Abstract
Background
Presbyopia occurs when the lens of the eyes loses its elasticity leading to loss of accommodation. The lens may also progress to develop cataract, affecting visual acuity and contrast sensitivity. One option of care for individuals with presbyopia and cataract is the use of multifocal or extended depth of focus intraocular lens (IOL) after cataract surgery. Although trifocal and bifocal IOLs are designed to restore three and two focal points respectively, trifocal lens may be preferable because it restores near, intermediate, and far vision, and may also provide a greater range of useful vision and allow for greater spectacle independence in individuals with presbyopia.
Objectives
To assess the effectiveness and safety of implantation with trifocal versus bifocal IOLs during cataract surgery among people with presbyopia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2022, Issue 3); Ovid MEDLINE; Embase.com; PubMed; ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on 31 March 2022.
Selection criteria
We included randomized controlled trials that compared trifocal and bifocal IOLs among participants 30 years of age or older with presbyopia undergoing cataract surgery.
Data collection and analysis
We used standard Cochrane methodology and graded the certainty of the body of evidence according to the GRADE classification.
Main results
We identified seven studies conducted in Europe and Turkey with a total of 331 participants. All included studies assessed visual acuity using a logarithm of the minimum angle of resolution (LogMAR chart). Of them, six (86%) studies assessed uncorrected distance visual acuity (the primary outcome of this review). Some studies also examined our secondary outcomes including uncorrected near, intermediate, and best‐corrected distance visual acuity, as well as contrast sensitivity.
Study characteristics
All participants had bilateral cataracts with no pre‐existing ocular pathologies or ocular surgery. Participants' mean age ranged from 55 to 74 years. Three studies reported on gender of participants, and they were mostly women. We assessed all of the included studies as being at unclear risk of bias for most domains. Two studies received financial support from manufacturers of lenses evaluated in this review, and at least one author of another study reported receiving payments for delivering lectures with lens manufacturers.
Findings
All studies compared trifocal versus bifocal IOL implantation on visual acuity outcomes measured on a LogMAR scale. At one year, trifocal IOL showed no evidence of effect on uncorrected distance visual acuity (mean difference (MD) 0.00, 95% confidence interval (CI) −0.04 to 0.04; I2 = 0%; 2 studies, 107 participants; low‐certainty evidence) and uncorrected near visual acuity (MD 0.01, 95% CI −0.04 to 0.06; I2 = 0%; 2 studies, 107 participants; low‐certainty evidence). Trifocal IOL implantation may improve uncorrected intermediate visual acuity at one year (MD −0.16, 95% CI −0.22 to −0.10; I2 = 0%; 2 studies, 107 participants; low‐certainty evidence), but showed no evidence of effect on best‐corrected distance visual acuity at one year (MD 0.00, 95% CI −0.03 to 0.04; I2 = 0%; 2 studies, 107 participants; low‐certainty evidence). No study reported on contrast sensitivity or quality of life at one‐year follow‐up. Data from one study at three months suggest that contrast sensitivity did not differ between groups under photopic conditions, but may be worse in the trifocal group in one of the four frequencies under mesopic conditions (MD −0.19, 95% CI −0.33 to −0.05; 1 study; I2 = 0%, 25 participants; low‐certainty evidence). One study examined vision‐related quality of life using the 25‐item National Eye Institute Visual Function Questionnaire (NEI‐VFQ‐25) at six months, and suggested no evidence of a difference between trifocal and bifocal IOLs (MD 1.41, 95% CI −1.78 to 4.60; 1 study, 40 participants; low‐certainty evidence).
Adverse events
Adverse events reporting varied among studies. Of five studies reporting information on adverse events, two studies observed no intraoperative and postoperative complications or no posterior capsular opacification at six months. One study reported that glare and halos were similar to the preoperative measurements. One study reported that 4 (20%) and 10 (50%) participants had glare complaints at 6 months in trifocal and bifocal group, respectively (risk ratio 0.40, 95% CI 0.15 to 1.07; 40 participants). One study reported that four eyes (11.4%) in the bifocal group and three eyes (7.5%) in the trifocal group developed significant posterior capsular opacification requiring YAG capsulotomy at one year. The certainty of the evidence for adverse events was low.
Authors' conclusions
We found low‐certainty of evidence that compared with bifocal IOL, implantation of trifocal IOL may improve uncorrected intermediate visual acuity at one year. However, there was no evidence of a difference between trifocal and bifocal IOL for uncorrected distance visual acuity, uncorrected near visual acuity, and best‐corrected visual acuity at one year. Future research should include the comparison of both trifocal IOL and specific bifocal IOLs that correct intermediate visual acuity to evaluate important outcomes such as contrast sensitivity, quality of life, and vision‐related adverse effects.
Keywords: Aged; Female; Humans; Male; Middle Aged; Capsule Opacification; Cataract Extraction; Lenses, Intraocular; Presbyopia; Presbyopia/surgery; Quality of Life; Randomized Controlled Trials as Topic
Plain language summary
Trifocal versus bifocal lenses implantation after cataract surgery
What did we want to find out?
We wanted to find out whether there is a difference in effectiveness and safety of implantation of a lens that contains three regions that correct for distance, intermediate, and near vision (trifocal) into the eyes during cataract surgery compared to a lens that contains two regions that correct for distance and near vision (bifocal) in people with cataract.
Key messages
We found that people who receive trifocal lens after their cataract surgery may experience improvement in uncorrected intermediate sharpness of vision (visual acuity) at one year compared with those who had received bifocal lens, but we have little confidence in the evidence. There was no evidence of a difference between trifocal and bifocal lenses for uncorrected distance visual acuity, uncorrected near visual acuity, and best‐corrected distance visual acuity at one year. The effects of trifocal and bifocal lenses on quality of life and the ability to distinguish between fine increments of light and dark (contrast sensitivity) remain uncertain.
What is presbyopia, and how is it treated?
Presbyopia is an age‐related condition of the lens of the eye that causes a gradual loss of the ability to focus on nearby objects. Further age‐related lens changes may lead to loss of clarity of the lens (cataract) causing loss of visual acuity and contrast sensitivity. Lenses with three or two regions (trifocal or bifocal, respectively) are a new technology intended to decrease dependence on eye glasses use after cataract surgery.
What did we do?
We searched for studies that examined trifocal versus bifocal lenses in people with presbyopia. We compared and summarized the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found seven relevant studies conducted in Europe and Turkey with a total of 331 people with presbyopia.
We found that the implantation of trifocal lens at the time of cataract surgery may improve intermediate visual acuity at one year. We found no evidence of a difference between trifocal and bifocal lens in uncorrected distance visual acuity, uncorrected near visual acuity, and best‐corrected distance visual acuity at one year. It is uncertain whether trifocal compared with bifocal lens implantation has any effect on quality of life or contrast sensitivity.
What are the limitations of the evidence?
We have little confidence in the evidence because we were unclear about how studies were conducted, and we found only small number of relevant studies.
How up‐to‐date is this review?
We searched for studies published up to 31 March 2022.
Summary of findings
Summary of findings 1. Trifocal intraocular lenses versus bifocal intraocular lenses after cataract extraction among participants with presbyopia.
| Trifocal intraocular lenses versus bifocal intraocular lenses after cataract extraction among participants with presbyopia | ||||||
| Patient or population: people (> 30 years) with cataract and presbyopia Setting: eye clinic Intervention: trifocal IOL Comparison: bifocal IOL | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with bifocal IOL | Risk with trifocal IOL | |||||
| Mean uncorrected distance visual acuity (LogMAR (1 year) |
The mean uncorrected distance visual acuity (LogMAR) at 1 year was −0.01 to 0.01 LogMAR. | MD 0 LogMAR (−0.04 to 0.04 ) | ‐ | 107 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Mean uncorrected near visual acuity (LogMAR) (1 year) |
The mean uncorrected near visual acuity (LogMAR) at 1 year was 0.13 to 0.19 LogMAR. | MD 0.01 LogMAR (−0.04 to 0.06 ) | ‐ | 107 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Mean uncorrected intermediate visual acuity (LogMAR) (1 year) |
The mean uncorrected intermediate visual acuity (LogMAR) at 1 year was 0.25 to 0.26 LogMAR. | MD −0.16 LogMAR (−0.22 to −0.10 ) | ‐ | 107 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Mean best‐corrected distance acuity (LogMAR) (1 year) |
The mean best‐corrected distance acuity (LogMAR) at 1 year was −0.03 to −0.01 LogMAR. | MD 0.00 LogMAR (−0.03 to 0.04 ) | ‐ | 107 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Mean contrast sensitivity (1 year) |
No study reported this outcome at 1 year. |
‐ | ‐ | 1 study reported that contrast sensitivity did not differ between groups under photopic conditions, but may be worse in the trifocal group in 1 of the 4 frequencies under mesopic conditions at 3 months (MD −0.19, 95% CI −0.33 to −0.05; n = 25). | ||
| Mean quality of life or visual function (measured using Visual Function Index‐14 tool) (1 year) |
No study reported this outcome at 1 year. | ‐ | ‐ | 1 study examined vision‐related quality of life using the NEI‐VFQ‐25 at 6 months and found no evidence of a difference between trifocal and bifocal IOLs (MD 1.41, 95% CI −1.78 to 4.60; 40 participants). | ||
| Adverse events (1 year) |
See comment | ‐ | ‐ | 129 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | 1 study reported no intraoperative or postoperative complications; in the other study, 4 eyes (11.4%) in the bifocal group and 3 eyes (7.5%) in the trifocal group developed significant posterior capsular opacification requiring YAG capsulotomy. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOL: intraocular lens; LogMAR: logarithm of the minimum angle of resolution; MD: mean difference; NEI‐VFQ‐25: 25‐item National Eye Institute Visual Function Questionnaire; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded for risk of bias (one level), as most domains were judged as at unclear risk of bias. 2Downgraded for imprecision (one level), as evidence was based on a small sample.
Background
Description of the condition
The lens is one of the most important tissues in the human eye. Located in the posterior chamber, it is the second most powerful refractive structure, and contributes 20% to 30% of the total refractive power. It is an elastic and transparent tissue that helps focus images onto the retina. The accommodation process causes the lens to change its anterioposterior length and is associated with convergence and miosis (Glasser 1999). This process allows adequate intermediate and near vision.
The broadly accepted theory of accommodation is the Helmholtz theory, in which accommodation is the result of elastic properties of the human lens and possibly the vitreous which allows the lens to increase its negative power when zonular tension is relieved, and vice versa. This movement is performed by the ciliary muscle. This property of the human lens is lost in a progressive manner during aging until the accommodation range is practically none (Glasser 1999; Torricelli 2012).
Accommodation decreases with aging in a process known as presbyopia. In individuals with presbyopia, the ability of the lens to accommodate is insufficient for near vision. This process generally occurs between age 40 and 50 years (Glasser 1999; Papadopoulos 2014), and if not corrected has a significant impact on quality of life (Torricelli 2012).
As presbyopia develops and elasticity is lost, the lens may become opaque. The loss of transparency of the lens is called cataract. There are several types of cataracts. The most common type is the senile (age‐related) cataract. There are several well‐known risk factors for developing this type of vision‐impairing lens opacity, including high sodium intake (Bae 2015), some systemic diseases such as diabetes mellitus (Li 2014), high body mass index (WHO 2015), exposure to ultraviolet B radiation, and smoking (Hodge 1995).
According to the World Health Organization, cataract accounts for 51% of worldwide blindness and affects about 20 million people around the world (WHO 2015).
Symptoms associated with this condition are myopia and decrease in contrast sensitivity and visual acuity. Cataract extraction by phacoemulsification followed by capsular bag implantation of an artificial intraocular lens (IOL) is one option of care for individuals with presbyopia and cataract (Carson 2014). Intraocular lens implantation does reduce spectacle dependence compared with aphakia, but many individuals may still need spectacle correction for near vision following cataract surgery.
Description of the intervention
Cataract surgery is performed to extract the cloudy lens material, while preserving some structures such as the capsular bag. An artificial IOL is then placed to restore vision in the eye (Kohnen 2009). The standard practice is usually the implantation of a monofocal IOL, which confers only one focal point on the retina (Carson 2014), typically to provide good distance vision. With a monofocal IOL, a pseudophakic patient thus continues to be presbyopic, and spectacles may still be needed after phacoemulsification surgery to restore vision at other distances. With the advancement of technology and increased expectations of better vision, the goal of cataract surgery is no longer only limited to restoring vision, but management of the refractive component is also important prior, during, and after surgery (Torricelli 2012). Multifocal lenses were therefore designed to give more than one focal point and provide spectacle independence to the patient.
With changes in social and work environments, especially with the use of computers, tablets, smartphones, etc., excellent intermediate distance vision has become more important. New types of IOL design feature a refractive and diffractive component and confer three focal points within the eye. These trifocal IOLs restore near, far, and intermediate vision (Gatinel 2013). Intraocular lenses with this design have been shown to achieve better patient satisfaction (Kretz 2015b).
How the intervention might work
Unlike monofocal IOLs, diffractive IOLs were originally designed using apodization and convolution technologies that cause light to divide, and produce two or more focal points. To restore near, intermediate, and far vision, three focal points may be preferable in an IOL.
Multifocal acrylic IOLs come in several designs. The goal of the first generation of multifocal lens design (bifocal IOL) was to restore two focal points: far and near vision (Voskresenskaya 2010). These bifocal IOLs, known by convention as a multifocal lens, have acceptable visual outcomes and give spectacles independence to many pseudophakic people (Calladine 2012; Torricelli 2012). The latest generation of multifocal IOLs are based on a diffractive/refractive technology design with the main objective of restoring intermediate vision (Papadopoulos 2014).
