduction in the amount of the fusion protein present in the extract. We concluded that the 100-codon region containing repeated sequences f1, f2, and f3 was sufficient for binding IgA, although the binding signal was greatly diminished compared to that of the fusion encoded by pCS7296 (Fig. 6A, lower panel, compare lane 5 to lane 3).
Stable multimerization of Eib proteins.
Fusions containing the C-terminal 141 (EibA) or 142 (EibF) residues of the protein produced a series of bands, some exceeding 200 kDa in size (Fig. 5B, lanes 2 and 6), indicating that this region was sufficient for multimerization. A fusion which contained only the last 92 amino acids of EibA (pDC2252) also produced large-molecular-mass bands (Fig. 5B, lane 5). In contrast, a fusion which contained only the last 60 amino acids of EibF (pCS7284), failed to produce high-molecular-mass bands (Fig. 6C, lane 6). The fusion encoded by pCS7284 differs in size from that encoded by pDC2252 by 33 amino acids, 26 residues from block 3 and 7 residues from block 4. This suggested that amino acids in this region contribute to multimerization. However, the fusion encoded by pCS7285, which contained these 33 amino acids, failed to produce high-molecular-mass forms (Fig. 6C, lane 2), indicating that this region alone, while necessary, is not sufficient for multimerization.
Trypsinization of intact cells.
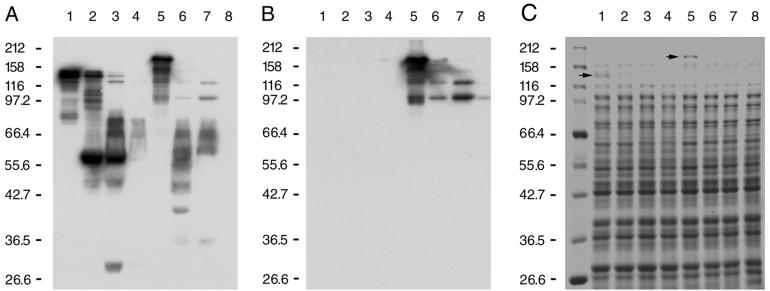
Ig binding material was previously shown to be exposed at the cell surface and accessible to trypsin in Ig binding ECOR strains (17). The outer membrane-targeting sequence identified in the eib gene sequences predicted that the Eib proteins expressed by the recombinant eib clones would also be exposed at the cell surface and be accessible to trypsin. This has been found to be the case for both EibA and EibF. Intact cells harboring eibF or eibA were treated with trypsin at various concentrations, harvested by centrifugation, and then fractionated on duplicate gels. One gel was used for immunoblotting, and one was stained with Coomassie brilliant blue. Figure 7 shows an immunoblot incubated sequentially with IgG Fc (Fig. 7A) and then IgA (Fig. 7B). The stained gel is shown in Fig. 7C. The residual Ig binding activities of cell-associated fragments of EibA are shown in Fig. 7A and B, lanes 1 through 4, and those of EibF in lanes 5 through 8. Trypsin at the highest concentration used (500 μg per ml) completely eliminated binding of IgG Fc to both proteins (Fig. 7A, lanes 4 and 8) and binding of IgA to EibF (Fig. 7B, lane 8). It also eliminated the Eib proteins observed by staining (Fig. 7C). Examination of the other proteins in the stained gel showed that most were unaffected by treatment with trypsin (Fig. 7C, compare lanes 2 through 4 to lane 1 and lanes 6 through 8 to lane 5). Trypsin at lower concentrations (5 or 50 μg per ml) produced an array of cell-associated Ig binding fragments of various sizes (Fig. 7A and B, lanes 2 and 3). Observation of these intermediate profiles provided important insights into EibF protein structure. Examination of Fig. 7 showed that EibF fragments of approximately 38 to 80 kDa bound IgG Fc (Fig. 7A, lanes 6 and 7) but did not bind IgA (Fig. 7B, lanes 6 and 7). Only the larger (>100-kDa) fragments bound both IgG Fc and IgA (Fig. 7A and B, lanes 5). If we make the assumption that the Eib proteins insert into the outer membrane by interactions involving their C termini, then we would anticipate that sequences closer to the N terminus would be more sensitive to trypsin. These results therefore supported those obtained with fusion proteins and were consistent with a model in which IgG Fc binding is specified by a region of EibF, which is closer to the C terminus than the region specifying IgA binding. Trypsin-treated cells expressing EibA also had relatively small (approximately 30- to 90-kDa) cell-associated fragments which bound IgG Fc (Fig. 7A, lanes 2 and 3). This would be expected, since the region responsible for binding IgG Fc was mapped to blocks 2 and 3, a region which is highly conserved between Eib proteins A and F.
