Abstract
Background:
Recent studies in both human and experimental animals have identified fragmented and unpredictable parental and environmental signals as a novel source of early-life adversity. Early-life unpredictability may be a fundamental developmental factor that impacts brain development, including reward and emotional memory circuits, affecting the risk for psychopathology later in life. Here, we tested the hypothesis that self-reported early-life unpredictability is associated with psychiatric symptoms in adult clinical populations.
Methods:
Using the newly validated Questionnaire of Unpredictability in Childhood, we assessed early-life unpredictability in 156 trauma-exposed adults, of which 65% sought treatment for mood, anxiety, and/or posttraumatic stress disorder (PTSD) symptoms. All participants completed symptom measures of PTSD, depression and anhedonia, anxiety, alcohol use, and chronic pain. Relative contributions of early-life unpredictability versus childhood trauma and associations with longitudinal outcomes over a 6-month period were determined.
Results:
Early-life unpredictability, independent of childhood trauma, was significantly associated with higher depression, anxiety symptoms, and anhedonia, and was related to higher overall symptom ratings across time. Early-life unpredictability was also associated with suicidal ideation, but not alcohol use or pain symptoms.
Conclusions:
Early-life unpredictability is an independent and consistent predictor of specific adult psychiatric symptoms, providing impetus for studying mechanisms of its effects on the developing brain that promote risk for psychopathology.
Keywords: anhedonia, anxiety, childhood trauma, depression, early-life adversity, posttraumatic stress, unpredictability
1 |. INTRODUCTION
The developing brain is highly sensitive to environmental signals, many of which have long-term effects on adult traits and neuropsychiatric risk. Environmental factors such as trauma, socioeconomic disadvantage, and nutritional deficits are well-established moderators of brain circuit development and subsequent behavioral functions (Frewen et al., 2012; Pechtel & Pizzagalli, 2013). Childhood adversity and trauma are associated with increased risk for mood and anxiety disorders in addition to physical health problems in adulthood (Agorastos et al., 2014). Recently proposed theoretical models suggest that differential forms of adversity may have distinct effects on outcomes relevant to psychopathology (McLaughlin & Sheridan, 2016). A more comprehensive understanding of the neuropsychiatric outcomes associated with particular forms of early-life adversity may aid in prevention and intervention efforts.
Integrated studies in both experimental systems and humans have identified a novel source of developmental effects on neural and behavioral functions: fragmented and unpredictable signals, particularly those derived from maternal care and the home environment (Davis et al., 2017; Glynn et al., 2018; Molet, Heins et al., 2016; Risbrough et al., 2018). Fragmented and unpredictable patterns of sensory signals from the mother were originally described in animal models of simulated early-life poverty. In these studies, observation and quantitative measures of maternal care demonstrated that while the quantity and typical qualitative measures of care were provided, the patterns of care behaviors (i.e., the sensory and tactile signals) received by the pups were disrupted via stress experienced by the mother (Chen & Baram, 2016; Ivy et al., 2008; Molet et al., 2016). These studies revealed that, in rodents, unpredictable care disrupts reward circuits and reward-seeking behaviors and predicts anhedonia-like behaviors (Bolton et al., 2017, 2018; Molet, Heins et al., 2016).
In humans, fragmented and unpredictable early-life experiences are measured across multiple domains, including maternal sensory signals, maternal mood, and caregiving and environmental experiences during childhood and adolescence. Exposure to higher unpredictability of maternal sensory signals in the first year of life predicts lower cognitive and language skills, poorer performance on a hippocampus-dependent memory task, and poorer effortful control across early and middle childhood (Davis et al., 2019, 2017). Furthermore, higher unpredictability of maternal mood during the prenatal period predicts lower cognitive development, lower expressive language, higher levels of child negative affectivity, and higher self-reported anxiety and depressive symptoms across childhood and early adolescence (Glynn et al., 2018; Howland et al., 2020). Self-reported exposure to unpredictability in social, emotional, and physical domains during childhood and adolescence predicts lower emotional control, as well as greater symptoms of anxiety, depression, and anhedonia in adolescents and adults (Glynn et al., 2019; McGinnis et al., 2022; Szepsenwol et al., 2021). Exposure to early-life unpredictability is also associated with an increased risk of later substance use, conduct problems, risk-taking behavior, and poor relationship quality (Doom et al., 2016; McGinnis et al., 2022; Simpson et al., 2012; Szepsenwol et al., 2021). Taken together, unpredictability across domains and age of exposure are associated with important cognitive, biological, affective, and social outcomes across the lifespan.
Importantly, many of these findings were in cohorts with relatively low trauma exposure, suggesting potential unique effects of early-life unpredictability in childhood. Contributions of early-life unpredictability on emotional and cognitive functions have now been replicated across laboratories and human cohorts internationally, suggesting that unpredictable signals from caregivers and the environment may tap into a fundamental developmental factor that shapes neural development and risk for psychopathology in adulthood (Davis et al., 2019; Doom et al., 2016; Glynn & Baram, 2019; Glynn et al., 2019; Granger et al., 2021; McGinnis et al., 2022; Risbrough et al., 2018). To date, most studies have used samples that were not selected for psychiatric risk or current symptoms. Therefore, there is little information about how early-life unpredictability contributes to symptoms in psychiatric populations. Filling this gap is an important step in understanding what dimensions of early-life adversity contribute to neuropsychiatric risk, and if these dimensions individually contribute to distinct or specific symptom patterns.
Here, we tested the hypothesis that early-life unpredictability explains unique variance in symptom domain and severity in adults with elevated mood and anxiety symptoms. We utilized a well-validated self-report measure of early-life unpredictability, the Questionnaire of Unpredictability in Childhood (QUIC; Glynn et al., 2019), and established clinical measures to assess psychiatric symptoms. To test specificity, we examined associations of early-life unpredictability with alcohol abuse and pain symptoms. To understand the relative contributions of different forms of early-life adversity, we compared the effects of early-life unpredictability with the effects of childhood abuse and neglect, as measured with the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1994). Finally, we tested the hypothesis that these associations may predict symptom change over time, by examining associations of QUIC scores, time, and their interaction on symptoms assessed 3- and 6-month intervals.