Different bifocal IOLs have different visual outcomes, mainly because of the different added power placed in the IOL to adjust for different near vision distances. Besides near and distance vision, good intermediate vision is needed to increase patient satisfaction with IOLs (Kretz 2015a; Kretz 2015b). A few trifocal IOLs are available. Excellent visual outcomes and high patient satisfaction scores have been reported with these lenses (Cochener 2012; Law 2014; Lesieur 2012; Sheppard 2013; Torricelli 2012; Voskresenskaya 2010; Vryghem 2013).
However, the most common adverse visual effects in a multifocal IOL are glare, halos, and loss of contrast sensitivity, which result in poor quality of vision during mesopic conditions (Carson 2014). These effects could be related to neuroadaptation when the brain and visual system adapt to the new way of vision. As this process evolves, patients become more comfortable with their new vision, and their perception of side effects decreases (Voskresenskaya 2010).
Why it is important to do this review
It is important to restore visual acuity at all distances in order to treat cataract and presbyopia satisfactorily. Adverse effects such as halos, glare, lowered contrast sensitivity, and dissatisfaction associated with IOLs seem to be inherent with the multifocal designs (bifocal or trifocal IOL). However, other visual and patient‐important benefits have been reported for both bifocal and trifocal IOLs (Cochener 2012; Law 2014; Lesieur 2012; Sheppard 2013; Voskresenskaya 2010; Vryghem 2013). Another Cochrane Review comparing multifocal and monofocal IOLs after cataract extraction was published in 2012 (Calladine 2012), but to our knowledge no high‐quality systematic review of evidence for the comparison of trifocal and bifocal IOLs has been published.
Objectives
To assess the effectiveness and safety of implantation with trifocal versus bifocal intraocular lenses (IOLs) during cataract surgery among people with presbyopia.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomized controlled trials (RCTs). We included all eligible trials regardless of their publication status or language of publication.
Types of participants
We included studies in which the participants were 30 years of age or older with cataract and presbyopia. We documented studies that included participants with other ocular comorbidities, such as pseudoexfoliation syndrome, glaucoma, diabetes mellitus, age‐related macular degeneration, retinal disease, optic nerve disease, or amblyopia in the eye undergoing cataract surgery or a history of intraocular surgery, pediatric cataract, or ocular trauma.
Types of interventions
We included studies in which implantation of trifocal IOLs was compared with implantation of bifocal IOLs during cataract surgery.
Types of outcome measures
Primary outcomes
Mean uncorrected (without the aid of spectacles or contact lenses) distance visual acuity measured by logarithm of the minimum angle of resolution (LogMAR) chart at one‐year follow‐up.
Secondary outcomes
Mean uncorrected distance visual acuity measured by LogMAR chart at three‐ and six‐month follow‐up.
Mean uncorrected near visual acuity at three‐month, six‐month, and one‐year follow‐up.
Mean uncorrected intermediate visual acuity at three‐month, six‐month, and one‐year follow‐up.
Mean best‐corrected distance visual acuity at three‐month, six‐month, and one‐year follow‐up.
Mean contrast sensitivity, measured by the FACT (Functional Acuity Contrast Test) chart (Pesudovs 2004), or by the Pelli‐Robson contrast sensitivity test (Mantyjarvi 2001), noted in logarithm of the contrast sensitivity (LogCS) at different cycles per grade in spatial frequencies at three‐month, six‐month, and one‐year follow‐up.
Mean quality of life or visual function evaluated by validated and comparable instruments (e.g. 25‐item National Eye Institute Visual Function Questionnaire (NEI‐VFQ‐25)) noted in numeric scores at three‐month, six‐month, and one‐year follow‐up.
Adverse outcomes
Visual disturbances such as glare, experienced when a source of light other than the main target image illuminates the retina, and halos, defined as visual disturbances related to the main target image that lower contrast sensitivity; these visual disturbances are only noted by proportions at three months, six months, and one year after surgery.
Opacification of the posterior capsule (proliferation of epithelial lens cells in the main visual axis that lowers visual acuity), with or without YAG laser capsulotomy, at three months, six months, and one year after surgery.
We assessed additional adverse effects related to IOLs mentioned in any of the included studies.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials. There were no restrictions on language or year of publication. The electronic databases were last searched on 31 March 2022.
Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (2022, Issue 3) (Appendix 1).
MEDLINE Ovid (1946 to 31 March 2022) (Appendix 2).
Embase.com (1980 to 31 March 2022) (Appendix 3).
PubMed (1948 to 31 March 2022) (Appendix 4).
LILACS (Latin American and Caribbean Health Science Information database) (1982 to 31 March 2022) (Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 31 March 2022) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; 31 March 2022) (Appendix 7).
Searching other resources
We searched the reference lists of included studies to identify additional relevant studies. We did not conduct specific searches on conference proceedings for this review because CENTRAL covers those abstracts. We did not contact any institution or personnel to identify potentially eligible trials.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of all records identified by the electronic and manual searches. Each review author reviewed and labeled each record as 'definitely relevant,' 'possibly relevant,' or 'definitely not relevant.' We retrieved the full‐text report for all records labeled as 'definitely relevant' or 'possibly relevant.' Two review authors independently assessed each full‐text report, classifying each as 'include,' 'exclude,' or 'awaiting classification.' Any differences between the two review authors at title, abstract, and full‐text screening stage were resolved by discussion. We documented the studies excluded after full‐text review and noted their reasons for exclusion.
Data extraction and management
Two review authors independently extracted the data from reports of the included studies using a data collection form developed by Cochrane Eyes and Vision and implemented in Covidence software (Covidence). Two review authors independently checked the data before entry into Review Manager 5 or Review Manager Web software (Review Manager 2020; RevMan Web 2022). We recorded the following characteristics of the included studies: study methods, participants, interventions, and outcomes. Where information about (or outcome data from) included studies was missing or unclear, we contacted the study investigators or organizations involved for additional data, confirmation, or clarification. We collected and used the most detailed numerical data available from the included studies to facilitate analyses. We attempted to obtain data from available reports, investigators, or organizations in preference to less precise methods such as extracting numeric data from graphs. When it was necessary to extract data available only in graphical displays, two review authors independently extracted the data, resolving any disagreements or discrepancies by discussion or by consulting a third review author.
Assessment of risk of bias in included studies
We used the Cochrane risk of bias tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias in the included studies (Higgins 2017). Two review authors independently assessed risk of bias for each included study, grading each risk of bias domain as low, high, or unclear. We evaluated the following risk of bias domains: selection bias (sequence generation and allocation concealment before assignment), performance bias (masking of participants and study personnel), detection bias (masking of outcome assessors), attrition bias (loss to follow‐up), reporting bias (selective outcome reporting), and other sources of bias. Any disagreements between review authors were resolved by discussion until consensus was reached or by consulting another review author.
Measures of treatment effect
Continuous outcomes
We had planned to use standardized mean differences (SMDs) and 95% confidence intervals (CIs) calculated for continuous data outcomes in anticipation of the use of different instruments of measurement in different studies. This statistic is used, for example, when distance and near visual acuity are reported on different scales (LogMAR, decimal, or Snellen fraction) in different studies to permit the analysis of effects on a uniform scale (Deeks 2017). However, because all visual acuity was reported as LogMAR in all studies that contributed data to the meta‐analysis, we estimated the overall effects as mean differences (MDs) and 95% CIs. Where possible, we checked for skewness using the methods outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Dichotomous outcomes
When data on adverse outcome such as 'glare,' 'halos,' 'spectacle independence,' 'posterior capsular opacification' (PCO), and 'glistenings' were available, we analyzed them as dichotomous outcomes, and calculated risk ratios (RRs) along with their 95% CIs to estimate effects.
Unit of analysis issues
The participant was the primary unit of analysis when only one eye per participant was enrolled in the study. We determined whether the included studies included one or both eyes from each participant and whether the study investigators randomized and analyzed data at the participant or eye level. We planned that when both eyes were randomized to the same treatment group (two‐eye design) or to different treatment groups (paired‐eye design), we would extract the results that had accounted for the correlation and refer to Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions for guidelines regarding considerations of including variants on randomized trials (Higgins 2019). When studies with more than two arms were included (e.g. two or more IOLs), we evaluated each relevant comparison separately, and selected one pair of intervention and comparison that were relevant to the review without double counting them in the analysis (Higgins 2019).
Dealing with missing data
We analyzed outcomes on an intention‐to‐treat basis. We contacted study authors when outcome data were missing. When no response was received within two weeks, we used the best information available to analyze the data. We only analyzed available data, and did not impute missing data for the purposes of this review.
Assessment of heterogeneity
We investigated clinical or methodological heterogeneity among studies by evaluating differences with respect to characteristics of participant populations, interventions, and outcome assessment. We evaluated statistical heterogeneity among outcomes by examining the overlap in CIs of forest plots and by using the Chi2 and the I2 statistic, as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). We used the I2 statistic to assess the proportion of total variability explained by heterogeneity among studies. If we observed substantial heterogeneity (I2 > 60%) or inconsistency among effect sizes estimated from individual studies contributing data to a meta‐analysis, we did not report a pooled analysis, and instead provided a narrative summary of the intervention effects estimated from the individual studies. However, if all estimates were in the same direction, we performed a meta‐analysis despite substantial statistical heterogeneity, and interpreted the findings taking account of the heterogeneity.
Assessment of reporting biases
We assessed selective outcome reporting for each study by comparing the outcomes specified in a protocol or clinical trial registry with the reported results. When protocols or clinical trial registry records were not available, we assessed selective outcome reporting based on the outcomes specified in the methods section of the study reports and on data collected and reported in the study. We planned to use funnel plots to assess small‐study effects that could result in publication bias when a sufficient number of trials (more than 10) were included in the review. However, we did not assess publication bias because the number of studies included in the review was less than 10.
Data synthesis
We analyzed data according to the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). If there was no statistical or clinical heterogeneity, or fewer than three trials contributed data to a meta‐analysis, we used a fixed‐effect model to estimate intervention effects; otherwise we used random‐effects models. When we detected substantial statistical heterogeneity (I2 > 50%), and the direction of treatment effects was not consistent across studies, we did not perform a meta‐analysis and instead presented a narrative summary.
Subgroup analysis and investigation of heterogeneity
We had planned to conduct subgroup analysis to investigate the reasons for any clinical or statistical heterogeneity according to outcomes within subgroups of participants defined by such factors as unilateral versus bilateral surgery and optical design in the IOLs. However, we did not conduct these analyses due to insufficient numbers of studies.
Sensitivity analysis
We had planned to conduct sensitivity analysis to examine the impact of excluding studies with high risk of bias, unpublished data, and industry‐funded studies to assess the robustness of estimates with respect to these factors. Due to insufficient numbers of included studies and the absence of unpublished studies, we did not perform these analyses. Although we had not planned at the protocol stage to conduct sensitivity analysis based on unit of analysis (participants versus eyes), we had planned post hoc to conduct additional sensitivity analyses to examine the impact of restricting our analyses to studies for which analysis was performed at the participant level. However, we did not conduct this analysis because only one study analyzed data at the participant level.
Summary of findings and assessment of the certainty of the evidence
We prepared a summary of findings table according to the methods described in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b), and presented the estimated effects of trifocal IOLs versus bifocal IOLs at one‐year follow‐up. We included the following outcomes in the summary of findings table.
Mean uncorrected distance visual acuity
Mean uncorrected near visual acuity
Mean uncorrected intermediate visual acuity
Mean best‐corrected distance visual acuity
Mean contrast sensitivity
Mean quality of life or visual function scores
Adverse events
Using the GRADE approach, two review authors independently judged the certainty of the evidence for each outcome as high, moderate, low, or very low (Langendam 2013). Any differences between review authors were resolved by discussion.
Results
Description of studies
Results of the search
Detailed results of the previous search were published in the original version of this review (Zamora‐de La Cruz 2020). Briefly, we included five studies (six reports), excluded 21 studies (21 reports), and categorized one study (one report) as awaiting classification from 8787 records identified by the database searches in September 2019.
We performed an updated electronic database searches on 31 March 2022, which yielded 1030 unique records. After title and abstract screening of 1030 records, we retrieved 21 full‐text reports for further review. We included two studies (two reports) and excluded 17 studies (17 reports). We listed two studies as awaiting classification because we were unclear about study design (Nava 2019; Song 2020). In addition, after review of the one study awaiting classification in the previous version of this review, we decided to exclude it because it was not an RCT (de Carneros‐Llorente 2019).
Overall, we included seven studies (eight reports), excluded 40 studies (40 reports), and categorized two studies (two reports) as awaiting classification. A study flow diagram is shown in Figure 1.
1.
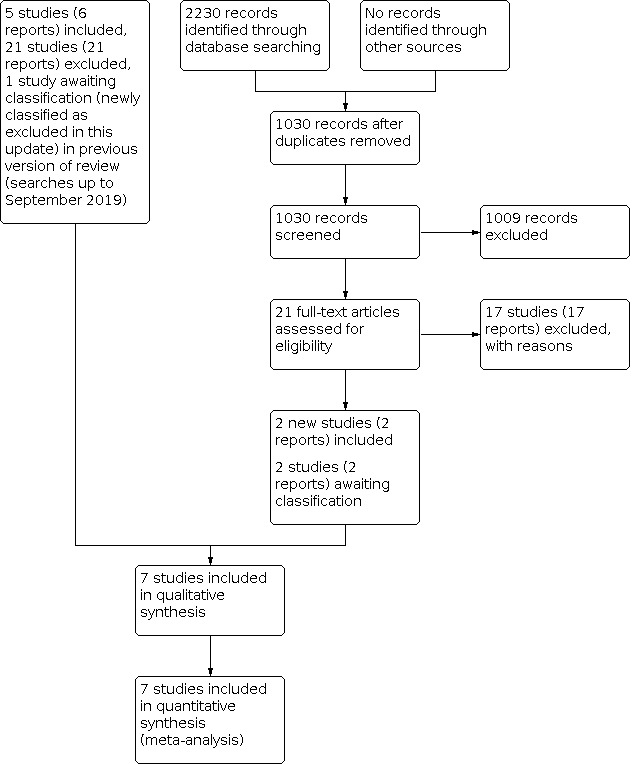
Study flow diagram.
