Abstract
Using two different animal models of Streptococcus pneumoniae infection, we have demonstrated that this organism is able to spread to the central nervous system and cause meningitis by bypassing the bloodstream. Following respiratory tract infection induced via intranasal inoculation, bacteria were rapidly found in the bloodstream and brains in the majority of infected mice. A similar pattern of dissemination occurred following otitis media infection via transbullar injection of gerbils. However, a small percentage of animals infected by either route showed no bacteria in the blood and yet did have significant numbers of bacteria in brain tissue. Subsequent experiments using a galU mutant of S. pneumoniae, which is impaired in its ability to disseminate to the bloodstream following infection, showed that this organism is able to spread to the brain and cerebrospinal fluid. These results demonstrate that, unlike many bacterial pathogens that cause meningitis, S. pneumoniae is able to do so independent of bloodstream involvement upon different routes of infection. This may address the difficulty in treating human infections caused by this organism.
Streptococcus pneumoniae causes a broad range of diseases and remains a formidable adversary in the fight against infectious disease worldwide. One feature of this gram-positive pathogen that makes it so dangerous is its ability to disseminate from localized infections in the middle ear and lungs to cause more serious invasive bacteremia and meningitis infections (1, 15). It is this ability that causes the most concern for clinicians and makes infections with this organism especially difficult yet imperative to treat.
This organism is responsible for causing the majority of cases of bacterial meningitis in U.S. children under 5 years of age (1). Yearly, there are thousands of cases of pneumococcal meningitis in the United States and one million cases worldwide, and the mortality rate varies between 30 and 80%, depending on the age and overall health of the patient (1, 9). In addition, seven million cases of otitis media are caused by this pathogen each year (1). It is widely believed that pneumococcal meningitis is acquired via colonization of the nasopharynx, followed by bacteremia and invasion of the central nervous system (CNS) (10). Even after antibiotic treatment, approximately 30% of patients are left with neurological complications (14).
Several animal models have been developed to study the course and treatment of meningitis. The two most widely used are the infant rat model and the adult rabbit model (7, 14). Neither replicates the human course of the disease, but they have been very valuable research tools nonetheless. The infant rat model initiates infection via either intranasal instillation or intraperitoneal injection, resulting in bacteremia and entry of the bacteria into the CNS (11, 14). Since bacteremia is a requirement of this model, it is difficult to obtain accurate bacterial counts in brain and cerebrospinal fluid (CSF) without contamination with infected blood.
It is believed that the bacteria gain entry into the CNS by breaching the blood-brain barrier (4) or the blood-CSF barrier either by local tissue damage or by transcytosis within infected leukocytes (6, 10, 12, 16). One report indicated that the blood-brain barrier damage is caused by pneumolysin produced by S. pneumoniae (17). The adult rabbit model relies on direct injection of bacteria into the CSF (14). The ease with which multiple CSF samples may be drawn is a major benefit of this model; however, its main drawback is the nonphysiological route of infection. Other models involve intracerebral injection of rats for examining the efficacy of therapies to clear the infection (13).
We report here two relevant, reproducible models for studying pneumococcal meningitis in mice and gerbils. We define meningitis in these models as the dissemination of bacteria from the primary infection site to either the brain or the CSF. Meningitis in these models is initiated via either intranasal or otitis media infection, more directly mimicking this pathogen's natural infection route in humans. In addition, we show that a bacteremic state is not necessary for the pneumococcus to gain access to CNS tissue. Meningitis following otitis media infection in gerbils has been reported previously (2, 8), and one report describes direct infection of the CNS from the middle ear (8). Here we have extended those studies to include an alternate infection route and a mutant S. pneumoniae strain that does not disseminate into the blood.
MATERIALS AND METHODS
Bacterial strains used for infections.
The S. pneumoniae strains used in these studies were the serotype 2 strain D39 and a galU mutant of this strain constructed by replacing the galU gene with a gene encoding spectinomycin resistance. Bacteria were routinely cultivated on tryptic soy agar (TSA) plates supplemented with 5% defibrinated sheep blood (BBL) and 500 μg of spectinomycin (for the galU mutant only) per ml at 37°C and 7.5% CO2. In preparation for an experiment, bacteria were inoculated from frozen stocks onto appropriate plates and incubated for 15 h at 37°C and 7.5% CO2. Bacterial growth was scraped from fresh plates and suspended in phosphate-buffered saline (PBS), and the optical density of a 1:10 dilution was measured at A600. Suspensions were diluted in PBS to the appropriate cell density, as described below.