2 |. METHODS
2.1 |. Participants
Participants (N = 156 at intake assessment, mean age = 43.03 years, 62% male) seeking treatment at Veterans Affairs (VA) clinics or interested in mental health research studies at the VA were referred by staff or were recruited through flyers and word of mouth to participate in the Center of Excellence for Stress and Mental Health (CESAMH) posttraumatic stress disorder (PTSD)/Traumatic Brain Injury(TBI) Biorepository Study at the San Diego Veterans Affairs Healthcare System (SDVAHS). Mental health symptoms were assessed via standardized self-report measures presented over an electronic tablet (eScreening; Pittman et al., 2017), as well as a brief interview with a research assistant to assess current treatment status. Participants provided tissue for banking (blood, urine, and saliva; tissue data not included in this analysis). Participants were assessed up to three times, with approximately 3 months between assessments to measure potential symptom changes over a 6-month period. Participants were recruited through behavioral health clinics or through collaborating mental-health treatment studies at the SDVAHS. The majority (N = 130) of the study population had past military service or were Veterans seeking treatment at the VA for mental health symptoms, although non-Veterans or those with no history of service (N = 26) and Veterans without mental health symptoms (N = 9) were also allowed to participate as comparison groups and to enhance the generalizability of study findings. This study used all available data collected at the time of analysis. Note that a limited dataset from this group (N = 36 at the intake assessment) was merged with other data and reported in a study that examined the validity of the QUIC across multiple age groups and populations (Glynn et al., 2019). Data utilized for the current analysis were from the initial baseline visit (N = 156, and n = 147 for all participants with both QUIC and CTQ measures), in addition to secondary analyses examining associations of early-life unpredictability and symptom change over time, which utilized data from up to three visits (Ns = 156, 100, 67, at visits 1, 2, 3). Race and ethnicity of the sample population were 8.3% American–Indian/Alaskan Native, 8.3% Asian, 21.8% Black, 2.6% Native Hawaiian/Pacific Islander, 3.2% other/unknown, 65.4% White, and of the total sample 21.2% were Hispanic/Latino (Table 1a). The CESAMH PTSD/TBI Biorepository Study was approved by the Veterans Affairs Institutional Review Board and all participants provided written, informed consent.
Table 1.
Descriptive statistics for symptoms, trauma exposure and population demographics.
|
1A. Descriptive statistics for early-life adversity and symptom endorsement in VA TBI/PTSD Biorepository Sample. Mean (Standard Deviation) per visit of self-reported measures of anhedonia, depression, anxiety, posttraumatic stress, alcohol use, pain, and suicidal ideation, as well as prior trauma exposures (LEC, DRRI CES and DRRI PBE). Assessment visits 2 and 3 were approximately 3 and 6 months after the initial assessment visit. See Methods section for instrument ranges and cutoff scores indicating symptom severity. | |||
| Number of subjects | 156 | 100 | 67 |
| Assessment Visit | 1 | 2 | 3 |
|
| |||
| Race/ethnicity | |||
| American Indian/Alaskan Native | 8.33% | 11.00% | 10.45% |
| Asian | 8.33% | 8.00% | 4.48% |
| Black | 21.79% | 24.00% | 23.88% |
| Native Hawaiian/Pacific Islander | 2.56% | 2.00% | 5.97% |
| White | 65.38% | 65.00% | 64.18% |
| Other/Unknown | 3.21% | 6.00% | 2.99% |
| Hispanic/Latino | 21.15% | 19.00% | 19.40% |
| Veteran | 77.56% | 74.00% | 65.67% |
| Life Exposure Checklist “happened to me” | 8.74 (5.74) | 9.24 (5.81) | 9.43 (5.63) |
| Combat Exposure Scale (DRRI-CES) | 27.75 (16.46)* | -- | -- |
| Post Battle Experiences Scale (DRRI-PBE) | 23.58 (12.97)** | -- | -- |
| Early-life unpredictability (QUIC) | 12.87 (9.13) | -- | -- |
| Childhood trauma (CTQ) | 51.80 (20.20) | -- | -- |
| Anhedonia (MASQ-22) | 70.28 (16.82) | 67.80 (18.10) | 72.08 (16.45) |
| PTSD (PCL-5) | 35.17 (21.03) | 31.35 (20.72) | 33.91 (22.39) |
| Depression (PHQ-9) | 10.96 (6.97) | 9.23 (6.55) | 10.38 (7.12) |
| Anxiety (GAD-7) | 9.14 (6.43) | 7.92 (6.07) | 8.62 (6.38) |
| Alcohol Use (AUDIT) | 3.39 (4.83) | 3.46 (5.19) | 4.87 (6.94) |
| Pain Intensity (PROMIS) | 7.87 (2.64) | 7.61 (2.72) | 7.65 (2.81) |
| Pain Interference (PROMIS) | 16.77 (6.94) | 15.16 (6.92) | 15.84 (7.40) |
| Suicidal Ideation Item (MASQ) | 1.53 (.93) | 1.55 (.98) | 1.65 (.98) |
| Suicidal Ideation Item (PHQ-9) | 0.37 (.68) | 0.33 (.71) | 0.35 (.72) |
|
1B. Education, employment, and income characteristics at each study visit. Data presented as percentage of total population at the specific visit reported. | |||
| Number of subjects | 156 | 100 | 67 |
| Assessment Visit | 1 | 2 | 3 |
|
| |||
| Education | |||
| Some High School | 1.92% | 2.00% | 2.99% |
| GED | 1.92% | 5.00% | 5.97% |
| High School Diploma | 7.69% | 7.00% | 8.96% |
| Some College | 32.69% | 32.00% | 26.87% |
| Associates Degree | 16.03% | 13.00% | 14.93% |
| 4-year College Degree | 25.00% | 28.00% | 26.87% |
| Master’s Degree | 12.82% | 10.00% | 10.45% |
| Doctoral Degree | 1.28% | 2.00% | 2.99% |
| Not reported | 0.64% | 1.00% | 0.00% |
| Employment status | |||
| Full time | 26.28% | 25.00% | 20.90% |
| Part time | 12.82% | 19.00% | 14.93% |
| Seasonally | 3.85% | 1.00% | 4.48% |
| Day labor | 0.64% | 1.00% | 2.99% |
| Unemployed | 55.77% | 53.00% | 56.72% |
| unknown | 0.64% | 1.00% | 0.00% |
| Income | |||
| Less than $15,000 | 22.44% | 26.00% | 23.88% |
| $15,000-$29,999 | 17.31% | 13.00% | 20.90% |
| $30,000-$44,999 | 16.03% | 22.00% | 13.43% |
| $45,000-$59,999 | 14.10% | 15.00% | 17.91% |
| $60,000-$74,999 | 7.69% | 7.00% | 4.48% |
| $75,000-$99,999 | 10.90% | 9.00% | 11.94% |
| $100,000+ | 10.26% | 6.00% | 4.48% |
n=130
n=129.