Included studies
Study design
Six included studies were conducted in Europe: two in the Czech Republic (Mojzis 2014; Mojzis 2017), one in France (Cochener 2016), one in the Netherlands (Jonker 2015), one in Spain (Gil 2019), and one multicenter study was conducted in Spain, Germany, and France (Kaymak 2017). The remaining study was conducted in Turkey (Bozkurt Gencer 2021). Further details of the included studies can be found in the Characteristics of included studies table. The included studies were published between 2014 and 2021. Follow‐up duration ranged from three months in Mojzis 2014 to one year in Kaymak 2017 and Mojzis 2017. All of the included studies randomized both eyes of the same participant to the same intervention. Two studies analyzed data at the participant level (Cochener 2016; Gil 2019). In one study it was unclear if the unit of analysis was the eye or individual, and the remaining four studies analyzed data at the eye level, but the investigators did not report whether they had accounted for the correlation between eyes in their analysis. Two studies were of multi‐arm design (Gil 2019; Kaymak 2017), and we included only the comparison relevant to this review. We included all seven studies in meta‐analysis. The authors of two studies reported receiving funding from manufacturers of the lens examined in this review (Jonker 2015; Kaymak 2017). In one study at least one author reported a conflict of interest with manufacturers of the lens examined (Jonker 2015).
Participants
The seven included studies enrolled a total of 331 participants. The studies varied in size from 27 in Cochener 2016 to 116 participants in Gil 2019. The mean age of participants ranged from 55 to 74 years. Three studies reported information on the gender of participants, and participants were predominantly women, ranging from 60% in Kaymak 2017 to 71% in Gil 2019. Diagnosis of cataract varied among participants, highlighting clinical heterogeneity. All studies involved participants with bilateral cataracts and no pre‐existing ocular pathologies or ocular surgery. Cochener 2016 included participants who had started to show clouding of the crystalline lens (Lens Opacities Classification System III classification global score 2 or greater) and corneal astigmatism of 1.00 diopter (D) or less. Jonker 2015 and Kaymak 2017 included participants with bilateral cataracts with less than 1.0 D corneal astigmatism in both eyes. The remaining two studies enrolled participants with cataracts and presbyopic or pre‐presbyopic requiring refractive lenses (Mojzis 2014; Mojzis 2017). Gil 2019 included participants with bilateral cataracts, good motivation for spectacle independence potential visual acuity (20/25 of better), and preoperative corneal astigmatism less or equal to 1.25D. Bozkurt Gencer 2021 included participants with low vision in both eyes and best‐corrected visual acuity of less than 0.5 and astigmatism > 1.0 D. They used the Verion digital system and the conventional corneal marking method.
Interventions
All seven included studies evaluated trifocal versus bifocal IOLs.
Outcomes
All seven included studies assessed visual acuity measured using a LogMAR chart, and three studies assessed contrast sensitivity (Bozkurt Gencer 2021; Jonker 2015; Mojzis 2017). Five studies examined vision‐related quality of life; Bozkurt Gencer 2021 used the 25‐item National Eye Institute Visual Function Questionnaire (NEI‐VFQ‐25); Gil 2019 evaluated patient satisfaction and quality of life using the Visual Function 14 (VF‐14) questionnaire; three studies assessed other visual functions such as spectacle independence, Cochener 2016; Jonker 2015, and reading performance (Jonker 2015; Kaymak 2017). Five studies reported adverse events (Bozkurt Gencer 2021; Cochener 2016; Gil 2019; Jonker 2015; Mojzis 2017).
Excluded studies
We excluded 40 studies after full‐text review. Seventeen of these studies did not address the comparisons of interest, and the remaining 23 studies were not reports of RCTs (see Characteristics of excluded studies table).
Ongoing studies and studies awaiting classification
We listed two studies as awaiting classification because it was unclear if participants had been randomly assigned to intervention groups (see Characteristics of studies awaiting classification table). We identified no ongoing studies.
Risk of bias in included studies
Risk of bias assessment for all seven studies is summarized in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Randomization sequence was adequately generated in three studies: participants were assigned to treatment groups using web‐based software in Cochener 2016, computer‐generated random sequence in Gil 2019, and by randomization code administered centrally using a vocal server in Kaymak 2017. We judged these three studies to be at low risk of bias for this domain. Four studies did not report the method of randomization (Bozkurt Gencer 2021; Jonker 2015; Mojzis 2014; Mojzis 2017), and were therefore judged as at unclear risk of bias.
Allocation concealment
We judged Kaymak 2017 to be at low risk of bias as the treatment allocation was accomplished centrally. The method of treatment allocation concealment was not reported in the remaining six studies, therefore they were judged as at unclear risk of bias.
Blinding
We judged most of the included studies as at unclear risk of performance and detection bias. Only one study masked both participants and investigators to the IOL implanted, and was judged as at low risk of performance bias (Jonker 2015). We judged Bozkurt Gencer 2021 as at high risk of bias because masking could not be done due to the nature of interventions (i.e. digital versus manual marking). We judged the remaining five studies as at unclear risk of bias for various reasons. Gil 2019 reported that their study was "double‐masked," but it was unclear who was masked and how th masking was conducted. Mojzis 2014 and Mojzis 2017 masked participants to IOL but did not report whether study personnel were masked. Cochener 2016 and Kaymak 2017 did not report if participants or study personnel were masked.
The primary outcome of this review was mean uncorrected distance visual acuity at one‐year follow‐up. Only one study reported that all postoperative outcome assessments were performed by an independent observer who was masked to the intervention (Kaymak 2017); we judged this study to be at low risk of detection bias. The remaining six studies did not report masking outcome assessors and were therefore judged as at unclear risk of detection bias.
Incomplete outcome data
There were no missing outcome data in Bozkurt Gencer 2021; we judged this study as at low risk of bias. Kaymak 2017 excluded six eyes, three (9%) of participants from the analysis, because participants withdrew after IOL implantation. Given that this proportion of the total eyes/participants was small, we judged Kaymak 2017 as at low risk of attrition bias. The remaining five studies did not report attrition information and were therefore judged as at unclear risk of attrition bias.
Selective reporting
In two studies some outcomes were reported incompletely (Bozkurt Gencer 2021; Gil 2019). We judged these studies as having unclear risk of reporting bias. Although the remaining five studies had no trial registration or published protocol, the authors reported all outcomes specified in the methods section of the study, therefore we judged these studies as at low risk of reporting bias.
Other potential sources of bias
In two of the seven included studies, at least one study investigator had a financial relationship with manufacturers of one of the intervention devices (Jonker 2015; Kaymak 2017). The remaining five studies appeared to be free from other sources of bias.
Effects of interventions
See: Table 1
The only comparison in this review was trifocal compared with bifocal IOL implantation during cataract surgery for visual acuity among presbyopic participants. All seven included studies reported data on at least one of our prespecified outcomes.
Mean uncorrected distance visual acuity (UDVA)
At three months, data were available from only two studies. Participants treated with trifocal IOL implantation probably experienced more of an improvement in UDVA than in those treated with bifocal IOL (mean difference (MD) −0.04, 95% confidence interval (CI) −0.08 to 0.00; I2 = 0%; 2 studies, 92 participants; Analysis 1.1; Figure 3). The certainty of the evidence was moderate, downgraded for risk of bias. The beneficial effect of trifocal IOL for this outcome persisted at six months (MD −0.04, 95% CI −0.07 to 0.00; I2 = 53%; 5 studies, 257 participants; Analysis 1.1; Figure 3). The certainty of the evidence was moderate, downgraded for risk of bias. However, the observed beneficial effect of trifocal IOL disappeared at one year (MD 0.00, 95% CI −0.04 to 0.04; I2 = 0%; 2 studies, 107 participants; Analysis 1.1; Figure 3), the primary outcome of our review. The certainty of the evidence was low, downgraded for imprecision and risk of bias.
1.1. Analysis.

Comparison 1: Trifocal versus bifocal intraocular lenses after cataract extraction, Outcome 1: Mean uncorrected distance visual acuity (LogMAR)
3.

Forest plot of comparison: 1 Trifocal versus bifocal intraocular lenses after cataract extraction, outcome: 1.1 Mean uncorrected distance visual acuity (LogMAR).
Mean uncorrected near visual acuity (UNVA)
At three months, pooled data from two studies suggested that trifocal IOL implantation had no effect on UNVA compared with bifocal IOL (MD −0.08, 95% CI −0.20 to 0.03; I2 = 84%; 2 studies, 92 participants; Analysis 1.2; Figure 4). There was considerable statistical heterogeneity, and the certainty of the evidence was very low, downgraded for risk of bias, imprecision, and inconsistency. We observed similar findings at six months (MD −0.01, 95% CI −0.05 to 0.02; I2 = 50%; 5 studies, 257 participants; Analysis 1.2; Figure 4) and at one year (MD 0.01, 95% CI −0.04 to 0.06; I2 = 0%; 2 studies, 107 participants; Analysis 1.2; Figure 4). Estimates at both time points were imprecise. The certainty of the evidence was very low at six months (downgraded for risk of bias, imprecision, and inconsistency) and low at one year (downgraded for imprecision and risk of bias).
1.2. Analysis.

Comparison 1: Trifocal versus bifocal intraocular lenses after cataract extraction, Outcome 2: Mean uncorrected near visual acuity (LogMAR)
4.

Forest plot of comparison: 1 Trifocal versus bifocal intraocular lenses after cataract extraction, outcome: 1.2 Mean uncorrected near visual acuity (LogMAR).
Mean uncorrected intermediate visual acuity (UIVA)
At three months, data were available for two studies. Participants treated with trifocal IOL implantation may experience more improvement in UIVA than those treated with bifocal IOL (MD −0.19, 95% CI −0.29 to −0.09; I2 = 52%; 2 studies, 92 participants; Analysis 1.3; Figure 5). The certainty of the evidence was low, downgraded for risk of bias and imprecision. Although the observed beneficial effect of trifocal IOL implantation disappeared at six months (MD −0.15, 95% CI −0.31 to 0.01; I2 = 97%; 5 studies, 256 participants; Analysis 1.3; Figure 5), the data should be interpreted with caution, as the I2 statistic indicates substantial heterogeneity. Data from two studies suggest that trifocal IOL implantation may improve UIVA more than bifocal IOL at one year (MD −0.16, 95% CI −0.22 to −0.10; I2 = 0%; 2 studies, 107 participants; Analysis 1.3; Figure 5). The certainty of the evidence was very low at six months (downgraded for risk of bias, imprecision, and inconsistency) and low at one year (downgraded for risk of bias and imprecision).
1.3. Analysis.

Comparison 1: Trifocal versus bifocal intraocular lenses after cataract extraction, Outcome 3: Mean uncorrected intermediate visual acuity (LogMAR)
5.

Forest plot of comparison: 1 Trifocal versus bifocal intraocular lenses after cataract extraction, outcome: 1.3 Mean uncorrected intermediate visual acuity (LogMAR).
Mean best‐corrected distance visual acuity (BCDVA)
At three months, available data from two studies suggested that trifocal IOL implantation had no effect on BCDVA compared to bifocal IOL (MD −0.03, 95% CI −0.06 to 0.01; I2 = 0%; 2 studies, 92 participants; Analysis 1.4; Figure 6). Pooled analysis of six studies suggested that trifocal IOL implantation may have a slight effect on BCDVA at six months compared with bifocal IOL (MD −0.01, 95% CI −0.02 to 0.00; I2 = 2%; 6 studies, 352 participants; Analysis 1.4; Figure 6). The estimates at both time points were also imprecise. The certainty of the evidence at both three months and six months was low, downgraded for risk of bias and imprecision. At one year, available data from two studies suggested no evidence of an effect of trifocal IOL compared with bifocal IOL (MD 0.00, 95% CI −0.03 to 0.04; I2 = 0%; 2 studies, 107 participants; Analysis 1.4; Figure 6). The certainty of the evidence was low, downgraded for risk of bias and imprecision.
1.4. Analysis.

Comparison 1: Trifocal versus bifocal intraocular lenses after cataract extraction, Outcome 4: Mean best‐corrected distance acuity (LogMAR)
6.

Forest plot of comparison: 1 Trifocal versus bifocal intraocular lenses after cataract extraction, outcome: 1.4 Mean best‐corrected distance visual acuity (LogMAR).
Contrast sensitivity
Three studies assessed contrast sensitivity. However, at three months only one study reported data on mean contrast sensitivity in log contrast sensitivity function (logCSF) scale under both photopic and mesopic conditions at four spatial frequencies: 3, 6, 12, and 18 cycles per degree (Jonker 2015). Point estimates suggested no evidence of a difference in contrast sensitivity between groups under photopic conditions at all four spatial frequencies. The certainty of the evidence was very low, downgraded for risk of bias and imprecision (−2). However, when contrast sensitivity was measured under mesopic conditions, point estimates suggested that trifocal IOL implantation showed no evidence of a difference in contrast sensitivity at all spatial frequencies (data not shown), except one (6 cycles per degree), where participants treated with trifocal IOL did worse compared with those in the bifocal IOL group (MD −0.19, 95% CI −0.33 to −0.05; 1 study, 25 participants; Analysis 1.5). The certainty of the evidence was low, downgraded for risk of bias and imprecision. At three months, Mojzis 2014 observed no evidence of a difference between groups for contrast sensitivity for most frequencies analyzed. The authors of Mojzis 2017 reported observing minimal difference in contrast sensitivity between groups, which was significant only for low to medium spatial frequencies, at six months but not at one year.