FIG. 7.
Trypsin treatment of cells expressing EibA or EibF. Whole cells containing cloned eibA (lanes 1 to 4) or eibF (lanes 5 to 8) were treated with trypsin at concentrations of 5 (lanes 2 and 6), 50 (lanes 3 and 7), or 500 (lanes 4 and 8) μg per ml (see Materials and Methods). Whole-cell extracts were prepared and fractionated on duplicate gels by SDS-PAGE (8% polyacrylamide) as described in the legend to Fig. 2. One gel was blotted to PVDF and then incubated sequentially with IgG Fc-HRP (A) and IgA-HRP (B). The second gel was stained with Coomassie brilliant blue (C). Arrows, EibA (lane 1) and EibF (lane 5) when trypsin was not added.
Prophage genes linked to eibF.
All four of the eib genes cloned from ECOR-9 were found to be located in the genomes of prophage. The respective prophages were designated P-EibA through P-EibE, according to the associated eib gene (16). To determine whether eibF was also part of a prophage, we analyzed the nucleotide sequences adjacent to eibF. Sequencing revealed that an organization identical to that previously found for the other four eib genes in the neighboring ORFs homologous to both ORF-191 (upstream) and ORF-156 (downstream) was present. The amino acid sequence predicted by eibF-linked ORF-191 was 93 to 94% identical to that predicted by homologs from P-EibC, P-EibD, and P-EibE. Comparison of this ORF-191 to the homolog found in P-EibA revealed 96% identity, but only for 113 amino acids beginning at amino acid 60. (ORF-191 of P-EibA is not homologous to the other versions of ORF-191 or to any sequences in the databases for the first 61 amino acids.) The amino acid sequence predicted by eibF-linked ORF-156 was 92 to 94% identical to that predicted by homologs from P-EibA, P-EibC, and P-EibD but only 69% identical to that from P-EibE. It is interesting that the ORF-156 associated with eibF contains a 117-bp sequence which is 94% identical to a sequence found in bacteriophage 933W (14) and bacteriophage VT2-Sa (12) of E. coli O157:H7.
As previously reported and in contrast to successes for eibC, eibD, and eibE, we were unable to demonstrate infectious transfer of eibA from ECOR-9 (16). Attempts to demonstrate infectious transfer of eibF from ECOR-2 were also negative, although we were able to demonstrate the infectious transfer of two other genes, both conferring binding activity for IgG Fc (but not IgA) on recipient E. coli (unpublished data). Our tentative conclusion, based on the similar gene organization and homology, is that eibF is part of a prophage genome, most likely a defective one. This new prophage has been designated P-EibF.
eibF attenuates serum sensitivity.