Murine RTI.
Bacteria for respiratory tract infections (RTI) were prepared as described above so that the A600 of a 1:10 dilution of the cell suspension was ∼0.3, corresponding to approximately 5 × 108 cells/ml. Six-week-old female CD-1 mice (Charles River Laboratories) were anesthetized with isoflurane (3% in O2) and infected with 50 μl of this suspension via intranasal instillation (approximately 107 bacteria/mouse). Generally, 5 to 10 mice were infected per group. Mice were allowed to recover from anaesthesia and given food and water ad libitum. At 48 h postinfection, mice were sacrificed, and blood was recovered via cardiac puncture; in addition, lungs and brains were harvested aseptically and homogenized in 1 ml of PBS using a stomacher (Labconco). Recovered tissues were serially diluted in PBS and plated on TSA–5% sheep blood plates for bacterial enumeration. To assess contamination of tissues with in situ blood, in some experiments, animals were perfused with PBS following cardiac puncture but prior to lung and brain harvest. All procedures were performed in accordance with Protein Design Labs, Institutional Animal Care and Use Committee Guidelines.
Otitis media infection of Mongolian gerbils.
Bacteria were prepared as described above and diluted to ∼5 × 105 bacteria/ml. Male Mongolian gerbils (Harlan), weighing 30 to 35 g, were anesthetized with isoflurane (4% in O2) and infected bilaterally via transbulla injection of 30 μl of bacterial suspension (approximately 104 organisms/bulla). Groups typically contained 3 to 10 gerbils, depending on the length of the experiment. Animals were allowed to recover from anesthesia and given food and water ad libitum. At designated time points, gerbils were sacrificed, and blood was collected via cardiac puncture into heparinized vials. Middle ear exudates were recovered by flushing each middle ear cavity with 100 μl of PBS through the tympanic membrane. In addition, CSF was collected using a 29-gause needle inserted directly into the cisterna magna. Samples of blood, exudates, and CSF were serially diluted in PBS, and dilutions were plated on TSA–5% sheep blood plates to determine bacterial numbers. Brain samples were harvested by aseptic techniques and homogenized in 1 ml of PBS in a stomacher, and dilutions were plated as above. All procedures were performed in accordance with Protein Design Labs' Institutional Animal Care and Use Committee Guidelines.
RESULTS
RTI leads to brain infection in mice.
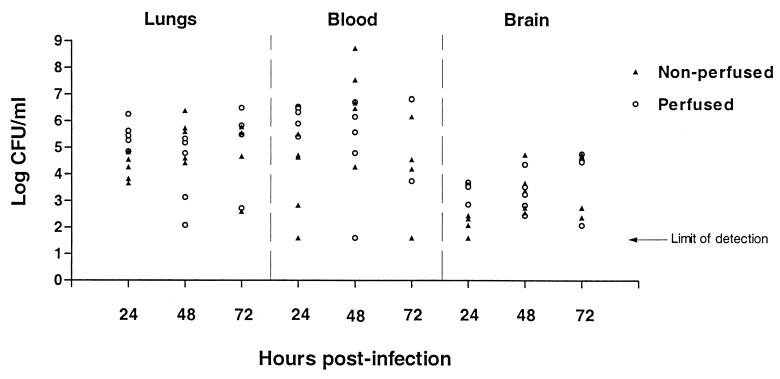
It had been observed that the D39 strain of S. pneumoniae was able to disseminate to the blood fairly rapidly after i.n. infection of mice. In such experiments it was not unusual to find upwards of 106 bacteria/ml of blood at 24 h postinfection. In addition, very few mice infected via this route remained alive at 72 h. We hypothesized that the high levels of bacteremia following intranasal inoculation were causing possible meningitis and death. Mice were inoculated with 107 bacteria intranasally, and bacterial numbers in the blood, lungs, and brains were determined over time. In order to avoid contamination of brain tissue, one set of mice was perfused with PBS prior to harvesting lungs and brains.