1.5. Analysis.

Comparison 1: Trifocal versus bifocal intraocular lenses after cataract extraction, Outcome 5: Mean contrast sensitivity
Quality of life
One study examined vision‐related quality of life using the NEI‐VFQ‐25 at six months (Bozkurt Gencer 2021). Analysis suggested no evidence of a difference between trifocal and bifocal IOLs (MD 1.41, 95% CI −1.78 to 4.60; 1 study, 40 participants; Analysis 1.6). One study evaluated patient satisfaction and quality of life using the VF‐14 questionnaire (Gil 2019), and three studies assessed other visual function such as reading speed (Jonker 2015), reading performance (Kaymak 2017), and spectacle independence (Cochener 2016; Jonker 2015). However, none of these studies provided data in a format that permitted formal analysis. In Jonker 2015, reading speed was assessed at six months using the Radner reading chart. Investigators found no evidence of a difference between groups in mean reading distance, mean reading speed, or maximum reading speed under 70% and 100% contrast. At six months, Kaymak 2017 observed no evidence of a difference between groups in reading performance. Similarly, investigators found no evidence of a difference between groups in spectacle dependence score at all distances (P ≥ 0.296) (Kaymak 2017). At six months, Cochener 2016 found that spectacle independence and patient satisfaction may be higher in the trifocal compared with the bifocal IOL group. In addition, Jonker 2015 observed that at six months all participants were spectacle‐free for distance, and 80% of participants in the trifocal group (12 participants) reported complete spectacle independence at six months compared to 50% (6 participants) in the bifocal group, suggesting good‐quality vision. The certainty of the evidence for these outcomes in each study at the time points examined was low, downgraded for risk of bias and imprecision.
1.6. Analysis.

Comparison 1: Trifocal versus bifocal intraocular lenses after cataract extraction, Outcome 6: Mean quality of life
Adverse events
One study reported that no intraoperative or postoperative complications had occurred (Cochener 2016). Jonker 2015 reported that "side effects of trifocal IOLs such as glare and halos was similar to preoperative measurements." Another study reported that four eyes (11.4%) in the bifocal group and three eyes (7.5%) in the trifocal group developed significant posterior capsular opacification requiring YAG capsulotomy (Mojzis 2017). In Bozkurt Gencer 2021, four (20%) participants in trifocal group and 10 (50%) participants in bifocal group had glare complaints (risk ratio 0.40, 95% CI 0.15 to 1.07; 40 participants; Analysis 1.7). Gil 2019 reported that no posterior capsular opacification was observed at six months. The remaining two studies did not report on adverse events such as glare/halos. The certainty of the evidence for adverse events was low, downgraded for risk of bias and imprecision.
1.7. Analysis.

Comparison 1: Trifocal versus bifocal intraocular lenses after cataract extraction, Outcome 7: Adverse outcomes
Discussion
Summary of main results
We identified two new trials in this update, for a total of seven studies with 331 participants that compared trifocal versus bifocal IOLs implantation during cataract surgery among participants 30 years or older with cataract and presbyopia. After reviewing the available evidence, we summarized our findings in Table 1 for the main comparison.
The evidence was not consistent across outcomes and time points. Moderate‐certainty evidence shows that trifocal IOL implantation probably results in more improvement in UDVA at three and six months after surgery than bifocal IOL, but with no evidence of an effect at one year. We found no evidence of a difference in UNVA between trifocal and bifocal IOL implantation at three months, six months, and one year after surgery. Trifocal IOL implantation may improve UIVA compared with bifocal IOL at three months and one year, but the evidence is very uncertain at six months after surgery. Trifocal IOL implantation showed a slight effect on BCDVA compared with bifocal IOL at six months, but not at three months and one year. There was also no evidence of a difference between trifocal and bifocal IOL implantation for contrast sensitivity, although the data from one study suggested that contrast sensitivity may be worse in the trifocal group at three months in one of the four spatial frequencies under mesopic conditions. There was no evidence of an important difference in vision‐related quality of life or visual function, except spectacle independence and patient satisfaction, which favored the trifocal IOL group. Treatment with trifocal IOL appears to be well tolerated, as fewer eyes in the trifocal compared with the bifocal IOL group developed complications such as glare complaints and significant posterior capsular opacification requiring YAG capsulotomy.
Overall completeness and applicability of evidence
The included studies differed in a number of characteristics. Participants were recruited predominantly in Europe, and most participants were women. The evidence from this review may not be applicable to certain racial groups or genders, as well as people with certain ocular pathologies, as these groups were either underrepresented or excluded in the primary studies that contributed data to the review.
Quality of the evidence
The certainty of the evidence was mostly low across the outcomes examined in this review. Most studies did not report how the random sequence was generated or the method of concealing treatment allocation. We assessed most studies as at unclear risk of performance and detection bias. Attrition bias was unclear for most studies. However, risk of bias for selective outcome reporting was low for most studies. Other limitations to the certainty of the evidence included wide confidence interval of the effect estimates, resulting in downgrading for risk of bias and imprecision. In addition to aspects of study design, we considered financial support as a potential source of bias. Two of the seven included studies were sponsored by manufacturers of one of the study lenses under investigation, and some study investigators reported that they had a financial relationship with industry that marketed the study lens.
Potential biases in the review process
We performed a very broad literature search of multiple electronic databases with the help of an Information Specialist. To reduce potential bias arising during study selection, risk of bias assessment, and data extraction, two review authors, working independently, completed all steps of the review process outlined in the Methods section of this review. We decided post hoc to include studies with a two‐eye design; none of these studies reported on how correlation between eyes from the two‐eye design was accounted for in their analysis. Analyses that do not account for the correlation between eyes may appear to have more information than there actually is, and can overestimate the treatment effect with a false increase in precision (Murdoch 1998). Our post hoc decision to include data from studies with a two‐eye design may have artificially overestimated the magnitude of the effect estimate, since four of the seven included studies in this review used a two‐eye design.
Agreements and disagreements with other studies or reviews
Our review is generally in agreement with other systematic reviews comparing bifocal and trifocal IOL. Jin 2019 included four RCTs and four cohort studies (489 eyes, 245 participants) that compared trifocal with bifocal IOL implantation among participants undergoing cataract surgery. The study authors found that trifocal IOLs improve intermediate visual acuity, but that there was no difference between groups in distance or near visual acuity. Similar results were reported by Xu and colleagues (Xu 2017), who found improvement in intermediate vision in favor of trifocal compared with bifocal IOL. They observed that distant and near vision did not differ between groups at six months postoperatively (Xu 2017). Yoon 2018 also reported improvement in intermediate vision and no evidence of a difference between trifocal and bifocal IOL implantation for distance and near vision. Additionally, evidence from Yang 2018 supported no evidence of a difference between trifocal and bifocal IOL in distance and near vision. Shen 2017 also observed results in favor of trifocal IOL implantation for intermediate vision.
Zhang 2021 included nine studies with 440 participants undergoing cataract surgery in a recent systematic review. They reported that the trifocal IOL group showed improvement compared with the bifocal IOL group in near and intermediate vision, but not distance vision (time points varied from three to 12 months). The difference in findings from our review was due to study selection. We excluded Postolache 2015 because it was not a RCT, and did not include Bilbao‐Calabuig 2016 because it applied mix‐and‐match implantation in the bifocal group (different bifocal in the same participant). Additionally, Zhang 2021 separately included Kaymak 2017 and Alió 2018 (under the study references of Kaymak 2017) in the meta‐analyses, although these two studies used the same cohorts.
Authors' conclusions
Implications for practice.
We found low‐certainty evidence for our comparison of trifocal versus bifocal intraocular lens (IOL) implantation among participants with presbyopia undergoing cataract surgery. Trifocal IOL may result in better intermediate distance visual acuity at one year when compared with bifocal IOLs. However, there was no evidence of a difference between trifocal and bifocal IOL for uncorrected distance visual acuity, uncorrected near visual acuity, and best‐corrected visual acuity at one year. Information on contrast sensitivity, quality of life, and adverse events was sparse. Caution is therefore advised in the use of the current evidence in clinical practice decisions, considering the above limitations of the evidence. Such decisions should be based on patient preferences and provider judgement, given the variability of the results and risk of bias in the studies relevant to this topic.
Implications for research.
Given the increasing interest in trifocal IOLs and in comparison with other presbyopic correcting options during cataract surgery, future research should compare trifocal IOL and specific bifocal IOLs that correct intermediate visual acuity in order to evaluate important outcomes such as visual acuity, contrast sensitivity, vision‐related adverse effects, and quality of life. As trifocal IOLs have rings to achieve far, intermediate, and near distances within the structure of the IOL, this may affect contrast sensitivity and increase the risk of adverse events such as halos. However, evidence regarding contrast sensitivity, quality of life, and adverse events is limited. We believe that evaluation of these outcomes is needed to permit definitive conclusions regarding the benefit and harm of trifocal compared with bifocal IOL in clinical practice. Future research should examine these outcomes as well as incidence of adverse events such as glare/halos. The methods for the evaluation of adverse effects and quality of life are diverse, which may imply a limitation for a clinical final decision between trifocal or bifocal IOL.
What's new
| Date | Event | Description |
|---|---|---|
| 31 March 2022 | New search has been performed | Issue 3, 2022: electronic searches were updated. |
| 31 March 2022 | New citation required but conclusions have not changed | We included two new studies (Bozkurt Gencer 2021; Gil 2019). |
History
Protocol first published: Issue 5, 2017 Review first published: Issue 6, 2020
Acknowledgements
We thank Lori Rosman, Information Specialist for Cochrane Eyes and Vision (CEV), who created and executed the electronic search strategies, and Lisa Winer, copy‐editor, for her valuable suggestions and edits. We also thank the CEV editorial team for their comments on the review; Anupa Shah, Managing Editor for CEV, for support and guidance in preparation of this review.
We are grateful to the following peer reviewers for their time and comments: Jithin Yohannan (Wilmer Eye Institute), Sandra Finestone (Association of Cancer Patient Educators), and one peer reviewer who wished to remain anonymous.
We would like to acknowledge the contributions of Drs Karla Zúñiga‐Posselt and Samuel A Abariga to the previous version of the review (Zamora‐de La Cruz 2020).