2.2 |. Measures
2.2.1 |. Early-life adversity
Early-life unpredictability was assessed using the QUIC (Glynn et al., 2019; https://contecenter.uci.edu/questionnaire-of-unpredictability-in-childhood/). The QUIC is a self-report measure that asks respondents about experiences related to caregiving and the household environment before age 18, with a subset of questions focusing on experiences more likely to occur in earlier childhood (before age 12). Example items include “I experienced changes in my custody arrangement,” “At least one of my parents regularly checked that I did my homework,” and “At least one of my parents was disorganized.” The scale is comprised of 38 items which are endorsed as either “yes” or “no” (some of which are reverse coded) and are then fit to five subscales: parental involvement (9 items), parental predictability (12 items), parental environment (7 items), physical environment (7 items), and safety and security (3 items). Total scores range from 0 to 38, with a higher score indicating greater exposure to unpredictability in the environment before 18 years of age. The QUIC total score has demonstrated strong internal consistency (α = .90–.92 among adults) and excellent test–retest reliability (r = 0.92; Glynn et al., 2019).
Participants also completed the CTQ (Bernstein et al., 1994), a 28-item scale that assesses child abuse and neglect (possible score from 5 to 125) and includes five subscales: physical, emotional and sexual abuse, and physical and emotional neglect. A higher score on the CTQ denotes greater endorsement of abuse and neglect before the age of 18. The CTQ total score has demonstrated strong internal consistency in community (α = .91, Scher et al., 2001) and clinical (α = .95, Bernstein et al., 1994) adult samples, as well as high test-retest reliability (r = 0.88, Bernstein et al., 1994).
2.2.2 |. Depression, anxiety and PTSD symptoms
Depression and anxiety symptoms in the past 2 weeks were assessed using the nine-item Patient Health Questionnaire-9 (PHQ-9; Kroenke et al., 2001) and the seven-item Generalized Anxiety Disorder Scale-7 (GAD-7; Spitzer et al., 2006), respectively. Both the PHQ-9 and GAD-7 have demonstrated strong internal consistency and test–retest reliability in medical and psychiatric patient samples (Beard & Björgvinsson, 2014; Beard et al., 2016; Kroenke et al., 2001; Spitzer et al., 2006). PHQ-9 scores of 0–4 indicate no symptoms, 5–9 mild symptoms, 10–14 moderate symptoms, 15–19 moderately-severe symptoms, and 20–27 severe depression symptoms. GAD-7 cutoff scores of 5, 10, and 15 also indicate mild, moderate, and severe anxiety symptoms. PTSD symptoms in the past month were assessed using the PTSD checklist for DSM-5 (PCL-5; Weathers et al. 2012). The PCL-5 has demonstrated strong internal consistency (α = .96) and test–retest reliability among Veterans (r = 0.84; Bovin et al., 2016). PCL-5 symptoms >33 suggest the likelihood of PTSD.
2.2.3 |. Anhedonia
To assess anhedonia, we used the Anhedonic Depression subscale of the Mood and Anxiety Symptoms Questionnaire (MASQ-AD; Watson & Clark, 1991). This 22-item scale measures low positive emotion and anhedonia in the past week and can be used independently of the full MASQ. The MASQ-AD has demonstrated strong internal consistency (α = .90; Kendall et al., 2016).
2.2.4 |. Suicidal ideation
To specifically assess QUIC relationships with suicidal ideation (SI), we used one item from the PHQ-9 (“thinking that you would be better off dead or that you want to hurt yourself in some way”) and one item from the MASQ-AD (“thought about death or suicide”). Each item was examined independently.
2.2.5 |. Substance use
Alcohol abuse was assessed using the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993). This scale allows for the assessment of both consumption and dependence behaviors. AUDIT scores >8 but <15 indicate hazardous drinking, while scores >14 indicate the likelihood of dependence.
2.2.6 |. Pain
To assess associations with pain symptoms, we used the nine-item Patient-Reported Outcomes Measurement Information System for pain (PROMIS; www.NIHPROMIS.org). The PROMIS measures were developed using item response theory and calibrated in a sample of 21,133 people, with the aim of providing highly reliable, precise measures of patient-reported health status for physical, mental, and social well-being. Here, we focused on the pain intensity (range of 3–15, a score of 8 is approximately equivalent to average pain intensity endorsed by the US population) and interference (possible range 6–30, a score of 8 is approximately equivalent to average pain interference endorsed by US population) subscales.
2.3 |. Analyses
All statistical analyses were performed using R Studio, version 1.2.5042. In Time 1 analyses which compare QUIC and CTQ total scores as predictors of symptom severity, due to the high covariance of QUIC and CTQ scores, we utilized a whitening technique (Kessy et al., 2018) to quantify QUIC associations with symptoms separate from CTQ contribution (n = 147 subjects completed both measures). We constructed separate linear regression models (lm function in R) with a fixed main effect of QUIC total, and nine dependent variables: total scores on the PCL-5 (PTSD symptoms), GAD-7 (anxiety symptoms), PHQ-9 (depression symptoms), MASQ-AD (anhedonia), AUDIT (alcohol use), and PROMIS current pain (interference and intensity scores). In the case of MASQ-AD Item 22 and PHQ-9 Item 9 (SI), we applied a Poisson distribution (glm function in R), as the variables were highly positively skewed. A Benjamini–Hochberg correction for multiple comparisons was applied for the single-level regressions (nine models). We repeated these analyses on whitened QUIC totals in regressions that were found to be significant (5 total). Finally, hierarchical regressions were also used to examine the specific contribution of the QUIC to R2 after accounting for the CTQ in measure variance.
To examine the effects of early-life unpredictability, time, and their interaction on measures of symptom severity, we constructed separate linear mixed effect models with a random intercept to account for within-subject correlation on the measures (lmer function in R). As in the single-level regressions, a Poisson distribution was used to model the SI item. For examination of the associations of QUIC with symptoms over time, we utilized participants in the sample with up to three visits. Fixed main effects included mean-centered QUIC total, visit number (i.e., −1, 0, 1), and a fixed interaction for mean-centered QUIC total and visit number. Time was coded as nonoverlapping ordinal levels (1 = baseline [n = 156]; 2 = 3.23 months [range = 1.40–4.70 months; n = 100]; 3 = 7.08 months [range = 4.80–10.27 months; n = 67]). Five predictor–outcome relationships were modeled independently (i.e, PCL-5 [PTSD symptoms], GAD-7 [anxiety symptoms], PHQ-9 [depression symptoms], MASQ-AD [anhedonia], as well as PHQ-9 Item 9 [SI]). The random effect variance–covariance matrix was unstructured with a random intercept. A Benjamini–Hochberg correction for multiple comparisons was applied for the associations of the QUIC with symptoms over time (five models).
3 |. RESULTS
3.1 |. Sample demographics
The participant population was majority male (62%), white (65%), and currently seeking treatment for PTSD and/or depression (65%). Participants ranged in age from 21 to 90 years old. They endorsed moderate levels of childhood unpredictability (Glynn et al., 2019) and were above clinical cutoffs for moderate childhood trauma (Bernstein & Fink, 1998; see Table 1). The majority reported they were in active treatment (behavioral and/or pharmacotherapy) for PTSD, depression, and/or substance use (Table 2, and see Supporting Information: Table S1 for a more detailed description of treatment types across the sample population).