At 24 h postinfection, in both perfused and nonperfused mice, bacteria were found in all three sites, lungs, blood, and brain (Fig. 1). All but three mice had bacteria in the blood (one at each time point), in some cases as high as 108/ml. The large variability between mice is typical of infection with this organism and probably reflects the competition between host clearance and bacterial seeding from the lungs. Overall, the log mean number of bacteria found in the lungs over the 3 days of the experiment remained fairly constant at ∼5.0, whereas the mean number in the brain increased steadily over time (Table 1). The increase in brain counts was observed in both perfused and nonperfused samples, indicating that the increase that we are seeing is not due simply to contamination of the tissue with infected blood. In fact, in two mice, bacteria were found in the brain tissue without concomitant bacterial infection of the blood. This led us to examine whether smaller inocula could still cause brain infection while resulting in decreased rates of bacteremia and increased survival rates over 3 days.
FIG. 1.
Time course of pneumococcal infection following RTI in mice. Mice were infected with 107 bacteria via intranasal instillation. At 24, 48, and 72 h following infection, animals were sacrificed and tissues were sampled for bacterial titration. Half of the animals were perfused with PBS prior to tissue harvest, and the remainder were not. Solid triangles, nonperfused samples; open circles, perfused samples. The limit of detection is 40 bacteria/ml. This experiment was performed twice, and the data shown are from a single representative experiment.
TABLE 1.
| Mice | Mean log no. of bacteria/ml ± SD
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lungs
|
Blood
|
Brain
|
|||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| All | 4.86 ± 0.26 | 4.72 ± 0.40 | 4.89 ± 0.52 | 4.99 ± 0.52 | 5.85 ± 0.62 | 5.09 ± 0.67 | 2.74 ± 0.27 | 3.35 ± 0.24 | 3.80 ± 0.42 |
| Nonperfused | 4.23 ± 0.22 | 5.35 ± 0.37 | 4.65 ± 0.72 | 3.86 ± 0.72 | 6.74 ± 0.73 | 4.13 ± 0.94 | 2.01 ± 0.18 | 3.42 ± 0.39 | 3.64 ± 0.63 |
| Perfused | 5.48 ± 0.23 | 4.10 ± 0.64 | 5.13 ± 0.83 | 6.13 ± 0.21 | 4.96 ± 0.89 | 6.05 ± 0.77 | 3.47 ± 0.16 | 3.28 ± 0.33 | 3.96 ± 0.63 |
