Abstract
We sought to determine which facets of sleep neurophysiology were most strongly linked to cognitive performance in 3,819 older adults from two independent cohorts, using whole-night electroencephalography (EEG). From over 150 objective sleep metrics, we identified 23 that predicted cognitive performance, and processing speed in particular, with effects that were broadly independent of gross changes in sleep quality and quantity. Metrics including REM duration, features of the EEG power spectra derived from multivariate analysis, and spindle and slow oscillation morphology and coupling. These metrics were further embedded within broader associative networks linking sleep with aging and cardiometabolic disease: individuals who, compared to similarly aged peers, had better cognitive performance tended to have profiles of sleep metrics more often seen in younger, healthier individuals. Taken together, our results point to multiple facets of sleep neurophysiology that track coherently with underlying, age-dependent determinants of cognitive and physical health trajectories in older adults.
Introduction
Both sleep problems1,2 and cognitive impairment3,4 increase with age. As well as disruptions of the sleep-wake cycle, polysomnographic studies have revealed marked age-dependent changes in sleep macro architecture (including stage duration5,6), micro architecture (including spindle activity7) and rates of sleep-disordered breathing8. Given the growing body of literature that points to a major role of sleep in supporting cognitive processing9–12, it is natural to ask whether changes in sleep may be associated with accelerated cognitive decline and impairment in older adults. A large, but not always consistent, literature has indeed addressed these issues from several perspectives, from large epidemiological studies of self-reported sleep, to focused laboratory studies of sleep-dependent memory consolidation and experimental sleep deprivation13. Observational studies have reported various aspects of sleep to be associated with cognitive performance, mild cognitive impairment and/or incident dementia in older adults, including sleep duration14–21 and quality22,23, excessive daytime sleepiness15,24–26, elements of non-rapid eye movement (NREM)27–31 as well as rapid eye movement (REM)28,32–36 sleep neurophysiology, and sleep-disordered breathing37. Many of the studies based on objective sleep measures suffered from small sample sizes and inadequate control of multiple testing, however, making it challenging to draw robust conclusions about population-level links between sleep and cognition.
In this study, we sought to identify the aspects of sleep neurophysiology most strongly associated with cognitive performance in older adults. Our data-driven approach aimed to assess a broad range of sleep measures, coupled with large sample sizes, control of multiple testing and replication across cohorts to ensure rigor. The initial cohort was the Multi-Ethnic Study of Atherosclerosis (MESA)38,39, an ethnically-diverse population-based sample of late-middle aged and elderly adults, recruited from six sites in the United States. The MESA Sleep Ancillary Study collected unattended level 2 polysomnography (PSG) and subjective reports of sleep quality on a subset of this sample40. We extended analyses to a second, independent cohort, the Osteoporotic Fractures in Men Study (MrOS), a study of community-dwelling men 65 years or older who underwent level 2 PSG.
For details of the sleep and cognitive measures assessed, see the Methods and Supplementary Methods sections below. In brief, we scored each individual’s sleep study on 173 metrics in MESA (168 in MrOS), organized in eleven domains: 1) subjective sleep problems, 2) sleep macro architecture, 3) chronotype, 4) spectral power metrics, 5) alternative time- and frequency-domain metrics, 6) a data-driven modeling of power spectra, labeled principal spectral component (PSC) analysis, 7) spindle occurrence, 8) spindle morphology, 9) slow oscillation (SO) occurrence, 10) SO morphology and 11) spindle/SO coupling. All objective PSG metrics – the primary focus of this report – were identically calculated in MESA and MrOS, whereas some self-report measures were not available in both studies. In both cohorts, neuropsychological measures were available that collectively indexed global cognitive functioning, processing speed, working memory, attention and psychomotor functioning. Specifically, MESA employed four measures: Digit Symbol Coding Test (DSCT)41, Cognitive Abilities Screening Instrument (CASI)42, and Digit Span Test (Forward and Backward, DSF and DSB)43. MrOS employed three: Trails B44, a modified, expanded version of the Mini-Mental State Examination (3MS)45, and the Digit Vigilance Test (DVT)46.
When considering age-dependent associations between sleep and cognition, a number of potential mediating or confounding factors should be borne in mind. Poor cardiometabolic health, including hypertension and diabetes, is associated with sleep architecture47 as well as cognitive decline48. Similarly, depressed mood is associated with sleep disturbances49 and considered a risk factor for dementia50. Common medications associated with these conditions, including antidepressants51,52 and beta blockers53, can also impact sleep. In some individuals, sleep apnea could be an underlying factor that connects poor sleep with cardiometabolic and cardiovascular health37. In secondary analyses, we therefore considered potential mediating and confounding factors that have been associated with sleep and/or cognition.
Results
The final analytic MESA sample comprised 1,595 adults between 54 and 93 years of age, of whom 759 (48%) were male; the MrOS sample comprised 2,224 males between 67 to 96 years of age (Supplementary Figure 1, Supplementary Tables 1 & 2). Sleep EEG signals were recorded centrally (C4/M1) in both studies. We removed individuals with high levels of artifact and set to missing statistical outliers (Supplementary Table 3, Supplementary Data 1 & 2). Below, all references to NREM sleep imply N2+N3 combined. Unless otherwise explicitly noted, all statistics are regression coefficients (b) and corresponding significance values (p) from multiple linear regressions, always including at least a ‘baseline’ set of covariates (age, sex in MESA, race/ethnicity, and collection site), and typically with cognition as the dependent variable, and a single sleep metric as a predictor.
As many of the objective sleep metrics have not been reported in these samples before, below we first describe the distributions and demographic associations of key metrics. We report associations between sleep metrics and cognitive performance, first in MESA and then expanding to MrOS. Finally, for the top cognition-associated objective sleep metrics, we characterize and contextualize their associations in a series of secondary analyses.
Demographic and other correlates of cognitive performance
As expected, cognitive performance was negatively associated with age in each cohort (Figure 1a, Supplementary Tables 1 and 2). In MESA, DSCT (b = −0.46 SD units per decade of age, 95% confidence interval (CI) −0.50, −0.41, p < 10−15) and CASI (b = −0.26, 95% CI −0.31, −0.21, p < 10−15) showed stronger age-related declines in performance compared to DSTF (b = −0.14, 95% CI −0.18, −0.09, p = 1×10−8) and DSTB (b = −0.16, 95% CI −0.21, −0.11, p = 3×10−10). In MrOS, all measures showed a marked decline in performance with higher age, with b = −0.63 (95% CI −0.70, −0.55), −0.57 (95% CI −0.65, −0.50) and −0.37 (95% CI −0.45, −0.30) for Trails B*, 3MS and DVT* respectively (all p < 10−15; note for timed measures, an asterisk (*) denotes the sign-reversed metric, such that negative coefficients indicate worse performance consistently for all tests). Other demographic factors (including sex, race/ethnicity and educational attainment) and health factors (including depressive symptoms and cardiometabolic disease) showed significant associations with several cognitive measures (Supplementary Tables 1 & 2), and along with collection site, these were included as covariates in subsequent secondary analyses.
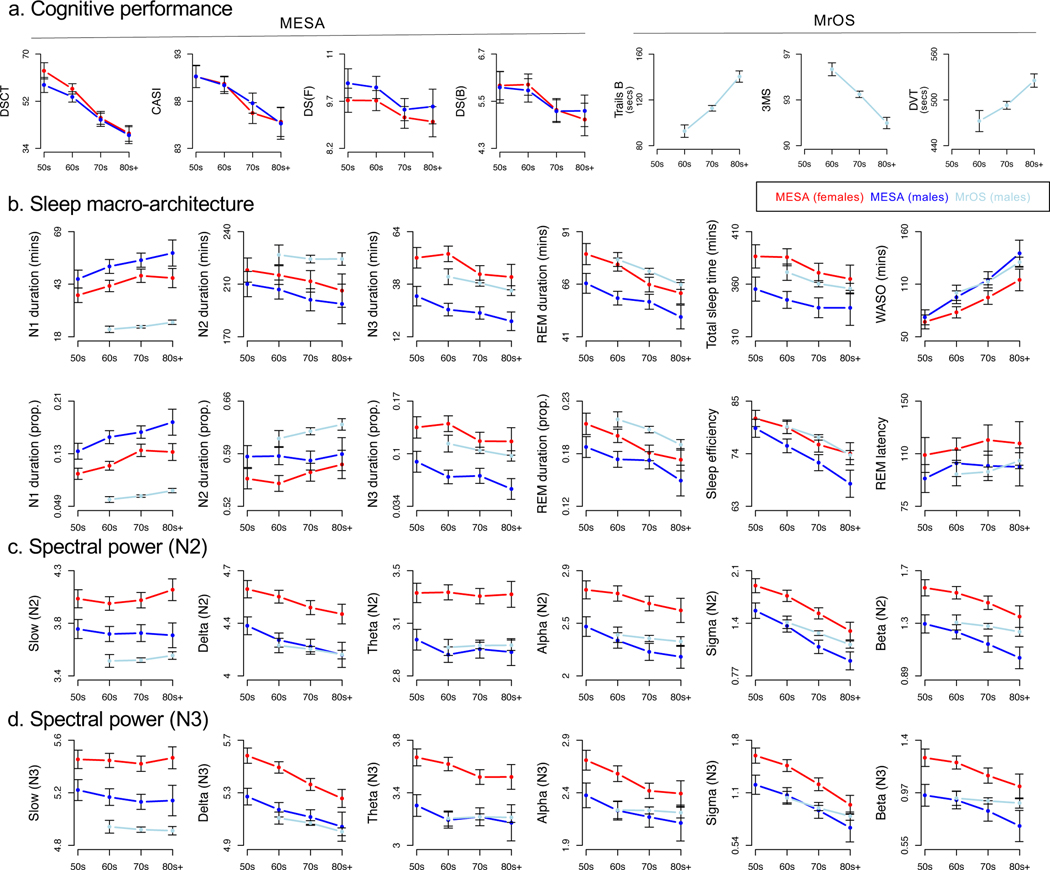
Figure 1. Demographic profiles of primary sleep and cognitive measures.
a) Mean cognitive measure values in MESA, stratified by decade of age and sex, for DSCT, CASI, DS(F) (=DSF) and DS(B) (=DSB), and mean cognitive measure values in MrOS stratified by decade of age, for Trails B, 3MS and DVT. Note that here we present the raw values of Trails B and DVT, but association results are given for Trails B* and DVT*, which are those variables multiplied by −1. Error bars represent 95% confidence intervals around the estimate of the mean. b) Key sleep macro-architecture mean values stratified by decade of age, study and sex for N1, N2, N3 and REM duration (in minutes, and as a proportion of TST), TST, WASO (in minutes), sleep efficiency and REM latency. c) Absolute log-scaled N2 spectral band power stratified by age decade, study and sex for slow (<1 Hz), delta (1–4 Hz), theta (4–8 Hz), alpha (8–11 Hz), sigma (11–15 Hz) and beta (15–30 Hz) power, each divided by total power to yield relative power metrics. d) As for c), but for N3 sleep.
Sleep macro-architecture and spectral band power
On average, MESA participants had longer N1 sleep than MrOS participants (Figure 1b, 47.7 versus 23.2 minutes) but shorter N2 sleep (206.4 versus 224.7 minutes), REM sleep (65.8 versus 71.2) and WASO (90.7 versus 112.5 minutes). Stage duration expressed as a percentage of total sleep time (TST) showed a similar pattern of differences, except the relative proportion of N2 increased with age (Figure 1b). Conditional on age and sex, MESA and MrOS showed broadly similar distributions for key stage-dependent micro-architecture metrics, including spectral power during N2 (Figure 1c) and N3 (Figure 1d), as well as spindle (Figures 2b), slow oscillation (Figure 2d) and spindle/SO coupling (Figures 2b, 2e) metrics, with the exception of slow (<1 Hz) power (Figures 1c, 1d) and SO duration (Figure 2d), potentially reflecting differential high-pass filtering during acquisition or export of the original MrOS recordings. These minor differences in stage and slow power distributions notwithstanding, we note that our current focus is on within-cohort association. That is, as none of the analyses below directly combine measurements across cohorts, systematic between-cohort differences cannot – by definition – induce sleep-cognition associations spuriously.
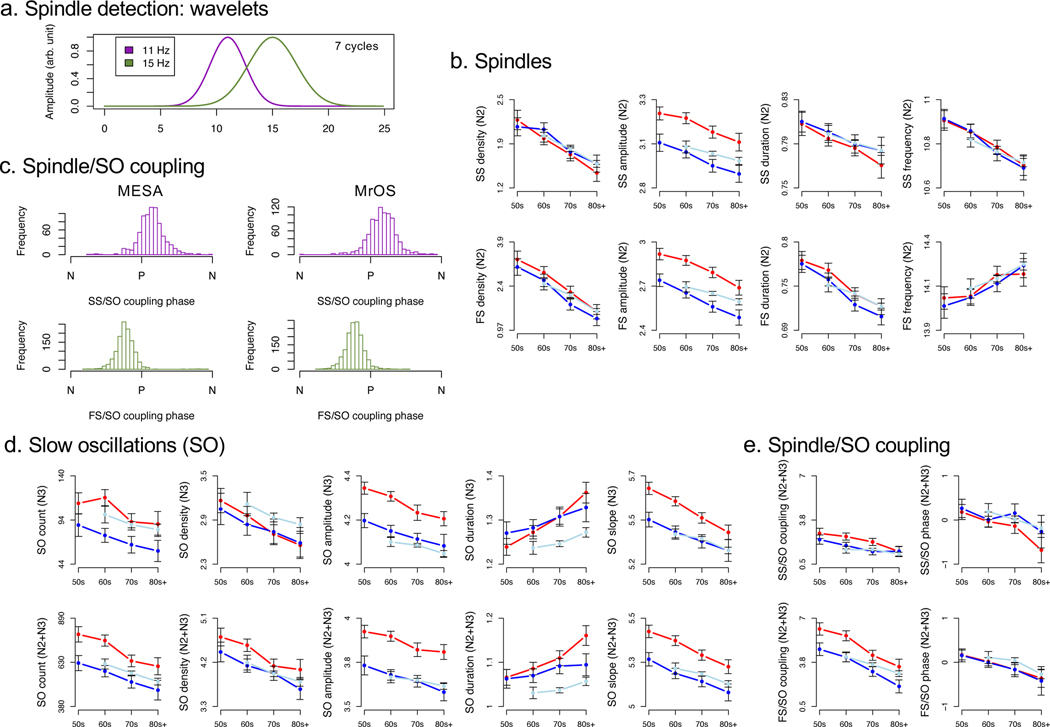
Figure 2. Spindles, slow oscillations and their coupling.
a) Spindle wavelet amplitude (in arbitrary units) when using the default bandwidth setting (7 cycles) for FC = 11 Hz and FC =15 Hz wavelets, where each wavelet detects spindles primarily within approximately +/− 2 Hz of the target frequency. b) Key N2 spindle metrics stratified by age (decade), cohort and sex for spindle density, amplitude, duration and frequency, separately for slow (top row) and fast (bottom row) spindles. c) Distributions of individuals’ mean SO phase at spindle peak for slow spindles (SS) and fast spindles (FS) in MESA (left) and MrOS (right). SO phase angle is oriented on the x-axis from one negative peak (N) to the subsequent one; spindles tend to cluster just after (slow spindles, SS) or just before (fast spindles, FS) the positive SO peak (P). d) Key SO occurrence and morphology mean values stratified by age (decade), cohort and sex for SO count, density, amplitude, duration and slope, in both N3 (top row) and N2+N3 combined (bottom row). e) Spindle/SO coupling metrics (magnitude Z score and normalized SO angle) for slow spindles (SS) and fast spindles (SS), in N2+N3 sleep.
Sleep spindles, slow oscillations and their coupling
We targeted fast (FC = 15 Hz) and slow (FC = 11 Hz) spindles in our primary analyses (Figure 2a, Supplementary Figures 2 & 3). On average, we detected 1.9 slow spindles and 2.46 fast spindles per minute of N2 sleep in MESA; in MrOS, these values were 1.75 and 2.03 respectively. Fast and slow spindle density estimates were significantly correlated with each other, although the majority (~90%) of variation in spindle density was unique to either fast or to slow spindles, rather than shared across types: adjusted for baseline covariates, r = 0.33 (95% CI 0.29, 0.38, p < 10−15) in MESA and r = 0.32 (95% CI 0.28, 0.36, p < 10−15) in MrOS.
We detected slow oscillations (SOs) based on two complementary heuristics, using either an adaptive or an absolute amplitude threshold. Metrics of SO morphology were similar and highly correlated between threshold definitions, and similar between cohorts and stages (Supplementary Table 4, Supplementary Figure 4). For example, the mean durations of SOs detected during N3 only varied between 1.24 seconds (0.81 Hz) and 1.28 seconds (0.78 Hz) across definitions and cohorts. During N2+N3 sleep combined, SO frequency was ~0.85 Hz under the absolute definition but above 0.9 Hz under the adaptive definition (Supplementary Table 4). Estimates of SO occurrence varied more so between the two definitions, however (Supplementary Table 4). For MESA and MrOS respectively, under the absolute definition there were 7.50 and 4.25 SO per minute during N3, compared to 1.95 and 1.02 during N2+N3 combined. In contrast, under the adaptive definition, which as expected, reduces between cohort differences somewhat, there were 2.83 and 2.90 SO per minute during N3 sleep, which rose to 4.26 and 3.96 during N2+N3 combined. That SO density counter-intuitively increased when N2 sleep was included reflected the implicit lowering of the adaptive threshold. (See the Supplementary Methods for a discussion of the complementary features and interpretative challenges of adaptive and absolute thresholds.)