This review update was managed by CEV@US and signed off for publication by Tianjing Li and Gianni Virgili.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Cataract] explode all trees #2 MeSH descriptor: [Cataract Extraction] explode all trees #3 MeSH descriptor: [Pseudophakia] explode all trees #4 pha?oemulsif* or (pha?o next/1 emulsif*) or Capsulorhexis or Capsulorrhexis #5 phakectom* or lensectom* #6 Pseudophak* #7 (extract* or aspirat* or operat* or remov* or surg* or excis* or implant*) near/4 (cataract*) #8 (extract* or aspirat* or operat* or remov* or surg* or excis*) near/4 (lens*) #9 {or #1‐#8} #10 MeSH descriptor: [Lens Implantation, Intraocular] explode all trees #11 MeSH descriptor: [Lenses, Intraocular] explode all trees #12 MeSH descriptor: [Pseudophakia] explode all trees #13 Pseudophak* #14 (artificial* or implant* or acrylic) near/4 (lens*) #15 artificial near/2 device* #16 (intra*ocular or intra ocular) near/3 lens* #17 (phakic near/3 lens*) #18 IOL* #19 {or #10‐#18} #20 multifocal* or (multi next/1 focal*) or bifocal* or (bi next/1 focal*) #21 trifocal* or (tri next/1 focal*) #22 diffractive* or refractive* #23 toric* or finevision or "AT LISA tri 839MP" or "AT.LISA tri 839 MP" or "MIOL‐Record" or MFIOL or "AcrySof IQ PanOptix" #24 {or #20‐#23} #25 #9 and #19 and #24
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Cataract Extraction/ 13. exp Cataract/ 14. exp Pseudophakia/ 15. (pha?oemulsif* or (pha?o adj1 emulsif*) or Capsulorhexis or Capsulorrhexis).tw. 16. (phakectom* or lensectom*).tw. 17. Pseudophak*.tw. 18. ((extract* or aspirat* or operat* or remov* or surg* or excis* or implant*) adj4 cataract*).tw. 19. ((extract* or aspirat* or operat* or remov* or surg* or excis*) adj4 lens*).tw. 20. or/12‐19 21. exp Lens Implantation, Intraocular/ 22. Lenses, Intraocular/ 23. exp Pseudophakia/ 24. Pseudophak*.tw. 25. ((artificial* or implant* or acrylic) adj4 lens*).tw. 26. (artificial adj2 device*).tw. 27. ((intra*ocular or "intra ocular") adj3 lens*).tw. 28. (phakic adj3 lens*).tw. 29. IOL*.tw. 30. or/21‐29 31. (multifocal* or (multi adj1 focal*) or bifocal* or (bi adj1 focal*)).tw. 32. (trifocal* or (tri adj1 focal*)).tw. 33. (diffractive* or refractive*).tw. 34. (toric* or finevision or "AT LISA tri 839MP" or "AT.LISA tri 839 MP" or "MIOL‐Record" or MFIOL or "AcrySof IQ PanOptix").tw. 35. or/31‐34 36. 20 and 30 and 35 37. 11 and 36
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'cataract'/exp #34 'cataract extraction'/exp #35 'pseudophakia'/exp #36 pha*oemulsif*:ab,ti OR (pha*o NEXT/1 emulsif*):ab,ti OR capsulorhexis:ab,ti OR capsulorrhexis:ab,ti #37 phakectom*:ab,ti OR lensectom*:ab,ti #38 pseudophak*:ab,ti #39 ((extract* OR aspirat* OR operat* OR remov* OR surg* OR excis* OR implant*) NEAR/4 cataract*):ab,ti #40 ((extract* OR aspirat* OR operat* OR remov* OR surg* OR excis*) NEAR/4 lens*):ab,ti #41 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 #42 'lens implantation'/exp #43 'lens implant'/exp #44 'pseudophakia'/exp #45 pseudophak*:ab,ti #46 ((artificial* OR implant* OR acrylic) NEAR/4 lens*):ab,ti #47 (artificial NEAR/2 device*):ab,ti #48 ((intra*ocular OR 'intra ocular') NEAR/3 lens*):ab,ti #49 (phakic NEAR/3 lens*):ab,ti #50 iol*:ab,ti #51 #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 #52 multifocal*:ab,ti OR (multi NEXT/1 focal*):ab,ti OR bifocal*:ab,ti OR (bi NEXT/1 focal*):ab,ti #53 trifocal*:ab,ti OR (tri NEXT/1 focal*):ab,ti #54 diffractive*:ab,ti OR refractive*:ab,ti #55 toric*:ab,ti OR finevision:ab,ti OR 'at lisa tri 839mp':ab,ti OR 'at.lisa tri 839 mp':ab,ti OR 'miol‐record':ab,ti OR mfiol:ab,ti OR 'acrysof iq panoptix':ab,ti #56 #52 OR #53 OR #54 OR #55 #57 #41 AND #51 AND #56 #58 #32 AND #57
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 phacoemulsif*[tw] OR phakoemulsif*[tw] OR phaco emulsif*[tw] OR phako emulsif*[tw] OR Capsulorhexis[tw] OR Capsulorrhexis[tw] #3 phakectom*[tw] OR lensectom*[tw] #4 Pseudophak*[tw] #5 (extract*[tw] OR aspirat*[tw] OR operat*[tw] OR remov*[tw] OR surg*[tw] OR excis*[tw] OR implant*[tw]) AND (cataract*[tw]) #6 (extract*[tw] OR aspirat*[tw] OR operat*[tw] OR remov*[tw] OR surg*[tw] OR excis*[tw]) AND (lens*[tw]) #7 #2 OR #3 OR #4 OR #5 OR #6 #8 Pseudophak*[tw] #9 (artificial*[tw] OR implant*[tw] OR acrylic[tw]) AND (lens*[tw]) #10 artificial[tw] AND device*[tw] #11 (intraocular[tw] or intra ocular[tw]) AND lens*[tw] #12 (phakic[tw] AND lens*[tw]) #13 IOL*[tw] #14 #8 OR #9 OR #10 OR #11 OR #12 OR #13 #15 multifocal*[tw] OR multi focal*[tw] OR bifocal*[tw] OR bi focal*[tw] #16 trifocal*[tw] OR tri focal*[tw] #17 diffractive*[tw] OR refractive*[tw] #18 toric*[tw] OR finevision[tw] OR "AT LISA tri 839MP"[tw] OR "AT.LISA tri 839 MP"[tw] OR "MIOL‐Record"[tw] OR MFIOL[tw] OR "AcrySof IQ PanOptix"[tw] #19 #15 OR #16 OR #17 OR #18 #20 #7 AND #14 AND #19 #21 #1 AND #20 #22 Medline[sb] #23 #21 NOT #22
Appendix 5. LILACS search strategy
(MH:C11.510.245$ OR MH:E04.540.825.249$ OR MH:C23.888.681$ OR phaco$ OR phako$ OR Capsulorhexis or Capsulorrhexis OR phakectom$ OR lensectom$ OR Pseudophak$ OR cataract$) AND (MH:E04.540.825.600$ OR MH:E07.632.500.460$ OR MH:E07.695.460$ OR MH:VS2.006.001.009.003$ OR MH:C23.888.681$ OR Pseudophak$ OR IOL$ OR ((artificial$ OR implant$ OR acrylic OR intraocular OR "intra ocular" OR phakic) AND lens$) OR "artificial device" OR "artificial devices") AND (multifocal$ OR "multi focal" OR "multi focals" OR bifocal$ OR "bi focal" OR "bi focals" OR trifocal$ OR "tri focal" OR "tri focals" OR diffractive$ OR refractive$ OR toric$ OR finevision OR "AT LISA tri 839MP" OR "AT.LISA tri 839 MP" OR "MIOL‐Record" OR MFIOL OR "AcrySof IQ PanOptix")
Appendix 6. ClinicalTrials.gov search strategy
(cataract OR phacoemulsification OR pseudophakia) AND (trifocal OR bifocal OR multifocal OR diffractive OR refractive OR toric OR finevision OR "AT LISA tri 839MP" OR "AT.LISA tri 839 MP" OR "MIOL‐Record" OR MFIOL OR "AcrySof IQ PanOptix")
Appendix 7. WHO ICTRP search strategy
Cataract AND trifocal OR cataract AND bifocal OR cataract AND multifocal OR cataract AND diffractive OR cataract AND refractive OR phacoemulsification AND trifocal OR phacoemulsification AND bifocal OR phacoemulsification AND multifocal OR phacoemulsification AND diffractive OR phacoemulsification AND refractive OR pseudophakia AND trifocal OR pseudophakia AND bifocal OR pseudophakia AND multifocal OR pseudophakia AND diffractive OR pseudophakia AND refractive OR Cataract AND toric OR cataract AND finevision OR cataract AND "AT LISA tri 839MP" OR cataract AND "AT.LISA tri 839 MP" OR cataract AND "MIOL‐Record" OR cataract AND MFIOL OR cataract AND "AcrySof IQ PanOptix" OR phacoemulsification AND toric OR phacoemulsification AND finevision OR phacoemulsification AND "AT LISA tri 839MP" OR phacoemulsification AND "AT.LISA tri 839 MP" OR phacoemulsification AND "MIOL‐Record" OR phacoemulsification AND MFIOL OR phacoemulsification AND "AcrySof IQ PanOptix" OR pseudophakia AND toric OR pseudophakia AND finevision OR pseudophakia AND "AT LISA tri 839MP" OR pseudophakia AND "AT.LISA tri 839 MP" OR pseudophakia AND "MIOL‐Record" OR pseudophakia AND MFIOL OR pseudophakia AND "AcrySof IQ PanOptix"
Data and analyses
Comparison 1. Trifocal versus bifocal intraocular lenses after cataract extraction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean uncorrected distance visual acuity (LogMAR) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 3 months | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.08, ‐0.00] |
| 1.1.2 6 months | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.00] |
| 1.1.3 12 months | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 1.2 Mean uncorrected near visual acuity (LogMAR) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 3 months | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.20, 0.03] |
| 1.2.2 6 months | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.02] |
| 1.2.3 12 months | 2 | 107 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 1.3 Mean uncorrected intermediate visual acuity (LogMAR) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 3 months | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.29, ‐0.09] |
| 1.3.2 6 months | 5 | 256 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.31, 0.01] |
| 1.3.3 12 months | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.22, ‐0.10] |
| 1.4 Mean best‐corrected distance acuity (LogMAR) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 3 months | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.06, 0.01] |
| 1.4.2 6 months | 6 | 352 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.00] |
| 1.4.3 12 months | 2 | 107 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
| 1.5 Mean contrast sensitivity | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.1 Mesopic: 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Mean quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.7 Adverse outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.1 Visual disturbance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bozkurt Gencer 2021.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 80 eyes of 40 participants in total; 40 eyes of 20 participants to each group Exclusions after randomization: none Losses to follow‐up: none Number analyzed (total and per group): 80 eyes of 40 participants in total; 40 eyes of 20 participants in each group Unit of analysis: participant How were the missing data handled?: not applicable Power calculation: no |
|
| Participants |
Country: Turkey Setting: university hospital Age: Trifocal toric IOL: mean (SD): 63.7 (11.11) years, range 47 to 81 years Bifocal toric IOL: mean (SD): 54.66 (11.98), range 36 to 74 years Sex: Trifocal toric IOL: 7 men and 13 women Bifocal toric IOL: 10 each Inclusion criteria: low vision in both eyes and best‐corrected distance visual acuity (CDVA) of ≤ 0.5 according to Snellen chart examination Exclusion criteria: diabetes mellitus, hypertension, optic neuritis, glaucoma, diabetic retinopathy, age‐related macular degeneration, pseudoexfoliation, pterygium, strabismus, corneal nephelion, or a history of ophthalmic surgery Equivalence of baseline characteristics: comparable in age and sex |
|
| Interventions |
Intervention 1 (Trifocal toric IOL): VERION digital microscope‐mounted marking system was applied with Acriva Reviol tri‐ED trifocal toric IOL (Amsterdam, the Netherlands) Intervention 2: Acriva Reviol bifocal toric IOL (Amsterdam, the Netherlands) was implanted with the conventional corneal marking method Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes, as defined:
Secondary outcomes: spherical mean values, cylindrical mean values, mean scores of visual function scale (VFQ25), glasses prescription, contrast sensitivity, Titmus Stereotest, IOL rotation |
|
| Notes |
Institution: Antalya Training and Research Hospital Email: mervebozkrt@gmail.com Address: Ophthalmology Clinic, Antalya Training and Research Hospital, Antalya 07100, Turkey Study period: September 2016 to May 2017 Funding sources: not reported Declarations of interest: authors declare none Reported subgroup analyses: not reported Trial registration number: the Universal Trial Number (UTN): U1111‐1264‐1020 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Masking could not be done: digital marking versus manual marking in different groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol or study registration available; however, the authors reported all outcomes specified in the methods section of the study. Contrast sensitivity results were displayed on the graph only. |
| Other bias | Low risk | No evidence of any other sources of bias detected. |
Cochener 2016.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: total 54 eyes of 27 participants; FineVision trifocal: 30 eyes of 15 participants; Tecnis bifocal: 24 eyes of 12 participants Exclusions after randomization: none Losses to follow‐up: none Number analyzed (total and per group): total 54 eyes of 27 participants; FineVision trifocal: 30 eyes of 15 participants; Tecnis bifocal: 24 eyes of 12 participants Unit of analysis: participant How were the missing data handled?: not reported Power calculation: yes, "to find a clinically significant difference between the two groups, investigators assumed a difference of 0.2 logMAR, and based on alpha of 0.05 and power of 0.8, it was determined that 12 patients were required for each group" |
|
| Participants |
Country: France Setting: not reported Age: FineVision trifocal: mean (SD): 58.7 (6.4) years Tecnis bifocal: mean (SD): 60.6 (9.1) years Sex: not reported Inclusion criteria: age > 55 years, starting to show clouding of the crystalline lens (Lens Opacities Classification System III classification global score 2 or greater), corneal astigmatism of 1.00 D or less Exclusion criteria: pre‐existing amblyopia, maculopathy, optic neuropathy or glaucoma, previous retinal detachment, or unrealistic expectations regarding the outcome of the surgery Equivalence of baseline characteristics: participants in the FineVision group were more myopic, whereas those in the Tecnis group were more hyperopic |
|
| Interventions |
Intervention 1: FineVision Micro F trifocal IOL (PhysIOL, Liege, Belgium) Intervention 2: TecnisZMB00 bifocal IOL (Abbott Medical Optics, Santa Ana, CA) Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes, as defined:
Secondary outcomes: not reported |
|
| Notes |
Institution: Morvan University Hospital Email: beatrice.cochener@ophtalmologie‐chu29.fr Address: Department of Ophthalmology, CHU Morvan, 2 Av Foch 29609 Brest Cedex, France Study period: not reported Funding sources: not reported Declarations of interest: authors declare no financial or proprietary interest in study Reported subgroup analyses: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization for the study was performed using the software provided by www.random.org, with the FineVision IOL assigned as 1 and the Tecnis IOL assigned as 2. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not describe treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants or study investigators was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not report any losses to follow‐up, and it was unclear whether all participants were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Low risk | No published protocol or study registration available; however, the authors reported all outcomes specified in the methods section of the study. |
| Other bias | Low risk | No evidence of any other sources of bias detected. |
Gil 2019.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 116 participants in total; 19 to SV25T0, 18 to ATLISA 809M, 19 to ATLISA Tri 839MP, 20 to ZKB00, 20 to ZLB00, and 20 to Symfony ZXR00. Exclusions after randomization: not explicitly reported Losses to follow‐up: not explicitly reported Number analyzed (total and per group): 116 participants in total; 19 to SV25T0, 18 to ATLISA 809M, 19 to ATLISA Tri 839MP, 20 to ZKB00, 20 to ZLB00, and 20 to Symfony ZXR00 Unit of analysis: participant (1 eye per participant was chosen for analysis) How were the missing data handled?: not reported Power calculation: no |
|
| Participants |
Country: Spain Setting: Ophthalmology Department, Santa Creu and Sant Pau Hospital Age: Trifocal toric IOL: ATLISATri 839MP: mean (SD): ATLISA 839M: 68.7 (10.3) years Bifocal toric IOL: AcrySof ReSTOR SV25T0: mean (SD): 74.3 (7.5) years Bifocal toric IOL: Tecnis ZKB00: mean (SD): 68.9 (12.9) years Bifocal toric IOL: Tecnis ZLB00: mean (SD): 73.3 (4.6) years Bifocal toric IOL: ATLISA 809M: mean (SD): 71.6 (7.1) years Sex: Trifocal toric IOL: ATLISATri 839MP: 4 men and 15 women Bifocal toric IOL: AcrySof ReSTOR SV25T0: 8 men and 11 women Bifocal toric IOL: Tecnis ZKB00: 5 men and 15 women Bifocal toric IOL: Tecnis ZLB00: 7 men and 13 women Bifocal toric IOL: ATLISA 809M: 4 men and 14 women Inclusion criteria: age over 60 years, bilateral cataract, good motivation for spectacle independence, potential visual acuity of 20/25 or better, and preoperative corneal astigmatism ≤ 1.25 D Exclusion criteria: preoperative exclusion criteria were ocular fundus abnormalities, such as retinal detachment, history of glaucoma, dry eye requiring ophthalmologic treatment, and corneal pathologies. Patients with a history of intraocular or corneal surgery, irresponsive or abnormal pupils, or diabetic retinopathy were also excluded. Intra‐operative exclusion criteria were any surgical complication, including pupillary trauma and loss of vitreous. Postoperatively, patients were excluded if the lens could not be placed in the capsular bag. Patients were also excluded if they reported critical visual demands, such as night drivers, and if they had difficulty attending the required follow‐up visits. Equivalence of baseline characteristics: "No statistically significant differences were found in any of these variables amongst groups." |