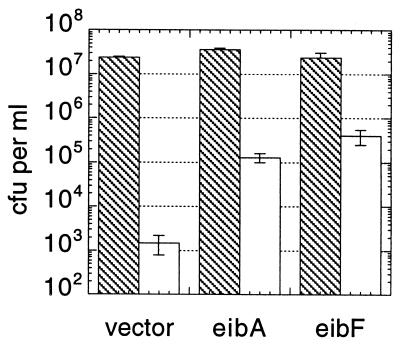
The eib genes cloned from ECOR-9 were previously shown to enhance the serum resistance of E. coli strain AB1157, a strain which is known to be particularly sensitive to serum (16). Cloned eibF was tested for this property and compared to eibA. Cells were exposed to 25% human serum or control PBS for 1 h, diluted, and plated. The results of a representative experiment are shown in Fig. 8. Only 0.006% of control cells harboring vector survived serum treatment, while 0.36% of cells harboring eibA and 1.67% of cells harboring eibF survived (Fig. 8). A survival ratio was calculated for cells harboring each gene by dividing its survival frequency by the survival frequency observed for the control (4). The survival ratios conferred by the eib genes in these experiments were 58 (eibA) and 267 (eibF), representing 58-fold and 267-fold protection of strain AB1157. By way of comparison, gene bor of bacteriophage lambda confers a 23-fold protection upon this strain, increasing the survival of this strain in 20% guinea pig serum from 0.0013 to 0.031% (4).
FIG. 8.
Attenuation of serum sensitivity imparted by eibF. E. coli AB1157 cells containing cloned eibF(pCS7216), eibA(pCS6379), or vector (pOK12) were treated with 25% human serum complement (open bars) or PBS (hatched bars) for 1 h. The cells were diluted and plated (see Materials and Methods). Each bar represents the mean of three (PBS) or five (serum) replicate treatments. Error bars indicate standard deviations.
DISCUSSION
This report documents the cloning of a new eib gene, eibF, from a second strain of E. coli, ECOR-2. EibF, the protein encoded by eibF, occurred as a multimer which bound human serum IgA in addition to human IgG Fc. EibF has many similarities to Eib proteins A, C, D, and E of ECOR-9. These include two sorting signals: a signal sequence for export (block 1) and a C-terminal outer membrane protein-targeting motif (block 5). It also includes a region of sequence homology (block 4) which defines a family of proteins that includes YadA, UspA2, and DsrA. EibF contains additional regions having homology to Eib proteins but not to other proteins in the database (blocks 2 and 3). Like the other Eib proteins, EibF has a sequence, block V, located between blocks 1 and 2 which shows greater diversity than other parts of the protein.
All Eib proteins so far studied share two features, binding of IgG Fc and stable multimerization. EibF, but not EibA, possesses an additional significant feature, that of IgA binding. All of these traits were retained by appropriate protein fusion constructs. By testing a series of protein fusions containing different portions of EibA and EibF, we have found that these three functional traits are encoded by different sequence segments and can be separated from one another. Both the C-terminal 141-amino-acid segment of EibA and the corresponding 142-amino-acid segment of EibF bound IgG Fc (Fig. 5, lanes 2 and 6). Since the two amino acid sequences are 98% identical, their similar activity in this test was not surprising. The 142-amino-acid segment from EibF did not impart IgA binding activity to the fusion (Fig. 6A, lane 4). A sequence nearer the N terminus was found necessary. A fusion bearing 100 amino acids derived from EibF was sufficient to permit IgA binding (Fig. 6A, lane 5). Significantly, the sequences sufficient for IgA binding (amino acids 181 to 280 of the EibF sequence) did not overlap the sequences sufficient for IgG Fc binding (amino acids 318 to 459). Consequently, the essential binding contacts of EibF for IgA and IgG Fc are completely different. This IgA specificity portion lies within block V of EibF and no significant sequence similarity of EibF block V to EibA is seen. The IgA specificity portion included a set of imperfect tandem repeats, f1, f2, and f3. Both f2 and f3 contain 26 amino acids, while f1 has only the last 11 residues of the repeat. Interestingly, EibC and EibD, which also bind IgA, each have a single copy of this repeat while EibA has none. The position of repeat copy d2 within the EibD sequence and its alignment with those of EibF are designated in Fig. 4. It seems quite possible that this repeat sequence plays a key role in IgA binding by those Eib proteins that possess it. Our studies of IgA binding do not permit conclusions regarding the portion (Fc or Fab) of the IgA molecule involved in binding, since all IgA binding studies were done with the whole molecule.