With respect to spindle/SO coupling, we observed a clear clustering of individuals’ mean phase angles, whereby fast spindles tended to have their peak on the rising slope of SO positive peaks (labeled P in Figure 2c), in both MESA and MrOS. In contrast, slow spindles tended to peak on or after the positive SO peak. For coupling magnitude metrics that were dependent on SO definitions, we observed generally high correlations and similar means between adaptive (the default) and absolute SO definitions, (Supplementary Table 5). We also considered spindle coupling with respect to continuous slow wave activity (SWA) irrespective of detected SOs (called spindle/SWA coupling below). When considering spindle/SWA coupling, 80% of MESA individuals had a significant (empirical p < 0.05, see Methods for details) fast spindle/SO phase coupling metric, whereas 52% did for slow spindles (Supplementary Table 6). In MrOS, these proportions were 76% and 41% for fast and slow spindles respectively. We observed similar patterns of spindle/SO coupling, considering only the subset of spindles that overlapped a detected SO, although proportions tended to be higher for the adaptive versus absolute SO definition (Supplementary Table 6). In terms of spindle/SO gross overlap, we observed above-chance (empirical p > 0.05) coupling in approximately ~40 to ~60% of individuals for both fast and slow spindles, based on the adaptive SO definition (the default in our primary spindle/SO coupling analyses); again, proportions tended to be significantly lower when using the absolute definition (Supplementary Table 6).
Although not included as a metric in our primary analyses, in order to assess the performance of asymptotic significance tests of non-random spindle/SO and spindle/SWA coupling, for each individual we calculated the proportion of shuffled replicates that were nominally p < 0.05 significant. Expected to be approximately 5% under the null, we observed many individuals for whom this proportion was at least doubled; in both MESA and MrOS, the median proportion of significant nulls could be as high as 10% for fast spindles, and 5% of individuals had type I error rates over 20% (Supplementary Table 6). These results underscore the potential for bias when relying on certain asymptotic statistical tests of coupling, and the need for robust approaches including non-parametric, empirical methods, as employed here.
Principal spectral component (PSC) analysis
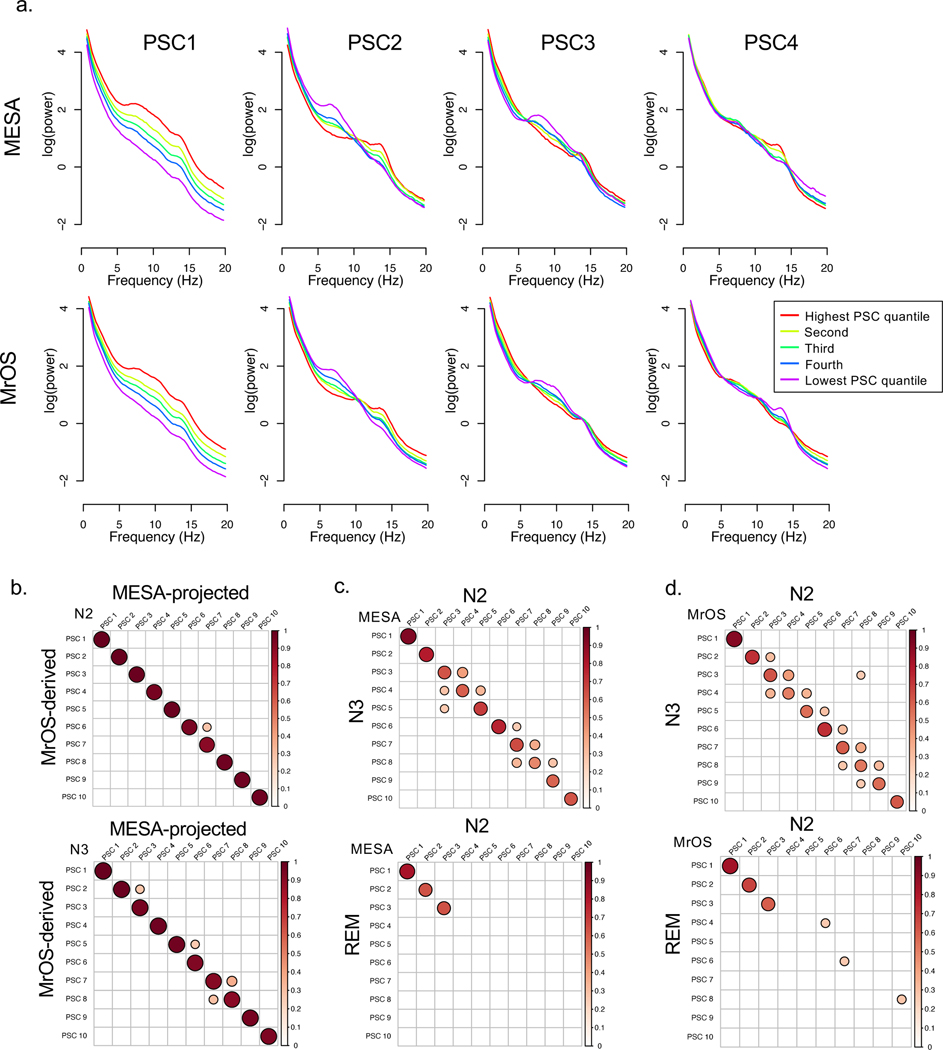
We also applied a multivariate statistical approach (PSC analysis) to capture some of the complex and inter-related features that biological signals often show, for example between spindle and other rhythmic activity54. Whereas standard measures of power primarily focus on predefined frequency intervals one at a time (e.g. 11–15 Hz “sigma” power), PSCs can capture coordinated changes across the entire power spectra in a more flexible, data-driven manner, as well as providing an alternative to normalization based on relative power (see Methods). Applied to N2 and N3 sleep separately, PSC analysis reduced the joint variation across all 1,595 MESA power spectra to ten orthogonal components that explained over 99% of the total variance (Supplementary Figure 5); similar curves were observed for MrOS (data not shown). Taking the ten largest components from analyses performed separately in MESA and MrOS, we observed a clear correspondence between the kth MESA component and the kth MrOS component (Figures 3a, 3b). Broadly speaking, the first components (PSC1) captured individual differences in total power, whereas the second components reflected differences in the general steepness of the 1/f slope (Figure 3a). The third and fourth components reflected individual differences yoked across alpha, sigma and beta bands (Supplementary Figures 6 and 7 show the equivalent plots for all N2 and N3 PSCs). Nonetheless, to ensure comparability between MESA and MrOS, our primary analysis used components based on a projection of the MESA PSC analysis to compute MrOS components. These ‘MESA-derived’ MrOS components were highly similar to the components obtained from a MrOS-only analysis (Figure 3b).
Figure 3. Principal spectral component (PSC) analysis.
a) Median absolute log-scaled power for five groups defined by the quintiles of the corresponding PSC component during N2 sleep, showing the first four components (see Supplementary Figures 6 and 7 for the first ten, in both N2 and N3 sleep). b) Absolute correlations from an intra-MrOS comparison of the top ten N2 PSC components, comparing those derived from analysis within MrOS versus those projected from the MESA-derived components. The results show a very strong correspondence between the PSC structures in MESA and MrOS. c) Within-cohort, between sleep stage comparisons for N2-N3 (top row) and N2-REM (bottom row) PSC for MESA, showing absolute Pearson correlation coefficients. Whereas N2 and N3 show a clear correspondence of PSCs as ordered by the rank of singular values, REM only shows strong correspondence for the first three PSCs. d) Same as c) but showing results from MrOS. For clarity of presentation, in all correlation plots (b-d), only correlations p <10−10 and |r| > 0.25 are shown.
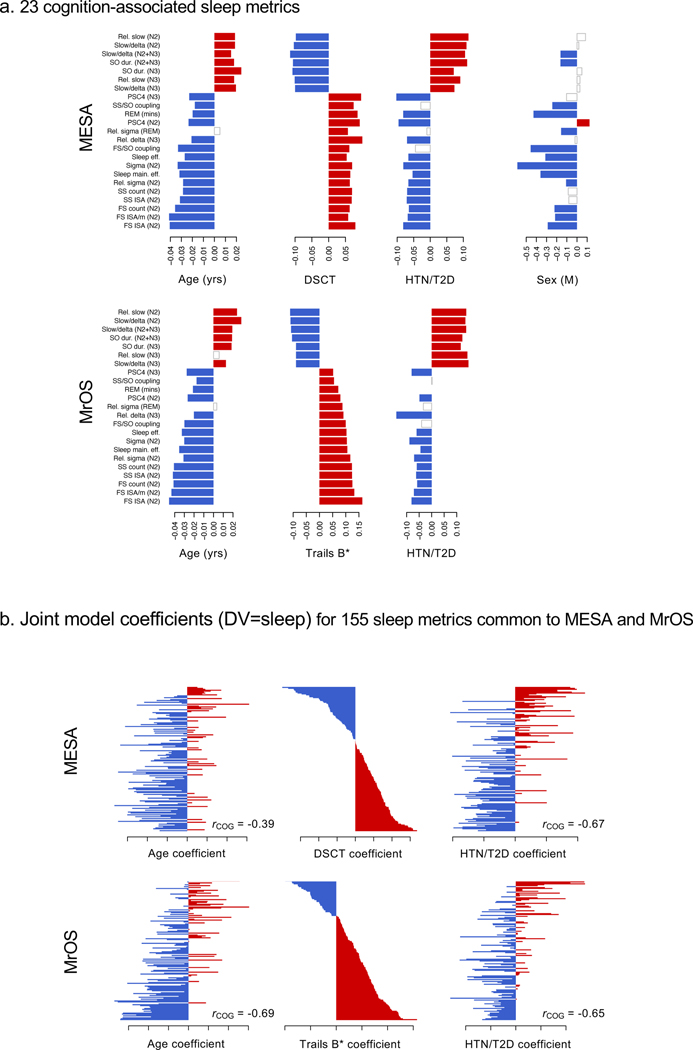
Figure 7. Patterns of associations between sleep metrics and age, cognition, cardiometabolic disease and sex.
a) Standardized regression coefficients for age (per year), cognitive performance (DSCT and Trails B* in MESA and MrOS respectively, per SD unit), a binary indicator variable denoting the presence of hypertension or diabetes, and sex (1 male, 0 female). Rows correspond to the 23 replicated cognition-associated sleep metrics, ordered by increasing effect size of the cognition/sleep association in MrOS. Positive effects are in red, negative effects are in blue; effects not nominally significant (p>0.05) are white/gray. For the DSCT/Trails B*, cognition was the dependent variable and the sleep metric was the predictor (i.e. as per the primary analyses). For the other variables, the sleep metric was the dependent variable. All models included baseline covariates. b) Results for all 155 sleep metrics in common between MESA and MrOS (all objective metrics plus ESS). Unlike a), here effect estimates for age, cognition and cardiometabolic disease were all jointly estimated in a single multiple linear regression, with the sleep metric as the dependent variable, and age, cognitive performance (DSCT or Trails B*) and cardiometabolic disease state as predictors (along with other baseline model covariates). All effect estimates are plotted in red/blue to denote the direction of effect (b>0 or b<0), regardless of statistical significance. Rows/sleep metrics are sorted by the cognitive effect estimate, separately for MESA and MrOS. Across all sleep metrics, effect estimates were highly negatively correlated for cognition and cardiometabolic in both MESA (Pearson’s r = −0.66, 95% CI −0.74, −0.57, p < 10−15) and MrOS (r = −0.65, 95% CI −0.73, −0.55, p < 10−15) as well as age in MESA (r = −0.39, 95% CI −0.52, −0.25, p < 10−15) and MrOS (r = −0.69, 95% CI −0.76, −0.59, p < 10−15) respectively.
Within the same cohort, there was clear correspondence between the kth N2 component and the kth N3 component (Figures 3c, 3d, top two plots). This pairwise correspondence of components did not arise obligatorily: for example, when applied to REM sleep spectra, fewer REM components strongly correlated with a single N2 component (Figures 3c, 3d, bottom two plots), reflecting the greater degree of structured, stereotypical waveforms that occur during NREM but not REM sleep, that are reflected by different PSCs. On the other hand, that the top three components showed greater concordance across REM and NREM sleep might indicate a lower likelihood of reflecting neural oscillatory activity that is specific to sleep, as opposed to general EEG phenomena including sources of gross anatomical or technical variability.
Supplementary Figure 8 shows all nominally (p < 0.05) significant correlations between N2 PSCs and other sleep metrics (after adjustment for age, sex, race and site). PSCs did not straightforwardly correspond to traditional band power metrics (either absolute or relative) in a one-to-one manner; different PSCs also showed qualitatively distinct patterns of correlation with spindle and slow oscillation metrics. For example, PSC 4 correlated positively with both fast and slow spindle density, and positively with slow spindle frequency, but negatively with fast spindle frequency, underscoring the complex patterns of individual differences in the EEG not necessarily captured by standard metrics. In general, Supplementary Figure 8 points to PSCs as reflecting a complex but data-driven re-parameterization of dozens of inter-related sleep metrics, and one that was stable between MESA and MrOS.
Correlational structure of sleep metrics
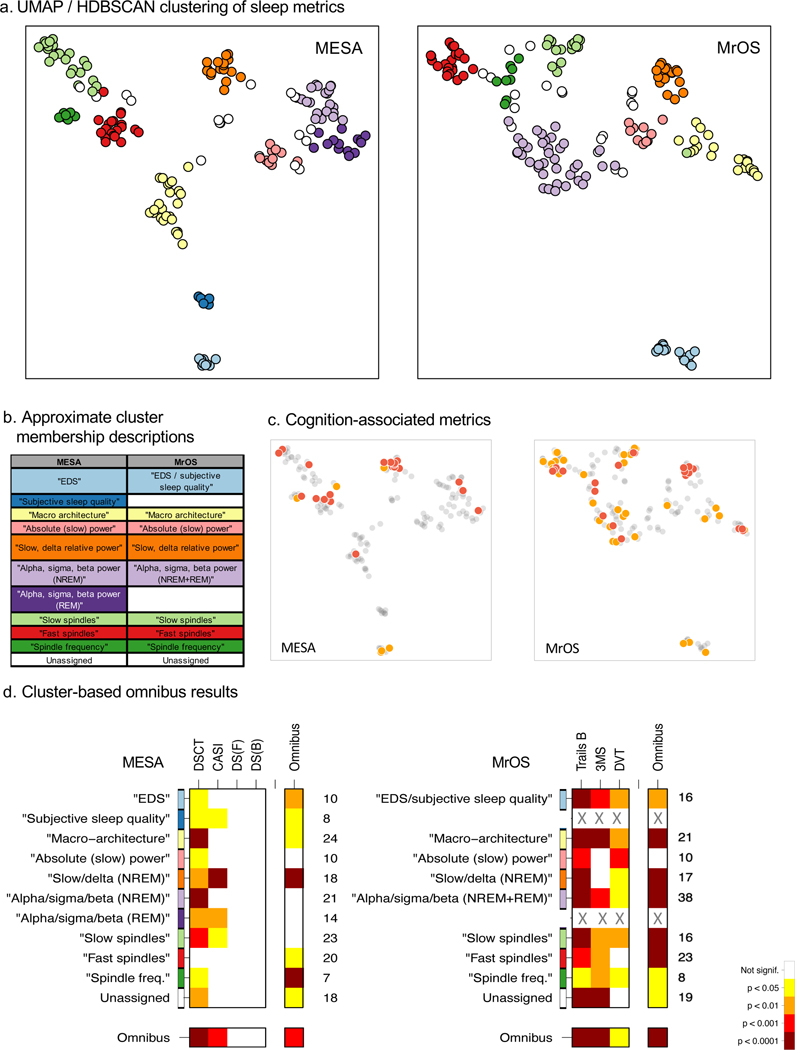
As described below, we originally organized sleep metrics into eleven prespecified ‘domains’, simply to provide some higher-order structure for evaluating association results (Figure 4a). An alternative approach – and one that may offer insight into how different aspects of sleep are interrelated – is to adopt a data-driven, empirical clustering of similar metrics. Based on distances derived from the correlation matrices for all metrics (Supplementary Figure 9), we applied dimension reduction for visualization and clustering (UMAP and HDBSCAN, see Methods) to MESA and MrOS separately. This analysis yielded 10 clusters in MESA plus a set of metrics not confidently assigned to any one cluster, and 8 clusters in MrOS plus a further ‘unassigned’ set (Supplementary Figure 10). We observed broadly comparable clustering between MESA and MrOS (Figures 5a, note, the precise, global locations of where each cluster appears are largely arbitrary and should not be directly compared between plots; rather the local grouping of metrics is the more relevant aspect). We gave descriptive labels to clusters (Figures 5b) based on the assigned metrics (Supplementary Figure 11) but note that these are necessarily approximations. Although pre-specified ‘domains’ did not relate in a one-to-one manner with empirical clusters, there were clear points of correspondence (Supplementary Figure 12). On the broadest level, subjective, macro-architecture and micro-architecture metrics formed largely distinct clusters (in particular, the independence of subjective and objective metrics was clear from the raw correlation matrices, Supplementary Figure 9). In terms of micro-architecture metrics, perhaps the biggest difference between classifications was that fast and slow spindles were shown to be separable categories in both MESA and MrOS (Figures 5a, 5b), in contrast to our primary partitioning by ‘occurrence’ and ‘morphology’. The PSC metrics spanned a broad range of the space of micro-architecture metrics, as one might expect given the orthogonality inherent in their definition (Supplementary Figure 11).