|
| Interventions |
Trifocal toric IOL: ATLISATri 839MP (trifocal, anterior surface with a diffractive profile) Bifocal toric IOL: AcrySof ReSTOR SV25T0 (bifocal, anterior aspheric apodized diffractive (3.4 mm) and refractive surface) Bifocal toric IOL: Tecnis ZKB00 (2.75 bifocal, anterior aspheric and posterior diffractive surface) Bifocal toric IOL: Tecnis ZLB00 (3.25 bifocal, anterior aspheric and posterior diffractive surface) Bifocal toric IOL: ATLISA 809M (bifocal, aspheric diffractive) Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes, as defined:
Secondary outcomes: defocus curves, photopic and mesopic pupil diameters, quality of life by the VF‐14 questionnaire |
|
| Notes |
Institution: Santa Creu and Sant Pau Hospital Email: genis.cardona@upc.edu Address: Ophthalmology Department, Santa Creu and Sant Pau Hospital, carrer de Sant Quintı´, 89, 08041 Barcelona, Spain Study period: not reported Funding sources: "This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors." Declarations of interest: "The authors declares that they have no conflict of interest." Reported subgroup analyses: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 6 IOL designs were assessed, including an extended depth of focus (EDOF) lens (Table 1). IOL implantation followed a 1:1:1:1:1:1 block randomization design determined with the IBM SPSS software 25.0 (IBM Corp, Armonk, NY) for Windows. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This study was a "double‐masked" design, but it is unclear who was masked and how masking was performed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The optometrist was masked to lens type." Masking of outcome assessor during the VF‐14 questionnaire assessment was not detailed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not report any losses to follow‐up, and it was unclear whether all participants were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Unclear risk | Uncorrected distance intermediate and near visions are not reported, VFQ14 quality of life outcome seems incomplete. |
| Other bias | Low risk | No evidence of any other sources of bias detected. |
Jonker 2015.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): total 56 eyes of 28 participants; FineVision trifocal: 30 eyes of 15 participants; AcrySof ReSTOR bifocal: 26 eyes of 13 participants Unit of analysis: eyes How were the missing data handled?: not reported Power calculation: yes, "to find a clinically significant difference between the two groups, investigators assumed a difference of 0.2 logMAR, and based on alpha of 0.05 and power of 0.8, it was determined that 12 patients were required for each group, while assuming a dropout rate of 15% on the primary outcome measure, this resulted in a total requirement of 28 patients" |
|
| Participants |
Country: the Netherlands Setting: University Eye Clinic Maastricht Age: FineVision trifocal: mean (SD): 62.6 (8.7) years AcrySof ReSTOR bifocal: mean (SD): 64.0 (8.8) years Sex: not reported Inclusion criteria: bilateral cataract, less than 1.0 D corneal astigmatism in both eyes, age > 42 years, and an expected postoperative corrected distance visual acuity of 0.3 LogMAR or less Exclusion criteria: combined ocular procedures, previous ocular surgery, ocular pathology that would limit postoperative visual outcome, suturing of the incision during surgery, and complications during surgery in the first eye Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: FineVision Micro F trifocal IOL (PhysIOL S.A.) Intervention 2: AcrySof ReSTOR IQ +3.0 bifocal IOL (Alcon Surgical Inc) Length of follow‐up: 6 months |
|
| Outcomes |
Primary outcomes, as defined: mean best‐corrected intermediate visual acuity at 6 months, LogMAR scale Secondary outcomes:
|
|
| Notes |
Institution: University Eye Clinic Maastricht Email: soraya.jonker@mumc.nl Address: University Eye Clinic Maastricht, Maastricht University Medical Center, P. Debyelaan 25, 6202 AZ Maastricht, the Netherlands Study period: not reported Funding sources: PhysIOL S.A., Liege, Belgium Declarations of interest: N Bauer received study grants from Alcon Laboratories, Carl Zeiss Meditec AG, and PhysIOL S.A. (study funder and creator of 1 of the tested lenses), and a lecture fee from Alcon Surgical (creator of 1 of the tested lenses). R Nuijts is a consultant to Alcon Surgical, Thea Pharma GmbH, and ASICO, and has received study grants from Acufocus, Alcon Surgical, Carl Zeiss Meditec AG, Ophtec BV, and PhysIOL S.A. Reported subgroup analyses: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors did not describe random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not describe treatment allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not report any losses to follow‐up, and it was unclear whether all participants were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Low risk | No published protocol or study registration available; however, the authors reported all outcomes specified in the methods section of the study. |
| Other bias | Unclear risk | At least 1 study investigator has a relationship with manufacturers of 1 of the intervention devices. |
Kaymak 2017.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: total 104 eyes of 52 participants; AT LISA bifocal: 38 eyes of 19 participants; AT LISA trifocal: 32 eyes of 16 participants, ReSTOR: 34 eyes of 17 participants Exclusions after randomization: not reported Losses to follow‐up: total 6 eyes of 3 participants; AT LISA bifocal: 4 eyes of 2 participants; AT LISA trifocal: 2 eyes of 1 participant, ReSTOR: none Number analyzed (total and per group): total 98 eyes of 47 participants; AT LISA bifocal: 34 eyes of 17 participants; AT LISA trifocal: 30 eyes of 15 participants, ReSTOR: 34 eyes of 17 participants Unit of analysis: eyes How were the missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: Spain, Germany, France Setting: Vissum Instituto (Spain), Breyer Kaymak und Klabe Augenchirurgie (Germany), Hopital Morvan (France) Age: AT LISA bifocal group: mean (SD): 64.4 (7.5) years AT LISA trifocal group: mean (SD): 62.5 (6.9) years ReSTOR group: mean (SD): 62.4 (8.9) years Sex AT LISA bifocal group: n (%): 11 females (57.9%) AT LISA trifocal group: n (%): 12 females (75.0%) ReSTOR group: n (%): 8 females (47.1%) Inclusion criteria: patients with cataract who seek spectacle independence, aged 50 to 80 years, pre‐existing refractive corneal astigmatism of less than 1.00 D Exclusion criteria: degenerative visual disorders that permanently limit the corrected distance visual acuity to 0.3 LogMAR (Snellen 20/40) or worse, glaucoma and/or intraocular pressure greater than 24 mmHg, intraoperative complications, and any other at‐risk pathology Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: AT LISA 809M bifocal IOL (Carl Zeiss Meditec) Intervention 2: AT LISA tri 839MP trifocal IOL (Carl Zeiss Meditec) Intervention 3: ReSTOR SN6AD1 bifocal IOL (Alcon Laboratories Inc) Length of follow‐up: 1 year |
|
| Outcomes |
Primary outcomes, as defined: not reported Secondary outcomes:
|
|
| Notes |
Institution: Breyer Kaymak und Klabe Augenchirurgie Email: h.kaymak@augenchirurgie.clinic Address: Berliner Allee 15, 40212 Dusseldorf, Germany Study period: not reported Funding sources: Carl Zeiss Meditec AG, Berlin, Germany Declarations of interest: authors state no proprietary or financial interest in study; however, the funder is the maker of AT LISA IOLs (Intervention 1 and Intervention 2) Reported subgroup analyses: none reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization code was administered centrally using a vocal server. |
| Allocation concealment (selection bias) | Low risk | "The randomization code was administered centrally using a vocal server and allocated to the patient in the chronological order of the surgery" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All participants were unaware of the implanted lens type, but the authors do not report whether investigators were masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent observer performed the postoperative measurements in each center. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors excluded 6 eyes of 3 (9%) of participants from the final analysis because the participants withdrew from study after surgery. We considered that excluding less than 10% of participants from the analysis may not introduce significant bias. |
| Selective reporting (reporting bias) | Low risk | No published protocol or study registration available; however, the authors reported all outcomes specified in the methods section of the study. |
| Other bias | Unclear risk | At least 1 study investigator has a relationship with manufacturers of 1 of the intervention devices. |
Mojzis 2014.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: total 60 eyes of 30 participants; AT LISA bifocal: 30 eyes of 15 participants; AT LISA trifocal: 30 eyes of 15 participants Exclusions after randomization: not reported Losses to follow‐up: none Number analyzed (total and per group): total 60 eyes of 30 participants; AT LISA bifocal: 30 eyes of 15 participants; AT LISA trifocal: 30 eyes of 15 participants Unit of analysis: eyes How were the missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: Czech Republic Setting: not reported Age: mean: 58.7 years AT LISA bifocal group: mean (SD): 62.3 (5.7) years AT LISA trifocal group: mean (SD): 55.2 (7.0) years Sex: not reported Inclusion criteria: patients with cataract or presbyopia/pre‐presbyopia suitable for refractive lens exchange seeking spectacle independence Exclusion criteria: patients with a history of glaucoma or retinal detachment, corneal disease, irregular corneal astigmatism, abnormal iris, macular degeneration or retinopathy, neuro‐ophthalmic disease, ocular inflammation, or previous ocular surgery Equivalence of baseline characteristics: IOL power was significantly higher and participants were significantly younger in the trifocal group |
|
| Interventions |
Intervention 1: AT LISA 809M bifocal IOL (Carl Zeiss Meditec) Intervention 2: AT LISA tri 839MP trifocal IOL (Carl Zeiss Meditec) Length of follow‐up: 3 months |
|
| Outcomes |
Primary outcomes, as defined: not reported Secondary outcomes:
|
|
| Notes |
Institution: University of Alicante Email: david.pinyero@ua.es Address: University of Alicante, Crta San Vicente del Raspeig s/n 03016, San Vicente del Raspeig, Alicante 03690, Spain Study period: not reported Funding sources: not reported Declarations of interest: authors state no financial or proprietary interest in study Reported subgroup analyses: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors did not describe random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not describe treatment allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors did not report masking of participants and study personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors did not reporting masking of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not report any losses to follow‐up, and it was unclear whether all participants were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Low risk | No published protocol or study registration available; however, the authors reported all outcomes specified in the methods section of the study. |
| Other bias | Low risk | No evidence of any other sources of bias detected. |
Mojzis 2017.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Setting: not reported Number randomized: total 75 eyes of 38 participants; AT LISA bifocal: 35 eyes of 15 participants; AT LISA trifocal: 40 eyes of 20 participants Exclusions after randomization: none Losses to follow‐up: not reported Number analyzed (total and per group): total 75 eyes of 38 participants; AT LISA bifocal: 35 eyes of 15 participants; AT LISA trifocal: 40 eyes of 20 participants Unit of analysis: eyes How were the missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: Czech Republic Age: 44 to 70 years Sex: not reported Inclusion criteria: visually significant cataract, presbyopic/pre‐presbyopic patients demanding refractive, and corneal astigmatism below 1.25 D Exclusion criteria: previous ocular surgery, antecedents of glaucoma, ocular inflammation or retinal detachment, active ocular disease, irregular corneal astigmatism, abnormal iris, macular degeneration or retinopathy, and neurophthalmic disease Equivalence of baseline characteristics: unclear |
|
| Interventions |
Intervention 1: AT LISA 809M bifocal IOL (Carl Zeiss Meditec) Intervention 2: AT LISA tri 839MP trifocal IOL (Carl Zeiss Meditec) Length of follow‐up: 1 year |
|
| Outcomes |
Primary outcomes, as defined: not reported Secondary outcomes:
|
|
| Notes |
Institution: University of Alicante Email: david.pinyero@ua.es Address: University of Alicante, Crta San Vicente del Raspeig s/n 03016, San Vicente del Raspeig, Alicante 03690, Spain Study period: not reported Funding sources: not reported Declarations of interest: authors report no conflicts of interest Reported subgroup analyses: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors did not describe random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not describe treatment allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants or study investigators was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not report any losses to follow‐up, and it was unclear whether all participants were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Low risk | No published protocol or study registration available; however, the authors reported all outcomes specified in the methods section of the study. |
| Other bias | Low risk | No evidence of any other sources of bias detected. |
D: diopter IOL: intraocular lens LogMAR: logarithm of the minimum angle of resolution SD: standard deviation VF‐14: Visual Function Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12607000601437 | Not the interventions of interest |
| Aose 2006 | Not the interventions of interest |
| Ban 2021 | Not the interventions of interest |
| Bilbao‐Calabuig 2016 | Not the interventions of interest |
| Bohm 2019 | Not a randomized clinical trial |
| ChiCTR2100042792 | Not a randomized clinical trial |
| CTRI/2021/07/035247 | Not a randomized clinical trial |
| de Carneros‐Llorente 2019 | Not a randomized controlled trial: "Finally, a limitation of our study is an ophthalmic technician randomly assigned the IOL rather than our using a software program to do so. Thus, strictly speaking, this was not a randomized study" |
| Doroodgar 2021 | Not a randomized clinical trial |
| Hayashi 2019 | Not a randomized clinical trial |
| Hovanesian 2021 | Not a randomized clinical trial |
| Hovanesian 2022 | Not a randomized clinical trial |
| ISRCTN64155646 | Not the interventions of interest |
| Kim 2019 | Not a randomized controlled trial |
| Labiris 2022 | Not a randomized clinical trial |
| Lesueur 2000 | Not the interventions of interest |
| Leyland 2002 | Not the interventions of interest |
| Liu 2018 | Not a randomized controlled trial |
| Maurino 2015 | Not the interventions of interest |
| NCT03117426 | Not the interventions of interest |
| NCT04156737 | Not the interventions of interest |
| NCT04907955 | Not a randomized clinical trial |
| NCT05186298 | Not the interventions of interest |
| NTR3556 | Not a randomized clinical trial |
| Paik 2020 | Not a randomized clinical trial |
| Palomino‐Bautista 2020 | Not a randomized clinical trial |
| Palomino‐Bautista 2021 | Not a randomized clinical trial |
| Paul 2016 | Not the interventions of interest |
| Postolache 2015 | Not a randomized clinical trial |
| Qu 2018 | Not the interventions of interest |
| Richter‐Mueksch 2002 | Not the interventions of interest |
| Ruiz‐Mesa 2017 | Not the interventions of interest |
| Schallhorn 2021 | Not a randomized clinical trial |
| Schmidinger 2006 | Not the interventions of interest |
| Skiadaresi 2015 | Not a randomized controlled trial |
| Szweda 1999 | Not a randomized controlled trial |
| Vargas 2021 | Not a randomized clinical trial |
| Yu 2016 | Not a randomized controlled trial |
| Zamora‐de‐la‐Cruz 2018 | Not a randomized controlled trial |
IOL: intraocular lens
Characteristics of studies awaiting classification [ordered by study ID]
Nava 2019.