Table 2. Treatment Seeking Characteristics.
The majority of Veterans (n=102/156) self-identified as currently receiving treatment at visit 1. The most common reasons for seeking treatment were PTSD, Depression and Anxiety.
| Overall percent of participants receiving current mental health treatment | 65.38% |
| PTSD | 72.55% |
| Depression | 64.71% |
| Anxiety | 55.88% |
| Schizophrenia/Psychosis | 3.92% |
| Bipolar Disorder | 7.84% |
| Substance/Alcohol Abuse | 4.90% |
| Other | 5.88% |
3.2 |. Association of early-life unpredictability with psychiatric symptoms and pain
The results of the linear regressions indicated that early-life unpredictability (QUIC total) significantly predicted greater baseline PTSD symptoms (PCL-5) (β = .214, t = 2.64, p = .009, adjusted [Adj.] R2 = 0.039, F(1,145) = 6.94, p = .009), anxiety (GAD-7) (β = .250, t = 3.10, p = .002, Adj. R2 = 0.056, F(1,145) = 9.63, p = .002), depression (PHQ-9) (β = .247, t = 3.07, p = .003, Adj. R2 = 0.055, F (1,145) = 9.43, p = .003), and anhedonia (MASQ-AD) (β = .258, t = 3.21, p = .002, Adj. R2 = 0.060, F(1,145) = 10.32, p = .002). See Figure 1 for correlation plots. We did not observe significant variance accounted for by early-life unpredictability (QUIC) on alcohol use (AUDIT scores) (F[1, 143] = 0.058, p = .809). Upon examination of the data, we did find a relatively high number of participants who endorsed no recent drinking (n = 64; 41%) likely due to participants’ enrollment in PTSD and/or alcohol treatment programs. However, we also did not detect a relation between alcohol use and unpredictability in the subset of participants who endorsed drinking (ρ = −0.002, p = .98). We also did not detect a relation between unpredictability and pain symptoms (PROMIS pain intensity and interference) (Fs[1, 144] ≤ 1.06, p’s > 0.31). We also tested for interactions between unpredictability and gender on these same outcome variables. There was a trend level interaction for an unpredictability by gender interaction on the PCL-5 (p = .054). When correlations were completed within each gender, only women showed a significant association between QUIC scores and PTSD symptom severity as measured by PCL-5 (Supporting Information: Figure S1; women: r = 0.40, p = .001; men: r = 0.08, p = .45). There were no significant gender differences in PCL-5 total scores (F (1,145) = 2.4, p = .12), although we did observe that women (n = 59) had slightly lower PCL-5 total scores than men (n = 88), (i.e., mean (SD)women = 31.95 (22.70), mean (SD)men = 37.45 (20.00) (see box-plots in Supporting Information: Figure S2). Komolgorov–Smirnov tests also indicated no difference between the sex-specific distributions (D = 0.180, p = .204). Furthermore, after controlling for the influence of CTQ total scores, the gender by QUIC interaction was significant (p = .04), and there was no interaction between CTQ and gender on PCL scores (p = .57). There were no significant interactions between gender and anxiety (GAD-7), depression (PHQ-9), or anhedonia (MASQ-AD). See Table 3 for all models.
FIGURE 1.
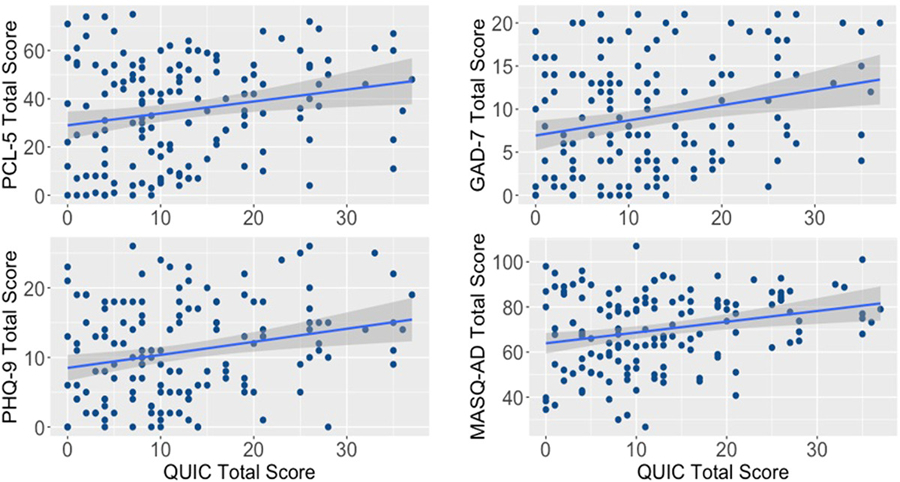
Early-life unpredictability is significantly positively associated with symptoms of anhedonia, depression, and anxiety. Scatterplots of and regression results of QUIC total score associations with PCL-5, GAD-7, PHQ-9, and MASQ-AD total scores:. Trauma-related symptoms (PCL-5 total scores) β = .214, t = 2.64, p = .009, Adj. R2 = 0.039, F(1,145) = 6.94, p = .009. Anxiety (GAD-7 total scores) β = .250, t = 3.10, p = .002, Adj. R2 = 0.056, F(1,145) = 9.63, p = .002. Depression (PHQ-9 total scores) β = .247, t = 3.07, p = .003, Adj. R2 = 0.055, F(1,145) = 9.43, p = .003. Anhedonic depression (MASQ-AD total scores) β = .258, t = 3.21, p = .002, Adj. R2 = 0.060, F(1,145) = 10.32, p = .002. Adj., adjusted; GAD-7, Generalized Anxiety Disorder Scale-7; MASQ-AD, Mood and Anxiety Symptom Questionnaire-Anhedonic Depression; PCL-5, PTSD Checklist for DSM-5; PHQ-9, Patient Health Questionnaire-9; PTSD, posttraumatic stress disorder; QUIC, Questionnaire of Unpredictability in Childhood.
Table 3. Statistical Results for Models Examining Early-life Unpredictability Associations with Psychiatric Symptoms.