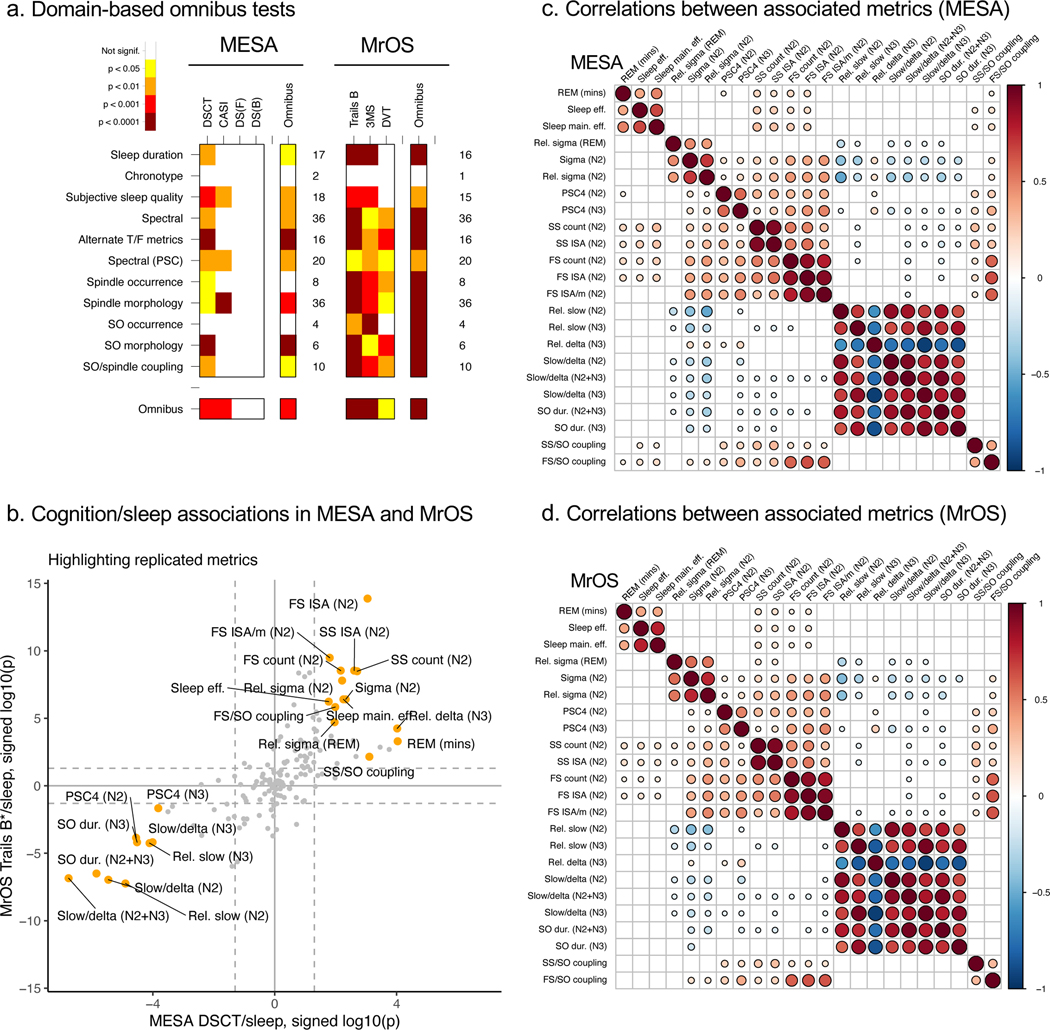
Figure 4. Primary sleep-cognition association results for omnibus tests and the 23 selected objective metrics, in MESA and MrOS.
a) Domain-based omnibus results in MESA and MrOS. Color-coded empirical significance values based on 20,000 permutations, calculated using the baseline covariate model and using the max test statistic to control for multiple testing. See Methods for further details. b) Correspondence of cognition/sleep associations in MESA and MrOS, showing log10-scaled p-values signed by the direction of effect, for DSCT in MESA (x-axis) versus Trails B* in MrOS (y-axis). Labeled orange points indicate the 23 metrics that replicated across cohorts. c) Correlation coefficients (Pearson’s r) in MESA between key sleep metrics, adjusted for baseline covariates, and only showing cells where p < 0.01 and |r| > 0.1. d) As above, for MrOS.
Figure 5. Empirical clustering of sleep metrics.
a) Results from UMAP dimension reduction (based on the 1-|rij| where rij is the Pearson correlation coefficient between the ith and jth sleep metrics), extracting 2 components for visualization, in MESA and MrOS separately. Colors correspond to the cluster assignment from HDBSCAN cluster analysis based on 10 UMAP components. b) Approximate labels of clusters from HDBSCAN analysis, see Supplementary Figure 10b for a full tabulation. MESA yielded a 10-cluster solution, MrOS an 8-cluster solution; clusters that are “similar” between MESA and MrOS (based on assigned metrics) are listed next to each other. c) Indication of cognition-associated sleep metrics in the UMAP 2-dimensional projection of MESA and MrOS sleep metrics. Red: one of the 23 replicated hits. Orange: metrics with p < 10−3 for DSCT (MESA) or Trails B (MrOS). d) Omnibus association empirical significance values for groupings of sleep metrics based on data-driven clustering, for the max-statistic.
Although, at the highest level, these cluster solutions arguably have face validity, there are limitations inherent in these analyses (outlined below in the Discussion) and it would be a mistake to place too much weight on the specifics of any one solution. We therefore use these empirically-derived clusters as well as the original ‘domains’ to structure the omnibus association testing, below.
Demographic correlates of sleep micro-architecture
As others have reported29,55–57, many sleep metrics showed marked age-related trends and sex differences. In general, objective sleep metrics showed larger demographic correlations compared to subjective measures. Age-related trends for objective sleep metrics were generally consistent between MESA and MrOS (Supplementary Figure 13a). Of all metrics, fast spindle density (including related metrics: spindle count, ISAM and ISAT, where ISA denotes Integrated Spindle Activity, see Methods) showed the largest age-related changes, in both MESA (b = −0.37 SD units per decade, 95% CI −0.42, −0.32, p < 10−15) and MrOS (b = −0.42, 95% CI −0.49, −0.34, p < 10−15), although multiple slow spindle occurrence, sigma power and spindle morphology metrics also showed substantial age-related changes in both cohorts (Supplementary Data 1 & 2). For example, both fast and slow spindles during N2 sleep showed reduced chirp (intra-spindle change in frequency) with increasing age, in both MESA (b = 0.22, 95% CI 0.17, 0.27, p < 10−15, and b = 0.12, 95% CI 0.06, 0.17, p = 1×10−5 for slow and fast spindles respectively) and MrOS (b = 0.33, 95% CI 0.25, 0.41, p < 10−15 and b = 0.17, 95% CI 0.09, 0.25, p = 3×10−5). (In all cases, mean spindle chirp was negative, meaning that over the course of a single spindle, oscillations tended to slow down; the positive coefficients above indicate that older individuals tended to have spindles that slowed down less.) A second age-related change in spindle morphology involved spindle frequency (Figure 2b). Although fast and slow spindles were defined in terms of fixed target FC (11 or 15 Hz), there was still considerable person-to-person variability in the observed frequencies of spindles detected for a given FC (e.g. slow spindles may have a mean frequency of 10.5 Hz in one individual, versus 11.5 Hz in another). In both MESA and MrOS, with increasing age, slow spindles grew slower (b = −0.28, 95% CI −0.34, −0.23, p < 10−15 and b = −0.26, 95% CI −0.34, −0.18, p = 2×10−10 for MESA and MrOS respectively) whereas fast spindles grew faster (b = +0.19, 95% CI 0.14, 0.24, p = 3×10−12 and b = +0.23, 95% CI 0.16, 0.31, p=5×10−9). Similar directional effects were observed during N3 sleep, and several PSCs exhibited large correlations of opposite signs between fast versus slow spindle frequency including PSC 4, the component with the strongest age association in both MESA and MrOS (Supplementary Figure 8).
Aside from spindle activity, a number of other sleep metrics showed marked age-related changes. In terms of sleep macro-architecture, WASO increased with age in both MESA (b = 19.3 minutes per decade, 95% CI 16.3, 22.2, p < 10−15) and MrOS (b = 21.0, 95% CI 16.3, 25.7, p < 10−15), with corresponding decreases in sleep efficiency. Both MESA and MrOS showed significant age-related increases in N1 sleep (absolute duration in minutes) as well as decreases in N3 and REM sleep duration. We observed marked age-related reductions in the magnitude of spindle/SO coupling, for both fast and slow spindles, as well as age-related shifts in the mean coupling phase angle, such that spindles occurred earlier in the SO in older individuals, in both MESA and MrOS (Supplementary Data 1 & 2). In contrast, although spindle/SO gross overlap decreased with age MESA, there was no evidence for association with age in MrOS (perhaps reflecting that coupling has already broken down for a greater proportion of individuals in the older MrOS sample, Supplementary Table 6).
Age-related changes in spectral power, including decreasing absolute sigma N2 power (b = −0.33 SD per decade, 95% CI −0.38, −0.29, p < 10−15 and b = −0.30, 95% CI −0.38, −0.22, p = 4×10−14 in MESA and MrOS respectively) were generally statistically stronger in MESA despite the smaller sample size (potentially reflecting its greater age range). We observed age-related reductions in delta power (e.g. b = −0.21, 95% CI −0.27, −0.15, p = 1×10−11 for relative delta power during N3 in MESA, and b = −0.20, 95% CI −0.29, −0.11, p = 6×10−6 in MrOS, with broadly similar results for N2 and absolute power metrics too). In contrast, we observed qualitatively different results for slow (<1 Hz) power, which did not exhibit credible evidence for age-related decline during NREM sleep. In fact, in only one instance was absolute slow power significantly associated with age, in which case it increased with increasing age (absolute slow power during N2 in MrOS, b = 0.12, 95% CI 0.05, 0.20, p = 0.002). Relative slow power also tended to increase with increasing age (e.g. b = +0.19, 95% CI 0.13, 0.24, p = 3×10−12 for relative slow power during N2 in MESA, and b = +0.24, 95% CI 0.16, 0.32, p = 1×10−9 in MrOS).
In terms of sex differences within MESA, the most marked reflected males having generally lower absolute spectral power during both N2 and N3, across all power bands. This association was captured by a large difference in PSC 1 (b = −0.78 SD units, 95% CI −0.87, −0.70, p < 10−15). Males also had shorter scored N3 (b = −23.2 minutes, 95% CI −26.1, −20.3, p < 10−15) and REM sleep (b = −12.6 minutes, 95% CI −15.3, −9.8, p < 10−15 ) as well as TST (b = −37.1 minutes, 95% CI −44.5, −29.8, p < 10−15). Consistent with our previous report in independent cohorts7, we observed sex differences in spindle density for fast (b = −0.18 SD units, 95% CI −0.27, −0.08, p = 2×10−4) but failed to reject the null hypothesis of no sex differences for slow (b = 0.07, 95% CI −0.02, 0.17, p = 0.12) spindles; a similar pattern generally held for most fast and slow spindle morphology metrics too. Fast spindles further showed a significant sex difference in spindle/SO gross overlap, with males showing reduced fast spindle overlap (b = −0.31 SD units, 95% CI −0.40, −0.21, p < 6×10−10), whereas we did not observe credible evidence of such an effect for slow spindles (b = −0.05, 95% CI −0.14, 0.05, p = 0.35). Finally, despite excellent power to detect relatively small effects (see Supplementary Methods), and the general tendency for males to have objectively ‘worse’ sleep (in the sense of male/female differences more often corresponding to old/young rather than young/old differences for a given metric), a number of metrics showed qualitatively distinct patterns of age and sex differences. For example, whereas the most statistically significant sex difference in MESA was for higher N2 absolute theta power in females (0.81 SD units, 95% CI 0.72, 0.90, p = 10−64), there was no credible evidence for association with age (b = −0.002, 95% CI −0.006, 0.003, p = 0.53). On the other hand, metrics including slow spindle density and frequency showed marked age-related change, but did not show credible evidence for any sex differences. Finally, as alluded to above, other metrics showed highly significant changes with respect to both age and sex, e.g. SO slope during N2+N3 sleep. Future work will be needed to resolve the extent of overlap in the mechanisms of age-related changes versus sex differences in sleep neurophysiology.
SO occurrence, morphology and coupling metrics showed generally similar profiles of demographic association under both adaptive and absolute threshold definitions (Supplementary Data 3 & 4), albeit with weaker and more variable age-related changes in MrOS compared to MESA. SO count, density, amplitude and slope all declined with increasing age, whereas SO duration increased, meaning that SOs became slower with age. Threshold definition did have a considerable impact on a handful of comparisons, however. Most prominently, in MESA whereas males had markedly lower SO density during N3 compared to females (b = −0.71 SD units, 95% CI −0.81, −0.60, p < 10−15) under the absolute threshold, there was no credible evidence for a similar effect under the adaptive definition (b = −0.045, 95% CI −0.16, 0.07, p = 0.43, Supplementary Data 3), precisely due to the within-individual normalization. In terms of precise spindle/SO phase coupling, all analyses (i.e. whether spindle/SWA coupling that include all spindles or spindle/SO coupling based on adaptive or absolute definitions that include only a subset of spindles, and whether for fast or slow spindles) tended to yield similar patterns of results, namely that the magnitude of coupling decreased with increasing age, and spindles tended to peak earlier during the SO in older individuals.
Primary sleep-cognition analyses
Statistical approach to association analysis: correction for multiple tests and replication
As the sleep-cognition analyses involved hundreds of significance tests, we ensured robustness using two approaches: 1) control for multiple testing by use of a permutation-based omnibus association framework, and 2) testing for replication in an independent sample. Specifically, for each cohort we generated 20,000 permuted datasets, in which individuals’ sleep metrics were randomly reassigned. In each null dataset, we refit all sleep-cognition regression models (e.g. 692 in MESA), recording both the most significant result and average result (in actuality, the maximum and sum of the 692 log-scaled p-values from the regressions). Considering all 20,000 randomized datasets, in this way we estimated the maximum and typical results that would be expected to occur by chance alone, whilst controlling for the correlations between tests. For each of the two aggregate metrics, the proportion of the 20,000 randomized datasets with equal or larger values than originally observed gives an empirical omnibus p-value, labelled below as max and sum (i.e. average) tests. As well as controlling for multiple testing, we used this framework to contextualize broad patterns of association between sleep and cognitive performance, by further considering subsets of tests (e.g. for one particular cognitive measure) rather than across all combinations of sleep metric and cognitive measure. With respect to replication, we initially applied this approach to MESA, and then considered replication in MrOS, at both the individual test and omnibus levels. Finally, we identified individual metrics showing significant and consistent patterns of association across both cohorts. See Methods and Supplementary Methods for more details.
Omnibus and domain-level analyses in MESA
In MESA, the global null hypothesis of no association with cognition for any sleep metrics was rejected under both max (omnibus empirical p = 0.001) and sum (omnibus empirical p = 0.0002) permutation-based omnibus statistics, adjusted for age, sex, race/ethnicity and field center (“site”) (Figure 4a, Supplementary Figure 12a). Of the eleven sleep domains tested, seven were globally significant at omnibus empirical p<0.05 under both statistics (Figure 4a, Supplementary Figure 12a): subjective sleep problems, sleep macro-architecture, spectral and alternate time/frequency metrics, PSC metrics, SO morphology and spindle/SO coupling. Additionally, spindle morphology had omnibus empirical p = 0.0002 for the max statistic, but omnibus empirical p = 0.08 for the sum statistic. In terms of cognitive measures, DSCT and CASI showed significant omnibus association across all sleep metrics (all omnibus empirical p < 0.05 for max and sum statistics), whereas neither digit span measure showed credible evidence for association (DSF and DSB, all omnibus empirical p > 0.15, Figure 4a, Supplementary Figure 12a). For the chronotype domain (which only comprised two metrics) and SO occurrence domain, we failed to reject the null hypothesis of omnibus association in MESA. However, the overwhelming picture, supported by a series of significant omnibus tests (Figure 4a, Supplementary Figure 12a) – from the global to subsets of sleep domains and cognitive measures – points to statistically robust evidence for correlation between sleep metrics and cognition, after controlling for age, sex, race/ethnicity and site.