| Methods | Unclear if study is an RCT |
| Participants |
Inclusion criteria: people who underwent sequential bilateral refractive lens exchange Exclusion criteria: people with any ocular pathology or previous surgery |
| Interventions |
Intervention 1: AcrySof IQ ReSTOR Intervention 2: AcrySof IQ PanOptix Intervention 3: FineVision Intervention 4: Tecnis Symfony Intervention 4: AT LISA tri Length of follow‐up: not clearly reported |
| Outcomes |
Primary outcome: visual acuities, refractive error, participant satisfaction, adverse events Secondary outcome: not reported Maximum follow‐up: not reported |
| Notes |
Start date: not reported Estimated end date: not reported |
Song 2020.
| Methods | Unclear if study is an RCT |
| Participants |
Inclusion criteria: adults 21 years or older at the time of preoperative examination, willingness to undergo surgery on their second eye within 7 days after their first eye, a postoperative visual potential of 20/25 or better, and a pre‐existing corneal astigmatism of less than 1.00 D Exclusion criteria: pregnant and lactating women, history of retinal disease, ocular trauma, or ocular surgery and with evidence of keratoconus or significant irregular astigmatism, worn rigid contact lenses within the past 6 months, gas‐permeable lenses within the past month, or longer wearing times or daily soft contact lenses within 7 days of scheduled surgery, other diseases affecting capsule stability such as pseudoexfoliation syndrome, glaucoma, traumatic cataract, or Marfan syndrome, and people who were not able to read and understand the informed consent |
| Interventions |
Intervention 1: Tecnis Symphony ZXR00 Intervention 2: Tecnis bifocal ZLB00+3.25D Intervention 3: Trifocal AcrySof IQ PanOptix Length of follow‐up: 1 week, 1 month, 6 months |
| Outcomes |
Primary outcome: UDVA, CDVA, uncorrected intermediate visual acuity, uncorrected near visual acuity, topography, corneal aberration, optical biometry, keratometry and funduscopy Secondary outcome: not reported Maximum follow‐up: 6 months |
| Notes |
Start date: not reported Estimated end date: not reported |
CDVA: corrected distance visual acuity CSF: contrast sensitivity function D: diopter IOL: intraocular lens LogMAR: logarithm of the minimum angle of resolution RCT: randomized controlled trial UDVA: uncorrected distance visual acuity
Differences between protocol and review
We decided post hoc to include results from studies that used a paired‐eye design without accounting for correlation in their analysis, and had planned to perform a sensitivity analysis to assess the impact of restricting our analysis to only studies that analyzed data at the participant level on the effect estimates when data were available.
Contributions of authors
Diego Zamora‐de la Cruz: screening search results, data extraction, risk of bias assessment, data verification, data interpretation, writing of the manuscript, final approval of manuscript.
John Bartlett: critical review of the clinical sections, final approval of manuscript.
Mario Gutierrez: critical review of the clinical sections, final approval of manuscript.
Sueko Matsumura Ng: screening search results, data extraction, risk of bias assessment, data entry, data analysis and interpretation, writing of the manuscript, final approval of manuscript.
Sources of support
Internal sources
-
None, Other
No internal source of support
External sources
-
National Eye Institute, National Institutes of Health, USA
Methodological support provided by the Cochrane Eyes and Vision US Project, supported by grant 1 U01 EY020522.
-
Queen's University Belfast, UK
Gianni Virgili, Co‐ordinating Editor for Cochrane Eyes and Vision’s work is funded by the Centre for Public Health, Queen’s University of Belfast, Northern Ireland.
Declarations of interest
Diego Zamora‐de la Cruz: none known. John Bartlett: none known. Mario Gutierrez: none known. Sueko Matsumura Ng: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bozkurt Gencer 2021 {published data only}
- Bozkurt Gencer M, Basmak H, Yasar E, Onal O. Comparison of trifocal toric and bifocal toric intraocular lens implantation in patients with cataract and high corneal astigmatism. International Journal of Ophthalmology 2021;14(12):1876-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochener 2016 {published data only}
- Cochener B. Prospective clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses. Journal of Refractive Surgery 2016;32(3):146-51. [DOI] [PubMed] [Google Scholar]
Gil 2019 {published data only}
- Gil MA, Varon C, Cardona G, Buil JA. Visual acuity and defocus curves with six multifocal intraocular lenses. International Ophthalmology 2020;40:393–401. [DOI] [PubMed] [Google Scholar]
Jonker 2015 {published data only}
- Jonker SM, Bauer NJ, Makhotkina NY, Berendschot TT, den Biggelaar FJ, Nuijts RM. Comparison of a trifocal intraocular lens with a +3.0 D bifocal IOL: results of a prospective randomized clinical trial. Journal of Cataract & Refractive Surgery 2015;41(8):1631-40. [DOI] [PubMed] [Google Scholar]
Kaymak 2017 {published data only}
- Alió JL, Kaymak H, Breyer D, Cochener B, Plaza‐Puche AB. Quality of life related variables measured for three multifocal diffractive intraocular lenses: a prospective randomised clinical trial. Clinical and Experimental Ophthalmology 2018;46(4):380-8. [DOI] [PubMed] [Google Scholar]
- Kaymak H, Breyer D, Alio JL, Cochener B. Visual performance with bifocal and trifocal diffractive intraocular lenses: a prospective three-armed randomized multicenter clinical trial. Journal of Refractive Surgery 2017;33(10):655-62. [DOI] [PubMed] [Google Scholar]
Mojzis 2014 {published data only}
- Mojzis P, Kukuckova L, Majerova K, Liehneova K, Pinero DP. Comparative analysis of the visual performance after cataract surgery with implantation of a bifocal or trifocal diffractive IOL. Journal of Refractive Surgery 2014;30(10):666-72. [DOI] [PubMed] [Google Scholar]
Mojzis 2017 {published data only}
- Mojzis P, Kukuckova L, Majerova K, Ziak P, Pinero DP. Postoperative visual performance with a bifocal and trifocal diffractive intraocular lens during a 1-year follow-up. International Journal of Ophthalmology 2017;10(10):1528-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12607000601437 {published data only}
- ACTRN12607000601437. A randomised, single centre study of the equivalence of two intraocular lenses used in cataract surgery [A randomised, single centre study of the equivalence in safety and efficacy of two intraocular lenses used in cataract surgery]. www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN12607000601437 (first received 1 June 2007).
Aose 2006 {published data only}
- Aose M, Matsushima H, Nagata M, Matui E, Obara Y, Yoshida S, et al. Characteristics of different types of hydrophobic acrylic intraocular lenses and clinical evaluation during early postoperative period. Investigative Ophthalmology and Visual Science 2006;47(13):610. [Google Scholar]
Ban 2021 {published data only}
- Ban JF, Li JK, Guo LX. Influence of three kinds of intraocular lens on vision and visual quality of patients with age-related cataract. International Eye Science 2021;21(1):106-10. [Google Scholar]
Bilbao‐Calabuig 2016 {published data only}
- Bilbao-Calabuig R, Gonzalez-Lopez F, Amparo F, Alvarez G, Patel SR, Llovet-Osuna F. Comparison between mix-and-match implantation of bifocal intraocular lenses and bilateral implantation of trifocal intraocular lenses. Journal of Refractive Surgery 2016;32(10):659-63. [DOI] [PubMed] [Google Scholar]
Bohm 2019 {published data only}
- Bohm M, Petermann K, Hemkeppler E, Kohnen T. Defocus curves of 4 presbyopia-correcting IOL designs: diffractive panfocal, diffractive trifocal, segmental refractive, and extended-depth-of-focus. Journal of Cataract & Refractive Surgery 2019;45(11):1625-36. [DOI] [PubMed] [Google Scholar]
ChiCTR2100042792 {published data only}
- ChiCTR2100042792. Visual quality after multifocal intraocular lens implantation. www.chictr.org.cn/hvshowproject.aspx?id=88342 (first received 10 May 2021).
CTRI/2021/07/035247 {published data only}
- CTRI/2021/07/035247. Reading performance of different multifocal lenses in various light and contrast conditions after cataract surgery. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=51594&EncHid=&userName=035247 (first received 28 July 2021).
de Carneros‐Llorente 2019 {published data only}
- Carneros-Llorente AM, Carneros AM, Carneros-Llorente PM, Jiménez-Alfaro I. Comparison of visual quality and subjective outcomes among 3 trifocal intraocular lenses and 1 bifocal intraocular lens. Journal of Cataract & Refractive Surgery 2019;45(5):587-94. [DOI] [PubMed] [Google Scholar]
Doroodgar 2021 {published data only}
- Doroodgar F, Niazi F, Sanginabadi A, Karimian F, Niazi S, Alinia C, et al. Visual performance of four types of diffractive multifocal intraocular lenses and a review of articles. International Journal of Ophthalmology 2021;14(3):356-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayashi 2019 {published data only}
- Hayashi K, Sato T, Igarashi C, Yoshida M. Comparison of visual outcomes between bilateral trifocal intraocular lenses and combined bifocal intraocular lenses with different near addition. Japanese Journal of Ophthalmology 2019;63(6):429-36. [DOI] [PubMed] [Google Scholar]
Hovanesian 2021 {published data only}
- Hovanesian JA, Jones M, Allen Q. The PanOptix trifocal IOL vs the ReSTOR 2.5 Active Focus and ReSTOR 3.0-Add multifocal lenses: a study of patient satisfaction, visual disturbances, and uncorrected visual performance. Clinical Ophthalmology 2021;15:983-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hovanesian 2022 {published data only}
- Hovanesian JA, Jones M, Allen Q. The Vivity extended range of vision IOL vs the PanOptix trifocal, ReStor 2.5 Active Focus and ReStor 3.0 multifocal lenses: a comparison of patient satisfaction, visual disturbances, and spectacle independence. Clinical Ophthalmology 2022;16:145-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN64155646 {published data only}
- ISRCTN64155646. Evaluation of post-operative dysphotopsia and spectacles independence after bilateral multifocal intraocular lens (IOL) implantation for cataract surgery and refractive lens exchange: Acri.LISA® 366D versus AcrySof® SN6AD1 randomised clinical trial. www.isrctn.com/ISRCTN64155646 (first received 1 September 2010).
Kim 2019 {published data only}
- Kim BH, Hyon JY, Kim MK. Effects of bifocal versus trifocal diffractive intraocular lens implantation on visual quality after cataract surgery. Korean Journal of Ophthalmology 2019;33(4):333-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Labiris 2022 {published data only}
- Labiris G, Panagiotopoulou EK, Perente A, Ntonti P, Delibasis K, Fotiadis I, et al. Premium monovision versus bilateral myopic monovision, hybrid monovision and bilateral trifocal implantation: a comparative study. Clinical Ophthalmology 2022;16:619-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lesueur 2000 {published data only}
- Lesueur L, Gajan B, Nardin M, Chapotot E, Arne JL. Comparison of visual results and quality of vision between two multifocal intraocular lenses. Multifocal silicone and bifocal PMMA [Resultats comparatifs visuels et qualite de vision de deux implants multifocaux. Silicone multifocal et PMMA bifocal]. Journal Francais d'Ophtalmologie 2000;23(4):355-9. [PubMed] [Google Scholar]
Leyland 2002 {published data only}
- Leyland MD, Langan L, Goolfee F, Lee N, Bloom PA. Prospective randomised double-masked trial of bilateral multifocal, bifocal or monofocal intraocular lenses. Eye 2002;16(4):481-90. [DOI] [PubMed] [Google Scholar]
Liu 2018 {published data only}
- Liu X, Xie L, Huang Y. Comparison of the visual performance after implantation of bifocal and trifocal intraocular lenses having an identical platform. Journal of Refractive Surgery 2018;34(4):273‐80. [DOI] [PubMed] [Google Scholar]
Maurino 2015 {published data only}
- Maurino V, Allan BD, Rubin GS, Bunce C, Xing W, Findl O. Quality of vision after bilateral multifocal intraocular lens implantation: a randomized trial—AT LISA 809M versus AcrySof ReSTOR SN6AD1. Ophthalmology 2015;122(4):700-10. [DOI] [PubMed] [Google Scholar]
NCT03117426 {unpublished data only}
- NCT03117426. A randomised evaluation of visual function after bilateral implantation of two types of presbyopia-correcting IOLs. clinicaltrials.gov/ct2/show/NCT03117426 (first received 18 April 2017).