The formation of highly stable multimers is a property of all Eib proteins as well as the related cell surface proteins YadA, UspA2, and DsrA. With each of these proteins, multimers are stable in SDS-PAGE sample buffer, and for all except DsrA, the multimers are stable even upon heating to 100°C. This family of proteins shares a region of sequence homology which we have designated block 4 for the Eib proteins. A fusion that retained only the C-terminal 92 amino acids of EibA (pDC2252) still formed stable multimers (Fig. 5B, lane 4). This fusion included block 4 and the outer membrane-targeting signal along with 26 additional amino acids proximal to the N terminus. Deletions within this homology block have shown its importance in the oligomerization of YadA (19). This region has been predicted to take the form of a membrane anchor domain formed by four amphipathic transmembrane β-strands in this family of proteins (9).
Formation of stable multimers is not essential for either IgA or IgG Fc binding. This was concluded from observations of a fusion involving EibA (pDC2283) which binds IgG Fc without stable multimerization (Fig. 5A, lane 3) and a fusion of EibF (pCS7285) which binds both IgA and IgG Fc without stable multimerization. Nevertheless, we found that constructs that did not form stable multimers yielded weaker binding signals for both Ig types. For example, the signal for binding IgG Fc was much stronger for a fusion containing the last 363 amino acids and which formed stable multimers than for one lacking the last 60 of these residues and which did not form multimers (Fig. 6B and C, compare lanes 1 and 2). Our data do not permit conclusions about the nature of stable multimerization, i.e., whether the multimers contain only Eib proteins or whether they contain additional components which have not yet been characterized. The binding signal was also diminished dramatically by the deletion of other regions not implicated in multimerization. For example, the signal for binding IgG Fc was much stronger for the fusion containing the last 363 residues of EibF than for one containing only the last 142, even though both formed multimers (Fig. 6B and C, compare lanes 1 and 4). It is possible that Ig binding requires the reassociation of monomers after they have been blotted to a membrane. Our data do not address this possibility.
Our original experiments using trypsin treatment were done with the ECOR strains themselves, and we sought to verify that the Eib proteins expressed after cloning were also expressed at the cell surface. The experiments shown in Fig. 7 not only supported the idea that cloned EibF is present on the cell surface but yielded results that suggested more detailed aspects of its exposure. Limited digestion produced an array of species that retained Ig binding. Although reduced in apparent size, these fragments were still significantly larger than the 49.5 kDa expected for the original EibF monomer (e.g., Fig. A, lane 7). This suggested that these various fragments all retained the potential for stable multimerization. Only relatively large fragments, migrating at positions indicating a size of 100 kDa or greater, bound IgA as well as IgG Fc (Fig. 7B, lane 7). Smaller fragments ranging from 60 to 70 kDa (apparent size) bound IgG Fc but not IgA. These results suggested to us that the N terminus of EibF is most exposed to trypsin, that the IgA binding sites are more susceptible than the IgG Fc binding sites, and that sites involved in multimerization are relatively protected. From the experiments using MBP fusions, we had concluded that multimerization requires Eib sequences near the C-terminal outer-membrane insertion signal, including most if not all of block 4.
In addition to nonimmune Ig binding, all of the Eib proteins identified so far attenuate the serum sensitivity of E. coli. All show regions of high sequence conservation, within both the N-terminal 18 codons and the C-terminal 167 codons of the eib gene sequences. In sharp contrast, sequence divergence characterizes block V, which lies between these regions of high sequence conservation. This pattern of highly conserved N and C termini, coupled with divergent subterminal sequences, is a feature shared with variants of UspA2 and the closely related UspA2H, as well as surface-exposed proteins of numerous other pathogens (11). All of the eib genes are associated with prophage, and this association provides a potential means for horizontal transfer. High sequence conservation is also maintained among the variants of ORF-191 and ORF-156 which adjoin the various eib genes. The presence of conserved flanking regions provides a potential site for recombination that would facilitate generation of new eib gene variants.