Table 3 depicts the original statistical model to examine contribution of early life unpredictability to psychiatric symptoms at Visit 1 (Table 3A) followed by models comparing contribution of unpredictability and childhood trauma at Visit 1 (Table 3B) and main effects of unpredictability across all 3 visits (Table 3C).
| A. Early-life Unpredictability Alone | ||||||
|---|---|---|---|---|---|---|
| QUIC | Overall Regression | |||||
| β | t | p | Adj. R2 | F | p | |
| Anxiety (GAD-7) | 0.25 | 3.10 | 0.002 | 0.056 | 9.63 | 0.002 |
| Depression (PHQ-9) | 0.247 | 3.07 | 0.003 | 0.055 | 9.43 | 0.003 |
| Anhedonia (MASQ-AD) | 0.258 | 3.21 | 0.002 | 0.06 | 10.3 | 0.002 |
| PTSD (PCL-5) | 0.214 | 2.64 | 0.009 | 0.039 | 6.94 | 0.009 |
| %ΔSI | z | p | ||||
| Suicidal Ideation (PHQ-9) | 4.4% (1.7–7.0) | 3.35 | 0.0008 | |||
| Suicidal Ideation (MASQ) | 1.68 | 0.094 | ||||
| B. Early-life Unpredictability Compared to Childhood Trauma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| QUIC | CTQ | Overall Regression | ||||||||
| β | t | p | β | t | p | Adj. R2 | F | p | ΔR2 | |
| Anxiety (GAD-7) | 0.176 | 2.18 | 0.031 | 0.185 | 2.30 | 0.023 | 0.052 | 5.02 | 0.008 | 0.031 |
| Depression (PHQ-9) | 0.159 | 1.976 | 0.05 | 0.206 | 2.55 | 0.01 | 0.055 | 5.21 | 0.007 | 0.025 |
| Anhedonia (MASQ-AD) | 0.192 | 2.39 | 0.018 | 0.176 | 2.18 | 0.03 | 0.055 | 5.24 | 0.006 | 0.037 |
| PTSD (PCL-5) | 0.128 | 1.57 | 0.118 | 0 .192 | 2.37 | 0.019 | 0.04 | 4.05 | 0.019 | NS |
| %ΔSI | z | p | %ΔSI | z | p | |||||
| Suicidal ideation (PHQ-9) | 2.15 (2.74-.58) | 1.74 | 0.082 | 4.0 (1.32–6.68) | 3.15 | 0.002 | NS | |||
| C. Early-life Unpredictability Associations with Symptoms When Collapsed Across 3 Visits | |||
|---|---|---|---|
| QUIC | |||
| beta* | t | p | |
| Anxiety (GAD-7) | 0.16 | 3.21 | 0.002 |
| Depression symptoms (PHQ-9) | 0.18 | 3.13 | 0.002 |
| Anhedonia (MASQ-AD) | 0.53 | 3.8 | 0.0002 |
| PTSD symptoms (PCL-5) | 0.51 | 2.82 | 0.005 |
| Suicidal ideation (PHQ-9 item 9) | 0.04 | 2.17 | 0.03 |
%ΔSI=percent change in suicidal ideation with for every unit increase in QUIC total
ΔR2= We conducted a series of hierarchical linear regressions in R Studio to inspect the change in R-square (R2) with the addition of the QUIC to the CTQ in explaining variance in our primary outcome variables. See results section for details. NS=Not significant.
the lmer function does not provide a standardized beta value
3.3 |. Association of early-life unpredictability with SI
To test the specific contribution of early-life unpredictability to current SI, we performed two Poisson regressions given that both measures of SI were highly positively skewed. The first regression examined the association between QUIC total and PHQ-9 Item 9 (“Thoughts that you would be better off dead, or thoughts of hurting yourself in some way?”). The percent change in SI was 4.4% (95% confidence interval [CI] = 1.7–7.0) higher for every unit increase in QUIC total (z = 3.35, p = .0008). We used the residual deviance to perform a goodness of fit test for the overall model. The goodness-of-fit χ2 test was not statistically significant (res. dev = 142.3719, p = .55) indicating good model fit (https://stats.idre.ucla.edu/r/dae/poisson-regression/). A second Poisson regression was run to predict SI as measured by MASQ-AD Item 22 (“Thought about death or suicide”) from QUIC total. The contribution of the MASQ-AD Item 22 to the overall model did not meet the conventional threshold for statistical significance (z = 1.68, p = .09). Finally, we applied a Benjamini–Hochberg correction for the nine models testing the association between QUIC and depression, anxiety, trauma symptoms, anhedonia, alcohol use, pain severity (two items), and suicidality (two items), and significant results were retained as reported (ps < .045). See Table 3a for regression coefficients and significance values.
3.4 |. Early-life unpredictability compared to childhood trauma
Our ability to tease apart the specific contribution of early-life unpredictability to the development of psychopathology may be limited by its high correlation with other measures of early adversity, including abuse and neglect. For example, we observe a correlation of r = .75 between QUIC and CTQ total scores. To address this issue, we performed a second set of regressions after conducting a statistical whitening procedure with the R package: whitening (Kessy et al., 2018). This approach transformed the original QUIC and CTQ variables into orthogonal variables (i.e., r-values between symptom clusters will be set to zero). This whitening approach also maximizes the correlation between the original variables and the newly transformed “whitened” variables (i.e., new r > .78). Therefore, the new whitened QUIC and CTQ totals continue to measure the same constructs, but they are no longer correlated with each other. This approach mitigates the issue of multicollinearity and allows us to test for specific incremental validity of the QUIC in understanding how early-life unpredictability contributes to adult psychopathology.
We reran regressions using both the whitened QUIC and CTQ total scores as simultaneous predictors of those factors that we found to be significantly predicted by the QUIC alone: PTSD symptoms (PCL-5), anxiety symptoms (GAD-7), depression symptoms (PHQ-9), anhedonia (MASQ-AD), and SI (PHQ-9 Item 9). The whitened QUIC total significantly predicted anxiety (GAD-7) (QUIC β = .176, t = 2.18, p = .031; CTQ β = .185, t = 2.30, p = .023; overall model Adj. R2 = 0.052, F(2,144) = 5.02, p = .008), depression (PHQ-9) (QUIC β = .159, t = 1.976, p = .05; CTQ β = .206, t = 2.55, p = .01; overall model Adj. R2 = 0.055, F(2,144) = 5.21, p = .007), and anhedonia (MASQ-AD) (QUIC β = .192, t = 2.39, p = .018; CTQ β = .176, t = 2.18, p = .03; overall model Adj. R2 = 0.055, F(2,144) = 5.24, p = .006). Interestingly, for the PCL-5, the QUIC was no longer a significant contributor to the overall model once CTQ was added, while CTQ was a significant predictor (QUIC β = .128, t = 1.57, p = .118; CTQ β = .192, t = 2.37, p = .019; overall model Adj. R2 = 0.040, F(2,144) = 4.05, p = .019). The contribution of early-life unpredictability (QUIC total score) to the overall model predicting PHQ-9 item 9 (SI) was diminished when considering the contribution of the CTQ. For the QUIC, the percent change in reported SI was 2.15% (95% CI = −2.74 to 4.58) higher for every unit increase (z = 1.74, p = .082). For the CTQ, the percent change in reported SI was 4.0% (95% CI = 1.32–6.68) higher for every unit increase (z = 3.15, p = .002). We conducted a series of hierarchical regressions in R Studio to inspect the change in R square (Δ R2) with the addition of the QUIC to the CTQ in explaining variance in our primary outcome variables. Consistent with the simultaneous regressions, we found that the addition of the QUIC significantly improved model fit for depression (PHQ-9 total; ΔR2 = 0.025), anxiety (GAD7; ΔR2 = 0.031), and anhedonia (MASQAD; ΔR2 = 0.037). Scores on the CTQ alone did not predict alcohol (AUDIT) or current pain (PROMIS pain intensity or interference), consistent with the QUIC findings. See Table 3b for regression coefficients and significance values.