Means were calculated from the total number of infected mice or from only nonperfused mice or perfused mice.
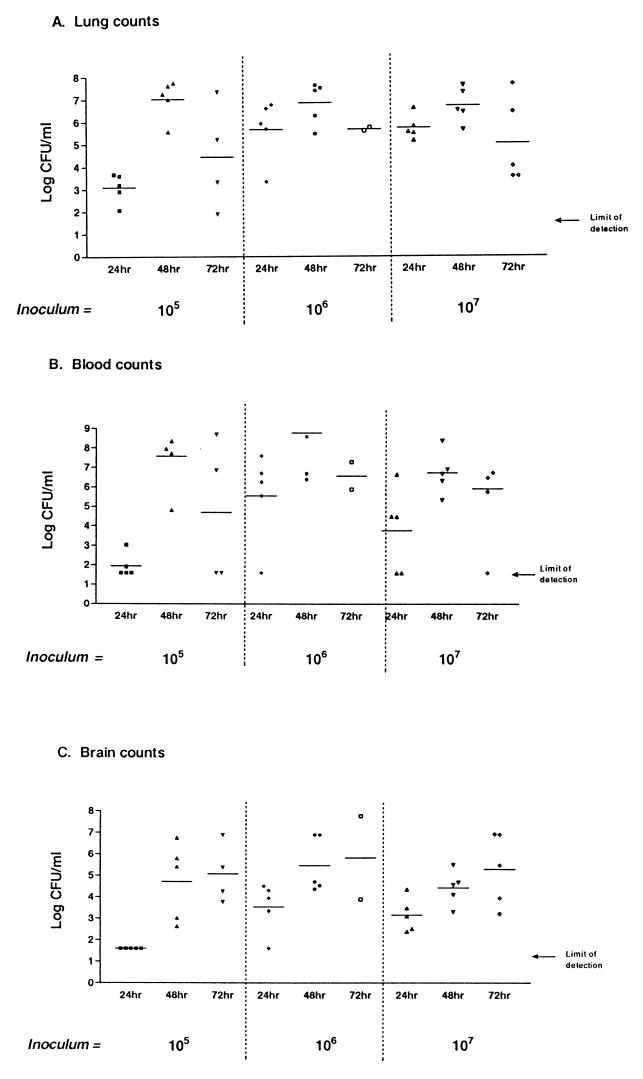
Figure 2 shows the results of using different inoculum sizes to induce RTI in mice, monitoring bacterial counts in lungs, blood, and brain over 3 days. Groups of mice were infected intranasally with either 105, 106, or 107 bacteria, and bacterial counts recovered from lungs at the three doses are shown in Fig. 2A. At all three doses, the number of recovered bacteria increased from 24 to 48 h and decreased slightly at 72 h. Similar results were seen with blood counts over the same time period (Fig. 2B). However, brain counts increased at each time point for all three doses (Fig. 2C); again, it was possible to recover bacteria from a number of mouse brains in the absence of blood infection. These results indicate that it is possible for S. pneumoniae to infect the brains of mice following RTI in the absence of bacteremia.
FIG. 2.
Time course of pneumococcal RTI with different inocula. Mice were infected with either 105, 106, or 107 organisms via intranasal instillation. At 24, 48, and 72 h postinfection, mice were sacrificed and tissues were sampled for bacterial titration. The limit of detection is 40 bacteria/ml. (A) Lung counts; (B) blood counts; (C) brain counts.
Otitis media leads to brain and CSF infection in gerbils.
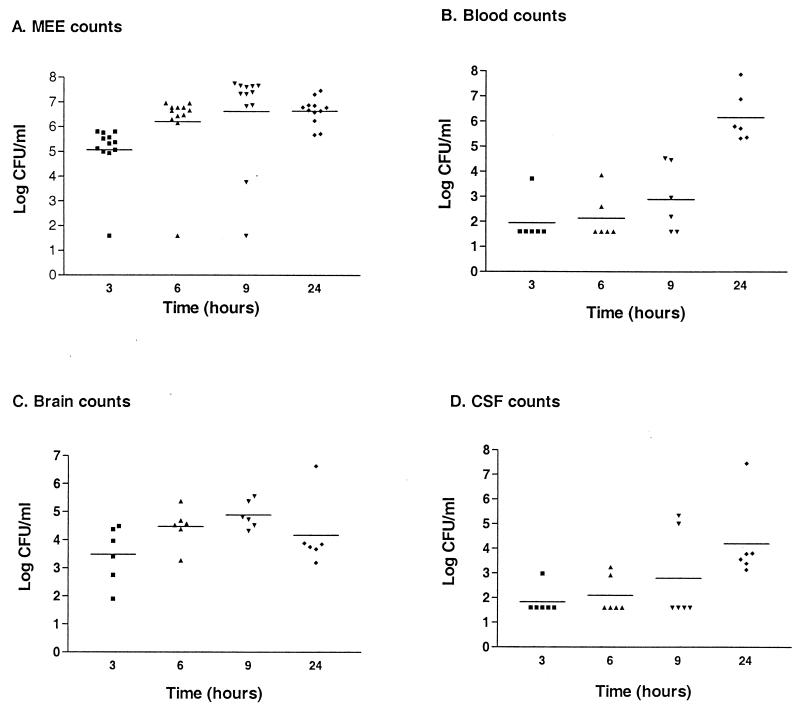
The gerbils' larger size compared to mice allowed us to cleanly recover CSF from infected animals as a further measure of bacterial entry into the CNS. As is true following murine RTI, S. pneumoniae D39 is able to disseminate into the bloodstream following otitis media infection in gerbils. Relatively small numbers of bacteria are used to initiate otitis media in gerbils, and bacteria can be found in the blood as early as 6 h postinfection (Fig. 3B). When CSF and brains are harvested at different times postinfection, bacteria are recovered by 6 and 3 h, respectively, and for the most part, bacterial numbers increase over time (Fig. 3C and D). It is not until 24 h postinfection, however, that we see bacteria in the blood of all infected gerbils (Fig. 3B).