Metric-level replication in MrOS
Given significant omnibus results across multiple domains, we next sought to identify and characterize associations at the level of individual sleep metrics. Guided by the omnibus tests, we identified 32 sleep metrics that were significant for either DSCT or CASI at p < 10−2 (based on nominal, asymptotic baseline model p-values) in MESA and present in the independent MrOS cohort for a replication attempt. Of the 32 significant metrics that spanned subjective, macro-architecture, spectral power, PSC, spindle and SO domains, 30 (94%) were nominally significant in MrOS for Trails B*, 3MS or DVT*, all of which had the same direction of effect (Supplementary Data 1 & 2). Overall, these results suggested a clear correspondence between associations in MESA and MrOS.
Omnibus and domain-level analyses in MrOS
Given the high rate of replication in MrOS, we next sought to characterize the patterns of association in MrOS more generally, adopting the same omnibus testing framework as applied to MESA (Figure 4a, Supplementary Figure 12a). The global omnibus test was significant for both max (omnibus empirical p = 5×10−5, implying that none of 20,000 null replicates equaled or exceeded the observed statistic) and sum (omnibus empirical p=5×10−5) statistics. Omnibus tests for all three cognitive measures were significant for both statistics, and ten of the eleven sleep domains were significant for both statistics, the exception being the (single-item) chronotype domain (Figure 4a, Supplementary Figure 12a). That is, we observed unambiguous statistical support at the omnibus level to suggest that sleep metrics across multiple domains of sleep were associated with the three cognitive measures in MrOS. Overall, results were statistically stronger in MrOS than MESA, potentially reflecting the larger sample size, different demographics (all male, on average 7.9 years older) and/or the different cognitive measures employed.
Omnibus cluster-level results in MESA and MrOS
Re-running the omnibus tests but with sleep metrics grouped by empirical cluster instead of prespecified ‘domain’, we observed broadly similar patterns of results (Figure 5d, Supplementary Figure 12b). For our primary test, based on max statistics, of the ten clusters and one unassigned set, all but one (“fast spindles”) were significantly associated with DSCT in MESA. In MrOS, all nine groups were significantly associated with Trails B (Figure 5d). That is, consistent with the domain-based grouping, testing based on empirical clusters pointed to multiple, independent aspects of sleep macro and micro architecture as being associated with cognitive performance, rather than only one or two tightly interconnected subsets of sleep metrics.
Identifying consistently associated sleep metrics across MESA and MrOS
As suggested by the above replication analyses, it seemed probable that many true MrOS associations would also be present in MESA and vice versa. To represent our final set of cognition-associated sleep metrics, we therefore identified all metrics that were consistently and significantly associated across both MESA and MrOS, as follows. For two of the four cognitive tests in MESA (DSF and DSB), the omnibus analyses failed to reject the global null hypothesis; we therefore did not consider those two tests further, to reduce the testing burden by a factor of two in this cohort. In contrast, associations appeared more diffusely spread with respect to type of sleep metric, in both MESA and MrOS, whether considering either domains or empirical clusters, for both max and sum statistics. As such, the omnibus results did not provide a clear basis for restricting the sets of sleep metrics to be advanced to individual testing. We therefore adopted a simple and stringent metric-level procedure to obtain a prioritized set of cognition-associated sleep metrics, independent of domain or cluster.
Specifically, we focused on 155 metrics that were common to MESA and MrOS (essentially all objective metrics plus the Epworth Sleepiness Scale [ESS]), for the two omnibus-significant cognitive measures in MESA (DSCT and CASI) and all three cognitive measures in MrOS (Trails B, 3MS and DVT). For this set, we identified metrics meeting Bonferroni-corrected significance at both discovery and replication. That is, for discovery we required p < 0.05 / (155 × t) for at least one cognitive measure in either MESA or MrOS, thereby allowing for 155 sleep metrics × t cognitive measures, where t = 2 in MESA (implying p < 1.6×10−4) and t = 3 in MrOS (p < 1.1×10−4). For metrics meeting the discovery threshold, we further required a replication p < 0.05/t and a directionally consistent association, with one or more cognitive measure in the second cohort. This yielded 23 sleep metrics, henceforth called the ‘associated metrics’ (Tables 1, 2, Figure 4b). Nonetheless, there are still likely to be true positives not included in this set of 23, selected only to represent a manageable subset with the strongest evidence for association.
Table 1. Metric-level cognition/sleep association results based on discovery in MESA, with replication attempted in MrOS.
Metric-level association statistics in MESA as the discovery sample, under the baseline covariate model, for the 13 metrics significant at p < 1.6×10−4 (0.05 Bonferroni corrected for 155 metrics present in both MESA and MrOS × 2 cognitive tests), considering only results for DSCT and CASI (i.e. based prior on omnibus results). Of these 13, 11 replicated in MrOS at p < 0.0166 (0.05 Bonferroni corrected for 3 cognitive tests). One subjective measure was not present in MrOS; the other objective measure (fast spindle frequency) was nominally significant in MrOS (p = 0.03) but did not meet the Bonferroni-adjusted threshold. All replicated associations showed the same direction of effect. Outcome indicates the most significant cognitive test (DSCT or CASI for MESA, or Trails B*, 3MS or DVT* for MrOS) and b and p indicate the corresponding coefficient and significance value. Note, Trails B* and DVT* are measures with the sign reversed, so that negative coefficients indicate worse cognitive performance for all measures.
| MESA, discovery p < 0.05/(155×2) | MROS, replication p < 0.05/3 | ||||||
|---|---|---|---|---|---|---|---|
| Sleep metric | Outcome | b (95% CI) | p | Replication? | Outcome | b (95% CI) | p |
| SWR (N2 & N3) | DSCT | –0.11 (−0.16,−0.072) | 2×10−7 | Yes | Trails B* | –0.11 (−0.15,−0.068) | 1×10−7 |
| SO wavelength (N2 & N3) | DSCT | –0.11 (−0.15,−0.063) | 1×10−6 | Yes | Trails B* | –0.1 (−0.14,−0.064) | 3×10−7 |
| SWR (N2) | DSCT | –0.1 (−0.15,−0.06) | 3×10−6 | Yes | Trails B* | –0.11 (−0.15,−0.07) | 1×10−7 |
| Slow (N2 relative) | DSCT | –0.098 (−0.14,−0.054) | 1×10−5 | Yes | Trails B* | –0.11 (−0.15,−0.071) | 6×10−8 |
| PSC 4 (N2) | DSCT | 0.092 (0.049,0.14) | 3×10−5 | Yes | Trails B* | 0.079 (0.039,0.12) | 0.00014 |
| SO wavelength (N3) | DSCT | –0.11 (−0.16,−0.057) | 3×10−5 | Yes | Trails B* | –0.089 (−0.13,−0.045) | 7×10−5 |
| Slow (N3 relative) | DSCT | –0.1 (−0.15,−0.051) | 8×10−5 | Yes | Trails B* | –0.089 (−0.13,−0.046) | 6×10−5 |
| REM duration (mins) | DSCT | 0.086 (0.043,0.13) | 9×10−5 | Yes | Trails B* | 0.071 (0.031,0.11) | 0.00050 |
| Delta (N3 relative) | DSCT | 0.1 (0.05,0.15) | 1×10−4 | Yes | Trails B* | 0.091 (0.047,0.13) | 6×10−5 |
| SWR (N3) | DSCT | –0.1 (−0.15,−0.05) | 1×10−4 | Yes | Trails B* | –0.089 (−0.13,−0.045) | 7×10−5 |
| PSC 4 (N3) | DSCT | 0.096 (0.047,0.15) | 0.00015 | Yes | DVT* | 0.067 (0.021,0.11) | 0.0040 |
| Fast spindle frequency (N2) | CASI | –0.1 (−0.15,−0.057) | 9×10−6 | No | Trails B* | –0.045 (−0.085,−0.0045) | 0.030 |
| Fall asleep while sitting and talking | DSCT | –0.087 (−0.13,−0.046) | 4×10−5 | N/A | . | . | . |
Table 2. Metric-level cognition/sleep association results based on discovery in MrOS, with replication attempted in MESA.
Similar results for presented in Table 1, except here with MrOS as the discovery cohort and MESA as the replication, with suitably adjusted significance thresholds representing the different number of cognitive tests considered in each study. Here, 39 metrics were significant after correction for multiple testing in the MrOS discovery cohort; of these, 20 were significant (corrected for the two tests in MESA) and had the same direction of effect in MESA. Of the remainder, 2 subjective metrics were not present in MESA and so could not be tested for replication; of the other 17, 7 were nominally significant at p<0.05 but did not meet the stricter threshold of 0.05/3. Note, Trails B* and DVT* are measures with the sign reversed, so that negative coefficients indicate worse cognitive performance for all measures. Overall, from both Tables 1 and 2 there are 23 unique sleep metrics that were discovered in one cohort and replicated in the second cohort.
| MrOS, discovery p < 0.05/(155×3) | MESA, replication p < 0.05/2 | ||||||
|---|---|---|---|---|---|---|---|
| Sleep metric | Outcome | b (95% CI) | p | Replication? | Outcome | b (95% CI) | p |
| Fast spindle total ISA (N2) | Trails B* | 0.16 (0.12,0.2) | 1×10−14 | Yes | DSCT | 0.079 (0.033,0.13) | 0.00090 |
| Fast spindle ISA per minute (N2) | Trails B* | 0.13 (0.091,0.17) | 3×10−10 | Yes | DSCT | 0.058 (0.011,0.1) | 0.016 |
| Fast spindle count (N2) | Trails B* | 0.12 (0.084,0.17) | 3×10−9 | Yes | DSCT | 0.062 (0.017,0.11) | 0.0068 |
| Slow spindle total ISA (N2) | Trails B* | 0.12 (0.083,0.16) | 3×10−9 | Yes | DSCT | 0.069 (0.024,0.11) | 0.0025 |
| Slow spindle count (N2) | Trails B* | 0.12 (0.083,0.16) | 3×10−9 | Yes | DSCT | 0.069 (0.026,0.11) | 0.0020 |
| Sigma (N2 relative) | Trails B* | 0.12 (0.077,0.16) | 2×10−8 | Yes | DSCT | 0.063 (0.018,0.11) | 0.0061 |
| Slow (N2 relative) | Trails B* | –0.11 (−0.15,−0.071) | 6×10−8 | Yes | DSCT | –0.098 (−0.14,−0.054) | 1×10−5 |
| Sleep efficiency | 3MS | 0.11 (0.069,0.15) | 9×10−8 | Yes | CASI | 0.059 (0.013,0.11) | 0.012 |
| SWR (N2) | Trails B* | –0.11 (−0.15,−0.07) | 1×10−7 | Yes | DSCT | –0.1 (−0.15,−0.06) | 3×10−6 |
| SWR (N2 & N3) | Trails B* | –0.11 (−0.15,−0.068) | 1×10−7 | Yes | DSCT | –0.11 (−0.16,−0.072) | 2×10−7 |
| SO wavelength (N2 & N3) | Trails B* | –0.1 (−0.14,−0.064) | 3×10−7 | Yes | DSCT | –0.11 (−0.15,−0.063) | 1×10−6 |
| Sleep maintenance efficiency | Trails B* | 0.11 (0.065,0.15) | 4×10−7 | Yes | DSCT | 0.064 (0.019,0.11) | 0.0056 |
| Sigma (N2 absolute) | Trails B* | 0.1 (0.064,0.14) | 4×10−7 | Yes | DSCT | 0.07 (0.021,0.12) | 0.0047 |
| FS/SO magnitude (N2+N3) | Trails B* | 0.1 (0.059,0.14) | 1×10−6 | Yes | DSCT | 0.061 (0.015,0.11) | 0.010 |
| SS/SO magnitude (N2+N3) | 3MS | 0.091 (0.051,0.13) | 8×10−6 | Yes | DSCT | 0.074 (0.031,0.12) | 0.00078 |
| Sigma (REM relative) | Trails B* | 0.087 (0.047,0.13) | 2×10−5 | Yes | DSCT | 0.057 (0.013,0.1) | 0.011 |
| Slow (N3 relative) | Trails B* | –0.089 (−0.13,−0.046) | 6×10−5 | Yes | DSCT | –0.1 (−0.15,−0.051) | 8×10−5 |
| Delta (N3 relative) | Trails B* | 0.091 (0.047,0.13) | 6×10−5 | Yes | DSCT | 0.1 (0.05,0.15) | 1×10−4 |
| SO wavelength (N3) | Trails B* | –0.089 (−0.13,−0.045) | 7×10−5 | Yes | DSCT | –0.11 (−0.16,−0.057) | 3×10−5 |
| SWR (N3) | Trails B* | –0.089 (−0.13,−0.045) | 7×10−5 | Yes | DSCT | –0.1 (−0.15,−0.05) | 1×10−4 |
| FS/SWA magnitude (N2+N3) | Trails B* | 0.12 (0.081,0.16) | 4×10−9 | No | DSCT | 0.042 (−0.0037,0.087) | 0.072 |
| Fast spindle density (N2) | Trails B* | 0.12 (0.083,0.16) | 4×10−9 | No | DSCT | 0.032 (−0.013,0.078) | 0.16 |
| Fast spindle ISA per spindle (N2) | Trails B* | 0.12 (0.079,0.16) | 8×10−9 | No | DSCT | 0.038 (−0.0078,0.084) | 0.10 |
| WASO | 3MS | –0.11 (−0.15,−0.067) | 2×10−7 | No | DSCT | –0.047 (−0.092,−0.0022) | 0.040 |
| Slow oscillation count (N2 & N3) | 3MS | 0.1 (0.065,0.14) | 3×10−7 | No | DSCT | 0.047 (0.002,0.091) | 0.041 |
| Fast spindle count (N3) | Trails B* | 0.11 (0.067,0.16) | 1×10−6 | No | DSCT | 0.033 (−0.021,0.086) | 0.23 |
| N1 duration (%) | Trails B* | –0.096 (−0.14,−0.056) | 2×10−6 | No | DSCT | –0.043 (−0.087,0.0016) | 0.059 |
| Slow spindle density (N2) | Trails B* | 0.097 (0.056,0.14) | 3×10−6 | No | DSCT | 0.046 (0.0014,0.091) | 0.043 |
| Slow spindle ISA per minute (N2) | Trails B* | 0.096 (0.055,0.14) | 4×10−6 | No | DSCT | 0.047 (0.0017,0.093) | 0.042 |
| Fast spindle ISA per minute (N3) | Trails B* | 0.1 (0.057,0.15) | 8×10−6 | No | DSCT | 0.051 (−0.0011,0.1) | 0.055 |
| Fast spindle total ISA (N3) | Trails B* | 0.099 (0.055,0.14) | 1×10−5 | No | DSCT | 0.058 (0.0042,0.11) | 0.035 |
| Fast spindle density (N3) | Trails B* | 0.093 (0.049,0.14) | 4×10−5 | No | DSCT | 0.023 (−0.028,0.074) | 0.38 |
| Slow oscillation density (N2 & N3) | 3MS | 0.083 (0.043,0.12) | 5×10−5 | No | CASI | 0.023 (−0.023,0.069) | 0.33 |
| Sigma (N3 relative) | Trails B* | 0.089 (0.046,0.13) | 6×10−5 | No | DSCT | 0.05 (−0.00016,0.1) | 0.051 |
| Beta (REM relative) | DVT* | 0.084 (0.043,0.13) | 8×10−5 | No | DSCT | 0.044 (0.00078,0.088) | 0.046 |
| Sigma (N3 absolute) | Trails B* | 0.087 (0.044,0.13) | 9×10−5 | No | DSCT | 0.056 (0.002,0.11) | 0.042 |
| FS/SWA angle (N2+N3) | 3MS | 0.089 (0.044,0.13) | 1×10−4 | No | DSCT | –0.036 (−0.084,0.013) | 0.15 |
| PSQI (efficiency) | Trails B* | –0.089 (−0.13,−0.049) | 1×10−5 | N/A | . | . | . |
| FOSQ (productivity) | Trails B* | 0.084 (0.045,0.12) | 3×10−5 | N/A | . | . | . |
Considering these 23 associated metrics, cognitive performance was related to sleep across macro-architecture and multiple spectral, spindle, SO and spindle/SO coupling domains. Based on a visual inspection their correlational structure (Figure 4c,d), associated metrics fell across at least three broad classes: 1) sleep duration and continuity, 2) spindle activity and spindle/SO coupling and 3) slow wave activity. Consistent with the omnibus results, these 23 metrics were also widely dispersed across UMAP component space (Figure 5c). Briefly, in terms of macro-architecture, increased REM duration, sleep efficiency and sleep maintenance efficiency were associated with better cognitive performance (Figure 4b, Tables 1 & 2). In terms of spindle activity, higher count and ISA were associated with better cognitive performance for both fast and slow N2 spindles, as well as higher fast spindle ISA/min, absolute and relative sigma power during N2, but also higher sigma power during REM. Higher values of PSC 4 (which was correlated with higher sigma but lower beta power, multiple of spindle metrics, and showed a marked age-related decrease) were also associated with better cognition during both N2 and N3 sleep (Figures 4b, 6a & Supplementary Figure 8). With respect to slow wave activity, we observed eight highly interrelated cognition-associated metrics (Figures 4c, 4d). Higher relative slow (<1 Hz) power (during both N2 and N3) but lower relative delta (1–4 Hz) power (N3) were associated with worse cognitive performance). Correspondingly, higher slow/delta ratios (for N2, N3 and N2+N3) also predicted worse cognitive performance. Based on individual SOs (detected under default, adaptive thresholds), decreased SO duration (for N3 as well as N2+N3) were associated with better cognitive performance. Associations for SO duration were similar when based on absolute thresholds (Supplementary Data 3 & 4). SO duration was very highly correlated with slow/delta ratio in both MESA (r = 0.947, 95% CI 0.942, 0.952, p<10−15 during N2+N3 sleep) and MrOS (r = 0.952, 95% CI 0.948, 0.956, p<10−15): as per-individual mean SO durations varied between approximately 0.8 and 1.5 seconds in both cohorts, with a mean around 1.1 seconds, slower SOs contribute relatively more to slow (<1 Hz) than to delta (>1 Hz) power. Finally, a stronger magnitude of spindle/SO coupling was associated with better cognitive performance, for both fast and slow spindles (Figure 5b).