3.5 |. Early-life unpredictability associations with symptoms across time
Finally, we explored whether the QUIC predicted symptom states in those measures that showed a significant relationship with the QUIC at Time 1 (i.e., PTSD, anxiety, depression, anhedonia, and suicidality [PHQ-9 SI]) over a 6-month period. Results of the linear mixed model with QUIC total and time as fixed effects indicated that QUIC total significantly predicted PTSD symptoms (β = .51, t = 2.82, p = .005), anxiety symptoms (β = .16, t = 3.21, p = .002), depression symptoms (β = .18, t = 3.13, p = .002), and anhedonia (β = .53, t = 3.80, p = .0002) across all three time points. In each case, the main effects signaled that higher QUIC total scores were related to consistently elevated scores on all outcome measures (and exceeded Bonferroni corrections of p ≤ .0125). There was also a main effect of time on PCL-5 scores (β = −1.71, t = −2.30, p = .02), such that PCL-5 total scores decreased overall across time, which would be expected given that the majority of patients were receiving active treatment (65%) (See Table 2 for treatment-seeking characteristics, and Table S1 for treatment modality characteristics). There were no other significant main effects of time, or interactions between QUIC and time, on our variables of interest. For SI, results of the generalized linear mixed models (R function “glmer,” family= Poisson) with QUIC total and time as fixed effects indicated that QUIC total significantly predicted SI on PHQ-9 Item 9 (β = .04, t = 2.17, p = .03). There were no main effects of time, or significant interactions of QUIC with time, for either SI outcome. These results survived correction for multiple comparisons (five variables) after applying a Benjamini–Hochberg adjustment (ps ≤ .03). See Table 3c for regression coefficients and significance values.
4 |. DISCUSSION
The principal findings of the current study testing associations between early-life unpredictability and psychiatric symptoms in a sample of adults with trauma exposure are: (1) greater early-life unpredictability is associated with greater PTSD symptoms, anxiety symptoms, depression symptoms, and anhedonia, and increased risk for SI at the baseline assessment. (2) These associations are specific, as the QUIC does not significantly associate with alcohol use or pain symptoms. (3) The use of a whitening approach to reduce multi-collinearity between measures of early-life unpredictability and childhood trauma, in conjunction with hierarchical regressions, revealed that early-life unpredictability significantly contributes to the variance of anxiety symptoms, depression symptoms, and anhedonia above and beyond the contribution of childhood trauma. (4) The described associations persisted for a minimum of 6 months, indicating a consistent positive association between early-life unpredictability and self-reported measures of PTSD symptoms, anxiety symptoms, depression symptoms, anhedonia, and SI. Taken together, these data suggest that early-life unpredictability is uniquely associated with anxiety and mood-related symptoms among adults with trauma exposure. While early-life unpredictability was also related to trauma-related symptoms and SI, childhood trauma was a stronger predictor, supporting a specific relationship between childhood traumatic experiences and adult trauma symptoms as well as SI (Angelakis et al., 2019; Brewin et al., 2000). Importantly, the data support accumulating evidence for conceptualizing adversity across distinct dimensions including unpredictability.
The current work provides additional evidence that early-life unpredictability may be an important type of early-life adversity that influences the risk for psychopathology. This study extends existing models of how childhood adversity may be linked to psychopathology (e.g., cumulative stress; Evans et al., 2013) by providing evidence that unpredictability is a rarely considered but impactful form of negative early-life experience. In other words, early-life unpredictability may help explain the powerful and pervasive association between adversity and psychopathology by highlighting that it is not only the quality of early life experiences but also, the underlying patterns of caregiving and environmental signals that are key (for review, see Glynn & Baram, 2019). As such, the addition of unpredictability to our existing models can enrich our understanding of how childhood adversity leads to psychopathology, and bolster our ability to predict who is at greatest risk for psychiatric outcomes and which outcomes may be most likely (e.g., Kessler et al., 2010), as well as guide the development of targeted interventions (McLaughlin et al., 2019).
In the current sample of adults with trauma exposure, we found that endorsement of higher levels of early-life unpredictability was associated with increased anhedonia, above and beyond childhood trauma. These data support the increasing evidence that unpredictability (as measured here by household routine and caregiving predictability) may be linked to disruption in reward processes (Birnie et al., 2020). Rodent models of fragmented maternal care consistently result in relatively specific anhedonia-like phenotypes, such as reduced interest in social and appetitive reward stimuli (Bolton et al., 2018; Molet, Heins, et al., 2016), that are causally linked to aberrant signaling in the amygdala-prefrontal cortex and amygdala-nucleus accumbens circuits (Birnie et al., 2020; Bolton et al., 2018). It will be critical for future studies to examine in what manner anhedonia may be most associated with early-life unpredictability to better understand the mechanisms that may underlie the relationship between this form of adversity and psychiatric symptoms.
There are a number of potential mechanisms through which early-life unpredictability may modify stress response systems and circuits. Exposure to higher unpredictability of maternal sensory signals in infancy is associated with blunted infant cortisol to a painful stressor at one year old (Noroña-Zhou et al., 2020), suggesting that this unpredictability can shape hypothalamic–pituitary–adrenal (HPA) axis responding in early development. In animals, fragmented early care or stress during early life consistently results in long-term disruption of the HPA axis and its primary signaling system corticotrophin-releasing factor, resulting in abnormal neuronal function across multiple corticolimbic circuits in adulthood (Chen & Baram, 2016; Gunn et al., 2013; Toth et al., 2016). In animals and humans, unpredictable sensory signals from caregivers alter the hippocampal structure, memory function, and executive function (Davis et al., 2019, 2017), as well as connectivity to cortical circuits (Granger et al., 2021). These structural changes are potentially linked to altered synapse number and function in the hippocampus, observed in animal models of unpredictable care (Ivy et al., 2008; Molet, Maras, et al., 2016). Reduced hippocampal function and abnormalities in structure and connectivity are a consistent phenotype for both depression and PTSD, with some indication that it may be a predisposing risk factor for these disorders (for review, see Acheson et al., 2012). An additional corticolimbic circuit that may be disrupted by unpredictable care is the uncinate fasciculus, the primary fiber bundle connecting the amygdala to the orbitofrontal cortex and a key component of the medial temporal lobe prefrontal cortex circuit. Exposures to higher levels of unpredictable maternal signals in infancy predicted greater generalized fractional anisotropy of the uncinate fasciculus in middle childhood, which in turn was associated with reduced episodic memory (Granger et al., 2021). Overall, these studies indicate that unpredictable care may disrupt the maturation of critical corticolimbic circuits mediating reward and memory functions, which may in turn confer risk for psychiatric symptomatology. Notably, the practice of family routines during the Covid-19 pandemic predicts lower levels of psychopathology among children (Glynn et al., 2021), suggesting that routine may buffer against the deleterious effects of unpredictability, though the precise mechanisms remain unclear. Aberrant social information and emotion processing, learning, and accelerated biological aging are proposed as other possible transdiagnostic mechanisms underlying associations between early adversity and psychopathology (McLaughlin et al., 2020, 2019). Further examination of the psychological and biological mechanisms underlying the association between early-life unpredictability and psychiatric outcomes is an important direction for future research because these processes may be effective targets of intervention.