ACKNOWLEDGMENTS
We thank Du Chungen for expert technical assistance.
This work was supported by Public Health Service grant GM16329 from the National Institutes of Health and an Innovative Biotechnology Seed Grant from the Pennsylvania State University Life Sciences Consortium.
REFERENCES
- 1.Aebi C, Lafontaine E R, Cope L D, Latimer J L, Lumbley S L, McCracken G H, Jr, Hansen E J. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun. 1998;66:3113–3119. doi: 10.1128/iai.66.7.3113-3119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi C, Maciver I, Latimer J L, Cope L D, Stevens M K, Thomas S E, McCracken G H, Jr, Hansen E J. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun. 1997;65:4367–4377. doi: 10.1128/iai.65.11.4367-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barondess J J, Beckwith J. A bacterial virulence determinant encoded by lysogenic coliphage l. Nature. 1990;346:871–874. doi: 10.1038/346871a0. [DOI] [PubMed] [Google Scholar]
- 5.Elkins C, Morrow K J, Jr, Olsen B. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect Immun. 2000;68:1608–1619. doi: 10.1128/iai.68.3.1608-1619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 7.Herzer P J, Inouye S, Inouye M, Whittam T S. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J Bacteriol. 1990;172:6175–6181. doi: 10.1128/jb.172.11.6175-6181.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill C W, Feulner G, Brody M S, Zhao S, Sadosky A B, Sandt C H. Correlation of Rhs elements with Escherichia coli population structure. Genetics. 1995;141:15–24. doi: 10.1093/genetics/141.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoiczyk E, Roggenkamp A, Reichenbecher M, Lupas A, Heesemann J. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 2000;19:5989–5999. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson J R, Delavari P, Kuskowski M, Stell A L. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J Infect Dis. 2001;183:78–88. doi: 10.1086/317656. [DOI] [PubMed] [Google Scholar]
- 11.Lafontaine E R, Cope L D, Aebi C, Latimer J L, McCracken J G H, Hansen E J. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J Bacteriol. 2000;182:1364–1373. doi: 10.1128/jb.182.5.1364-1373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto H, Nakai W, Yajima N, Fujibayashi A, Higuchi T, Sato K, Matsushiro A. Sequence analysis of Stx2-converting phage VT2-Sa shows a great divergence in early regulation and replication regions. DNA Res. 1999;6:235–240. doi: 10.1093/dnares/6.4.235. [DOI] [PubMed] [Google Scholar]
- 13.Ochman H, Selander R K. Standard reference strains of Escherichia coli from natural populations. J Bacteriol. 1984;157:690–693. doi: 10.1128/jb.157.2.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plunkett G, III, Rose D J, Durfee T J, Blattner F R. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J Bacteriol. 1999;181:1767–1778. doi: 10.1128/jb.181.6.1767-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosqvist R, Skurnik M, Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988;334:522–525. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- 16.Sandt C H, Hill C W. Four different genes responsible for nonimmune immunoglobulin-binding activities within a single strain of Escherichia coli. Infect Immun. 2000;68:2205–2214. doi: 10.1128/iai.68.4.2205-2214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandt C H, Wang Y-D, Wilson R A, Hill C W. Escherichia coli strains with nonimmune immunoglobulin-binding activity. Infect Immun. 1997;65:4572–4579. doi: 10.1128/iai.65.11.4572-4579.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skurnik M, Wolf-Watz H. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol Microbiol. 1989;3:517–529. doi: 10.1111/j.1365-2958.1989.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 19.Tamm A, Tarkkanen A-M, Korhonen T K, Kuusela P, Toivanen P, Skurnik M. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol. 1993;10:995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 20.Vieira J, Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]
- 21.Zhao S, Sandt C H, Feulner G, Vlazny D A, Gray J A, Hill C W. Rhs elements of Escherichia coli K-12: complex composites of shared and unique components that have different evolutionary histories. J Bacteriol. 1993;175:2799–2808. doi: 10.1128/jb.175.10.2799-2808.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]