FIG. 3.
Short time course of pneumococcal otitis media infection in gerbils. Gerbils were infected via direct intrabullar injection, and groups were sacrificed at 3, 6, 9, and 24 h for tissue sampling and bacterial enumeration. The limit of detection is 40 bacteria/ml. This experiment was performed twice, and data were combined to produce this graph. (A) Bacterial counts recovered from middle ear exudates (MEE); (B) blood counts; (C) brain counts; (D) counts recovered from CSF.
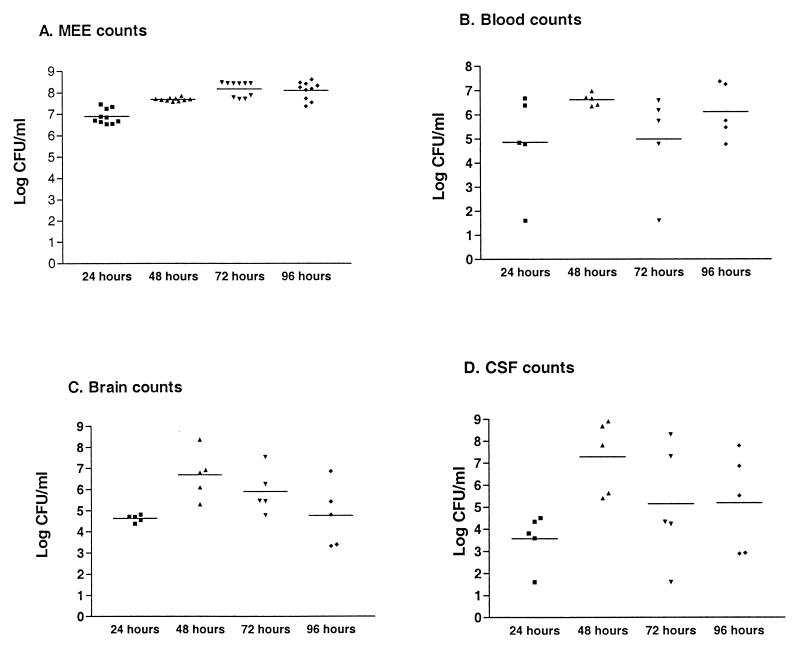
When bacterial numbers in various sites were monitored over a longer time period, the following observations were made (Fig. 4): bacterial counts in middle ear exudates remained fairly constant over time, reaching ∼108/ml of fluid by 48 h and staying at that level for up to 4 days (Fig. 4A); bacterial counts in the brain peaked at 48 h but then decreased so that numbers at 96 h were similar to those seen at 24 h postinfection (Fig. 4C); and bacterial counts in the CSF paralleled counts in the blood, increasing between 24 and 48 h but then dropping at 72 and 96 h (Fig. 4B and D). Again, the correlation can be made that in the absence of blood infection, no bacteria can be found in the CSF. In this experiment we again saw a small number of infected gerbils with undetectable numbers of bacteria in the blood (Fig. 4B, 24 and 72 h) that nonetheless had significant numbers of bacteria in the brain.
FIG. 4.
Long time course of pneumococcal otitis media infection in gerbils. Gerbils were infected via direct intrabullar injection, and groups were sacrificed at 24, 48, 72, and 96 h postinfection. Tissues were recovered for bacterial enumeration, and the limit of detection was 40 bacteria/ml. This experiment was performed twice, and data were combined to produce this graph. (A) Bacterial counts recovered from middle ear exudates (MEE); (B) blood counts; (C) brain counts; (D) counts recovered from CSF.
A galU mutant of S. pneumoniae is defective in dissemination to the bloodstream yet can still gain entry into CNS.