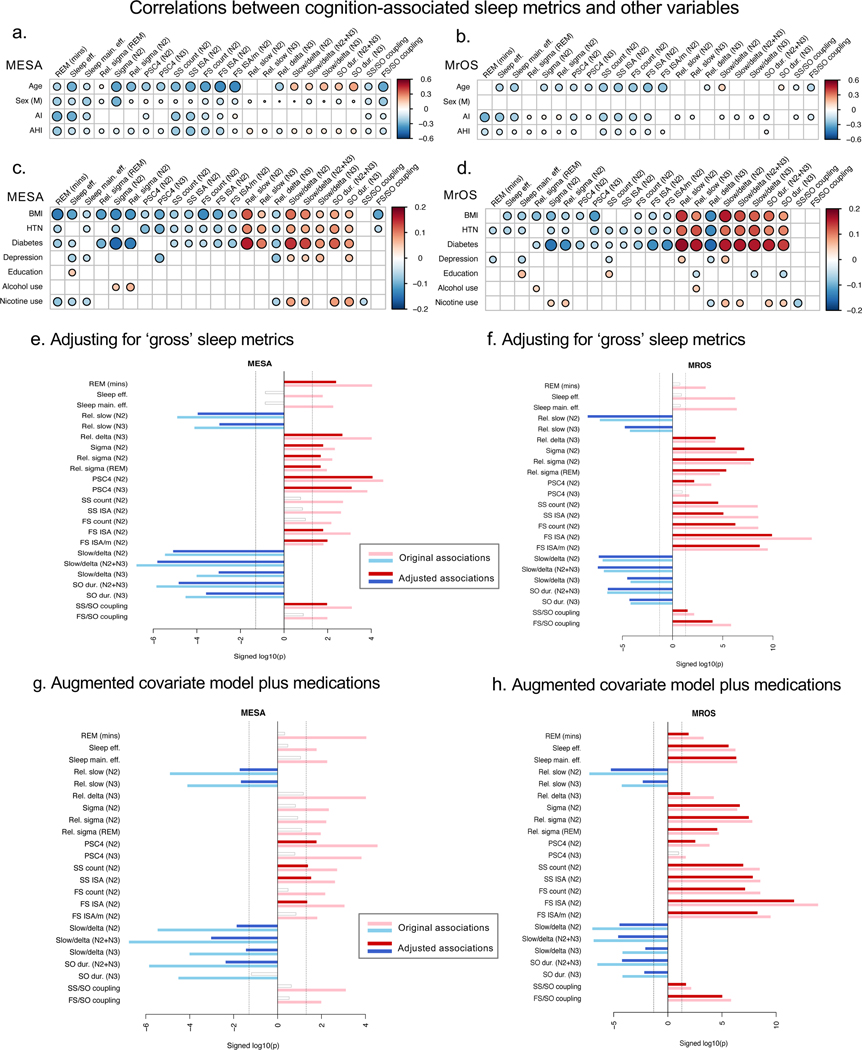
Figure 6. Correlations between cognition-associated metrics and other putative confounding and mediating variables.
Pearson correlations between sleep metrics with age and sex, and other factors. Correlations with variables other than age and sex are adjusted for age, sex, race/ethnicity and collection site (being based on the baseline model residuals rather than raw sleep metrics). Only significant (p<0.05) correlations are shown. a) Correlations with age, sex, AI and AHI in MESA. b) As above, for MrOS. c) Correlations with other health and behavioral measures in MESA. Note, for clarity of presentation, as age, sex, AI and AHI show large correlations with some sleep metrics, the scale is reduced here (+/−0.2 versus +/−0.6). d) As above, for MrOS. e) Association results (direction-of-effect signed -log10 p-values) in MESA for the 23 selected metrics, adjusted for ‘gross’ sleep metrics, namely Epworth Sleepiness Scale (ESS)96, the Women’s Health Initiative Insomnia Rating Scale (WHIIRS)97, and two MESA Sleep Questionnaire items in MESA (see Supplementary Methods). f) As above, in MrOS, adjusting for ESS, Pittsburgh Sleep Quality Index99 and the Functional Outcomes of Sleep Questionnaire100. g) Fully adjusted association results for the selected 23 metrics under the augmented covariate model and including medication-use covariates in MESA. h) As above, for MrOS.
Considering these hits, in almost all cases, DSCT and Trails B were the most highly associated cognitive measure in MESA and MrOS, respectively (Tables 1, 2, Supplementary Data 1 & 2). To simplify subsequent follow-up analyses, we therefore concentrate primarily on DSCT and Trails B.
Joint analyses of associated metrics
That a particular metric was associated independently of the others is challenging to state definitively. We fit penalized Lasso regression models to all 23 metrics, with DSCT and Trails B as the dependent variable for MESA and MrOS respectively; all models were forced to include baseline covariates (see Supplementary Methods). For MESA (N=945 due listwise deletion for missing data and the presence of N3 metrics), ten metrics with non-zero Lasso coefficients were: REM duration, sleep maintenance efficiency, relative slow power (N2), relative sigma power (REM), PSC4 (both N2 and N3), slow spindle count (N2), fast spindle ISA (N2), SO duration (N2+N3) and slow spindle coupling magnitude (N2+N3). For MrOS (N = 1,571 due listwise deletion for missing data), fifteen selected metrics were: REM duration, sleep efficiency and sleep maintenance efficiency, relative slow power (N2), relative delta power (N3), relative sigma power (REM), PSC4 (both N2 and N3), slow spindle ISA (N2), fast spindle ISA and ISA/min (N2), slow/delta ratio (N2), SO duration (N2+N3), fast and slow spindle/SO coupling magnitude (N2+N3).
While unlikely to truly prioritize optimal metrics, the above analyses indicate independent contributions of sleep macro-architecture as well as spectral features of the sleep EEG, spindle activity, SO activity and spindle/SO coupling in predicting cognitive performance. To address this more directly, we asked whether EEG-based micro-architecture metrics (spectral metrics and spindle/SO activity and coupling) were informative over and above ‘simpler’ indices that could be assayed via actigraphy or self-report. We fit a series of baseline regression models additionally controlling for TST, WASO, sleep efficiency and subjectively reported typical sleep duration and sleep quality (Figure 6e & 6f). When predicting cognitive performance controlling for these indices of sleep continuity, satisfaction, disturbance and sleepiness, we found that, as one might expect, metrics that by definition depend on TST (i.e. REM duration, spindle count and total ISA) showed p-values that were orders-of-magnitude smaller, in both MESA and MrOS (Figure 6e, 6f). In MESA, both sleep efficiency metrics, SS count and total ISA, FS count, but also FS/SO coupling magnitude were no longer nominally significant (p>0.05) in the adjusted model; in MrOS, REM duration, both sleep efficiency metrics and PSC4 (N3) were no longer nominally significant. However, for all other metrics, association statistics remained highly significant, suggesting that most micro-architecture metrics captured more than simply reflecting sleep duration or ‘poor sleep’ on a gross level (Figures 6e, 6f).
Profiles of age-related and cognition-related sleep metric associations
Many cognition-associated metrics also showed marked age-related changes (Figures 7a). Furthermore, even though all models controlled for chronological age, associations between objective sleep metrics and cognitive performance appeared to be yoked inversely to their associations with age in both MESA (Figure 7a & Supplementary Figure 13a) and MrOS (Figure 7a & Supplementary Figure 13b). That is, if higher values were associated with increased age (e.g. SO duration), then higher values were also associated with decreased cognitive performance, even after controlling for age. Conversely, if lower values were associated with increased age (i.e. REM duration), then lower values were associated with decreased cognitive performance. More broadly, across all objective sleep metrics and irrespective of statistical significance we observed considerable negative correlations between coefficients for age and cognition from a joint model with the sleep metric as the dependent variable, along with baseline covariates and cardiometabolic disease status (Figure 7b).
Correlates of cognition-associated sleep metrics
Other factors linked to both sleep and cognition may account for one or more of the associations presented above. Across MESA and MrOS, we observed that depressed mood, hypertension, diabetes, alcohol consumption and smoking – but not arousal index (AI), apnea hypopnea index (AHI) or BMI – showed significant associations with cognitive performance (Supplementary Tables 1 & 2). Many of the 23 associated sleep metrics were also correlated with one or more of these factors (Figures 6a–d). In particular, AI, AHI and BMI, although not highly correlated with cognition in this sample, were quite highly correlated with several macro- and micro-architecture metrics. Between cohorts, we also saw consistent patterns of associations for other putative confounders and/or mediators and a number of sleep metrics. For example, in MESA longer SO duration was associated with type 2 diabetes (b = 0.31 SD units, 95% CI 0.18, 0.43, p = 1×10−6) and hypertension (b = 0.20, 95% CI 0.10, 0.30, p = 1×10−4), these associations being of comparable statistical significance to the association between SO duration and DSCT, with all analyses controlling for baseline covariates. SO duration continued to predict DSCT when additionally controlling for diabetes and hypertension status, however, albeit with a wider confidence interval and higher estimated effect size (b = −0.68 SD units, 95% CI −1.0, −0.35, p = 8×10−5), and in this joint model both diabetes (b = −0.17, 95% CI −0.29, −0.06, p = 2×10−3) and hypertension (b = −0.20, 95% CI −0.29, −0.11, p = 2×10−5) remained significant. We observed a similar pattern of associations between SO duration, diabetes/hypertension and Trails B in MrOS also, suggesting that although inter-related, these aspects of cardiometabolic health did not substantively confound or mediate this particular relationship between sleep neurophysiology and cognitive performance. More generally, the patterns of associations between objective metrics and diabetes/hypertension reflected age-related changes in those metrics (Figure 7a, Supplementary Table 7). That is, based on models that regressed each sleep metric on disease state, controlling for age and other baseline covariates, individuals with diabetes/hypertension tended to have sleep metrics that looked more similar to those seen in older but healthier individuals, particular for the NREM slow activity metrics. Even considering all 155 sleep metrics common to MESA and MrOS, and estimating regression coefficients from a series of joint models with the sleep metric as the dependent variable, and cognition (DSCT or Trails B*) and cardiometabolic disease state (hypertension or diabetes) as predictors along with baseline covariates, we observed strong negative correlations between the sleep-cognition associations on one hand, versus sleep-age and sleep-cardiometabolic disease associations on the other hand (Figure 7b). There were also numerous correlations between commonly used medications (including sleeping pills, antidepressants and diabetes/hypertension medications) and the 23 sleep metrics (Supplementary Figure 14a). For example, beta-blocker use was associated with significantly longer SO duration in both MESA (b = 0.23 SD units, 95% CI 0.09, 0.36, p = 0.001) and MrOS (b = 0.14, 95% CI 0.05, 0.24, p = 0.004) controlling for diabetes, hypertension and other baseline covariates. In general, however, it will be challenging to disentangle the effects of medications from the underlying conditions which they treat (Supplementary Figure 14b).
To establish whether the 23 associated sleep metrics showed associations with cognitive performance that were statistically independent of these other factors, we re-ran the regressions with an augmented covariate model, including the factors considered in Figures 6a–d and Supplementary Figure 14 (i.e. educational attainment, AI, AHI, BMI, diabetes, hypertension, depression symptoms, alcohol/nicotine use as well as common medication use, see Supplementary Methods). In MESA, only 10 of the 23 metrics remained a nominally significant predictor of DSCT independent of these factors, including SO duration, slow power metrics, PSC 4 and fast and slow spindle ISA, although even these associations were attenuated (Figure 6g). In MrOS, however, 22 of the 23 metrics remained significant predictors of Trails B after adjustment, albeit with slight reductions in statistical support (Figure 6h). That the MrOS sample was more homogeneous, larger and potentially better-powered to detect associations might account for this apparent difference. In MESA, there was no single additional covariate that clearly accounted for the attenuated sleep-cognition associations (Supplementary Figure 15). Educational attainment, in particular, is often used to adjust neurocognitive test scores; we found that adjusting for educational attainment had little or no impact on our primary baseline results in either MESA (Supplementary Figure 15b) or MrOS, however. Unlike cardiometabolic disease, educational attainment did not show credible evidence of association with most of the 23 sleep metrics (Supplementary Table 7). Overall, further work will be needed to unpack this set of inter-related associations between cognition, cardiometabolic health and medication, and specific elements of sleep neurophysiology, including SO morphology.
Sleep duration: objective and subjective metrics and inverted-U association models
Objectively measured total sleep time (TST) did not meet criteria for association in either study, although there was a nominally significant result for DSCT in MESA (b=0.06, 95% CI 0.01, 0.10, p=0.01). Nonetheless, it is important to note that objectively measured TST tends to capture qualitative as well as quantitative differences in sleep (Supplementary Figure 16). For example, presumably reflecting the typical structure of successive sleep cycles, individuals with longer TST had less % N1 (r=−0.13, 95% CI −0.18, −0.08, p=2×10−7 in MESA, r = −0.20, 95% CI −0.24, −0.16, p < 10−15 in MrOS) but more % REM sleep (r = 0.22, 95% CI 0.18, 0.27, p < 10−15 in MESA and r = 0.17, 95% CI 0.13, 0.21, p < 10−15 in MrOS). Such distinctions may be significant when interpreting studies of self-reported or actigraphy-based sleep duration, and may also have implications when considering putative interventions or behavioral modifications centered around sleep duration: that is, the quality or micro-architecture of sleep, and not simply the quantity, may be the causally relevant factor.
In our cohorts, the available self-report measures of typical sleep duration were only moderately correlated with objective TST from the PSG night, in both MESA (r=0.21, 95% CI 0.16, 0.25, p < 10−15) and MrOS (r=0.17, 95% CI 0.13, 0.21, p < 10−15). As others have noted58, objective TST was also substantially shorter (~6 hours in both MESA and MrOS) compared to self-reported sleep duration (7.8 and 8.1 hours for weekdays and weekends respectively in MESA, and 6.7 hours in MrOS based on a 4-point scale of <5, 5–6, 6–7, and >7 hours coded as 4.5, 5.5, 6.5 and 7.5 hours respectively) – differences which may arise for multiple reasons including reporting bias and misclassification as well as the atypical context of having a PSG administered58.
Comparing objective and subjective sleep duration, we also observed differences in the MESA associations with DSCT: as noted above, longer objective TST showed a modest association with improved DSCT performance, whereas longer subjective sleep duration was modestly associated with worse DSCT (b = −0.04, 95% CI −0.09, −0.0004, p = 0.048 for weekday sleep) and CASI (b = −0.05, 95% CI −0.10, −0.01, p = 0.02 and b = −0.06, 95% CI −0.10, −0.01, p = 0.013 for weekday and weekend sleep respectively) performance. One explanation for this apparent discrepancy is the possibility of nonlinear effects: whereas our primary analyses considered only linear associations, there have been reports of nonlinear, inverted-U associations between sleep duration and poorer cognitive outcomes20,21. As linear models may obscure estimates of such effects, for both subjective and objective measures of sleep duration, we fit additional models that included an orthogonal second-order polynomial term for sleep duration as a predictor. In MrOS, for both subjective and objective sleep duration measures we observed significant quadratic terms in models predicting Trails B, 3MS and DVT (Supplementary Figure 17b), although results were less clear for MESA (Supplementary Figure 17a). In both MESA and MrOS, objective and subjective sleep time showed significant nonlinear associations with other measures of sleep quality, including ESS and PSG-derived sleep efficiency and WASO (Supplementary Figure 18). In general, compared to individuals with a typical self-reported sleep duration, those who reported either shorter or longer sleep tended to have worse quality sleep. As others have suggested59, this makes interpretation of associations with sleep duration, nonlinear or otherwise, hard to interpret without more detailed knowledge of sleep architecture.