This study has a number of strengths and limitations. The strengths are in the relatively large sample size as compared to earlier inquiries of the measure, associations with a longitudinal assessment of psychiatric symptoms, and the wide range of early-life unpredictability and symptom severity in the study population. These strengths support the generalizability of the findings. Limitations are that (1) this is a majority Veteran (77%), White (65%), and male (62%) population, (2) the study relied on a retrospective measurement of early-life unpredictability, (3) there was significant attrition over study visits, (4) we did not find associations between QUIC and alcohol-related problems and pain ratings. Therefore, (1) additional work with a more diverse range of individuals is necessary to assess how the QUIC performs depending on a variety of personal characteristics. (2) While the study relied on a retrospective measurement of early-life unpredictability, it should be noted this instrument has been validated using prospective observational data of early-life unpredictability as well as prospective validation of items on the QUIC (e.g., moved frequently; Glynn et al., 2019), supporting its reliability in measuring this newly conceptualized dimension of early-life adversity. (3) While there was significant participant attrition over time, sample attributes remained steady across visits (see Table 1), suggesting overall stability of participant characteristics. (4) Finally, null associations between QUIC and alcohol use and pain should be interpreted cautiously given the relatively low base rates of these symptoms in this sample (only about half the sample currently consumed alcohol); pain intensity scores were similar to the average score for the US population indicating a potentially restricted range within which to detect associations.
5 |. CONCLUSION
These findings indicate that early-life unpredictability is associated with mood and anxiety symptoms, as well as comorbid SI and anhedonia, in individuals with clinical levels of mood and anxiety symptoms. These relations are stable over time and are selectively above and beyond contributions of other forms of adversity such as childhood trauma. These findings support the examination of the unique contribution of unpredictability to the development and maintenance of these symptoms across the lifespan.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIMH P50MH096889 (Andrea D. Spadoni, Laura M. Glynn, Elysia P. Davis, Tallie Z. Baram, Dewleen G. Baker, and Victoria B. Risbrough), VA Merit Awards (Andrea D. Spadoni 5I01CX001762 and Victoria B. Risbrough 5I01BX004312), the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs (Meghan Vinograd), and the Center of Excellence for Stress and Mental Health (Andrea D. Spadoni, Meghan Vinograd, Dewleen G. Baker, Caroline M. Nievergelt, and Victoria B. Risbrough).
Funding information
Veteran Affairs, Grant/Award Numbers: 5I01BX004312, 5I01CX001762; National Institute of Mental Health, Grant/Award Number: P50MH096889
Footnotes
CONFLICT OF INTEREST
Victoria B. Risbrough has received consulting fees from Engrail, Jazz Pharmaceuticals, and Fallon Capital in the last 36 months.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Acheson DT, Gresack JE, & Risbrough VB (2012). Hippocampal dysfunction effects on context memory: Possible etiology for posttraumatic stress disorder. Neuropharmacology, 62(2), 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Pittman JOE, Angkaw AC, Nievergelt CM, Hansen CJ, Aversa LH, Parisi SA, Barkauskas DA, & Baker DG (2014). The cumulative effect of different childhood trauma types on self-reported symptoms of adult Male depression and PTSD, substance abuse and health-related quality of life in a large active-duty military cohort. Journal of Psychiatric Research, 58, 46–54. [DOI] [PubMed] [Google Scholar]
- Angelakis I, Gillespie EL, & Panagioti M (2019). Childhood maltreatment and adult suicidality: A comprehensive systematic review with meta-analysis. Psychological Medicine, 49(7), 1057–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C, & Björgvinsson T (2014). Beyond generalized anxiety disorder: Psychometric properties of the GAD-7 in a heterogeneous psychiatric sample. Journal of Anxiety Disorders, 28(6), 547–552. 10.1016/j.janxdis.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Beard C, Hsu KJ, Rifkin LS, Busch AB, & Björgvinsson T (2016). Validation of the PHQ-9 in a psychiatric sample. Journal of Affective Disorders, 193, 267–273. 10.1016/j.jad.2015.12.075 [DOI] [PubMed] [Google Scholar]
- Bernstein D, & Fink. (1998). Childhood trauma questionnaire: A retrospective self-report San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, & Ruggiero J (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry, 151(8), 1132–1136. 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- Birnie MT, Kooiker CL, Short AK, Bolton JL, Chen Y, & Baram TZ (2020). Plasticity of the reward circuitry after early-life adversity: Mechanisms and significance. Biological Psychiatry, 87(10), 875–884. 10.1016/j.biopsych.2019.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Molet J, Ivy A, & Baram TZ (2017). New insights into early-life stress and behavioral outcomes. Current Opinion in Behavioral Sciences, 14, 133–139. 10.1016/j.cobeha.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, Yang DZ, Obenaus A, & Baram TZ (2018). Anhedonia following Early-Life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biological Psychiatry, 83(2), 137–147. 10.1016/j.biopsych.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, & Keane TM (2016). Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychological Assessment, 28(11), 1379–1391. 10.1037/pas0000254 [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, & Valentine JD (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology, 68(5), 748–766. [DOI] [PubMed] [Google Scholar]
- Chen Y, & Baram TZ (2016). Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology, 41(1), 197–206. 10.1038/npp.2015.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Korja R, Karlsson L, Glynn LM, Sandman CA, Vegetabile B, Kataja EL, Nolvi S, Sinervä E, Pelto J, Karlsson H, Stern HS, & Baram TZ (2019). Across continents and demographics, unpredictable maternal signals are associated with children’s cognitive function. EBioMedicine, 46, 256–263. 10.1016/j.ebiom.2019.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Stout SA, Molet J, Vegetabile B, Glynn LM, Sandman CA, Heins K, Stern H, & Baram TZ (2017). Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proceedings of the National Academy of Sciences of the United States of America, 114(39), 10390–10395. 10.1073/pnas.1703444114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Vanzomeren-Dohm AA, & Simpson JA (2016). Early unpredictability predicts increased adolescent externalizing behaviors and substance use: A life history perspective. Development and Psychopathology, 28(4 Pt 2), 1505–1516. 10.1017/S0954579415001169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Li D, & Whipple SS (2013). Cumulative risk and child development. Psychological Bulletin, 139(6), 1342–1396. 