Previous experiments have shown that a galU replacement mutant of S. pneumoniae shows very strong attenuation in the murine RTI model, colonizing the lungs at levels about 10,000-fold lower than the parental strain D39 (unpublished observations). During the course of these experiments, we never observed the galU mutant in the bloodstream in this model, either because it is unable to disseminate, it is very rapidly cleared, or it disseminates at levels below the limit of detection.
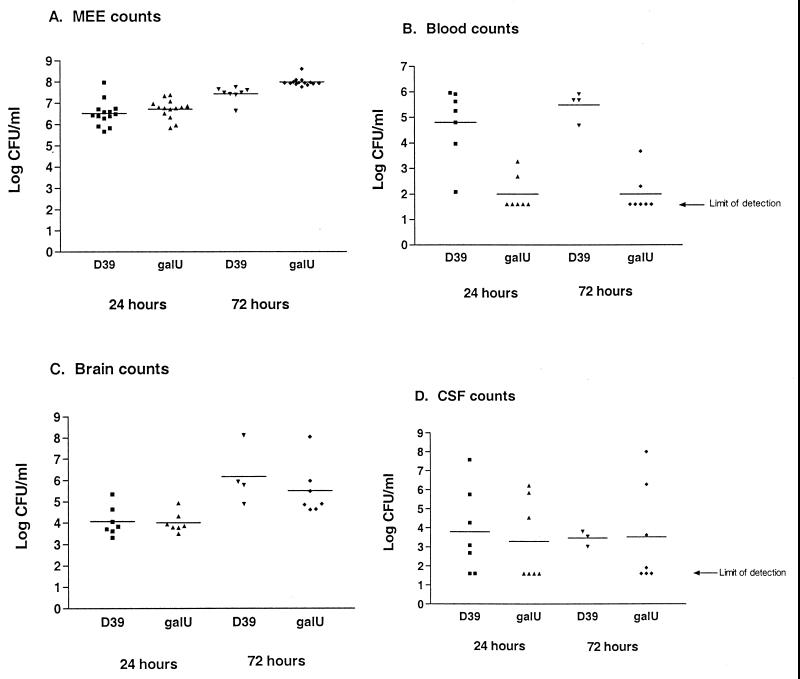
When the galU mutant was used to infect gerbils by an intrabulla injection, it proliferated as well as the parent strain D39 in the middle ear cavity (Fig. 5A), at both 24 and 72 h postinfection. However, the galU mutant showed a dramatic defect in dissemination into the bloodstream at both time points following infection. The parent strain D39 was found in the blood at 105 to 106/ml at both 24 and 72 h (Fig. 5B). Despite its decreased dissemination to the blood, the galU mutant was found in the brains and CSF of infected gerbils at levels comparable to those of D39. Especially noteworthy is the finding that at both 24 and 72 h postinfection, only two of six gerbils infected with the galU strain had bacteria in the blood, and yet all six had bacteria in the brain. In contrast, all D39-infected gerbils have bacteria in both brain and blood at both time points. The galU mutant was found in the CSF at nearly wild-type levels, reaching high cell densities in some animals at 72 h. Together these results suggest that the pathway for pneumococcal invasion of the CNS can be independent of bacteremia.
FIG. 5.
Comparison of D39 and galU mutant in otitis media infection in gerbils. Gerbils were infected via direct intrabullar injection with either D39 or the galU mutant. At 24 and 72 h postinfection, groups of gerbils were sacrificed, and tissues were recovered for bacterial quantitation. The limit of detection was 40 bacteria/ml. This experiment was performed twice, and the data shown are from a single representative experiment. (A) Bacterial counts recovered from middle ear exudates (MEE); (B) blood counts; (C) brain counts; (D) counts recovered from CSF.
DISCUSSION
Traditionally, animal models for studying bacterial meningitis have suffered from the need to establish infection via a nonphysiological, nonrelevant route, intraperitoneal, intracerebral, or intra-CSF injection (11, 13, 14). Only sporadic reports in the literature have described more relevant approaches to examining this disease, either by intranasal or otitis media infection (2, 8). Whereas these methods more closely reproduce the course of infection in humans, they have not been pursued as rigorously or with adequate numbers of animals to justify their use in the study and treatment of meningitis. Our observations and analyses of CNS infection by the pneumococcus following either intranasal or otitis media infection aim to establish these as relevant animal models for meningitis infection.