Testing for effect modification by sex and APOE genotype
Finally, in MESA we tested the 23 associated metrics for evidence of effect modification by sex or APOE genotype. There was no credible evidence for interaction with sex (all p > 0.1). Previous analyses in MESA identified effect modification by APOE (carriers versus non-carriers of an E4 allele) of the association between DSCT and ESS60. We confirmed this result in our analytic sample and testing framework, controlling for ancestry by inclusion of the first ten principal components61 as well as self-reported race/ethnicity (p = 2×10−3 for ESS×APOE interaction on DSCT), whereby a significant DSCT/ESS association was observed in carriers (N = 406, b = −0.14, 95% CI −0.22, −0.06, p = 5×10−4) but not non-carriers (N = 1,118, b = 0.005, 95% CI −0.05, 0.06, p = 0.86). We also observed a significant ESS×APOE interaction on CASI (p = 4×10−4), although unlike DSCT this was primarily driven by a positive association in non-carriers (b = 0.11, 95% CI 0.05, 0.16, p = 1×10−4) combined with the absence of a nominally significant association in carriers. Beyond ESS, however, there was no strong and consistent evidence to suggest either association with or effect moderation by APOE genotype for any of the 23 cognition-associated sleep metrics on DSCT or CASI. Reduced power to detect interactions compared to main effects makes it difficult to definitively rule out possible involvement of APOE in the links between sleep and cognition, however.
Discussion
Sleep quality and quantity change markedly over the life course. Emerging evidence suggests that these changes are predictive of — and potentially causally related to — age-related cognitive decline and impairment. Here, we adopted an explicitly data-driven and exploratory approach with the aim of broadly cataloging individual differences in sleep neurophysiology and their relationships to cognition. In two independent community samples of older adults, we identified multiple inter-related properties of sleep — in terms of macro-architectural as well as spectral, spindle and SO micro-architectural elements — that were associated with cognitive performance, and with processing speed and executive functioning in particular. Whereas multiple other studies have reported associations between aspects of sleep microarchitecture and cognitive performance, our study is distinguished by the scope of metrics comprehensively tested. Despite evaluating a large number of metrics, we attempted to ensure the rigor of our data-driven testing paradigm by the use of large samples, formal control of multiple testing, and insistence on replication in an independent sample.
Several aspects of our results are worth highlighting. First, the dozens of inter-related sleep metrics measured exhibited a higher-order underlying structure that was broadly consistent between MESA and MrOS, with different aspects being apparent through dimension reduction/cluster analysis and principal spectral components (PSC) analysis. To a first approximation, subjective metrics were independent of objective PSG metrics. Of the objective metrics, macro-architecture metrics including sleep duration and efficiency, clustered separately from micro-architecture metrics. Of the micro-architecture metrics, analyses in both MESA and MrOS pointed to at least six further subdivisions, including “absolute total (slow) power”, “slow, delta relative power”, “alpha, sigma, beta power”, “slow spindles” and “fast spindles”. These results suggest that many of the different metrics deployed in our study are capturing unique aspects of the sleep process.
Second, multiple metrics were robustly associated with cognition, spanning a number of domains/clusters. The 23 flagged metrics included REM duration, sleep efficiency, PSC 4 (“sigma/beta ratio”), both fast and slow spindle activity, SO duration (and slow/delta ratio) and the strength of spindle/SO coupling. We observed independent associations with cognition across multiple of these metrics, suggesting that studies of sleep and cognition should be careful not to focus on only one aspect of sleep. Third, spectral/spindle/SO micro-architecture metrics tended to predict cognitive performance independently of more routinely collected measures of sleep duration and quality. Indeed, overall, the subjective measures of sleep were only loosely correlated with objective metrics, and could not be seen as meaningful ‘proxies’ for the cognition-relevant information contained in the sleep EEG. Fourth, all cognition-associated objective sleep metrics showed marked age-dependent trends, such that objective sleep metrics more commonly observed in younger individuals tended to be associated with better cognitive health, even after adjusting for chronological age. Indeed, one could relatively well predict whether a sleep metric would associate with cognition (independent of age), and in which direction, solely on the basis of its association with age. Fifth, sleep metrics associated with advanced age and worse cognitive performance tended to also be independently associated with poor cardiometabolic health, namely diabetes and hypertension.
One possibility is that multiple facets of sleep neurophysiology may reflect more general biological aging processes that mediate age-dependent cognitive decline, rather than being specific to sleep per se. One approach that captures the spirit of these observation is to estimate a so-called ‘brain age index’ from sleep signal data, whereby accelerated aging (as estimated by sleep microstructure relative to chronological age) may be a general marker of pathophysiology62. Our results support this general concept, although identifying the points at which a unidimensional construct such as this loses explanatory value will be an empirical question. For example, although males indeed ‘look like older females’ for many sleep metrics in our data, there are many other sleep metrics for which this pattern clearly doesn’t hold, suggesting at least one other pertinent (sex-specific) dimension, and potentially many more, that may not be optimally captured by a single number.
Impairment on standard cognitive tests can precede the onset of dementia by several years 63. Indeed, there is an increasing interest in differentiating between normal aging versus emerging dementia64,65, and consideration of sleep may be fruitful in this regard. Aging and Alzheimer disease (AD) are characterized by sleep disturbances and brain regions important for sleep and wake mechanisms are affected in early AD 66,67. Inadequate sleep duration, increased fragmentation, decreased depth and increased daytime sleepiness have been associated with a heightened risk of developing AD independent of co-existing sleep disorders 16,18,68,69. Excessive daytime sleepiness has also been found to be a marker of cognitive decline and dementia 15,25, and in brain imaging studies, changes in default mode network connectivity were associated with daytime sleepiness, distinct from the effects of aging 70,71. With respect to total sleep duration, we found partial support for a nonlinear, inverted-U association with cognition, primarily in MrOS. Although inverted-U models have been reported for sleep duration and a range of health outcomes, subjectively-reported sleep duration is a poor proxy for a complex set of underlying factors, which makes nonlinear association hard to interpret if duration is not a unidimensional construct59,72–74. Our results are consistent with a model in which (self-reported) sleep duration captures variation in sleep quality as well as sleep duration, and may include increased WASO.
Primary transient elements of the sleep EEG including spindles and slow oscillations are known to vary with age and correlate with cognitive functions. For example, patients with AD performed better on memory testing if a greater number of fast spindles were present75. Though the exact neural underpinnings of fast versus slow spindles remain unclear, their differential occurrence during the slow oscillation cycle has pointed to separate generation mechanisms76. The occurrence of fast and slow spindles also differ over the life course: although spindle density declines with age, relatively more fast spindles are observed in older individuals compared to younger individuals7; here we further observed that whereas fast spindles tend to become faster with age, slow spindles become slower.
Much attention has been given to the link between cognitive decline/dementia and N3 or slow wave sleep (SWS) and/or slow wave activity (SWA), based on human and animal studies demonstrating that newly learned memory traces are strengthened through an active neuronal replay of these memory representations during SWS10,77,78. Furthermore, disruption of SWA is associated with increased CSF amyloid-β and, vice versa, Aβ pathology has been linked to a reduced generation of slow wave oscillation and sleep-related memory consolidation79–81. We did not observe associations with N3 duration and our cognitive measures, whereas SO wavelength, the balance of slow (<1 Hz) to delta (1–4 Hz) spectral power and spindle/SO(SWA) coupling predicted cognitive performance. Whereas some studies define SWA to encompass 0.5 – 4 Hz79, we observed qualitative differences in the associations of < 1 Hz versus > 1 Hz SWA. This distinction is reflected in the metric used here and by others30,80, that explicitly indexes the proportion of SWA that is < 1 Hz (‘<1 Hz relative SWA’), labeled here as the ‘slow/delta ratio’. However, seemingly at odds with previous reports of reduced < 1 Hz relative SWA predicting increased β-amyloid burden30,80, we instead observed that increased < 1 Hz relative SWA was associated with poorer cognitive performance. Consistent with this, increased SO duration (which is highly correlated with increased <1 Hz relative SWA), similarly predicted poorer cognitive performance, and both measures increased with increasing age in both cohorts. As others have noted, increased SO duration and slope may reflect changes in synaptic density and white matter integrity that accumulate with age56.
Our findings with respect to REM duration strengthen early polysomnographic studies82,83 and more recent epidemiological reports on cognitive performance16,18,28,84,85 and incident dementia33, as well as animal studies86,87. Cholinergic neurons are important modulators of REM sleep, and AD pathology is associated with impairment in cholinergic networks and imbalance between cholinergic and orexinergic systems; it has been suggested that, in AD, these impairments may be responsible for sleep fragmentation and changes in NREM/REM sleep architecture resulting in decreased REM sleep 88. REM sleep, as well as others aspects of sleep neurophysiology including spindles, is under partial circadian control89, although in this study the REM effects on cognition were independent of chronotype metrics available.
From a methodological perspective, we applied principal spectral component (PSC) analysis to complement traditional band power metrics, uncovering dimensions of individual variation in the sleep EEG that were highly preserved across cohorts. Of ten retained components, the top three were common to both REM and NREM sleep, whereas subsequent components were more specific to NREM sleep. In both MESA and MrOS, and in both N2 and N3 sleep, the fourth component (PSC 4, higher values of which primarily indexed relatively greater sigma and lower beta power) was consistently and significantly associated with poorer cognitive performance. These effects persisted when additionally controlling for sigma power by itself (data not shown). Of all principal spectral components, PSC 4 also showed the strongest age-related decline, in both MESA and MrOS and in both N2 and N3. PSCs provide an efficient partitioning of individual differences based on empirical patterns of variation, although they do not necessarily map directly and uniquely onto distinct underlying physiological processes any more than a gross measure such as sigma band power does. In the future, application of this approach to sleep studies with multiple EEG channels (potentially including cross-channel coherence spectral terms) may be particularly attractive. Following from our use of MESA as a reference (i.e. a set of singular values and vectors used to impute PSCs into MrOS participants), a further possible extension is to develop a standardized, “universal” reference, so that other investigators could impute the equivalent set of PSCs into new studies. Ideally, such a reference panel (or panels) would be based on data from a demographically and medically diverse range of individuals and across different stages of sleep; likewise, separate single-channel and multi-channel references could be created for particular montages. Inasmuch as PSCs prove to reflect an efficient summary of power spectra, this would provide a means by which large-scale studies could empower smaller ones.
Our study has a number of further limitations that limit its scope, generalizability and basis for inference. First, we only considered cognition cross-sectionally, rather than assessing cognitive decline per se. Second, the characterization of sleep was based on a limited EEG montage, reducing our ability to detect spatially specific associations or consider functional connectivity during sleep.
Third, we do not know the extent to which our findings on sleep in older adults will generalize in different populations, including younger participants. Although our results may have implications for AD and clinically recognized mild cognitive impairment, the present study primary reflects variation within the normal range of cognitive performance in older adults. As we were not able to assay EEG or other brain biomarkers during the waking state, the extent to which our findings reflect sleep-dependent processes specifically, versus more general neuropsychological or neurodegenerative features cannot be directly inferred. Nonetheless, it may still be the case that the sleep EEG provides a particularly good index of these more general factors.
Fourth, although the cognitive tests used were valid and sensitive measures within their domains, the cognitive battery was not comprehensive, and it did not assess all relevant cognitive domains. The lack of a learning task that measures longer term memory retention is a weakness, as this domain is known to change with age and serve as a differential marker of normal age-related decline versus dementia. Our primary measures of executive functioning and processing speed (DSCT and Trails B) may also reflect psychomotor and visual problems. Nonetheless, our findings suggested some measures may be less sensitive sleep-dependent processes, namely Digit Span in MESA.
Fifth, unraveling the precise causal relationships between sleep and cognitive functioning – which may likely include bidirectional effects and pleiotropic genetic factors – is beyond the current scope. As well as a baseline covariate model, we applied an augmented covariate model, and specifically considered the role of educational attainment on the reported associations. Controlling for educational attainment itself, although strongly correlated with cognitive performance, had little impact on results, in both MESA and MrOS. In MESA, a number of metrics that were significant under the baseline model were no longer significant under the broader augmented model. In contrast, almost all metrics remained significant under both baseline and augmented covariate models in MrOS. Interpreting such models is challenging when key aspects of the underlying causal models are unknown. In particular, the addition of an extra covariate may over-control, if it is on the causal path from predictor to outcome, or if it is itself a parallel outcome.
Sixth, although chronotype metrics did not show associations with cognition, chronotype was far from comprehensively measured in this study, with only a single objective metric: sleep midpoint from a single night of in-home PSG. Nonetheless, sleep midpoint was still highly correlated with MEQ morningness in MESA (r = −0.42, 95% CI −0.46, −0.38, p < 10−15) and so can be taken as a rough index of chronotype, as participants were instructed to go to bed at their usual time.
Seventh, our characterization of sleep was by no means fully comprehensive: for example, as well as topographical differences and cross-channel coherence, which were excluded due to the limited montage, we did not consider nocturnal (ultradian) dynamics, cross-frequency coupling, or specific transient features of REM sleep.
Finally, although our top-down ‘domain-based’ approach to testing provided a useful framework for organizing our work (as well as a granular but rigorous statistical control of multiple testing, an issue which is sometimes afforded scant regard in the literature), the precise assignment of metrics to domains was in many ways arbitrary. There are other reasonable ways to structure domains: for example, instead of spindle occurrence and morphology, we could instead have tested fast and slow spindles as two separate domains. In recognition of this, we also performed a parallel, empirical clustering to group sleep metrics (which did in fact show fast and slow spindles to be separable features). Nonetheless, this approach is not without drawbacks either: there are myriad approaches to clustering and/or dimension reduction, and different approaches will give different sets of clusters. There is no gold standard to adjudicate between competing solutions, and exhaustive comparison is beyond our present scope. Furthermore, dimension reduction/cluster solutions will be different in different samples, potentially making it difficult to compare between studies. For these reasons, although the broad picture is clear, we advise that not too much weight be placed on the specifics of any single grouping presented here.
In conclusion, this study contributes to a body of evidence, including strong animal and emerging human work, that indicates that sleep disturbances are associated with cognitive impairment. Identifying the specific elements of sleep neurophysiology that are associated with cognitive impairment in older adults has a broad range of potential benefits: to elucidate pathogenic mechanisms of cognitive decline, to provide intermediate phenotypes (for example, outcomes in intervention or genetic studies), and to predict high-risk individuals. At the same time, our study also highlights some of the challenges inherent in applying sleep neurophysiology metrics as clinically relevant biomarkers. While statistically highly significant, most effect sizes for individual metrics were modest, with standardized beta coefficients typically under 0.1, meaning that their predictive power is individually limited. Many metrics are inter-related with each other and can be calculated in a number of different ways, meaning it is often difficult to determine the optimal and independent set of metrics. Furthermore, we demonstrated a number of demographic differences in some key sleep metrics considered here. Although it was possible for us to control for these factors in the context of an epidemiological study, in the context of using sleep metrics from individual patients as biomarkers, it will be crucial to adjust raw metrics to account for such effects, or to identify metrics that are less susceptible to technical measurement factors, for example. Still, identifying which facets of sleep neurophysiology are relevant for cognitive performance retains the potential to provide therapeutic targets including the modulation of sleep oscillations. As potential therapeutic targets either through lifestyle intervention and cognitive behavioral therapies, pharmacological approaches90, or closed-loop auditory stimulation and tDCS/TMS91–95, multiple aspects of sleep behavior and neurophysiology may be modifiable, pointing to the possibility of future sleep-based initiatives to maintain and improve cognitive health. Future longitudinal and interventional studies will be needed, however, to address the extent to which these putative biomarkers have direct causal influences on cognition and whether they are modifiable in ways that impact cognition, or whether they instead secondarily reflect a more general accelerated biological aging that manifests as worse cognitive functioning as well as poorer cardiometabolic health.
Methods
Cohorts and cognitive measures
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective observational cohort study consisting of a diverse community-based sample (38% white, 28% African-American, 22% Hispanic, and 12% Chinese-American) of 6,814 men and women. Details on the study design for MESA have been previously published38,39. The current analysis used data from the MESA Sleep ancillary study, conducted in proximity to the 5th MESA follow-up examination, in which 2,048 individuals underwent PSG conducted between 2010 and 2013. (The clinic exams occurred 2010–2012; the PSG were scheduled at that time but occurred between 2010–2013.) Three neuropsychological tests were selected to assess cognitive function during the Visit 5 examination (2010–12), the Cognitive Abilities Screening Instrument (CASI)42, Digit Symbol Coding Test (DSCT)41 and Digit Span Test (Forward and Backward, DSF and DSB)43. Key covariates included self-reported educational attainment and income, arousal index (AI) and apnea-hypopnea index (AHI) from the PSG, body mass index (BMI), self-reported diagnoses of hypertension or diabetes, depression symptoms from the Center for Epidemiologic Studies Depression Scale (CESD), current alcohol and nicotine use, and APOE4 genotype.
The MrOS Sleep Study enrolled 3,135 participants (2003 to 2005) for in-home PSG. Cognitive measures and key covariates were available that were similar to, but not identical to, those in MESA. Cognitive data included in this report were collected during Sleep Visit 1 (2003–2005): Trails B44, a modified, expanded version of the Mini-Mental State Examination (3MS)45 as well as the Digit Vigilance Test (DVT)46. Along with age, collection site and ethnicity (white/non-white), key covariates were educational attainment, AI, AHI, self-reported sleep apnea or any sleep disorder, BMI, self-reported hypertension and diabetes, symptoms of depression and anxiety rated by the Geriatric Depression Scale (GDS) and the Goldberg depression and anxiety scales, and current alcohol and nicotine use. Comparing cognitive measures in MESA and MrOS for which we might expect a broad correspondence of results, we note that DSCT and Trails B are both measures of processing speed and executive functioning, whereas CASI and 3MS are both designed to be global indices sensitive to dementia. Note that Trails B and DVT were based on timed performance; for consistency of reporting, Trails B* and DVT* refer to the corresponding measure with sign reversed, such that a negative coefficient implies worse performance across all seven cognitive measures. See the Supplementary Methods for details on cohorts and measures.