10.1037/a0031808 [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, & Lanius RA (2012). Assessment of anhedonia in psychological trauma: Psychometric and neuroimaging perspectives. European Journal of Psychotraumatology, 3, 8587. 10.3402/ejpt.v3i0.8587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, & Baram TZ (2019). The influence of unpredictable, fragmented parental signals on the developing brain. Frontiers in Neuroendocrinology, 53, 100736. 10.1016/j.yfrne.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Luby JL, Baram TZ, & Sandman CA (2021). A predictable home environment May protect child mental health during the COVID-19 pandemic. Neurobiology of Stress, 14, 100291. 10.1016/j.ynstr.2020.100291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Howland MA, Sandman CA, Davis EP, Phelan M, Baram TZ, & Stern HS (2018). Prenatal maternal mood patterns predict child temperament and adolescent mental health. Journal of Affective Disorders, 228, 83–90. 10.1016/j.jad.2017.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Stern HS, Howland MA, Risbrough VB, Baker DG, Nievergelt CM, Baram TZ, & Davis EP (2019). Measuring novel antecedents of mental illness: The Questionnaire of Unpredictability in Childhood. Neuropsychopharmacology, 44(5), 876–882. 10.1038/s41386-018-0280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger SJ, Glynn LM, Sandman CA, Small SL, Obenaus A, Keator DB, Baram TZ, Stern H, Yassa MA, & Davis EP (2021). Aberrant maturation of the uncinate fasciculus follows exposure to unpredictable patterns of maternal signals. Journal of Neuroscience, 41(6), 1242–1250. 10.1523/JNEUROSCI.0374-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, & Belelli D (2013). Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: Relevance to neurosteroids and programming of the stress response. Journal of Neuroscience, 33(50), 19534–19554. 10.1523/jneurosci.1337-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland MA, Sandman CA, Davis EP, Stern HS, Phelan M, Baram TZ, & Glynn LM (2020). Prenatal maternal mood entropy is associated with child neurodevelopment. Emotion, 21, 489–498. 10.1037/emo0000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, & Baram TZ (2008). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience, 154(3), 1132–1142. 10.1016/j.neuroscience.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall AD, Zinbarg RE, Bobova L, Mineka S, Revelle W, Prenoveau JM, & Craske MG (2016). Measuring positive emotion with the mood and anxiety symptom questionnaire: Psychometric properties of the anhedonic depression scale. Assessment, 23(1), 86–95. 10.1177/1073191115569528 [DOI] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, Angermeyer M, Benjet C, Bromet E, Chatterji S, de Girolamo G, Demyttenaere K, Fayyad J, Florescu S, Gal G, Gureje O, … Williams DR (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. British Journal of Psychiatry, 197(5), 378–385. 10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessy A, Lewin A, & Strimmer K (2018). Optimal whitening and decorrelation. The American Statistician, 72(4), 309–314. 10.1080/00031305.2016.1277159 [DOI] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis EW, Sheridan M, & Copeland WE (2022). Impact of dimensions of early adversity on adult health and functioning: A 2-decade, longitudinal study. Development and Psychopathology, 10.1017/S095457942100167X [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Colich NL, Rodman AM, & Weissman DG (2020). Mechanisms linking childhood trauma exposure and psychopathology: A transdiagnostic model of risk and resilience. BMC Medicine, 18, 96. 10.1186/s12916-020-01561-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, DeCross SN, Jovanovic T, & Tottenham N (2019). Mechanisms linking childhood adversity with psychopathology: Learning as an intervention target. Behaviour Research and Therapy, 118, 101–109. 10.1016/j.brat.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Sheridan MA (2016). Beyond cumulative risk: A dimensional approach to childhood adversity. Current Directions in Psychological Science, 25(4), 239–245. 10.1177/0963721416655883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, & Stern H (2016). Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Translational Psychiatry, 6, e702. 10.1038/tp.2015.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Maras PM, Kinney-Lang E, Harris NG, Rashid F, Ivy AS, Solodkin A, Obenaus A, & Baram TZ (2016). MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus, 26(12), 1618–1632. 10.1002/hipo.22661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noroña-Zhou AN, Morgan A, Glynn LM, Sandman CA, Baram TZ, Stern HS, & Davis EP (2020). Unpredictable maternal behavior is associated with a blunted infant cortisol response. Developmental Psychobiology, 62(6), 882–888. 10.1002/dev.21964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2013). Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse: A high-density event-related potential study. JAMA Psychiatry, 70(5), 499–507. 10.1001/jamapsychiatry.2013.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JOE, Floto E, Lindamer L, Baker DG, Lohr JB, & Afari N (2017). VA escreening program: Technology to improve care for post-9/11 veterans. Psychological Services, 14(1), 23–33. 10.1037/ser0000125 [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Glynn LM, Davis EP, Sandman CA, Obenaus A, Stern HS, Keator DB, Yassa MA, Baram TZ, & Baker DG (2018). Does anhedonia presage increased risk of posttraumatic stress disorder? Current Topics in Behavioral Neuroscience, 38, 249–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, & Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction, 88(6), 791–804. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, & Forde DR (2001). The Childhood Trauma Questionnaire in a community sample: Psychometric properties and normative data. Journal of Traumatic Stress, 14(4), 843–857. 10.1023/a:1013058625719 [DOI] [PubMed] [Google Scholar]
- Simpson JA, Griskevicius V, Kuo SI, Sung S, & Collins WA (2012). Evolution, stress, and sensitive periods: The influence of unpredictability in early versus late childhood on sex and risky behavior. Developmental Psychology, 48(3), 674–686. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Lowe B (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- Szepsenwol O, Simpson JA, Griskevicius V, Zamir O, Young ES, Shoshani A, & Doron G (2021). The effects of childhood unpredictability and harshness on emotional control and relationship quality: A life history perspective. Development and Psychopathology, 10.1017/S0954579421001371 [DOI] [PubMed] [Google Scholar]
- Toth M, Flandreau EI, Deslauriers J, Geyer MA, Mansuy IM, Merlo Pich E, & Risbrough VB (2016). Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology, 41(6), 1681–1690. 10.1038/npp.2015.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1991). The Mood and Anxiety Symptom Questionnaire Department of Psychology, University of Iowa. [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2012). Clinician-administered PTSD scale for DSM-5 National Center for PTSD. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