Initially we sought to determine the cause of death of mice following intranasal infection. Rapid spread of bacteria into the bloodstream was found to result in systemic infection, with bacteria seen in liver, kidney, spleen, lungs, brain, and blood (data not shown). A threshold number of D39 bacteria in the lungs (∼104) seems to be required for dissemination to the blood (personal observation), irrespective of the size of the inoculating dose. Given these observations, it was of interest to determine whether the bacteria were spreading from the lungs to the blood and then to the CNS, resulting in meningitis infection.
Our results suggest that, in the RTI model, there is no correlation between bacterial load in the lungs and the presence of organisms in the brain, as noted above for blood dissemination. The results with the RTI model indicate that S. pneumoniae can disseminate from the lungs directly to the brain, at least in the rare instances when we observed brain infection with undetectable blood counts.
In order to reduce the likelihood that the observed brain counts were due to blood contamination of the samples, we perfused one set of mice with PBS and saw similar bacterial numbers in the brains and lungs with and without perfusion. It was expected that nonperfused animals would have larger bacterial loads in the lungs and brains than perfused animals, since the blood in these mice carries large numbers of bacteria. That perfused mice had similar bacterial counts in the lungs and brains compared with nonperfused mice added further evidence that meningitis was occurring following RTI without a bloodstream intermediate.
We next examined CNS dissemination in the otitis media model in order to determine whether the CNS involvement we were seeing was unique to the RTI model and if otitis media can also lead to meningitis in the same manner. Previous reports have demonstrated that meningitis often follows otitis media infection in gerbils (2, 8). It is clear from our short time course study that bacteremia is not necessary for brain infection. The course of CSF infection in this model roughly parallels that of blood infection; indeed, it appears that in the otitis media model there is constant seeding of the CSF with bacteria from the blood, and gerbils that did not have bacteria in the blood also did not have bacteria in the CSF.
We next infected gerbils with a galU mutant of S. pneumoniae and compared its growth and spread with those of its wild-type parent strain D39. This mutant has been shown to be attenuated for lung colonization in the RTI model and, more importantly, is unable to infect the blood following intranasal instillation. It is also true that whereas this mutant multiplies to high numbers in the middle ear cavity in an otitis media infection, it has diminished capacity to disseminate to the blood. It is not known whether the inability to recover galU organisms from the blood is due to a defect in this mutant's ability to disseminate to or to survive in the blood. Experiments are currently in progress to address this issue. In addition, it is not clear from our data whether these organisms are replicating in the CNS sites or are being continuously seeded from the primary infection sites. In any event, the data obtained with the galU strain lend further evidence that S. pneumoniae is able to infect the CNS without reaching detectable levels in the bloodstream.
In both the RTI and the otitis media models, we have observed increased variability in recovered bacterial numbers in several sites at later time points. We suspect that this is due to varied rates of clearance of bacteria concomitant with bacterial multiplication and seeding of organisms from the primary infection sites. In the case of wild-type-infected animals, there are often deaths due to the infection, so that the number of animals sampled decreases, perhaps indicating that the bacterial load in the surviving animals is lower. Since we have observed this phenomenon in more than one model involving different animals and infection routes, it is unlikely that CNS infection is due to accidental inoculation during otitis media injections.
The data presented indicate that in mice following RTI and in gerbils following otitis media infection, S. pneumoniae is able to enter the CNS, usually within 24 h postinfection. The spread to the CNS can occur in the absence of blood infection, and our results suggest that there is a direct route from the lungs (or nasopharyngeal area) to the brain in mice and the middle ear cavity to the brain in gerbils. There has been at least one report of this course of pneumococcal infection in the literature (8). An alternative route in gerbils appears to be from the blood directly to the CSF. However, it appears that most bacteria that cause meningitis do so via breaching the blood-brain barrier or the blood-CSF barrier (3, 5–7). It is not known whether the phenomenon that we report here occurs in humans or if it is unique to these animal models. However, it is interesting to speculate that this course of infection may occur in humans and, therefore, that inhibition of bacterial replication at primary infection sites is of utmost importance very early in infection to prevent constant seeding of the CNS with bacteria.