Subjective sleep metrics
In both MESA and MrOS, daytime sleepiness was measured by the Epworth Sleepiness Scale (ESS)96, which consists of eight items each scored 0 to 3. The summed total score (range 0 to 24) can be dichotomized such that scores of 10 or more indicate excessive daytime sleepiness. In MESA only, insomnia symptoms were assessed with the Women’s Health Initiative Insomnia Rating Scale (WHIIRS)97, a five-item scale that rates sleep latency, sleep maintenance insomnia, early morning awakening and sleep quality, and circadian preference towards morningness and eveningness was assessed by a five-item modified Horne-Ostberg Morningness-Eveningness Questionnaire (MEQ)98, where higher scores indicate a preference for morningness. Additionally, in MESA we included two other self-report items from the MESA sleep questionnaire, asking whether the participant had sleep difficulties causing irritability, and whether they felt overly sleepy during the day (each question asked with respect to the past four weeks). MrOS participants also completed the Pittsburgh Sleep Quality Index99 and the Functional Outcomes of Sleep Questionnaire100.
Objective sleep metrics
All PSG studies were manually scored by trained staff using standard criteria101, and low quality studies were identified by scorers and removed from subsequent data analysis. Epochs containing manually annotated arousals or movements were removed. All EEG analysis used the Luna C/C++ pipeline, developed by one of us (S.M.P., URL: http://zzz.bwh.harvard.edu/luna/). In both cohorts, EEG signals from C4/M1 were resampled at 100 Hz and segmented into 30-second epochs, and then band-pass filtered with transition frequencies at 0.3 and 35Hz, using a Kaiser window zero-phase filter. We extracted epochs corresponding to four sets of stages (N2, N3, N2+N3 and REM) and, within each set, applied two consecutive approaches to artifact detection, as well correction for potential cardiac contamination (see Supplementary Methods). Separately for each stage (or for N2+N3 combined), we required at least 10 epochs (5 minutes) of artifact-free sleep for that individual to be included in further spectral, spindle and slow oscillation analyses (Supplementary Table 3).
We derived a panel of metrics, grouped into a number of ‘domains’ (fully enumerated in Supplementary Methods): sleep macro-architecture, chronotype, spectral power, alternative time/frequency metrics, principal spectral components, spindle occurrence, spindle morphology, slow oscillation (SO) occurrence, SO morphology, and spindle/SO coupling (which encompass the so-called ‘spindle/SWA’ coupling metrics also). Based on a priori considerations, not all metrics were calculated for all stage sets as described below, e.g. we did not consider spindles or slow oscillations during REM sleep (see the Supplementary Methods for more details). We also derived, separately for MESA and MrOS empirical groupings of sleep metrics based on cluster analysis, described below. Note that these domains and clusters were only used during omnibus testing, to provide a high-level view of the patterns of results: neither domains nor empirically-derived clusters were used in the selection of the final set of associated metrics.
Sleep macro-architecture
The sleep architecture domain comprised 15 metrics based on manual staging: total sleep time (TST), stage N1, N2, N3 and REM duration (minutes and %), number and mean duration of NREM cycles (defined as per ref. 102), sleep efficiency (total sleep time as a proportion of total recording time), sleep maintenance efficiency (which excludes the sleep latency period from the denominator), WASO (mins), and REM latency (mins) (time from sleep onset to first REM epoch). In MESA this domain included self-reported typical weekday and weekend sleep durations, whereas in MrOS this domain included a single measure of self-reported sleep duration.
Chronotype
The chronotype domain comprised a single objective metric: sleep midpoint (middle point between sleep onset and offset based on the PSG, coded as hours past 5pm). In MESA, this domain also included self-reported chronotype based on a modified Horne-Ostberg Morningness/Eveningness Questionnaire (MEQ)98.
Spectral power
We calculated spectral power using the Welch algorithm and fast Fourier transformation (FFT) applied to 4 second windows, shifted by 2 second increments and tapered with a Tukey window function (taper length 50%), yielding a frequency resolution of 0.25 Hz. Relative band power was obtained by dividing absolute band power by total power, where total power is based on the band [0.5, 30) Hz. This domain comprised 36 metrics: absolute log-scale and relative spectral power for slow, delta, theta, alpha, sigma and beta bands – defined as [0.5–1), [1,4), [4,8), [8,12), [12, 15) and [15,30) Hz respectively – for N2, N3 and REM sleep (2 definitions × 6 bands × 3 stages = 36).
Alternative time/frequency metrics
A second domain of alternative EEG summaries comprised 16 metrics: three Hjorth parameters (activity, mobility and complexity)103, an index of EEG slowing for N2, N3 and REM sleep and, following ref. 80, the absolute slow (S, 0.5–1Hz) power normalized by the sum of slow and delta (D, 1–4 Hz) power, labeled here as the “slow/delta ratio” – although note this quantity is S/(S+D) not S/D. For comparability with other SO metrics (below), the slow/delta ratio was also calculated for N2+N3 sleep combined (i.e. 4 metrics × 3 stages + 1 metric × 4 stages = 16).
Principal spectral component (PSC) analysis
In addition to summarizing power spectra in traditional bands, we applied an alternative, data-driven decomposition of power spectra, labelled principal spectral components (PSC) analysis. This approach uses singular value decomposition (SVD) to define empirical bases of variation in power spectra between individuals; others have used similar approaches in different contexts104–106. Here, we used PSC analysis to reduce 77 correlated absolute power measures (0.75 to 20 Hz in 0.25 Hz intervals) to a smaller number of orthogonal components that captured the majority of the variation observed within each cohort. PSC analysis provides a natural way to quantify coordinated changes across power spectra, and may be expected to better capture subtle individual differences, such as a shift in peak spindle frequency within the sigma band, which will be reflected in the correlational structure of power measures. Inasmuch as broad individual differences in total power, or overall ~1/f slope, map onto specific components, PSC analysis also effectively normalizes power spectra, such that the remaining orthogonal components will more directly index specific oscillatory activity with aperiodic background activity subtracted out.
We applied PSC analysis to N2 and N3 power spectra separately. Based on visual review of the cumulative total variation explained, we selected the number of components to retain. As the sign/polarity of SVD components is arbitrary, we constrained comparable (highly correlated) N2 and N3 components to be positively correlated with each other, to aid the interpretation of results. If PSC analyses were applied to MESA and MrOS separately, one challenge would be to establish the comparability of the resulting components: that is, whether the PSC 4 in MESA, for example, measured a similar thing as PSC 4 in MrOS. For the primary analyses, rather than perform PSC analysis separately in MESA and MrOS, we therefore projected the observed power spectra in MrOS into the reduced dimensional space defined by the MESA PSC analysis, to ensure comparability of PSCs between cohorts. See Supplementary Methods for details. We compared the resulting “MESA-derived” MrOS components to those from a MrOS-specific analysis. To describe each component (labeled PSC1, PSC2, …) in terms of the original power spectra, we plotted median power (at each 0.25 Hz frequency bin) for groups stratified according to quintiles of that component.
Spindles
We detected spindles using a previously published wavelet-based approach7, modified to determine a suitable detection threshold empirically, based on Otsu’s method107 of maximizing between-class variance in the wavelet coefficient, comparing above-threshold (putative “spindle”) and below-threshold (“non-spindle”) intervals (Supplementary Figure 2), which selected a multiplicative threshold of 6 times the individual’s median wavelet power. Based on prior work108–110, we assumed that a frequency-dependent dichotomization between ‘fast’ and ‘slow’ spindles captured important distinctions in spindle properties. Indeed, this assumption was supported by an analysis in which we fit, separately for each individual, a Gaussian mixture model to the multi-modal frequency distribution of spindles detected under an untargeted, frequency-agnostic approach (see Supplementary Methods and Supplementary Figures 3a, 3b).
Our primary analyses targeted two classes of spindle: fast (center frequency FC=15 Hz) and slow (FC=11 Hz), in each case detecting spindles within approximately +/− 2 Hz of the target frequency (Figures 2a). Given these two sets of detected spindles, we estimated each individual’s mean for several spindle morphology metrics. The spindle occurrence domain comprised 8 metrics: spindle count and density for fast and slow spindles during either N2 or N3 sleep (2 measures × 2 spindle types × 2 stages = 8). The spindle morphology domain comprised 36 metrics: mean spindle duration, frequency, number of oscillations, an index of integrated spindle activity per spindle (ISAS), per minute (ISAM) or in total (ISAT), intra-spindle frequency change (“chirp”), symmetry and asymmetry indices, for fast and slow spindles during N2 and N3 sleep (9 measures × 2 spindle types × 2 stages = 36). See Supplementary Methods for details.
Slow oscillations (SO)
We used a heuristic to detect individual slow oscillations (SO) in the sleep EEG. Multiple quantifications of SO have been used in the literature28,111,112 and there is no consensus on optimal definitions, especially in elderly individuals who tend to have decreased slow wave sleep, potentially leading to methodological difficulties113. We therefore adopted two approaches – using either adaptive (relative) or absolute (fixed) amplitude thresholds – and assessed the robustness of results to this choice. In primary analyses, we used adaptive thresholds, defined relative to each individual’s baseline level of slow activity, see Supplementary Methods. We initially focused on SO during N3 sleep only but extended this to consider N2+N3 sleep combined, as a nontrivial number of older individuals did not have a sufficient duration of N3 sleep. The SO occurrence domain comprised four metrics: SO count and density (counts per minute) during either N3 sleep or N2+N3 sleep combined. The SO morphology domain comprised six metrics: mean peak-to-peak amplitude (μV, log-scaled), duration or “wavelength” (secs), and upward slope of the negative peak (μV/sec, log-scaled) during either N3 sleep or N2+N3 sleep combined (3 measures × 2 stage groups = 6).
Spindle/SO coupling
The primary quantification of spindle/SO coupling was based on the phase of slow wave activity at spindle ‘peaks’, the points of maximal spindle oscillation (peak-to-peak amplitude), typically near the spindle’s center. We estimated three measures of coupling: gross overlap, coupling magnitude and coupling phase angle. As defined below, ‘overlap’ measured whether spindles and SO tended to occur concurrently, whereas ‘phase coupling’ measured whether spindles tended to peak at particular times during the SO, thereby indexing a more precise temporal relationship.
Overlap was defined as the number of spindle peaks that fell within a detected SO; we determined the null distribution of this metric empirically, by randomly shuffling spindle peaks and recalculating overlap 100,000 times. For each individual, we normalized the overlap metric as a Z score, given the mean and standard deviation of the null distribution; we also calculated an empirical p-value for above-chance overlap. One potential concern when using this approach, especially when considering N2+N3 sleep combined, is that apparent above-chance spindle/SO overlap may simply reflect that relatively more spindles occur during N2 but relatively more SO occur during N3, whereas stage-agnostic shuffling does not preserve this aspect of the data. We therefore used a modified scheme, whereby each spindle peak was randomly shuffled only within the 30-second epoch that spanned it.
Coupling magnitude and phase angle metrics were based on the instantaneous phase from a Hilbert transform of EEG after bandpass-filtering in the 0.3 to 4 Hz range. Although we were primarily interested in SO in the ~1 Hz frequency range, using a broader bandpass (up to 4 Hz) helped to not impose an artificially sinusoidal shape on SO waveforms. That is, the criteria to define SOs identified waveforms that were, on average, of <1 Hz frequency (i.e. Supplementary Figure 4, Supplementary Table 4). We calculated intra-trial phase consistency (ITPC) 114 as a measure of the strength of spindle/SO coupling. Briefly, if the Hilbert-derived phase angle at each spindle peak is assumed to be a unit vector on a circle, with an angle matching the phase angle, then the ITPC is the magnitude of the average of these complex vectors. Under the null hypothesis of no coupling, an asymptotic p-value can be computed as exp(−n * ITPC2). An individual’s coupling phase angle is given by the angle of the complex mean of these vectors. Separately for fast and slow spindles, we calculated two ITPC statistics: a) for all spindles, b) only for spindles with a peak that overlapped a SO. We refer to these two classes of coupling metrics as spindle/SWA and spindle/SO respectively. As ITPC statistics and asymptotic p-values can show bias or noise when based on a small number of spindle/SO events, or on non-sinusoidal waveforms, our primary analyses generated empirical null distributions to normalize them. Under the former spindle/SWA scenario, we used the within-epoch shuffling scheme described above. Under the latter spindle/SO scenario, that only considered spindles overlapping detected SOs, in order to ensure the phase coupling metric was not confounded by differences in gross spindle/SO overlap, we required null replicates to contain the same number of SO-overlapping spindle peaks, i.e. to have an identical extent of overlap. We therefore shuffled each spindle peak by a random offset, between o seconds and the duration of the spanning SO, wrapping as necessary. This ‘within-SO’ shuffling scheme preserved the total number of spindles, SO and their gross overlap in each null replicate, but randomized only the precise relationships between spindle peaks and SO phase.
Spindle/SWA and spindle/SO analyses were restricted to N2+N3 sleep combined, because spindles are predominant during N2 whereas SO are predominant during N3. Many individuals had too few N3 spindles to assess coupling in N3 alone (Supplementary Table 3). For primary association analyses with cognitive measures, the spindle/SO coupling domain comprised 10 metrics: overlap, phase coupling magnitude, and mean phase at spindle peak, separately for fast and slow spindles; furthermore, phase coupling magnitude and angle metrics were calculated separately for a) all spindles, i.e. spindle/SWA coupling, versus b) only spindles whose peak overlapped a detected SO, i.e. spindle/SO coupling (1 measure × 2 spindle types + 2 measures × 2 spindle types × 2 spindle filters = 10).
Empirical clustering of sleep metrics
To complement the prespecified domains, we also sought to determine groupings of sleep metrics empirically in MESA and MrOS. We defined the distance between metrics i and j as 1-|rij|, and applied Uniform Manifold Approximation and Projection for Dimension Reduction (UMAP)115 as implemented in the umap R package, allowing for 9 nearest neighbors, a minimum distance of 0.1, and extracting either 10 components (for clustering) or 2 components (for visualization). We then applied Hierarchical Density-Based Spatial Clustering of Applications with Noise (HDBSCAN)116 as implemented in the dbscan R package, setting the minimum size of clusters to 6. We manually labelled clusters by visual inspection of the assigned metrics. Note that UMAP dimension reduction does not fully preserve the global structure of the data (i.e. versus local clustering) and the orientation of components/axes is arbitrary, meaning that direct comparisons between MESA and MrOS projections are not necessarily meaningful.
Statistical approach to association analysis: correction for multiple tests and replication
As enumerated above, in MESA alone we tested 173 sleep metrics × 4 cognitive measures, yielding 692 tests. Performing multiple tests necessitates consideration of statistical false positives. We therefore adopted a permutation-based, top-down approach to control false positive rates while accounting for the correlational structure between sleep predictors and between cognitive outcomes. We generated 20,000 permuted datasets, in which individuals’ sleep metrics were randomly reassigned. In each null dataset, we refit the 692 linear regression models of covariate-residualized cognitive measure on sleep metric, and recorded the –log10(p-value) from each. For each replicate (randomized dataset), we calculated two additional ‘omnibus’ statistics based on these asymptotic p-values: the maximum and the sum of the -log10(p), labeled max and sum respectively. We rejected the global null hypothesis that all sleep metrics were unassociated with all cognitive measures given an empirical p<0.05 for either the max or sum statistic, based on their null distributions. Specifically, if R is the number of times a statistic from a null replicate was equal to or exceeded the observed statistic, the empirical p-value is (R+1)/(N+1), where N = 20,000 replicates. The max statistic is expected to be more powerful under the scenario in which a small number of metrics are highly associated with cognitive performance, whereas the sum statistic will be more powerful when a larger proportion of sleep metrics show more modest associations with cognitive performance.
Following a top-down approach, and contingent on a significant global omnibus test, we next asked whether specific subsets of tests had significant max or sum statistics, considering each of the 11 sleep metric domains (e.g. all spindle morphology metrics for all cognitive measures) and each of the 4 MESA cognitive measures (e.g. all sleep metrics for DSCT). Finally, we tested each sleep domain for each cognitive measure separately (e.g. all spindle morphology metrics for DSCT). In this way, as well as controlling for multiple testing, this framework helped to contextualize broader patterns of association between sleep and cognitive performance.
We initially designed this study to focus only on the MESA cohort, but subsequently sought to replicate our findings in the MrOS cohort. Respecting this context, we first applied the omnibus testing framework to MESA. Second, we attempted replication in the independent MrOS sample, for the specific metrics that showed the strongest association with cognitive performance in MESA. Third, we repeated omnibus testing for MrOS, and considered the broader correspondence of results between cohorts. Fourth, with a focus on objective rather than subjective sleep metrics, we identified those showing significant and consistent patterns of association across both cohorts. Finally, for the set of replicated cognition-associated sleep metrics, we considered potential confounder or mediator variables in a series of secondary analyses.