NCT04156737 {published data only}
- NCT04156737. A comparative study of TECNIS Symfony Plus IOL and a trifocal IOL. clinicaltrials.gov/show/NCT04156737 (first received 7 November 2019).
NCT04907955 {published data only}
- NCT04907955. Visual performance following implantation of presbyopia correcting IOLs. clinicaltrials.gov/ct2/show/NCT04907955 (first received 1 June 2021).
NCT05186298 {published data only}
- NCT05186298. Prospective evaluation and comparison of bilateral synergy implants and bilateral Panoptix implants. clinicaltrials.gov/ct2/show/NCT05186298 (first received 11 January 2022).
NTR3556 {published data only}
- NTR3556. A randomized, subject-blinded evaluation of visual function after bilateral implantation of two types of presbyopia-correcting multifocal IOLs. trialsearch.who.int/Trial2.aspx?TrialID=NTR3556 (first received 2 August 2012).
Paik 2020 {published data only}
- Paik DW, Park JS, Yang CM, Lim DH, Chung TY. Comparing the visual outcome, visual quality, and satisfaction among three types of multi-focal intraocular lenses. Scientific Reports 2020;10(1):14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palomino‐Bautista 2020 {published data only}
- Palomino-Bautista C, Sanchez-Jean R, Carmona-Gonzalez D, Pinero DP, Molina-Martin A. Subjective and objective depth of field measures in pseudophakic eyes: comparison between extended depth of focus, trifocal and bifocal intraocular lenses. International Ophthalmology 2020;40(2):351-9. [DOI] [PubMed] [Google Scholar]
Palomino‐Bautista 2021 {published data only}
- Palomino-Bautista C, Sanchez-Jean R, Carmona-Gonzalez D, Pinero DP, Molina-Martin A. Depth of field measures in pseudophakic eyes implanted with different type of presbyopia-correcting IOLS. Scientific Reports 2021;11(1):12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2016 {published and unpublished data}
- Paul R, Rayside M. Comparison of 3 presbyopic correcting intraocular lenses. Clinical and Experimental Ophthalmology 2016;44(Suppl 1):71. [Google Scholar]
Postolache 2015 {published and unpublished data}
- Postolache C, Postolache O. Comparation of refractive results with bifocal implants at LISA 809 and trifocal at LISA TRI839. Romanian Journal of Ophthalmology 2015;59(2):100-2. [PMC free article] [PubMed] [Google Scholar]
Qu 2018 {published data only}
- Qu S, Chen C, Lin S, Di H, Li L, Wang YF, et al. Comparison on the visual quality after different multifocal lens implantation in cataract patients. International Eye Science 2018;18(3):486-90. [Google Scholar]
Richter‐Mueksch 2002 {published data only}
- Richter-Mueksch S, Weghaupt H, Skorpik C, Velikay-Parel M, Radner W. Reading performance with a refractive multifocal and a diffractive bifocal intraocular lens. Journal of Cataract & Refractive Surgery 2002;28(11):1957-63. [DOI] [PubMed] [Google Scholar]
Ruiz‐Mesa 2017 {published data only}
- Ruiz-Mesa R, Abengozar-Vela A, Aramburu A, Ruiz-Santos M. Comparison of visual outcomes after bilateral implantation of extended range of vision and trifocal intraocular lenses. European Journal of Ophthalmology 2017;27(4):460-5. [DOI] [PubMed] [Google Scholar]
Schallhorn 2021 {published data only}
- Schallhorn JM. Multifocal and extended depth of focus intraocular lenses: a comparison of data from the United States Food and Drug Administration premarket approval trials. Journal of Refractive Surgery 2021;37(2):98-104. [DOI] [PubMed] [Google Scholar]
Schmidinger 2006 {published data only}
- Schmidinger G, Geitzenauer W, Hahsle B, Klemen UM, Skorpik C, Pieh S. Depth of focus in eyes with diffractive bifocal and refractive multifocal intraocular lenses. Journal of Cataract & Refractive Surgery 2006;32(10):1650-6. [DOI] [PubMed] [Google Scholar]
Skiadaresi 2015 {published data only}
- Skiadaresi E. Re: Maurino et al.: Quality of vision after bilateral multifocal intraocular lens implantation: a randomized trial—AT LISA 809M versus AcrySof ReSTOR SN6AD1 (Ophthalmology 2015;122:700-10). Ophthalmology 2015;122(9):e56-7. [DOI] [PubMed] [Google Scholar]
Szweda 1999 {published data only}
- Szweda E, Olejarz E, Kaluzny J, Czajkowski G, Seredyka-Burduk M. Comparative evaluation of visual acuity after cataract extraction with monofocal, bifocal or progressive lenses implantation [Ocena porownawcza ostrosci wzroku po operacji zacmy z wszczepem soczewek tylnokomorowych jednoogniskowych, dwuogniskowych i progresywnych]. Klinika Oczna 1999;101(6):437-9. [PubMed] [Google Scholar]
Vargas 2021 {published data only}
- Vargas V, Alio JL, Oliveira RF, Renna A, Yebana P. Long-term objective and subjective outcomes following bilateral implantation of diffractive bifocal or trifocal intraocular lenses. European Journal of Ophthalmology 2021;31(3):1014-20. [DOI] [PubMed] [Google Scholar]
Yu 2016 {published and unpublished data}
- Yu S, Kim YI, Ha SW, Lee GJ, Lee KW, Park YJ. Comparison of the visual outcomes after cataract surgery with implantation of a bifocal and trifocal diffractive intraocular lens. Journal of the Korean Ophthalmological Society 2016;57(3):405-12. [Google Scholar]
Zamora‐de‐la‐Cruz 2018 {published data only}
- Zamora-de-la-Cruz D, Garzón M, Chávez-Mondragón E. Comparison of visual results and quality of vision after bilateral implantation of trifocal intraocular lenses versus bifocal intraocular lenses. Revista Mexicana de Oftalmologia 2018;92(2):62-9. [Google Scholar]
References to studies awaiting assessment
Nava 2019 {published data only}
- Nava JA, Morales-Mancillas NR, Valdez JE. Quality of vision after bilateral multifocal intraocular lens implantation in pregeriatric Hispanic population, after refractive lens exchange. Investigative Ophthalmology & Vision Science 2019;60(9):2063. [iovs.arvojournals.org/article.aspx?articleid=2742315] [Google Scholar]
Song 2020 {published data only}
- Song JE, Khoramnia R, Son HS, Knorz MC, Choi CY. Comparison between bilateral implantation of a trifocal IOL and mix-and-match implantation of a bifocal IOL and an extended depth of focus IOL. Journal of Refractive Surgery 2020;36(8):528‐35. [DOI] [PubMed] [Google Scholar]
Additional references
Bae 2015
- Bae JH, Shin DS, Lee SC, Hwang IC. Sodium intake and socioeconomic status as risk factors for development of age-related cataracts: the Korea National Health and Nutrition Examination Survey. PLOS ONE 2015;10(8):e0136218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calladine 2012
- Calladine D, Evans JR, Shah S, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD003169. [DOI: 10.1002/14651858.CD003169.pub3] [DOI] [PubMed] [Google Scholar]
Carson 2014
- Carson D, Hill WE, Hong X, Karakelle M. Optical bench performance of AcrySof® IQ ReSTOR®, AT LISA® tri, and FineVision® intraocular lenses. Clinical Ophthalmology 2014;8:2105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochener 2012
- Cochener B, Vryghem J, Rozot P, Lesieur G, Heireman S, Blanckaert JA, et al. Visual and refractive outcomes after implantation of a fully diffractive trifocal lens. Clinical Ophthalmology 2012;6:1421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 10 November 2018. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Gatinel 2013
- Gatinel D, Houbrechts Y. Comparison of bifocal and trifocal diffractive and refractive intraocular lenses using an optical bench. Journal of Cataract and Refractive Surgery 2013;39(7):1093-9. [DOI] [PubMed] [Google Scholar]
Glasser 1999
- Glasser A, Campbell MC. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Research 1999;39(11):1991-2015. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2019
- Higgins JPT, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Hodge 1995
- Hodge WG, Whitcher JP, Satariano W. Risk factors for age-related cataracts. Epidemiologic Reviews 1995;17(2):336-46. [DOI] [PubMed] [Google Scholar]
Jin 2019
- Jin S, Friedman DS, Cao K, Yusufu M, Zhang J, Wang J, et al. Comparison of postoperative visual performance between bifocal and trifocal intraocular lens based on randomized controlled trials: a meta-analysis. BMC Ophthalmology 2019;19(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohnen 2009
- Kohnen T, Baumeister M, Kook D, Klaproth OK, Ohrloff C. Cataract surgery with implantation of an artificial lens. Deutsches Arzteblatt International 2009;106(43):695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kretz 2015a
- Kretz FT, Breyer D, Diakonis VF, Klabe K, Henke F, Auffarth GU, et al. Clinical outcomes after binocular implantation of a new trifocal diffractive intraocular lens. Journal of Ophthalmology 2015;2015:962891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kretz 2015b
- Kretz FT, Muller M, Gerl M, Gerl RH, Auffarth GU. Binocular function to increase visual outcome in patients implanted with a diffractive trifocal intraocular lens. BMC Ophthalmology 2015;15:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI: 10.1186/2046-4053-2-81] [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2014
- Law EM, Aggarwal RK, Kasaby H. Clinical outcomes with a new trifocal intraocular lens. European Journal of Ophthalmology 2014;24(4):501-8. [DOI] [PubMed] [Google Scholar]
Lesieur 2012
- Lesieur G. Outcomes after implantation of a trifocal diffractive IOL [Resultats apres implantation d'un implant trifocal diffractif]. Journal Francais d'Ophtalmologie 2012;35(5):338-42. [DOI] [PubMed] [Google Scholar]
Li 2014
- Li L, Wan XH, Zhao GH. Meta-analysis of the risk of cataract in type 2 diabetes. BMC Ophthalmology 2014;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantyjarvi 2001
- Mantyjarvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. Journal of Cataract & Refractive Surgery 2001;27(2):261-6. [DOI] [PubMed] [Google Scholar]
Murdoch 1998
- Murdoch IE, Morris SS, Cousens SN. People and eyes: statistical approaches in ophthalmology. British Journal of Ophthalmology 1998;82(8):971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papadopoulos 2014
- Papadopoulos PA, Papadopoulos AP. Current management of presbyopia. Middle East African Journal of Ophthalmology 2014;21(1):10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pesudovs 2004
- Pesudovs K, Hazel CA, Doran RM, Elliott DB. The usefulness of Vistech and FACT contrast sensitivity charts for cataract and refractive surgery outcomes research. British Journal of Ophthalmology 2004;88(1):11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.4.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Shen 2017
- Shen Z, Lin Y, Zhu Y, Liu X, Yan J, Yao K. Clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses: a systematic review and meta-analysis. Scientific Reports 2017;7:45337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheppard 2013
- Sheppard AL, Shah S, Bhatt U, Bhogal G, Wolffsohn JS. Visual outcomes and subjective experience after bilateral implantation of a new diffractive trifocal intraocular lens. Journal of Cataract & Refractive Surgery 2013;39(3):343-9. [DOI] [PubMed] [Google Scholar]
Torricelli 2012
- Torricelli AA, Junior JB, Santhiago MR, Bechara SJ. Surgical management of presbyopia. Clinical Ophthalmology 2012;6:1459-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Voskresenskaya 2010
- Voskresenskaya A, Pozdeyeva N, Pashtaev N, Batkov Y, Treushnicov V, Cherednik V. Initial results of trifocal diffractive IOL implantation. Graefe's Archive for Clinical and Experimental Ophthalmology 2010;248(9):1299-306. [DOI] [PubMed] [Google Scholar]
Vryghem 2013
- Vryghem JC, Heireman S. Visual performance after the implantation of a new trifocal intraocular lens. Clinical Ophthalmology 2013;7:1957-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. Prevention of blindness and visual impairment: priority eye diseases. Cataract. www.who.int/blindness/causes/priority/en/index1.html (accessed 31 October 2015).
Xu 2017
- Xu Z, Cao D, Chen X, Wu S, Wang X, Wu Q. Comparison of clinical performance between trifocal and bifocal intraocular lenses: a meta-analysis. PLOS ONE 2017;12(10):e0186522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2018
- Yang JJ, Liu QP, Li JM, Qin L. Comparison of visual outcomes with implantation of trifocal versus bifocal intraocular lens after phacoemulsification: a meta-analysis. International Journal of Ophthalmology 2018;11(3):484-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2018
- Yoon CH, Shin IS, Kim MK. Trifocal versus bifocal diffractive intraocular lens implantation after cataract surgery or refractive lens exchange: a meta-analysis. Journal of Korean Medical Science 2018;33(44):e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2021
- Zhang Z, Jiang H, Zhou H, Zhou F. Comparative efficacy between trifocal and bifocal intraocular lens among patients undergoing cataract surgery: a systematic review and meta-analysis. Frontiers in Medicine 2021;8:647268. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Zamora‐De la Cruz 2017
- Zamora‐De la Cruz D, Garzón M, Pulido‐London D, Jimenez‐Corona A, Zúñiga‐Posselt K, Bartlett J, et al. Trifocal intraocular lenses versus bifocal intraocular lenses after cataract extraction. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012648. [DOI: 10.1002/14651858.CD012648] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamora‐de La Cruz 2020
- Zamora-de La Cruz D, Zúñiga-Posselt K, Bartlett J, Gutierrez M, Abariga SA. Trifocal intraocular lenses versus bifocal intraocular lenses after cataract extraction among participants with presbyopia. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD012648. [DOI: 10.1002/14651858.CD012648.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