ACKNOWLEDGMENTS
We thank Magda Bartilson for generating and providing us with the galU mutant and Stacey Lawson, Roman Moniz, and James Gamez for technical assistance.
REFERENCES
- 1.Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Morb Morta Wkly Rep. 2000;49:1–8. [PubMed] [Google Scholar]
- 2.Barry B, Muffat-Joly M, Gehanno P, Pocidalo J-J. Pathogenicity and meningeal spread of Streptococcus pneumoniae serotypes 19 and 23 with varying susceptibility to penicillin in experimental acute otitis media. In: Lim D J, Bluestone C, Casselbrant M, Klein J, Ogra P, editors. Recent advances in otitis media: proceedings of the sixth international symposium. B.C. Hamilton, Ontario, Canada: Decker; 1986. pp. 520–521. [Google Scholar]
- 3.Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–336. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury M W. The structure and function of the blood-brain barrier. Fed Proc. 1984;43:186–190. [PubMed] [Google Scholar]
- 5.Drevets D A, Jelinek T A, Freitag N E. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect Immun. 2001;69:1344–1350. doi: 10.1128/IAI.69.3.1344-1350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drevets D A, Leenen P J M. Leukocyte-facilitated entry of intracellular pathogens into the central nervous system. Microbes Infect. 2000;2:1609–1618. doi: 10.1016/s1286-4579(00)01317-4. [DOI] [PubMed] [Google Scholar]
- 7.Moxon E R, Smith A L, Averill D R, Smith D H. Haemophilus influenzae meningitis in infant rats after intranasal inoculation. J Infect Dis. 1974;129:154–162. doi: 10.1093/infdis/129.2.154. [DOI] [PubMed] [Google Scholar]
- 8.Muffat-Joly M, Barry B, Henin D, Fay M, Gehanno P, Pocidalo J-J. Otogenic meningoencephalitis induced by Streptococcus pneumoniae in gerbils. Arch Otolaryngol Head Neck Surg. 1994;120:925–930. doi: 10.1001/archotol.1994.01880330015004. [DOI] [PubMed] [Google Scholar]
- 9.Quagliarello V, Scheld W M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 10.Ring A, Weiser J N, Tuomanen E I. Pneumococcal trafficking across the blood-brain barrier. J Clin Investig. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez A F, Kaplan S L, Hawkins E P, Mason E O. Hematogenous pneumococcal meningitis in the infant rat: description of a model. J Infect Dis. 1991;164:1207–1209. doi: 10.1093/infdis/164.6.1207. [DOI] [PubMed] [Google Scholar]
- 12.Saez-Llorens X, Ramilo O, Mustafa M M, Mertsola J, McCracken G H. Molecular pathophysiology of bacterial meningitis: current concepts and therapeutic implications. J Pediatr. 1990;116:671–684. doi: 10.1016/s0022-3476(05)82647-2. [DOI] [PubMed] [Google Scholar]
- 13.Strake J G, Mitten M J, Ewing P J, Alder J D. Model of Streptococcus pneumoniae meningitis in adult rats. Lab Anim Sci. 1996;46:524–529. [PubMed] [Google Scholar]
- 14.Tauber M G, Zwahlen A. Animal models for meningitis. Methods Enzymol. 1994;235:93–106. doi: 10.1016/0076-6879(94)35134-1. [DOI] [PubMed] [Google Scholar]
- 15.Watson D A, Musher D M, Verhoef J. Pneumococcal virulence factors and host immune responses to them. Eur J Clin Microbiol Infect Dis. 1995;14:479–490. doi: 10.1007/BF02113425. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J-R, Tuomanen E. Molecular and cellular mechanisms for microbial entry into the CNS. J Neuro virol. 1999;5:591–603. doi: 10.3109/13550289909021288. [DOI] [PubMed] [Google Scholar]
- 17.Zysk G, Schneider-Wald B K, Hwang J H, Bejo L, Kim K S, Mitchell T J, Hakenbeck R, Heinz H-P. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect Immun. 2001;69:845–852. doi: 10.1128/IAI.69.2.845-852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]