Data Availability Statement
All PSG data are freely available via the National Sleep Resource (NSRR, http://sleepdata.org). The MESA dataset (DOI: 10.25822/n7hq-c406) URL is https://sleepdata.org/datasets/mesa. The MrOS dataset (DOI: 10.25822/kc27-0425) URL is https://sleepdata.org/datasets/mros, respectively. Full polysomnography and clinical/covariate data are available for all interested parties, pending completion of a Data Access and Use Agreement and IRB, as outlined on the NSRR website.
Code Availability Statement
Sleep EEG data were processed using the Luna package, developed by S.M.P. (http://zzz.bwh.harvard.edu/luna/). The C/C++ code is available at the following Github repository: http://github.com/remnrem/luna-base/. Specifically, the analysis presented in this manuscript used Luna to derive measures of sleep architecture (HYPNO command),and perform epoch-level artifact detection (SIGSTATS), signal filtering (FILTER), spectral estimation (PSD) and spindle/SO detection (SPINDLES).
Supplementary Material
Acknowledgements
MESA and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NHLBI, and by grants UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, DK063491, K24AG045334, P30AG059303, and R01AG058969. Funding support for the Sleep Polysomnography dataset was provided by grant HL56984. Funding for SHARe genotyping was provided by NHLBI Contract N02-HL-64278. Genotyping was performed at Affymetrix (Santa Clara, California, USA) and the Broad Institute of Harvard and MIT (Boston, Massachusetts, USA) using the Affymetrix Genome-Wide Human SNP Array 6.0. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
In addition, this work was also supported by NIH/NIMH grant R03 MH108908 (S.M.P.), NIH/NHLBI grant R01 HL146339 (S.M.P.), NIH/NHLBI grant R21 HL145492 (S.M.P.), NIH/NIMHD grant R21 MD012738 (S.M.P.), NIH grant K23AG049955 (J.M.D.), NIH/NHLBI grant K01HL138211 (D.J), NIH/NHLBI grant R35 HL135818 (S.R.), NIH/NINDS grant R01 NS096177 (M.J.P.), NIH/NIA grant R01 AG054081 (M.J.P.), K24 AG045334 (J.A.L), a Neurology Departmental Grant, Beth Israel Deaconess Medical Center (I.D.) and NIH/NHLBI grant R24 HL114473 (S.R., S.M. and S.M.P.).
This work is, in part, also a publication of the US Department of Agriculture, Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine (Houston, TX) funded in part by the USDA/ARS (Cooperative Agreement 58-3092-5-001). The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement from the US government.
None of the funders had any role in study design, data collection and analysis, decision to publish or preparation of this manuscript.
Footnotes
Competing Interests
No competing interests to declare.
References
- 1.Foley DJ et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep 18, 425–432 (1995). [DOI] [PubMed] [Google Scholar]
- 2.Foley D, Ancoli-Israel S, Britz P.& Walsh J.Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J. Psychosom. Res 56, 497–502 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC et al. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol 56, 303–308 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Li S-C et al. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol. Sci 15, 155–163 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Dijk DJ, Duffy JF & Czeisler CA Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int 17, 285–311 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Ohayon MM, Carskadon MA, Guilleminault C.& Vitiello MV Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27, 1255–1273 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Purcell SM et al. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat. Commun 8, 15930 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redline S.et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch. Intern. Med 164, 406–418 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Diering GH et al. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355, 511–515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson MA & McNaughton BL Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Stickgold R.& Walker MP Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 8, 331–343 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vivo L.et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355, 507–510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scullin MK & Bliwise DL Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect. Psychol. Sci 10, 97–137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohayon MM & Vecchierini M-F Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep 28, 981–989 (2005). [PubMed] [Google Scholar]
- 15.Keage HAD et al. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 13, 886–892 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Song Y.et al. Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. Sleep 38, 411–421 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavuoto MG et al. Objective but not subjective sleep predicts memory in community-dwelling older adults. J Sleep Res 25, 475–485 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Blackwell T.et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J. Am. Geriatr. Soc 59, 2217–2225 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spira AP et al. Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep 40, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devore EE et al. Sleep duration in midlife and later life in relation to cognition. J. Am. Geriatr. Soc 62, 1073–1081 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos AR et al. Sleep duration and neurocognitive function in the hispanic community health study/study of latinos. Sleep 39, 1843–1851 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackwell T.et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep 34, 1347–1356 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potvin O.et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep 35, 491–499 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaussent I.et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep 35, 1201–1207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley D.et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J. Am. Geriatr. Soc 49, 1628–1632 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Carvalho DZ et al. Association of Excessive Daytime Sleepiness With Longitudinal β-Amyloid Accumulation in Elderly Persons Without Dementia. JAMA Neurol. 75, 672–680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mander BA, Winer JR, Jagust WJ & Walker MP Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of alzheimer’s disease? Trends Neurosci. 39, 552–566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lafortune M.et al. Sleep spindles and rapid eye movement sleep as predictors of next morning cognitive performance in healthy middle-aged and older participants. J Sleep Res 23, 159–167 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Helfrich RF, Mander BA, Jagust WJ, Knight RT & Walker MP Old Brains Come Uncoupled in Sleep: Slow Wave-Spindle Synchrony, Brain Atrophy, and Forgetting. Neuron 97, 221–230.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winer JR et al. Sleep as a Potential Biomarker of Tau and β-Amyloid Burden in the Human Brain. J. Neurosci 39, 6315–6324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muehlroth BE et al. Precise Slow Oscillation-Spindle Coupling Promotes Memory Consolidation in Younger and Older Adults. Sci. Rep 9, 1940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prinz PN et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol. Aging 3, 361–370 (1982). [DOI] [PubMed] [Google Scholar]
- 33.Pase MP et al. Sleep architecture and the risk of incident dementia in the community. Neurology 89, 1244–1250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga AW et al. Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. J. Neurosci 34, 14571–14577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorness TE, Riley BT, Tysor MK & Poe GR REM restriction persistently alters strategy used to solve a spatial task. Learn. Mem 12, 352–359 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith C.& Rose GM Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol. Behav 59, 93–97 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Yaffe K.et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306, 613–619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke G, Lima J, Wong ND & Narula J.The multiethnic study of atherosclerosis. Glob Heart 11, 267–268 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Bild DE et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am. J. Epidemiol 156, 871–881 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Chen X.et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 38, 877–888 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wechsler D.Adult Intelligence Scale-III (WAIS-III). (Psychological Corporation/Harcourt, Inc, 1996). [Google Scholar]
- 42.Teng EL et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr 6, 45–58; discussion 62 (1994). [DOI] [PubMed] [Google Scholar]
- 43.Fitzpatrick AL et al. Sociodemographic Correlates of Cognition in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Geriatr. Psychiatry 23, 684–697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reitan RM Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Skills 8, 271–276 (1958). [Google Scholar]
- 45.Teng EL & Chui HC The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry 48, 314–318 (1987). [PubMed] [Google Scholar]
- 46.Kelland DZ & Lewis RF The Digit Vigilance Test: reliability, validity, and sensitivity to diazepam. Arch Clin Neuropsychol 11, 339–344 (1996). [PubMed] [Google Scholar]
- 47.Cappuccio FP & Miller MA Sleep and Cardio-Metabolic Disease. Curr. Cardiol. Rep 19, 110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Köhler S.et al. Temporal evolution of cognitive changes in incident hypertension: prospective cohort study across the adult age span. Hypertension 63, 245–251 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Roberts RE & Duong HT The prospective association between sleep deprivation and depression among adolescents. Sleep 37, 239–244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byers AL & Yaffe K.Depression and risk of developing dementia. Nat. Rev. Neurol 7, 323–331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kluge M, Schüssler P.& Steiger A.Duloxetine increases stage 3 sleep and suppresses rapid eye movement (REM) sleep in patients with major depression. Eur. Neuropsychopharmacol 17, 527–531 (2007). [DOI] [PubMed] [Google Scholar]
- 52.DeMartinis NA & Winokur A.Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol Disord Drug Targets 6, 17–29 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Scheer FAJL et al. Repeated melatonin supplementation improves sleep in hypertensive patients treated with beta-blockers: a randomized controlled trial. Sleep 35, 1395–1402 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laventure S.et al. Beyond spindles: interactions between sleep spindles and boundary frequencies during cued reactivation of motor memory representations. Sleep 41, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubé J.et al. Cortical thinning explains changes in sleep slow waves during adulthood. J. Neurosci 35, 7795–7807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carrier J.et al. Sleep slow wave changes during the middle years of life. Eur. J. Neurosci 33, 758–766 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Martin N.et al. Topography of age-related changes in sleep spindles. Neurobiol. Aging 34, 468–476 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Jackson CL, Patel SR, Jackson WB, Lutsey PL & Redline S.Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep 41, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bianchi MT, Thomas RJ & Westover MB An open request to epidemiologists: please stop querying self-reported sleep duration. Sleep Med. 35, 92–93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson DA et al. Greater Cognitive Deficits with Sleep-disordered Breathing among Individuals with Genetic Susceptibility to Alzheimer Disease. The Multi-Ethnic Study of Atherosclerosis. Annals of the American Thoracic Society 14, 1697–1705 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y.et al. Methylomics of gene expression in human monocytes. Hum. Mol. Genet 22, 5065–5074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun H.et al. Brain age from the electroencephalogram of sleep. Neurobiol. Aging 74, 112–120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jorm AF, Masaki KH, Petrovitch H, Ross GW & White LR Cognitive deficits 3 to 6 years before dementia onset in a population sample: the Honolulu-Asia aging study. J. Am. Geriatr. Soc 53, 452–455 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Brody JA & Schneider EL Diseases and disorders of aging: an hypothesis. J. Chronic Dis 39, 871–876 (1986). [DOI] [PubMed] [Google Scholar]
- 65.Toepper M.Dissociating Normal Aging from Alzheimer’s Disease: A View from Cognitive Neuroscience. J. Alzheimers Dis 57, 331–352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang JL et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann. Neurol 78, 317–322 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swaab DF, Fliers E.& Partiman TS The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 342, 37–44 (1985). [DOI] [PubMed] [Google Scholar]
- 68.Blackwell T.et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J. Gerontol. A, Biol. Sci. Med. Sci 61, 405–410 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Lim ASP et al. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain 137, 2847–2861 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ward AM et al. Daytime sleepiness is associated with decreased default mode network connectivity in both young and cognitively intact elderly subjects. Sleep 36, 1609–1615 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carvalho DZ et al. Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Med. 32, 236–243 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knutson KL & Turek FW The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep 29, 878–879 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Patel SR, Malhotra A, Gottlieb DJ, White DP & Hu FB Correlates of long sleep duration. Sleep 29, 881–889 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grandner MA & Drummond SPA Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med. Rev 11, 341–360 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rauchs G.et al. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport 19, 1159–1162 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mölle M, Bergmann TO, Marshall L.& Born J.Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep 34, 1411–1421 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feld G.& Diekelmann S.Sleep smart – Optimizing sleep for declarative learning and memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huber R, Ghilardi MF, Massimini M.& Tononi G.Local sleep and learning. Nature 430, 78–81 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Ju Y-ES et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 140, 2104–2111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mander BA et al. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat. Neurosci 18, 1051–1057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang C.& Holtzman DM Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45, 104–120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feinberg I, Koresko RL & Heller N.EEG sleep patterns as a function of normal and pathological aging in man. J. Psychiatr. Res 5, 107–144 (1967). [DOI] [PubMed] [Google Scholar]
- 83.Spiegel R, Herzog A.& Köberle S.Polygraphic sleep criteria as predictors of successful aging: an exploratory longitudinal study. Biol. Psychiatry 45, 435–442 (1999). [DOI] [PubMed] [Google Scholar]
- 84.Kim SJ, Lee JH, Lee DY, Jhoo JH & Woo JI Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am. J. Geriatr. Psychiatry 19, 374–381 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Della Monica C, Johnsen S, Atzori G, Groeger JA & Dijk D-J Rapid Eye Movement Sleep, Sleep Continuity and Slow Wave Sleep as Predictors of Cognition, Mood, and Subjective Sleep Quality in Healthy Men and Women, Aged 20–84 Years. Front. Psychiatry 9, 255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Markowska AL et al. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol. Aging 10, 31–43 (1989). [DOI] [PubMed] [Google Scholar]
- 87.Stone WS, Altman HJ, Berman RF, Caldwell DF & Kilbey MM Association of sleep parameters and memory in intact old rats and young rats with lesions in the nucleus basalis magnocellularis. Behav. Neurosci 103, 755–764 (1989). [DOI] [PubMed] [Google Scholar]
- 88.Liguori C.et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 71, 1498–1505 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Czeisler CA, Zimmerman JC, Ronda JM, Moore-Ede MC & Weitzman ED Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep 2, 329–346 (1980). [PubMed] [Google Scholar]
- 90.Roh JH et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J. Exp. Med 211, 2487–2496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Astori S, Wimmer RD & Lüthi A.Manipulating sleep spindles--expanding views on sleep, memory, and disease. Trends Neurosci. 36, 738–748 (2013). [DOI] [PubMed] [Google Scholar]
- 92.Marshall L, Helgadóttir H, Mölle M.& Born J.Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Wilckens KA, Ferrarelli F, Walker MP & Buysse DJ Slow-Wave Activity Enhancement to Improve Cognition. Trends Neurosci. 41, 470–482 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bellesi M, Riedner BA, Garcia-Molina GN, Cirelli C.& Tononi G.Enhancement of sleep slow waves: underlying mechanisms and practical consequences. Front. Syst. Neurosci 8, 208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Papalambros NA et al. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front. Hum. Neurosci 11, 109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johns MW A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545 (1991). [DOI] [PubMed] [Google Scholar]
- 97.Levine DW et al. Validation of the Women’s Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosom. Med 67, 98–104 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Horne JA & Ostberg O.A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4, 97–110 (1976). [PubMed] [Google Scholar]
- 99.Buysse DJ, Reynolds CF, Monk TH, Berman SR & Kupfer DJ The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989). [DOI] [PubMed] [Google Scholar]
- 100.Weaver TE et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 20, 835–843 (1997). [PubMed] [Google Scholar]
- 101.Rechtschaffen A.& Kales A.A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. (1968). [DOI] [PubMed] [Google Scholar]
- 102.Feinberg I.& Floyd TC Systematic trends across the night in human sleep cycles. Psychophysiology 16, 283–291 (1979). [DOI] [PubMed] [Google Scholar]
- 103.Hjorth B.EEG analysis based on time domain properties. Electroencephalogr. Clin. Neurophysiol 29, 306–310 (1970). [DOI] [PubMed] [Google Scholar]
- 104.Tenke CE & Kayser J.Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA). Clin. Neurophysiol 116, 2826–2846 (2005). [DOI] [PubMed] [Google Scholar]
- 105.Miller KJ et al. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput. Biol 8, e1002655 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duffy FH & Als H.A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls - a large case control study. BMC Med. 10, 64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Otsu N.A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern 9, 62–66 (1979). [Google Scholar]
- 108.Ayoub A.et al. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep 36, 905–911 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeitlhofer J.et al. Topographic distribution of sleep spindles in young healthy subjects. J Sleep Res 6, 149–155 (1997). [DOI] [PubMed] [Google Scholar]
- 110.De Gennaro L.& Ferrara M.Sleep spindles: an overview. Sleep Med. Rev 7, 423–440 (2003). [DOI] [PubMed] [Google Scholar]
- 111.Massimini M, Huber R, Ferrarelli F, Hill S.& Tononi G.The sleep slow oscillation as a traveling wave. J. Neurosci 24, 6862–6870 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dang-Vu TT et al. Spontaneous neural activity during human slow wave sleep. Proc. Natl. Acad. Sci. USA 105, 15160–15165 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muehlroth BE & Werkle-Bergner M.Understanding the interplay of sleep and aging: Methodological challenges. Psychophysiology 57, e13523 (2020). [DOI] [PubMed] [Google Scholar]
- 114.Cohen MX Analyzing neural time series data: Theory and practice. (The MIT Press, 2014). [Google Scholar]
- 115.McInnes L, Healy J.& Melville J.Umap: Uniform manifold approximation and projection for dimension reduction. arXiv preprint arXiv:1802.03426 (2018). [Google Scholar]
- 116.Campello RJGB, Moulavi D.& Sander J.in Advances in Knowledge Discovery and Data Mining (eds. Pei J, Tseng VS, Cao L, Motoda H.& Xu G) 7819, 160–172 (Springer Berlin; Heidelberg, 2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All PSG data are freely available via the National Sleep Resource (NSRR, http://sleepdata.org). The MESA dataset (DOI: 10.25822/n7hq-c406) URL is https://sleepdata.org/datasets/mesa. The MrOS dataset (DOI: 10.25822/kc27-0425) URL is https://sleepdata.org/datasets/mros, respectively. Full polysomnography and clinical/covariate data are available for all interested parties, pending completion of a Data Access and Use Agreement and IRB, as outlined on the NSRR website.